Abstract
Immunoglobulin (Ig) A in the mucus of the intestinal tract plays an important role in preventing the invasion of pathogenic microorganisms and regulating the composition of the gut microbiota. Several strains of probiotic lactic acid bacteria (LAB) are known to promote intestinal IgA production. Bacteria are also known to naturally release spherical membrane vesicles (MVs) that are involved in various biological functions such as quorum sensing, pathogenesis, and host immunomodulation. However, the production of MVs by LAB and their effects on host immunity remain poorly understood. In this study, we investigated the MV production by Lactobacillus sakei subsp. sakei NBRC15893 isolated from kimoto, the traditional seed mash used for brewing sake. MVs were separated from the culture broth of L. sakei NBRC15893 through filtration and density gradient ultracentrifugation and were observed by transmission electron microscopy. The MVs showed a spherical morphology, with a diameter of 30–400 nm, and contained proteins and nucleic acids. In addition, both the LAB cells and purified MVs promoted IgA production by murine Peyer’s patch cells. This MV- and cell-induced IgA production was suppressed by neutralization of Toll-like receptor (TLR) 2, which recognizes cell wall components of gram-positive bacteria, using an anti-TLR2 antibody. Collectively, our results indicate that MVs released from L. sakei NBRC15893 enhance IgA production by activating host TLR2 signaling through its cell wall components. Thus, it is important to consider novel interactions between gut microbiota and hosts via MVs, and MVs derived from probiotic bacteria could have promising applications as safe adjuvants.
Keywords: lactic acid bacteria, membrane vesicle, gut immunity, IgA
INTRODUCTION
The intestinal tract is equipped with multilevel barriers to prevent the invasion of pathogenic microorganisms, including chemical barriers such as the mucus and antimicrobial peptides, a physical barrier created by epithelial cells, and a biological barrier established by intestinal microflora and immune cells [1]. The gut mucosal immune system is characterized by the production of immunoglobulin (Ig) A, which not only plays an important role in preventing the invasion of pathogenic microorganisms into epithelial cells [2] but also regulates the composition of the gut microbiota [3, 4]. Therefore, enhancement of IgA production in the gut through dietary components and probiotics such as lactic acid bacteria (LAB) and bifidobacteria can be an effective strategy for enhancing the immune system, thereby preventing infectious diseases and maintaining the optimal composition of the gut microbiota.
IgA production in the gut involves the induction of IgA class-switch recombination (CSR) in which IgM+ B cells change to IgA+ B cells in gut-associated lymphatic tissues, including Peyer’s patches (PPs). This step is followed by the differentiation of the IgA+ B cells into IgA-producing plasma cells in the intestinal mucosal lamina propria. During this process, CD4+ T cells, which are activated by antigen-specific dendritic cells (DCs), induce the IgA CSR of IgM+ B cells in the presence of stimuli such as transforming growth factor (TGF)-β, interleukin (IL)-4, and IL-6 [5]. IgA CSR is also induced by retinoic acid, a proliferation-inducing ligand, or B-cell activating factor, which are produced by DCs in the PPs through a route independent of T cells [6]. Indeed, several reports have demonstrated the IgA-enhancing effects of LAB strains in the gut [7], including Lactobacillus pentosus strain b240 [8], L. plantarum AYA [9], and L. gasseri SBT 2055 [10]. These strains activate DCs in the PPs via Toll-like receptors (TLRs) and induce the production of retinoic acid, IL-6, and TGF-β, which in turn enhance IgA production in vitro.
Bacteria also naturally release membrane vesicles (MVs) with diameters of 20–500 nm under various growth conditions. Although the majority of studies on MVs have focused on gram-negative bacteria, recent studies have also reported MV production by gram-positive bacteria with thick peptidoglycan (PG). Moreover, the mechanism of production and functions of MVs have been predominantly examined in pathogenic strains. MVs contains bacterial components such as nucleic acids, proteins, and cell wall components and are involved in processes such as quorum sensing, relief of environmental stresses, delivery of pathogenic factors, and host immunomodulation [11,12,13]. For example, MVs produced by gram-negative bacteria Neisseria meningitidis [14] and Helicobacter pylori [15] act on macrophages to induce the inflammatory cytokine IL-6 and exert immunomodulatory effects. Similar mechanisms have been demonstrated for gram-positive bacteria. For instance, MVs of Mycobacteria tuberculosis inhibit the antigen presentation of DCs and macrophages [16], and the MVs of Clostridium perfringens induce IL-6 production from macrophage-like cells [17]. However, there are few reports on the production of MVs by nonpathogenic and probiotic bacteria, including LAB [18, 19]. The MVs of L. rhamnosus JB-1 were found to modulate regulatory T cells [18], and those of L. plantarum WCFS1 were reported to enhance the immune response against vancomycin-resistant enterococci [19]. Besides these examples, it remains unclear whether other LAB strains produce MVs or show immunomodulatory effects, including IgA-enhancing effects.
We found that the cells of Lactobacillus sakei subsp. sakei NBRC15893, which was isolated from kimoto, the traditional seed mash used for brewing sake, and the microcomponents obtained from the culture supernatant by ultracentrifugation promote IgA production from PP cells in vitro. In the present study, we aimed to elucidate whether L. sakei NBRC15893 produces MVs and to identify specific microcomponents that mediate the IgA-enhancing effect.
MATERIALS AND METHODS
Bacterial strain and culture conditions
L. sakei subsp. sakei NBRC15893 was purchased from Biological Resource Center, NITE (Tokyo, Japan). The strain was precultured anaerobically in deMan, Rogosa and Sharpe (MRS) medium (Becton Dickinson and Company [BD], Tokyo, Japan) using an AnaeroPack system (Mitsubishi Gas Chemical Company, Tokyo, Japan) and then cultured statically in MRS medium at 30°C. The optical density at 660 nm (OD660) of the culture broth was measured using a UV-1850 spectrophotometer (Shimadzu, Kyoto, Japan).
Preparation of MVs
The microcomponents in the culture broth were purified according to standard purification procedures for MVs [17]. The culture broth was centrifuged (8,500 × g, 5 min, 4°C), and the precipitated LAB cells were autoclaved at 121°C for 15 min and then dried under a reduced pressure at 22–25°C. The supernatant was filtered (0.45 μm, Thermo Fisher Scientific, Waltham, MA, USA) and then ultracentrifuged (100,000 × g, 1 hr, 4°C). The precipitate was washed with 10 mM HEPES containing 0.85% NaCl, pH 6.8 (hereafter HEPES-NaCl), and ultracentrifuged again (100,000 × g, 1 hr, 4°C). For investigation of MV, the precipitate obtained from 400 ml of culture broth was resuspended in 50 µl of HEPES-NaCl (the suspension is hereafter referred to as the crude MV fraction). For purification of MVs, the precipitate obtained from 2.2 l of culture broth at 24 hr was suspended in 1 ml of 45% (v/v) OptiPrep (iodixanol; Axis-Shield, Dundee, Scotland) in HEPES-NaCl and then overlaid by 1-ml aliquots of 40%, 35%, 30%, 25%, 20%, 15%, and 10% (v/v) OptiPrep in HEPES-NaCl. After ultracentrifugation (100,000 × g, 6 hr, 4°C), 1.5-ml fractions were collected from the tops of the ultracentrifuge tubes (Fr. 1–5). Each fraction was washed with HEPES-NaCl by ultracentrifugation (100,000 × g, 1 hr, 4°C), and each precipitate was resuspended in 50 µl of the buffer.
Absorbance at 260 and 280 nm was measured using a NanoDrop ND-1000 system (Thermo Fisher Scientific). Protein concentrations were determined with a bicinchoninic acid protein assay reagent kit (Thermo Fisher Scientific).
Transmission electron microscopy (TEM)
Fractionated samples were stained with 2% sodium phosphotungstate on a collodion-coated grid (150-mesh, Okenshoji, Tokyo, Japan) and observed by TEM (JEM-1400, JOEL, Tokyo, Japan) at 80 kV.
Preparation and culture of PP cells
The experimental protocols used in this study followed the Guide for the Care and Use of Experimental Animals issued by the Prime Minister’s Office of Japan and were reviewed and approved by the Animal Ethics Committee of Kansai University (Approval No. 1706). The breeding room was maintained under the following conditions: temperature, 21–23°C; humidity, 55–65%; light period, 08:00 am to 08:00 pm. Female BALB/c mice (7–14 weeks old, Japan SLC, Shizuoka, Japan) were used to obtain murine PP cells. PPs were harvested from the small intestine of mice and then incubated in RPMI 1640 medium (Sigma-Aldrich, Tokyo, Japan) containing 0.5 mg/ml collagenase, 2% (v/v) fetal bovine serum (FBS; Thermo Fisher Scientific), 100 U/ml penicillin, and 100 µg/ml streptomycin for 30 min. The PP cells were filtered through a cell strainer (100 μm; BD) and washed with the medium without collagenase. The cells were suspended in RPMI 1640 medium containing 10% (v/v) FBS, 55 µM 2-mercaptoethanol, 100 U/ml penicillin, and 100 µg/ml streptomycin and then cultured at 1.0 × 105 cells/well in a U-bottom 96-well plate with or without LAB cells or fractionated samples for 4 days at 37°C under 5% CO2 in air.
Neutralization of TLR2 in PP cells
Murine PP cells were preincubated with 0.5 µg/ml anti-TLR2 antibody (purified anti-mouse/human CD282, clone T2.5, Biolegend, San Diego, CA, USA) or isotype antibody (purified mouse IgG1, clone MOPC-21, Biolegend) at 37°C for 30 min and then cultured with or without the purified MVs (Fr. 3 obtained by the density gradient ultracentrifugation) or L. sakei NBRC15893 (50 µg/well) cells for 4 days at 37°C under 5% CO2 in air.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of IgA were determined by ELISA. In brief, 96-well plates (MaxiSorp; Thermo Fisher Scientific) were coated with 50 µl of 10 µg/ml goat anti-mouse IgA (Bethyl Laboratories, Montgomery, TX, USA) for 1 hr at 22–25°C. After washing with phosphate-buffered saline (PBS) containing 0.05% (w/v) Tween 20, the wells were blocked with 300 µl PBS containing 1% bovine serum albumin (Sigma-Aldrich) at room temperature for 1 hr, and 50 µl of the diluted samples was added to each well and incubated at room temperature for 1 hr. After washing, 50 µl of 25 ng/ml goat anti-mouse horseradish-peroxidase-conjugated IgA (Bethyl Laboratories) was added, and the wells were further incubated at room temperature for 1 hr. After washing, 50 µl of the TMB microwell peroxidase substrate system (SeraCare, Milford, MA, USA) was added and incubated at room temperature. After the addition of 25 µl of 1 M sulfuric acid, the absorbance at 450 nm was measured using an infinite F200 microplate reader (Tecan, Zürich, Switzerland). Purified mouse IgA (κ isotype control; BD) was used as a standard.
The concentrations of IL-6 were determined by ELISA using a Mouse IL-6 DuoSet ELISA (R&D Systems, Minneapolis, MN, USA).
RESULTS
Production of MVs by L. sakei NBRC15893
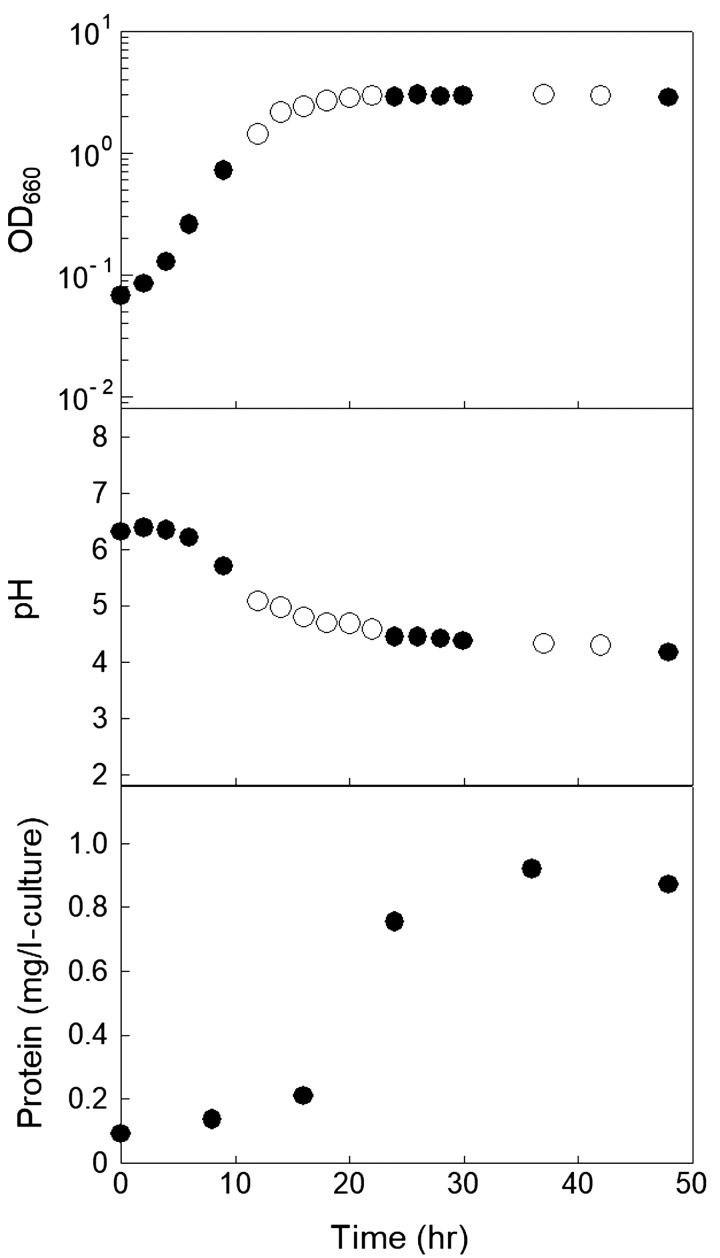
To demonstrate whether L. sakei subsp. sakei NBRC15893 produces MVs, the microcomponents in the culture supernatant (crude MV fraction) were first recovered by ultracentrifugation. The time courses of the OD660 and pH of the culture and the amount of protein recovered as the crude fraction are shown in Fig. 1. The amount of recovered protein increased with the increase in bacterial concentration. A typical TEM image of the crude MV fraction at 24 hr is shown in Fig. 2A. MV-like spherical structures with a diameter of 30–400 nm, which were similar to the MVs produced by other bacteria, were observed. This indicated that L. sakei NBRC15893 releases MVs. The MVs were purified using a routine purification method. The protein concentrations and absorbance at 260 and 280 nm of each fraction (Fr. 1–5) obtained by density gradient ultracentrifugation are shown in Table 1. Brown and white bands were observed in Fr. 2 and 3, respectively, and the protein concentration of Fr. 3 was higher than that of the other fractions. TEM observation of each fraction further revealed that the MVs were much more heavily concentrated in Fr. 3 than in the other fractions and that Fr. 2 and Fr. 4 contained only a small number of MVs (Fig. 2B–F). These results indicated that the MVs derived from L. sakei NBRC15893 contained proteins and nucleic acids, similar to other bacterial MVs.
Fig. 1.
Time course of the growth of L. sakei NBRC15893, pH of the culture broth, and MV production.
Protein concentration is expressed as the amount of protein obtained from 1 l of the culture supernatant.
Open circles and closed circles indicate the values of OD660 and pH of the culture broth in different runs, respectively. The values are the average of three measurements, and the measurement error was within approximately 5%. Repeatability was confirmed by repeating the experiments at least three times, and typical results are shown.
Fig. 2.

Typical transmission electron microscope images of MVs.
(A) Crude membrane vesicle fraction precipitated by the ultracentrifugation and the fractions obtained by the density-gradient ultracentrifugation of the precipitate: (B) Fr. 1, (C) Fr. 2, (D) Fr. 3, (E) Fr. 4, (F) Fr. 5. Scale bars, 200 nm. Repeatability was confirmed by repeating the similar experiment at least three times, and typical results are shown.
Table 1. Concentrations of cytoplasmic constituents of fractions obtained by density gradient ultracentrifugation.
| Fraction No. | Protein (mg/ml) | A260 | A280 |
|---|---|---|---|
| 1 | 0.02 | 0.07 | 0.02 |
| 2 | 0.11 | 0.29 | 0.14 |
| 3 | 3.42 | 1.59 | 0.92 |
| 4 | 0.30 | 1.26 | 0.76 |
| 5 | 0.01 | 0.53 | 0.11 |
The values are the average of three measurements, and the measurement error was within approximately 5%. Repeatability was confirmed by repeating the experiments at least three times, and typical results are shown.
IgA-enhancing effects of L. sakei NBRC15893 MVs
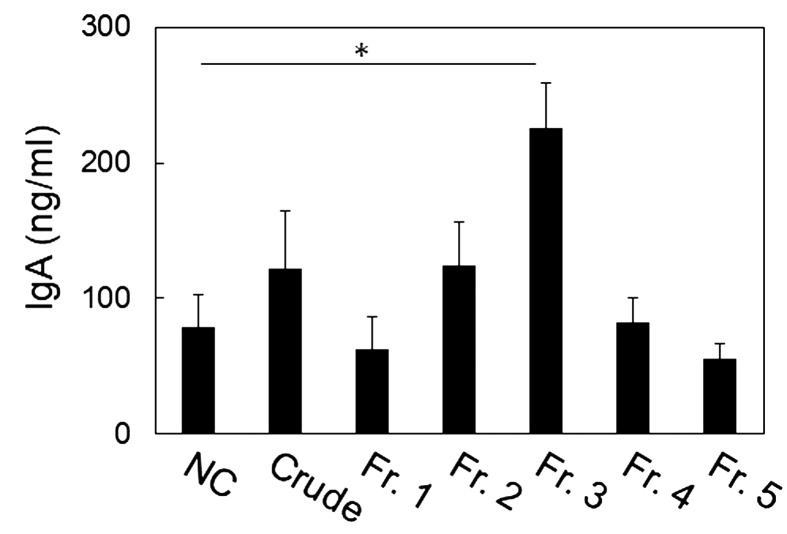
IgA production from the PP cells was evaluated by ELISA with the crude MV fraction at 24 hr and the fractions obtained by the density gradient ultracentrifugation. The crude MV fraction enhanced IgA production by the PP cells, showing that the microcomponents derived from L. sakei NBRC15983 were involved in the IgA-enhancing effects. As shown in Fig. 3, only the IgA concentration of the cells cultured with Fr. 3 was significantly increased compared with that of the negative control group (p=0.021). These results indicated that MVs derived from L. sakei NBRC15893 in the culture broth enhanced IgA production by the PP cells.
Fig. 3.
IgA-enhancing effects of fractions obtained by density gradient ultracentrifugation in Peyer’s patch (PP) cells.
The PP cells (1.0 × 105 cells/well) were cultured with the crude membrane vesicle (MV) fraction (protein concentration, 37 µg/ml) or equal volumes of each fraction obtained by the density-gradient ultracentrifugation of 10–45% (v/v) OptiPrep (one-hundredth the protein concentration of each fraction shown in Table 1) for 4 days. NC, negative control. Crude, crude MV fractions. The data are presented as means ± standard deviations of triplicate samples. *p<0.05, Student’s t-test.
Involvement of TLR2 in the IgA-enhancing effect of MVs
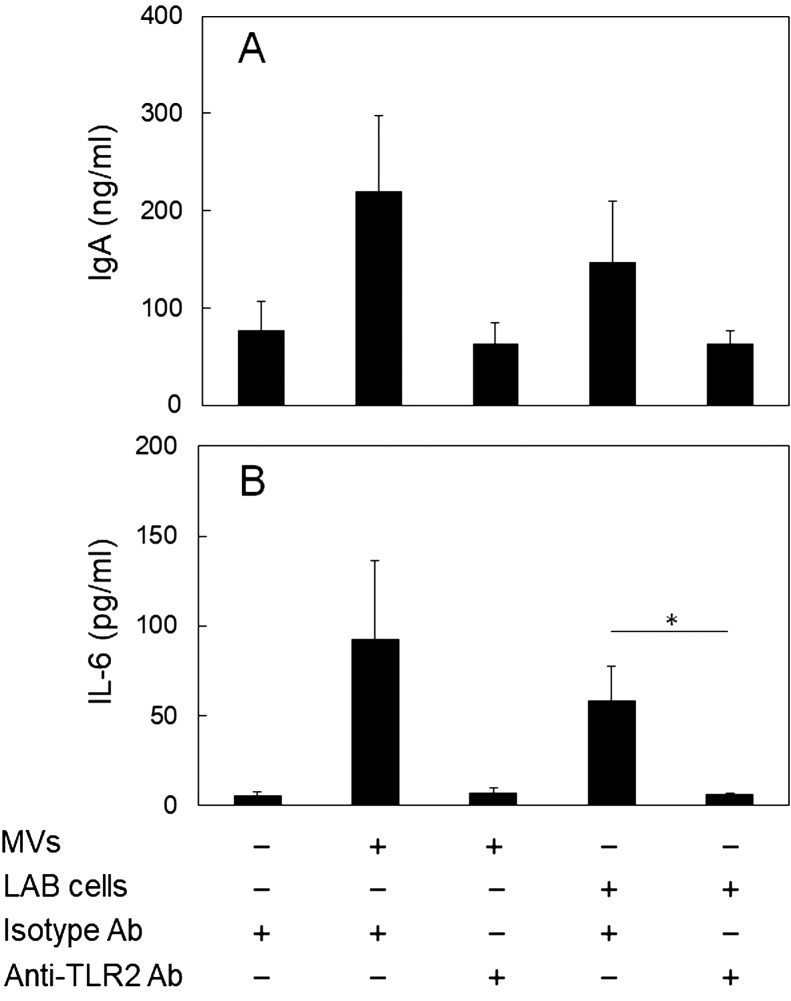
The IgA-enhancing effects of several LAB have been shown to be mediated by TLR2 expressed on DCs, which recognize the cell wall components of gram-positive bacteria [8, 10]. Thus, the involvement of TLR2 in the IgA-enhancing effects of the MVs derived from L. sakei NBRC15893 and the LAB cells was investigated using an anti-TLR2 antibody for neutralization of TLR2. The increases in the IgA production induced by the addition of purified MVs (Fr. 3) and the LAB cells were completely inhibited by neutralization of TLR2 with anti-TLR2 (Fig. 4A), indicating that both L. sakei NBRC15893 cells and the MVs they release enhance IgA production from PP cells via TLR2. In addition, since it was reported that several LAB strains induce IL-6 production from DCs [8,9,10], which activates B cells to produce IgA, the IL-6 concentration in the culture supernatant was measured. MVs and LAB cells induced IL-6 production from PP cells, and the induction by MVs and LAB cells was suppressed by addition of anti-TLR2 antibody (p=0.071 and 0.045, respectively; Fig. 4B), similar to that in IgA production. It was revealed that MVs of the strain induced IL-6 production from PP cells via TLR2. These results suggested that both MVs and LAB cells enhance IL-6 production via TLR2, resulting in promotion of IgA production.
Fig. 4.
Involvement of TLR2 in the IgA-enhancing effects of the purified MVs and the cells of L. sakei NBRC15893.
Peyer’s patch (PP) cells (1.0 × 105 cells/well) were cultured in the presence or absence of the anti-TLR2 antibody for 4 days with or without the MVs (protein concentration; 34 µg/ml) or L. sakei NBRC15893 cells (50 µg/ml). (A) IgA concentration and (B) IL-6 concentration in the culture supernatant was measured by ELISA. The data are expressed as means ± standard deviations of triplicate samples. *p<0.05, Student’s t-test.
DISCUSSION
We demonstrated that the nonpathogenic gram-positive bacterium L. sakei NBRC15893 produced MVs, which, along with the cells themselves, exhibit IgA-enhancing effects in PP cells via TLR2.
Envelope structures of gram-negative bacteria are stabilized via protein cross-links that reach from the inner membrane (IM) through the PG to the outer membrane (OM). MVs are produced through liberation of the OM from the covalent and noncovalent OM-PG-IM cross-links without concomitant damage and loss of membrane integrity either due to the loss of OM proteins, periplasmic lipoproteins, and PG or through the accumulation of misfolded proteins in the periplasm [20, 21]. In addition, it has been reported that explosive cell lysis produces membrane fragments that rapidly form MVs through the activity of a cryptic prophage endolysin [22]. Although little is known about the mechanism of MV production of gram-positive bacteria, it is predicted that an increase in the turgor pressure of the cytoplasm, followed by distortion of the cell wall, and presence of a channel that penetrates the cell wall result in MV release [23]. Recently, Toyofuku et al. reported that the cell membrane was extruded from the pores of PG degraded by endolysin to release MVs in Bacillus subtilis [24].
According to the Kyoto Encyclopedia of Genes and Genomes database (KEGG; http://www.genome.jp/kegg/), several lactobacilli such as L. plantarum WCFS1, L. casei BL23, L. salivarius JCM1046, and L. rhamnosus GG possess genes encoding endolysin. Although we did not confirm whether L. sakei NBRC15893 also possesses an endolysin-encoding gene, it is possible that endolysin is also involved in the MV production by the LAB and that MV production is not limited to certain strains but occurs widely in most LAB strains.
Cell wall components such as lipoteichoic acid of L. casei YIT 9029 and L. fermentum YIT 0159 [25], teichoic acid of L. plantarum ATCC 14917T [26], PG of L. pentosus strain b240 [8] and L. casei YIT 9029 [27], and lipoprotein of L. acidophilus X37 [28] have been reported to exert immunomodulatory effects via TLR2. MVs generally contain these cell wall components in addition to nucleic acids and proteins. For example, the lipoproteins contained in the MVs of M. tuberculosis were shown to inhibit the antigen presentation of DCs and macrophages via TLR2 [16], and components considered to be lipoproteins contained in the MVs of C. perfringens also induced the IL-6 production of macrophage-like cells [17]. Here, we found that the whole cells of L. sakei NBRC15893 also enhanced IgA production via TLR2 signaling, suggesting that IgA production by the MVs may be stimulated by the TLR2 ligands that are derived from the cells and also contained in the MVs.
Regarding the induction of IgA production from PP cells, it has been reported that several LAB cells produce IL-6 and other cytokines via TLR2 of DCs [8,9,10], resulting in activation of B cells promoting IgA production. In this study, it was suggested that IL-6 induced by MVs of L. sakei NBRC15893 promote IgA production as well as the LAB cells. It is necessary to clarify the detailed mechanism of the IgA-enhancing effect of MVs derived from the strain.
Furthermore, the MVs of L. sakei NBRC15893 may be taken up by PP cells in vivo, resulting in enhancement of IgA production in the gut, as Al-Nedawi et al. [18] demonstrated that MVs were taken into the DCs of the PP after oral administration of the MVs derived from L. rhamnosus JB-1. With increasing recognition of the influence of gut microbiota on health and diseases of the host, the finding that LAB in the host microbiota produce MVs with immunomodulatory effects, including an IgA-enhancing effect, indicates an important role of MVs in mediating interactions between the host and bacteria. Moreover, MVs are frequently isolated from biofilms and have been related to the aggregation of bacteria and biofilm formation [29, 30]. When LAB are used as probiotics, they are expected to become established in the intestinal tract of the host. Since the aggregation of bacterial cells is an important step leading to the successful establishment [31], gaining a detailed understanding of the mechanism of MV production and its functions is very important to ensure a beneficial effect.
In summary, we found that the MVs of L. sakei NBRC15893 could activate the mucosal immune system via enhanced IgA production. Moreover, antigen-specific secretion of IgA to the mucosal surface can be induced by the nasal immunization of MVs derived from pathogenic bacteria such as Porphyromonas gingivalis, indicating that MVs are effective agents for the control of the mucosal immune system [32]. Our findings highlight the potential of the application of MVs derived from probiotics with established safety based on food experience for overall health benefits via control of the mucosal immune system and their potential as a vaccine adjuvant via the mucosa.
Acknowledgments
We thank Dr. Yoshihiro Ojima (Osaka City University) for valuable discussions. We also gratefully acknowledge the Japan Society for the Promotion of Science (JSPS) KAKENHI grant received (Nos. 16K18302 and 18K04857 [to S.Y.Y]; 15H05790, 16H01373, 17H04134, and 26293111 [to J.K.]) for support of this study. This research was also supported by a Strategic Project to Support the Formation of Research Bases at Private Universities: Matching Fund Subsidy from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), 2013-2017 (to Y.K.), by the Kansai University Fund for Supporting Young Scholars, 2015 (to S.Y.Y.), and by the Research Group Fund of Kansai University, 2018 (to S.Y.Y.). This work was also supported in part by the Ministry of Health, Labour and Welfare of Japan (to J.K.) and the Japan Agency for Medical Research and Development (Nos. JP17fk0108223h0002, JP17ek0410032s0102, JP17fk0108207h0002, JP17ek0210078h0002, JP17ak0101068h0001, and JP17gm1010006s0101 [to J.K.]), the Terumo Foundation for Life Sciences and Arts (to J.K.), ONO Medical Research Foundation (to J.K.), and the Canon Foundation (to J.K.).
References
- 1.Chassaing B, Kumar M, Baker MT, Singh V, Vijay-Kumar M. 2014. Mammalian gut immunity. Biomed J 37: 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooper LV, Macpherson AJ. 2010. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 10: 159–169. [DOI] [PubMed] [Google Scholar]
- 3.Pabst O. 2012. New concepts in the generation and functions of IgA. Nat Rev Immunol 12: 821–832. [DOI] [PubMed] [Google Scholar]
- 4.Okai S, Usui F, Ohta M, Mori H, Kurokawa K, Matsumoto S, Kato T, Miyauchi E, Ohno H, Shinkura R. 2017. Intestinal IgA as a modulator of the gut microbiota. Gut Microbes 8: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerutti A. 2008. The regulation of IgA class switching. Nat Rev Immunol 8: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T. 2011. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 34: 247–257. [DOI] [PubMed] [Google Scholar]
- 7.Perdigón G, Fuller R, Raya R. 2001. Lactic acid bacteria and their effect on the immune system. Curr Issues Intest Microbiol 2: 27–42. [PubMed] [Google Scholar]
- 8.Kotani Y, Kunisawa J, Suzuki Y, Sato I, Saito T, Toba M, Kohda N, Kiyono H. 2014. Role of Lactobacillus pentosus Strain b240 and the Toll-like receptor 2 axis in Peyer’s patch dendritic cell-mediated immunoglobulin A enhancement. PLoS One 9: e91857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi Y, Kunitoh-Asari A, Hayakawa K, Imai S, Kasuya K, Abe K, Adachi Y, Fukudome S, Takahashi Y, Hachimura S. 2014. Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PLoS One 9: e86416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai F, Hosoya T, Ono-Ohmachi A, Ukibe K, Ogawa A, Moriya T, Kadooka Y, Shiozaki T, Nakagawa H, Nakayama Y, Miyazaki T. 2014. Lactobacillus gasseri SBT2055 induces TGF-β expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS One 9: e105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathirana RD, Kaparakis-Liaskos M. 2016. Bacterial membrane vesicles: Biogenesis, immune regulation and pathogenesis. Cell Microbiol 18: 1518–1524. [DOI] [PubMed] [Google Scholar]
- 12.Eberlein C, Baumgarten T, Starke S, Heipieper HJ. 2018. Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl Microbiol Biotechnol 102: 2583–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyofuku M, Morinaga K, Hashimoto Y, Uhl J, Shimamura H, Inaba H, Schmitt-Kopplin P, Eberl L, Nomura N. 2017. Membrane vesicle-mediated bacterial communication. ISME J 11: 1504–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirlashari MR, Høiby EA, Holst J, Lyberg T. 2001. Outer membrane vesicles from Neisseria meningitidis: effects on cytokine production in human whole blood. Cytokine 13: 91–97. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto M, Ohno T, Graham DY, Yamaoka Y. 2011. Helicobacter pylori outer membrane proteins on gastric mucosal interleukin 6 and 11 expression in Mongolian gerbils. J Gastroenterol Hepatol 26: 1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman-Freedman A, Jacobs WR, Jr, Porcelli SA, Casadevall A. 2011. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest 121: 1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obana N, Nakao R, Nagayama K, Nakamura K, Senpuku H, Nomura N. 2017. Immunoactive clostridial membrane vesicle production is regulated by a sporulation factor. Infect Immun 85: e00096–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Nedawi K, Mian MF, Hossain N, Karimi K, Mao YK, Forsythe P, Min KK, Stanisz AM, Kunze WA, Bienenstock J. 2015. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J 29: 684–695. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Lee K, Hsu M, Nau G, Mylonakis E, Ramratnam B. 2017. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol 17: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwechheimer C, Sullivan CJ, Kuehn MJ. 2013. Envelope control of outer membrane vesicle production in Gram-negative bacteria. Biochemistry 52: 3031–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jan AT. 2017. Outer Membrane Vesicles (OMVs) of Gram-negative bacteria: a perspective update. Front Microbiol 8: 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK, Osvath SR, Cárcamo-Oyarce G, Gloag ES, Shimoni R, Omasits U, Ito S, Yap X, Monahan LG, Cavaliere R, Ahrens CH, Charles IG, Nomura N, Eberl L, Whitchurch CB. 2016. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun 7: 11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown L, Wolf JM, Prados-Rosales R, Casadevall A. 2015. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13: 620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L. 2017. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat Commun 8: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, Yoshikai Y. 2003. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol 10: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaji R, Kiyoshima-Shibata J, Nagaoka M, Nanno M, Shida K. 2010. Bacterial teichoic acids reverse predominant IL-12 production induced by certain Lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J Immunol 184: 3505–3513. [DOI] [PubMed] [Google Scholar]
- 27.Shida K, Kiyoshima-Shibata J, Kaji R, Nagaoka M, Nanno M. 2009. Peptidoglycan from lactobacilli inhibits interleukin-12 production by macrophages induced by Lactobacillus casei through Toll-like receptor 2-dependent and independent mechanisms. Immunology 128 Suppl: e858–e869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeuthen LH, Fink LN, Frøkiaer H. 2008. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 124: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Chanda W, Zhong M. 2015. The relationship between biofilm and outer membrane vesicles: a novel therapy overview. FEMS Microbiol Lett 362: fnv117. [DOI] [PubMed] [Google Scholar]
- 30.Liao S, Klein MI, Heim KP, Fan Y, Bitoun JP, Ahn SJ, Burne RA, Koo H, Brady LJ, Wen ZT. 2014. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol 196: 2355–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiyama K, Sugiyama M, Mukai T. 2016. Adhesion properties of lactic acid bacteria on intestinal mucin. Microorganisms 4: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakao R, Hasegawa H, Ochiai K, Takashiba S, Ainai A, Ohnishi M, Watanabe H, Senpuku H. 2011. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS One 6: e26163. [DOI] [PMC free article] [PubMed] [Google Scholar]