Abstract
Obesity is a disease with a rapidly increasing prevalence all over the world in recent years. Genetic and environmental factors are involved in the etiology of obesity, and the effect of microbiota on obesity is becoming increasingly clear. Obesity treatment has various treatment modalities such as behavior modification, medical nutrition therapy, physical activity enhancement, and surgical intervention. When other treatment methods are not successful, bariatric surgery is usually resorted to as the treatment method. Some changes such as food choices, the level of hormones and enzymes due to anatomical changes, pH of the stomach, and microbiota are observed after bariatric surgery. Alteration in the microbiota composition after bariatric surgery has also been reported to be important in achieving body weight loss and preserving body weight loss.
Keywords: obesity, bariatric surgery, microbiota
INTRODUCTION
Obesity is defined by the World Health Organization (WHO) as an accumulation of excess fat in the body to the extent that it can impair health [1]. Although excessive food intake and physical inactivity are usually thought of as the cause of obesity, the etiology of obesity is quite complex. There are many factors in the etiology of obesity, such as environmental, genetic, and lifestyle [2]. Besides, the microbiota has recently been reported as an important component in obesity etiology [3,4,5,6]. There are various treatment modalities such as medical nutrition therapy, medical treatment, and physical activity enhancement for obesity [2]. Besides these treatments, if the appropriate indications are present, bariatric surgery as a surgical intervention would be another treatment [7]. After the application of bariatric surgery, body weight loss occurs with changes in the metabolism of bile acids (BAs), in gastric pH, in the metabolism of hormones, and in microbiota [8].
OBESITY
Obesity is defined as a condition of abnormal or excessive fat accumulation in adipose tissue, to the extent that health is impaired [1]. This is considered a risk factor for a number of chronic diseases including cardiovascular diseases, hypertension, type 2 diabetes, nonalcoholic fatty liver disease, and colon cancer. Over the past 20 to 30 years, the prevalence of obesity has also been increasing rapidly in not only developed countries but also in developing countries. This is related to lifestyle changes, which include decreased physical activity and a Western-type diet with a high energy content [9]. In addition to physiological regulatory mechanisms, numerous complex factors that affect each other, such as environmental and genetic factors and psychological and cultural conditions, are among the causes of obesity [10]. Obesity is one of the most easily diagnosed but difficult to treat diseases. However, it should be treated to prevent various health problems. Obesity management should be planned specifically for each individual [11]. Nowadays, treatment methods for obesity as follows; diet therapy, behavior modification therapy, medical treatment, and surgical treatment [12].
BARIATRIC SURGERY
Bariatric surgery is one of the treatment methods that are effective in the treatment of obesity and complications [13]. Thanks to bariatric surgery, long-term permanent body weight loss is achieved, metabolic effects of obesity are reduced, many diseases are prevented, and quality of life is increased [14]. Body weight loss with bariatric surgery is achieved through reduction of nutrient digestion, alteration of food preferences, acceleration of gastric emptying, regulation of hormonal changes (e.g. glucagon-like peptide 1 [GLP-1] and peptide YY [PYY]) and alterations in the metabolism of BAs. Although bariatric surgery is suitable for obesity treatment, some complications such as gastric outlet obstruction, mesh erosion, slippage, gastroesophageal reflux, nutritional deficiencies, internal herniation, and marginal ulcerations can occur rarely [15]. Indications for bariatric surgery were established by the US National Institutes of Health in 1991 (Table 1) [16]. There are various bariatric surgical methods according to their effect mechanisms (Table 2) [17].
Table 1. Indications for surgical operation [16].
| • BMI=40 kg/m2 or additional disease (type 2 diabetes, hypertension, sleep apnea, hyperlipidemia) together with BMI >35 kg/m2 |
| • Acceptance of surgical risk |
| • Failure of nonsurgical treatments |
| • Psychiatric stability, no alcohol and drug dependence |
| • Patients is well-motivated, knowing the operation and sequelae |
| • No medical problems that will harm the surgeon |
| • No uncontrolled psychotic and depressive disorder |
| • Complete family and social support |
Table 2. Methods of surgical intervention [17].
| Restrictive |
|---|
| Laparoscopic adjustable gastric banding (LAGB) |
| Sleeve gastrektomy (SG) |
| Vertical banded gastroplasty (VBG) |
| Malabsorptive |
| Biliopancreatic diversion (BPD) |
| Jejunoileal bypass (JIB) |
| Combined restrictive and Malabsorptive |
| Roux-en-Y gastric bypass (RYGB) |
| Duodenal switch (DS) with BPD |
BARIATRIC SURGICAL METHODS
Roux-en-Y gastric bypass (RYGB) is the gold standard and the most commonly practiced bariatric surgery in the world [18]. This method consists of two steps. In the first step, the stomach capacity is left to be about 30 cm3. Roux sputum can then be pulled up from the stomach, the front of the colon and back of the stomach, or behind the colon and stomach for gastrojejunostomy [19]. Intake of food and energy decreases due to the reduction in stomach volume. A small amount of fat malabsorption also occurs [20].
Laparoscopic sleeve gastrectomy (LSG) is the removal of the remaining long, 80% of lateral aspect of the stomach in a vertical fashion, leaving a long, tubuler gastric tube [21]. LSG is preferred for patients who have super obesity and a BMI <50 kg/m2 [22]. Due to the reduction of gastric volume, nutrient intake and energy intake are restricted. However, there is a reduction in plasma levels of ghrelin [23].
Laparoscopic adjustable gastric banding (LAGB) involves the placement of an adjustable silicone band around the upper part of the stomach, thus forming a small gastric space over the gastric band. The size of the gap between the upper stomach space and the back of the stomach can be adjusted by filling it with sterile saline injected through the abdominal wall. Adjustment of the band can be done gradually over time during postoperative follow-up [24]. This method provides body weight loss by reducing nutrient uptake by a completely restrictive effect [17].
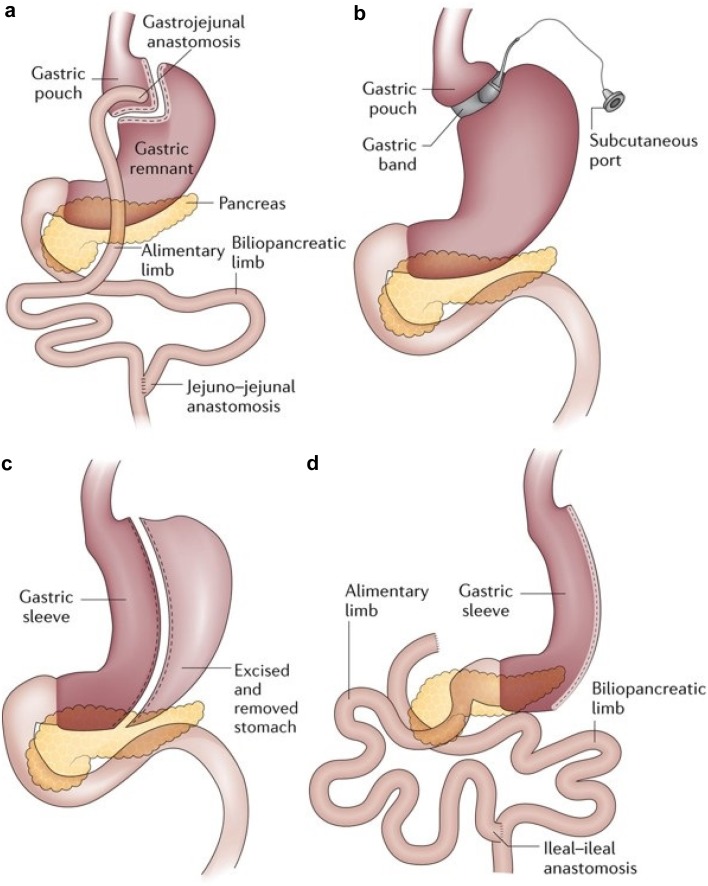
Biliopancreatic diversion (BPD) consists of three main components: a tube stomach with preserved pylorus, distal ileoanal anastomosis, and anastomosis of the proximal duodenal bile duct. Body weight loss is provided by the reduction of gastric volume and decrease of ghrelin hormone, increasing peptide-YY. In this technique, hormonal changes with anatomical changes are thought to lead to body weight loss [25]. Common bariatric surgical procedures are shown in Fig. 1 [19].
Fig. 1.
Common bariatric surgical procedures [19].
a: Roux-en-Y gastric bypass; b: adjustable gastric banding; c: sleeve gastrectomy; d: biliopancreatic diversion with duodenal switch.
Hormones known to generate hunger, and reduce satiation and satiety, adapt to weight loss in a manner encouraging hunger and weight gain. There are sustained reductions in leptin, insulin, and peptide YY while levels of ghrelin and pancreatic polypeptide increase with intentional weight loss. The hormonal changes after the different techniques of the bariatric surgeon are summarized in Table 3 [26]. Surgery induces changes in both environmental and systemic factors, as well as anatomical changes in the digestive tract (Table 4) [27].
Table 3. A summary of the changes in key hormones related to energy balance and weight loss for each of the established surgical procedures and for intentional dietary behavioral weight loss [26].
| RYGB | LSG | LAGB | BPD | Behavioral weight loss | |
|---|---|---|---|---|---|
| Leptin | ▼ | ▼ | ▼ | ▼ | ▼ |
| Insulin | ▼ | ▼ | ▼ | ▼ | ▼ |
| Adiponectin | ▲ | ▲ | ▲ | ▲ | ▲ |
| Glucagon | ▲ | ? | ▬ | ? | ▼ |
| Ghrelin | ▲, ▼, ▬ | ▼ | ▲, ▬ | ▲, ▬ | ▲ |
| GLP-1 | ▲ | ▲ | ▬ | ? | ▬ |
| PYY | ▲ | ▲ | ? | ▲ | ▬ |
▲: a substantial number of studies indicate an increase;▼: a substantial number of studies indicate a decrease; ▬: a substantial number of studies found no change; ?: too few data.
RYGB: Roux-en-Y gastric bypass; LSG: Laparoscopic sleeve gastrectomy; LAGB: Laparoscopic adjustable gastric banding; BPD: Biliopancreatic diversion.
Table 4. Dietary and digestive changes induced by different types of bariatric surgery [27].
| Changes | LAGB | LSG | RYGB |
|---|---|---|---|
| Time spent chewing | Increased | Increased | Increased |
| Food intake | Decreased | Decreased | Decreased |
| Food transit time | Decreased | No change | Increased |
| Food choices and preferences | Preference for pureed food and fewer fibre-containing foods | No change | Reduced preference for high-fat or high-sugar foods |
| Acid production | No change | No change | Disrupted |
| Ghrelin levels | No change | Decreased | No change |
| GLP1 and PYY levels | No change | No change | Increased |
LAGB: Laparoscopic adjustable gastric banding; LSG: Laparoscopic sleeve gastrectomy; RYGB: Roux-en-Y gastric bypass.
GUT MICROBIOTA
The microbiota is defined as a community of microorganisms located in a prominent ecological environment or place. It comprises commensal, symbiotic, and pathogenic microorganisms living in the human body. Microbiome is defined as the genetic pool of microbiota living in a specific place and their relation with the environment [28]. It is estimated that there are about 1,014 microorganisms in the human body, more than seventy percent of which are in the colon, and more than 35,000 bacterial strains in the gastrointestinal tract [29]. The microbiota is influenced by many factors such as delivery type, breastfeeding time, transition time to complementary feeding, diet, and use of antibiotics from birth to death [30]. The intestinal microbiota, composed mainly of anaerobic bacteria in which Bacteroides and Firmicutes are involved, has physiological, metabolic, immunological, and neural functions in the body [31]. It has been reported that the human intestinal microbiota is composed of more than 1,000 species. It can be classified into 6 bacterial clusters in healthy individuals. These include Firmicutes (including gram-positive strains of Clostridium, Eubacterium, Ruminococcus, Butyrivibrio, Anaerostipes, Roseburia, Faecalibacterium, etc.), Bacteroidetes (including gram-negative strains of Bacteroides, Porphyromonas, Prevotella, etc.), Proteobacteria (including gram-negative strains such as Enterobacteriaceae), Actinobacteria (including the gram-positive Bifidobacterium genus), Fusobacteria, and Verrucomicrobia (including Akkermansia, etc.) [32]. Bacteroidetes and Firmicutes constitute more than 90% of total intestinal microbiota. The most important components of the human intestinal microbiota are the obligate anaerobes of the genus Bacteroides, Eubacterium, Clostridium, Ruminococcus, Peptococcus, Peptostreptococcus, Bifidobacterium, and Fusobacterium and facultative anaerobes such as Escherichia, Enterobacter, Enterococcus, Klebsiella, Lactobacillus, and Proteus. Methanogenic archaea have also been reported extensively [33]. The most important Methanogenic archaeon in the human gut is Methanobrevibacter smithii.
Changes in microbiota content affect human health at a significant level. It is reported that many noncommunicable diseases such as obesity, type 2 diabetes, asthma, allergies, and atopic diseases, inflammatory bowel disease, metabolic syndrome, necrotizing enterocolitis, and atherosclerosis are closely associated with the intestinal microbiota [34].
GUT MICROBIOTA IN PATIENTS WITH OBESITY
It is reported that genetic and environmental factors influence the etiology of obesity. Researchers have also reported that intestinal microbiosis contributes to the regulation of energy and fat metabolism and that it affects obesity and its complications [35]. It has been reported that patients with obesity have less variability in the intestinal microbiota than thin individuals [36]. The important function that separates microbial strains from obese and thin individuals is the inability to produce fermentation. Another difference is that short-chain fatty acids cannot be produced from indigestable food items [37].
Intestinal microbiota studies in both humans and animal models have helped clarify the role of microbial activity in the etiology of obesity. It is reported that patients with obesity have fewer Bacteroides and more Firmicutes in their microbiota than normal-weight people. It has been stated that diets rich in saturated fatty acids lead to the development of hepatic steatosis and obesity, increasing the amounts of Firmicutes/Bacteroidetes ratio in the intestinal microbiota [38]. Fat and carbohydrate-restricted diets and body weight loss cause the amount of Bacteroidetes to increase [39]. On the other hand, some studies show that there is no relationship between body mass index and Firmicutes/Bacteroidetes [40, 41]. However other studies show an increase in Firmicutes/Bacteroidetes ratio in obesity and insulin resistance. Reduction of carbohydrate intake in patients with obesity decreases the butyrate levels in feces and level of Roseburia spp. and Eubacterium rectale [42]. The microbiota is affected by the loss of body weight caused by diet and exercise. It has been reported that the amounts of Bacteroides and Lactobacillus increase as a result of energy restriction and exercise in patients with obesity. However, no changes were seen in overweight adolescents who lost less than 2 kg in body weight [43]. In view of such information, intestinal microbiota modification may be a potential therapeutic treatment for prevention or reversal of obesity.
GUT MICROBIOTA AFTER BARIATRIC SURGERY
Significant changes are reported in the intestinal microbiota after bariatric surgery. Possible mechanisms for the changes in the intestinal microbiota include food choices and preferences, reduction of food consumption, and nutrient malabsorption [44]. Short-term dietary changes may cause rapid changes in the intestinal microbiota composition. Prevotella enterotypes have been reported to be associated with both complex carbohydrate-rich and simple carbohydrate-rich diets, while the Bacteroides enterotype is associated with a typical “Western diet” rich in animal protein and saturated fat [45]. In particular, low-fat, high-carbohydrate diets and high-carbohydrate, low-glycemic-indexed diets affect the amounts of specific strains differently in the intestinal microbiota [46]. Diet therapy after bariatric surgery also causes changes in microbiota due to these reasons.
A second factor affecting the change in microbiota after bariatric surgery has been reported to be BAs [8]. BAs can autoregulate their synthesis and intestinal reabsorption through modulation of the nuclear-located farnesoid X receptor (FXR). Another pathway of autoregulation is the G-linked protein TGR5, but this pathway is not yet fully understood [47]. Recently, the physiological role of BAs has been linked to the control of glucose homeostasis, beta-cell function, and energy consumption. These roles of BAs are associated with FXR and TGR5 [8]. Biliopancreatic extract biliary fluid and nutrients are separated from each other in RYGB. BAs come together with nutrients in the lower parts of the intestine. The distal jejunum and proximal ileum are excesseively exposed to the nutrients. Dietary lipids are surrounded by the Bas, while BAs cycling in the upper intestine becomes blunted. As a result, increased plasma BAs and FGF15/19 levels normalize the postprandial BAs response after surgery [48]. The mechanism underlying the beneficial effects of bariatric surgery has been reported to be alterations in BAs metabolism [43]. The change in BAs flow has a definite effect on the changes in microbiota after bariatric surgery, too. In the proximal jejunum, the absence of nutrient transit and decreased mobility lead to an increase in the number of bacteria [27]. The changes in BA flow also alter the 7α-dehydroxylation capacity of the intestinal microbiota, which is involved in the synthesis of the secondary (intermediate) BAs. Administration of a diet supplemented with the primary BAs colic acid to rats increase the presence of Firmicutes, which contains the enzyme 7α-hydroxylase such as Clostridium spp. [49].
Hormones such as leptin and ghrelin may change after bariatric surgery. The change in hormones is related to both energy metabolism and microbiota [50]. Although the relationship between the intestinal microbiota and ghrelin is not fully understood, it is reported that prebiotics modulates the intestinal microbiota and prebiotics decrease circulating levels of ghrelin [51].
Serum leptin levels in circulation have been reported to be positively correlated with Mucispirillum, Lactococcus, and the high amount of Lachnospiraceae which cannot be classified. Another study reported that leptin has a negative correlation with Bacteroides, Clostridium, and Prevotella, and a positive correlation with Bifidobacterium and Lactobacillus [52]. Studies have emphasized that further research is needed, although hormones have been reported to affect the intestinal microbiota [38, 53,54,55].
Another factor affecting microbiota is reported to be changes in pH. After surgery, pH increases as the volume of the stomach shrinks. The changing pH affects every part of the digestive system after the stomach. Increased pH can affect microbiota at a significant level. It has been reported that Bacteroidetes decrease due to pH changes after bariatric surgery, while Firmicutes and Actinobacteria increase [56].
After bariatric surgery, microbiota diversity changes due to the reasons mentioned above. Table 5 summarizes the way in which the numbers of microorganisms are affected after bariatric surgery [8].
Table 5. Taxonomic profile of the gut microbiota after bariatric surgery [8].
| Microbial richness | Reported change |
|---|---|
| Microbial richness | ↑ (h) |
| Firmicutes | ↓ (a,h) |
| Erysipelotrichales (Or) | ↓ (a) |
| Lactobacillales (Or)/Lactobacillus spp. | ↓ (a) |
| Lactobacillus reuteri | ↓ (h) |
| Clostridiales (Or) | ↓ (h) |
| Clostridium difficile | ↓ (h) |
| Clostridium hiranonis | ↓ (h) |
| Blautia spp. | ↓ (h) |
| Dorea spp. | ↓ (h) |
| Streptococcus spp. | ↑ (h) |
| Staphylococcus epidermis | ↓ (h) |
| Roseburia intestinalis | ↓ (h) |
| Eubacterium rectale | ↓ (h) |
| Dialister invisus | ↓ (h) |
| Coprococcus comes | ↓ (h) |
| Anaerostipes caccae | ↓ (h) |
| Gemellasanguinis | ↓ (h) |
| Faecalibacterium prausnitzii | ↓ (h) |
| Veillonella spp. | ↑ (h) |
| Veillonella parvula | ↑ (h) |
| Veillonella dispar | ↑ (h) |
| Bacteroidetes | ↓ (h) |
| Bacteroides/Prevotella spp. | ↑ (h) |
| Alistipes spp. | ↑ (a,h) |
| Actinobacteria | |
| Bifidobacterium spp. | ↓ (h) ↑ (h) |
| Nakamurella spp. | ↓ (h) |
| Mycobacterium kansaii | ↓ (h) |
| Chloroflexi | |
| Thermomicrobium roseum | ↓ (h) |
| Fibrobacteres | |
| Fibrobacter succinogenes | ↓ (h) |
| Verrucomicrobia | |
| Akkermansia muciniphila | ↑ (a,h) |
| Proteobacteria | ↑ (a,h) |
| Gammaproteobacteria (Cl) | ↑ (a,h) |
| Escherichia coli | ↑ (a,h) |
| Klebsiella pneumoniae | ↑(h) |
| Shigella boydii | ↑ (h) |
| Salmonella enterica | ↑ (h) |
| Enterobacter cancerogenus | ↑ (h) |
| Enterobacter hormaechei | ↑ (a) |
| Citrobacter spp. | ↑ (h) |
| Pseudomonas spp. | ↑ (h) |
| Enterococcus faecalis | ↑ (h) |
| Epsilonproteobacteria (Cl) | |
| Helicobacter spp. | ↓ (h) |
| Spirochaetes | |
| Treponema pallidum | ↓ (h) |
| Brachyspira hyodysenteriae | ↓ (h) |
| Fusobacteria | |
| Fusobacterium nucleatum | ↑ (h) |
| Fusobacterium periodonticum | ↓ (h) |
| Archae (K) | ↓ (h) ↑ (a) |
a: animal studies; h: human studies.
CONCLUSION
Bariatric surgery is an important method in the treatment of obesity. It is quite effective in achieving and protecting weight loss. This effectiveness of obesity treatment after bariatric surgery is not only related to food consumption. The altered microbiota after bariatric surgery als have an impact on its effectiveness. Malabsorption status after bariatric surgery, changes in the metabolism of bile acids, changes in gastric pH, and changes in the metabolism of hormones lead to gut microbiota changes. Changes in microbiota also affect energy homeostasis. Because of these reasons, body weight loss is achieved after bariatric surgery.
REFERENCES
- 1.WHO: http://www.who.int/topics/obesity/en/.
- 2.Panuganti KK, Lenehan CP. 2017. Obesity. StatPearls. StatPearls Publishing, Florida. [Google Scholar]
- 3.Duranti S, Ferrario C, van Sinderen D, Ventura M, Turroni F. 2017. Obesity and microbiota: an example of an intricate relationship. Genes Nutr 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seganfredo FB, Blume CA, Moehlecke M, Giongo A, Casagrande DS, Spolidoro JVN, Padoin AV, Schaan BD, Mottin CC. 2017. Weight-loss interventions and gut microbiota changes in overweight and obese patients: a systematic review. Obes Rev 18: 832–851. [DOI] [PubMed] [Google Scholar]
- 5.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. 2016. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang B, Yao M, Ling Z, Li L. 2017. The human microbiota in health and disease. Engineering (Beijing) 3: 71–82. [Google Scholar]
- 7.Dyson PA. 2010. The therapeutics of lifestyle management on obesity. Diabetes Obes Metab 12: 941–946. [DOI] [PubMed] [Google Scholar]
- 8.Anhê FF, Varin TV, Schertzer JD, Marette A. 2017. The gut microbiota as a mediator of metabolic benefits after bariatric surgery. Can J Diabetes 41: 439–447. [DOI] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Kit BK, Flegal KM. 2012. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- 10.Eker E, Şahin M. 2002. Birinci basamakta obeziteye yaklaşım. Sürekli Tıp Eğitim Dergisi. 11: 246. [Google Scholar]
- 11.Nelson JM, Vos MB, Walsh SM, O’Brien LA, Welsh JA. 2015. Weight management-related assessment and counseling by primary care providers in an area of high childhood obesity prevalence: current practices and areas of opportunity. Child Obes 11: 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baysal A., Baş M. 2008. Yetişkinlerde Ağırlık Yönetimi (Yazarlar. M. Baş ve ark.) 17-34 Ekspres Baskı İstanbul.
- 13.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lönroth H, Narbro K, Näslund I, Olbers T, Svensson PA, Carlsson LM. 2012. Bariatric surgery and long-term cardiovascular events. JAMA 307: 56–65. [DOI] [PubMed] [Google Scholar]
- 14.Brethauer SA. 2011. Sleeve gastrectomy. Surg Clin North Am 91: 1265–1279, ix. [DOI] [PubMed] [Google Scholar]
- 15.Miras AD, le Roux CW. 2013. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 10: 575–584. [DOI] [PubMed] [Google Scholar]
- 16.1992. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 55 Suppl: 615S–619S. [DOI] [PubMed] [Google Scholar]
- 17.Sağlam F., Güven H. 2014. Obezitenin Cerrahi Tedavisi. Okmeydanı Tıp Dergisi. 30: 60–65. [Google Scholar]
- 18.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. 2015. Bariatric surgery worldwide 2013. Obes Surg 25: 1822–1832. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen NT, Varela JE. 2017. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 14: 160–169. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, Lieske JC, Collazo-Clavell ML, Sarr MG, Olson ER, Vrtiska TJ, Bergstralh EJ, Li X. 2011. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 149: 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regan JP, Inabnet WB, Gagner M, Pomp A. 2003. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg 13: 861–864. [DOI] [PubMed] [Google Scholar]
- 22.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. 2008. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 247: 401–407. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Liu J. 2009. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obes Surg 19: 357–362. [DOI] [PubMed] [Google Scholar]
- 24.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. 2008. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299: 316–323. [DOI] [PubMed] [Google Scholar]
- 25.Jain-Spangler K, Portenier D, Torquati A, Sudan R. 2013. Conversion of vertical banded gastroplasty to stand-alone sleeve gastrectomy or biliopancreatic diversion with duodenal switch. J Gastrointest Surg 17: 805–808. [DOI] [PubMed] [Google Scholar]
- 26.Dixon JB, Lambert EA, Lambert GW. 2015. Neuroendocrine adaptations to bariatric surgery. Mol Cell Endocrinol 418: 143–152. [DOI] [PubMed] [Google Scholar]
- 27.Aron-Wisnewsky J, Doré J, Clement K. 2012. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol 9: 590–598. [DOI] [PubMed] [Google Scholar]
- 28.Rautava S. 2016. Early microbial contact, the breast milk microbiome and child health. J Dev Orig Health Dis 7: 5–14. [DOI] [PubMed] [Google Scholar]
- 29.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. 2015. The infant microbiome development: mom matters. Trends Mol Med 21: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. 2012. Host-gut microbiota metabolic interactions. Science 336: 1262–1267. [DOI] [PubMed] [Google Scholar]
- 31.Ottman N, Smidt H, de Vos WM, Belzer C. 2012. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol 2: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308: 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel RM, Denning PW. 2015. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res 78: 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. 2013. Assessing the human gut microbiota in metabolic diseases. Diabetes 62: 3341–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. 2009. A core gut microbiome in obese and lean twins. Nature 457: 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. 2005. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 40.Mai V, McCrary QM, Sinha R, Glei M. 2009. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutr J 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. 2007. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 73: 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. 2016. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7: 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, Moreno LA, Veiga O, Redondo-Figuero C, Garagorri JM, Azcona C, Delgado M, García-Fuentes M, Collado MC, Sanz Y, EVASYON Study Group. 2009. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 17: 1906–1915. [DOI] [PubMed] [Google Scholar]
- 44.Peat CM, Kleiman SC, Bulik CM, Carroll IM. 2015. The intestinal microbiome in bariatric surgery patients. Eur Eat Disord Rev 23: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. 2013. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes 37: 216–223. [DOI] [PubMed] [Google Scholar]
- 46.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ, Turnbaugh PJ. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. 2009. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89: 147–191. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad NN, Pfalzer A, Kaplan LM. 2013. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes 37: 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. 2011. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773–1781. [DOI] [PubMed] [Google Scholar]
- 50.Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL. 2012. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 20: 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cani PD, Delzenne NM. 2009. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 15: 1546–1558. [DOI] [PubMed] [Google Scholar]
- 52.Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ. 2013. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One 8: e65465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. 2016. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes 2016: 7353642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, 4th, Taylor CM, Welsh DA, Berthoud HR. 2015. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. 2014. The intestinal microbiome in early life: health and disease. Front Immunol 5: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. 2017. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg 27: 917–925. [DOI] [PubMed] [Google Scholar]