Abstract
Peptide infusions of peptides the corticotropin releasing factor family, including urocortin 2, stresscopin, and urocortin 3 (UCn3), have favorable acute effects in clinical heart failure (HF), but their short half-lives make them unsuitable for chronic therapy. This study asked whether UCn3 gene transfer, which provides sustained elevation of plasma UCn3 levels, increases the function of the failing heart. HF was induced by transmural left ventricular (LV) cryoinjury in mice. LV function was assessed 3 weeks later by echocardiography. Those with ejection fractions (EF) <40% received intravenous saline or intravenous adeno-associated virus type-8 encoding murine UCn3 (AAV8.mUCn3; 1.9 × 1013 genome copies/kg). Five weeks after randomization, repeat echocardiography, assessment of LV function (+dP/dt, −dP/dt), and quantification of Ca2+ transients and sarcomere shortening in isolated cardiac myocytes were conducted, and assessment of LV Ca2+ handling and stress proteins was performed. Three weeks after myocardial infarction, prior to treatment, EFs were reduced (mean 31%, from 63% in sham-operated animals). Mice randomized to receive UCn3 gene transfer showed increased plasma UCn3 (from 0.1 ± 0.01 ng/mL in the saline group to 5.6 ± 1.1 ng/mL; n = 12 each group; p < 0.0001). Compared to mice that received saline, UCn3 gene transfer was associated with higher values for EF (p = 0.0006); LV +dP/dt (p < 0.0001), and LV −dP/dt (p < 0.0001). Cardiac myocytes from mice that received UCn3 gene transfer showed higher peak Ca2+ transients (p = 0.0005), lower time constant of cytosolic Ca2+ decline (tau, p < 0.0001), and higher rates of sarcomere shortening (+dL/dt, p = 0.03) and lengthening (–dL/dt, p = 0.04). LV samples from mice that received UCn3 gene transfer contained higher levels of SERCA2a (p = 0.0004 vs. HF) and increased amounts of phosphorylated troponin I (p = 0.04 vs. HF). UCn3 gene transfer is associated with improved Ca2+ handling and LV function in mice with HF and reduced EF.
Keywords: gene therapy, heart failure with reduced EF, SERCA2a, AAV8
Introduction
Cardiovascular disease is the most common cause of death in the United States. Heart failure (HF) affects 6 million people in the United States and has a dismal outlook, despite optimal therapy.1 Because of such a poor prognosis, new approaches, including gene transfer, are warranted. Urocortin 3 (UCn3) is a 38-amino acid peptide in the corticotropin-releasing factor (CRF) family that binds with high affinity to corticotropin-releasing hormone receptor-2 (CRHR2). Intravenous infusion of urocortin 2 (UCn2) peptide, which has 42% sequence homology with UCn3,2 has beneficial cardiovascular effects,3 but UCn3 has not been studied as extensively for its cardioprotective activity. In preclinical studies, UCn3 peptide infusion provided cardiac protection against ischemia–reperfusion injury.4 Stresscopin, which has 96% sequence homology with UCn3, is efficacious when briefly infused in clinical HF.5 A recent study in healthy individuals and HF patients showed that UCn2 and UCn3 peptide infusions have beneficial effects, including reduced systemic vascular resistance, and increased cardiac output.6
The major impediment to the translation of these approaches to use in clinical HF is the short half-life (10 min) of these peptides.7 Sustained hemodynamic effect of these short-acting peptides would require continuous intravenous (i.v.) infusion, which hampers their clinical usefulness in chronic therapy for HF. A solution for such a problem would be using gene transfer to provide sustained plasma levels of the peptide. In animal studies, such an approach has been used to achieve sustained increases in plasma peptide concentration and increases in cardiac function.8,9 Preclinical studies have shown that such an approach, using i.v. delivery of an adeno-associated virus type 8 (AAV8) encoding UCn2, provided sustained increases in plasma UCn2 and is also beneficial for the normal and failing heart.8,9
Data were recently published comparing the long-term cardiovascular effects of chronic exposure to sustained high plasma levels of UCn2 versus UCn3 using gene transfer in normal animals.10 Similar beneficial effects were found on cardiac function. However, UCn2 gene transfer increased glucose disposal and resulted in a significant decline in fasting glucose—an effect not shared by UCn3 gene transfer. These data indicate that UCn2 gene transfer may be an optimal selection for patients with diabetes and HF, while UCn3 may be better suited for the majority of patients with HF who are not diabetic. However, no previous studies have established that chronic elevation of plasma UCn3 is effective in the treatment of HF. The present study tested the hypothesis that UCn3 gene transfer would improve function of the failing heart.
Materials and Methods
AAV8.UCn3 vector
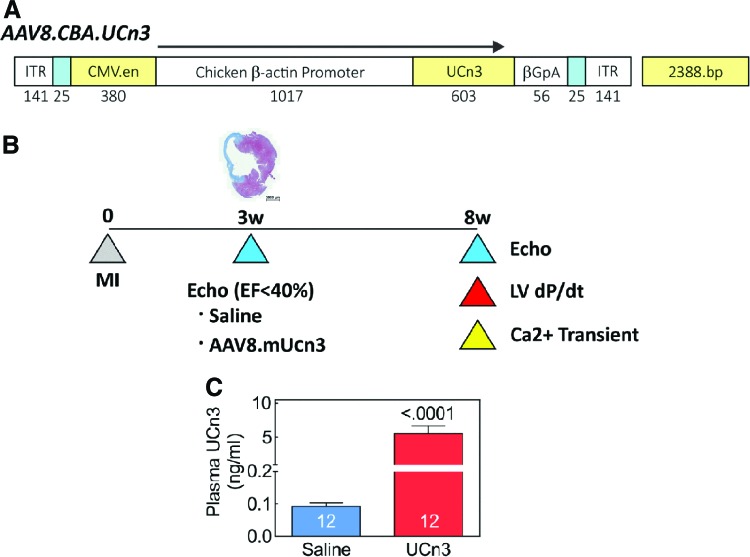
HEK293T cells were transfected with the pRep2/Cap8 and pAd-Helper plasmid11 to produce a helper-virus free AAV8 vector encoding murine urocortin-3 (UCn3) driven by a chicken β-actin (CBA) promoter (AAV8.CBA.mUCn3; Fig. 1). Plasmid pRep2/Cap8 was obtained from the University of Pennsylvania Vector Core. Virus vectors were then purified and concentrated, as previously described.9,12 Virus titers were determined by quantitative real-time polymerase chain reaction (RT-PCR), with virus genome DNA prepared from purified virus.
Figure 1.
Urocortin 3 (UCn3) transgene and study design. (A) AAV8.CBA.mUCn3 vector map. ITR, inverted terminal repeat; CMV.en, cytomegalovirus enhancer; CBA, chicken β-actin promoter; UCn3, murine urocortin 3; βGpA, β globin polyadenylation signal. (B) Study design. A representative cross-section of the left ventricle (LV) following cryoinjury, which resulted in transmural infarction of 27 ± 2% of the LV (n = 5). (C) UCn3 plasma levels in mice with heart failure (HF) 5 weeks after intravenous (i.v.) saline (Sal) or i.v. AAV8.mUCn3 (UCn3 ). Mean ± standard error (SE) are shown. Numbers in bars denote group size. p-Value is from a Student's t-test (unpaired, two-tailed). Color images available online at www.liebertpub.com/hum
Animal use
The Animal Use and Care Committee of the VA San Diego Healthcare System approved the studies. Eighty-eight C57BL/6J mice (51 males) aged 8–12 weeks and weighing 24.5 ± 0.4 g were obtained (Jackson Laboratories, Bar Harbor, ME). Of these, 20 (9 males) underwent thoracotomy and heart manipulation but no cryoinjury; 68 underwent cryoinjury to induce HF.
HF model
Cryoinjury was used to induce anterior wall myocardial infarction (MI) and subsequent left ventricular (LV) chamber dilation and reduced function, as previously described.13 Animals were intubated and mechanically ventilated with oxygen and 1.5% isoflurane. To expose the heart, a thoracotomy was performed at the fourth left intercostal space, and the pericardium was opened. A cryoprobe 3.5 mm in diameter (Brymill, Ellington, CT) was applied to the LV anterior free wall for 10 s, followed by rinsing (25°C saline) to avoid traumatic detachment from the LV. This process was then repeated. Three weeks after cryoinjury, echocardiography was performed to assess heart function. Those with LV ejection fraction (EF) <40% were included in the study and received i.v. saline or i.v. AAV8.UCn3 (1.9 × 1013 genome copies [gc]/kg).
UCn3 gene transfer
Under anesthesia (1.5% isoflurane via nosecone), the jugular vein was exposed, and a syringe with a 31-gauge needle was inserted to deliver AAV8.UCn3 (1.9 × 1013 gc/kg in 100 μL) or a similar volume of saline.
Echocardiography
Echocardiography was performed, as previously described.14 Three weeks after MI, mice were anesthetized initially with 5% and then maintained at 1–1.5% isoflurane, and using a Vevo 3100 ultrasound system (FUJIFILM Visualsonics, Toronto, Canada), echocardiography was performed to document reduced LV function (EF <40%) and to record LV chamber dimensions. Echocardiographic assessment of heart function was then repeated 5 weeks after randomization of mice to receive i.v. delivery of AAV8.UCn3 or saline.
LV systolic and diastolic function
Sodium pentobarbital (80 mg/kg administered intraperitoneally [i.p.]) was used to anesthetize the animals, and a 1.4F micromanometer catheter (SPR 839; Millar Instruments, Houston, TX) was advanced via the right carotid artery across the aortic valve and into the LV cavity. LV pressure was recorded, stored digitally, and then processed (IOX V2.9.5; Emka Technologies, Christchurch, VA), as previously reported.14 Subsequently, blood and tissue samples were obtained. The first derivatives of LV pressure development (LV +dP/dt) and decline (LV −dP/dt) were used to assess LV systolic and diastolic function, respectively, in a manner that was relatively load independent and a more accurate estimate of contractility than EF.
RT-PCR, immunoblotting
LV and liver samples were collected and stored at −80°C for quantitative RT-PCR and Western blotting. Total RNA was isolated and reverse transcribed into cDNA that was used in quantitative PCR, as previously described.8 The gene-specific primers used in PCR are listed in Table 1. Immunoblotting was performed, as described previously.8 The following antibodies were used: Phospho-PKA-RIIa, phosphor-PKA-RIIb, CamKII, phosphor-CamKII, and cardiac ankyrin repeat protein (CARP; Santa Cruz Biotechnology, Santa Cruz, CA); phospho-PKA catalytic subunit, total PKA, p-TnI, total TnI (Cell Signaling Technology, Danvers, MA); PLB (Thermo Fisher Scientific, Waltham, MA); Ser 16 and Thr 17-phospho-PLB (Badrilla Ltd., Leeds, United Kingdom); SERCA2a (Enzo Life Sciences, Farmingdale, NY); and phospho-RYR2 and MYLK3 (Abcam, San Francisco, CA).
Table 1.
Primers used in qRT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| α-MHC | 5′-AAAGGCTGAGAGGAACTACC | 5′-ACCAGCCTTCTCCTCTGC |
| β-MHC | 5′-GCTGAAAGCAGAAAGAGATTATC | 5′-TGGAGTTCTTCTCTTCTGGAG |
| α -SK-actin | 5′-GTGTCACCCACAACGTGC | 5′-AGGGCCACATAGCACAGC |
| ANF | 5′-CCTCGTCTTGGCCTTTTGG | 5′-CATCTTCTACCGGCATCTTC |
| BNP | 5′-GAAGTCCTAGCCAGTCTCC | 5′-CAGCTTGAGATATGTGTCACC |
| CARP | 5′-CTGAACCTGTGGATGTGCC | 5′-GGCTCCAGCCTCCATTAACT |
| FHL-1 | 5′-TGCAACAAGTGCGCTACTCG | 5′-CAATGTTTGGCGAACTTGGTC |
| Coll1α1 | 5′-GCCAAGAAGACATCCCTGAAG | 5′-GGGTCCCTCGACTCCTAC |
| Coll3α1 | 5′-GCACAGCAGTCCAACGTAGA | 5′-TCTCCAAATGGGATCTCTGG |
| MMP8 | 5′-GACTCTGGTGATTTCTTGCTAAC | 5′-CACCATGGTCTCTTGAGACG |
| MMP9 | 5′-CGTCGTGATCCCCACTTACT | 5′-GAACACACAGGGTTTGCCTTC |
| TIMP1 | 5′-GACAGCTTTCTGCAACTCGG | 5′-CTTGTGGACATATCCACAGAGG |
| IL-1b | 5′-CCCATCCTCTGTGACTCATG | 5′-AAGGCCACAGGTATTTTGTCG |
| IL-6 | 5′-CGGAGAGGAGACTTCACAG | 5′-TTCTGCAAGTGCATCATCGTC |
| IL-10 | 5′-GCTCTTACTGACTGGCATGAG | 5′-CGCAGCTCTAGGAGCATGTG |
| UCn3 | 5′- ACGCACCTCCAGATCAAAAG | 5′- AATTCTTGGCCTTGTCGATG |
qRT-PCR, quantitative real-time polymerase chain reaction; α-MHC, alpha-myosin heavy chain; β-MHC, beta-myosin heavy chain; α-SK-actin, alpha-skeletal actin; ANF, atrial natriuretic peptide; BNP, brain natriuretic peptide; CARP, cardiac ankyrin repeat protein; FHL-1, four and a half LIM domains protein 1; Coll, collagen; MMP, matrix metalloproteinase; TIMP1, tissue inhibitor of metalloproteinase 1; IL, interleukin; UCn3, urocortin 3.
Cardiac myocyte isolation and Ca2+ transients
Cardiac myocytes were isolated, as previously described.14 Mice were heparinized (5,000 IU/kg i.p.), and then anesthetized with sodium pentobarbital (150 mg/kg i.p.). Hearts were extracted and quickly cannulated via the aorta for perfusion. Using a peristaltic pump, a calcium-free medium containing Joklik-modified minimal essential medium (Gibco-BRL; Thermo Fisher Scientific) was perfused for 3 min (3 mL/min), followed by perfusion of the same buffer with the addition of 20 μM of CaCl2 and collagenase type II (Worthington Biochemical Corp., Lakewood, NJ) for another 12–13 min. Hearts were then placed into a beaker with digestion solution, atria were removed, and ventricles were sliced into pieces and mixed gently with a wide-nozzle pipette (3–4 min) to dissociate the myocytes. Isolated cardiac myocytes were plated onto laminin pre-coated glass coverslips and loaded with Indo-1/AM (with 0.02% pluronic F-127) for 20 min. Coverslips then were placed in a field stimulation perfusion chamber, rinsed to remove excess stain, and mounted on a Nikon TMD inverted microscope (Nikon, Melville, NY) equipped with a 40 × objective interfaced to a photomultiplier-based fluorescence measuring system (PTI, Edison, NJ). During the measurements, cardiac myocytes were continuously perfused with KRH solution (125 mM of NaCl, 5 mM of KCl, 1.2 mM of NaH2PO4, 6 mM of glucose, 1.2 mM of MgCl2, and 25 mM of HEPES, pH 7.37–7.38) containing 2 mM of CaCl2, and they were field stimulated at 0.3 Hz. Using an excitation wavelength of 365 nm via a monochromator, fluorescent emission was directed to two photomultiplier tubes through 20 nm band-pass filters centered at 405 and 485 nm, respectively. Indicator of the [Ca2+]i is the ratio F405/485. Multiple cardiac myocytes from each heart of three to five mice per group were used to obtain the Ca2+ transient data.
Quantification of fibrosis, LV apoptosis, and infarct size
Fibrosis
Samples of liver and viable (uninfarcted) transmural LV samples were formalin fixed and paraffin embedded. Five-micron sections were mounted and counterstained with hematoxylin and eosin and Masson's trichrome.
Infarct size
A separate group of animals (n = 5) underwent cryoinjury. Eight days later, hearts were arrested in diastole (0.6% KCl) and the right ventricle removed, and LV (including septum) was pressed between two microscopic slides (1.2 mm apart) and scanned using an EPSON V850 pro scanner (Epson, Nagano, Japan). Using ImageJ v1.49 software, the area of transmural infarct was measured and recorded as percent of total LV.
Apoptosis
Paraffin-embedded tissue sections were rehydrated and then microwaved for 5 min in 10 mM of sodium citrate buffer (pH 6.0) for epitope retrieval. After quenching of endogenous peroxidase activity and blocking with normal goat serum, the tissue sections were incubated (18 h at 4°C) with anti-cleaved caspase-3 antibody (1:200; Cell Signaling Technology) to assess apoptosis. After washing, immunostaining was performed using an ImmPRESS HRP kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions.
Statistical analysis
Data acquisition and analysis were performed without knowledge of group identity. Group sizes were determined by power calculations, and male and female mice were used. Since the hypothesis was that UCn3 gene transfer would improve function of the failing heart, the key statistical comparison was between mice with HF that received i.v. saline and those that received UCn3 gene transfer, and Student's t-test was used (unpaired, two-tailed). In order to confirm the presence of LV dysfunction, a sham-operated age- and sex-matched control group was included and, when appropriate, additional between-group t-tests (control vs. HF) were performed, with Bonferroni correction. Data represent mean ± standard error (SE). Analyses were performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). The null hypothesis was rejected when p < 0.05.
Results
Plasma UCn3 levels
Plasma levels of UCn3 were measured 5 weeks after gene transfer. Mice that received UCn3 gene transfer showed increased plasma UCn3 concentrations (HF: 0.1 ± 0.01 ng/mL, n = 12; HF + UCn3: 5.6 ± 1.1 ng/mL, n = 12; p < 0.0001; Fig. 1). There was no sex difference in plasma UCn3 concentrations in HF saline-injected mice (p = 0.5), but in mice that received UCn3 gene transfer, male mice had higher plasma UCn3 levels than female mice (male: 8.5 ± 1 ng/mL, n = 6; female: 2.7 ± 0.8 ng/mL; n = 6; p = 0.001).
MI-induced HF
Sixteen mice died in the first 3 weeks after MI, and 12 did not show EF <40%. Thus, of 68 mice that underwent infarction by cryoinjury, 40 met the enrollment criteria 3 weeks later, giving a yield of 59%. This method is superior to coronary occlusion, where the yield can be <10%.15 No mice died in the 5-week interval after treatment.
Infarct size
Cryoinjury resulted in transmural infarction of 27 ± 2% of the LV (n = 5). Figure 1B shows a representative cross-section of the LV following cryoinjury infarction.
Echocardiography
Three weeks after MI (before randomization), reductions in EF (p < 0.0001) and velocity of circumferential fiber shortening were seen (corrected for heart rate, VCFc; p < 0.0001; Table 2). In addition, end-systolic dimensions (ESD) and end-diastolic dimensions (EDD) were increased among HF mice compared to controls (Table 2), confirming that cryoinjury-induced MI resulted in LV chamber dilation and dysfunction. There were no pretreatment group differences in HF mice in EF, VCFc, EDD, or ESD. However, 8 weeks after MI (5 weeks after saline or UCn3 gene transfer,) beneficial between-group differences conferred by UCn3 gene transfer on EF were seen. Indeed, the final EF was nine percentage units higher (a 32% relative increase) in mice that received UCn3 gene transfer (HF: 28 ± 1%, n = 13; HF + UCn3: 37 ± 1%, n = 13; p = 0.0006; Table 3). In addition, UCn3 gene transfer was associated with higher VCFc (p = 0.0001; Table 3).
Table 2.
Echocardiography 3 weeks after MI (no treatment)
| Control (16) | HF (13) | HF + UCn3 (13) | p, control vs. HF | |
|---|---|---|---|---|
| HR (bpm) | 541 ± 6 | 535 ± 5 | 544 ± 6 | 0.5 |
| EDD (mm) | 3.7 ± 0.1 | 4.5 ± 0.1 | 4.6 ± 0.1 | <0.0001 |
| ESD (mm) | 2.4 ± 0.1 | 3.8 ± 0.1 | 4.0 ± 0.1 | <0.0001 |
| IVSd (mm) | 0.7 ± 0.03 | 0.8 ± 0.02 | 0.8 ± 0.03 | <0.0001 |
| LVEF (%) | 63 ± 2 | 31 ± 2 | 30 ± 2 | <0.0001 |
| VCFc (circ/s) | 22.2 ± 0.9 | 9.6 ± 0.6 | 9.8 ± 0.6 | <0.0001 |
p-Values from student's t-tests (unpaired, two-tailed) with Bonferroni correction for multiple comparisons; numbers in parentheses indicate group size.
MI, myocardial infarction; HF, heart failure; HR, heart rate; bpm, beats per minute; EDD, LV end – diastolic diameter; ESD, LV end – systolic diameter; IVSd, interventricular septum thickness in diastole; LVEF, left ventricular ejection; VCFc, velocity of circumferential fiber shortening (corrected for heart rate); control, sham-operated animals.
Table 3.
Echocardiography 5 weeks after UCn3 gene transfer
| p | |||||
|---|---|---|---|---|---|
| Control (16) | HF (13) | HF + UCn3 (13) | Control vs. HF | HF vs. HF + UCn3 | |
| HR (bpm) | 544 ± 6 | 535 ± 7 | 539 ± 6 | 0.6 | 1 |
| EDD (mm) | 3.6 ± 0.1 | 4.6 ± 0.1 | 4.6 ± 0.2 | <0.0002 | 1 |
| ESD (mm) | 2.4 ± 0.1 | 4.0 ± 0.1 | 3.8 ± 0.2 | <0.0002 | 0.4 |
| IVSd (mm) | 0.7 ± 0.02 | 0.9 ± 0.05 | 0.8 ± 0.06 | <0.0002 | 0.8 |
| LVEF (%) | 62 ± 3 | 28 ± 1 | 37 ± 1 | <0.0002 | 0.0006 |
| VCFc (circ/s) | 21.8 ± 1 | 9.3 ± 0.5 | 12.3 ± 0.5 | <0.0002 | 0.001 |
p-Values from a Student's t-test (unpaired, two-tailed) with Bonferroni correction for multiple comparisons; numbers in parentheses indicate group size.
LV function
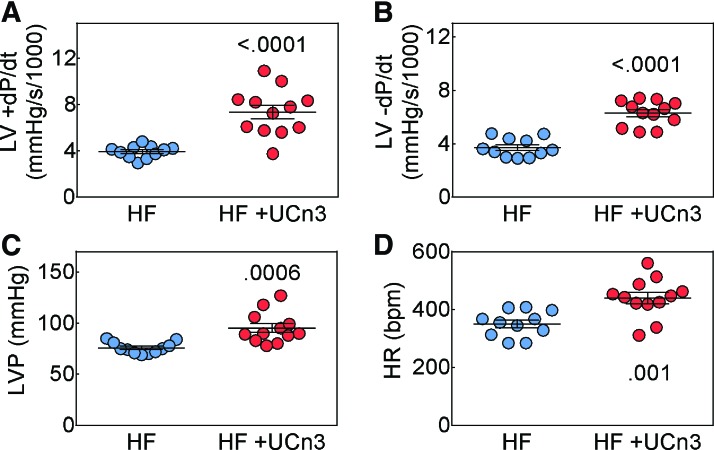
Eight weeks after MI, mice with HF that received UCn3 gene transfer showed an elevation in LV peak +dP/dt (HF: 3,941 ± 159 mmHg/s, n = 11; HF + UCn3: 7,353 ± 582 mmHg/s, n = 12; p < 0.0001; Fig. 2A), LV peak −dP/dt (HF: −3,713 ± 211 mmHg/s, n = 11; HF + UCn3: −6,302 ± 270 mmHg/s, n = 12; p < 0.0001; Fig. 2B) and developed LV pressure (HF: 76 ± 2 mmHg/s, n = 11; HF + UCn3: 95 ± 4 mmHg/s, n = 12; p = 0.0006; Fig. 2C). Heart rates of the HF saline-treated group were lower than the HF UCn3-treated group (HF: 350 ± 13 mmHg/s, n = 11; HF + UCn3: 440 ± 20 mmHg/s, n = 12; p = 0.001; Fig. 2D). After UCn3 gene transfer, there were no sex differences in LV peak +dP/dt (male: 7,288 ± 586 mmHg/s, n = 7; female: 7,444 ± 1,228 mmHg/s, n = 5; p = 0.9) or in LV peak −dP/dt (male: −6,457 ± 303 mmHg/s, n = 7; female: −6,086 ± 519 mmHg/s, n = 5; p = 0.5).
Figure 2.
In vivo assessment of LV function. Eight weeks after myocardial infarction (MI) 5 weeks after i.v. delivery of AAV8.UCn3 (1.9 × 1013 gc/kg; HF + UCn3) or saline (HF), mice underwent physiological studies to assess LV function. (A and B) Peak rate of LV pressure development (A, +dP/dt) and peak rate of LV pressure decline (B, −dP/dt). These data indicate UCn3 gene transfer increased both peak +dP/dt and peak −dP/dt in mice with HF, indicating increased systolic and diastolic LV function. (C) LV developed pressure (LVP) was increased by UCn3 gene transfer. (D) Heart rate (HR) in anesthetized animals was lower in the saline group. Individual data are shown (mean ± SE are indicated); p-values are from a Student's t-test (unpaired, two-tailed). Color images available online at www.liebertpub.com/hum
Cytosolic Ca2+ transients and sarcomere length
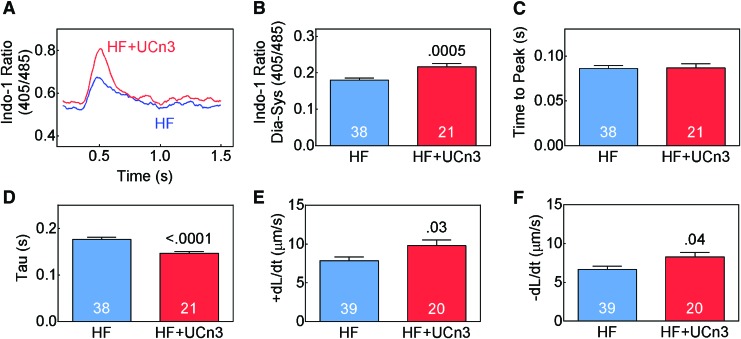
Cardiac myocytes were isolated 8 weeks after MI (5 weeks after randomization to saline or UCn3 gene transfer) to assess Ca2+ transients. Cardiac myocytes from mice that received UCn3 gene transfer showed higher peak cytosolic Ca2+ concentration (Fig. 3A and B; p = 0.0005) and lower Tau (Fig. 3D; p < 0.0001). Time to peak Ca2+ concentration showed no group difference. Sarcomere shortening was also measured in these cardiac myocytes, and it was found that UCn3 gene transfer was associated with a higher shortening rate (+dL/dt; HF: 7.8 ± 0.5 μm/s, n = 39; HF + UCn3: 9.8 ± 0.7 μm/s, n = 20; p = 0.03; Fig. 3E) and a higher lengthening rate (–dL/dt; HF: 6.7 ± 0.4 μm/s, n = 39; HF + UCn3: 8.3 ± 0.6 μm/s, n = 20; p = 0.04; Fig. 3F). These data are consistent with group differences in Ca2+ transients and pressure development and decline in vivo (Fig. 2A–C).
Figure 3.
Cytosolic Ca2+ transients. (A) Representative Indo-1 Ca2+ transient recordings from cardiac myocytes from one heart in each group. (B) Mean peak Ca2+ transients from multiple cardiac myocytes from each group, showing increased peak Ca2+ transients in cardiac myocytes isolated form mice with HF versus HF mice treated with UCn3 gene transfer (HF + UCn3). (C) Time-to-peak cytosolic Ca2+ concentration showed no group difference between mice with HF that received saline versus UCn3 gene transfer. (D) Time constant of cytosolic Ca2+ decline (tau) showed shorter tau (more rapid decline) in cardiac myocytes isolated from mice following UCn3 gene transfer. (E) Rate of sarcomere shortening in systole (+dL/dt) was higher in HF animals that received UCn3 gene transfer. (F) Rate of sarcomere lengthening in diastole (–dL/dt) was higher in HF animals that received UCn3 gene transfer. In (B–F), summary data are from cardiac myocytes isolated from three to five mice per group. Mean ± SE are shown; p-values are from a Student's t-test (unpaired, two-tailed). Color images available online at www.liebertpub.com/hum
Necropsy
Body and liver weights showed no group differences (Table 4). HF mice compared to control mice showed higher weights of LV (p = 0.002) and LV/body weight ratio (p < 0.0002), which were increased similarly after MI in HF and HF + UCn3 groups (Table 4).
Table 4.
Necropsy data 5 weeks post saline or AAV8.UCn3 administration in mice with HF
| p | |||||
|---|---|---|---|---|---|
| Con (15) | HF (16) | HF + UCn3 (13) | Control vs. HF | HF vs. HF + UCn3 | |
| BW (g) | 26.9 ± 1.1 | 26.5 ± 0.7 | 26.7 ± 0.9 | 1 | 1 |
| LV (mg) | 84 ± 4 | 110 ± 6 | 102 ± 5 | 0.002 | 0.8 |
| LV/BW (mg/g) | 3.1 ± 0.1 | 5.0 ± 0.2 | 4.8 ± 0.3 | <0.0002 | 1 |
| Liver/BW (mg/g) | 45 ± 1 | 45 ± 1 | 43 ± 1 | 1 | 0.8 |
p-Values from a Student's t-test (unpaired, two-tailed) with Bonferroni correction for multiple comparisons; numbers in parentheses indicate group size.
BW, body weight.
LV Ca2+ handling protein expression
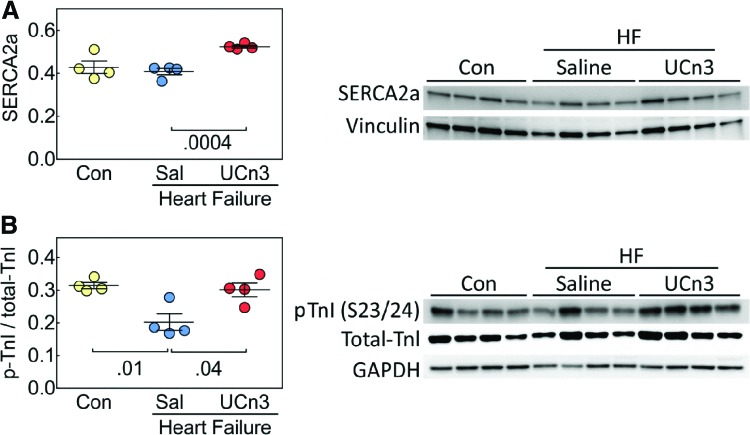
After MI, LV content of sarco/endoplasmic reticulum Ca2+ ATPase (SERCA2a) protein was higher after UCn3 gene transfer (HF: 0.41 ± 0.02, n = 4; HF + UCn3: 0.52 ± 0.01, n = 4; p = 0.0004; Fig. 4A). LV content of phosphorylated troponin I (p-TnI) was lower after MI (p = 0.01) but was normalized after UCn3 gene transfer. It was found that p-TnI was 50% higher in animals with HF that received UCn3 (control: 0.31 ± 0.01, n = 4; HF: 0.20 ± 0.03, n = 4; HF + UCn3: 0.30 ± 0.02, n = 4; p = 0.04; Fig. 4B). Selected additional proteins relevant to Ca2+ handling showed reduced phosphorylation (p-RYR2, p-16-PLB, and p17-PLB) after MI, but no HF versus HF + UCn3 group differences were seen (Table 5).
Figure 4.
LV SERCA2a and phosphorylation of troponin I (TnI) in mice with HF 5 weeks after UCn3 gene transfer. (A) LV SERCA2a content was higher in mice with HF that received UCn3 gene transfer (p = 0.0004 vs. HF). (B) Troponin I (TnI) phosphorylation at serine 23 and 24 was higher in HF mice that received UCn3 gene transfer (p = 0.04 vs. HF). Individual mouse data are shown (mean ± SE are indicated); Con, sham-operated animals. p-Values are from a Student's t-test (unpaired, two-tailed) with Bonferroni correction for multiple comparisons. Color images available online at www.liebertpub.com/hum
Table 5.
LV protein levels (immunoblotting)
| p | |||||
|---|---|---|---|---|---|
| Protein | Con (4) | HF (4) | HF + UCn3 (4) | Control vs. HF | HF vs. HF + UCn3 |
| p-PKA-RIIa | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 1 | 0.6 |
| p-PKA-RIIb | 0.3 ± 0.15 | 0.3 ± 0.03 | 0.2 ± 0.03 | 1 | 0.8 |
| Total-PKA | 0.4 ± 0.01 | 0.3 ± 0.02 | 0.3 ± 0.02 | 1 | 0.8 |
| p-PKA/Total | 1.8 ± 0.3 | 2.0 ± 0.10 | 1.9 ± 0.1 | 1 | 0.8 |
| CamKII | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 1 | 0.8 |
| p-CamKII | 0.5 ± 0.1 | 0.4 ± 0.02 | 0.6 ± 0.1 | 0.8 | 0.2 |
| p-CamKII/Total | 0.9 ± 0.15 | 0.9 ± 0.1 | 1.1 ± 0.2 | 1 | 1 |
| p-RYR2/Total | 2.2 ± 0.4 | 0.7 ± 0.1 | 1.2 ± 0.1 | 0.01 | 0.06 |
| p16-PLB | 0.1 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.11 | 1 | 0.8 |
| p16-PLB/Total | 3.7 ± 0.6 | 1.1 ± 0.2 | 2.1 ± 0.5 | 0.01 | 0.2 |
| p17-PLB | 6.3 ± 0.7 | 2.4 ± 0.8 | 2.2 ± 0.4 | 0.02 | 1 |
| p17-PLB/Total | 28.6 ± 3.5 | 3.5 ± 1.1 | 5.4 ± 1.5 | 0.001 | 0.6 |
| CARP | 10.3 ± 1 | 14 ± 1.8 | 14 ± 1.1 | 0.2 | 1 |
| MYLK3 | 0.6 ± 0.1 | 1.0 ± 0.3 | 0.7 ± 0.1 | 0.4 | 0.8 |
Immunoblotting data presenting protein levels normalized for GAPDH or vinculin. Data are mean ± SE. p-Values from a Student's t-test (unpaired, two-tailed) with Bonferroni correction for multiple comparisons; numbers in parentheses indicate group size.
p-TnI, S23/24 phosphorylated troponin I; PKA-RIIa, protein kinase A IIa; CamKII, Ca2+/calmodulin-dependent protein kinase II; RYR, ryanodine receptor; PLB, phospholamban; MYLK3, myosin light chain kinase 3.
LV mRNA expression of markers of hypertrophy and fibrosis
As expected, there were group differences in LV mRNA expression of several markers of LV stress 8 weeks after MI. However, brain natriuretic peptide (BNP; p = 0.006), atrial natriuretic peptide (ANF; p = 0.05), α-skeletal actin (p = 0.02), and CARP (p = 0.01) were lower in HF + UCn3 than in HF mice. The levels of LV beta-myosin heavy chain (β-MHC) mRNA were lower in HF + UCn3 versus HF mice (p < 0.01; Table 6). Although several genes related to fibrosis showed increased LV mRNA expression in HF versus control mice, their expression was not altered by UCn3 gene transfer.
Table 6.
LV mRNA expression of markers of hypertrophy and fibrosis
| Gene | HF (4), fold control | HF + UCn3 (4), fold control | p |
|---|---|---|---|
| α-MHC | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.40 |
| β-MHC | 25 ± 5 | 6.6 ± 0.5 | 0.01 |
| α-skeletal actin | 5.3 ± 1 | 2.3 ± 0.3 | 0.02 |
| ANF | 15 ± 3.8 | 5.6 ± 0.6 | 0.05 |
| BNP | 3.4 ± 0.4 | 1.6 ± 0.2 | 0.006 |
| CARP | 3.5 ± 0.3 | 2.3 ± 0.04 | 0.01 |
| FHL-1 | 1.3 ± 0.2 | 1.6 ± 0.1 | 0.16 |
| Col1 | 3.8 ± 0.7 | 2.7 ± 0.2 | 0.21 |
| Col3 | 3.8 ± 0.6 | 3.0 ± 0.3 | 0.30 |
| MMP8 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.89 |
| MMP9 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.74 |
| TIMP1 | 0.7 ± 0.01 | 0.7 ± 0.1 | 0.46 |
| IL-1b | 0.4 ± 0.02 | 0.4 ± 0.1 | 0.89 |
| IL-6 | 3.9 ± 0.4 | 4 ± 1.3 | 0.93 |
| IL-10 | 0.8 ± 0.1 | 1.2 ± 0.3 | 0.17 |
Data are mean ± SE. p-Values are from a Student's t-test (unpaired, two-tailed); numbers in parentheses indicate group size.
Col1, collagen 1; col3, Collagen 3; MMP8, matrix metalloproteinase 8; MMP9, matrix metalloproteinase 9; IL-1b, interleukin 1b; IL-6, interleukin 6; IL-10, interleukin 10.
Fibrosis and LV apoptosis
Fibrosis
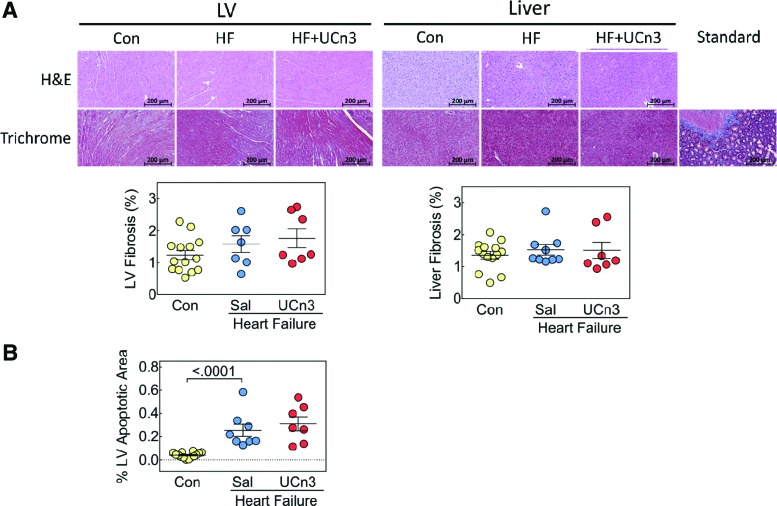
There were no group differences in fibrosis in the liver or in the uninfarcted region of LV, and there were no differences in inflammatory infiltrates (Fig. 5A).
Figure 5.
Histological analysis of LV and liver and LV apoptosis 5 weeks after UCn3 gene transfer. (A) Hematoxylin and eosin (H&E) and Masson's trichrome staining were performed on liver and transmural sections of LV (original magnification 20 × ; presented image magnification H&E and Masson's trichrome: 7 × ), no evidence of inflammatory infiltrates was seen. LV fibrosis of the viable (non-infarcted LV) was similar in all three groups 8 weeks after MI. There were no group differences in liver fibrosis. (B) LV apoptosis was assessed using active caspase-3 staining on transmural LV sections, which showed a group difference between control and HF groups. Standard, control for Masson's trichrome. Individual data are shown (mean ± SE); p-values are from a Student's t-test (unpaired, two-tailed). Color images available online at www.liebertpub.com/hum
LV apoptosis
Quantification of active caspase-3 staining showed more apoptosis after MI (p < 0.0001), with similar amounts of apoptosis in HF and HF + UCn3 mice (control: 0.04 ± 0.01%, n = 14; HF: 0.26 ± 0.05%, n = 8; HF + UCn3: 0.31 ± 0.06%, n = 7; Fig. 5B).
Discussion
The major finding of this study is that UCn3 gene transfer increased the function of the failing heart. Three weeks after MI, LV EF was reduced from a mean value of 63% (control) to 30–31%. Five weeks later, mice that received UCn3 gene transfer showed increased EF, while those that received saline showed a further decline. The final EF was nine percentage units higher (32% relative increase) in mice that received UCn3 gene transfer (p = 0.0006; Table 3). Furthermore, LV peak +dP/dt showed a 1.9-fold increase in HF + UCn3 versus HF (p < 0.0001; Fig. 2A). Finally, LV diastolic function after MI was also enhanced by UCn3 gene transfer. LV peak −dP/dt, a measure of diastolic function, showed a 1.7-fold increase in HF + UCn3 versus HF (p < 0.0001; Fig. 2B).
What explains these improvements in function of the failing heart associated with UCn3 gene transfer? If the evidence for improved function resided in EF alone, one could propose that the benefit might reflect a vasodilating effect of UCn35,6 because a reduction in systemic pressure would be expected to enhance EF. However, the favorable effects of UCn3 gene transfer also were seen in LV peak +dP/dt, an event that occurs prior to aortic valve opening, and therefore is not as affected by systemic pressure. Likewise, isolated cardiac myocytes from HF mice that received UCn3 gene transfer showed a higher sarcomere shortening rate (+dL/dt) versus HF alone, confirming that the benefits reside within the cardiac myocyte per se and do not reflect vasodilatory enhancement of LV function.
The group difference in HR during measures of LV peak +dP/dt (Fig. 2) may have contributed to the increase in LV peak +dP/dt via a positive force–frequency relationship. However, LV peak +dP/dt was increased 90% in mice with HF treated with UCn3 gene transfer, and the force–frequency effect would account for a much smaller change.16 Finally, the increase in VCFc (at matched HR) and the increase in isolated cardiac myocyte shortening rates indicate changes in function not related to HR.
What then might explain intrinsic improvement in function of the LV and cardiac myocytes isolated from failing hearts treated with UCn3 gene transfer? The rate of cardiac myocyte shortening during systole (+dL/dt, p = 0.03; Fig. 3E) and lengthening during diastole (–dL/dt, p = 0.04; Fig. 3F) were increased in the HF + UCn3 group versus the HF group. These findings show tight correlations with LV peak +dP/dt and peak −dP/dt, physiological measures of the intact heart. UCn3 gene transfer increases Ca2+ handling, and subsequently increases LV systolic and diastolic function. It was found that cardiac myocytes from HF + UCn3 mice showed increased peak Ca2+ transients (p = 0.0005; Fig. 3B) and reduced time constant of Ca2+ decline (tau, p < 0.0001; Fig. 3D) compared to those from HF mice. This may explain the increases in LV systolic and diastolic function.
To determine molecular underpinnings for increased LV Ca2+ handling, LV protein expression of key Ca2+ related proteins was assessed. LV SERCA2a returns cytosolic Ca2+ to the sarcoplasmic reticulum, and consequently, increased expression of this protein would increase cytosolic Ca2+ decline, as was observed (Fig. 3D). An increase in cytosolic Ca2+ decline, by increasing Ca2+ availability to myofilaments, could also increase systolic function. It was found that LV SERCA2a protein levels were similar in control and HF mice but were higher after UCn3 gene transfer (p = 0.0004 vs. HF; Fig. 4A). Upregulation of SERCA2a would be anticipated to increase peak systolic Ca2+ transient amplitude and rate of Ca2+ decline (Fig 3A, B, and D), and subsequently increase systolic and diastolic function of isolated cardiac myocytes (Fig. 3E and F) and the intact heart (Fig. 2A and B). Cardiac myocytes would no longer be exposed to sustained high plasma levels of UCn3 once isolated. However, one would anticipate that increases in SERCA2a protein, which has a half-life of 2–3 days,17 would endure for the 2 h interval required for cell isolation and study.
Improved Ca2+ handling continues to be seen in the cardiac myocytes that were isolated from animals that received UCn3 gene transfer. Although increased LV SERCA2a provides a potential mechanism for UCn3's effects on LV Ca2+ handling, it does not elucidate the molecular mechanism by which this occurs, which will require further studies.
Troponin I phosphorylation plays a role in cardiac contractility and relaxation and is impaired under ischemic conditions. In the current study, phosphorylation of TnI at Serine residues 23 and 24 was reduced in the HF group but restored to normal by UCn3 gene transfer (HF vs. HF + UCn3, p = 0.04; Fig. 4B). The implications of the restoration of LV p-TnI to normal levels vis-à-vis cardiac function and the mechanism by which UCn3 affected this change were not established in the present study. However, reduction in TnI phosphorylation at Ser 23/24 is seen in explanted hearts from patients with end-stage HF,18 and Ser 23/24 phosphorylation of TnI is associated with more rapid relaxation,19 and therefore may be of mechanistic importance for the enhanced diastolic function conferred by UCn3 gene transfer in the present study.
There were increases in the HF group (vs control) in ANF, BNP, α-skeletal actin, and β-MHC, which are molecular markers of hypertrophy and stress.20–22 These markers were significantly less increased after UCn3 gene transfer (Table 6). These group difference likely are a consequence of improved LV function in HF after UCn3 gene transfer. LV CARP expression is increased in clinical HF and in many animal models of HF, and may exacerbate adverse cardiac remodeling and apoptosis.23,24 Increased LV CARP mRNA was seen in HF mice, as was somewhat less of an increase in the HF + UCn3 group (p = 0.01; Table 6). LV mRNA expression of Col 1, Col3, and IL-6 were increased similarly in HF and HF + UCn3 mice (Table 6).
HF is associated with protean abnormalities, and it is uncertain whether the benefits seen in mice after UCn3 gene transfer would also be seen in clinical HF. The present study tested UCn3 gene transfer in HF with reduced EF on endpoints obtained 5 weeks after initiation of therapy. Will UCn3 gene transfer have enduring effects, and will such effects be seen in models of HF with preserved EF? Despite these limitations, it is promising that a simple i.v. injection of AAV8.UCn3 has such benefits on function of the failing heart.
AAV8 was selected because the target was the liver, and the beneficial effects reside in increased plasma concentrations and do not depend on increasing LV expression per se. In head-to-head experiments of various vectors and promoters, AAV8.CBA was superior to others.8 After i.v. AAV8 vector delivery, the primary organ in which one detects transgene expression is the liver,8,10 which is the target of this paracrine-based gene transfer. Another potential advantage is less pre-existing anti-AAV8 antibodies in humans versus AAV9.25
The National Institutes of Health has requested that preclinical studies be conducted in similar proportions of male and female animals when possible,26 a requirement embraced by the Food and Drug Administration for Investigational New Drug applications. In the current study, the same amount of AAV8.UCn3 (1.9 × 1013 gc/kg i.v.) was associated with threefold higher plasma UCn3 concentrations in male vesus female mice. Even so, the mean plasma UCn3 concentration in females after gene transfer was >20-fold higher than that of mice that did not receive vector and was a sufficient amount to evoke equivalent LV +dP/dt and LV −dP/dt as was found in male mice. These data indicate that lower doses of AAV8.UCn3 than used in the current study will suffice to obtain a maximal effect. The dose–response effect of AAV8.UCn3 in each sex will be addressed in separate studies.
In conclusion, UCn3 gene transfer increases systolic and diastolic function of the failing heart. These beneficial effects of UCn3 gene transfer reverberate from Ca2+ transients and sarcomere shortening in isolated cardiac myocytes to measures of LV function in vivo. The mechanism for these benefits can be attributed to improved LV Ca2+ handling, which directly affects sarcomere shortening, and is possibly a consequence of increased expression of LV SERCA2a and perhaps restoration of TnI phosphorylation. These studies indicate that chronically increasing plasma levels of UCn3 may be beneficial in HF with reduced EF. Translating this to possible clinical application will require proof of safety and efficacy and in other models of HF, and studies in larger mammals.
Acknowledgments
This work was supported by National Institutes of Health grant (P01 HL66941) and Merit grants from the Department of Veteran's Affairs (1101 BX001515 and 1I01 BX003774).
Author Disclosure
H.K.H. is a founder, board member, and unpaid consultant of Renova Therapeutics. Renova redundant as stated played no role in the studies. None of the other authors have any disclosures.
References
- 1. Lloyd-Jones D, Adams RJ, Brown TM, et al. . Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010;121:948–954 [DOI] [PubMed] [Google Scholar]
- 2. Lewis K, Li C, Perrin MH, et al. . Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A 2001;98:7570–7575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan WY, Frampton CM, Crozier IG, et al. . Urocortin-2 infusion in acute decompensated heart failure: findings from the UNICORN study. JACC Heart Fail 2013;1:433–441 [DOI] [PubMed] [Google Scholar]
- 4. Brar BK, Jonassen AK, Egorina EM, et al. . Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology 2004;145:24–35 [DOI] [PubMed] [Google Scholar]
- 5. Gheorghiade M, Greene SJ, Ponikowski P, et al. . Haemodynamic effects, safety, and pharmacokinetics of human stresscopin in heart failure with reduced ejection fraction. Eur J Heart Fail 2013;15:679–689 [DOI] [PubMed] [Google Scholar]
- 6. Stirrat CG, Venkatasubramanian S, Pawade T, et al. . Cardiovascular effects of urocortin 2 and urocortin 3 in patients with chronic heart failure. Br J Clin Pharmacol 2016;82:974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel K, Rademaker MT, Kirkpatrick CM, et al. . Comparative pharmacokinetics and pharmacodynamics of urocortins 1, 2 and 3 in healthy sheep. Br J Pharmacol 2012;166:1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao MH, Lai NC, Miyanohara A, et al. . Intravenous adeno-associated virus serotype 8 encoding urocortin-2 provides sustained augmentation of left ventricular function in mice. Hum Gene Ther 2013;24:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lai NC, Gao MH, Giamouridis D, et al. . Intravenous AAV8 encoding urocortin-2 increases function of the failing heart in mice. Hum Gene Ther 2015;26:347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giamouridis D, Gao MH, Lai NC, et al. . Effects of urocortin 2 versus urocortin 3 gene transfer on left ventricular function and glucose disposal. JACC Basic Transl Sci 2018;3:249–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 1998;72:2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao G, Qu G, Burnham MS, et al. . Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum Gene Ther 2000;11:2079–2091 [DOI] [PubMed] [Google Scholar]
- 13. van den Bos EJ, Mees BM, de Waard MC, et al. . A novel model of cryoinjury-induced myocardial infarction in the mouse: a comparison with coronary artery ligation. Am J Physiol Heart Circ Physiol 2005;289:1291–1230 [DOI] [PubMed] [Google Scholar]
- 14. Gao MH, Lai NC, Roth DM, et al. . Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation 1999;99:1618–1622 [DOI] [PubMed] [Google Scholar]
- 15. Bayat H, Swaney JS, Ander AN, et al. . Progressive heart failure after myocardial infarction in mice. Basic Res Cardiol 2002;97:206–213 [DOI] [PubMed] [Google Scholar]
- 16. Palakodeti V, Oh S, Oh BH, et al. . Force-frequency effect is a powerful determinant of myocardial contractility in the mouse. Am J Physiol 1997;273:H1283–H1290 [DOI] [PubMed] [Google Scholar]
- 17. Louch WE, Hougen K, Mørk HK, et al. . Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout. J Physiol 2010;588:465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol 2007;42:247–259 [DOI] [PubMed] [Google Scholar]
- 19. Salhi HE, Hassel NC, Siddiqui JK, et al. . Myofilament calcium sensitivity: mechanistic insight into TnI Ser-23/24 and Ser-150 phosphorylation integration. Front Physiol 2016;7:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Battistoni A, Rubattu S, Volpe M. Circulating biomarkers with preventive, diagnostic and prognostic implications in cardiovascular diseases. Int J Cardiol 2012;157:160–168 [DOI] [PubMed] [Google Scholar]
- 21. Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol 2004;44:2390–2397 [DOI] [PubMed] [Google Scholar]
- 22. Copeland O, Nowak KJ, Laing NG, et al. . Investigation of changes in skeletal muscle alpha-actin expression in normal and pathological human and mouse hearts. J Muscle Res Cell Motil 2010;31:207–214 [DOI] [PubMed] [Google Scholar]
- 23. Chen C, Shen L, Cao S, et al. . Cytosolic CARP promotes angiotensin II- or pressure overload-induced cardiomyocyte hypertrophy through calcineurin accumulation. PLoS One 2014;9:e104040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen L, Chen C, Wei X, et al. . Overexpression of ankyrin repeat domain 1 enhances cardiomyocyte apoptosis by promoting p53 activation and mitochondrial dysfunction in rodents. Clin Sci (Lond) 2015;128:665–678 [DOI] [PubMed] [Google Scholar]
- 25. Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712 [DOI] [PubMed] [Google Scholar]
- 26. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014;509:282–283 [DOI] [PMC free article] [PubMed] [Google Scholar]