Abstract
Uterine quiescence during pregnancy is maintained by progesterone primarily via signaling mediated by the type-B progesterone receptor (PR-B) in myometrial cells. Withdrawal of PR-B-mediated progesterone activity is a principal trigger for labor. One mechanism for PR-B withdrawal is by inhibition of its activity by the type-A PR (PR-A) isoform in myometrial cells. We hypothesized that human parturition involves hormonal interactions that induce the capacity for PR-A to inhibit PR-B in myometrial cells and that pro-inflammatory cytokines are major regulators of this process. We tested this hypothesis in an immortalized human myometrial cell line, hTERT-HMA/B, in which levels of PR-A and PR-B can be experimentally controlled. We found that the capacity for PR-A to repress PR-B, assessed by activity of a transiently transfected reporter DNA controlled by the progesterone response element, and expression of FK506 binding protein 5 (FKBP5) an endogenous PR-B responsive gene, was increased by serum supplementation and interleukin-1β. In pregnant uterus, FKBP5 was detected exclusively in myometrial cells and its expression decreased with advancing gestation and in association with the onset of labor at term. These findings suggest that in myometrial cells the repressive activity of PR-A on PR-B increases with advancing gestation and is induced by pro-inflammatory cytokines. This may be a key mechanism linking inflammation with the onset of labor.
Keywords: progesterone receptor, myometrium, PR-A, control of functional progesterone withdrawal
Introduction
Progesterone is essential for the establishment and maintenance of pregnancy and does so primarily by promoting myometrial relaxation and cervical closure.1–3 In all viviparous species studied so far, disruption of progesterone signaling induces parturition, and it is now generally accepted that parturition is triggered by progesterone withdrawal. In most species, the progesterone withdrawal trigger for parturition is caused by a systemic decrease in circulating progesterone levels.3 This does not occur in women; instead, progesterone levels continue to rise throughout gestation and during labor and delivery.4–6 Nonetheless, treatment of pregnant women with nuclear progesterone receptor (PR) antagonists such as mifepristone and onapristone increases uterine contractility, and in most cases, induces the full parturition cascade at all stages of pregnancy.7–9 These observations suggest that human parturition is triggered by a functional, rather than systemic, progesterone withdrawal via changes in PR-mediated progesterone actions in uterine cells. One such mechanism is by modulation of the functional interaction between the 2 nuclear PR isoforms, PR-A and PR-B, leading to a net decrease in progesterone responsiveness.
The human PR isoforms are encoded by a single gene controlled by 2 promoters to produce the full-length PR-B and the truncated (by 164 N-terminal amino acids) PR-A.10,11 Both PRs are transcriptionally active and mediate distinct genomic actions of progesterone in a cell-type and context-specific manner.12–14 Interestingly, in some cells, PR-A inhibits the transcriptional activity of PR-B and progesterone responsiveness decreases as the PR-A:PR-B ratio increases.15–21 This observation led us to propose that increased PR-A abundance and transrepressive activity in uterine cells is a mechanism for the functional progesterone withdrawal trigger for human parturition.21–23 Consistent with this hypothesis, studies of PR abundance assessed by immunoblotting show that the level of PR-A in the human myometrium at term increases in association with the onset of labor at term,21,24 and studies in cultured human myometrial cells show that increased abundance of PR-A relative to PR-B (ie, PR-A:PR-B ratio >1) decreases PR-B transcriptional activity.18,21,23 A key question arising from this hypothesis is how the transrepressive activity of PR-A is controlled in the context of human pregnancy and parturition.
The control of PR-A transrepressive activity in the setting of human pregnancy and parturition is not clearly defined. Recently, we found that the capacity for PR-A to inhibit the transcriptional activity of PR-B in myometrial cells is increased by hormonal factors, especially pro-inflammatory stimuli, and is independent of the PR-A:PR-B ratio.25,26 To further test this hypothesis, we used a human myometrial cell line to examine factors that control PR-A transrepressive activity assessed by expression of an endogenous PR-B-responsive gene, FK506 binding protein 5 (FKBP5), as a readout for PR-B transcriptional activity.
Methods
Myometrium
Women undergoing cesarean section (c-section) delivery at MacDonald Women’s Hospital, University Hospitals Cleveland Medical Center, Cleveland, Ohio were consented (institutional review board approval #11-06-04) to provide a biopsy of full-thickness uterus (1-2 cm3) from upper margin of the lower uterine segment incision. Tissue was excised after delivery of the placenta and was immediately washed in ice-cold phosphate-buffered saline (PBS). Myometrium was carefully isolated from connective tissue, perimetrium, and decidua by microscope-aided dissection, and fragments were snap frozen in liquid nitrogen and stored at −80°C and fixed in 4% paraformaldehyde in PBS.
Tissue was stratified into 3 groups: (1) term not in labor: comprising elective c-sections without complications and with intact membranes, a closed cervix, and a quiescent uterus at term; (2) preterm not in labor: comprising c-sections performed at before the 36th week of gestation for indications such as breech presentations and fetal distress; and (3) term in labor: comprising c-sections preformed at term in women in active labor exhibiting regular and forceful contractions coupled with documented cervical change with effacement and dilation greater than 4 cm.
Cell Culture
An immortalized human myometrial cell line, hTERT-HMA/B, stably transfected with PR-A and PR-B transgenes such that each can be experimentally controlled using independent inducers (doxycycline [DOX] for PR-A and diacylhydrazine [DAH] for PR-B), was used as an in vitro model for the pregnancy myometrium.23,27 Cell cultures were performed at 37°C in a 5% CO2 humidified incubator in DMEM/Ham’s F12 (1:1) supplemented with low (0%-0.5%) or high (5%-10%) levels of charcoal-stripped fetal bovine serum (FBS), 1% penicillin–streptomycin, 0.1 mg/mL geneticin, and 2 mM l-glutamine (Life Technologies, Carlsbad, California).
Transfection
In some experiments, hTERT-HMA/B cells were transiently transfected with a progesterone/PR responsive reporter DNA that expresses luciferase (LUC) under the control of a promoter containing tandem progesterone response elements (PRE-LUC; provided by provided by Dr Zafar Nawaz; University of Miami Sylvester; Braman Family Breast Cancer Institute) and a renilla-LUC (REN-LUC) DNA (Promega, Madison, Wisconsin) at a 10:1 molar ratio. Cells were transfected using the Amaxa Nucleofector system (Lonza, Basel, Switzerland). Briefly, cells collected by trypsinization were resuspended in Nucleofector solution for primary mammalian smooth muscle cells (Lonza, Cat #: VPI-1004) at a concentration of 1 × 106 cells/100 μL containing DNA to be transfected. Nucleofection was performed using the program A033 in the nucleofector device. The cells were then replated and allowed to recover for a minimum of 16 hours.
Luciferase Assay
Lysate was prepared from PRE-LUC transfected hTERT-HMA/B cells, and LUC activity measured with the Dual-Luciferase Reporter Assay (Promega) with luminosity assayed using a GloMax 20/20 Luminometer (Promega). Data were normalized to REN-LUC activity.
RNA and Protein Extraction
Total RNA was isolated utilizing Nucleospin RNA kits (Machery-Nagel, Bathlehem, Pennsylvania). Myometrial tissue was pulverized in liquid nitrogen and the frozen powder suspended in kit lysis buffer and subjected to bead-mill homogenization (Bullet Blender, Next Advanced, Averill Park, New York) and centrifuged (16 000g for 10 minutes at 4°C). hTERT-HMA/B cells were lysed on the plate with kit lysis buffer and centrifuged (16 000g for 10 minutes at 4°C). Supernatant from tissue and cell lysates were passed through Nucleospin RNA binding columns by centrifugation to bind RNA to the silica membrane. DNA was digested on the column, and the silica membrane with bound RNA sequentially washed and the RNA eluted with H2O and quantified by light absorption at 260 nm.
Total cell protein lysates were prepared using the RIPA extraction buffer (Sigma, St Louis, Missouri), supplemented with protease and phosphatase inhibitors (Roche Indianapolis, Indianapolis; final concentrations: 0.5 mmol/L phenylmethylsulfonyl fluoride, 86 μmol/L leupeptin, 77 μg/mL aprotinin, 1.4 μmol/L pepstatin A, and 100 μg/mL bacitracin) on ice. Myometrial tissue was pulverized in liquid nitrogen and the frozen powder resuspended in RIPA extraction buffer, subjected to bead mill homogenization centrifuged (16 000g for 10 minutes at 4°C), and the supernatant collected. hTERT-HMA/B cells were collected by scraping, lysed in RIPA buffer, centrifuged (16 000g for 10 minutes at 4°C), and the supernatant collected. Protein concentration was assessed by the bicinchoninic acid method (Thermo Scientific, Rockford, Illinois).
RNA Analysis by Quantitative Real-Time Polymerase Chain Reaction
Total RNA (300-600 ng) was reverse transcribed with random primers using Superscript II reverse transcriptase (Life Technologies). Paired oligonucleotide primers were designed using the Primer Express software (Applied Biosystems, Foster City, California) based on published sequences. Polymerase chain reaction primers for FKBP5 (Fwd: ATGCCATTTACTGTGCAAACCAG; Rev: AAGAGAGTTGCATTCGAGGGAA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Fwd: TTGCCATCAATGACCCCTTCA; Rev: CGCCCCACTTGATTTTGGA) were used. Assays were optimized and validated as previously described23 by confirming that single amplicons of appropriate size and sequence were generated and that the priming and amplification efficiencies of all primer pairs were identical. Polymerase chain reaction was performed in the presence of SYBR Green (Applied Biosystems) in an ABI PRISM 7500 Sequence Detector (Applied Biosystems). The cycling conditions were 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. The cycle at which the fluorescence reached a preset threshold (cycle threshold: CT) was used for quantitative analyses. The threshold in each assay was set at a level where the rate of exponential increase in amplicon abundance was approximately parallel between all samples. Messenger RNA (mRNA) abundance data were expressed relative to the abundance of the constitutively expressed GAPDH mRNA using the ΔCT method (ie, relative mRNA abundance = 2−(CT FKBP5 − CT GAPDH)].
Immunoblotting
Lysates containing equal amounts of protein were diluted in gel loading buffer (375 mM Tris-HCl, 6% sodium dodecyl sulfate [SDS], 48% glycerol, 9% β-mercaptoethanol, and 0.03% bromophenol blue, pH 6.8), heated for 5 minutes at 100°C, and subjected to denaturing SDS polyacrylamide gel electrophoresis on precast 4% to 20% tris-glycine polyacrylamide gels with the Novex electrophoresis system (Life Technologies). Proteins were then transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, Massachusetts). For imumunodetection, membranes were first incubated in blocking buffer (5% nonfat milk in tris-buffered saline [TBS] containing 0.1% tween-20 [TBST]) at room temperature for 1 hour and then with primary antibodies (PR-A/B: Dako, catalog number M3568, 1:750; FKBP5: Cell Signaling Technology, catalog number 12210, 1:1000; GAPDH: Santa Cruz Biotechnology, catalog number sc-32233, 1:100 000) overnight at 4°C. The following day, membranes were washed 3 times with TBST and incubated at room temperature for 1 hour with horseradish peroxidase-conjugated anti-mouse IgG (Cell Signaling Technology, catalog number 7076, 1:3000) or anti-rabbit IgG (Cell Signaling Technology, catalog number 7074, 1:3000) antibodies. Immunoreactive proteins were visualized using the HyGlo Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific, South Plainfield, New Jersey). Chemiluminescence was quantified with the FluorChem E processor (ProteinSimple, San Jose, California).
Immunohistochemistry
Immunocytochemistry (IHC) was performed using the Millipore IHC select kit (Millipore immunoperoxidase secondary detection system cat. #DAB150) on formalin-fixed paraffin-embedded sections (5 μm) of term myometrium. Tissue sections were deparaffinized and rehydrated in graded ethanol and then subjected to antigen unmasking by incubation in 10 mM sodium citrate buffer pH 6.0 for 10 minutes at 100°C. After cooling to room temperature, sections were washed in TBS and incubated in blocking solution (5% BSA in TBST) for 1 hour. Tissue sections were then incubated with primary antibody (FKBP5: Cell Signaling Technology, catalog number 12210) diluted 1:200 in blocking solution (Signal Stain Antibody Diluent: Cell Signaling catalog number 8112) or the equivalent amount of nonimmune IgG overnight at 4°C in a humidified chamber. The next day, the sections were washed 3 × 5 minutes in TBS and incubated with biotin-conjugated secondary antibody for 30 minutes at room temperature, washed in TBS, incubated with streptavidin horseradish peroxidase solution for 30 minutes, washed in TBS, and incubated in 3,3′-diaminobenzidine (DAB) for 10 minutes. Sections were then washed in TBS, mounted, and examined by light microscopy for immunoreactive signal indicated by brown DAB staining.
Statistical Analyses
All experiments were performed in triplicate. Data were assessed for normalcy using the Kolmogorov-Smirnov test, and all normal data were analyzed with Student t test or analysis of variance for multiple comparisons. Differences were considered statistically significant with a P < .05.
Results
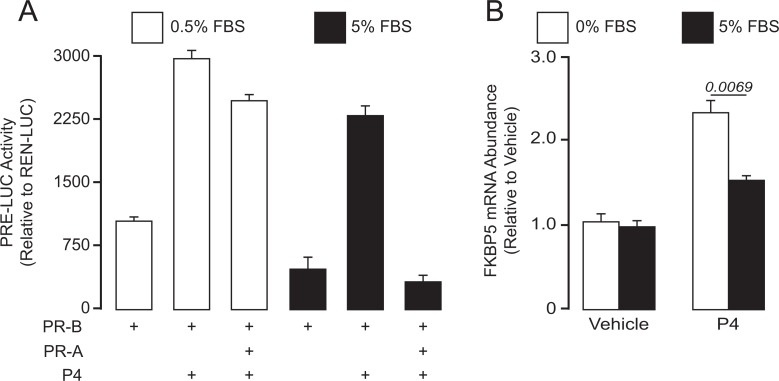
The effect of FBS on the transrepressive activity of PR-A was examined in hTERT-HMA/B cells induced to express PR-B alone or PR-B and PR-A (by exposure to DAH and/or DOX) and cultured in media containing 5.0% FBS or 0.5% FBS for 24 hours with and without progesterone. Progesterone receptor transcriptional activity was measured by the expression of the transiently transfected PRE-LUC reporter (Figure 1A). In cells expressing only PR-B, progesterone increased PRE-LUC activity under both FBS conditions. As expected, increased expression of PR-A repressed PR-B-mediated transcriptional activity. However, PR-A transrepression of PR-B was more prominent in cells cultured in 5.0% FBS compared to cells cultured in 0.5% FBS. Parallel experiments were performed to assess the effects of FBS on the transcriptional activity of FKPB5, an endogenous progesterone/PR-B-responsive gene. We previously showed that FKBP5 expression is stimulated by progesterone via PR-B but not by PR-A in hTERT-HMA/B cells.23,26 In cells conditioned to express PR-A and PR-B, exposure to progesterone for 6 hours (sufficient time to detect changes in expression of genes directly affected by progesterone/PR23) increased abundance of mRNA-encoding FKBP5. However, the magnitude of the increase was significantly decreased by the addition of 5% FBS (compared to serum-free media) to the culture media (Figure 1B). In low serum, PR-A had no effect on progesterone/PR-B-induced FKBP5 expression,23 and serum-free media was essentially identical to media containing 0.5% FBS (data not shown). The data suggest that PR-A transrepression of PR-B is increased by FBS-derived factors.
Figure 1.
Regulation of PR-A/B-mediated progesterone responsiveness in human myometrial cells by serum factors. A, Effect of FBS (5.0% or 0.5%) on progesterone (P4)-induced PRE-LUC activity in hTERT-HMA/B cells conditioned to express PR-A (with DOX) and/or PR-B (with DAH). Cell were transiently transfected with PRE-LUC expression DNA and condition to express PR-A, PR-B, or both overnight and then exposed to progesterone for 24 hours in various FBS media levels. Fetal bovine serum increased the capacity for PR-A to transrepress the activity of PR-B. B, Effect of FBS on progesterone (P4)-induced expression of FKBP5 in hTERT-HMA/B cells. Cells were conditioned to express PR-A and PR-B overnight in media containing 5% FBS. The following morning, the cells were washed and cultured in 5% FBS or serum-free (0% FBS) media containing progesterone (P4; 100 nM) or vehicle (ethanol) for 6 hours. The capacity for progesterone to increase FKBP5 mRNA abundance in cells expressing PR-A and PR-B was significantly decreased by FBS. All data are mean ± standard error of the mean (SE) (n = 3). FBS, fetal bovine serum; FKBP5, FK506 binding protein; mRNA, messenger RNA; PRE-LUC, progesterone response element–luciferase.
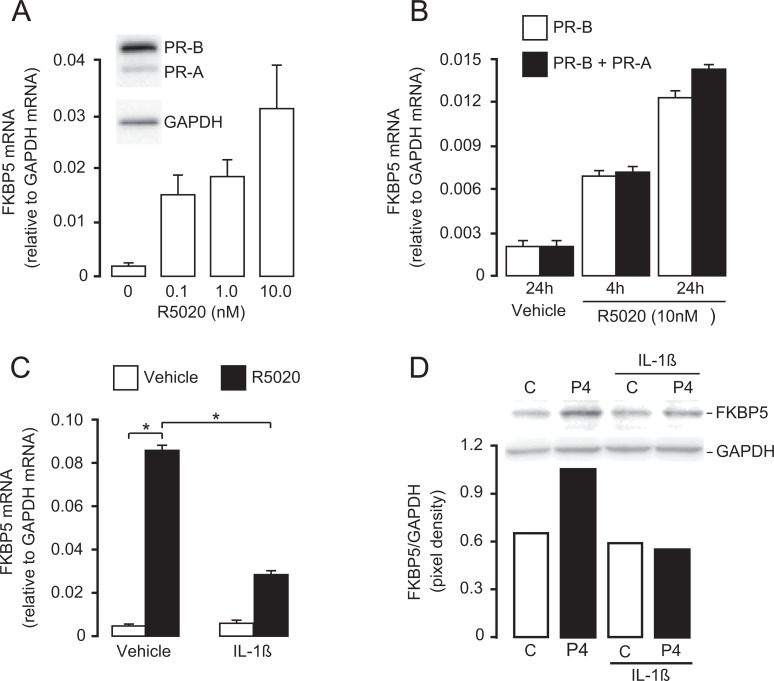
As human parturition is associated with tissue-level inflammation within the uterine compartments (myometrium, decidua, and cervix),28 we hypothesize that inflammatory cytokines induce PR-A-mediated functional progesterone withdrawal.26,29 This hypothesis predicts that inflammatory stimuli increase the transrepressive activity of PR-A. To test this hypothesis, hTERT-HMA/B cells were induced to express PR-A and PR-B and then exposed to R5020, a PR agonist, for 24 hours in media containing 0.5% FBS. This exposure time was chosen to mimic the long-term exposure to progesterone in vivo during pregnancy. As expected, R5020 increased FKBP5 expression in a dose- (Figure 2A) and time- (Figure 2B) dependent manner (reaching a maximum at 24 hours). This effect was mediated by PR-B and not by PR-A.23 To mimic an inflammatory challenge during pregnancy (eg, intrauterine infection), some cultures were exposed to interleukin (IL)-1β or vehicle for the final 4 hours of the 24-hour period. Interleukin-1β significantly decreased R5020-induced abundance of FKBP5 mRNA only in cells coexpressing PR-A and PR-B. Interleukin-1β had no effect on basal- (ie, no R5020) or R5020-induced abundance of FKBP5 mRNA in cells expressing only PR-B.26 In parallel studies, hTERT-HMA/B cells were induced to express equivalent levels of PR-A and PR-B (verified by immunoblotting) and treated with progesterone or vehicle with and without IL-1β for 24 hours. Interleukin-1β decreased progesterone-induced FKBP5 protein levels (Figure 2D). These data support the hypothesis that inflammatory stimuli inhibit responsiveness of myometrial cells to progesterone and that this is likely mediated by increased PR-A transrepression of PR-B.
Figure 2.
Effect of progestins (R5020 or progesterone [P4]) acting via PR-A and PR-B on FKBP5 expression in hTERT-HMA/B cells, and how this is modulated by IL-1β. A, Dose–response effect of R5020 on FKBP5 expression (assessed by mRNA abundance) in hTERT-HMA/B cell condition to express PR-A and PR-B (representative PR-A/B immunoblot shown) and then exposed to various amounts of R5020 for 16 hours. B, Time-course effect of R5020 (10 nM) on FKBP5 mRNA abundance in cells conditioned to express only PR-B or PR-A and PR-B. C, Effect of IL-1β on R5020-induced FKBP5 expression (assessed by mRNA abundance) in hTERT-HMA/B cells conditioned to express PR-A and PR-B. Cells expressing both PRs were exposed to R5020 (10 nM) or vehicle (ethanol) for 24 hours. Some cells were exposed to IL-1β (1 ng/mL) or vehicle (PBS) for the final 4 hours. Interleukin-1β decreased R5020-induced FKBP5 expression in cells coexpressing PR-A and PR-B. D, Effect of IL-1β on P4-induced FKBP5 protein in hTERT-HMA/B cells expressing PR-A and PR-B. Cells expressing PR-A and PR-B were exposed to P4 and IL-1β for 24 hours. Representative immunoblot and densitometry data are shown. All data are mean ± standard error of the mean (SE) (n = 3). *P < .05; Student t test. FKBP5 indicates FK506 binding protein; IL-1β, interleukin-1β; mRNA, messenger RNA; PBS, phosphate-buffered saline; PR, progesterone receptor.
Immunoreactive FKBP5 was detected in the cytoplasm and nucleus of myometrial cells in paraffin sections of term lower-segment uterus (Figure 3A). Abundance of FKBP5 protein assessed by immunoblot analysis in term lower-segment uterus decreased significantly in association with the onset of labor (Figure 3B). Similarly, abundance of FKBP5 mRNA in lower-segment uterus decreased with advancing gestation and with the onset of labor at term (Figure 3C).
Figure 3.
FKBP5 expression in the human pregnancy myometrium. A, Immunostaining with anti-FKBP5 and nonimmune IgG in term myometrium. FKBP5 immunoreactive staining was mainly in the cytoplasm of myometrial cells (examples are indicated by arrows). Bar = 18 μm. B, Immunoblot analysis of FKBP5 abundance in term myometrium before and after active labor. Relative abundance of FKBP5 was lower in laboring compared with nonlaboring term myometrium. C, Abundance of mRNA encoding FKBP5 in preterm and term myometrium (± labor). FKBP5 mRNA abundance decreased with advancing gestation and the onset of labor. P values <.05 determined by ANOVA are shown. ANOVA indicates analysis of variance; BV, blood vessel; FKBP5, FK506 binding protein; mRNA, messenger RNA.
Discussion
The PR-A/PR-B hypothesis for functional progesterone withdrawal posits that human parturition is, at least in part, triggered by functional progesterone withdrawal caused by increased transrepressive activity of PR-A in uterine cells (especially myometrial cells).22 Data from in vivo and in vitro studies indirectly support this hypothesis.18,21,22,24 A logical prediction of the PR-A/PR-B hypothesis is that PR-A transrepressive activity is controlled as part of the hormonal cascade initiating human parturition. The present study addressed factors that affect PR-A transrepressive activity in a human myometrial cells.
We found that in hTER-HMA/B cells PR-A transrepressive activity was dependent on FBS supplementation in the culture media (Figure 1). This effect was detected by PRE-LUC activity and by expression of an endogenous PR-B-responsive gene, FKBP5 (Figure 1B). FKBP5 expression in hTERT-HMA/B cells is increased by progesterone via PR-B but not via PR-A,23 and therefore, its level of expression reflects progesterone/PR-B transcriptional activity. In cells conditioned to produce equivalent levels of PR-A and PR-B (PR-A:PR-B ∼1), progesterone-induced FKBP5 expression was significantly decreased in 5% FBS compared to serum-free conditions (Figure 1B). Thus, FBS supplementation appears to increase PR-A transrepressive activity in hTERT-HMA/B cells or alternatively decrease PR-B-mediated induction of FKBP5 expression. Taken together, the data support the hypothesis that net PR-A/B-mediated transcriptional activity is affected by the hormonal milieu and that this may reflect the hormonal control of PR-A transrepressive activity.
Studies on breast cancer cells showed that PR-A transrepressive activity is affected by posttranslational modifications, especially phosphorylation and SUMOylation.30 Site-specific serine phosphorylation of PRs is ligand dependent and induced by mitogen-activated protein kinases (MAPKs),31–33 many of which are activated in cultured cells by FBS. Thus, it is possible that FBS supplementation activated specific MAPK-directed posttranslational modification(s) of PR-A that increased its transrepressive activity. This hypothesis is supported by our recent finding that phosphorylation of PR-A at serine-344/345 is required for PR-A to inhibit the anti-inflammatory activity of PR-B in hTERT-HMA/B cells.25 Thus, the hormonal control of PR-A transrepressive activity in myometrial cells may involve the activation of specific protein kinases, which phosphorylate PR-A leading to activation of its transrepressive activity.
Human parturition is an inflammatory process in which the uterine tissues (myometrium, decidua, and cervix) exhibit a sterile tissue-level inflammatory phenotype.28,34 We found that exposure of hTERT-HMA/B cells to IL-1β, a potent pro-inflammatory stimulus, increased the capacity for PR-A to transrepress progesterone/PR-B-induced FKBP5 expression (Figure 2). We previously reported that prostaglandin-F2α increased expression of PR-A but not PR-B (estimated by quantitative real-time polymerase chain reaction) in PHM1-31 human myometrial cells,29 and recently, we reported that pro-inflammatory stimuli, including IL-1β, increase the abundance of PR-A in hTER-HMA/B cells by augmenting its stability.26 Taken together, the data suggest that pro-inflammatory stimuli induce parturition by increasing the transrepressive activity of PR-A in myometrial cells. Importantly, this could be the mechanism for inflammation-induced parturition and explain the strong correlation between intrauterine infection and preterm birth.
Based on our in vitro data with hTERT-HMA/B cells, we reasoned that FKBP5 expression could be used as a marker for PR-B transcriptional activity in pregnancy myometrium. Using immunohistochemistry, we found that FKBP5 protein localized to myometrial cells (Figure 3A). Interestingly, FKBP5 expression in lower segment uterus decreased with advancing gestation and the onset of active labor at term (Figure 3B and C). Those data suggest (assuming that expression of FKBP5 is exclusively controlled by progesterone/PR-B) that PR-B transcriptional activity in the myometrium is lower in term tissue compared with preterm tissue and is decreased further in association with the onset of labor. We propose that the decrease in PR-B transcriptional activity may be due to increased transrepressive activity of PR-A with advancing gestation and the onset of labor, possibly in response to accumulating pro-inflammatory stimuli. Our FKBP5 mRNA data suggest that PR-A-mediated functional progesterone withdrawal occurs gradually during the prelude to parturition. At the biochemical level, this is likely since transformation of the myometrium from the quiescent to the laboring phenotype is thought to require changes in the expression of specific genes in response to progesterone withdrawal. Thus, progesterone withdrawal, and specifically decreased PR-B activity due to PR-A transrepression, would be expected to precede the onset of active labor.
The key concept supported by this study is that PR-A-mediated functional progesterone withdrawal in the human pregnancy myometrium is a hormonally controlled process that is induced by pro-inflammatory stimuli. Our working model is that throughout pregnancy the uterine tissues are exposed to an “inflammatory load” derived from multiple pro-labor/pro-inflammatory stimuli (eg, uterine wall distention, fetal membrane senescence, and intrauterine infection) and that it increases with advancing gestation. We propose that a threshold exists above which inflammatory load induces sufficient PR-A-mediated functional progesterone withdrawal to trigger the parturition cascade and that this is a key functional interaction linking inflammation with the onset of labor. Further studies to elucidate the molecular mechanisms for this effect may reveal therapeutic targets to prevent preterm birth.
Footnotes
Authors’ Note: Bansari Patel and Gregory A. Peters contributed equally to the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding to SM from the March of Dimes Prematurity Center, Ohio Collaborative and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (HD069819).
References
- 1. Csapo A. Progesterone block. Am J Anat. 1956;98(2):273–291. [DOI] [PubMed] [Google Scholar]
- 2. Csapo A. The “seesaw” theory of the regulatory mechanism of pregnancy. Am J Obstet Gynecol. 1975;121(4):578–581. [PubMed] [Google Scholar]
- 3. Young IR, Renfree MB, Mesiano S, et al. The comparative physiology of parturition in mammals: hormones and parturition in mammals In: Norris D, Lopez K, eds. Hormones and Reproduction in Vertebrates. London, United Kingdom: Academic Press; 2010. [Google Scholar]
- 4. Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112(8):1095–1100. [DOI] [PubMed] [Google Scholar]
- 5. Boroditsky RS, Reyes FI, Winter JS, Faiman C. Maternal serum estrogen and progesterone concentrations preceding normal labor. Obstet Gynecol. 1978;51(6):686–691. [PubMed] [Google Scholar]
- 6. Walsh SW, Stanczyk FZ, Novy MJ. Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab. 1984;58(4):629–639. [DOI] [PubMed] [Google Scholar]
- 7. Frydman R, Fernandez H, Pons JC, Ulmann A. Mifepristone (RU486) and therapeutic late pregnancy termination: a double-blind study of two different doses. Hum Reprod. 1988;3(6):803–806. [DOI] [PubMed] [Google Scholar]
- 8. Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. Mifepristone (RU486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril. 1991;56(3):385–393. [DOI] [PubMed] [Google Scholar]
- 9. Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. [DOI] [PubMed] [Google Scholar]
- 10. Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9(5):1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wen DX, Xu YF, Mais DE, Goldman ME, McDonnell DP. The A and B isoforms of the human progesterone receptor operate through distinct signaling pathways within target cells. Mol Cell Biol. 1994;14(12):8356–8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. [DOI] [PubMed] [Google Scholar]
- 13. Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68(10-13):771–778. [DOI] [PubMed] [Google Scholar]
- 14. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100(17):9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993;7(10):1256–1265. [DOI] [PubMed] [Google Scholar]
- 16. Vegeto E, Shahbaz MM, Wen DX, et al. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7(10):1244–1255. [DOI] [PubMed] [Google Scholar]
- 17. Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20(9):3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interaction between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7(9):875–879. [DOI] [PubMed] [Google Scholar]
- 19. Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20(4):764–775. [DOI] [PubMed] [Google Scholar]
- 20. Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20(11):2724–2733. [DOI] [PubMed] [Google Scholar]
- 21. Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92(5):1927–1933. [DOI] [PubMed] [Google Scholar]
- 22. Mesiano S, Chan EC, Fitter JT, et al. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87(6):2924–2930. [DOI] [PubMed] [Google Scholar]
- 23. Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97(5):E719–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ke W, Chen C, Luo H, et al. Histone deacetylase 1 regulates the expression of progesterone receptor A during human parturition by occupying the progesterone receptor A promoter. Reprod Sci. 2016;23(7):955–964. [DOI] [PubMed] [Google Scholar]
- 25. Amini P, Michniuk D, Kuo K, et al. Human parturition involves progesterone receptor-A phosphorylation at serine-345 in myometrial cells. Endocrinology. 2016;157(11):4434–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peters GA, Yi L, Skomorovska-Prokvolit Y, et al. Inflammatory stimuli increase progesterone receptor-A stability and transrepressive activity in myometrial cells. Endocrinology. 2016;158(1):158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Condon J, Yin S, Mayhew B, et al. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67(2):506–514. [DOI] [PubMed] [Google Scholar]
- 28. Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236. [PubMed] [Google Scholar]
- 29. Madsen G, Zakar T, Ku CY, et al. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab. 2004;89(2):1010–1013. [DOI] [PubMed] [Google Scholar]
- 30. Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277(37):33950–33956. [DOI] [PubMed] [Google Scholar]
- 31. Hagan CR, Daniel AR, Dressing GE, Lange CA. Role of phosphorylation in progesterone receptor signaling and specificity. Mol Cell Endocrinol. 2012;357(1-2):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abdel-Hafiz HA, Horwitz KB. Post-translational modifications of the progesterone receptors. J Steroid Biochem Mol Biol. 2014;140:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hagan CR, Lange CA. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med. 2014;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]