Abstract
Introduction:
Selective hippocampal (HC) subfield atrophy has been reported in older adults with mild cognitive impairment and Alzheimer’s disease. The goal of this study was to investigate the associations between the volume of hippocampal subfields and visual and verbal episodic memory in cognitively normal older adults.
Methods:
This study was conducted on a subset of 133 participants from the Einstein Aging Study (EAS), a community-based study of non-demented older adults systematically recruited from the Bronx, N.Y. All participants completed comprehensive EAS neuropsychological assessment. Visual episodic memory was assessed using the Complex Figure Delayed Recall subtest from the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). Verbal episodic memory was assessed using Delayed Recall from the Free and Cued Selective Reminding Test (FCSRT). All participants underwent 3T MRI brain scanning with subsequent automatic measurement of the hemispheric hippocampal subfield volumes (CA1, CA2- CA3, CA4-dente gyrus, presubiculum, and subiculum).Weused linear regressions to model the association between hippocampal subfield volumes and visual and verbal episodic memory tests while adjusting for age, sex, education, and total intracranial volume.
Results:
Participants had a mean age of 78.9 (SD = 5.1) and 60.2% were female. Total hippocampal volume was associated with Complex Figure Delayed Recall (β = 0.31, p = 0.001) and FCSRT Delayed Recall (β = 0.27, p = 0.007); subiculum volume was associated with Complex Figure Delayed Recall (β = 0.27, p = 0.002)and FCSRT Delayed Recall ( β = 0.24, p = 0.010); CA1 was associated with Complex Figure Delayed Recall ( β = 0.26, p < 0.002) and FCSRT Delayed Recall ( β = 0.20, p = 0.025).
Conclusions:
Our findings confirm previous research on the specific roles of CA1 and subiculum in episodic memory. Our results suggest that hippocampal subfields have sensitive roles in the process of visual and verbal episodic memory.
Keywords: Hippocampal subfields, Subiculum, CA1, Visual episodic memory, Verbal episodic memory, Older adults
1. Introduction
The association between the hippocampus and episodic memory is well established [1–4]; however, within the hippocampus, the roles of task-specific structures are still emerging, and only a few studies have investigated the association between hippocampal subfields and psychometric tests of memory in healthy older adults.
Hippocampal (HC) subfields include the cornu ammonis (CA1, CA2–3), CA4-dente gyrus (DG), the presubiculum and the subicu-lum. Previous research suggests that CA2, CA3, and DG are input structures, responsible for encoding, while the CA1 and subiculum are output structures, responsible for retrieval [5–9]. fMRl studies have further suggested specialized function for each subfield in hippocampal formation. Zeineh et al. [8] used a face-name associated task to study mnemonic processing in 10 healthy young adults; results showed that the CA2, CA3 and DG were involved in encoding whereas the subiculum showed more activation during recall, thus suggesting a double dissociation of activation patterns and tasks. In a similar experiment, Nauer et al. [9] assessed 34 healthy young adults on their working memory abilities for visuospa- tial information using complex visual outdoor scenes in delayed match-to-sample (DMS) tasks; results in this study showed that the hippocampal subfields CA1, CA3, DG and subiculum remained activated well into the delayed period, suggesting an ongoing mechanism of long-term information processing. Nauer et al. [9] refer to this as “ongoing encoding”, in which the immediate and delayed tasks are explained as one continuous process of activity reflecting ongoing encoding of stimuli.
Structural MRl studies have also shown these associations in case-control studies [10–12]. Muller et al. [12] explored verbal episodic memory using the California Verbal Learning Test Il (CVLT- ll) in individuals with temporal lobe deficits and healthy controls; they found that immediate verbal recall was associated with larger CA3 and DG volumes, while delayed verbal recall was associated with larger CA1 volumes. They also found similar results in another study using the same test on cognitively impaired older adults [11]. Research has also shown that CA1 volume declines with increasing age [13], and that compared to age-matched controls, Alzheimer’s disease (AD) subjects show more atrophy in the CA1 and subiculum [14,15]. CA1 has also been associated with delayed verbal recall in AD [10], further suggesting CA1’s role in retrieval that is associated with AD [15]. These findings have been extended to younger healthy adults in a study [16] that applied structural MRl to the study of hippocampal formation in younger adults; results showed that CA1, CA2/CA3 and DG played significant roles in verbal and visual memory retrieval.
ln this manuscript, we assess the relationships of HC subfield volume with standardized neuropsychological tests of visual and verbal memory. ldentifying neurocognitive measures that tap onto hippocampal subfields may provide a more efficient method of targeting individuals at risk of AD. Psychometric tests that are associated with specific hippocampal subfields may suggest atrophy and consequently ensure a more proactive approach of referring at-risk individuals for imaging procedures and early diagnosis. Thus, the aim of our study was to explore the role of hippocampal subfields in delayed verbal and delayed visual recall in a sample of community-dwelling older adults without dementia. We hypothesized that CA1 and subiculum would be associated with performance on tests of visual and verbal memory recall.
2. Methods
2.1. Sample
This cross-sectional study was conducted on a subset of 133older adults from the Einstein Aging Study (EAS). The EAS studydesign and methods have been described previously [17]. Briefly,the EAS is an ongoing community-based volunteer sample of indi-viduals over the age of 70 living in the Bronx, New York. Participants are systematically recruited from Medicare and from voter registration lists from Bronx County, New York City Board of Elections. Participants with visual and/or auditory impairment that interfere with neuropsychological testing, psychiatric symptomatology that interferes with test completion, non-English speakers, a nonambulatory status, and a dementia diagnosis were excluded from the study. In addition, participants did not participate in the study if they were ineligible for an MRI (e.g. due to metallic implants, claustrophobia, etc.). Written informed consent was obtained on their first clinical visit. The study protocol was approved by the local institutional review board. In this study, we only selected participants who have participated in MRI studies betweenJuly 2011 and October 2014.
2.2. Visual and verbal episodic memory assessment
We used two tests that contained three measures of visual episodic memory:
-
i)
Complex Figure subtest from the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) - copy and delayed recall [18]: This test assesses immediate and delayed visual and spatial ability and visual episodic memory and is relatively language free. The first part of this test involves the immediate free-hand copying of a very detailed line drawing. In the second part, which is the delayed part, the participant is required to recall and reproduce the figure from memory after a 20 min delay. Scores are based on accuracy of drawing and placement. Possible scores range from 0 to 20 for each test condition. This test is frequently used to test for dementia or neuropsychological impairment. In this study we only assessed the delayed condition since our interest is in visual episodic memory.
-
ii)
The Free and Cued Selective Reminding Test (FCSRT) [19] is an episodic memory test, which includes learning of 16 pictures by identifying and naming each picture. It also consists of three trials of immediate free recall, each of which is followed by cued recall in which a category cue is given to the subject to facilitate recall of the items not freely recalled. It also consists of a delayed free recall trail given after a 20 min delay. Delayed free recall (range 0–16) was used in these analyses.
2.3. MRI image processing
Imaging was performed using a 3.0T MRI scanner (Achieva Quasar TX; Philips Medical Systems, Best, the Netherlands) with a 32-channel head coil (Sense Head Coil; Philips Medical Systems, Best, the Netherlands). T1-weighted whole-head structural imaging was performed using sagittal three-dimensional magnetization-prepared rapid acquisition gradient echo (MP- RAGE) with TR/TE 9.9/4.6ms; 240 mm2 FOV; 240 × 240 matrix; partition thickness, 1 mm; and parallel acceleration factor 2.0. Furthermore, a 3D T2-weighted fluid-attenuated inversion recovery (T2W-FLA1R) acquisition was obtained with the following pulse sequence parameters: TR/TE/T111000/120/2800 ms; 240 × 240 mm FOV; 240 × 240 matrix; 1 mm partition thickness and parallel acceleration factor 2.0.
MR1 data was processed using the FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu/). Image processing methods in the EAS have been previously described in detail [20]. Briefly, the processing stream starts with a hybrid watershed algorithm, which removes non-brain tissue, automated transformation to the Talairach reference space and segmentation of the subcortical white matter and deep gray matter. All volumes including cortical GM volume, total cerebral WM volume, ventricular volume, and total hippocampal volume (HV) were segmented usingFreeSurfer’s standard segmentation procedure using a probabilistic brain atlas [21]. Additionally, for each subject, the estimated intracranial volume (TICV) was calculated by the procedure described by Buckner et al. [22]. Subsequently, we performed automated subfield segmentation of the hippocampus using another procedure within the FreeSurfer suite. This procedure uses Bayesian inference and a probabilistic atlas of the hippocampal formation, which is based on manual delineations of subfields in T1- weighted MRI scans from a number of different subjects [23]. Using this method, seven subfield volumes were calculated for each side of the hippocampus: CA1, CA2–3, CA4-DG, presubiculum, subiculum, fimbria, and hippocampal fissure. The larger subfields are shown to correlate well with manual volume estimates, with an average dice coefficient of around 0.7 for CA1, CA2–3, CA4-DG, presubiculum, and subiculum [23]. Thus, for the purpose of this study, we chose larger subfields of CA1, CA2–3, CA4-DG, presubiculum, and subiculum due to their strong reliability of measurement. Automated volume estimates of these subfields are shown to correlate well between different MRI scanners [24]. Segmentation results were also visually inspected for errors in all datasets, but no manual edits were needed.
2.4. Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY: IBM Corp). In order to decrease Type I error by performing multiple comparisons, we combined left and right hippocampal subfields. Pearson correlation coefficients were utilized to examine associations between visual and verbal episodic memory tests and hippocampal volume including hippocampal subfields (CA1, CA2/3, CA4/DG, presubiculum, subiculum). Separate linear regression analyses were performed to examine hippocampal subfields as independent variables and delayed visual and verbal recall tests as dependent variables. Age, gender, race, education and TICV were used a potential covariates. We also used a Sidak correction [25] factor with an adjusted p- value of 0.01 for the hippocampal subfields (alpha = 0.05, five HC subfields). Subfields that were significantly associated with visual and verbal episodic memory were then analyzed for laterality.
3. Results
3.1. Demographic and sample characteristics
The mean age of the sample was 78.9 years, 39.8% were male,and 60% were Caucasian. Average total number of years in edu-cation was 14.4 years. Global cognitive function, as measured bythe the Mini Mental State Examination was on average 26.5 (SD = 1.7). The mean scores on the Complex Figure Delayed Recall conditions were 11.6 (SD = 3.8). The mean score on the delayed recall from the FCSRT was 11.0 (SD = 3.7). Sample characteristics, visual and verbal episodic memory, and MRI-derived volumes of hippocampal sub-regions are presented in Table 1.
Table 1.
Sample characteristics, including correlations between hippocampal subfields and Complex Figure Delayed Recall and Free and Cued Selective Reminding Test Delayed Free Recall.
| Variables and units | Total sample (n = 133) | Complex Figure Delayed Recall |
FCSRT Delayed Free Recall |
||
|---|---|---|---|---|---|
| r | p-value | r | p-value | ||
| Age, years (SD) | 78.9 (5.1) | −0.23 | 0.008 | −0.13 | 0.153 |
| Males (%) | 45 (39.8) | −0.13 | 0.150 | 0.11 | 0.260 |
| Education, years (SD) | 14.4 (3.4) | 0.19 | 0.028 | −0.00 | 0.990 |
| White (%) | 60 (53.1) | 0.04 | 0.620 | 0.01 | 0.892 |
| Mini Mental State Examination | 26.5(1.7) | −0.36 | <0.001 | −0.25 | 0.004 |
| R Bans Complex Figure − Copy (SD) | 17.4(2.0) | 0.32 | <0.001 | −0.06 | 0.487 |
| R Bans Complex Figure − Recall (SD) | 11.6 (3.8) | - | - | 0.37 | <0.001 |
| FCSRT delayed free recall | 25.1 (9.3) | 0.37 | <0.001 | - | - |
| Total hippocampal volume, cm3 (SD) | 6.5 (8.1) | 0.30 | 0.001 | 0.23 | 0.008 |
| CA1, cm3 (SD) | 0.6 (7.2) | 0.28 | 0.001 | 0.20 | 0.023 |
| CA2/3, cm3 (SD) | 0.1 (2.1) | 0.19 | 0.030 | 0.12 | 0.158 |
| CA4/DG, cm3 (SD) | 0.9 (1.2) | 0.17 | 0.049 | 0.13 | 0.126 |
| Pre-subiculum, cm3 (SD) | 0.7 (1.02) | 0.20 | 0.018 | 0.16 | 0.066 |
| Subiculum, cm3 (SD) | 0.1 (1.3) | 0.28 | 0.001 | 0.23 | 0.008 |
| Total intracranial volume, cm3, (SD) | 1358(213) | 0.17 | 0.051 | −0.09 | 0.300 |
3.2. Correlational analysis
In correlational analyses (Table 1), age correlated negatively with Complex Figure Delayed Recall, and FCSRT Delayed Recall. Education correlated positively with Complex Figure Delayed Recall but was not significantly linked with FCSRT Delayed Recall. Total hippocampal volume was positively associated with both Complex Figure Delayed Recall (r = 0.31, p <0.001) and FCSRT Delayed Recall (r=0.23, p = 0.008). Volumes of all hippocampal subfields were directly correlated with performance on Complex Figure Recall and FCSRT Delayed Recall (Figs. 1 and 2).
Fig. 1.
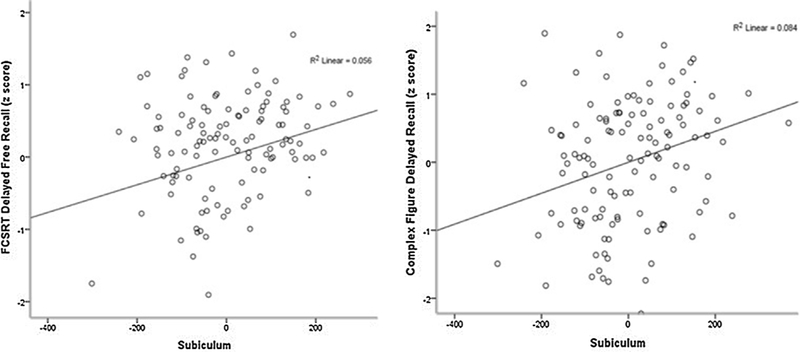
Figures displaying correlation between subiculum and Complex Figure Delayed Recall and Free and Cued Selective Reminding Test Delayed Free Recall.
Fig. 2.
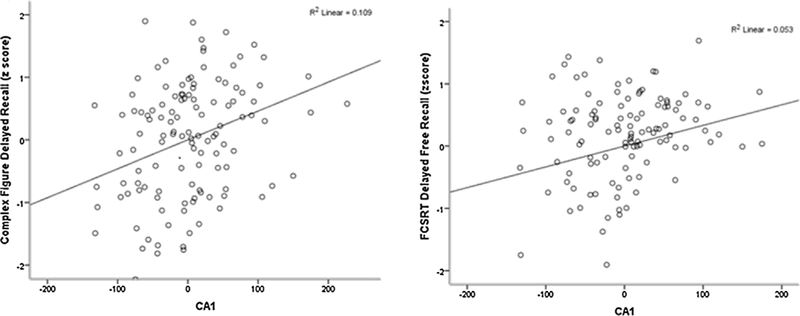
Figure showing relationship between CA1 and Complex Figure Delayed Recall and Free and Cued Selective Reminding Test Delayed Free Recall.
3.3. Hippocampal volumes and visual and verbal memory
In linear regression analysis adjusted for age, sex, race, years of education, and TICV, total HV was positively associated with Complex Figure Delayed Recall (β = 0.31, p = 0.001) and FSCRT Delayed Recall (β=0.27, p = 0.007).
Table 2 shows that within the hippocampal subfields, larger CA1 was associated with Complex Figure Delayed Recall (β = 0.26, β = 0.002) and with FCSRT Delayed Recall (β = 0.20, p = 0.025). The subiculum showed a positive independent association with Complex Figure Delayed Recall (β = 0.27, p = 0.002) and FCSRT Delayed Recall (β = 0.24 p = 0.010). Figs. 1 and 2 illustrate these results.
Table 2.
Linear regression models for the effect of hippocampal subfields on Complex Figure Delayed Recall and Free and Cued Selective Reminding Test Delayed Free Recall.
| Hippocampal subfields | Figure Delayed Recall |
FCSRT Delayed Free Recall |
||||
|---|---|---|---|---|---|---|
| β | t | p | β | t | p | |
| CA1 | 0.26 | 3.13 | 0.002 | 0.20 | 2.67 | 0.025 |
| CA2/CA3 | 0.16 | 1.92 | 0.058 | 0.11 | 1.28 | 0.204 |
| CA4/DG | 0.17 | 2.02 | 0.045 | 0.13 | 1.41 | 0.160 |
| Pre-Subiculum | 0.17 | 1.92 | 0.057 | 0.16 | 1.80 | 0.074 |
| Subiculum | 0.27 | 3.12 | 0.002 | 0.24 | 2.63 | 0.010 |
Note: FCSRT = Free and Cued Selective Reminding test. Models adjusted for age, sex, education, total intracranial volume. Corrected for multiple testing (p <0.01 was considered statistically significant). Bold indicates significant associations.
4. Discussion
In this cross-sectional study we found strong associations between total hippocampal volume and the performance on tests of visual and verbal episodic memory. Within the hippocampal structure, CA1 and subiculum were strongly associated with both visual and verbal episodic memory as represented by Complex Figure Delayed Recall and FCSRT Delayed Recall. Specifically, smaller CA1 and smaller subiculum were associated with poorer performance on both these tests.
Within the hippocampus, the subiculum is the main output component, projecting CA1’s information to cortical and subcortical targets [26]. Previously, the subiculum has either been given little attention in the literature or treated as an ambiguous set of structures [27]; however, recently some focus has been attributed to it due to its emerging importance in spatial processing and performance [26,28], delayed verbal recall [29], and visual memory [8,9]. The subiculum encodes a representation of task relevant information for a relatively short time, whereas the CA1 cells become progressively engaged in retrieval processes [26]. Studies on rodents have further shown that CA1 and subiculum also work conjointly to encode information in visual delayed activities [30]. In human studies, subiculum and CA1 results also suggest involvement in immediate and delayed visual memory tests [8,9,16] and delayed verbal episodic memory tests [29]. Our results highlighting an association between verbal and visual episodic memory and CA1 and subiculum are in line with previous findings on increased CA1 and subiculum activity during delayed periods of visual [9,16,29,30] and verbal tasks [29]. This further enforces the hypothesis that CA1 and subiculum structures in the hippocampus are responsible for retrieval processes. We urge other researchers to further expand on these findings by using tests of spatial episodic memory to find out if results are replicated in this cognitive domain, and if laterality is present in other tasks of spatial episodic memory that are known for their association with the hippocampus [31]. Studying the hippocampus in a detailed approach across age groups will help to sensitively map its distinct functions; in the case of pathology and cognitive decline this approach also helps target more selectively specific areas that are sensitive to atrophy and impairment.
Studies investigating hippocampal subfields in relation to AD have consistently shown that the CA1 and subiculum are the most affected areas [13,14,32,33], with slight disagreements on whether the neuronal loss is also significant in presymptomatic AD [14]. Some studies have shown that conversion from cognitively normal to MCI is associated with hippocampal atrophy specific to the subiculum and CA1 three years prior to a diagnosis, with conversion from MCI to AD showing further atrophy in the CA2-CA3 subfields [33]. Other studies have failed to find a significant association between the presymptomatic phase of AD and CA1 atrophy [14]. Identifying neurocognitive tests that are specifically associated with these regions in the presymptomatic phase (i.e. even before MCI diagnosis) will serve as attainable and inexpensive measures in clinical settings that will conveniently help target individuals at higher risks of memory impairment. We urge future research to study the sensitivity of these neurocognitve tests in relation to hippocampal subfield atrophy and to incident MCI and AD.
Although there are numerous studies that have investigated the association between total hippocampal volume and neurocognitive tests, research on associations between hippocampal subfields and neurocognitive measures are sparse. In one study Lim and colleagues [34], reported associations between atrophy in the CA1 and subiculum and verbal immediate recall, verbal delayed recall, verbal recognition memory, and constructional recall in 51 AD patients [34]. In another study, investigating hippocampal atrophy and onset of dementia, Costafreda et al. [35] found that by analyzing the level of hippocampal atrophy alone the authors accurately categorized participants into MCI vs no MCI groups. Hippocampal morphology in MCls was also associated with poorer global cognition (MMSE) and verbal memory (CERAD memory). In their study, CA1 was the area most specifically associated with risk of MCI conversion after 12 months of follow-up [35]. Although our study was cross-sectional, and thus we were unable to find out rates of conversion based on HC atrophy and neurocognitive tests, our findings are consistent with previous literature reporting CA1 and subicu- lum’s specific involvement in visual and verbal episodic memory in older adults.
Despite novel results, our study has some noteworthy limitations. Within the hippocampus itself, the subfields are not distinctively separated, and due to their proximity some subfield boundaries in FreeSurfer, such as CA1 and the subiculum may have some overlap since it is possible that some areas of the CA1 may also be assigned to the subiculum and CA2/CA3 [26,36]. In fact, Freesurfer’s accuracy in detecting some of the hippocampal subfields has been questioned [36], therefore further studies using manual methods or newer automated methods are warranted to confirm our findings. We applied Sidak correction for multiple testing; like most other adjustments for multiple comparisons, this test can be somewhat conservative; the correction may also lead to false negatives, hence reducing statistical power. Due to the high correlations (r = 0.37) between FCSRT delayed recall and Complex Figure delayed recall, the association between these tests and CA1 and subiculum may be reflective of the variance between the cognitive tests. Although the number of subjects in this study is fairly large in comparison with other MRl studies, some of the associations marginally missed significance (CA1 and FCSRT Delayed Recall, p = 0.025), therefore larger population-based studies might be required to detect smaller associations. The crosssectional nature of this study precludes conclusions about causality between imaging measures and the performance on visual and verbal tests and MCI/AD incidence. Further longitudinal studies are required to investigate these associations over time and to find out whether these verbal and visual tests have the same power in predicting MCI and AD as CA1 and subiculum atrophy. We only used single tests to represent verbal and visual episodic memory; we urge other researches to explore these associations with other tests representing these domains to generalize findings to visual and verbal episodic memories.
5. Conclusion
ln this study we explored associations between delayed visual and verbal recall measures using the RBANS Complex Figure subtest and the FCSRT Delayed Recall subtest and hippocampal sub-regions in healthy older adults. Our results showed CA1 and subiculum subfields as strongly associated with both verbal and visual episodic memory. Our results suggested that hippocampal atrophy, specifically, CA1 and subiculum may be markers of normative cognitive decline, and that the R-BANS Complex Figure and the FCSRT delayed recall may provide quick, sensitive insights into the aging brain and its cognitive function.
Our results offer clinical applicability by encouraging the use of these neurocognitive tests as prognostic measures in clinical settings to encourage watchful waiting in individuals whose scores are below age-adjusted means, and thus denote risk or even possible impairment, in which case a more active approach and thorough assessment that resorts to imaging procedures as a confirmatory approach for diagnosis may proceed. We suggest future research to use these and other psychometric tests to aid in developing a better understanding of the hippocampus, its subfields, and their functions relating to cognition in humans.
HIGHLIGHTS.
Neurocognitive tests tapping specific HC subfields can help target at-risk individuals.
Subiculum was associated with verbal and visual episodic memory.
CA1 was associated with verbal and visual episodic memory.
No other subfields were associated with verbal or visual episodic memory.
Our results suggest that CA1 and subiculum are responsible for retrieval.
Acknowledgements
We thank the EAS research participants. We thank Charlotte Magnotta, Diane Sparracio and April Russo for assistance in participant recruitment; Betty Forro, Wendy Ramratan, and MaryJoan Sebastian for assistance in clinical and neuropsychological assessments; Michael Potenza for assistance in data management.
Funding sources
This research was supported by the Einstein Aging Study (PO1 AG03949) from the National Institutes on Aging program; the National Institutes of Health CTSA (1UL1TR001073) from the National Center for Advancing Translational Sciences (NCATS), the Sylvia and Leonard Marx Foundation, and the Czap Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
AZ, EZ, MZ, ML and MK declare that there are no financial, personal, or other potential conflicts of interest. RBL serves on the editorial boards of Neurology and as senior advisor to Headache. He has reviewed for the NIA and NINDS, holds stock options in eNeura Therapeutics; serves as consultant, advisory board member, or has received honoraria from: Alder, Allergan, American Headache Society, Amgen, Autonomic Technologies, Avanir, Boehringer- Ingelheim, Boston Scientific, Bristol-Myers Squibb, Colucid, Dr. Reddy’s, Electrocore, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, Teva, Vedanta. He receives royalties from Wolff’s Headache, 8th Edition, Oxford Press University, 2009 and Informa.
References
- [1].Van Petten C, Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis, Neuropsychologia 42 (10) (2004) 1394–1413. [DOI] [PubMed] [Google Scholar]
- [2].Burgess N, Maguire EA,O’Keefe J, The human hippocampus and spatial and episodic memory, Neuron 35 (4) (2002) 625–641. [DOI] [PubMed] [Google Scholar]
- [3].Manns JR, Hopkins RO, Squire LR, Semantic memory an the human hippocampus, Neuron 38(1)(2003) 127–133. [DOI] [PubMed] [Google Scholar]
- [4].Chen KH, et al. , Hippocampal region-specific contributions to memory performance in normal elderly, Brain Cogn. 72 (3) (2010) 400–407. [DOI] [PubMed] [Google Scholar]
- [5].Eldridge LL, et al. , A dissociation of encoding and retrieval processes in the human hippocampus,J. Neurosci. 25 (13) (2005) 3280–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Preston AR, et al. , High-resolution fMRI of content-sensitive subsequent memory responses in human medial temporal lobe,J. Cogn. Neurosci. 22(1) (2009)156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Suthana N, et al. , Dissociations within human hippocampal subregions during encoding and retrieval of spatial information, Hippocampus 21 (7) (2011)694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zeineh MM, et al. , Dynamics ofthe hippocampus during encoding and retrieval of face-name pairs, Science 299 (5606) (2003) 577–580. [DOI] [PubMed] [Google Scholar]
- [9].Nauer RK, et al. , Hippocampal subfield and medial temporal cortical persistent activity during working memory reflects ongoing encoding, Front. Syst. Neurosci. 9 (2015) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kerchner GA, et al. , Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer’s disease, Neuroimage 63 (1)(2012) 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mueller SG,et al. , Evidence forfunctionalspecializationofhippocampal subfields detected by MR subfield volumetry on high resolution images at 4 T, Neuroimage 56 (3) (2011) 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mueller SG, et al. , Different structural correlates forverbal memory impairment in temporal lobe epilepsy with and without mesial temporal lobe sclerosis, Hum. Brain Mapp. 33 (2) (2012) 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mueller SG, et al. , Measurement ofhippocampal subfields and age-related changes with high resolution MRI at 4T, Neurobiol. Aging 28 (5) (2007) 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].West MJ, et al. , Hippocampal neurons in pre-clinical Alzheimer’s disease, Neurobiol. Aging 25 (9) (2004) 1205–1212. [DOI] [PubMed] [Google Scholar]
- [15].West MJ, et al. , Differences in the pattern ofhippocampal neuronal loss in normal ageing and Alzheimer’s disease, Lancet 344 (8925) (1994) 769–772. [DOI] [PubMed] [Google Scholar]
- [16].Travis SG, et al. , High field structural MRI reveals specific episodic memory correlates inthe subfields ofthe hippocampus, Neuropsychologia 53 (2014) 233–245. [DOI] [PubMed] [Google Scholar]
- [17].Katz MJ, et al. ,Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study, Alzheimer Dis. Assoc. Disord. 26 (4)(2012)335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Randolph C, Repeatable Battery forthe Assessment ofNeuropsychological Status Manual, Psychological Corporation, San Antonio, TX, 1998. [Google Scholar]
- [19].Buschke H, Selective reminding foranalysis of memoryand learning,J. Verbal Learn. Verbal Behav. 12(1973)543–550. [Google Scholar]
- [20].Ezzati A, et al. , Hippocampal subfields differentially correlate with chronic pain in older adults, Brain Res. 1573 (2014) 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fischl B, et al. , Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain, Neuron 33 (3) (2002) 341–355. [DOI] [PubMed] [Google Scholar]
- [22].Buckner RL, et al. , A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement oftotal intracranial volume, Neuroimage 23 (2) (2004) 724–738. [DOI] [PubMed] [Google Scholar]
- [23].Van Leemput K, et al. , Automated segmentation ofhippocampal subfields from ultra-high resolution In vivo MRI, Hippocampus 19 (6) (2009) 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Krogsrud SK, et al. , Development ofhippocampal subfield volumes from 4 to 22 years, Hum. Brain Mapp. 35(11) (2014) 5646–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sidak Z, Rectangularconfidence regions forthe means of multivariate normal distributions,J. Am. Stat. Assoc. 62 (318) (1967) 626–633. [Google Scholar]
- [26].O’Mara SM, et al. , Roles forthe subiculum in spatial information processing: memory, motivation and the temporal control ofbehaviour, Prog. Neuro Psychopharmacol. Biol. Psychiatry 33 (5) (2009) 782–790. [DOI] [PubMed] [Google Scholar]
- [27].Amaral DG, Dolorfo C, Alvarez-Royo P, Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat, Hippocampus 1 (4) (1991) 415–435. [DOI] [PubMed] [Google Scholar]
- [28].de la Prida LM, et al. , The subiculum comes of age, Hippocampus 16(11) (2006) 916–923. [DOI] [PubMed] [Google Scholar]
- [29].Travis SG, et al. , High field structural MRI reveals specific episodic memory correlates inthe subfields ofthe hippocampus, Neuropsychologia 53 (2014) 233–245. [DOI] [PubMed] [Google Scholar]
- [30].Deadwyler SA, Hampson RE, Differential but complementary mnemonic functions ofthe hippocampus and subiculum, Neuron 42 (3) (2004) 465–476. [DOI] [PubMed] [Google Scholar]
- [31].Maguire EA, Woollett K, Spiers HJ, London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis, Hippocampus 16(12) (2006) 1091–1101. [DOI] [PubMed] [Google Scholar]
- [32].Bobinski M, et al. , Atrophy ofhippocampal formation subdivisions correlates with stage and duration ofAlzheimerdisease, Dementia 6(4) (1995) 205–210. [DOI] [PubMed] [Google Scholar]
- [33].Apostolova LG, et al. , Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal, Neurobiol. Aging 31(7) (2010) 1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lim HK, et al. , Relationships between hippocampal shape and cognitive performances in drug-na’ive patients withAlzheimer’s disease, Neurosci. Lett. 516 (1)(2012) 124–129. [DOI] [PubMed] [Google Scholar]
- [35].Costafreda SG, et al. , Automated hippocampal shape analysis predicts the onset ofdementia in mild cognitive impairment, Neuroimage 56 (1) (2011) 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wisse LEM, Biessels GJ, Geerlings MI, A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer, Front. Aging Neurosci. 6 (2014) 261. [DOI] [PMC free article] [PubMed] [Google Scholar]


