Abstract
HIV-1 subtype and viral load set point have been implicated as strong predictors of HIV-1 disease progression; however, the relationship between these two variables has not been investigated. We used data from the Rakai Community Cohort Study to investigate whether the association between viral load set point and disease progression is modified by HIV subtype. Time to AIDS or AIDS-related death was estimated by Kaplan–Meier survival analysis stratified by subtype and viral set point, and Cox proportional hazards regression with an interaction term between viral load set point and HIV subtype. The interaction term did not indicate effect measure modification between viral load set point and progression to AIDS by HIV-1 subtype [adjusted hazard ratio (aHR), 0.99; 95% confidence interval (CI) 0.61–1.61; p = .968]. Stratifed analysis by subtype was also not indicative of a difference in relationship between viral load set point and time to AIDS with overlapping 95% CIs between subtypes A and D (subtype A aHR: 2.40, 95% CI 1.45–3.99, subtype D aHR: 1.96, 95% CI 1.60–2.40). These results indicate that the higher mortality in subtype D-infected individuals is independent of viral load set point.
Keywords: Uganda, HIV subtype, viral load, disease progression
There is wide-ranging genetic diversity of HIV-1, with the majority of infections worldwide, as represented by subtypes within HIV-1 group M, differing by as much as 35% in terms of viral protein sequences.1 Genotypic differences between HIV-1 subtypes can result in differences in pathogenicity between subtypes.2 In Rakai, Uganda, HIV-1 viral subtypes A and D are predominant.3 Previous studies have shown that subtype D is associated with faster disease progression than subtype A.4 In addition, higher HIV-1 viral load is associated with faster progression to AIDS and increased mortality.5 Although HIV-1 subtype and viral load set point have been implicated as among the strongest predictors of HIV-1 disease progression,6 the relationship between these two variables and their joint impact on disease progression has not been investigated. We assessed HIV disease progression in individuals infected with subtypes A or D, and investigated whether the impact of HIV viral load is differential across HIV-1 subtypes through assessing interaction of these predictors on both the multiplicative and additive scales.
The data obtained in this study were from the Rakai Community Cohort Study. Consenting participants of ages from 15 to 49 years were enrolled and followed up at ∼12–18 months intervals from January 1995 to October 2007.4 Venous blood samples were collected for HIV testing and it was assumed that the date of infection was the midpoint between the last negative and first positive test. The analysis included individuals infected with either subtypes A or D and excluded recombinant and/or multiple infections. Laboratory methods used to determine HIV-1 subtype are described in detail elsewhere.3,4,7 Of the 375 HIV-1 seroconverters infected with subtypes A or D in this analysis, 78 (20.8%) were infected with subtype A and 297 (79.2%) were infected with subtype D. Age at HIV-1 seroconversion [subtype A: 30 (38.5%) <25 years, 32 (41.0%) 25–34 years, 16 (20.5%) ≥35 years; subtype D: 105 (35.4%) <25 years, 138 (46.5%) 25–34 years, 54 (18.2%) ≥35 years] and gender [subtype A: 51 (65.4%) female; subtype D: 177 (59.6%) female] did not differ significantly by infecting subtype. Median set point viral load, as defined using the time points after the initial positive and before AIDS, was similar between subtypes [subtype A: 4.4 log10 copies/mL (interquartile range, IQR: 4.1–5.1); subtype D: 4.6 log10 copies/mL (IQR: 4.1–5.1), p = .47].
Time from seroconversion to AIDS or death from AIDS-related causes was analyzed using Kaplan–Meier survival analysis and differences were assessed using the log-rank test for equality of survivor functions. Cox proportional hazards regression analyses were used to assess adjusted differences in disease progression.8,9 The proportional hazards assumption was confirmed with a goodness-of-fit analysis of the Schoenfeld residuals. Continuous log10-transformed HIV-1 set point viral load and categorical HIV-1 subtype A or D were the primary exposure variables with progression to AIDS or AIDS-related death as the primary outcome. AIDS was defined as a CD4+ cell count of ≤250 cells/mm3 or death due to AIDS-related causes ascertained by a verbal autopsy survey. Observations were censored at the last follow-up date or the end of the study. Initiation of antiretroviral (ART) and death from non-AIDS-related causes were censored at time of occurence. Statistical analyses were performed using Stata, version 15.0.
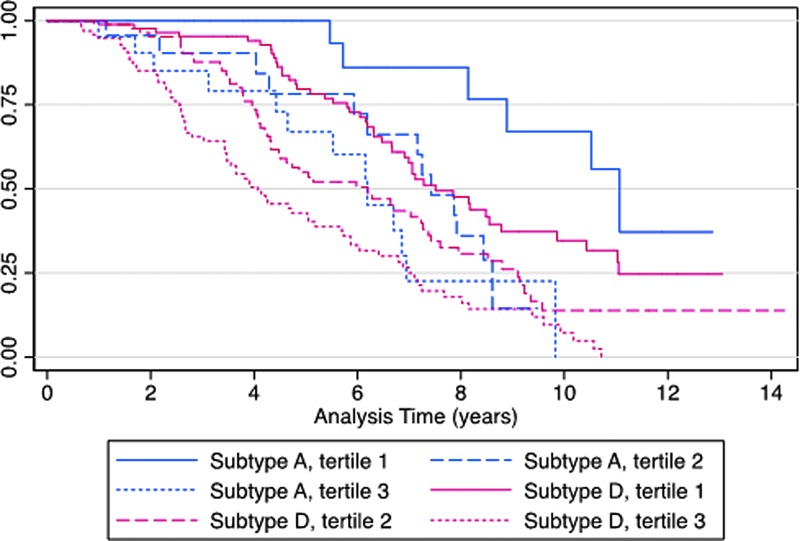
Median time to progression to AIDS or AIDS-related death was longer with subtype A (median: 5.7 years; IQR: 2.6–8.0 years) versus subtype D (median: 4.7 years; IQR: 2.8–7.1, p = .017). The number of participants who died from AIDS was 8 (10%) with subtype A and 82 (28%) with subtype D, p = .001. In addition, 24 (31%) of subtype A and 93 (31%) of subtype D-infected indiviuals had AIDS during follow-up. Figure 1 shows the Kaplan–Meier survival curves stratified by HIV-1 subtype and viral load tercile. Both increasing viral load and infection with subtype D were associated with a shorter time to AIDS or AIDS-related death (p < .001).
FIG. 1.
Kaplan–Meier survival by HIV-1 subtype, stratified by viral load tercile. p < .001, by the log-rank test. Subtype A: tercile 1: ≤4.1 log10 copies/mL, tercile 2: 4.2–4.8 log10 copies/mL, tercile 3: ≥5.0 log10 copies/mL; subtype D: tercile 1: ≤4.3 log10 copies/mL, tercile 2: 4.3–5.0 log10 copies/mL, tercile 3: ≥5.0 log10 copies/mL.
Age and gender were not statistically signficiantly associated with disease progression in the Cox regression univariable analysis [centered-age per year increase adjusted hazard ratio (aHR), 1.01; 95% confidence interval (CI) 0.99–1.03, p = .099; gender male vs. female aHR, 0.79; 95% CI 0.60–1.05, p = .099], but were included in the final model as they have been previously identified to be predictors of HIV-1 disease progression.4 Controlling for age and gender, each log10 increase of viral load was associated with an increased risk of progression to AIDS or death (aHR, 2.01; 95% CI 1.67–2.43; p < .001) as was infection with subtype D (aHR, 1.63; 95% CI 1.11–2.37; p = .012). These findings are consistent with previous studies.4,5 Stratifed analysis using Cox regression showed no statistically significant difference in the relationship between viral load set point and time to AIDS by subtype (subtype A aHR: 2.40, 95% CI 1.45–3.99; subtype D aHR: 1.96, 95% CI 1.60–2.40). The interaction term between subtype and viral load in multivariable Cox proportional hazards regression was not statistically significant (aHR, 0.99; 95% CI 0.61–1.61; p = .968).
Additive interaction between HIV-1 subtype and viral load set point was estimated by calculating the relative excess risk due to interaction (RERI) from relative risks derived from a log-binomial model at yearly time points from 4 to 8 years after HIV-1 seroconversion. CIs were derived using the delta method estimate of the variance.10 There was no evidence of significant additive interaction at any of the five time points analyzed [year 4 (RERI A n = 78, D n = 297; 5.78; 95% CI −5.57 to 17.13; p = .318); year 5 (RERI A n = 72, D n = 301; 1.01; 95% CI −2.87 to 4.89; p = .610); year 6 (RERI A n = 68, D n = 265; 0.78; 95% CI −0.84 to 2.40; p = .347); year 7 (RERI A n = 64, D n = 246; −1.23; 95% CI −3.77 to 1.32; p = .346); year 8 (RERI A n = 58, D n = 221; −0.92; 95% CI −2.56 to 0.72; p = .272)].
The presence of multiplicative or additive interaction between HIV-1 subtype and viral load set point would indicate that these predictors work together to increase disease progression, resulting in a greater risk of progression to AIDS than expected. Based on this analysis, there did not appear to be any significant excess risk resulting from the pathogenic effects of having both a subtype D HIV-1 infection and a high set point viral load. This indicates that the dose–response effect seen in progression to clinical AIDS by viral load set point is not differential across HIV-1 subtypes. The higher mortality in subtype D-infected individuals is not mediated through viral load set point.
Acknowledgments
This study was supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Original data were obtained with the following support: U.S. Army Medical Research and Material Command, Department of the Army (cooperative agreement DAMD17-98-2-8007); Henry M. Jackson Foundation [grants 5D43TW00010 and 2D43TW000010-19 from the Fogarty Foundation, National Institutes of Health (NIH)]; Fogarty AIDS International Training and Research Program, Case Western Reserve University. This study was approved by the National Institute of Allergy and Infectious Diseases, Johns Hopkins Medical Institutions, Western IRB (Olympia, WA), the Scientific and Ethics Committee of the Uganda Virus Research Institute, and the Uganda National Council for Science and Technology institutional review boards. The studies were conducted according to the ethical standards set forth by the institutional review boards of the participating institutions. All participants provided written informed consent.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Subbarao S, Schochetman G: Genetic variability of HIV-1. AIDS 1996;10:S13–S23 [DOI] [PubMed] [Google Scholar]
- 2. Kaleebu P, French N, Mahe C, et al. : Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J Infect Dis 2002;185:1244–1250 [DOI] [PubMed] [Google Scholar]
- 3. Conroy SA, Laeyendecker O, Redd AD, et al. : Changes in the distribution of HIV type 1 subtypes D and A in Rakai District, Uganda between 1994 and 2002. AIDS Res Hum Retroviruses 2010;26:1087–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiwanuka N, Laeyendecker O, Robb M, et al. : Effect of human immunodeficiency virus type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis 2008;197:707–713 [DOI] [PubMed] [Google Scholar]
- 5. Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC: Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001;344:720–725 [DOI] [PubMed] [Google Scholar]
- 6. Eller MA, Opollo MS, Liu M, et al. : HIV type 1 disease progression to AIDS and death in a rural Ugandan cohort is primarily dependent on viral load despite variable subtype and T-cell immune activation levels. J Infect Dis 2015;211:1574–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arroyo MA, Hoelscher M, Sanders-Buell E, et al. : HIV type 1 subtypesamong blood donors in the Mbeya region of southwest Tanzania. AIDS Res Hum Retroviruses 2004;20:895–901 [DOI] [PubMed] [Google Scholar]
- 8. Kaplan EL, Meier P: Nonparametric estimation from incomplete observtions. J Am Stat Assoc 1958;53:457–481 [Google Scholar]
- 9. Cox DR: Regression models and life-tables. J R Stat Soc B 1972;34:187–220 [Google Scholar]
- 10. Assmann SF, Hosmer DW, Lemeshow S, Mundt KA: Confidence intervals for measures of interaction. Epidemiology 1996;7:286–290 [DOI] [PubMed] [Google Scholar]