Abstract
Background:
Despite strong and consistent prospective associations of elevated low-density lipoprotein-cholesterol (LDL-C) concentration with incident coronary and cerebrovascular disease (CCVD), data for incident peripheral artery disease (PAD) are less robust. Atherogenic dyslipidemia characterized by increased small LDL particle concentration (LDL-P), rather than total LDL cholesterol content, along with elevated triglyceride-rich lipoproteins and low high-density lipoprotein-cholesterol (HDL-C) may be the primary lipid driver of PAD risk.
Methods:
The study population was a prospective cohort study of 27,888 women ≥45 years old free of cardiovascular disease at baseline and followed for a median of 15.1 years. We tested whether standard lipid concentrations as well as nuclear magnetic resonance (NMR) spectroscopy-derived lipoprotein measures were associated with incident symptomatic PAD (n=110) defined as claudication and/or revascularization.
Results:
In age-adjusted analyses, while LDL-C was not associated with incident PAD, we found significant associations for increased total and small LDL-P concentrations, triglycerides, and concentrations of very low-density lipoprotein particle subclasses (VLDL-P), increased total cholesterol (TC):HDL-C, low HDL-C, and low HDL particle concentration (HDL-P) (all P for extreme tertile comparisons <0.05). Findings persisted in multivariable-adjusted models comparing extreme tertiles for elevated total LDL-P (HRadj 2.03; 95% CI, 1.14 to 3.59), small LDL-P [HRadj 2.17 (1.10 to 4.27)], very large VLDL-P (HRadj 1.68 (1.06 to 2.66)], medium VLDL-P (HRadj 1.98 (1.15 to 3.41)], and TC:HDL-C [HRadj, 3.11 (1.67 to 5.81)]. HDL was inversely associated with risk; HRadj for extreme tertiles of HDL-C and HDL-P were 0.30 (P-trend <0.0001) and 0.29 (P-trend <0.0001), respectively. These components of atherogenic dyslipidemia, including small LDL-P, medium and very large VLDL-P, TC:HDL-C, HDL-C, and HDL-P, were more strongly associated with incident PAD than incident CCVD. Finally, the addition of LDL-P and HDL-P to TC:HDL-C measures identified women at heightened PAD risk.
Conclusions:
In this prospective study, NMR-derived measures of LDL particle concentration, but not LDL-C, were associated with incident PAD. Other features of atherogenic dyslipidemia, including elevations in TC:HDL-C, elevations in triglyceride-rich lipoproteins, and low standard and NMR-derived measures of HDL, were significant risk determinants. These data help clarify prior inconsistencies and may elucidate a unique lipoprotein signature for PAD compared to CCVD.
Keywords: peripheral artery disease, coronary artery disease, lipoproteins, magnetic resonance spectroscopy
Introduction
Atherogenic dyslipidemia, which comprises a triad of increased blood concentrations of small, dense low-density lipoprotein (LDL) particles, decreased high-density lipoprotein (HDL) particles, and increased triglyceride-rich lipoproteins, has been linked to both coronary artery disease and cerebrovascular disease (CCVD).1 However, the specific lipoprotein components that contribute to peripheral artery disease (PAD) risk are less clear. In contrast to CCVD, the epidemiologic data supporting a link between LDL cholesterol (LDL-C) and incident PAD are limited, especially among women.2, 3 Additionally, individuals with heterozygous familial hypercholesterolemia (FH) and genetically elevated levels of LDL-C have notably lower rates of PAD compared to coronary artery disease (CAD).4 Instead, studies suggest that dyslipidemia parameters such as an elevated ratio of total cholesterol:high-density lipoprotein cholesterol (TC:HDL-C), mixed dyslipidemia and hypertriglyceridemia may be the strongest lipid risk factors for incident PAD and PAD progression.2, 5–8
One means of delineating the lipid-related risk in PAD is by using more detailed lipoprotein measures derived from proton nuclear magnetic resonance (NMR) spectroscopy. Standard lipid panels measure the entire plasma cholesterol or triglyceride content in concentration per deciliter of each lipoprotein class. In contrast, NMR spectroscopy quantifies both the number and size of lipoprotein particles.9 Plasma cholesterol concentration can differ among individuals due to both variations in particle size as well as metabolic processes that regulate the cholesterol and triglyceride content of the lipoprotein particle core, and these differences often lead to discrepant risk estimates based on traditional versus NMR-derived lipoprotein measures.10 NMR-derived lipoprotein measures are associated with future myocardial infarction (MI),11–16 stroke,11, 14, 16 diabetes,17, 18 and hypertension.19 To our knowledge, this methodology has not yet been applied to PAD.
Given the lack of robust data showing a link between LDL-C and PAD, we hypothesized that NMR-derived lipoprotein subclass abnormalities associate with incident PAD and would be distinct from those previously described in other cardiovascular disorders.11 Therefore, in the current study, we evaluated baseline NMR lipoprotein particles and conventional lipid concentrations in a prospective cohort of middle-aged and older American women free of PAD, MI, and stroke at baseline and measured the association of these lipid measures with incident PAD.
Methods
Data Availability
The data will not be made available to other researchers for purposes of reproducing the results. However, the methods used in the analysis are available upon request.
Study Population
Participants were identified from the Women’s Health Study (WHS), a previously completed randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease.20 From 1992–1995, the study enrolled a total of 39 876 female healthcare professionals in the US without a history of cancer, MI, stroke, coronary revascularization or peripheral artery revascularization. At the time of enrollment, women completed questionnaires on baseline demographics, anthropometrics, medical history, and lifestyle factors. Following completion of the trial, willing individuals were consented to participate in a longitudinal observational component of the WHS. All participants provided written informed consent, and the study was approved by the institutional review board at Brigham and Women’s Hospital.
Prior to randomization, 28 345 of the participants consented to provide blood samples, and 98.9% (n = 28 024) of these samples underwent NMR lipoprotein profiling. Individuals missing baseline demographic data on body mass index (BMI) as well as history of smoking, hypertension or hormonal therapy were excluded from the analysis. In addition, subjects with confirmed pre-randomization PAD (n = 30) were excluded from the present analysis. The final study population (n =27 888) was followed for a median of 15.1 years.
Outcome Ascertainment
Health outcomes of WHS participants were ascertained using annual questionnaires. The primary outcome of interest for the present study was symptomatic lower extremity PAD defined as intermittent claudication and/or peripheral artery revascularization (surgical or percutaneous). To validate reported events, PAD outcomes were initially identified through annual questionnaires and then confirmed through physician interview and medical records review. For cases of claudication, confirmation was performed using the Edinburgh Claudication Questionnaire which was administered during telephone interviews conducted by a physician adjudicator. The Edinburgh Claudication Questionnaire is an accepted tool for the detection of PAD that is commonly used in clinical research and has been validated against in-office physician-diagnosed intermittent claudication with a sensitivity of 91.3% and a specificity of 99.3%.21 These values for sensitivity and specificity are similar to and, in some cases, higher than those for reported resting ankle-brachial index (ABI).22 If patients reported lower extremity revascularization on their questionnaire, these events were confirmed by cardiologist review of primary medical records. CCVD was defined as non-fatal MI, percutaneous coronary intervention, coronary artery bypass grafting, non-fatal stroke, or coronary-related death, and these endpoints were adjudicated as previously described.23 Utilizing these criteria, we confirmed 130 cases of incident PAD. The most common causes for non-ischemic leg pain in disconfirmed cases were venous disease, lower extremity arthritis, lumbar disk disease, and peripheral neuropathy.
Laboratory Analysis
Blood samples were stored in liquid nitrogen (−150°C to −180°C) until analysis. Samples were thawed, aliquoted, and shipped in 200μL frozen aliquots to LipoScience (now LabCorp) (Raleigh, NC) for analysis. The lipoprotein analysis used in the present study is the NMR LipoProfile 4 panel. In this panel, the concentration of each lipoprotein particle subclass is calculated from the NMR signal of terminal methyl groups, and these same NMR signals are used to help calculate weighted-average lipoprotein particle sizes.10 Particles are classified based on size into the following categories: LDL-P, HDL-P, and very low-density lipoprotein (VLDL-P). Supplemental Table 1 lists lipoprotein particle diameters.
A core laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program measured standard lipids and apolipoproteins. LDL-C was measured using a homogeneous direct method with a Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, Ind). HDL-C was measured using a direct enzymatic colorimetric assay, and triglycerides were measured enzymatically with correction for endogenous glycerol. Coefficients of variation were <3% for all standard lipids. Non-HDL-C was calculated by subtracting HDL-C from TC. Apolipoproteins B100 and A-1 were measured using immunoturbidometric assays (DiaSorin, Stillwater, Minn) with coefficients of variation of 5% and 3%, respectively. High-sensitivity C-reactive protein (hsCRP) was measured by a high-sensitivity immunoturbidimetric assay (Denka Seiken, Niigata, Japan).
Statistical Analysis
Continuous data are summarized as either mean ± standard deviation or median with interquartile range depending on normality of the distributions. Categorical data are listed as percentages. Between group differences were assessed by the Wilcoxon rank-sum test for continuous data and the χ2 test for categorical data. Lipid biomarkers were divided into tertiles. Cox proportional-hazards models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for each biomarker tertile, and results are presented as top tertile compared to bottom tertile; similar analyses were performed per standard deviation increase of each biomarker. Tests of linear trend across tertiles were performed using the median value from each tertile. We also calculated Spearman rank correlation coefficients to test the relationships between standard and NMR-derived lipoprotein measures.
As preventive therapies instituted at diagnosis of MI or stroke may dramatically alter the subsequent risk of vascular events, we censored women having non-PAD vascular events (CCVD) at the time of diagnosis. Thus, within these models, follow up time was censored at the time of the PAD event except in situations in which a CCVD event occurred first, in which case censoring occurred at the time of the CCVD event. There were a total of 130 confirmed PAD cases, and in 20 of these cases, a CCVD event occurred prior to the PAD event. Thus, the final population in the current analysis was 110 cases of incident PAD.
Regression models were sequentially adjusted for age followed by smoking pack-years (Model 1). Fully-adjusted models (Model 2) were adjusted for age, smoking pack-years, metabolic syndrome, hypertension, postmenopausal hormone therapy (HT), hsCRP, lipid lowering therapy, and BMI. Data on smoking pack years was collected as a categorical variable within WHS, and this variable was divided into the following categories for the purposes of regression modeling: 0, 1–10, 11–29, ≥30. All regression results in the text are presented for Model 2 unless otherwise noted. Additionally, all models were adjusted for randomized treatment within the WHS trial. Measures of serum triglycerides were log-transformed for P-trend analysis due to a right-skewed distribution. Models of LDL particle size were also adjusted for total LDL particle concentration as previously described.24 Given the inverse correlation of LDL-P subclasses,24 models assessing each LDL-P subclass were additionally adjusted for the remaining LDL-P subclasses to delineate independent risk associations. The likelihood ratio χ2 statistic was used to assess model fit. To evaluate the joint effects of LDL-C concentration with LDL-P as well as TC:HDL-C with LDL-P, HDL-P, and VLDL-P, individuals were classified into 4 groups based on the values of each biomarker relative to the population median. Kaplan-Meier survival curves were plotted based on these strata and analyzed using a logrank test for trend with three degrees of freedom. All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC). All 95% CIs are 2-tailed, and the P value cutoff for all analyses was 0.05.
Results
As shown in Table 1, women with incident PAD were more likely to be older, current smokers and have higher rates of baseline hypertension. In this population with a low prevalence of baseline diabetes, there was no significant difference in diagnosed diabetes, although individuals with incident PAD were more likely to have a history of metabolic syndrome. Baseline levels of triglycerides, apolipoprotein B100, non-HDL-C, TC:HDL-C, and hsCRP were all higher in individuals who developed PAD. The baseline levels of HDL-C and apolipoprotein A-1 were lower in individuals with incident PAD. There was no statistically significant difference in TC or LDL-C.
Table 1.
Baseline Characteristics of the Study Population
| Women Remaining Free of PAD Events (n = 27 778)* | Women Developing PAD Events (n = 110)† | P | |
|---|---|---|---|
| Age, mean (SD), y | 54.7 (7.1) | 59.2 (7.5) | <0.0001 |
| BMI, mean (SD), kg/m2 | 25.9 (5.0) | 25.7 (4.5) | 0.75 |
| Non-Hispanic white, % | 95.3 | 98.2 | 0.25 |
| Current smoking, % | 11.5 | 47.3 | <0.0001 |
| Prior smoking, % | 36.6 | 36.4 | 1.00 |
| Pack-years, % | |||
| 0 | 52.3 | 16.5 | <0.0001 |
| 1–10 | 15.3 | 6.4 | |
| 11–29 | 22.6 | 32.1 | |
| ≥30 | 9.8 | 45.0 | |
| Diabetes, % | 2.5 | 3.6 | 0.35 |
| Metabolic syndrome, % | 24.6 | 33.6 | 0.03 |
| Hypertension, % | 25.1 | 39.1 | 0.001 |
| Treatment for hypercholesterolemia, % | 3.2 | 5.5 | 0.17 |
| Family history of premature CAD, % | 14.4 | 18.5 | 0.22 |
| Exercise ≥1 time/wk, % | 43.2 | 38.2 | 0.33 |
| Current HT use, % | 42.6 | 36.4 | 0.21 |
| WHS trial assignment to vitamin E, % | 50.1 | 49.1 | 0.85 |
| WHS trial assignment to aspirin, % | 50.1 | 50.9 | 0.92 |
| hsCRP, mg/L | 2.0 (0.8–4.4) | 2.8 (1.6–6.6) | <0.0001 |
| Standard chemical lipids, mg/dL | |||
| Total cholesterol | 208 (184–235) | 214 (185–246) | 0.13 |
| LDL cholesterol | 121 (101–144) | 130 (103–153) | 0.05 |
| HDL cholesterol | 52 (43–62) | 44 (37–55) | <0.0001 |
| Triglycerides | 118 (84–175) | 146 (104–218) | 0.0001 |
| Apolipoproteins, mg/dL | |||
| Apolipoprotein B100 | 100 (84–121) | 115 (91–133) | <0.0001 |
| Apolipoprotein A-1 | 149 (132–168) | 138 (124–152) | <0.0001 |
| Non-HDL cholesterol, mg/dL | 154 (129–182) | 172 (137–200) | 0.0007 |
| Total cholesterol: HDL cholesterol | 3.97 (3.23–4.92) | 4.66 (3.87–6.02) | <0.0001 |
Values are median (25th-75th percentile) unless otherwise indicated. P values for continuous variables were obtained from the Wilcoxon rank sum test. P values for categorical variables were obtained using the Chi-square test.
PAD refers to peripheral artery disease; CAD indicates coronary artery disease; HT indicates hormonal therapy; WHS refers to Women’s Health Study; hsCRP refers to high-sensitivity C-reactive protein; SD refers to standard deviation.
Number missing: 234 for Race; 227 for Pack-years; 15 for Diabetes, 49 for Metabolic syndrome; 20 for Treatment for hypercholesterolemia; 462 for Family history of premature CAD; 10 for Exercise; 86 for hsCRP; 87 each for Total cholesterol and HDL cholesterol; 86 each for LDL cholesterol and Triglycerides; 224 for Apolipoprotein B100; 220 for Apolipoprotein A-1; 88 each for Non-HDL cholesterol and Total cholesterol: HDL cholesterol
Number missing: 1 for Race; 1 for Pack-years; 2 for Family history of premature CAD; 4 each for Apolipoprotein B100 and Apolipoprotein A-1
Table 2 shows median concentrations of NMR-derived lipoprotein particles according to case status. Total LDL-P as well as small LDL-P subclass concentration were higher in women with PAD whereas large LDL-P, medium LDL-P, and total HDL-P were significantly lower. Among HDL-P subclasses, all but small HDL-P were lower in individuals with PAD. Total VLDL-P, very large VLDL-P, large VLDL-P, and medium VLDL-P particle concentrations were higher in those with PAD. Differences in small and very small VLDL-P concentrations did not reach statistical significance. Consistent with data for particle subclass concentrations, women developing PAD had smaller average LDL-P and HDL-P size and a larger average VLDL-P size.
Table 2.
Baseline NMR Lipoprotein Profile
| Women Remaining Free of PAD Events (n = 27 778)* |
Women Developing PAD Events (n = 110)† | P | |
|---|---|---|---|
| NMR lipoprotein particle concentrations, nmol/L | |||
| LDL particles | |||
| Total | 1567 (1329–1839) | 1723 (1495–1989) | <0.0001 |
| Large | 306 (160–467) | 233 (69–474) | 0.02 |
| Medium | 156 (0–347) | 128 (0–285) | 0.03 |
| Small | 953 (685–1336) | 1208 (875–1599) | <0.0001 |
| VLDL particles | |||
| Total | 166.5 (130.3–208.4) | 180.1 (145.3–221.9) | 0.005 |
| Very Large | 0.1 (0.1–0.2) | 0.2 (0.1–0.5) | 0.003 |
| Large | 1.6 (0.3–4.3) | 2.9 (0.6–5.5) | 0.004 |
| Medium | 15.8 (8.5–25.5) | 20.8 (13.2–31.9) | 0.0009 |
| Small | 55.4 (34.0–81.8) | 62.2 (36.3–85.2) | 0.22 |
| Very Small | 84.3 (58.9–114.8) | 87.8 (64.9–118.9) | 0.18 |
| HDL particles | |||
| Total | 24400 (22000–27000) | 23150 (20950–25250) | 0.0009 |
| Large | 2100 (1300–3300) | 1600 (1050–2500) | 0.0002 |
| Medium | 5300 (3700–7200) | 3850 (2600–6200) | <0.0001 |
| Small | 16300 (14100–18700) | 16750 (15150–18650) | 0.11 |
| NMR average particle size, nm | |||
| LDL particles | 20.9 (20.6–21.2) | 20.7 (20.4–21.1) | 0.003 |
| VLDL particles | 42.5 (38.6–47.9) | 44.0 (39.1–50.8) | 0.04 |
| HDL particles | 8.9 (8.7–9.2) | 8.7 (8.6–9.1) | 0.0001 |
Values are median (25th-75th percentile). P values were obtained from the Wilcoxon rank sum test.
PAD refers to peripheral artery disease.
Number missing: 581
Number missing: 6
Supplemental Table 2 shows Spearman correlation coefficients for NMR lipoproteins with standard lipid and apolipoprotein measures in the total sample. Total LDL-P concentration correlated strongly with LDL-C (r = 0.71) as well as apolipoprotein B-100 (r = 0.86), non-HDL-C (r = 0.78), and TC/HDL-C (r = 0.66). Large and small LDL-P concentration correlated modestly with LDL-C (r = 0.27 and 0.25, respectively). Large HDL-P correlated strongly with HDL-C (r = 0.75), but total HDL-P (r = 0.52) and the medium HDL-P subclass (r = 0.50) showed more modest correlations. Supplemental Table 3 lists Spearman correlation coefficients for NMR lipoproteins with themselves. Total LDL-P strongly correlated with small LDL-P (r = 0.63), and total VLDL-P most strongly correlated with very small VLDL-P (r = 0.69). HDL-P had similar positive correlations with large, medium, and small HDL-P subclasses (r = 0.35, 0.49, and 0.51 respectively).
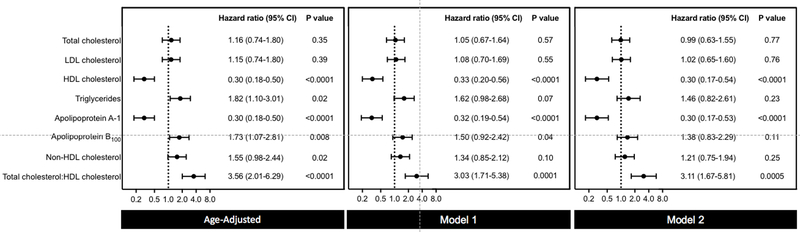
Figure 1 and Supplemental Table 4 show the results from Cox regression analyses adjusted for both age and non-lipid risk factors in women classified based on standard lipid and apolipoprotein tertiles. The strongest positive risk association was with TC:HDL-C (multivariable-adjusted HR 3.11; 95% CI 1.67 to 5.81; P for trend 0.0005). Serum triglyceride concentration was strongly associated with incident PAD in age-adjusted but not multivariable-adjusted models (adjusted HR 1.46; 95% CI, 0.82 to 2.61; P for trend 0.22). Significant findings were also seen for both apolipoprotein B100 and non-HDL-C in age-adjusted but not multivariable-adjusted models. Importantly, no significant associations were seen for TC or LDL-C. In both age-adjusted and multivariable-adjusted models, HDL-C concentration was inversely associated with PAD with a 70% lower relative risk for the lowest tertile versus the highest (adjusted HR, 0.30; 95% CI, 0.17 to 0.54; P for trend <0.0001). There was a similar strong inverse association seen with apolipoprotein A-1 in fully-adjusted models (HR 0.30; 95% CI, 0.17 to 0.53; P for trend <0.0001). Overall, similar results were seen for incident PAD per standard deviation increase of each biomarker (Supplemental Table 5), although the association for apolipoprotein B100 reached statistical significance in the fully-adjusted model (HR 1.23; 95% CI, 1.02 to 1.49; P for trend 0.03).
Figure 1. Risk Associations Between Standard Lipid and Apolipoprotein Measures and Incident PAD.
Hazard ratios and 95% CIs for the top versus bottom tertile of standard lipid and apolipoprotein measures. Model 1 adjusted for age and smoking pack-years. Model 2 adjusted for age, smoking pack-years, metabolic syndrome, hypertension, hormonal therapy, high-sensitivity C-reactive protein, lipid lowering therapy, randomized treatment assignment, and body mass index.
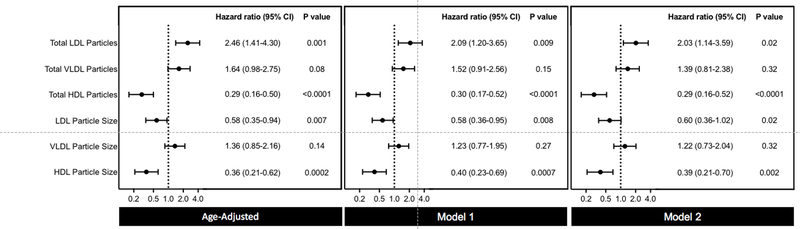
Similarly, age-adjusted and multivariable-adjusted analyses for total NMR-derived lipoprotein particle concentrations are displayed in Figure 2 and Supplemental Table 6. In contrast to the null association of LDL-C with PAD, total LDL-P was the strongest positive lipoprotein risk factor (adjusted extreme tertile HR 2.03; 95% CI 1.14 to 3.59; P for trend 0.02). Both LDL-P size (adjusted extreme tertile HR 0.60; 95% CI 0.36 to 1.02; P for trend 0.02) and HDL-P size (adjusted extreme tertile HR 0.39; 95% CI 0.21 to 0.70; P for trend 0.002) were inversely associated with incident PAD. No significant association was seen for total VLDL-P concentration or VLDL particle size. Total HDL-P was inversely associated with PAD (adjusted HR 0.29; 95% CI, 0.16 to 0.52; P for trend <0.0001). The associations between each biomarker analyzed per standard deviation and incident PAD are displayed in Supplemental Table 7.
Figure 2. Risk Associations Between NMR Lipoprotein Particle Concentrations and Size and Incident PAD.
Hazard ratios and 95% CIs for the top versus bottom tertile of NMR lipoprotein particle concentrations and sizes. Model 1 adjusted for age and smoking pack-years. Model 2 adjusted for age, smoking pack-years, metabolic syndrome, hypertension, hormonal therapy, high-sensitivity C-reactive protein, lipid lowering therapy, randomized treatment assignment, and body mass index.
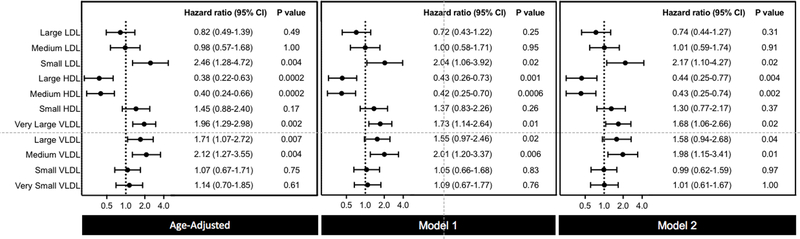
In multivariable models that evaluated lipoprotein particle subclass concentrations, small LDL-P remained significantly associated with incident PAD (adjusted HR 2.17; 95% CI 1.10 to 4.27; P for trend 0.02) (Figure 3, Supplemental Table 8). No residual association was seen for large or medium LDL-P. Both large and medium HDL-P were associated with protection against PAD (adjusted HR 0.44; 95% CI 0.25 to 0.77; P for trend 0.004; and adjusted HR 0.43; 95% CI 0.25 to 0.74; P for trend 0.002, respectively). Of VLDL-P subclasses, very large (size range: 90–240nm), large (size range: 50–89nm), and medium VLDL-P (size range: 37–49nm) were significantly associated with incident PAD (adjusted HR 1.68; 95% CI 1.06 to 2.66; P for trend 0.02; adjusted HR 1.58; 95% CI 0.94 to 2.68; P for trend 0.04; and adjusted HR 1.98; 95% CI 1.15 to 3.41; P for trend 0.01, respectively). Supplemental Table 9 shows the association per standard deviation increase in each biomarker.
Figure 3. Risk Associations Between NMR Lipoprotein Particle Subclasses and Incident PAD.
Hazard ratios and 95% CIs for the top versus bottom tertile of NMR lipoprotein particle subclasses. Model 1 adjusted for age and smoking pack-years. Model 2 adjusted for age, smoking pack-years, metabolic syndrome, hypertension, hormonal therapy, high-sensitivity C-reactive protein, lipid lowering therapy, randomized treatment assignment, and body mass index.
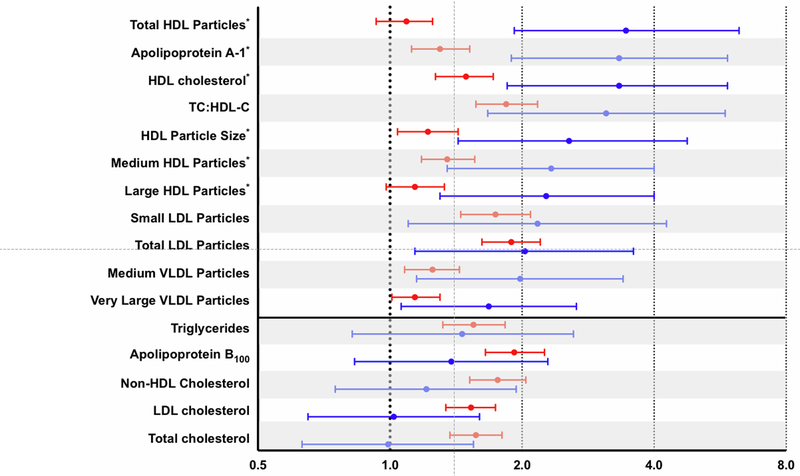
Figure 4 (Supplemental Figure 1, Supplemental Table 10) displays multivariable-adjusted risk associations for standard lipid, apolipoprotein, and NMR lipoprotein measures for both incident PAD ranked by magnitude of the risk estimate. Results are displayed comparing highest versus lowest tertile except for biomarkers associated with protection against incident disease, in which case results are presented as lowest versus highest tertile to facilitate comparisons. Of all measures analyzed, HDL-P, apolipoprotein A-1, HDL-C, and TC:HDL-C had the largest HRs for incident PAD (Figure 4). Statistically significant associations were also seen for HDL particle size, medium and large HDL-P, small and total LDL-P, and medium, large, and very large VLDL-P.
Figure 4. Risk Associations Between NMR Lipoprotein and Standard Lipid Measures with Incident PAD Versus Incident CCVD.
Hazard ratios and 95% CIs for the top versus bottom tertile of incident PAD (blue) and CCVD (red), adjusted for age, smoking pack-years, metabolic syndrome, hypertension, hormonal therapy, high-sensitivity C-reactive protein, lipid lowering therapy, randomized treatment assignment, and body mass index. Measures displayed include all standard lipid and apolipoprotein assays as well as NMR-derived measures with a statistically significant association for incident PAD. Horizontal line separates markers of atherogenic dyslipidemia from other measures without statistical significance for incident PAD.
Although TC and LDL-C were not associated with incident PAD, both were associated with incident CCVD (adjusted HR 1.57; 95% CI 1.37 to 1.80; P for trend <0.0001 and adjusted HR 1.53; 95% CI 1.34 to 1.74; P for trend <0.0001, respectively) (Figure 4, Supplemental Figure 2, Supplemental Table 10). Among standard lipid and apolipoprotein measures, adjusted HRs for apolipoprotein B100 and non-HDL-C were nominally larger and statistically significant only for incident CCVD, while HDL-C, apolipoprotein A-1, and TC:HDL-C were more strongly associated with incident PAD. Of the NMR-derived lipoprotein measures, total LDL-P, small LDL-P, large and medium subclasses of VLDL-P, and total HDL-P appeared more strongly associated with incident PAD than incident CCVD.
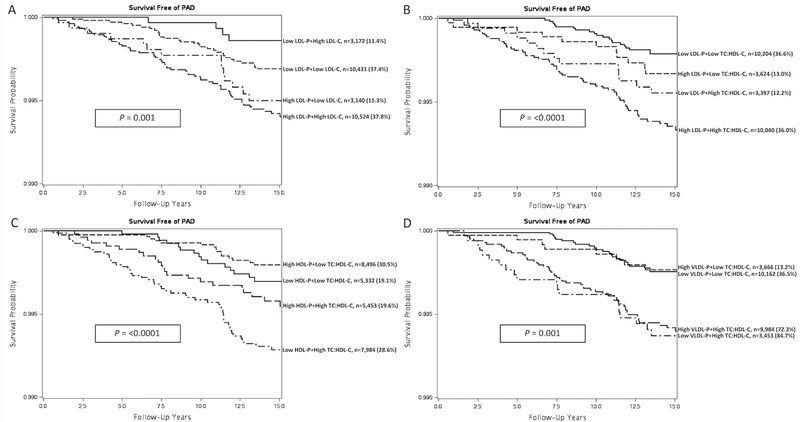
Women were categorized based on both LDL-C and total LDL-P concentration (above or below median) to evaluate the joint role of these biomarkers in PAD risk prediction (Figure 5A). Overall, women with total LDL-P values above the population median were at highest risk of incident PAD irrespective of their LDL-C measure. Additionally, we reclassified women based on total LDL-P, HDL-P, and VLDL-P concentrations and TC:HDL-C (above or below median) (Figure 5B-D). Even among those with elevated TC:HDL-C, the addition of total LDL-P or total HDL-P particle concentration further differentiated individuals based on their risk of incident PAD. VLDL-P levels above the population median did not increase the incidence off PAD beyond the risk of an elevated TC:HDL-C.
Figure 5. Joint Effects of NMR Lipoprotein and Standard Lipid Measures with Incident PAD.
A, PAD survival curve according to LDL-C and LDL-P particle concentration (above or below population median). B, PAD survival curve according to LDL-P particle concentration and TC:HDL-C (above or below population median). C, PAD survival curve according to HDL-P particle concentration and TC:HDL-C (above or below population median). D, PAD survival curve according to VLDL-P particle concentration and TC:HDL-C (above or below population median).
Discussion
In this prospective evaluation comparing standard lipid and NMR-derived lipoprotein measures, we found that TC:HDL-C as well as total and small LDL-P concentration had strong positive associations for incident PAD, particularly in comparison with LDL-C, non-HDL-C, and apoB100, which had no significant associations in multivariable models. Medium, large, and very large VLDL-P were also significantly associated with PAD, while plasma triglycerides were a significant risk predictor in age-adjusted models only. In aggregate, these findings provide evidence that the atherogenic dyslipidemia profile is an important determinant of PAD risk in women, and this profile appears more strongly linked to incident PAD than to CCVD. Although small, retrospective studies have shown an association between atherogenic dyslipidemia and PAD,25, 26 the current data provide a more robust evaluation of this important issue including lipid subclassification which may explain prior inconsistencies. Our data also show a joint association of total LDL-P particle concentration with measures of both LDL-C and TC:HDL-C, such that women with elevations of total LDL-P were at higher risk of PAD irrespective of these traditional lipid measures alone. Thus, if confirmed in other cohorts, our data suggest that LDL particle number may provide important prognostic information for PAD incidence in women, among whom few prospective data currently exist.
In contrast to well established associations for CCVD, the link between LDL-C and PAD is not robust. Few published studies have demonstrated that elevations of LDL-C are associated with incident PAD.2, 3 In the Physicians’ Health Study, which was restricted to men, elevated LDL-C was a risk factor for developing PAD but had no added value beyond the association with TC:HDL-C in models adjusting for both.2 Data from the Cardiovascular Health Study showed that LDL-C measures within the highest quartile were associated with incident PAD in both men and women.3 Of note, this cohort comprised older individuals (> 65 years, mean baseline age ~74 years) and included both subjects with prior cardiovascular disease as well as men. Risk associations were not provided separately for women. Indeed, in contrast to CAD, published data on the link between LDL-C and PAD are notably absent from several large prospective cohorts having measured lipid levels and follow-up for PAD, including the Framingham Offspring Study,27 Multi-Ethnic Study of Atherosclerosis (MESA),5 and the Edinburgh Artery Study.28
Studies of patients with FH and, thus, hereditary elevations in LDL-C, may also be informative. In these studies, prevalence of clinical coronary and cerebrovascular disease is higher than clinical PAD. In a large cohort of 2,752 individuals with molecularly-confirmed heterozygous FH, 0.5% had a history of peripheral revascularization while 9.0% had undergone coronary revascularization.4 Other cohorts have also noted a lower incidence of PAD compared to CAD in individuals with heterozygous FH.29, 30 LDL particle concentrations were not reported in any of these investigations.
In addition to a risk association with TC:HDL-C, the present study found that both total LDL-P and small LDL-P concentrations were linked to PAD in women, and LDL particle size was inversely associated with incident PAD. Even among women with TC:HDL-C measures above the median, the addition of total LDL-P concentration values identified women at even greater risk of PAD. There are several potential explanations for these findings. Importantly, with even modestly elevated levels of serum triglycerides, triglyceride-rich lipoproteins (such as VLDL) exchange triglyceride molecules with cholesterol from large LDL particles with cholesterol-rich cores.10 As a result, large LDL particles become enriched for triglycerides and undergo subsequent hydrolysis and conversion to small LDL. Individuals with smaller LDL particles also tend to have greater concentrations of LDL particles, which may further explain the risk association seen in our analysis.24 Although prospective data for PAD are sparse, it is interesting to note that metabolic syndrome has been linked to heightened risk of PAD,31 and the predominant dyslipidemia pattern in these individuals is elevated small LDL-P and relatively normal LDL-C.32
Our findings pertaining to triglyceride-rich lipoproteins, such as VLDL, are of particular interest. These lipoproteins can cause increased inflammation, monocyte activation, and endothelial dysfunction.33 In addition, the size of triglyceride-rich lipoproteins may be important. As previously discussed, large VLDL particles serve as a reservoir for triglyceride exchange with cholesterol-rich large LDL particles, thus facilitating their transition from large to highly atherogenic small LDL particles.10 As a potential second mechanism, partially hydrolyzed VLDL particles in the ≤70nm range (which includes large and medium VLDL particles in the current analysis) are small enough to traverse the endothelial barrier.34 These cholesterol ester-enriched VLDL remnants may bind to and be retained by the connective tissue matrix where uptake by arterial macrophages leads to foam cell formation. Previous studies have also shown triglyceride levels to be associated with PAD risk,7, 8 and trial data suggest both triglyceride lowering and raising of HDL-C with fibrate therapy may reduce claudication severity.35 We found stronger risk associations for triglyceride-rich very large (90–240nm), large (50–89nm), and medium (37–49nm) VLDL particles than for triglyceride level alone or even non-HDL cholesterol concentration suggesting that packaging in these triglyceride-rich lipoprotein particles may be the more potent driver of PAD risk.
Our findings with regard to HDL-C are not new, and several previous studies have found a negative correlation between HDL-C concentration and PAD.36,37 Indeed, given the null risk association between TC and incident PAD, HDL-C (along with triglyceride-rich VLDL) is the primary driver of risk for TC:HDL-C in our study. However, we also note that concentrations of apolipoprotein A-1, HDL-C, and total HDL-P as well as HDL particle size were inversely associated with PAD. Additionally, low levels of HDL-C identified women at heightened risk for PAD beyond that of TC:HDL-C. HDL particle subclasses vary in terms of both cholesterol and apolipoprotein A-1 composition,38 and some data suggest that large HDL particles are protective against coronary artery disease.39 Increased concentration of small HDL-P is associated with pre-diabetes,40 insulin-resistance,41 and abdominal obesity,42 again suggesting a potential mechanistic link between atherogenic dyslipidemia and PAD.
These findings have several important clinical implications. First, they add to the growing body of evidence that the atherogenic dyslipidemia phenotype is a precursor to PAD. Second, our findings suggest that focus on LDL-C as a clinical risk factor for PAD, at least in women, may be insufficient and that further characterization of LDL and VLDL particle concentrations may identify women at heightened risk of PAD who would otherwise remain undetected. Our data also suggest there may be important differences in the development of atherosclerosis and thrombosis in different arterial beds. Indeed, in clinical practice, many patients develop severe manifestations of PAD but never exhibit overt evidence of CCVD such as angina or MI. This was also seen in our analysis, in which 95 of the 110 total individuals with incident PAD never suffered a CCVD event (data not shown). Although LDL-C may be an important risk factor for subclinical atherosclerosis in PAD as it is in CAD, our findings suggest that a lipoprotein profile of elevated triglyceride-rich lipoproteins, increased LDL particle (in particular small particle) concentration, and low HDL particle concentration may be more important in the pathogenesis of symptomatic PAD.
Our findings may be relevant for therapeutic trials in PAD patients. Observational studies have suggested a benefit of statin therapy on limb outcomes.43,44 Arya et al. found that among 155 647 patients with incident PAD in the Veterans Affairs health system, statin utilization within the first year was associated with large and significant reductions in lower extremity amputation compared to individuals prescribed anti-platelet therapy alone.44 However, because of statin pleiotropy,45–47 it remains unclear whether this benefit was due to LDL-C reduction, inflammation reduction, or improvement in atherogenic dyslipidemia. Finally, it remains possible that individuals receiving statins in this observational study were also benefitting from more guideline-directed therapy overall. Data from the FOURIER randomized clinical trial of evolocumab, a PCSK9 inhibitor with potent LDL-C lowering effects, modest improvements in triglyceride and HDL-C levels, but no substantive hsCRP reduction, add some clarity in this regard.48 A 42% reduction in major adverse limb events was observed.49 However, treatment effects on LDL particle number and other components of atherogenic dyslipidemia are currently unavailable and may yet explain these findings. Whether treatment of atherogenic dyslipidemia per se improves limb-related vascular outcomes will be assessed in the recently initiated PROMINENT trial (NCT03071692) of pemafibrate to reduce cardiovascular events in patients with elevated triglycerides and low HDL-C.
Strengths of the present study include the prospective design, large sample size, long-term follow-up, and homogeneity of our study population, which may reduce confounding. However, several potential limitations should be considered. First, the WHS has no male enrollees, and the majority of participants were Caucasian and healthy at baseline. Thus, our conclusions may not be generalizable to other groups. It is unclear from our data whether NMR lipoprotein profiling would be beneficial in high-risk individuals or in those receiving lipid-lowering therapy, since the study population was comprised of relatively healthy women enrolled in 1992–1995. Second, because our study is observational, residual unmeasured confounding may be present. However, data collected on a broad range of established cardiovascular risk factors were available for multivariable adjustment. Third, the use of symptomatic PAD as the primary endpoint by definition excludes subclinical disease that might otherwise have been detected with the use of ABI or abnormal pulse examination; however, we believe that our data are not only mechanistically relevant but also clinically important because claudication and ischemia requiring limb revascularization are the principal clinical manifestations of PAD. Importantly, each case included in this analysis was confirmed through rigorous methods with the use of a validated claudication questionnaire, cardiovascular physician interview, and medical record review. In addition, women enrolled in the WHS are female health professionals and are therefore less likely to encounter barriers to medical care, which may otherwise have led to underdiagnosis. Furthermore, although potential misclassification resulting from atypical or occult disease may have occurred, this, if anything, would have biased our results toward the null by inclusion of potentially misclassified cases in the event-free group. In terms of the traditional lipid measures used in the present study, more refined methods of calculating LDL-C have been developed.50 However, the performance of the calculated LDL-C variable in this study was likely adequate given that LDL-C was associated with incident CCVP, as expected. Finally, given our relatively small sample size, the study may be underpowered to detect risk associations for some biomarkers, although numerous statistically significant associations were identified.
In summary, our data show that both standard lipid as well as NMR-derived lipoprotein measures indicative of atherogenic dyslipidemia are associated with PAD in women, whereas LDL-C, non-HDL-C, and apoB100 were not. Measures of total and small LDL-P concentration further identified women at heightened risk of PAD beyond standard lipid measures. Importantly, our data also indicate that this lipoprotein signature may be unique to PAD in comparison to coronary or cerebrovascular atherosclerosis. Our results require confirmation, yet the biologic construct is not only plausible but raises the intriguing possibility that therapeutic modulation of these lipoprotein abnormalities may have clinical benefits in patients at risk for PAD for whom few medical treatment options currently exist.
Supplementary Material
Clinical Perspective.
What is new?
Among women age 45 and older without cardiovascular disease at baseline, elevated levels of low-density lipoprotein cholesterol (LDL-C) were not associated with future peripheral artery disease (PAD).
Using both standard lipids and nuclear magnetic resonance-derived lipoprotein measures, we found strong associations of an atherogenic dyslipidemia profile, including small, dense LDL particle concentration, triglyceride-rich lipoproteins, high-density lipoprotein cholesterol (HDL-C) and particle concentration, and total cholesterol:HDL-C with incident PAD.
These same components of atherogenic dyslipidemia were more strongly associated with PAD than with a composite of cardiovascular and cerebrovascular disease, suggesting a unique lipoprotein profile for incident PAD.
What are the clinical implications?
Focus on LDL-C in terms of atherosclerotic risk prediction underestimates the risk of PAD among middle-aged, low-risk women.
The addition of nuclear magnetic resonance-derived lipoprotein measures to traditional lipid measures may improve risk assessment for PAD, and importantly may elucidate a novel therapeutic strategy for PAD prevention.
Ongoing clinical trials are investigating whether treating atherogenic dyslipidemia, rather than elevations in LDL-C alone, is beneficial in preventing PAD.
Acknowledgments
The authors wish to thank statistical programmers Rimma Dushkes, Eunjung Kim, Chunying Li, and M. Vinayaga Moorthy for their efforts.
Sources of Funding
This work was supported by the National Institutes of Health under Award Number 5T32HL007575–33 (Drs. Aday and Lawler). Dr. Mora received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL117861. Dr. Pradhan received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01HL111156. The Women’s Health Study was funded by grants CA047988, HL043851, HL080467, HL099355, and UM1 CA182913. Additional funding was provided by American Heart Association for NMR measurements.
Disclosures
P. Ridker: Is listed as a coinventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease, which have been licensed to AstraZeneca and Siemens, has received investigator research support from Kowa Research Institute, Novartis, Pfizer, and Astra-Zeneca, has served as a consultant to Jannsen, Novartis, and Sanofi-Regenerson, and serves as co-Principal Investigator of the PROMINENT trial (NCT03071692). S. Mora: Receives research grant support from Atherotech Diagnostics for research outside the current work, and served as a consultant to Amgen, Lilly, Pfizer, and Quest Diagnostics, and is co-inventor on a patent on the use of NMR-measured GlycA for predicting risk of colorectal cancer. A. Pradhan: Receives investigator-initiated research support from Kowa Research Institute and serves as co-Principal Investigator of the PROMINENT trial (NCT03071692). The remaining authors report no conflicts.
Clinical Trial Registration: http://clinicaltrials.gov/ct/show/NCT00000479; Unique Identifier NCT00000479
References
- 1.Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological Targeting of the Atherogenic Dyslipidemia Complex: The Next Frontier in CVD Prevention Beyond Lowering LDL Cholesterol. Diabetes 2016;65:1767–1778. doi: 10.2337/db16-0046. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy M, Solomon C, Manolio TA, Criqui MH, Newman AB, Polak JF, Burke GL, Enright P, Cushman M. Risk factors for declining ankle-brachial index in men and women 65 years or older: the Cardiovascular Health Study. Arch Intern Med 2005;165:1896–1902. doi: 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 4.Pérez de Isla L, Alonso R, Mata N, Saltijeral A, Muñiz O, Rubio-Marin P, Diaz-Diaz JL, Fuentes F, de Andrés R, Zambón D, Galiana J, Piedecausa M, Aguado R, Mosquera D, Vidal JI, Ruiz E, Manjón L, Mauri M, Padró T, Miramontes JP, Mata P, SAFEHEART Investigators. Coronary Heart Disease, Peripheral Arterial Disease, and Stroke in Familial Hypercholesterolaemia: Insights From the SAFEHEART Registry (Spanish Familial Hypercholesterolaemia Cohort Study). Arterioscler Thromb Vasc Biol 2016;36:2004–2010. doi: 10.1161/ATVBAHA.116.307514. [DOI] [PubMed] [Google Scholar]
- 5.Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, Kori S. The Effect of Novel Cardiovascular Risk Factors on the Ethnic-Specific Odds for Peripheral Arterial Disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol 2006;48:1190–1197. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 6.Criqui MH, Aboyans V. Epidemiology of Peripheral Artery Disease. Circ Res 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 7.Bainton D, Sweetnam P, Baker I, Elwood P. Peripheral vascular disease: consequence for survival and association with risk factors in the Speedwell prospective heart disease study. Br Heart J 1994;72:128–132. doi: 10.1136/hrt.72.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith I, Franks PJ, Greenhalgh RM, Poulter NR, Powell JT. The influence of smoking cessation and hypertriglyceridaemia on the progression of peripheral arterial disease and the onset of critical ischaemia. Eur J Vasc Endovasc Surg 1996;11:402–408. [DOI] [PubMed] [Google Scholar]
- 9.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin Lab Med 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Am J Cardiol 2002;90:22i–29i. doi: 10.1016/S0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 11.Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Harchaoui K, van der Steeg WA, Stroes ESG, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJP, Khaw K-T, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 13.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol 2002;22:1175–1180. doi: 10.1161/01.ATV.0000022015.97341.3A. [DOI] [PubMed] [Google Scholar]
- 14.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation 2002;106:1930–1937. doi: 10.1161/01.CIR.0000033222.75187.B9. [DOI] [PubMed] [Google Scholar]
- 15.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 16.Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, Wilson PW, D’Agostino RB. LDL Particle Number and Risk of Future Cardiovascular Disease in the Framingham Offspring Study - Implications for LDL Management. J Clin Lipidol 2007;1:583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein Particle Size and Concentration by Nuclear Magnetic Resonance and Incident Type 2 Diabetes in Women. Diabetes 2010;59:1153–1160. doi: 10.2337/db09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dugani SB, Akinkuolie AO, Paynter N, Glynn RJ, Ridker PM, Mora S. Association of Lipoproteins, Insulin Resistance, and Rosuvastatin With Incident Type 2 Diabetes Mellitus. JAMA Cardiol 2016;1:136. doi: 10.1001/jamacardio.2016.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paynter NP, Sesso HD, Conen D, Otvos JD, Mora S. Lipoprotein Subclass Abnormalities and Incident Hypertension in Initially Healthy Women. Clin Chem 2011;57:1178–1187. doi: 10.1373/clinchem.2011.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Cook NR, Lee I-M, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 21.Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol 1992;45:1101–1109. [DOI] [PubMed] [Google Scholar]
- 22.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FGR, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HEJ, Treat-Jacobson D; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Manson JE, Buring JE, Shih J, Matias M, Hennekens CH. Homocysteine and risk of cardiovascular disease among postmenopausal women. JAMA 1999;281:1817–1821. doi: 10.1001/jama.281.19.1817. [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, O’Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007;192:211–217. doi: 10.1016/j.atherosclerosis.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo M, Pernice V, Frasheri A, Berneis K. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis 2008;197:237–241. doi: 10.1016/j.atherosclerosis.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 26.O’Neal DN, Lewicki J, Ansari MZ, Matthews PG, Best JD. Lipid levels and peripheral vascular disease in diabetic and non-diabetic subjects. Atherosclerosis 1998;136:1–8. doi: 10.1016/S0021-9150(97)00175-5. [DOI] [PubMed] [Google Scholar]
- 27.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PWF. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J 2002;143:961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 28.Fowkes FG, Housley E, Riemersma RA, Macintyre CC, Cawood EH, Prescott RJ, Ruckley CV. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am J Epidemiol 1992;135:331–340. [DOI] [PubMed] [Google Scholar]
- 29.Pereira C, Miname MH, Makdisse MRP, Watanabe C, Pesaro AE, Jannes CE, Kalil Filho R, Pereira AC, Santos RD. Peripheral arterial disease in heterozygous familial hypercholesterolemia. Atherosclerosis 2015;242:174–178. doi: 10.1016/j.atherosclerosis.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Beaumont V, Jacotot B, Beaumont JL. Ischaemic disease in men and women with familial hypercholesterolaemia and xanthomatosis. A comparative study of genetic and environmental factors in 274 heterozygous cases. Atherosclerosis 1976;24:441–450. doi: 10.1016/0021-9150(76)90136-2. [DOI] [PubMed] [Google Scholar]
- 31.Conen D, Rexrode KM, Creager MA, Ridker PM, Pradhan AD. Metabolic Syndrome, Inflammation, and Risk of Symptomatic Peripheral Artery Disease in Women: A Prospective Study. Circulation 2009;120:1041–1047. doi: 10.1161/CIRCULATIONAHA.109.863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PWF, D’Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation 2006;113:20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 33.Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Nordestgaard BG, Ray KK, Reiner Z, Taskinen M-R, Tokgözoglu L, Tybjaerg-Hansen A, Watts GF, European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J 2011;32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiner Z Hypertriglyceridaemia and risk of coronary artery disease. Nat Rev Cardiol 2017;14:401–411. doi: 10.1038/nrcardio.2017.31. [DOI] [PubMed] [Google Scholar]
- 35.Meade T, Zuhrie R, Cook C, Cooper J. Bezafibrate in men with lower extremity arterial disease: randomised controlled trial. BMJ 2002;325:1139. doi: 10.1136/bmj.325.7373.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation 1997;96:44–49. doi: 10.1161/01.CIR.96.1.44. [DOI] [PubMed] [Google Scholar]
- 37.Meijer WT, Grobbee DE, Hunink MG, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: the Rotterdam study. Arch Intern Med 2000;160:2934–2938. doi: 10.1101/archinte.160.19.2934. [DOI] [PubMed] [Google Scholar]
- 38.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 39.Arsenault BJ, Lemieux I, Després J-P, Gagnon P, Wareham NJ, Stroes ESG, Kastelein JJP, Khaw K-T, Boekholdt SM. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Atherosclerosis 2009;206:276–281. doi: 10.1016/j.atherosclerosis.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 40.Festa A, Williams K, Hanley AJG, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 41.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 42.Pascot A, Lemieux I, Prud’homme D, Tremblay A, Nadeau A, Couillard C, Bergeron J, Lamarche B, Després JP. Reduced HDL particle size as an additional feature of the atherogenic dyslipidemia of abdominal obesity. J Lipid Res 2001;42:2007–2014. [PubMed] [Google Scholar]
- 43.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith J, Sidney C, Goto S, Ohman EM, Elbez Y, Sritara P, Baumgartner I, Banerjee S, Creager MA, Bhatt DL. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J 2014;35:2864–2872. doi: 10.1093/eurheartj/ehu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arya S, Khakharia A, Binney ZO, DeMartino RR, Brewster LP, Goodney PP, Wilson PWF. Association of Statin Dose With Amputation and Survival in Patients With Peripheral Artery Disease. Circulation 2018;137:1435–1446. doi: 10.1161/CIRCULATIONAHA.117.032361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawler PR, Akinkuolie AO, Chu AY, Shah SH, Kraus WE, Craig D, Padmanabhan L, Glynn RJ, Ridker PM, Chasman DI, Mora S. Atherogenic Lipoprotein Determinants of Cardiovascular Disease and Residual Risk Among Individuals With Low Low-Density Lipoprotien Cholesterol. J Am Heart Assoc 2017;6: e005549. doi: 10.1161/JAHA.117.005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berthold HK, Rizzo M, Spenrath N, Montalto G, Krone W, Gouni-Berthold I. Effects of lipid-lowering drugs on high-density lipoprotein subclasses in healthy men-a randomized trial. PLoS One 2014;9:391565. doi: 10.1371/journal.pone.0091565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Trial Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 48.Bohula EA, Giugliano RP, Leiter LA, Verma S, Park JG, Sever PS, Pineda AL, Honarpour N, Wang H, Murphy SA, Keech A, Pedersen TR, Sabatine MS. Inflammatory and Cholesterol Risk in the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Patients With Elevated Risk). Circulation 2018;138:131–140. doi: 10.1161/CIRCULATIONAHA.118.034032. [DOI] [PubMed] [Google Scholar]
- 49.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgözoglu L, Somaratne R, Sever PS, Pedersen TR, Sabatine MS. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation 2018;137:338–350. doi: 10.1161/CIRCULATIONAHA.117.032235. [DOI] [PubMed] [Google Scholar]
- 50.Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a Novel Method vs the Friedewald Equation for Estimating Low-Density Lipoprotein Cholesterol Levels From the Standard Lipid Profile. JAMA 2013;310:2061–2068. doi: 10.1001/jama.2013.280532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will not be made available to other researchers for purposes of reproducing the results. However, the methods used in the analysis are available upon request.