Abstract
Heavy alcohol drinking causes alterations in the metabolism of fatty acids and zinc that participate in inflammation and liver injury. HIV infection has been reported to cause dysregulated polyunsaturated fatty acid (PUFA) and zinc metabolism. In this pilot study, we examined the role of dysregulated PUFA metabolism and zinc deficiency in the liver injury occurring in heavy drinkers with early-stage HIV diagnosis. Fourteen heavy drinking alcohol-dependent (AD) patients [seven with treatment-naive HIV diagnosis (AD+HIV) and seven without HIV infection (AD)] participated in this study. Liver injury, serum zinc, PUFAs, viral load, CD4+ count, and drinking measures using lifetime drinking history (LTDH), and timeline follow-back past 90 days (TLFB90) were evaluated. Liver injury was also assessed in seven age- and gender-matched socially drinking HIV treatment-naive patients who served as disease controls. HIV viral load by itself did not show any correlation with liver injury. Liver enzymes were significantly elevated in both AD+HIV and AD patients, and AD+HIV patients had significantly higher alanine aminotransferase (ALT) levels than did AD patients, even with lower drinking. Serum zinc was significantly lower in AD+HIV patients. Only AD+HIV patients showed a significant elevation in linoleic acid (LA) and alpha-linoleic acid (ALA) levels. Serum zinc and ALT, LA and ALT, and ALA and ALT were significantly associated only in AD+HIV patients. The association between LA and ALT showed a higher effect than did the ALA and ALT association in the AD+HIV patients. Interestingly, AD+HIV subjects (who drank less), nevertheless, showed more liver injury compared with AD patients, who reported heavier drinking. We speculate that the underlying proinflammatory response resulting from zinc deficiency and an elevation in serum LA likely contributed to liver injury in AD+HIV patients, even with a comparatively lower degree of heavy drinking.
Keywords: alcohol dependent, CD4+, HIV, liver injury, PUFAs, serum zinc
Introduction
Liver injury in HIV patients due to antiretroviral therapy (ART) can lead to significant morbidity and mortality,1,2 and a significant proportion of these patients with ART hepatotoxicity also are coinfected with Hepatitis B and/or Hepatitis C viruses.3 Thus, the occurrence of a confounding condition that impacts the liver is of concern. Alcohol consumption in patients with HIV infection is one such concerning circumstance. Both alcohol consumption and HIV infection have, individually, been extensively studied in terms of prevalence, medical management, and prognosis.4–6 Heavy alcohol drinking, itself, has been associated with negative multiorgan and systemic consequences,7,8 including alcohol-associated liver disease (AALD).9 Susceptibility to liver damage due to very heavy drinking and its metabolic manifestations is not well defined during the early-stage asymptomatic HIV infection. In this study, we investigated liver injury in heavy drinking alcohol-dependent (AD) patients who were diagnosed with HIV during admission to an alcohol treatment program, and who were asymptomatic and HIV treatment naive.
HIV infection alone is not a major cause of liver injury, and only 0.5% of treatment-naive HIV-infected patients have reported liver injury of unknown origin.10,11 However, HIV infection plus a second factor, such as ART therapy, viral infection with Hepatitis B/C, and/or heavy alcohol intake can cause liver injury. Zinc deficiency is a frequent complication of alcohol abuse and can also occur in patients with HIV infection. Zinc is involved in the conversion of linoleic acid (LA) to γ-LA, and mobilization of dihomogammalinolenic acid (DGLA).12 Zinc deficiency is known to cause inflammation through multiple mechanisms, including the upregulation of free fatty acids (FAs), and this can result in oxidative stress and inflammation.13 More severe liver injury in AALD has been reported with changes in ω-3 and ω-6 FAs, showing a proinflammatory response in both experimental models and patients.14,15 However, there is limited information about the role of serum zinc and FAs in liver injury in patients with HIV infection who are also heavy drinkers. Thus, we aimed to characterize the association of specific FAs (those that participate in inflammation) and serum zinc levels with liver injury in HIV-infected patients and appropriate controls.
Materials and Methods
Patient recruitment
This HIV study was approved by the Office of Human Safety and Research Protection (NIH-OHSRP-12176) at the National Institutes of Health, Bethesda, MD. Twenty-one men and women aged 21–65 years were included in this study (Table 1). Among them, 14 patients were AD patients diagnosed by DSM-IV criteria for alcohol use disorder (AUD), based on the alcohol dependence module of the SCID I-interview. The AUD patients were evaluated under a larger clinical assessment and treatment protocol (15-AA-0121) approved by the NIH Addictions Institutional Review Board at NIH (indexed at the National Clinical Trial website; www.clinicaltrials.gov: NCT00001673). Seven among these 14 patients were also diagnosed with HIV infection (first-time diagnosis) at the time of screening/admission. Individuals were excluded from the study if they were identified with illicit drug use either in laboratory testing at the time of screening or self-reported history. Pregnancy or active breastfeeding were also exclusions. Details of eligibility criteria for study participation related to alcohol dependence and liver diseases are described extensively in a previous publication.16 In brief, patients were excluded from the study if they had clinical manifestations of liver injury/disease or any biochemical evidence for any liver injury other than that associated with alcohol. Patients were also excluded from the study if they exhibited any severe psychiatric illness such as dementia or active psychotic disorder, including delirium, psychotic phase of bipolar disorder at the screening intake. Patients were also excluded if they were currently suicidal or violent, if they had agitation requiring immediate clinical treatment, and/or if they had other kind of clinically significant psychiatric condition.
Table 1.
Demographics, Drinking Profile, and HIV-CD Panel for the Three Study Groups: AD, AD+HIV, and HIV-Only Patients
| Measures | AD (n = 7) | AD + HIV (n = 7) | HIV social drinkers (n = 7) |
|---|---|---|---|
| Demographic measures | |||
| Age | 36.1 ± 6.8 | 41.9 ± 10.9a | 30.7 ± 9.4 |
| Gender: men, n (%) | 4 (57) | 4 (57) | 7 (100) |
| BMI | 26.9 ± 4.1 | 27.3 ± 6.9 | 25.8 ± 2.7 |
| Drinking history markers | |||
| LTDH | 11.4 ± 8.2 | 20.8 ± 8.7b | NA |
| TD90 | 1286.7 ± 761.8 | 508.0 ± 313.0b | NA |
| NDD90 | 75.2 ± 21.5 | 72.8 ± 22.2 | NA |
| AvgDPD90 | 19.0 ± 12.2 | 6.7 ± 2.7b | NA |
| HDD90 | 72.8 ± 21.7 | 53.8 ± 30.6 | NA |
| HIV-CD panel | |||
| CD3+ | NA | 1639.6 ± 645.5 | 1337.4 ± 421.2 |
| CD4+ | NA | 569.4 ± 312.6 | 504.3 ± 150.8 |
| CD8+ | NA | 1014.8 ± 333.8 | 775.4 ± 393.4 |
Data were represented as mean ± SD and statistical significance was set at p < .05.
Indicates a p-value between .05 and .1 when comparing AD+HIV versus HIV only.
Indicates a p-value between .05 and .1 when comparing AD+HIV versus AD.
LTDH, lifetime drinking history; timeline follow-back (TLFB for 90 days): total drinks (TD90), drinking days (NDD90), average drinks per drinking day (AvgDPD90), and heavy drinking days (HDD90); CD, clusters of differentiation [Department of Laboratory Medicine (NIH) normal ranges: CD3: 714–2,266 cells/mL3, CD4: 334–1,556 cells/mL3, CD8: 149–787 cells/mL3].
Females, n = 3 each in AD and AD + HIV group.
AD, alcohol dependent; BMI, body mass index.
The third group of seven patients were treatment-naive socially drinking HIV positive individuals. These patients were included only if they did not report moderate or heavy drinking during the intake, and they resembled AD+HIV patients in their asymptomatic stage of HIV presentation. These patients were social drinkers and did not report any past or present drug or alcohol addiction or tested negative at the time of enrollment. These HIV patients reported practicing unsafe sex, which could be a likely cause of transmission of disease. These patients did not have a preexisting diagnosis or clinical evidence of liver disease.
Demographics, drinking, and laboratory assessments
Demographics [age, gender, and body mass index (BMI)] were collected. Drinking measures were collected using timeline follow-back (TLFB),17 and LTDH questionnaires.18 TLFB included total drinks past 90 days (TD90), number of drinking days past 90 days (NDD90), number of nondrinking days past 90 days (NNDD90), average drinking days (AvgDD90), and heavy drinking days past 90 days (HDD90). The primary measure for LTDH used in this study was “number of years of drinking.”
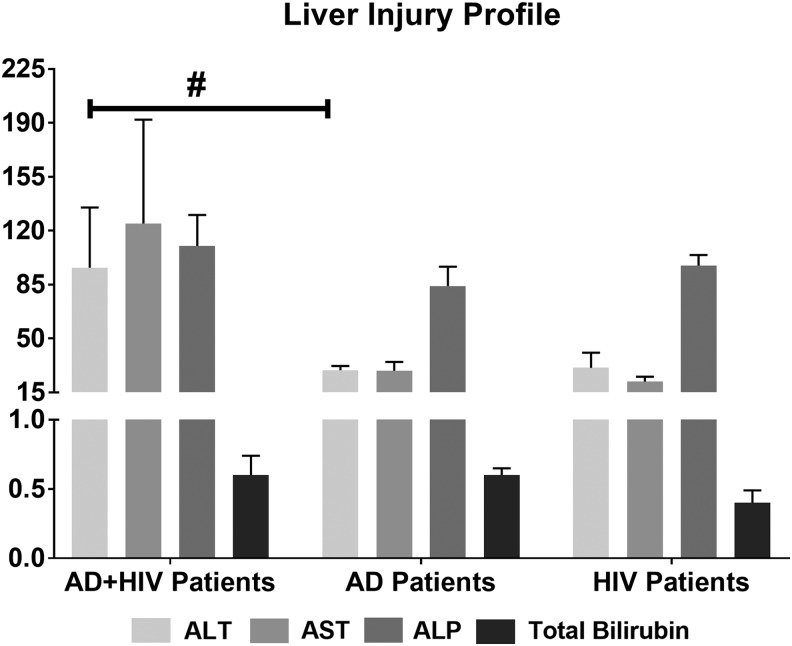
Patients were grouped by HIV diagnosis. Blood samples were drawn and analyzed for liver tests using a comprehensive metabolic panel, serum zinc (using atomic absorption spectrophotometry), and comprehensive FA panel (specific and total ω-3 and ω-6 FA levels using gas chromatography/mass spectrometry). CD cell panel testing was also conducted (cell surface immunophenotyping technique). Zinc levels were assigned as follows: normal, ≥71 mcg/dL; low, <71 mcg/dL. All tests were performed by the Department of Laboratory Medicine of the NIH, Bethesda, MD (Fig. 1 and Table 2). Alanine aminotransferase (ALT) level was used as a reference to assess liver injury (MedlinePlus-National Institutes of Health, 2014); 40 IU/L for ALT was used as the upper limit of normal, and values ≥41 IU/L indicated liver injury.
FIG. 1.
Assessment of liver injury in AD and AD+HIV patients. AD+HIV patients showed significantly elevated liver injury markers. ALT in AD+HIV patients was higher compared with the AD patients (#p = .054), when adjusted for lifetime drinking history. Data are presented as mean ± SE. Statistical significance was set at p ≤ .05. ALT/GPT (bar 1): alanine aminotransferase (normal range: 6–41 U/L); AST/GOT: aspartate transaminase (normal range: 9–34 U/L); ALP: alkaline phosphatase (normal range: 37–116 U/L); total bilirubin normal range: 0.1–1.0 mg/dL. AD, alcohol dependent.
Table 2.
ω-3 and ω-6 Fatty Acids in Inflammation and Resolution Pathways in AD and AD+HIV Patients
| Measures | AD | AD + HIV | Normal range |
|---|---|---|---|
| ω-3 | |||
| α-Linolenic acida | 81.3 ± 33.1 | 221.6 ± 402.2 (H) | 50–130 |
| EPA | 96.4 ± 50.6 | 85.0 ± 55.07 | 14–100 |
| DPA5 3ωb | 139.0 ± 75.5 | 74.3 ± 48.3 | 20–210 |
| DHA | 230.9 ± 126.9 | 183.7 ± 104.9 | 30–250 |
| ω-6 | |||
| LAc | 3387.6 ± 1060.2 | 4125.6 ± 2115.2 (H) | 2,270–3,850 |
| γ-Linolenic acid | 80.1 ± 29.2 | 85.3 ± 75.2 | 16–150 |
| DGLA | 113.1 ± 22.4 | 123.7 ± 43.3 | 50–250 |
| Arachidonic acid | 1369.0 ± 763.6 | 1174.9 ± 329.6 | 520–1,490 |
| DTA | 39.0 ± 27.7 | 38.0 ± 28.1 | 10–80 |
| DPA5 6ω | 58.6 ± 39.1 | 36.3 ± 18.6 | 10–70 |
| ω-6/ω-3 ratio | 9.4 ± 1.7 | 11.6 ± 5.4 | 10–15 |
AD+HIV patients showed clinically relevant elevation in α-LA and LA levels. Unit for FAs: nmol/mL.
Elevated α-LA in AD+HIV patients was clinically significant.
DPA3ω was significantly lower p = .029 in AD+HIV group, when covaried with LTDH at moderate effect, unadjusted R2 = 0.426.
Elevated LA was clinically high in AD+HIV patients. Data represented as mean ± SD. Statistical significance at p ≤ .05.
DGLA, dihomogammalinolenic acid; DHA, docosahexaenoic acid; DPA5 3ω, docosapentaenoic acid; DPA5 6ω, docosapentaenoic acid; DTA, docosatetraenoic acid; EPA, eicosapentaenoic acid; FA, fatty acid; H, high; LA, linoleic acid.
Viral load and infection
All patients were tested for HIV diagnosis before admission/screening. None of the AD patients reported any positive HIV infection diagnosis before admission. We conducted Western blot analysis to verify HIV positive screening test.
Statistical analysis
Differences among demographics, drinking history markers, zinc levels, FA levels, and ALT levels were analyzed using one-way ANOVA for three groups and Student's t-test for two groups. Fisher's exact test was used to compare group differences for categorical variables (e.g., gender). Univariate and multivariate linear regression analyses were conducted to analyze associations between one variable versus one or multiple variables, respectively. Drinking history was used as a covariate where applicable. The significance level was set at p < .05, association analysis described goodness of model fit (adjusted R2). Data are presented as mean ± standard deviation (M ± SD) for continuous variables and count and percentage for categorical variables in tables, and mean ± standard error (M ± SE) for continuous variables in figures, unless otherwise indicated.
Results
Patient demographics, drinking profile, HIV status, and liver injury
There were no significant group differences in the demographic measures and drinking history markers (Table 1). AD+HIV patients were relatively older and both groups were in the overweight range of BMI. AD patients drank more than twice as much compared with the AD+HIV patients in the past 90 days, although both groups of patients were classified as heavy drinkers. The lifetime duration of drinking (LTDH) was longer in AD+HIV patients. Average CD4+ count in the AD+HIV patients was 569.4 ± 312.6 cells/μL (mean ± SD). Furthermore, these patients were largely asymptomatic. Mean CD4+ count in HIV patients (disease control group), without any significant drinking history, was 504.3 ± 150.8 cells/μL (Table 1) and was not statistically different from the AD+HIV group (Table 1). The disease control patients were also largely asymptomatic. None of these patients exhibited staging/symptoms of AIDS or a clinical stage of primary HIV infection (acute retroviral syndrome). As assessed by their levels of CD4+, both AD+HIV and HIV disease control patients had a reasonably low risk of opportunistic infection.19
AD+HIV patients exhibited a mild form of liver disease. The ALT levels in AD+HIV patients were about two-fold greater than in the AD patients (Fig. 1). The higher ALT levels approached statistical significance in AD+HIV patients versus AD patients, when adjusted for the LTDH, p = .054. No other liver injury markers showed a statistical difference (likely due to variability and small sample), although AST levels in AD+HIV patients were higher. AD patients did not show any obvious clinical signs of liver injury, despite drinking more (>2-fold) than the AD+HIV group patients. In HIV patients, who did not report any significant drinking history (Fig. 1), we also did not find any liver injury—ALT levels were normal [18.67 ± 9.4 IU/L (mean ± SD)]. Since this group of HIV patients did not show any liver injury, we pursued within-group assessments and comparisons for AD and AD+HIV patients only.
Changes in serum zinc and association with liver injury in HIV-diagnosed AD patients
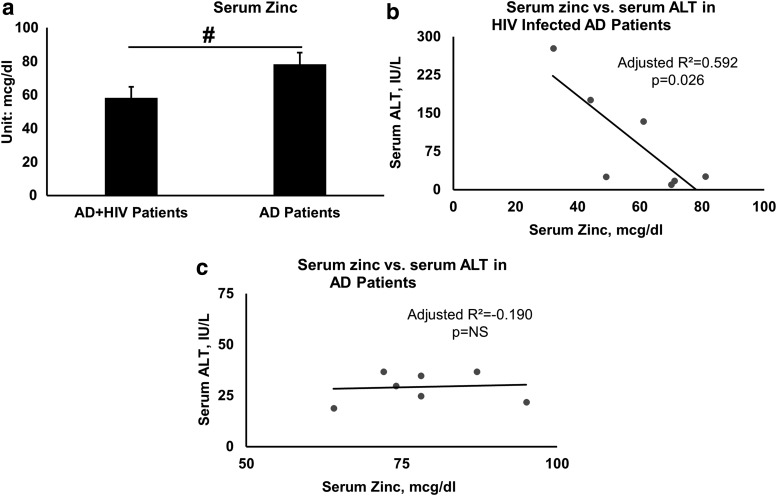
HIV-diagnosed AD patients showed a significantly lower serum zinc (mean serum zinc = 58.3 ± 17.3 μg/dL, p = .022, R2 = 0.314) compared with normal serum zinc in AD patients (mean serum zinc = 78.3 ± 10.1 μg/dL) (Fig. 2a), although the mean serum zinc level was low-normal. We further reviewed the association of serum zinc with ALT levels within each group. In HIV+AD patients, we found a significant moderate effect, adjusted R2 = 0.592, p = .026 (Fig. 2b); in AD patients, this association did not show any effect, adjusted R2 = −0.190, p = .848 (Fig. 2c).
FIG. 2.
Serum zinc in AD+HIV and AD patients and association with liver injury. (a) Serum zinc was significantly lower in AD+HIV patients. (b) An inverse significant association was present between serum zinc (hypozincemia) and the liver injury marker, ALT, in AD+HIV patients. (c) There was no association between serum zinc (hypozincemia) and ALT in AD patients. Data presented in (a) are mean ± SE. Between group ANOVA was used for (a). Linear regression analysis was used as shown in (b, c). Statistical significance was set at p ≤ .05.
Changes in proinflammatory FAs and association with liver injury
A high ω-6:ω-3 ratio is associated with the promotion of the pathogenesis of several diseases. The ω-6:ω-3 ratio was numerically higher in the serum of AD+HIV patients compared with the AD patients (11.6 ± 5.4 vs. 9.4 ± 1.7, respectively), but that difference was not statistically significant (Table 2). Nor did we find any statistically significant differences in total ω-3, and total ω-6 levels between the two groups (AD vs. AD+HIV patients). However, AD+HIV patients showed an elevation in alpha-linoleic acid (ALA) and LA levels (Table 2). We used a linear regression model to evaluate the association of FA levels and drinking history markers (in both AD and AD+HIV patients), and CD4+ (in AD+HIV) levels as individual independent variables; or both (individual drinking history markers and CD4+ count) as multiple independent variables to estimate a possible dual role in AD+HIV patients. We did not find any statistically significant association in this comparison in either the AD+HIV or the AD patient group.
Several FAs, which do not necessarily participate in inflammation, were included in the total ω-3 or total ω-6 categories20 while calculating the overall concentrations (Table 2). Therefore, the difference in total ω-3 or ω-6 levels might not have direct relevance to inflammation. We found no statistically significant differences in FA analysis between the AD and AD+HIV groups, probably because any changes could be subtle at the onset of inflammation/injury and we had small sample sizes. Specific ω-3, or ω-6 FAs that participate in anti-inflammatory or proinflammatory responses could influence inflammation in these patients. Thus, to identify any relations between FAs and liver injury within each group, we evaluated the cluster of FAs and specific FAs that participate in inflammation. In the AD+HIV group, we found a significant association between total ω-6 FA level and ALT, adjusted R2 = 0.561, p = .032; however, total ω-3 FA level and ALT did not show any statistically significant association in that group. There was no association between ω-3 polyunsaturated fatty acids (PUFAs) and ALT, or ω-6 PUFAs and ALT in the AD group. Nor did we find any association between the ω-6:ω-3 ratio and ALT in either the AD+HIV group or the AD group.
In the AD+HIV group, we found a close association between LA and ALT, and between ALA and ALT (Table 3). LA and ALT, and ALA and ALT were also associated in the AD group; however, this association was not significant (Table 3). This finding suggests a possible involvement of specific FAs that could have an important role in liver injury in these specific patients.
Table 3.
Degree of Association of Liver Injury and Fatty Acids in AD+HIV and AD Patients
| ALT, U/L | ||
|---|---|---|
| Variables/groups | AD+HIV patients | AD patients |
| LA, nmol/mL | Adjusted R2 = 0.598, p = .025; B = 0.045 ± 0.014; CI (95%) for B: 0.008–0.082 | Adjusted R2 = 0.018, p = .341; B = −0.003 ± 0.003; CI (95%) for B: −0.010–0.004 |
| A-LA, nmol/mL | Adjusted R2 = 0.505, p = .044; B = 0.197 ± 0.074; CI (95%) for B: 0.007–0.387 | Adjusted R2 = 0.366, p = .088; B = −0.153 ± 0.072; CI (95%) for B: −0.339–0.033 |
Bold, indicates effect size; italics, likely/no significance.
The significant moderate effect in the association of FAs and liver injury was observed in AD+HIV patients only. Statistical significance was set at p ≤ .05.
B = unstandardized coefficients presented as B ± SE.
ALT, alanine aminotransferase; CI, confidence interval; SE, standard error.
Association of serum zinc and FAs involved in inflammation
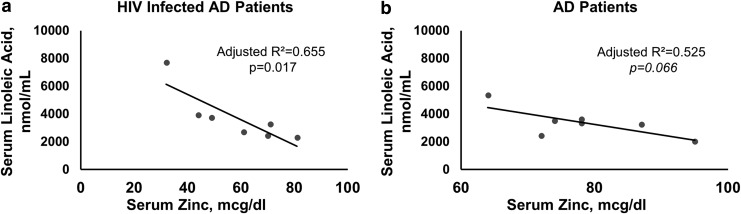
We also evaluated the association between serum zinc and LA, and between serum zinc and ALA in both disease groups. In the AD+HIV group, an inverse relation between serum zinc and LA was of higher effect, adjusted R2 = 0.655 and significant, p = .017 (Fig. 3a). In AD patients, this association showed a comparatively lower effect that was also inverse and was not significant (Fig. 3b), possibly due to the fact that six of the seven patients had normal zinc levels. The association of ALA and serum zinc was not significant in either the AD or the AD+HIV group.
FIG. 3.
Association of LA and serum zinc in AD+HIV and AD patient groups. (a) A significant inverse moderate association was present between serum LA and serum zinc in AD+HIV patients. (b) An inverse association that was not significant was observed in AD patients. Linear regression analysis was used as shown in (a, b). Statistical significance was set at p ≤ .05. LA, linoleic acid.
Discussion
Our first aim was to characterize potential liver injury in heavy alcohol drinking HIV-infected patients. These patients showed more liver injury, notably even with a lower drinking profile, compared with AD patients. We do not know why this group of patients, while still drinking heavily, drank considerably less than the AD group, and this is an area for further investigation. It could be that they were already feeling less well and cut back on drinking to compensate, or another explanation might be involved.
HIV infection generally does not cause liver injury by itself, although HAART/ART medications are known causes of hepatotoxicity.21,22 Heavy alcohol drinking places the liver in a vulnerable position to further injury.23 In our study, we also noted that LTDH was higher despite comparatively lower recent drinking pattern in HIV-infected AD patients. Again, we do not know if this finding is an artifact of this small study, or if it is important; further study is needed. Another study reported that lifetime drinking and Hepatitis C virus infection play roles in the progression of liver disease.24
Heavy alcohol drinking and HIV infection are both known to cause alterations in zinc and FA metabolism, promoting inflammation.25–29 Zinc supplementation has been used to prevent/diminish CD4+ cell loss in patients with HIV.30 The impact on liver health from the comorbid conditions of heavy alcohol drinking and HIV infection has not been adequately studied in treatment-naive HIV-diagnosed AD patients. A significant decrease in serum zinc and an increase in serum LA in the AD+HIV patients, even with recent lower drinking history, was a new finding in our study. Furthermore, this alteration of serum zinc and LA was closely associated with liver injury. Evaluating LA levels in AD+HIV patients showed that the association with ALT was not only larger, but was also statistically significant, compared with ALA. This suggests a possible role of an elevated proinflammatory response, characterized by elevated LA and low zinc in these patients (Fig. 2b and Table 3). This finding supported our second hypothesis that low zinc and elevated levels of specific FAs that participate in inflammation might be involved in liver injury in AD+HIV patients.
HIV infection, itself, generally does not cause liver injury, but other types of viral infection do adversely affect the liver.31–33 Thus, when the liver is injured (in our study due to heavy drinking), HIV infection may contribute to much lower zinc levels due to involvement in both a proinflammatory mechanism and a potential redistribution of zinc.30 Similarly, dysregulation of serum zinc34 and LA levels35 involved in a proinflammatory response is enhanced, due to the dual hits of alcohol drinking and altered metabolic proinflammatory response in HIV infection, thereby contributing to liver injury.
Low zinc has been recently reported to cause oxidative stress and more rapid progression of fibrosis in an HIV cohort.36 Similarly, another study showed the involvement of LA in T cell apoptosis in an HIV-infected human T cell line, and altered LA metabolism may contribute to the depletion of CD4+ T cells.37 One published recent review has described the interaction of LA and zinc in detail, explaining the role of zinc deficiency in the modulation of desaturase activity involved in FA absorption, oxidation, metabolism, and incorporation.38 The interaction of hypozincemia and proinflammatory FAs in our disease cohort likely is involved in liver injury. ART medication compliance could be a challenge, due to potential hepatotoxicity, when the liver is already vulnerable due to preexisting liver injury in HIV patients (e.g., from active heavy drinking).
Our study has several limitations. Although we had a roughly equal distribution of men and women in each study group, we did not have adequate numbers to analyze gender-based differences within each group or between groups. Moreover, we had small numbers of patients in each of our groups and we recognize that this is a small pilot study. However, several factors showed significance (e.g., zinc, LA). Assessments were conducted simultaneously with the diagnosis of HIV in this unique patient sample, who may not otherwise be tested for other organ-/system-based laboratory evaluations. HIV+AD patients recently drank fewer standard drinks per day than the AD patients; however, lifetime drinking in years was greater in HIV+AD patients and we did not determine the reason for this from the patients. Furthermore, we could not conduct extensive statistical analyses adjusting for multiple variables due to small sample size. Therefore, the results may be limited in scope. In treatment-naive HIV patients (disease controls), who did not drink heavily, we did not conduct any further tests, since there was no liver injury by our initial assessments, which was our primary question. We did not find any close association between CD4+ and zinc levels in our study patients. However, our study demonstrated that hypozincemia and a proinflammatory response in HIV+AD patients were closely related to liver injury.
We conclude that making informed decisions for the selection of treatment regimens for HIV-infected individuals who drink heavily is important. Nutritional intervention, such as zinc supplementation and dietary fat intake regulation, may help reduce liver inflammation/injury. In this group of heavy drinking and/or HIV-infected patients, nutrition intervention could be potentially useful in their medical management and might be a secondary therapeutic strategy.
Acknowledgments
Authors thank BTRIS at NIH for their support in data assembly for this project and Ms. Marion McClain for editorial support for this article. Trial registration: ClinicalTrials.gov identifier number NCT00106106. Proprietorship: this work was supported by intramural and extramural NIH and the University of Louisville Alcohol Research Center and is in the public domain in the USA. Financial/Grant Support: study was supported by NIH-OD-OHSRP-12176 and Z99-AA999999 (V.V.); U01AA021901, U01AA021893-01, U01AA022489-01A1, R01AA023681-01 (C.J.M.); Z1A AA000466 (V.A.R.); K23AA018339 (M.C.C.), U01AA026222-01, U01AA026225-01, R01AG061065, U01AA022618-03, P50AA024337-03, R01AA024405-04, P20GM113226-03 (S.S.B.). Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM113226 (C.J.M.), and the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number P50AA024337 (C.J.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support was also provided by the VA (1I01BX002996, C.J.M.).
Authors' Contributions
V.V. is the project PI and designed the study. M.L.S. provided data management and processing. V.V. and S.K. analyzed the data. V.V., C.J.M., M.C.C., R.K., and S.S. interpreted the outcomes. V.V., C.J.M., R.K., and S.S. wrote the article. C.J.M., A.B.J., M.C.C., S.S.B., and V.A.R. contributed scientifically. All the authors have approved the submission version of this article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Crum NF, Riffenburgh RH, Wegner S, et al. : Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr 2006;41:194–200 [DOI] [PubMed] [Google Scholar]
- 2. Wilcox CM, Saag MS: Gastrointestinal complications of HIV infection: Changing priorities in the HAART era. Gut 2008;57:861–870 [DOI] [PubMed] [Google Scholar]
- 3. Weber R, Sabin CA, Friis-Møller N, et al. : Liver-related deaths in persons infected with the human immunodeficiency virus. Arch Intern Med 2006;166:1632–1641 [DOI] [PubMed] [Google Scholar]
- 4. Rosenbloom MJ, Sullivan EV, Sassoon SA, et al. : Alcoholism, HIV infection, and their comorbidity: Factors affecting self-rated health-related quality of life. J Stud Alcohol Drugs 2007;68:115–125 [DOI] [PubMed] [Google Scholar]
- 5. Schneider M, Chersich M, Neuman M, Parry C: Alcohol consumption and HIV/AIDS: The neglected interface. Addiction 2012;107:1369–1371 [DOI] [PubMed] [Google Scholar]
- 6. Lucas GM, Gebo KA, Chaisson RE, Moore RD: Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS 2002;16:767–774 [DOI] [PubMed] [Google Scholar]
- 7. Meyerhoff DJ, Bode C, Nixon SJ, de Bruin EA, Bode JC, Seitz HK: Health risks of chronic moderate and heavy alcohol consumption: How much is too much? Alcohol Clin Exp Res 2005;29:1334–1340 [DOI] [PubMed] [Google Scholar]
- 8. Ji C: Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem Res Int 2012;2012:216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McClain CJ, Song Z, Barve SS, Hill DB, Deaciuc I: Recent advances in alcoholic liver disease IV. Dysregulated cytokine metabolism in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2004;287:G497–G502 [DOI] [PubMed] [Google Scholar]
- 10. Mallet V, Blanchard P, Verkarre V, et al. : Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS 2007;21:187–192 [DOI] [PubMed] [Google Scholar]
- 11. Maida I, Núñez M, Ríos MJ, et al. : Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr 2006;42:177–182 [DOI] [PubMed] [Google Scholar]
- 12. Horrobin DF, Cunnane SC: Interactions between zinc, essential fatty acids and prostaglandins: Relevance to acrodermatitis enteropathica, total parenteral nutrition, the glucagonoma syndrome, diabetes, anorexia nervosa and sickle cell anaemia. Med Hypotheses 1980;6:277–296 [DOI] [PubMed] [Google Scholar]
- 13. Cunnane SC, Wahle KW: Zinc deficiency increases the rate of Δ 6 desaturation of linoleic acid in rat mammary tissue. Lipids 1981;16:771–774 [DOI] [PubMed] [Google Scholar]
- 14. Nanji AA, French SW: Dietary linoleic acid is required for development of experimentally induced alcoholic liver injury. Life Sci 1989;44:223–227 [DOI] [PubMed] [Google Scholar]
- 15. Ramsden CE, Ringel A, Feldstein AE, et al. : Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids 2012;87:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vatsalya V, Song M, Schwandt ML, et al. : Effects of sex, drinking history, and omega‐3 and omega‐6 fatty acids dysregulation on the onset of liver injury in very heavy drinking alcohol‐dependent patients. Alcohol Clin Exp Res 2016;40:2085–2093 [Google Scholar]
- 17. Sobell LC, Agrawal S, Sobell MB, et al. : Comparison of a quick drinking screen with the timeline followback for individuals with alcohol problems. J Stud Alcohol 2003;64:858–861 [DOI] [PubMed] [Google Scholar]
- 18. Skinner HA, Sheu WJ: Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol 1982;43:1157–1170 [DOI] [PubMed] [Google Scholar]
- 19. Lang W, Perkins H, Anderson RE, Royce R, Jewell N, Winkelstein W, Jr.: Patterns of T lymphocyte changes with human immunodeficiency virus infection: From seroconversion to the development of AIDS. J Acquir Immune Defic Syndr 1989;2:63–69 [PubMed] [Google Scholar]
- 20. Djemli-Shipkolye A, Raccah D, Pieroni G, Vague P, Coste TC, Gerbi A: Differential effect of w3 PUFA supplementations on Na, K-ATPase and Mg-ATPase activities: Possible role of the membrane w6/w3 Ratio. J Membr Biol 2003;191:37–47 [DOI] [PubMed] [Google Scholar]
- 21. Sulkowski MS: Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clin Infect Dis 2004;38:S90–S97 [DOI] [PubMed] [Google Scholar]
- 22. Martín-Carbonero L, Núñez M, González-Lahoz J, Soriano V: Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine. HIV Clin Trials 2003;4:115–120 [DOI] [PubMed] [Google Scholar]
- 23. Dey A, Cederbaum AI: Alcohol and oxidative liver injury. Hepatology 2006;43:S63–S74 [DOI] [PubMed] [Google Scholar]
- 24. Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV: Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology 1998;27:1730–1735 [DOI] [PubMed] [Google Scholar]
- 25. Kupka R, Fawzi W: Zinc nutrition and HIV infection. Nutr Rev 2002;60:69–79 [DOI] [PubMed] [Google Scholar]
- 26. Graham NM, Sorensen D, Odaka N, et al. : Relationship of serum copper and zinc levels to HIV-1 seropositivity and progression to AIDS. J Acquir Immune Defic Syndr 1991;4:976–980 [PubMed] [Google Scholar]
- 27. Farahani M, Novitsky V, Wang R, et al. : Prognostic value of HIV-1 RNA on CD4 trajectories and disease progression among antiretroviral-naïve HIV-infected adults in Botswana: A joint modeling analysis. AIDS Res Hum Retroviruses 2016;32:573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wannamethee G, Shaper AG: Blood lipids: The relationship with alcohol intake, smoking, and body weight. J Epidemiol Commun Health 1992;46:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koutkia P, Meininger G, Canavan B, Breu J, Grinspoon S: Metabolic regulation of growth hormone by free fatty acids, somatostatin, and ghrelin in HIV-lipodystrophy. Am J Physiol Endocrinol Metab 2004;286:E296–E303 [DOI] [PubMed] [Google Scholar]
- 30. Baum MK, Shor-Posner G, Campa A: Zinc status in human immunodeficiency virus infection. J Nutr 2000;130:1421S–1423S [DOI] [PubMed] [Google Scholar]
- 31. Feld JJ, Liang TJ: Hepatitis C—Identifying patients with progressive liver injury. Hepatology 2006;43:S194–S206 [DOI] [PubMed] [Google Scholar]
- 32. Maini MK, Boni C, Lee CK, et al. : The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 2000;191:1269–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vatsalya V, Barve SS, McClain CJ, Ramchandani VA: Elevated linoleic acid (a pro-inflammatory PUFA) and liver injury in a treatment naive HIV-HCV co-infected alcohol dependent patient. J Biosci Med 2016;4:23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McClain C, Vatsalya V, Cave M: Role of zinc in the development/progression of alcoholic liver disease. Curr Treat Options Gastroenterol 2017;15:285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cunnane SC, Wahle KWJ: Zinc deficiency increases the rate of w6 desaturation of linoleic acid in rat mammary tissue. Lipids 1981;16:771–774 [DOI] [PubMed] [Google Scholar]
- 36. Martinez SS, Campa A, Li Y, et al. : Low plasma zinc is associated with higher mitochondrial oxidative stress and faster liver fibrosis development in the Miami adult studies in HIV cohort. J Nutr 2017;147:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sandstrom PA, Tebbey PW, Van Cleave S, Buttke TM: Lipid hydroperoxides induce apoptosis in T cells displaying a HIV-associated glutathione peroxidase deficiency. J Biol Chem 1994;269:798–801 [PubMed] [Google Scholar]
- 38. Knez M, Stangoulis JC, Glibetic M, Tako E: The linoleic acid: dihomo-γ-linolenic acid ratio (LA: DGLA)—An emerging biomarker of Zn status. Nutrients 2017;9:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]