Abstract
Aims
We aimed to assess the relation between number of pregnancies and cardiac structure, function, and arrhythmic events in women with arrhythmogenic cardiomyopathy (AC).
Methods and results
We included female AC patients in a cross-sectional study. Number of pregnancies and pregnancy related symptoms were recorded. Ventricular arrhythmias were defined as aborted cardiac arrest, sustained ventricular tachycardia, or appropriate implantable cardioverter-defibrillator therapy. Right and left ventricular dimensions and function, including strain analyses, were assessed by echocardiography and magnetic resonance imaging. We created a new AC severity score to grade the severity of AC disease. We included 77 women (age 47 ± 16, 43 probands and 34 AC mutation positive female relatives), 19 ± 14 years after last pregnancy. Median number of pregnancies was 2 (0–4); 19 had no previous pregnancies, 16 had 1 pregnancy, 30 had 2, and 12 had ≥3 pregnancies. Presence of a definite AC diagnosis (P = 0.36), severity of AC disease (P = 0.53), and arrhythmic events (P = 0.25) did not differ between groups of pregnancies. Number of pregnancies was related to increased right ventricular outflow tract diameter in single variable analyses [odds ratio (OR) 1.76, 95% confidence interval (CI) 1.08–2.87; P = 0.02], but not when adjusted for body surface area and age (OR 1.56, 95% CI 0.91–2.66; P = 0.11). The number of pregnancies was not associated with any other measures of cardiac structure and function.
Conclusion
Higher number of pregnancies did not seem to relate to a worse phenotype in women with AC.
Keywords: Arrhythmogenic right ventricular cardiomyopathy, Pregnancy , Right ventricle , Cardiomyopathy
Introduction
Arrhythmogenic cardiomyopathy (AC) is an inheritable, chronic, and progressive cardiomyopathy, and one of the leading causes of sudden cardiac death in young individuals.1,2 The most common forms of AC follow autosomal dominant inheritance patterns and affect cardiac desmosomes leading to progressive loss of cardiomyocytes, followed by fibrofatty replacement.1,3 The cardiac phenotype presents as right ventricular (RV) dilatation, wall thinning, aneurysms and impaired function, with increased occurrence of life-threatening ventricular arrhythmias (VA). Pathology is not restricted to the right ventricle as left ventricular involvement is commonly recognized.1,2
Recent reports have indicated that occurrence of VA and disease progression in AC is worsened by vigorous exercise.1,4–7 Pregnancy could be regarded as a comparable state of prolonged haemodynamic stress with increased wall stress due to volume expansion, rise in stroke volume and heart rate, in addition to sympathetic stimulation and hormonal changes.8,9 Thus, pregnancy might affect disease progression in AC. As the disease is frequently diagnosed in women of childbearing age,10 data regarding the effect of pregnancy on disease progression is needed to improve patient advice.11 However, such data are sparse with only two published studies on the effect of pregnancy in 6 and 26 women with AC,12,13 respectively, in addition to case reports.14,15 We aimed to investigate the relation between parity and cardiac structure, function, arrhythmias, and clinical course in female AC patients.
Methods
Study population
In this cross-sectional study, consecutive female AC patients were recruited from the Unit for genetic cardiac diseases, Oslo University Hospital, Rikshospitalet, Norway. The AC diagnosis was based on the 2010 Task Force Criteria (TFC).16 Patients with cardiopulmonary comorbidity were excluded. All included women were genetically tested for AC-related mutations. Medical history, including the number of pregnancies and miscarriages was collected from patient interview and medical records. Women with previous pregnancies were additionally contacted by telephone to collect specific pregnancy related information, including age at pregnancies, awareness of AC diagnosis in pregnancy, AC related symptoms and use of medications before or during pregnancy, and vaginal delivery or caesarian section. An echocardiographic study was performed in all patients.
All participants gave written informed consent. The study complied with the Declaration of Helsinki and was approved by the Regional Committees for Medical Research Ethics.
Arrhythmogenic cardiomyopathy patients followed during pregnancy and delivery
A subgroup of AC mutation positive female relatives was followed during pregnancy and delivery at our hospital. In this subgroup, echocardiography was performed before pregnancy and within 6 months post-partum. Findings during pregnancy and after delivery in these women were reported in detail.
Arrhythmogenic cardiomyopathy severity score
To evaluate disease severity in each patient, we introduced an AC disease severity score yielding one point for each minor criterion and two points for each major criterion according to the 2010 TFC. If a patient fulfilled a major criterion, points for minor criteria in the same category were not included. Therefore, a maximum score across 6 diagnostic categories was 12, and patients with definite AC had ≥ 4 points (2 major, or 1 major and 2 minor, or 4 minor criteria).
Electrocardiography and arrhythmias
A 12-lead electrocardiogram was obtained in all participants at the same visit as the echocardiographic examination. Signal averaged electrocardiograms (SAECGs) were recorded and analysed as previously reported.17 Ambulatory 24 h Holter registrations at AC diagnosis were analysed when available. Ventricular arrhythmias were defined as arrhythmic events, and included aborted cardiac arrest, sustained ventricular tachycardia/ventricular fibrillation or appropriate therapy from an implantable cardioverter-defibrillator (ICD). Appropriate ICD therapy was defined as anti-tachycardia pacing (ATP) or ICD shock due to correct recognition of ventricular tachycardia or ventricular fibrillation. Non-arrhythmic death or heart transplantation was not considered an arrhythmic event.
Echocardiographic study
2D echocardiographic studies were performed in all patients on Vivid 7 or 9 scanners with off-line data analyses (EchoPAC version 112, GE Healthcare, Horten, Norway). From 2D echocardiography, we assessed proximal right ventricular outflow tract (RVOT) diameter in the parasternal short- and long-axis views. We assessed RV basal diameter and right ventricular fractional area change (RVFAC) from the RV focused four-chamber view.18 Regional RV akinesia, dyskinesia, or aneurisms were detected in parasternal long- or short-axis view and RV focused four-chamber view. Left ventricular (LV) ejection fraction was calculated by the modified Simpson’s biplane method.19 Longitudinal strain was assessed by speckle tracking technique20 at frame rates >50/s. Left ventricular global longitudinal strain (LVGLS) was defined as the average of peak negative longitudinal from a 16 segments LV model.20 Right ventricular free wall longitudinal strain was defined as an average peak negative longitudinal strain from three RV segments.21,22 Right ventricular mechanical dispersion was calculated as the standard deviation of time from the onset of the Q/R wave in the electrocardiogram to the peak negative longitudinal strain from six RV segments.22 All measurements were performed by an independent observer blinded to patients’ previous pregnancies.
Cardiac magnetic resonance
Cardiac magnetic resonance was performed in subset of patients. The RV free wall was imaged by axial and sagittal breath-hold cine sequences. Parameters from the TFC 201016 together with LV volumes and function were analysed as previously described in Ref.17
Genetic analyses
Genetic testing was performed as part of the diagnostic work-up in women with suspected AC as described previously,23 including genes encoding plakophilin-2 (PKP2), desmoplakin (DSP), desmoglein-2 (DSG2), and desmocollin-2 (DSC2), as well as 29 of the 105 exons of the gene encoding the ryanodine receptor-2. Cascade genetic screening was performed in family members of AC probands with confirmed pathogenic mutations.
Statistical analyses
Data were presented as mean ± standard deviation, median with range, or frequencies with percentages, as appropriate. Comparisons of continuous data were performed by the unpaired Student’s t-test (SPSS 21.0, SPSS Inc., Chicago, IL, USA) or by the analysis of variance (ANOVA) F-test and the Bonferroni post hoc correction when >2 groups were compared. Categorical variables were compared by the χ2 test or Fisher’s exact test as appropriate. Bivariate correlations were assessed to test for linear trends. Multivariable logistic and multiple linear regression models were performed to investigate the impact of number of pregnancies, age, body surface area (BSA), and AC severity score on measures of RV and LV dimensions and function. To avoid collinearity, the AC severity score was not added to models with the dependent variable from the TFC. Kaplan–Meier curves were constructed and log-rank test was performed to assess cumulative lifetime arrhythmia-free survival. Cox regression analyses were performed to assess risk markers of age at VA, adjusted for number of pregnancies. P-values were two sided and values ≤0.05 were considered statistically significant.
Results
Clinical and electrocardiogram characteristics
We included 77 caucasian women (age 47 ± 16 years) with AC; 43 (56%) were probands [21 (27%) with a definite AC diagnosis, 9 (12%) with a borderline diagnosis, and 13 (17%) with a possible diagnosis] and 34 (44%) were AC mutation positive female relatives [23 (30%) with definite diagnosis, 4 (5%) with borderline AC, and 7 (9%) with possible AC diagnosis]. A table with the description of major and minor criteria of AC women grouped for number of pregnancies is reported (see Supplementary data online, Table S1). Pathogenic mutations were found in 61 of which the majorities were found in PKP2 gene (51 PKP2, 4 DSP, 5 DSG2, and 1 DSC2). Among the 77 AC women, 23 (30%) had a history of syncope, 31 (40%) had documented VA, and 23 (30%) had an ICD. Twenty-four hours Holter registrations were available in 26 patients (Table 1). Twelve had >500 premature ventricular contractions (10 definite, 1 borderline, and 1 possible diagnosis). Two AC women died: 1 (2%) from non-AC related death; 1 (2%) with primary preventive ICD died for unknown reasons.
Table 1.
Clinical characteristics and cardiac imaging parameters in AC women grouped by number of pregnancies
| 0 pregnancies (n = 19) | 1 pregnancy (n = 16) | 2 pregnancies (n = 30) | ≥3 pregnancies (n = 12) | P-value from F-test | >0 pregnancies (n = 58) | P-value 0 pregnancies vs. >0 pregnancies | |
|---|---|---|---|---|---|---|---|
| BSA (m2) | 1.7 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.2 | 2.9 ± 4.3 | 0.25 | 2.1 ± 2.2 | 0.47 |
| Age at examination (years) | 36 ± 18 | 48 ± 16a | 51 ± 13a | 55 ± 13a | <0.01 | 52 ± 13 | <0.01 |
| Age at arrhythmic event (years) | 35 ± 17 | 48 ± 16a | 48 ± 13a | 54 ± 13a | 0.02 | 49 ± 14 | <0.01 |
| Probands | 9 (47) | 5 (31) | 17 (57) | 4 (33) | 0.33 | 25 (43) | 0.50 |
| AC severity score | 4.3 ± 2.8 | 3.4 ± 1.6 | 4.7 ± 2.8 | 4.3 ± 2.3 | 0.53 | 4.2 ± 2.4 | 0.83 |
| Definite AC by TFC | 11 (58) | 6 (38) | 19 (63) | 8 (66) | 0.36 | 33 (57) | 0.58 |
| Syncope | 8 (42) | 2 (13) | 11 (37) | 2 (17) | 0.17 | 15 (26) | 0.16 |
| VA | 9 (47) | 4 (25) | 15 (50) | 3 (25) | 0.25 | 22 (38) | 0.34 |
| ICD | 6 (32) | 2 (13) | 11 (37) | 4 (33) | 0.38 | 16 (28) | 0.50 |
| ICD discharge | 1 (5) | 0 (0) | 6 (20) | 3 (25) | 0.36 | 10 (17) | 0.16 |
| ATP | 0 (0) | 1 (6) | 5 (17) | 2 (17) | 0.97 | 8 (14) | 0.05 |
| TWI major criteria | 7 (37) | 5 (31) | 10 (33) | 4 (33) | 0.99 | 19 (33) | 0.74 |
| Epsilon waves | 1 (5) | 0 (0) | 4 (13) | 0 (0) | 0.37 | 4 (7) | 0.64 |
| SAEG pathological | 4 (21) | 6 (38) | 10 (33) | 2 (17) | 0.54 | 18 (31) | 0.27 |
| Echocardiographic findings | |||||||
| RVOT PLAX (mm/m2) | 19 ± 5 | 18 ± 2 | 20 ± 4 | 21 ± 6 | 0.23 | 20 ± 4 | 0.82 |
| RVOT PSAX (mm/m2) | 19 ± 5 | 17 ± 3 | 19 ± 3 | 22 ± 6b | 0.06 | 19 ± 4 | 0.96 |
| RVD (mm) | 41 ± 8 | 39 ± 4 | 43 ± 10 | 42 ± 9 | 0.62 | 42 ± 8 | 0.98 |
| RVFAC (%) | 39 ± 9 | 37 ± 7 | 34 ± 12 | 39 ± 11 | 0.37 | 36 ± 10 | 0.27 |
| RV free wall LS (%) | −23.6 ± 6.1 | −26.1 ± 6.0 | −21.9 ± 5.8 | −24.0 ± 9.0 | 0.25 | −21.2 ± 4.8 | 0.82 |
| RVMD (ms) | 41 ± 22 | 32 ± 21 | 53 ± 23 | 40 ± 21 | 0.07 | 44 ± 28 | 0.60 |
| LVEF (%) | 56 ± 7 | 56 ± 6 | 54 ± 7 | 58 ± 7 | 0.42 | 55 ± 6 | 0.88 |
| LVGLS (%) | −19.3 ± 2.8 | −18.9 ± 2.8 | −19.9 ± 2.9 | −20.4 ± 1.9 | 0.50 | −19.7 ± 2.7 | 0.56 |
| CMR findings | n = 17 | n = 10 | n = 25 | n = 8 | n = 43 | ||
| CMR EDV RV (mL) | 199 ± 71 | 138 ± 29 | 192 ± 63 | 216 ± 127 | 0.26 | 184 ± 75 | 0.64 |
| CMR EDV LV (mL) | 146 ± 24 | 135 ± 22 | 149 ± 35 | 137 ± 36 | 0.74 | 144 ± 32 | 0.87 |
| CMR EF RV (%) | 46 ± 12 | 56 ± 4 | 47 ± 12 | 43 ± 18 | 0.23 | 48 ± 12 | 0.64 |
| CMR EF LV (%) | 60 ± 6 | 63 ± 6 | 56 ± 7 | 56 ± 15 | 0.28 | 57 ± 10 | 0.50 |
Data are represented as n (%) and mean ± SD unless otherwise stated.
P-value from ANOVA F-test with the post hoc Bonferroni correction, χ2 test, or Fisher’s exact test as appropriate.
AC, arrhythmogenic cardiomyopathy; ATP, anti-tachycardia therapy; BSA, body surface area; CMR, cardiac magnetic resonance; EDV, end-diastolic volume; EF, ejection fraction; ICD, implantable cardioverter-defibrillator; LS, longitudinal strain; LV, left ventricular; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; MR, magnetic resonance; PLAX, parasternal long-axis view; PSAX, parasternal short-axis view; RV, right ventricular; RVD, right ventricular basal diameter; RVFAC, right ventricular fractional area change; RVMD, right ventricular mechanical dispersion; RVOT, right ventricular outflow tract; SAECG, signal average ECG; TFC, Task Force Criteria; TWI, T-wave inversion; VA, ventricular arrhythmias.
Post hoc P < 0.05 vs. 0 pregnancy.
Post hoc P < 0.05 vs. 1 pregnancy.
Nineteen (24%) patients had no previous pregnancies, 16 (21%) had one pregnancy, 30 (39%) had 2 pregnancies, and 12 (16%) had ≥3 pregnancies (Table 1). As expected, age at examination was significantly lower in women without previous pregnancies (Table 1). In those with previous pregnancies, time since last pregnancy was 19 ± 14 years. All patients were in sinus rhythm at inclusion. There were no differences between women without previous pregnancies and women with previous pregnancies regarding ratio of probands, definite AC diagnosis, presence of AC symptoms, AC severity score, arrhythmic events, ICD implantations, ICD shock, ATP, or heart transplantations, indicating similar disease burden in all groups (Table 1). Two (5%) probands patients with definite diagnosis needed heart transplantation. One woman (3%) was transplanted twice, 17 and 20 years after the first and second pregnancy, respectively; one woman (5%) had no previous pregnancies. Electrocardiogram and SAECG parameters did not show significant differences with increased number of pregnancy (Table 1).
Pregnancy characteristics
Among the 58 AC women with previous pregnancies, age at first pregnancy was 29 ± 6 years. Six (10%) women knew they had AC or AC-associated mutations at the time of their first pregnancy. Five (8%) patients were on beta-blocker medication during pregnancy, four on metoprolol, and one on sotalol. Symptoms during pregnancy were reported by 12 (21%) patients (6 with palpitations, 3 symptomatic ventricular extrasystoles, 1 syncope, 1 atrial fibrillation, and 1 dizziness due to low blood pressure). Six (10%) patients reported that their symptoms had worsened during pregnancy, including palpitations and dizziness, but none reported an increase in serious events. Among a total of 88 deliveries, 83 (94%) were vaginal and 6 (6%) were by caesarian sections. The majority of deliveries [n = 85, (97%)], were reported as uncomplicated. The 3 (3%) complicated deliveries included 2 (2%) cases of breech birth and 1 (1%) case of caesarian section due to preeclampsia. No delivery was complicated by AC disease or symptoms. A deep venous thrombosis occurred in 1 (1%) patient post-partum. Two (3%) women reported miscarriages: 1 (2%) with definite and 1 (5%) with possible AC diagnosis, but both miscarriages occurred early in pregnancy and were therefore not included in number of pregnancies. Both women experienced other pregnancies, 2 and 3, respectively, with uncomplicated pregnancies and vaginal deliveries.
Imaging findings and comparisons based on number of pregnancies
For the total population of patients with AC, RVOT parasternal long-axis was increased (19.5 ± 4.2 mm/m2) and RV basal diameter was at the upper limit of the reference values (41 ± 8 mm). Right ventricular function was reduced when measured by RVFAC (37 ± 10%). Left ventricular function was preserved based on measurements of LV ejection fraction (56 ± 6%) and LVGLS (−19.6 ± 2.8%).
Separate analyses of women with AC who had not been pregnant compared to women with AC who had ≥1 pregnancy did not show any differences in echocardiographic measurements (Table 1).
By dividing into four groups according to number of pregnancies, we observed a linear trend towards increased RVOT with multiple pregnancies (linear trend P = 0.02 and ANOVA F-test, P = 0.05) (Table 1). However, in multiple linear regression, the number of pregnancies was not a marker of increased RVOT diameter, adjusted for age and BSA [beta value 0.87, 95% confidence interval (CI) −0.76 to 2.5; P = 0.29]. No other measurements of RV or LV structure or function were associated with the number of pregnancies (Tables 1 and 2). As expected, AC severity score was an independent marker of reduced LV ejection fraction (<54%) [odds ratio (OR) 1.35, 95% CI 1.05–1.74; P = 0.02] and of dilated RV basal diameter (>41 mm) (OR 1.93, 95% CI 1.41–2.64; P < 0.01).
Table 2.
Markers of pathological cardiac structure or function by echocardiography in 77 AC women
| Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Markers of LVEF <54% (n = 25) | |||||
| Age (years) | 1.04 (1.01–1.07) | 0.03 | 1.02 (0.98–1.05) | 0.42 | |
| Number of pregnancies (n) | 1.76 (1.08–2.87) | 0.24 | 1.67 (0.96–2.92) | 0.71 | |
| AC severity score | 1.37 (1.07–1.75) | 0.01 | 1.35 (1.05–1.74) | 0.02 | |
| Markers of LVGLS >−18 (n = 20) | |||||
| Age (years) | 1.00 (0.97–1.03) | 0.98 | 1.00 (0.97–1.04) | 0.89 | |
| Number of pregnancies (n) | 0.79 (0.48–1.31) | 0.37 | 0.77 (0.44–1.34) | 0.36 | |
| AC severity score | 1.1 (0.93–1.38) | 0.23 | 1.13 (0.92–1.39) | 0.25 | |
| Markers of RVOT PSAX ≥32 mm (n = 45) | |||||
| Age (years) | 1.04 (1.00–1.01) | 0.03 | 1.02 (0.98–1.05) | 0.40 | |
| Number of pregnancies (n) | 1.76 (1.08–2.87) | 0.02 | 1.56 (0.91–2.66) | 0.11 | |
| BSA (m2) | 18.3 (0.99–340.58) | 0.05 | 12.58 (0.54–292.44) | 0.12 | |
| Markers of RVD >41 mm (n = 25) | |||||
| Age (years) | 1.01 (0.98–1.05) | 0.40 | 0.99 (0.95–1.04) | 0.86 | |
| Number of pregnancies (n) | 0.95 (0.59–1.50) | 0.81 | 0.89 (0.48–1.64) | 0.70 | |
| AC severity score | 1.91 (1.41–2.58) | <0.01 | 1.93 (1.41–2.64) | <0.01 | |
| Markers of RVFAC ≤40% (n = 41) | |||||
| Age (years) | 1.02 (0.99–1.05) | 0.16 | 1.02 (0.98–1.05) | 0.27 | |
| Number of pregnancies (n) | 0.99 (0.64–1.55) | 0.98 | 0.85 (0.51–1.42) | 0.53 | |
| BSA (m2) | 5.23 (0.38–72.34) | 0.22 | 3.61 (0.24–54.39) | 0.35 | |
Univariable and multivariable logistic regression, retaining all potential confounders in multivariable analyses.
AC, arrhythmogenic cardiomyopathy; BSA, body surface area; CI, confidence interval; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; PSAX, parasternal short axis view; RVD, right ventricular basal diameter; RVFAC, right ventricular fractional area change; RVOT, right ventricular outflow tract.
Relation between pregnancies, arrhythmic events, and cardiac transplantation
As expected, AC women with a history of arrhythmic events had more frequently a pathological SAECG, regional akinesia, increased RV dimensions, as well as reduced RVFAC and RV free wall longitudinal strain compared to AC women without arrhythmic events (Table 3). Furthermore, AC severity score was a marker of VA as expected (P < 0.001) (Table 3). The number of pregnancies was not associated with VA in univariable analyses, nor when adjusted for severity of AC disease (Table 3). The Kaplan–Meier curves showed earlier onset of VA in those with 0 pregnancies compared with those with 1 pregnancy (P = 0.04) (Figure 1). No differences in onset of VA were observed between the other groups of pregnancies.
Table 3.
Comparisons of female AC patients without and with history of ventricular arrhythmias, and markers for age at ventricular arrhythmias adjusted for number of pregnancies
| AC without ventricular arrhythmias (n = 46) | AC with ventricular arrhythmias (n = 31) | P-value | Risk markers of ventricular arrhythmias multivariable HR (95% CI) | Multivariable P-value | |
|---|---|---|---|---|---|
| Age (years) | 46 ± 18 | 49 ± 12 | 0.45 | ||
| AC severity score | 3.1 ± 1.7 | 6.0 ± 2.5 | <0.001 | 1.31 (1.15–1.50)a | <0.001 |
| Number of pregnancies (n) | 1.5 ± 1.1 | 1.4 ± 1.1 | 0.69 | 0.72 (0.51–1.03)b | 0.08 |
| SAECG pathological | 7 (15) | 15 (48) | <0.001 | 0.76 (0.53–1.09)a | 0.14 |
| TWI major criteria | 7 (15) | 19 (61) | <0.01 | 0.74 (0.52–1.06)a | 0.10 |
| Epsilon waves | 3 (7) | 19 (61) | 0.99 | ||
| RVOT PSAX (mm) | 32 ± 6 | 36 ± 6 | 0.14 | ||
| RVD (mm) | 38 ± 4 | 46 ± 10 | <0.001 | 1.09 (1.05–1.14)a | <0.001 |
| RVFAC (%) | 40 ± 9 | 33 ± 11 | 0.01 | 0.98 (0.95–1.01)a | 0.22 |
| RV free wall LS (%) | −22.3 ± 4.5 | −19.6 ± 4.0 | <0.001 | 1.09 (1.03–1.17)a | 0.006 |
| LVEF (%) | 56 ± 7 | 55 ± 5 | 0.46 | ||
| LVGLS (%) | −19.7 ± 2.7 | −19.6 ± 3.0 | 0.90 | ||
| CMR EDV RV (mL) | 168 ± 70 | 213 ± 71 | 0.07 | ||
| CMR EDV LV (mL) | 138 ± 30 | 153 ± 30 | 0.14 | ||
| CMR EF RV (%) | 50 ± 12 | 45 ± 12 | 0.15 | ||
| CMR EF LV (%) | 58 ± 8 | 58 ± 10 | 0.96 | ||
| Regional akinesia RV | 7 (15) | 18 (58) | <0.01 | 3.01 (1.38–6.60)a | 0.006 |
Data are represented as mean ± SD or n (%).
P-value from the Student’s t-test or χ2 test as appropriate.
Multivariable Cox regression of age-adjusted incident VA.
AC, arrhythmogenic cardiomyopathy; CI, confidence interval; CMR, cardiac magnetic resonance; EDV, end-diastolic volume; EF, ejection fraction; HR, hazard ratio; MR, magnetic resonance; LS, longitudinal strain; LV, left ventricular; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; PSAX, parasternal short-axis view; RMS, root-mean-square voltage; RV, right ventricular; RVD, right ventricular basal diameter; RVFAC, right ventricular fractional area change; RVOT, right ventricular outflow tract; SAECG, signal averaged electrocardiogram; TWI, T-wave inversion.
Adjusted for number of pregnancies.
Adjusted for AC severity score.
Figure 1.
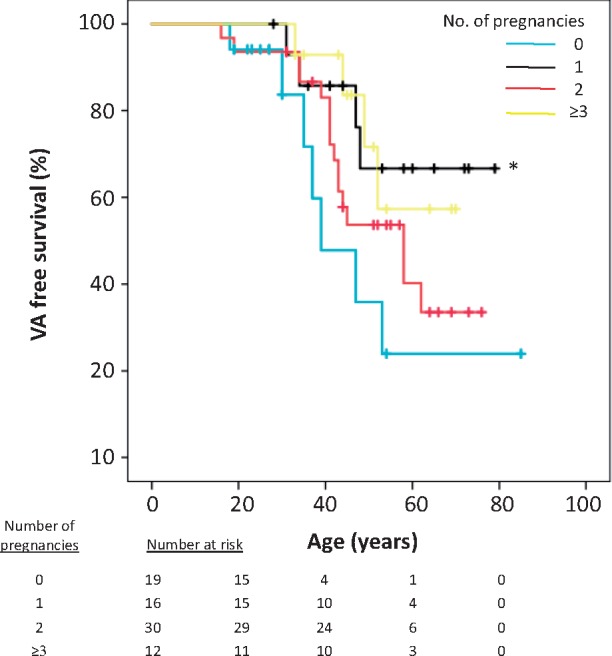
The Kaplan–Meier analyses of 77 female AC patients and their mutation positive female relatives. Women with 0 pregnancies had ventricular arrhythmias at younger ages compared with women with 1 pregnancy (*log rank = 0.04). No significant differences were observed in arrhythmic events and prognosis between 1, 2, and ≥3 pregnancies. AC, arrhythmogenic cardiomyopathy; VA, ventricular arrhythmias.
Two (3%) patients needed cardiac transplantation: One 27-year-old woman without previous pregnancy had severely affected right ventricle and was transplanted due to recurrent and refractory VA. One 50-year-old woman with two previous pregnancies was transplanted due to severe RV heart failure and arrhythmic storms.
Patients followed during pregnancy and delivery
Five AC mutation positive female relatives (4 plakophilin-2, 1 desmoglein-2) were followed with serial echocardiographic examinations during a total of six pregnancies at our department (1 patient had 2 pregnancies, and 4 had 1 pregnancy). Age at AC genetic diagnosis was 31 ± 3 years and age at first pregnancy was 34 ± 3 years. Arrhythmogenic cardiomyopathy severity score was 2.5 ± 1.0. No arrhythmias were reported during pregnancy, delivery or postpartum. Importantly, there were no indications of changes in RVOT diameter, RVFAC, RV free wall longitudinal strain, LVGLS, or LV ejection fraction within 6 months after pregnancy (Table 4).
Table 4.
Serial investigations in five AC mutation positive female relatives before pregnancy and within 6 months post-partum
| Before pregnancy | Within 6 months post-partum | P-value | |
|---|---|---|---|
| RVOT PSAX (mm) | 33 ± 6 | 32 ± 7 | 0.52 |
| RVFAC (%) | 46 ± 7 | 41 ± 7 | 0.08 |
| RV free wall LS (%) | −24.3 ± 3.1 | −21.9 ± 2.3 | 0.55 |
| LVEF (%) | 54 ± 2 | 54 ± 2 | 0.36 |
| LVGLS (%) | −19.5 ± 2.7 | −17.9 ± 4.6 | 0.44 |
Data represented as mean ± SD.
P-value from the Student’s t-test.
AC, arrhythmogenic cardiomyopathy; LS, longitudinal strain; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; PSAX, parasternal short-axis view; RV, right ventricular; RVFAC, right ventricular fractional area change; RVOT, right ventricular outflow tract.
Intra- and inter-observer variability for RV and LV strain and for RV mechanical dispersion was previously reported.17,23
Discussion
This is the first larger study exploring the effect of previous pregnancies on outcome in women with AC. The number of previous pregnancies in AC women did not seem to aggravate RV and LV structure or function, or increase the occurrence of VA in long-term follow up. Furthermore, the majority of the women reported their pregnancies as uncomplicated with no increase of serious adverse events during pregnancy.
Cardiac effects of pregnancy
In our AC population, RVOT diameter tended to increase with the number of previous pregnancies. However, number of previous pregnancies was not a marker of dilated RVOT when adjusted for age and BSA. Other markers of RV and LV structure and function did not show any relation to the number of pregnancies, even when adjusted for age and AC severity score. Although a small number, no changes were observed in RV diameters and function during pregnancy in the five AC women followed during pregnancy and delivery at our hospital. Overall, our findings indicated that pregnancy did not affect cardiac structure or function in AC, supporting previous case series and reports of well tolerated pregnancies in this patient group.12,13,15 Our results indicated that the long-term effect of pregnancy is well tolerated and safe in women with AC. Nevertheless, we suggest that these patients should be referred to a high experience centre for structured follow-up from early pregnancy until after delivery. A recent large study on pregnancy in hypertrophic cardiomyopathy indicated overall good survival, but cardiovascular complications during pregnancy were not uncommon. The results highlight that pregnancy alters haemodynamic and arrhythmic risk and requires appropriate follow up and management.24
The rationale for this study was the recently reported negative effects of repeated haemodynamic stress due to athletic activity on cardiac function and arrhythmias in AC.4,5 Pregnancy shares some physiological aspects with exercise and represents several months of continuous haemodynamic stress. The normal physiological changes in the cardiovascular system during pregnancy includes an increase in maternal blood volume by 40%, resulting in a 30–50% increase in cardiac output8 achieved by a rise in stroke volume and heart rate.8,9 Based on our results, the effects of haemodynamic stress during pregnancy seem to differ from the effects of exercise. Several explanations are possible; previous reports have reported doses of harmful athletic activity to range from 1 to 4 h activity per week for one to several years.4,5,25 Pregnancy, however, is limited to a 9 months period and the largest increase in cardiac stress is on lower intensity and spans an even shorter time period than 9 months. Furthermore, during pregnancy there is a decrease in vascular resistance by approximately 30%, causing peripheral vasodilatation, unlike exercise where there is an exercise induced increase in afterload. Other possible speculations may include hormonal and adaptive mechanisms involved during pregnancy, which may protect the myocardium.26,27
Arrhythmic events and outcome
Arrhythmic events were equally prevalent in women without and with previous pregnancies, and multivariable analyses showed no influence of pregnancies on VA outcome. As expected, AC severity score was highly predictive for the occurrence of VA and echocardiographic and SAECG were markers of VA. Arrhythmogenic cardiomyopathy women without previous pregnancies had experienced arrhythmic events at younger age compared with those with previous pregnancies as also shown in the Kaplan–Meier plot. This could indicate a possible confounding factor as women with severe arrhythmias at young age might have decided not to become pregnant and reflect more severe disease in the end. However, less than half of patients without pregnancy had experienced arrhythmic events, indicating limited confounding effect. Furthermore, no differences were found in age at arrhythmic events between those with 1, 2, and >3 pregnancies, and proband status and AC severity score were not different between the groups based on number of pregnancies.
Limitations
This study had a cross-sectional design, while data on symptoms during pregnancy were collected retrospectively with the inherent limitations. We cannot exclude survival bias and disease is underestimated in the older groups. We included AC mutation positive family members with early disease, and our population was therefore relatively healthy. We cannot exclude the possibility that women with symptomatic AC avoided pregnancy on the basis of diagnosis, a potential bias that is difficult to explore and control. Although being the largest study on this topic, the numbers of events were limited and larger studies are needed to confirm our findings.
Conclusions
Higher number of pregnancies was not associated with worse outcome in women with AC or in mutation positive female relatives. Serious cardiac symptoms did not worsen during pregnancy and number of pregnancies was not associated with arrhythmic events.
Supplementary Material
Acknowledgements
We would like to thank Margareth Ribe, RN, for her help with the telephone calls.
Funding
This work was supported by a public research grant from the South-Eastern Norway Regional Health Authority, Oslo, Norway, and the Center for Cardiological Innovation funded by the Norwegian Research Council.
Conflict of interest: none declared.
References
- 1. Sen-Chowdhry S, Morgan RD, Chambers JC, McKenna WJ.. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu Rev Med 2010;61:233–53. [DOI] [PubMed] [Google Scholar]
- 2. Thiene G, Nava A, Corrado D, Rossi L, Pennelli N.. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988;318:129–33. [DOI] [PubMed] [Google Scholar]
- 3. Xu T, Yang Z, Vatta M, Rampazzo A, Beffagna G, Pilichou K. et al. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2010;55:587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ. et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H. et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heidbuchel H, Hoogsteen J, Fagard R, Vanhees L, Ector H, Willems R. et al. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J 2003;24:1473–80. [DOI] [PubMed] [Google Scholar]
- 7. Kirchhof P, Fabritz L, Zwiener M, Witt H, Schafers M, Zellerhoff S. et al. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation 2006;114:1799–806. [DOI] [PubMed] [Google Scholar]
- 8. Krul SP, van der Smagt JJ, van den Berg MP, Sollie KM, Pieper PG, van Spaendonck-Zwarts KY.. Systematic review of pregnancy in women with inherited cardiomyopathies. Eur J Heart Fail 2011;13:584–94. [DOI] [PubMed] [Google Scholar]
- 9. Estensen ME, Beitnes JO, Grindheim G, Aaberge L, Smiseth OA, Henriksen T. et al. Altered maternal left ventricular contractility and function during normal pregnancy. Ultrasound Obstet Gynecol 2013;41:659–66. [DOI] [PubMed] [Google Scholar]
- 10. Corrado D, Basso C, Thiene G.. Arrhythmogenic right ventricular cardiomyopathy: diagnosis, prognosis, and treatment. Heart 2000;83:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murray B, Tichnell C, James C, Tandri H, Calkins H.. Pregnancy in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol 2013;62:1910–1. [DOI] [PubMed] [Google Scholar]
- 12. Bauce B, Daliento L, Frigo G, Russo G, Nava A.. Pregnancy in women with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Obstet Gynecol Reprod Biol 2006;127:186–9. [DOI] [PubMed] [Google Scholar]
- 13. Hodes AR, Tichnell C, Te Riele AS, Murray B, Groeneweg JA, Sawant AC. et al. Pregnancy course and outcomes in women with arrhythmogenic right ventricular cardiomyopathy. Heart 2016;102:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iriyama T, Kamei Y, Kozuma S, Taketani Y.. Management of patient with arrhythmogenic right ventricular cardiomyopathy during pregnancy. J Obstet Gynaecol Res 2013;39:390–4. [DOI] [PubMed] [Google Scholar]
- 15. Agir A, Bozyel S, Celikyurt U, Argan O, Yilmaz I, Karauzum K. et al. Arrhythmogenic right ventricular cardiomyopathy in pregnancy. Int Heart J 2014;55:372–6. [DOI] [PubMed] [Google Scholar]
- 16. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saberniak J, Leren IS, Haland TF, Beitnes JO, Hopp E, Borgquist R. et al. Comparison of patients with early-phase arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract ventricular tachycardia. Eur Heart J Cardiovasc Imaging 2017;18:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badano LP, Miglioranza MH, Edvardsen T, Colafranceschi AS, Muraru D, Bacal F. et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur Heart J Cardiovasc Imaging 2015;16:919–48. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 20. Edvardsen T, Haugaa KH.. Imaging assessment of ventricular mechanics. Heart 2011;97:1349–56. [DOI] [PubMed] [Google Scholar]
- 21. Sarvari SI, Haugaa KH, Anfinsen OG, Leren TP, Smiseth OA, Kongsgaard E. et al. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J 2011;32:1089–96. [DOI] [PubMed] [Google Scholar]
- 22. Haugaa KH, Basso C, Badano LP, Bucciarelli-Ducci C, Cardim N, Gaemperli O. et al. Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy-an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:237–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leren IS, Saberniak J, Haland TF, Edvardsen T, Haugaa KH.. Combination of ECG and echocardiography for identification of arrhythmic events in early ARVC. JACC Cardiovasc Imaging 2017;10:503–13. [DOI] [PubMed] [Google Scholar]
- 24. Goland S, van Hagen IM, Elbaz-Greener G, Elkayam U, Shotan A, Merz WM. et al. Pregnancy in women with hypertrophic cardiomyopathy: data from the European Society of Cardiology initiated Registry of Pregnancy and Cardiac disease (ROPAC). Eur Heart J 2017;38:2683–90. [DOI] [PubMed] [Google Scholar]
- 25. Mazzanti A, Ng K, Faragli A, Maragna R, Chiodaroli E, Orphanou N. et al. Arrhythmogenic right ventricular cardiomyopathy: clinical course and predictors of arrhythmic risk . J Am Coll Cardiol 2016;68:2540–50. [DOI] [PubMed] [Google Scholar]
- 26. Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH. et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol 2013;61:196–206. [DOI] [PubMed] [Google Scholar]
- 27. Leo CH, Jelinic M, Ng HH, Marshall SA, Novak J, Tare M. et al. Vascular actions of relaxin: nitric oxide and beyond. Br J Pharmacol 2017;174:1002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


