Abstract
Background
Patients with suspected colorectal cancer (CRC) usually undergo colonoscopy. Flexible sigmoidoscopy (FS) may be preferred if proximal cancer risk is low. We investigated which patients could undergo FS alone.
Methods
Cohort study of 7375 patients (≥55 years) referred with suspected CRC to 21 English hospitals (2004–2007), followed using hospital records and cancer registries. We calculated yields and number of needed whole-colon examinations (NNE) to diagnose one cancer by symptoms/signs and subsite. We considered narrow (haemoglobin <11 g/dL men; <10 g/dL women) and broad (<13 g/dL men; <12 g/dL women) anaemia definitions and iron-deficiency anaemia (IDA).
Results
One hundred and twenty-seven proximal and 429 distal CRCs were diagnosed. A broad anaemia definition identified 80% of proximal cancers; a narrow definition with IDA identified 39%. In patients with broad definition anaemia and/or abdominal mass, proximal cancer yield and NNE were 4.8% (97/2022) and 21. In patients without broad definition anaemia and/or abdominal mass, with rectal bleeding or increased stool frequency (41% of cohort), proximal cancer yield and NNE were 0.4% (13/3031) and 234.
Conclusion
Most proximal cancers are accompanied by broad definition anaemia. In patients without broad definition anaemia and/or abdominal mass, with rectal bleeding or increased stool frequency, proximal cancer is rare and FS should suffice.
Subject terms: Colorectal cancer, Physical examination, Digestive signs and symptoms, Anaemia, Endoscopy
Introduction
Patients referred to hospital with suspected colorectal cancer (CRC) typically undergo whole-colon investigation (WCI), predominantly colonoscopy or computed tomography (CT) colonography, in line with National Institute for Health and Care Excellence (NICE) guidelines.1 In 2015, NICE issued a guideline on referral criteria, including symptoms and signs conferring a positive predictive value for cancer of 3%.2 Consequently, large numbers of patients are undergoing WCI for suspected CRC, placing pressure on endoscopy and radiology services and incurring substantial costs to the National Health Service (NHS).3,4 Reducing the burden of symptomatic referrals on diagnostic services is recognised as a priority area for research.5
Flexible sigmoidoscopy is quicker, safer, less complicated, and cheaper than colonoscopy. Intravenous sedation is usually not needed and enemas used for preparation are associated with fewer side effects and greater acceptability than oral preparations used for WCI.6 Flexible sigmoidoscopy can be performed competently by non-physician endoscopists7 and has high sensitivity for CRCs in the distal colon and rectum8–10; however, it can only reach the splenic flexure at best and so abnormalities in the proximal colon are only found if distal findings precipitate subsequent WCI.
Previous research has demonstrated that presenting symptoms/signs are associated with CRC location.8–14 In the largest previous study, Thompson et al. defined a patient subgroup at low risk of proximal cancer, for whom they deemed examination by flexible sigmoidoscopy alone appropriate.8 However, subsequent studies reached variable conclusions regarding sole use of flexible sigmoidoscopy in any patient subgroup,9–14 and the current NICE guidelines only recommend it for patients with major comorbidity, in association with barium enema.1 The aim of the present SOCCER (Symptoms of Colorectal Cancer Evaluation Research) study15 was to further investigate whether presenting symptoms/signs could be used to identify patients at low risk of proximal cancer, for whom flexible sigmoidoscopy would suffice. This would help alleviate the burden of WCI on patients and endoscopy and radiology services.
Methods
Study design and participants
We included patients who had been referred to 1 of the 21 English NHS hospitals from 2004 to 2007 for investigation of symptoms/signs suggestive of CRC. Of these hospitals, 8 were general and 13 were teaching hospitals, and they varied from <40 to >1000 beds in size.
We retrospectively identified patients from those who were assessed for eligibility for the SIGGAR (Special Interest Group in Gastrointestinal and Abdominal Radiology) trials. The SIGGAR trials were two parallel randomised controlled trials assessing the clinical and cost-effectiveness of CT colonography vs. barium enema and colonoscopy for CRC diagnosis.16,17 To be eligible for the SIGGAR trials, patients had to be ≥55 years and judged to be in need of, and fit enough for, a WCI with full bowel preparation. Patients were ineligible if they were in follow-up for CRC, had undergone WCI within the previous 6 months, had a known diagnosis of familial adenomatous polyposis or Lynch syndrome, or had previously been diagnosed with inflammatory bowel disease (IBD).
Research nurses undertook the eligibility assessment, checking endoscopy and radiology databases, and patient records and notes. Only patients who were deemed eligible, gave informed consent, and had a consultant consent to their participation were randomised in the SIGGAR trials. Reasons for patient- and consultant-declined consent included patients wanting to have or avoid a specific type of WCI, consultants requesting a specific procedure, and prior cancer diagnoses.
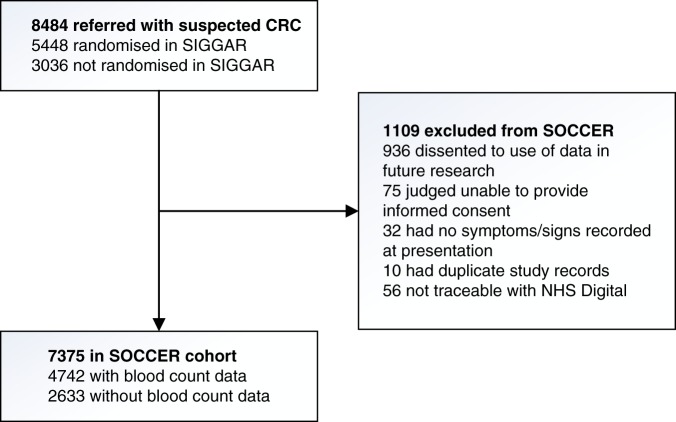
All patients who met the SIGGAR trial eligibility criteria (regardless of whether they proceeded to randomisation) were eligible for the SOCCER study, unless they were judged incapable of giving informed consent, had dissented to use of their data for research, had no symptoms/signs documented at presentation, had duplicate study records, or were untraceable through NHS Digital (Fig. 1).
Fig. 1. Flow chart of study participants CRC.
colorectal cancer, SIGGAR Special Interest Group in Gastrointestinal and Abdominal Radiology, SOCCER Symptoms of Colorectal Cancer Evaluation Research. The SOCCER study included patients who had been referred to hospital with suspected CRC and assessed for eligibility for the SIGGAR trials. All patients who met the SIGGAR trials eligibility criteria (regardless of whether they proceeded to randomisation) were eligible for the SOCCER study, unless they had dissented to use of their data in future research, were judged unable by a clinician to provide informed consent for the use of their data in future research, had no symptoms/signs recorded at presentation, had duplicate study records, or were not traceable with NHS Digital. Among those included in the SOCCER study, data on haemoglobin and mean red cell corpuscular volume were available for 4742 of the 7375 patients
Data collection and management
Research nurses or colorectal administrative assistants examined patient records and notes, including referral letters, and recorded demographic and clinical details on bespoke SIGGAR trial pro-formas, including age, sex, general practitioner (GP)-reported symptoms, route and urgency of referral, and planned diagnostic investigations. There were tick boxes for ‘abdominal pain’, ‘anaemia’, ‘change in bowel habit (CIBH)’, ‘positive FOBT’ (faecal occult blood test), ‘rectal bleeding’, and ‘weight loss’. Free text fields were included to record additional symptoms/signs that were coded and classified, and characteristics of reported CIBHs, classified as ‘more frequent’, ‘less frequent’, ‘variable’, or ‘unspecified’. Blood count data, additional clinical features, diagnostic investigations performed, and diagnoses during the hospital episode were ascertained through hospital record review.
For patients with blood count data, anaemia status was determined according to the results of blood tests performed within 6 months before and up to 3 months after referral. We examined the prevalence of anaemia and iron-deficiency anaemia (IDA) using two anaemia definitions: haemoglobin (Hb) <13 g/dL in men and <12 g/dL in women (broad definition anaemia), used by the World Health Organisation; and Hb < 11 g/dL in men and <10 g/dL in women (narrow definition anaemia), as in the 2005 NICE referral guidelines.18,19 IDA was classified on the basis of microcytosis (mean red cell corpuscular volume [MCV] <80 fL) or serum ferritin <20 μg/L. For patients without blood count data, we defined anaemia based on whether the investigation of anaemia was indicated as a reason for referral on the pro-forma.
CRC diagnoses occurring within 3 years of referral were obtained from cancer registries, via NHS Digital, and hospital records. CRC sites were defined by International Classification of Diseases, tenth revision codes: proximal cancer included C18.0–C18.5 (caecum to splenic flexure) and distal cancer included C18.6, C18.7, C19, C20, and C21 (descending colon to anus). CRC morphologies were defined by International Classification of Diseases for Oncology, second edition codes; we included codes related to invasive and in situ carcinomas (8000/3, 8010/3, 8070/3, 8123/3, 8140/2, 8140/3, 8144/3, 8210/3, 8261/2, 8261/3, 8263/2, 8263/3, 8480/3, 8481/3, 8490/3, 8510/3, and 8560/3).
Statistical analysis
The primary outcome was the yield of proximal vs. distal cancer within 3 years of referral by presenting symptom/sign. Yields were calculated as the number of patients with proximal or distal cancer divided by the number of patients with a particular symptom/sign, presented as percentages. Secondary outcomes included the number of needed whole-colon examinations (NNE) to detect one proximal vs. distal cancer by presenting symptom/sign, calculated by inverting the yield and presented with binomial exact 95% confidence intervals (CIs). For this calculation, we made the assumption that WCI has perfect sensitivity for detecting proximal and distal cancers.
We calculated the sensitivity of symptoms/signs for proximal and distal cancer and the proportion of patients with CRC who had proximal vs. distal cancer by presenting symptom/sign. We also calculated the proportion of CRCs that would have been missed if certain symptoms/signs were investigated by flexible sigmoidoscopy alone, with binomial exact 95% CIs, assuming that flexible sigmoidoscopy would have detected all distal cancers but not proximal cancers.
We estimated that, with a sample size of 8,484 patients, giving rise to an estimated 68 proximal and 421 distal cancers, we could estimate with sufficient precision the proportion of CRCs that would have been missed if certain symptoms/signs were investigated by flexible sigmoidoscopy alone. Data analyses were conducted using Stata/IC 13.1 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
A total of 8484 patients referred to hospital with suspected CRC were assessed for eligibility for the SIGGAR trials. Of these, 5448 were randomised to the SIGGAR trials and 3036 were not (Fig. 1). Comparing randomised and non-randomised patients, the former were younger and less likely to have been referred from a colorectal surgical outpatient clinic and via the urgent pathway (data not shown).
Of the total 8484 patients, all of whom were deemed eligible for the SOCCER study, 1109 were subsequently excluded, primarily due to patient dissent to use of their data for research. This left 7375 patients for our cohort analysis (Fig. 1). The median age of included patients was 69 years (interquartile range: 62–76) and 59.0% were women. The majority of patients were referred from a colorectal surgical outpatient clinic (84.5%) and via the urgent pathway (71.7%) (Table 1), and 1483 (20.1%) had undergone flexible sigmoidoscopy at the time of referral (data not shown).
Table 1.
Patient characteristics (N = 7375)
| Characteristic | N | % |
|---|---|---|
| Sex | ||
| Men | 3023 | 41.0 |
| Women | 4352 | 59.0 |
| Age (years) | ||
| 55–64 | 2407 | 32.6 |
| 65–74 | 2739 | 37.1 |
| 75–84 | 1896 | 25.7 |
| ≥85 | 333 | 4.5 |
| Route of referral | ||
| Colorectal surgical outpatient clinic | 6231 | 84.5 |
| Other outpatient clinic | 688 | 9.3 |
| Straight to test | 396 | 5.4 |
| Hospital admission | 33 | 0.4 |
| Not recorded | 27 | 0.4 |
| Urgency of referral | ||
| Urgent | 5290 | 71.7 |
| Soon | 660 | 9.0 |
| Routine | 914 | 12.4 |
| Not recorded | 511 | 6.9 |
| Availability of blood count dataa | ||
| Hb and MCV | 4742 | 64.3 |
| Serum ferritin | 1157 | 15.7 |
| None available | 2633 | 35.7 |
aAll patients with serum ferritin had a haemoglobin (Hb) and mean red cell corpuscular volume (MCV) count
In total, 556 CRCs were diagnosed in 551 of the 7375 patients (7.5%) within 3 years following referral (Table 2). Of the 551 patients with CRC, 522 (94.7%) were diagnosed within 6 months post-referral (data not shown). There were 127 proximal and 429 distal cancers (5 patients had synchronous proximal and distal cancer), giving diagnostic yields of 1.7% for proximal cancer and 5.8% for distal cancer (Table 2). Detailed subsite information is presented in Supplementary Table 1.
Table 2.
Yield of distal and proximal cancer overall and according to anaemia status (N = 7375)
| Group | Anaemia status | All referred patients | Distal cancera | Proximal cancera | ||||
|---|---|---|---|---|---|---|---|---|
| n | % of group | Patients with distal cancer, n | Diagnostic yield, % | Patients with proximal cancer, n | Diagnostic yield, % | Proportion of all proximal cancers in the group, % | ||
| All patients | 7375 | 100 | 429 | 5.8 | 127 | 1.7 | 100 | |
| Patients with blood count data available | Any | 4742 | 100 | 240 | 5.1 | 97 | 2.0 | 100 |
| Anaemia: narrow definition | ||||||||
| Hb < 11 g/dL in men and <10 g/dL in women | ||||||||
| Total | 672 | 14.2 | 38 | 5.7 | 51 | 7.6 | 52.6 (51/97) | |
| With IDAb | 363 | 7.7 | 22 | 6.1 | 38 | 10.5 | 39.2 (38/97) | |
| Without IDA | 309 | 6.5 | 16 | 5.2 | 13 | 4.2 | 13.4 (13/97) | |
| Anaemia: broad definition (WHO) | ||||||||
| Hb < 13 g/dL in men and <12 g/dL in women | ||||||||
| Total | 1660 | 35.0 | 106 | 6.4 | 78 | 4.7 | 80.4 (78/97) | |
| With IDAb | 567 | 12.0 | 36 | 6.3 | 48 | 8.5 | 49.5 (48/97) | |
| Without IDA | 1093 | 23.0 | 70 | 6.4 | 30 | 2.7 | 30.9 (30/97) | |
| No anaemia | ||||||||
| Hb ≥ 13 g/dL in men and ≥12 g/dL in women | ||||||||
| Total | 3082 | 65.0 | 134 | 4.3 | 19 | 0.6 | 19.6 (19/97) | |
| With IDAb | 66 | 1.4 | 2 | 3.0 | 2 | 3.0 | 2.1 (2/97) | |
| Without IDA | 3016 | 63.6 | 132 | 4.4 | 17 | 0.6 | 17.5 (17/97) | |
| Patients with no blood count data available | Any | 2633 | 100 | 189 | 7.2 | 30 | 1.1 | 100 |
| Anaemia indicated as a reason for referral | 229 | 8.7 | 19 | 8.3 | 9 | 3.9 | 30.0 (9/30) | |
| Anaemia not indicated as a reason for referral | 2404 | 91.3 | 170 | 7.1 | 21 | 0.9 | 70.0 (21/30) | |
| All patients (with and without blood count data) | Anaemia (by definition used in paperc) | 1889 | 25.6 | 125 | 6.6 | 87 | 4.6 | 68.5 (87/127) |
| No anaemiac | 5486 | 74.4 | 304 | 5.5 | 40 | 0.7 | 31.5 (40/127) | |
aFive patients had both a distal and proximal cancer diagnosed. These patients are included in both the distal and proximal cancer columns
bIron-deficiency anaemia (IDA) was classified on the basis of microcytosis [mean red cell corpuscular volume (MCV) <80 fL] or serum ferritin <20 μg/L. We did not have serum ferritin measurements for all patients with haemoglobin (Hb) counts
cIn patients with blood count data, anaemia was defined by the WHO (broad) definition (Hb level <13 g/dL in men or <12 g/dL in women). In patients without blood count data, anaemia was defined by whether the investigation of anaemia was indicated as a reason for referral
Blood count data were not available for all patients. Data on Hb and MCV were available for 4742 of 7375 patients (64.3%). There were 1157 patients (15.7%) with serum ferritin in addition to Hb and MCV counts (Table 1). Comparing the 4742 patients with Hb and MCV data to the 2633 patients without, those with data available were older and less likely to have been referred from a colorectal outpatient clinic (Supplementary Table 2).
Among the 4742 patients with blood count data, narrow definition anaemia was present in 672 (14.2%) and narrow definition anaemia with IDA was present in 363 (7.7%). Broad definition anaemia was present in 1660 (35.0%) and broad definition anaemia with IDA in 567 (12.0%) (Table 2). The prevalence of anaemia increased with age among men and women. Broad definition anaemia was present in 23.8% (131/551) of men and in 18.6% (161/867) of women aged 55–64 years and in 73.3% (66/90) of men and 57.2% (83/145) of women aged ≥85 years. This pattern of anaemia increasing with age was also observed when considering the other anaemia definitions and IDA (data not shown). Among the 2633 patients without blood count data, investigation of anaemia was a reason for referral in 229 (8.7%) (Table 2).
Anaemia and cancer site
Among the 4742 patients with blood count data, there were 97 proximal and 240 distal cancers. While yields of distal cancer did not vary by anaemia status, anaemia was strongly associated with proximal cancer (Table 2). Proximal cancer yield was 0.6% (19/3082) in patients without anaemia, increasing to 10.5% (38/363) in patients with narrow definition anaemia and IDA. Broadening the definition of anaemia and removing the requirement for IDA reduced the yield to 4.7% (78/1660). Among the 2633 patients without blood count data, yield of proximal cancer was 3.9% (9/229) in patients referred for investigation of anaemia vs. 0.9% (21/2404) in those who were not (Table 2).
Although the yield of proximal cancer was highest in patients with narrow definition anaemia and IDA, this criterion identified 39.2% (38/97) of patients with proximal cancer, while the broad definition of anaemia (with or without IDA) identified 80.4% (78/97) (Table 2). We therefore used the broad definition in all subsequent analyses of patients with blood count data (n = 4742) to ensure high sensitivity of anaemia for proximal cancer. For patients without blood count data (n = 2633), we defined anaemia based on whether the investigation of anaemia was indicated as a reason for referral. Among all 7375 patients, 1889 (25.6%) had either broad definition anaemia (n = 1660) or were referred for investigation of anaemia (n = 229) (Table 2).
Symptoms and signs related to cancer site
Among all patients, the most common symptoms/signs were a CIBH (73.0%, n = 5382), rectal bleeding (37.6%, n = 2773), abdominal pain (28.8%, n = 2126), weight loss (15.6%, n = 1148), and anaemia (25.6%, n = 1889) (Table 3). Most patients presented with more than one symptom/sign (Supplementary Table 3).
Table 3.
Yield of distal and proximal cancer by combinations of symptoms and signs (N = 7375)
| Total patients | Additional NICE 2015 guideline symptoms or signsa | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Change in bowel habit | Rectal bleeding | Abdominal pain | Weight loss | Anaemia | Abdominal mass | Rectal mass | |||||||||||||||||||||||||||||||||||||||
| Cancers | Cancers | Cancers | Cancers | Cancers | Cancers | Cancers | Cancers | Cancers | ||||||||||||||||||||||||||||||||||||||
| Total | Distal | Proximal | Total | Distal | Proximal | Total | Distal | Proximal | Total | Distal | Proximal | Total | Distal | Proximal | Total | Distal | Proximal | Total | Distal | Proximal | Total | Distal | Proximal | Total | Distal | Proximal | ||||||||||||||||||||
| n | % | n | % | n | % | n | n | % | n | % | n | n | % | n | % | n | n | % | n | % | n | n | % | n | % | n | n | % | n | % | n | n | % | n | % | n | n | % | n | % | n | n | % | n | % | |
| Symptoms | ||||||||||||||||||||||||||||||||||||||||||||||
| Change in bowel habit | 5382 | 73.0 | 308 | 5.7 | 70 | 1.3 | 1589 | 33 | 2.1 | 7 | 0.4 | — | — | — | — | — | 1674 | 196 | 11.7 | 18 | 1.1 | 1602 | 79 | 4.9 | 29 | 1.8 | 920 | 62 | 6.7 | 18 | 2.0 | 1085 | 77 | 7.1 | 39 | 3.6 | 134 | 13 | 9.7 | 10 | 7.5 | 97 | 34 | 35.1 | 0 | 0 |
| More frequent | 2862 | 38.8 | 159 | 5.6 | 36 | 1.3 | 908 | 17 | 1.9 | 2 | 0.2 | — | — | — | — | — | 811 | 100 | 12.3 | 13 | 1.6 | 811 | 35 | 4.3 | 15 | 1.8 | 525 | 40 | 7.6 | 7 | 1.3 | 565 | 45 | 8.0 | 18 | 3.2 | 63 | 4 | 6.3 | 4 | 6.3 | 36 | 10 | 27.8 | 0 | 0 |
| Less frequent | 865 | 11.7 | 43 | 5.0 | 13 | 1.5 | 189 | 5 | 2.6 | 2 | 1.1 | — | — | — | — | — | 307 | 26 | 8.5 | 2 | 0.7 | 277 | 12 | 4.3 | 6 | 2.2 | 171 | 6 | 3.5 | 4 | 2.3 | 211 | 12 | 5.7 | 8 | 3.8 | 32 | 3 | 9.4 | 1 | 3.1 | 17 | 6 | 35.3 | 0 | 0 |
| Variable | 648 | 8.8 | 26 | 4.0 | 8 | 1.2 | 183 | 8 | 4.4 | 1 | 0.5 | — | — | — | — | — | 188 | 8 | 4.3 | 1 | 0.5 | 235 | 7 | 3.0 | 4 | 1.7 | 103 | 2 | 1.9 | 5 | 4.9 | 113 | 8 | 7.1 | 4 | 3.5 | 11 | 2 | 18.2 | 2 | 18.2 | 9 | 2 | 22.2 | 0 | 0 |
| Unspecified | 1007 | 13.7 | 80 | 7.9 | 13 | 1.3 | 309 | 3 | 1.0 | 2 | 0.6 | — | — | — | — | — | 368 | 62 | 16.8 | 2 | 0.5 | 279 | 25 | 9.0 | 4 | 1.4 | 121 | 14 | 11.6 | 2 | 1.7 | 196 | 12 | 6.1 | 9 | 4.6 | 28 | 4 | 14.3 | 3 | 10.7 | 35 | 16 | 45.7 | 0 | 0 |
| Rectal bleeding | 2773 | 37.6 | 291 | 10.5 | 30 | 1.1 | 618 | 47 | 7.6 | 3 | 0.5 | 1674 | 196 | 11.7 | 18 | 1.1 | — | — | — | — | — | 622 | 57 | 9.2 | 6 | 1.0 | 300 | 37 | 12.3 | 5 | 1.7 | 549 | 66 | 12.0 | 16 | 2.9 | 48 | 7 | 14.6 | 3 | 6.3 | 95 | 43 | 45.3 | 1 | 1.1 |
| Abdominal pain | 2126 | 28.8 | 96 | 4.5 | 42 | 2.0 | 192 | 2 | 1.0 | 0 | 0 | 1602 | 79 | 4.9 | 29 | 1.8 | 622 | 57 | 9.2 | 6 | 1.0 | — | — | — | — | — | 355 | 26 | 7.3 | 12 | 3.4 | 384 | 24 | 6.3 | 25 | 6.5 | 93 | 8 | 8.6 | 13 | 14.0 | 26 | 9 | 34.6 | 0 | 0 |
| Weight loss | 1148 | 15.6 | 77 | 6.7 | 28 | 2.4 | 18 | 0 | 0 | 0 | 0 | 920 | 62 | 6.7 | 18 | 2.0 | 300 | 37 | 12.3 | 5 | 1.7 | 355 | 26 | 7.3 | 12 | 3.4 | — | — | — | — | — | 425 | 35 | 8.2 | 22 | 5.2 | 58 | 5 | 8.6 | 5 | 8.6 | 13 | 1 | 7.7 | 0 | 0 |
| Other symptomsb | 479 | 6.5 | 20 | 4.2 | 10 | 2.1 | — | — | — | — | — | 373 | 15 | 4.0 | 9 | 2.4 | 127 | 11 | 8.7 | 1 | 0.8 | 177 | 5 | 2.8 | 2 | 1.1 | 140 | 8 | 5.7 | 3 | 2.1 | 117 | 8 | 6.8 | 7 | 6.0 | 23 | 3 | 13.0 | 1 | 4.3 | 11 | 1 | 9.1 | 0 | 0 |
| Signs | ||||||||||||||||||||||||||||||||||||||||||||||
| Anaemiac | 1889 | 25.6 | 125 | 6.6 | 87 | 4.6 | 404 | 13 | 3.2 | 24 | 5.9 | 1085 | 77 | 7.1 | 39 | 3.6 | 549 | 66 | 12.0 | 16 | 2.9 | 384 | 24 | 6.3 | 25 | 6.5 | 425 | 35 | 8.2 | 22 | 5.2 | — | — | — | — | — | 83 | 8 | 9.6 | 10 | 12.0 | 31 | 4 | 12.9 | 1 | 3.2 |
| Abdominal mass | 216 | 2.9 | 19 | 8.8 | 20 | 9.3 | 12 | 1 | 8.3 | 4 | 33.3 | 134 | 13 | 9.7 | 10 | 7.5 | 48 | 7 | 14.6 | 3 | 6.3 | 93 | 8 | 8.6 | 13 | 14.0 | 58 | 5 | 8.6 | 5 | 8.6 | 83 | 8 | 9.6 | 10 | 12.0 | — | — | — | — | — | 3 | 2 | 66.7 | 0 | 0 |
| Rectal mass | 165 | 2.2 | 47 | 28.5 | 1 | 0.6 | 19 | 1 | 5.3 | 0 | 0 | 97 | 34 | 35.1 | 0 | 0 | 95 | 43 | 45.3 | 1 | 1.1 | 26 | 9 | 34.6 | 0 | 0 | 13 | 1 | 7.7 | 0 | 0 | 31 | 4 | 12.9 | 1 | 3.2 | 3 | 2 | 66.7 | 0 | 0 | — | — | — | — | — |
| Other signsd | 265 | 3.6 | 13 | 4.9 | 5 | 1.9 | — | — | — | — | — | 153 | 8 | 5.2 | 2 | 1.3 | 77 | 6 | 7.8 | 0 | 0 | 68 | 4 | 5.9 | 3 | 4.4 | 34 | 4 | 11.8 | 0 | 0 | 72 | 6 | 8.3 | 4 | 5.6 | 2 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
Percentages for distal and proximal cancers are yields. Five patients had both a distal and proximal cancer diagnosed. These patients are included in both the distal and proximal cancer columns. The symptoms occurring in these patients were: anaemia and rectal bleeding; anaemia, change in bowel habit (CIBH) (more frequent) and weight loss; rectal bleeding, CIBH (more frequent), abdominal pain and weight loss; CIBH (more frequent) and bloating; and CIBH (unspecified) alone
aNICE 2015 guideline symptoms/signs included CIBH, rectal bleeding, abdominal pain, weight loss, anaemia and abdominal or rectal mass without any restriction by age; the broad definition of anaemia was used. Patients may have had multiple symptoms/signs
bOther symptoms include bloating/flatulence (n = 203), tiredness/weakness (n = 152), anal symptoms (n = 97), nausea/vomiting (n = 44), back pain (n = 13), and upper gastrointestinal symptoms (n = 10)
cIn patients with blood count data, anaemia was defined by the WHO (broad) definition [haemoglobin (Hb) level <13 g/dL in men or <12 g/dL in women]. In patients without blood count data, anaemia was defined by whether the investigation of anaemia was indicated as a reason for referral
dOther signs include faecal occult blood test (FOBT) positivity (n = 113), family history (n = 117), history of polyps (n = 23), cancer antibodies (n = 3), elevated C-reactive protein (n = 4), and liver problems (n = 9)
Yields of proximal and distal cancer varied considerably by symptom/sign (Table 3). The highest yields of distal cancer were among patients with rectal mass (28.5%, 47/165) and rectal bleeding (10.5%, 291/2773). Yields of proximal cancer were generally lower than for distal cancer, with the highest yields among patients with abdominal mass (9.3%, 20/216) and anaemia (4.6%, 87/1889). Yields of proximal and distal cancer were generally higher when patients presented with a combination of symptoms/signs (Table 3).
The location of diagnosed cancers was also influenced by symptom/sign (Table 4). Rectal bleeding was associated with an approximate 90% chance that cancer was distal, irrespective of additional symptoms. Anaemia and abdominal mass were associated with a high probability that cancer was proximal (41.4% and 51.3%, respectively), irrespective of additional symptoms.
Table 4.
Proportion of all cancers located distally and proximally by combinations of symptoms and signs (N = 7375)
| Total patients | Additional NICE 2015 guideline symptoms or signsa | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Change in bowel habit | Rectal bleeding | Abdominal pain | Weight loss | Anaemiab | Abdominal mass | Rectal mass | |||||||||||||||||||||||||||||||
| Cancers | Cancers | Cancers | Cancers | Cancers | Cancers | Cancers | Cancers | Cancers | ||||||||||||||||||||||||||||||
| Total | Distal | Proximal | Distal | Proximal | Distal | Proximal | Distal | Proximal | Distal | Proximal | Distal | Proximal | Distal | Proximal | Distal | Proximal | Distal | Proximal | ||||||||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Symptoms | ||||||||||||||||||||||||||||||||||||||
| Change in bowel habit | 5382 | 73.0 | 308 | 82.3 | 70 | 18.7 | 33 | 86.8 | 7 | 18.4 | — | — | — | — | 196 | 92.0 | 18 | 8.5 | 79 | 73.8 | 29 | 27.1 | 62 | 79.4 | 18 | 23.1 | 77 | 67.0 | 39 | 33.9 | 13 | 56.5 | 10 | 43.5 | 34 | 100 | 0 | 0 |
| More frequent | 2862 | 38.8 | 159 | 82.8 | 36 | 18.8 | 17 | 94.4 | 2 | 11.1 | — | — | — | — | 100 | 89.3 | 13 | 11.6 | 35 | 71.4 | 15 | 30.6 | 40 | 88.9 | 7 | 15.6 | 45 | 72.6 | 18 | 29.0 | 4 | 50.0 | 4 | 50.0 | 10 | 100 | 0 | 0 |
| Less frequent | 865 | 11.7 | 43 | 76.8 | 13 | 23.2 | 5 | 71.4 | 2 | 28.6 | — | — | — | — | 26 | 92.9 | 2 | 7.1 | 12 | 66.7 | 6 | 33.3 | 6 | 60.0 | 4 | 40.0 | 12 | 60.0 | 8 | 40.0 | 3 | 75.0 | 1 | 25.0 | 6 | 100 | 0 | 0 |
| Variable | 648 | 8.8 | 26 | 76.5 | 8 | 23.5 | 8 | 88.9 | 1 | 11.1 | — | — | — | — | 8 | 88.9 | 1 | 11.1 | 7 | 63.4 | 4 | 36.4 | 2 | 28.6 | 5 | 71.4 | 8 | 66.7 | 4 | 33.3 | 2 | 50.0 | 2 | 50.0 | 2 | 100 | 0 | 0 |
| Unspecified | 1007 | 13.7 | 80 | 87.0 | 13 | 14.1 | 3 | 75.0 | 2 | 50.0 | — | — | — | — | 62 | 96.9 | 2 | 3.1 | 25 | 86.2 | 4 | 13.8 | 14 | 87.5 | 2 | 12.5 | 12 | 57.1 | 9 | 42.9 | 4 | 57.1 | 3 | 42.9 | 16 | 100 | 0 | 0 |
| Rectal bleeding | 2773 | 37.6 | 291 | 91.2 | 30 | 9.4 | 47 | 94.0 | 3 | 6.0 | 196 | 92.0 | 18 | 8.5 | — | — | — | — | 57 | 91.9 | 6 | 9.7 | 37 | 90.2 | 5 | 12.2 | 66 | 81.5 | 16 | 19.8 | 7 | 70.0 | 3 | 30.0 | 43 | 97.7 | 1 | 2.3 |
| Abdominal pain | 2126 | 28.8 | 96 | 70.1 | 42 | 30.7 | 2 | 100 | 0 | 0 | 79 | 73.8 | 29 | 27.1 | 57 | 91.9 | 6 | 9.7 | — | — | — | — | 26 | 70.3 | 12 | 32.4 | 24 | 49.0 | 25 | 51.0 | 8 | 38.1 | 13 | 61.9 | 9 | 100 | 0 | 0 |
| Weight loss | 1148 | 15.6 | 77 | 74.8 | 28 | 27.2 | 0 | 0 | 0 | 0 | 62 | 79.5 | 18 | 23.1 | 37 | 90.2 | 5 | 12.2 | 26 | 70.3 | 12 | 32.4 | — | — | — | — | 35 | 62.5 | 22 | 39.3 | 5 | 50.0 | 5 | 50.0 | 1 | 100 | 0 | 0 |
| Other symptomsb | 479 | 6.5 | 20 | 69.0 | 10 | 34.5 | — | — | — | — | 15 | 65.2 | 9 | 39.1 | 11 | 91.7 | 1 | 8.3 | 5 | 71.4 | 2 | 28.6 | 8 | 72.7 | 3 | 27.3 | 8 | 53.3 | 7 | 46.7 | 3 | 75.0 | 1 | 25.0 | 1 | 100 | 0 | 0 |
| Signs | ||||||||||||||||||||||||||||||||||||||
| Anaemiac | 1889 | 25.6 | 125 | 59.5 | 87 | 41.4 | 13 | 35.1 | 24 | 64.9 | 77 | 67.0 | 39 | 33.9 | 66 | 81.5 | 16 | 19.8 | 24 | 49.0 | 25 | 51.0 | 35 | 62.5 | 22 | 39.3 | — | — | — | — | 8 | 44.4 | 10 | 55.6 | 4 | 80.0 | 1 | 20.0 |
| Abdominal mass | 216 | 2.9 | 19 | 48.7 | 20 | 51.3 | 1 | 20.0 | 4 | 80.0 | 13 | 56.5 | 10 | 43.5 | 7 | 70.0 | 3 | 30.0 | 8 | 38.1 | 13 | 61.9 | 5 | 50.0 | 5 | 50.0 | 8 | 44.4 | 10 | 55.6 | — | — | — | — | 2 | 100 | 0 | 0 |
| Rectal mass | 165 | 2.2 | 47 | 97.9 | 1 | 2.1 | 1 | 100 | 0 | 0 | 34 | 100 | 0 | 0 | 43 | 97.7 | 1 | 2.3 | 9 | 100 | 0 | 0 | 1 | 100 | 0 | 0 | 4 | 80.0 | 1 | 20.0 | 2 | 100 | 0 | 0 | — | — | — | — |
| Other signsd | 265 | 3.6 | 13 | 72.2 | 5 | 27.8 | — | — | — | — | 8 | 80.0 | 2 | 20.0 | 6 | 100 | 0 | 0 | 4 | 57.1 | 3 | 42.9 | 4 | 100 | 0 | 0 | 6 | 60.0 | 4 | 40.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Percentages are the proportion of all cancers that are located in that site. Five patients had both a distal and proximal cancer diagnosed. These patients are included in both the distal and proximal cancer columns. The symptoms occurring in these patients were: anaemia and rectal bleeding; anaemia, change in bowel habit (CIBH) (more frequent) and weight loss; rectal bleeding, CIBH (more frequent), abdominal pain and weight loss; CIBH (more frequent) and bloating; and CIBH (unspecified) alone
aNICE 2015 guideline symptoms/signs included CIBH, rectal bleeding, abdominal pain, weight loss, anaemia and abdominal or rectal mass without any restriction by age; the broad definition of anaemia was used. Patients may have had multiple symptoms/signs
bOther symptoms include bloating/flatulence (n = 203), tiredness/weakness (n = 152), anal symptoms (n = 97), nausea/vomiting (n = 44), back pain (n = 13), and upper gastrointestinal symptoms (n = 10)
cIn patients with blood count data, anaemia was defined by the WHO (broad) definition [haemoglobin (Hb) level <13 g/dL in men or <12 g/dL in women]. In patients without blood count data, anaemia was defined by whether the investigation of anaemia was indicated as a reason for referral
dOther signs include faecal occult blood test (FOBT) positivity (n = 113), family history (n = 117), history of polyps (n = 23), cancer antibodies (n = 3), elevated C-reactive protein (n = 4), and liver problems (n = 9)
We evaluated the combination of anaemia and/or abdominal mass in further detail, given the strong association with proximal cancer. Among the 2022 patients with anaemia and/or abdominal mass, proximal cancer yield was 4.8%, the NNE was 21 (95% CI 18–26), and 42.0% of the patients with cancer had proximal cancer. The sensitivity of anaemia and/or abdominal mass for proximal cancer was 76.4% (97/127) (Table 5).
Table 5.
Symptom combination at presentation and yield of distal and proximal cancer (N = 7375)
| Symptom/signs combinations | Total patients | Distal cancersa | Proximal cancersa | All sites | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Patients with distal cancer, n | Proportion of distal cancers, % | Diagnostic yield, % | No. needed to examine to diagnose one cancer (95% CI) | Patients with proximal cancer, n | Proportion of proximal cancers, % | Diagnostic yield, % | No. needed to examine to diagnose one cancer (95% CI) | Patients with cancer, n | Proportion with a distal cancer, % | Proportion with a proximal cancer, % | |
| Total | 7375 | 100 | 429 | 100.0 | 5.8 | 18 (16, 19) | 127 | 100.0 | 1.7 | 59 (49, 70) | 551 | 77.9 | 23.0 |
| Anaemiab and/or abdominal mass | 2022 | 27.4 | 136 | 31.7 | 6.7 | 15 (13, 18) | 97 | 76.4 | 4.8 | 21 (18, 26) | 231 | 58.9 | 42.0 |
| Anaemiab, no abdominal mass | 1806 | 24.5 | 117 | 27.3 | 6.5 | 16 (13, 19) | 77 | 60.6 | 4.3 | 24 (19, 30) | 192 | 60.9 | 40.1 |
| Abdominal mass, no anaemiab | 133 | 1.8 | 11 | 2.6 | 8.3 | 13 (7, 24) | 10 | 7.9 | 7.5 | 14 (8, 28) | 21 | 52.4 | 47.6 |
| Anaemiab and abdominal mass | 83 | 1.1 | 8 | 1.9 | 9.6 | 11 (6, 24) | 10 | 7.9 | 12.0 | 9 (5, 17) | 18 | 44.4 | 55.6 |
| No anaemiab or abdominal mass | |||||||||||||
| Total | 5353 | 72.6 | 293 | 68.3 | 5.5 | 19 (17, 21) | 30 | 23.6 | 0.6 | 179 (126, 265) | 320 | 91.6 | 9.4 |
| Rectal bleeding | |||||||||||||
| Total | 2195 | 29.8 | 221 | 51.5 | 10.1 | 10 (9, 12) | 13 | 10.2 | 0.6 | 169 (99, 317) | 233 | 94.8 | 5.6 |
| Rectal bleeding alone | 571 | 7.7 | 43 | 10.0 | 7.5 | 14 (10, 19) | 3 | 2.4 | 0.5 | 191 (66, 922) | 46 | 93.5 | 6.5 |
| Rectal bleeding and CIBH | 1345 | 18.2 | 156 | 36.4 | 11.6 | 9 (8, 11) | 10 | 7.9 | 0.7 | 135 (74, 281) | 165 | 94.5 | 6.1 |
| Rectal bleeding and weight loss or abdominal pain, no CIBH | 202 | 2.7 | 8 | 1.9 | 4.0 | 26 (14, 58) | 0 | 0.0 | 0 | — | 8 | 100 | 0.0 |
| Rectal bleeding and only other symptoms/signsc | 77 | 1.0 | 14 | 3.3 | 18.2 | 6 (4, 10) | 0 | 0.0 | 0 | — | 14 | 100 | 0.0 |
| CIBH, no rectal bleeding | |||||||||||||
| Total | 2866 | 38.9 | 68 | 15.9 | 2.4 | 43 (34, 55) | 17 | 13.4 | 0.6 | 169 (106, 290) | 83 | 81.9 | 20.5 |
| CIBH alone | 1464 | 19.9 | 31 | 7.2 | 2.1 | 48 (34, 70) | 5 | 3.9 | 0.3 | 293 (126, 902) | 35 | 88.6 | 14.3 |
| More frequent | 836 | 11.3 | 15 | 3.5 | 1.8 | 56 (34, 100) | 0 | 0.0 | 0 | — | 15 | 100 | 0.0 |
| Less frequent | 174 | 2.4 | 5 | 1.2 | 2.9 | 35 (16, 107) | 2 | 1.6 | 1.1 | 87 (25, 717) | 7 | 71.4 | 28.6 |
| Variable | 166 | 2.3 | 8 | 1.9 | 4.8 | 21 (11, 48) | 1 | 0.8 | 0.6 | 166 (31, 6558) | 9 | 88.9 | 11.1 |
| Unspecified | 288 | 3.9 | 3 | 0.7 | 1.0 | 96 (34, 465) | 2 | 1.6 | 0.7 | 144 (41, 1188) | 4 | 75.0 | 50.0 |
| CIBH and weight loss or abdominal pain | 1253 | 17.0 | 34 | 7.9 | 2.7 | 37 (27, 54) | 10 | 7.9 | 0.8 | 126 (69, 261) | 44 | 77.3 | 22.7 |
| CIBH and only other symptoms/signsc | 149 | 2.0 | 3 | 0.7 | 2.0 | 50 (18, 240) | 2 | 1.6 | 1.3 | 75 (21, 614) | 4 | 75.0 | 50.0 |
| No rectal bleeding or CIBH | |||||||||||||
| Abdominal pain or weight loss | 241 | 3.3 | 2 | 0.5 | 0.8 | 121 (34, 994) | 0 | 0.0 | 0 | — | 2 | 100 | 0.0 |
| Only other symptoms/signsc | 51 | 0.7 | 2 | 0.5 | 3.9 | 26 (8, 209) | 0 | 0.0 | 0 | — | 2 | 100 | 0.0 |
| Suggested criteria for flexible sigmoidoscopy | |||||||||||||
| No anaemia or abdominal mass | |||||||||||||
| Rectal bleeding | 2195 | 29.8 | 221 | 51.5 | 10.1 | 10 (9, 12) | 13 | 10.2 | 0.6 | 169 (99, 317) | 233 | 94.8 | 5.6 |
| CIBH to more frequent alone | 836 | 11.3 | 15 | 3.5 | 1.8 | 56 (34, 100) | 0 | 0.0 | 0 | — | 15 | 100 | 0.0 |
| Total | 3031 | 41.1 | 236 | 55.0 | 7.8 | 13 (12, 15) | 13 | 10.2 | 0.4 | 234 (137, 438) | 248 | 95.2 | 5.2 |
aFive patients had both a distal and proximal cancer diagnosed. These patients are included in both the distal and proximal cancer columns. Of these five patients, two had anaemia, one had rectal bleeding and a change in bowel habit (CIBH) (more frequent), one had a CIBH (more frequent) and bloating, and one had a CIBH (unspecified) alone
bIn patients with blood count data, anaemia was defined by the WHO (broad) definition [haemoglobin (Hb) level <13 g/dL in men or <12 g/dL in women]. In patients without blood count data, anaemia was defined by whether the investigation of anaemia was indicated as a reason for referral
cIncludes bloating/flatulence (n = 203), tiredness/weakness (n = 152), anal symptoms (n = 97), nausea/vomiting (n = 44), back pain (n = 13), upper gastrointestinal symptoms (n = 10), faecal occult blood test (FOBT) positivity (n = 113), family history (n = 117), history of polyps (n = 23), cancer antibodies (n = 3), elevated C-reactive protein (n = 4), and liver problems (n = 9)
Among the 5353 patients without anaemia and/or abdominal mass, the yield of proximal cancer was 0.6% (NNE 179, 95% CI 126–265). Proximal cancer yield was also 0.6% (NNE 169, 95% CI 99–317) among the 2195 patients without anaemia and/or abdominal mass who had rectal bleeding. No proximal cancers were found in 836 patients without anaemia and/or abdominal mass who presented with a CIBH to increased frequency alone (Table 5).
There were 3031 patients without anaemia and/or abdominal mass who had rectal bleeding or solely a CIBH to increased frequency, accounting for 41.1% of the cohort. Among these patients, 236 distal cancers were diagnosed (yield of 7.8%; NNE 13, 95% CI 12–15) while only 13 proximal cancers were diagnosed (yield of 0.4%; NNE 234, 95% CI 137–438) (Table 5). Yields of proximal cancer were <1% for all age ranges in this patient subgroup (data not shown). Of the 13 proximal cancer patients, 6 had distal findings that would warrant WCI (Supplementary Table 4). Therefore, if this patient subgroup were investigated by flexible sigmoidoscopy alone, 7 of the total 556 cancers (1.3%, 95% CI 0.5–2.6%) would have been missed. For five of these seven cases, blood count data were not available and so anaemia at presentation cannot be ruled out.
Flexible sigmoidoscopy is considered complete if the sigmoid–descending colon junction is reached20; therefore, cancers in the descending colon may be missed. Descending colon cancers were rare in our study (1.4%, 8/556). Of the eight patients with descending colon cancers, two had the symptom profile of rectal bleeding or solely a CIBH to increased frequency without anaemia and/or abdominal mass; one of these had a synchronous cancer in the sigmoid colon while the other had no important distal findings (Supplementary Table 5).
Discussion
For patients referred to hospital with suspected CRC, an important decision is whether to offer WCI or flexible sigmoidoscopy. NHS clinics offering rapid access flexible sigmoidoscopy have been shown to be suitable for evaluation of urgently referred patients, with a low miss rate of proximal cancer.9,11 However, fear of missing proximal cancers remains, with 20–70% of patients proceeding to WCI following flexible sigmoidoscopy.8,9,11,21 This negates the convenience and cost-effectiveness of flexible sigmoidoscopy, particularly as subsequent WCI has a very low cancer yield.8,9 To address the fear of missing cancers in patients examined solely by flexible sigmoidoscopy, we sought to determine in which patients the yield of proximal cancer and the probability of missing proximal cancer is low.
This multicentre cohort study of 7375 patients referred with suspected CRC to 21 hospitals throughout England validates previous studies that showed how yields of proximal and distal cancer vary by presenting symptom/sign.8–14 The largest previous study of 16,433 patients referred to hospital from 1986 to 2001 concluded that flexible sigmoidoscopy alone was sufficient for patients with a CIBH, rectal bleeding, and/or abdominal pain but without IDA, abdominal mass, severe symptoms, or significant flexible sigmoidoscopy findings. This was based on the finding that proximal cancer was very rare (<1%) in such patients.8 In an extension of this study, including data collected from 1986 to 2007, proximal cancer was again associated with IDA and abdominal mass but not with a CIBH or rectal bleeding.22
Our study confirms the strong association between anaemia and abdominal mass with proximal cancer and newly demonstrates the importance of adopting a broad definition of anaemia. We found that 80% of proximal cancer cases could be identified using the broad definition (with or without IDA), compared to 39% using the narrow definition with requirement for IDA. The broad definition should therefore be adopted when selecting patients for flexible sigmoidoscopy since there is a greater likelihood of missing proximal cancers with the narrower definition. Given the importance of anaemia as a marker for proximal cancer, we also recommend that all patients referred with suspected CRC have a full blood count, unless an emergency investigation is required.
Through closer examination of cancer yield by combinations of symptoms and signs, we were able to define the criteria for flexible sigmoidoscopy more specifically. Proximal cancer risk was particularly low in patients without anaemia and/or abdominal mass who presented with a CIBH to increased frequency alone. Similarly, very few proximal cancers were detected in patients without anaemia and/or abdominal mass who presented with rectal bleeding, alone or with other symptoms/signs. These novel findings led us to conclude that flexible sigmoidoscopy alone is sufficient for patients without .anaemia and/or abdominal mass who present with any rectal bleeding or solely a CIBH to increased frequency, unless there are significant distal findings that warrant WCI (i.e. large or multiple adenomas, IBD) or the examination is incomplete. Patients fitting these criteria could be reassured that their risk of proximal cancer is very low but that they should return to their GP if symptoms continue. In our study, 41% of patients fulfilled these criteria for flexible sigmoidoscopy alone.
The probability of missing a proximal cancer is an important consideration. In the study by Thompson et al., 131 proximal cancers were diagnosed.8 Of the 37 cases without anaemia and/or abdominal mass, 20 would have undergone subsequent WCI because of severe symptoms or significant flexible sigmoidoscopy findings. Thus 17 proximal cancers (13%) would have been missed if only flexible sigmoidoscopy was undertaken. With our criteria for flexible sigmoidoscopy, 10 proximal cancers (8%) would have been missed in the Thompson data, and probably fewer if our broad anaemia definition had been considered. Adoption of our flexible sigmoidoscopy criteria would therefore minimise the probability of missing proximal cancers.
There is a small risk that distal cancers could go undetected if flexible sigmoidoscopy is undertaken alone. While flexible sigmoidoscopy, in theory, can reach the splenic flexure, the descending colon sometimes goes unexamined as there are no anatomical markers that the splenic flexure has been reached.20,23 Few cancers are, however, located in the descending colon.24 In our study, 8 of the 429 distal cancers were in the descending colon and only 1 of the 8 patients would have undergone flexible sigmoidoscopy alone with our criteria.
There is also a small risk of missing cancers in the sigmoid colon with flexible sigmoidoscopy. Examinations failing to reach the sigmoid–descending colon junction are, however, typically deemed incomplete,20,23,25 prompting subsequent whole-colon completion examination.8,9,21 It is worth noting that, although studies have reported high rates of incomplete examinations due to pain, faeces, or scope looping, these were based on flexible sigmoidoscopy performed over 10 years ago.23,25 There are now remedies to avoid these scenarios. Premixed 50% nitrous oxide and oxygen (Entonox, BOC Healthcare, Guildford, Surrey, UK) has been shown to be as effective as conventional sedation for colonoscopy and is used frequently for flexible sigmoidoscopy screening in the English Bowel Cancer Screening programme.26 Preparation for flexible sigmoidoscopy usually involves a single phosphate enema,6 although oral preparations are occasionally used. If the distal bowel is not adequately cleared by an initial enema, a second can be administered via the endoscope. This has proved highly effective in patients having inadequate bowel preparation at colonoscopy.27
Isolated proximal non-cancerous abnormalities could also be missed by undertaking flexible sigmoidoscopy alone. Few studies, however, have addressed this.28,29 One retrospective study of 1766 patients who had undergone colonoscopy for rectal bleeding found isolated proximal colitis in 26 (1.5%) patients.28 Similarly, in a study of 625 patients aged <50 years with diarrhoea, 10 (1.6%) had isolated proximal IBD or colitis.29 Microscopic colitis, a common cause of unexplained chronic diarrhoea, presents a particular challenge as it can only be diagnosed on colonic biopsy and the vast majority of patients who receive a diagnosis have macroscopically normal colons.30 Although limited, the current evidence indicates that, when microscopic colitis is present, it is present throughout the colon.31,32 It is therefore likely that distal biopsies taken during flexible sigmoidoscopy would be sufficient for diagnosis.
Our study has several strengths, including recruitment from multiple hospitals, detailed baseline patient information, and follow-up using cancer registries for 3 years post-referral. We are confident that the follow-up period was sufficiently long to capture all incident CRC cases as the vast majority of CRCs were diagnosed within 6 months, with very few diagnosed in the ensuing 3 years.
Limitations include a possible selection bias since all patients were aged ≥55 years, considered fit for WCI, and 72% were urgently referred. Some patients did not proceed to undergo WCI but we were unable to accurately identify these patients from hospital records; nevertheless, we are confident that we did not miss cancers because we had cancer registry data for 3 years post-referral. Reporting bias is possible as the recording of symptoms by medical staff may have been influenced by what investigations were planned or by the presumed significance of the symptoms.33
A further limitation is that we did not have blood count data on all patients; for those patients without this data, anaemia was defined by whether it was indicated as a reason for referral. With full blood count data, it is possible that even fewer proximal cancer cases would have been missed by our flexible sigmoidoscopy criteria, as a greater proportion may have had anaemia. Finally, it is important to note that the cohort was gathered before the Bowel Cancer Screening Programme was fully rolled out in England. It is now common in clinical practice to consider the screening history of patients along with how recently any colonic examinations have taken place when selecting the most appropriate diagnostic investigation.
Conclusion
Our findings confirm the strong association between anaemia and proximal cancer, with 80% of proximal cancer patients in our cohort meeting the criteria for broad definition anaemia. All patients with suspected CRC should therefore have a full blood count, unless an emergency investigation is needed, and patients identified as anaemic according to the broad definition of anaemia should be referred for WCI. Conversely, the risk of proximal cancer is very low in patients without broad definition anaemia and/or abdominal mass but who present with any rectal bleeding or solely a CIBH to increased frequency. Patients with this symptom profile can be examined safely by flexible sigmoidoscopy alone. If incorporated into guidelines, this strategy would greatly reduce the number of WCIs performed in patients at low risk of proximal cancer; this would alleviate the burden of WCI on patients and endoscopy and radiology services.
Electronic supplementary material
Acknowledgements
The investigators are grateful to the people listed below for their involvement in this study. In addition to those named, we would like to thank all of the personnel involved in the SIGGAR trials. CSPRG study staff: Mrs Elizabeth Coles (research assistance); Dr Paula Kirby (project management); Dr Fiona Lucas (literature review and editorial assistance); Miss Bhavita Patel (data collection, acquisition, cleaning and coding); Mr Iain Stenson (data collection and cleaning and information governance); and Dr Laura Turner (information governance, ethical approvals and project management). SOCCER steering committee: Professor Greg Rubin - Chair, independent (professor of general practice and primary care, University of Durham); Mr Omar Faiz – Non-independent (consultant colorectal surgeon, Imperial College London); Dr Pawan Randev – Independent (GP/primary care lead, London Cancer Alliance); Dr John De Caestecker – Independent (consultant gastroenterologist, Leicester General); Professor Richard Logan – Independent (professor of epidemiology/consultant gastroenterologist, University of Nottingham); Helen Watson – patient representative, Independent (arranged through Bowel Cancer UK). A special thank you to all of the patients who contributed data to the study.
Funding
This is a summary of independent research funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme and the Bobby Moore Fund for Cancer Research UK. The funders had no involvement in study design, data collection, data analysis, manuscript preparation, or publication decisions. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Cancer Research UK. Infrastructure support for this work was provided by the NIHR Imperial Biomedical Research Centre (BRC).
Author contributions
W.A., W.H., and S.H. were responsible for study design. A.J.C. and W.A. were responsible for oversight of data analysis. K.P. acquired supplemental raw data K.W. did the statistical analyses and A.J.C., W.A., and K.W. were responsible for data interpretation. E.C.R., J.P.B., M.R.T., S.H., S.T.-G., and B.P.S. critically appraised the data. A.J.C., W.A., K.W., E.C.R., and J.P.B. drafted the manuscript. K.P.,W.H., M.R.T., K.G.F., S.H., S.T.-G., M.V., and B.P.S. critically appraised the final manuscript. All authors gave final approval on the version to be published.
Availability of data and materials
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Ethics approval and consent to participate
The SIGGAR trials were registered (ISRCTN95152621) and received ethics approval from the Northern and Yorkshire Multicentre Research Ethics Committee (MREC/3/3/075). Written informed consent was obtained from all patients randomised in the SIGGAR trials. Permission to obtain cancer and mortality data for patients not randomised in the SIGGAR trials was granted by the National Information Governance Board (ref. ECC 5–04(E)/2011). Further ethics approval for the SOCCER study was granted by the National Research Ethics Service (NRES) Committee North East – York. Approval for the processing of patient identifiable information without consent under Section 251 of the NHS Act 2006 was granted by the NHS Health Research Authority Confidentiality Advisory Group (ref. 14/CAG/1043).
Competing interests
S.T.-G. reports fees from PENTAX Medical and Olympus for equipment loans and from Fujifilm and Aquilant for educational course sponsorship and support. The other authors declare no competing interests.
Footnotes
These authors contributed equally: Amanda J Cross, Kate Wooldrage
Electronic supplementary material
Supplementary information is available for this paper at 10.1038/s41416-018-0335-z.
References
- 1.National Institute for Health and Clinical Excellence. Colorectal Cancer: Diagnosis and Management. NICE Guidelines (CG131). (Last Update 2014) 1–25 (NICE, London, 2011).
- 2.National Institute for Health and Care Excellence. Suspected Cancer: Recognition and Referral. NICE Guidelines (NG12). (Last Update. 2017) 1–79 (NICE, London, 2015).
- 3.Brown, H. et al. Scoping the Future: An Evaluation of Endoscopy Capacity Across the NHS in England 1–90 (Cancer Research UK, London, UK, 2015).
- 4.Bending MW, et al. Estimating the direct costs of bowel cancer services provided by the National Health Service in England. Int. J. Technol. Assess. Health Care. 2010;26:362–369. doi: 10.1017/S0266462310001078. [DOI] [PubMed] [Google Scholar]
- 5.Lawler M, et al. Critical research gaps and recommendations to inform research prioritisation for more effective prevention and improved outcomes in colorectal cancer. Gut. 2018;67:179–193. doi: 10.1136/gutjnl-2017-315333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkin WS, et al. Single blind, randomised trial of efficacy and acceptability of oral picolax versus self administered phosphate enema in bowel preparation for flexible sigmoidoscopy screening. BMJ. 2000;320:1504–1508. doi: 10.1136/bmj.320.7248.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maslekar S, Hughes M, Gardiner A, Monson JR, Duthie GS. Patient satisfaction with lower gastrointestinal endoscopy: doctors, nurse and nonmedical endoscopists. Colorectal Dis. 2010;12:1033–1038. doi: 10.1111/j.1463-1318.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- 8.Thompson MR, et al. Flexible sigmoidoscopy and whole colonic imaging in the diagnosis of cancer in patients with colorectal symptoms. Br. J. Surg. 2008;95:1140–1146. doi: 10.1002/bjs.6234. [DOI] [PubMed] [Google Scholar]
- 9.Royle TJ, et al. Same-day assessment and management of urgent (2-week wait) colorectal referrals: an analysis of the outcome of 1606 patients attending an endoscopy unit-based colorectal clinic. Colorectal Dis. 2014;16:O176–O181. doi: 10.1111/codi.12508. [DOI] [PubMed] [Google Scholar]
- 10.Couch DG, Murphy JH, Boyle KM, Hemingway DM. Straight to flexible sigmoidoscopy: rationalization of 2-week wait referrals in suspected colorectal cancer. Colorectal Dis. 2015;17:980–983. doi: 10.1111/codi.12988. [DOI] [PubMed] [Google Scholar]
- 11.Badiani S, Desai A, Chapman MA. Is whole colonic imaging necessary for symptoms of change in bowel habit and/or rectal bleeding? Colorectal Dis. 2012;14:1197–1200. doi: 10.1111/j.1463-1318.2011.02918.x. [DOI] [PubMed] [Google Scholar]
- 12.Kent AJ, Woolf D, McCue J, Greenfield SM. The use of symptoms to predict colorectal cancer site. Can we reduce the pressure on our endoscopy services? Colorectal Dis. 2010;12:114–118. doi: 10.1111/j.1463-1318.2009.01770.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhangu A, et al. Detection and survival of colorectal cancer from a 2 week wait service. Surgeon. 2011;9:78–82. doi: 10.1016/j.surge.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald S, Radhakrishnan S, Seward E. Is flexible sigmoidoscopy ever enough? An audit of the rates of proximal disease during colonoscopy. Gut. 2013;62(Suppl 1(abstract)):A222–A223. [Google Scholar]
- 15.Atkin W, et al. Is whole-colon investigation by colonoscopy, computerised tomography colonography or barium enema necessary for all patients with colorectal cancer symptoms, and for which patients would flexible sigmoidoscopy suffice? A retrospective cohort study. Health Technol. Assess. 2017;21:1–80. doi: 10.3310/hta21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halligan S, et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1185–1193. doi: 10.1016/S0140-6736(12)62124-2. [DOI] [PubMed] [Google Scholar]
- 17.Atkin W, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1194–1202. doi: 10.1016/S0140-6736(12)62186-2. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organisation. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity 1–6 (World Health Organisation, Geneva, Switzerland, 2011).
- 19.National Institute for Health and Clinical Excellence. Referral Guidelines for Suspected Cancer. NICE Guidelines (CG27) 1–98 (NICE, London, 2005).
- 20.Lehman GA, Buchner DM, Lappas JC. Anatomical extent of fiberoptic sigmoidoscopy. Gastroenterology. 1983;84:803–808. doi: 10.1016/0016-5085(83)90149-X. [DOI] [PubMed] [Google Scholar]
- 21.Pullens HJ, Joosten M, Siersema PD, Brink MA. Open-access flexible sigmoidoscopy frequently leads to additional colonoscopy in symptomatic patients over 50 years. J. Gastrointestin. Liver. Dis. 2014;23:153–159. doi: 10.15403/jgld.2014.1121.232.1hjmp. [DOI] [PubMed] [Google Scholar]
- 22.Thompson MR, et al. Clinical assessment to determine the risk of bowel cancer using Symptoms, Age, Mass and Iron deficiency anaemia (SAMI) Br. J. Surg. 2017;104:1393–1404. doi: 10.1002/bjs.10573. [DOI] [PubMed] [Google Scholar]
- 23.Painter J, et al. Depth of insertion at flexible sigmoidoscopy: implications for colorectal cancer screening and instrument design. Endoscopy. 1999;31:227–231. doi: 10.1055/s-1999-13673. [DOI] [PubMed] [Google Scholar]
- 24.Cancer Research UK. Bowel cancer incidence statistics. [Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence (accessed August 2018).
- 25.Eloubeidi MA, Wallace MB, Desmond R, Farraye FA. Female gender and other factors predictive of a limited screening flexible sigmoidoscopy examination for colorectal cancer. Am. J. Gastroenterol. 2003;98:1634–1639. doi: 10.1111/j.1572-0241.2003.07480.x. [DOI] [PubMed] [Google Scholar]
- 26.NHS England. NHS Public Health Functions Agreement 2017-18. Service Specification No. 26A NHS Bowel Scope Screening Programme 1–38 (NHS England, London, UK, 2017).
- 27.Sim JS, Koo JS. Predictors of inadequate bowel preparation and salvage options on colonoscopy. Clin. Endosc. 2016;49:346–349. doi: 10.5946/ce.2016.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulcahy HE, et al. Yield of colonoscopy in patients with nonacute rectal bleeding: a multicenter database study of 1766 patients. Am. J. Gastroenterol. 2002;97:328–333. doi: 10.1111/j.1572-0241.2002.05465.x. [DOI] [PubMed] [Google Scholar]
- 29.Shale MJ, Walters JR, Westaby D. Adequacy of flexible sigmoidoscopy with biopsy for diarrhea in patients under age 50 without features of proximal disease. Gastrointest. Endosc. 2011;73:757–764. doi: 10.1016/j.gie.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Storr MA. Microscopic colitis: epidemiology, pathophysiology, diagnosis and current management-an update 2013. ISRN Gastroenterol. 2013;2013:352718. doi: 10.1155/2013/352718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genta RM, Sonnenberg A. The yield of colonic biopsy in the evaluation of chronic unexplained diarrhea. Eur. J. Gastroenterol. Hepatol. 2015;27:963–967. doi: 10.1097/MEG.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 32.Macaigne G, et al. Microscopic colitis or functional bowel disease with diarrhea: a French prospective multicenter study. Am. J. Gastroenterol. 2014;109:1461–1470. doi: 10.1038/ajg.2014.182. [DOI] [PubMed] [Google Scholar]
- 33.Price SJ, Stapley SA, Shephard E, Barraclough K, Hamilton WT. Is omission of free text records a possible source of data loss and bias in Clinical Practice Research Datalink studies? A case-control study. BMJ Open. 2016;6:e011664. doi: 10.1136/bmjopen-2016-011664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.