Abstract
The UV-filter benzophenone and the anti-inflammatory diclofenac are commonly detected in the environment. The aim of this study was to assess the multigenerational effects of chronic exposure to low concentrations of these chemicals on toxicity and DNA methylation levels in the copepod Gladioferens pectinatus. Acute toxicity tests were conducted to determine the sensitivity of G. pectinatus to the chemicals. All chemicals impacted breeding, hatching and egg viability. Diclofenac (1 mg.L-1) reduced the number of eggs per gravid female. Benzophenone (0.5 mg.L-1) decreased egg hatching success. Exposure to the reference toxicant copper (0.02 mg.L-1) led to unsuccessful hatching. Effects on DNA methylation was estimated by the percentage of 5- methylcytosine. The treatments resulted in strong differences in DNA methylation with increased methylation in the exposed animals. The two chemicals impacted both egg viability and the induction of differential DNA methylation, suggesting potential intra- and trans-generational evolutionary effects.
Key words: Gladioferens pectinatus, Toxicity, Multigenerational effects, Reproduction, Epigenetic, Emerging contaminants
Introduction
The accumulation of chemicals from consumer products, and industrial and domestic waste in the aquatic environment is a key environmental issue facing humanity. 1 Many pollutants that originate from personal care products and medicines are collectively known as Emerging Organic Contaminants (EOCs).2 EOCs are ubiquitous, persistent and can impact ecosystem and human health. There is a lack of information on the presence and fate of these chemicals and the risk they potentially pose.
Epigenetics is the study of heritable changes in gene function that can occur without a change in the DNA sequence which forms the underlying genetic code. Toxicant or contaminant exposure can perturb the epigenetic status of cells and organisms, as known from several studies on model organisms. Epigenetic mechanisms (e.g., DNA methylation) can increase, decrease, or silence the activity of genes, leading to novel phenotypic variation.3 They can respond to environmental cues and be passed on to offspring. Therefore, they may play a key role in rapid adaptation to environmental stressors.4,5
The aim of this study was to assess the effects of chronic exposure to low concentrations of the UV-filter benzophenone and the anti-inflammatory drug diclofenac, both commonly detected in the environment and classified as EOCs,6,7 on the modulation of toxicity and DNA-methylation in the pelagic copepod Gladioferens pectinatus.
Materials and Methods
Gladioferens pectinatus, a pelagic species of calanoid copepod commonly found in New Zealand, was kept in the laboratory in monoculture as described previously. 8 Acute toxicity tests (48hr) were performed to assess the sensitivity (effective concentration; ECx) of G. pectinatus to the target chemical compounds at the beginning and end of the exposure period.8 Dissolved oxygen, salinity, and pH were measured with a multi-parameter (HQ40d, Hach®) before and after the replacement of the test solutions.
For the multi-generational test, G. pectinatus were exposed to concentrations of 0.02, 0.5, 1.0 mg.L-1 of copper, benzophenone and diclofenac, respectively. Neonates (<24hr) from two gravid-females were loaded to vessel (100 mL) containing 60 mL of the test solution. For following generations, neonates from parental generation were sieved (55 μm), collected and transferred to test vessels containing fresh solutions at the same concentrations. The test was conducted over 84 days corresponding to 4 generations. Each test vessel was covered with a lid to avoid evaporation and incubated at 20 ± 1°C under a 12h/12h light-darkness cycle. Copepods were fed twice a week with 1×104 cells.mL-1 of microalgae (Isochrisis galabana and Chaetoceros muelleri). After the second generation (G2), copepods were transferred into larger vessels (750 mL). Reproductive output was evaluated at the end of each generation (21 days).
At the end of the test DNA was extracted from a pool of 100 copepods using the CTAB buffer method.9 The percentage of 5- methylcytosine was estimated, relative to the input DNA quantity for each copepod sample, using enzyme-linked immunosorbent assays as per the manufacturer’s instruction (5-mC DNA ELISA kit, Zymo Research, USA).
Data were checked for normality (Shapiro Wilkes test) and homogeneity of variance (Levene’s Test). A one-way ANOVA was applied to detect any effects due to treatment. Statistics were carried out with Statistica 13.3 (Tibco Software LTD).
Paired student t-tests (Microsoft Excel, Office 365) were used to determine statistically significant differences in the percentage of 5-methylcytosine for copepods from each treatment.
Results and Discussion
The acute toxicity results of the copepod G. pectinatus to copper, benzophenone and diclofenac exposure using are presented in Table 1. The EC50 values are similar to those reported for the copepod Acartia tonsa for copper (0.031 mg.L-1)10 and benzophenone (2.6 mg.L-1).7 The ranking EC50 values were copper < benzophenone < diclofenac. Of the doses used for the multigenerational exposure, copper resulted in malformation of the eggs and reduced hatching and not enough offspring were produced to complete the full experiment. Diclofenac reduced the number of eggs per sac (Figure 1). Both diclofenac and benzophenone reduced egg hatching success. The toxicity of G. pectinatus to the 2 chemicals did not significantly changed following the long-term exposure.
Table 1.
Acute toxicity of copper, benzophenone and diclofenac to G. pectinatus with 95% confidence intervals.
| Parameters | Copper | Benzophenone | Diclofenac |
|---|---|---|---|
| EC10 (mg.L-1) | 0.004 ± 0.003 | 0.33 (0.13-0.53) | 6.60 (2.79-10.41) |
| EC50 (mg.L-1) | 0.038 ± 0.009 | 1.51 (1.18-1.82) | 15.66 (12.99-18.32) |
| EC90 (mg.L-1) | 0.157 ± 0.057 | 3.99 (3.03-4.94) | 27.15 (16.15-38.15) |
Figure 1.
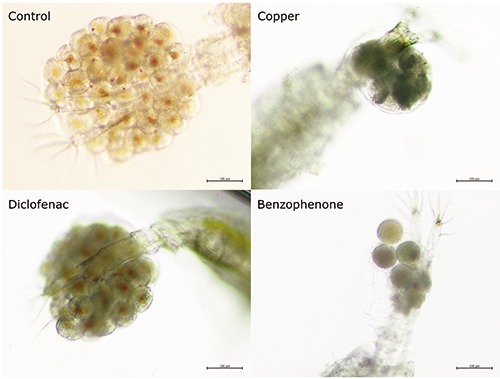
Pictures of copepods showing the effects of exposures to copper, benzophenone, and diclofenac compared to control.
In addition to the reduced reproductive output, both toxicants induced an increase in DNA methylation compared to controls but not significantly different (P<0.05) (Figure 2). Long-term exposure to stressors, including chemical stress, has been shown to result in altered epigenetic profiles which can correspond to increased resilience.3 Further determination of the extent, heritability, and longevity of these epigenetic changes within the genome of G. pectinatus in response to EOCs is warranted. Identifying the genomic location and resulting transcriptional control throughout whole genomes is a critical next step in assessing the role of epigenetic processes for adaptation to new stressors.3,4 Additionally, knowledge of the effects of persistent EOCs on species at the genomic and epigenomic levels will lead to improved characterization of impacts on marine ecosystems and public health.
Figure 2.
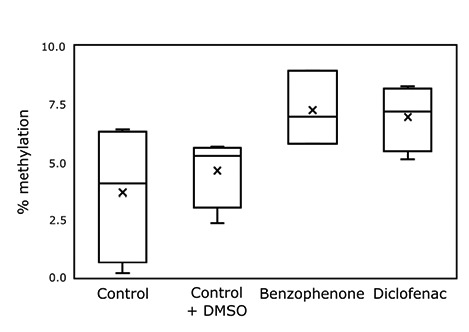
DNA methylation (% of total DNA) of G. pectinatus measured by ELISA exposed to the 2 chemicals over four generations (84 days).
Conclusions
This preliminary experiment with the copepod G. pectinatus showed potential as a model to assess the multigenerational impacts of EOCs. The treatments resulted in strong increases in the levels of global DNA methylation in the exposed animals but they were not significantly different. Further research is on-going to address the relationship between the DNA methylation changes and the modulation of the expression of specific genes and their implications in responding to low-level exposures to chemical stressors.
Acknowledgments
The authors thank Laura Biessy (Cawthron Institute) for assistance with the DNA extraction and ELISA work.
Funding Statement
Funding: this study was funded by the Centre for Integrated Biowaste Research, Institute of Environment Science and Research (ESR) Limited Strategic Science Investment Fund (SSIF), the NZ Ministry of Business, Innovation and Employment (contract no CAWX1708), and Boffa Miskell Limited, New Zealand. Callaghan Innovation R&D Student Fellowship Grant (contract no. BMISK1401) to MPC.
References
- 1.Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, Von Gunten U, Wehrli B. The challenge of micropollutants in aquatic systems. Science 2006;313:1072-7. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Xagoraraki I. Pharmaceuticals, personal care products and endocrine-disrupting chemicals in U.S. surface and finished drinking waters: A proposed ranking system. Sci Total Environ 2010;408:5972-89. [DOI] [PubMed] [Google Scholar]
- 3.Vandegehuchte MB, Janssen CR. Epigenetics in an ecotoxicological context. Mutation Res-Gen Toxicol Environ Mutagen 2014;764:36-45. [DOI] [PubMed] [Google Scholar]
- 4.Hawes NA, Fidler AE, Tremblay LA, Pochon X, Dunphy BJ, Smith KF. Understanding the role of DNA methylation in successful biological invasions: a review. Biol Invas 2018;1-16. [Google Scholar]
- 5.Eirin-Lopez JM, Putnam HM. Marine environmental epigenetics. Ann Rev Mar Sci 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Brozinski JM, Lahti M, Meierjohann A, Oikari A, Kronberg L. The anti-inflammatory drugs diclofenac, naproxen and ibuprofen are found in the bile of wild fish caught downstream of a wastewater treatment plant. Environ Sci Technol 2013;47:342-8. [DOI] [PubMed] [Google Scholar]
- 7.Kusk KO, Avdolli M, Wollenberger L. Effect of 2,4-dihydroxybenzophenone (BP1) on early life-stage development of the marine copepod Acartia tonsa at different temperatures and salinities. Environ Toxicol Chem 2011;30:959-66. [DOI] [PubMed] [Google Scholar]
- 8.Charry MP, Keesing V, Costello M, Tremblay LA. Assessment of the ecotoxicity of urban estuarine sediment using benthic and pelagic copepod bioassays. PeerJ 2018;6:e4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winnepenninckx B, Backeljau T, De Wachter R. Complete small ribosomal subunit RNA sequence of the chiton Acanthopleura japonica (lischke, 1873) (mollusca, polyplacophora). Nucl Acid Res 1993;21:1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosnowski SL, Gentile JH. Toxicological comparison of natural and cultured populations of Acartia tonsa to cadmium, copper, and mercury. J Fish Res Board Can 1978;35:1366-9. [Google Scholar]


