Abstract
Background:
Many parallels exist between growth and development of the placenta and that of cancer. One parallel is shared expression of antigens that may have functional importance and may be recognized by the immune system. Here, we characterize expression and regulation of one such antigen, Trophoblast glycoprotein (TPGB; also called 5T4), in the placenta across gestation, in placentas of preeclamptic (PE) pregnancies, and in purified microvesicles and exosomes.
Methods:
Trophoblast glycoprotein expression was analyzed by real-time reverse transcription-polymerase chain reaction (RT-PCR), Western blot, and immunohistochemistry. Regulation of 5T4 in cytotrophoblast cells was examined under either differentiating conditions of epidermal growth factor or under varying oxygen conditions. Microvesicles and exosomes were purified from supernatant of cultured and perfused placentas.
Results:
Trophoblast glycoprotein expression was prominent at the microvillus surface of syncytiotrophoblast and on the extravillous trophoblast cells, with minimal expression in undifferentiated cytotrophoblasts and normal tissues. Trophoblast glycoprotein expression was elevated in malignant tumors. In cytotrophoblasts, 5T4 was induced by in vitro differentiation, and its messenger RNA (mRNA) was increased under conditions of low oxygen. PE placentas expressed higher 5T4 mRNA than matched control placentas. Trophoblast glycoprotein was prominent within shed placental microvesicles and exosomes.
Conclusion:
Given the potential functional and known immunological importance of 5T4 in cancer, these studies reveal a class of proteins that may influence placental development and/or sensitize the maternal immune system. In extravillous trophoblasts, 5T4 may function in epithelial-to-mesenchymal transition during placentation. The role of syncytiotrophoblast 5T4 is unknown, but its abundance in shed syncytial vesicles may signify route of sensitization of the maternal immune system.
Keywords: placenta, trophoblast, cancer, 5T4, exosomes, microvesicles
Introduction
The placenta is a transient organ that supports the growth and development of the fetus in pregnancy. In humans, the development of the placenta is characterized by the extensive proliferation of cytotrophoblast cells coupled with their differentiation into several subpopulations, each with distinct anatomical location and highly specialized function. The syncytiotrophoblast, a multinucleate cell type that arises from fusion of single cytotrophoblast cells, lines the outermost aspect of the chorionic villi, which develop as a result of branching morphogenesis. Ultimately, the syncytiotrophoblast interfaces directly with maternal blood and therefore defines the nutritional, endocrinological, and immunological interface between the mother and the fetus. Concurrently, extravillous trophoblast cells exit the placenta and invade the uterine wall up to the inner third of the myometrium.1 Some extravillous trophoblast cells penetrate the walls of the maternal spiral arteries that traverse the decidua, leading to their replacement of maternal vascular endothelial cells. This, together with the movement of smooth muscle cells away from vessel walls and the breakdown of associated extracellular matrix, results in remodeling of the spiral arteries from a narrow, vasoreactive vessel, to a wider, low-pressure conduit for maternal blood that supplies the placenta and fetus with nutrient and oxygen-rich maternal blood.2–4
The placenta has been called a “pseudomalignant tissue” because of its multiple parallels with metastatic tumor cells. Both tissue types have a high propensity for proliferation and invasion and utilize overlapping pathways for migration and invasion. It has been postulated that tumor cells revert to primitive developmental processes representative of trophoblast cells by commandeering genes used by these cells to acquire metastatic potential.5,6 Prototypical epithelial-to-mesenchymal transition (EMT) occurs during early implantation/placentation and as tumor cells break away from the primary tumor and invade secondary organs. In each case, cells lose polarity, downregulate the expression of E-cadherin, and gain migratory function.7 During decidual invasion by trophoblast cells, changes in expression of integrins and matrix metalloproteases, which link cells to extracellular matrix and degrade extracellular matrix, respectively, can be mirrored by cancer cells.5 Furthermore, these changes appear to be regulated by similar transcription factors, signaling pathways, and epigenetic events.7,8 Finally, modulation of the immunological micro- and macroenvironment is hallmark of both placentation and cancer development.9 Together, these lines of evidence suggest that comparable biochemical mediators, signaling events, and functions are present in some cancers and the normal trophoblast. Notably, the strict temporal and spatial regulation that occurs to prevent malignant transformation of trophoblast cells is not mirrored in cancer cells.
In addition to these functional overlaps in cell processes, a number of proteins, many with unknown functions, are expressed in the feto-placental unit and reexpressed in a variety of cancers. Many of these “oncofetal antigens” are unexpressed or have only limited expression in normal, nonmalignant tissues. Examples include Carcinoembryonic Antigen 1 (CEACAM1), PLAC1, osteopontin, and several cancer-testes antigens including NY-ESO-1, MAGE-A3, and MAGE-A4.10,11 Some oncofetal antigens may naturally elicit immunity; in other cases, targeted antigenic responses can be elicited when administered together with autologous antigen presenting cells or adjuvant.12
Trophoblast glycoprotein is prominently expressed in the placenta and various cancers but appears to be rarely expressed in other tissues. It was first identified using a mouse monoclonal antibody raised against isolated glycoproteins from the microvillus membranes of human placental syncytiotrophoblast.13 Trophoblast glycoprotein is a 72 kDa transmembrane molecule with a short cytoplasmic region and N-glycosylated extracellular domain containing multiple leucine-rich repeat regions.14,15 Leucine-rich repeat regions are conserved 22 to 26 amino acid motifs that mediate protein–protein interactions, such as those associated with cellular adhesion and attachment to the extracellular matrix.16 In line with this, 5T4 expression has been shown to influence epithelial–mesenchymal transition, cytoskeletal organization, and motility, possibly through modulation of Wnt signaling.17–19 Finally, the restricted expression of 5T4 in cancer cells has raised interest in using this protein as a diagnostic marker and/or a molecular target of anticancer vaccines.20
Together, the antigenicity of 5T4 and its potential role in EMT raises interest in this protein in the context of placental biology. First, the placenta expresses a number of paternally inherited antigens that can elicit maternal immunity, but whether placenta-specific proteins have the potential to prime maternal lymphocytes is unknown. Second, novel modulators of trophoblast invasion may add insight into placental development as well as diseases of pregnancy that result from placental malinvasion and lead to heightened maternal inflammation. In this report, we begin investigations into the expression and regulation of 5T4 in the placentas of normal and preeclamptic (PE) pregnancies, as well as its presence in trophoblastic vesicular material shed from the placenta.
Materials and Methods
Tissue Collection
All tissues were collected in accordance with protocols approved by the human subjects committee at the University of Kansas Medical Center, the Mount Sinai Hospital, Sparrow Hospital, Michigan State University, and University of Oxford. Term placentas (n = 16) with no associated pathologies of pregnancy were collected after Cesarean delivery, and first- (gestation age 7 to 9 weeks; n = 4) and second-trimester (gestation age 13 and 16 weeks; n = 2) placentas were collected following elective termination of pregnancy. Additional first-trimester tissues (gestation age 4 to 5 weeks), as well as placentas from gestational age- and delivery-matched healthy and PE pregnancies (n = 10 each) were procured from the Research Center for Women’s and Infant’s Health BioBank at the Samuel Lunenfeld Research Institute (Mount Sinai Hospital, Toronto, Ontario, Canada); these samples were used for comparison of 5T4 messenger RNA (mRNA) and protein expression in whole placental tissue. Mode of delivery-matched normal (n = 5) and PE (n = 3) placentas was also collected from the Women’s Centre, John Radcliffe Hospital (Oxford, United Kingdom); these samples were used for analysis of microvesicles and exosomes from placental perfusate. For extraction of RNA and protein, villous placental tissues were snap-frozen in liquid nitrogen and stored at −80°C until used.
Trophoblast Isolation, Purification, and Culture
Term trophoblast cells were isolated and cultured as previously described.21 In brief, the basal plate was removed from the maternal side of the placenta and 40 to 80 g of villous tissue was dissected, rinsed with physiologic saline, and minced. The tissue was dissociated using a mixture of trypsin (Invitrogen, Carlsbad, California) and DNase (Sigma, St Louis, Missouri) and the resulting cell suspension was layered over a 5% to 70% Percoll (GE Healthcare, Pittsburgh, Pennsylvania) gradient followed by centrifugation. Enriched trophoblast cells were depleted of human leukocyte antigen (HLA) class I positive cells by consecutive labeling with anti-mouse immunoglobulin G (IgG) and W6/32 antibodies (American Type Culture Collection, Manassas, Virginia) coupled to magnetic microsphere beads (Miltenyi Biotec, Auburn, California). Viability was determined by trypan blue dye exclusion and purity was evaluated by labeling trophoblast cells with cytokeratin-7 antibody (DAKO, Carpinteria, California) followed by flow cytometry. For trophoblast cell culture, 2 × 105 cells/cm2 were cultured in medium containing Iscove’s Modified Dulbecco’s Medium, 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B, and 2 mM L-glutamine. To study the influence of epidermal growth factor (EGF; Peprotech, Rocky Hill, New Jersey) on 5T4 expression, 5 ng/mL of EGF was added to medium at 24 hours and cultured further for 24 hours or 48 hours. To observe the effects of hypoxia on cytotrophoblast cells, 70% to 80% confluent cells were placed in a Whitley H35 hypoxystation low oxygen incubator 2% O2 and 5% CO2 at 37°C (Hypoxygen, Frederick, Maryland). All culture media were preequilibrated to the appropriate oxygen concentration before adding cells.
To assess biochemical and morphological differentiation of trophoblast cells in culture, cells were seeded into glass chamber slides and treated with medium alone or EGF for 24 or 48 hours. Human chorionic gonadotropin-β (CGB) was measured in the culture supernatant using a commercially available ELISA kit (DRG International, Inc; Mountainside, New Jersey) according to the manufacturer’s instructions. To determine trophoblast fusion, desmoplakin immunoreactivity was determined by immunofluorescence as previously described (clone ZK-31, 1:400 dilution; Sigma, St Louis, Missouri).22 Digital images were captured at random locations within each well on a Nikon Eclipse Ti-U microscope, and a minimum of 100 nuclei were manually counted, and data are expressed as a percentage of cells containing 2 or more nuclei.
Cell Line and Explant Cultures
Cell lines and explants were cultured at 37°C under 5% CO2. BeWo and JEG3 cell lines were obtained from American Type Culture Collection (Rockville, Missouri). The first-trimester human cytotrophoblast cell lines, HTR-8/Svneo, and Swan 71 were generously donated by Dr Charles Graham from Queens University and Dr Gil Mor from Yale University, respectively.23,24 JEG3 and HTR-8/SVneo cells were cultured in RPMI-1640 medium and Swan 71 and BeWo cells were cultured in DMEM/F-12 medium supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. First-trimester and term explants were cultured in 12-well plates on Netwell inserts with 74 μm pores (Corning, Tewksbury, Massachusetts). First-trimester explants were cultured for 24 hours in DMEM/F-12 medium containing 10% FBS, 5 ng/mL EGF, 5 μg/mL insulin, 10 μg/mL transferrin, 20 nM sodium selenite, 400 U/L human chorionic gonadotropin, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B. The term placental tissue explants were cultured in RPMI-1640 medium containing 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B.
Microvesicle/Exosome Isolation
Explants from first trimester and term placentas were cultured in exosome-free medium containing additives as described above. Following culture, exosomes were isolated using previously described methods.25,26 Supernatants were cleared by sequential centrifugation at 300g, 2000g, and 10 000g for 10, 20, and 30 minutes, respectively. Following each centrifugation, pellets containing dead cells and debris were discarded. Supernatant was then ultracentrifuged at 100 000g for 1.5 hours at 4°C to pellet small vesicles, which includes exosomes. The pellets were washed in a large volume of phosphate buffered saline (PBS) to remove contaminating proteins and centrifuged at the same high speed for an additional 1.5 hours. Pellets were resuspended in PBS and protein concentrations were determined using the detergent compatible (DC) protein assay (Bio-Rad Laboratories, Hercules, California) according to the manufacturer’s instructions.
For density gradient analysis of exosomes, pellets were dissolved in 100 μL PBS and loaded onto a continuous sucrose gradient (2.0 to 0.25 M in 20 mM HEPES, pH 7.4), which was prepared using a Hoefer SG30 gradient maker (GE Healthcare). The gradients were ultracentrifuged at 210 000g for 15 hours at 4°C using a SW41Ti swinging bucket rotor in an Optima-Max ultracentrifuge. Eleven 1-mL fractions were collected from the top of the gradients, and densities of each fraction were measured using a refractometer (VeeGee Scientific, Kirkland, Washington). Each fraction was washed with 20 mM HEPES in PBS and ultracentrifuged at 110 000g for 1.5 hours using a TLA-100.4 rotor. The pellets were resuspended in PBS and used for further analysis on a NanoSight LM10 nanoparticle tracking analyzer (Malvern, Worcestershire, United Kingdom) and Western blot. The NanoSight and associated software provides the direct and real-time visualization and analysis of nanoparticles between 10 nm and 1 μm in liquids.27 Syncytiotrophoblast-derived microvesicle and exosome (10 000g and 150 000g, respectively) fractions were isolated from dual placental perfusion maternal side perfusate, as described recently by Dragovic et al28
RNA Extraction and Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
For extraction of RNA and protein, villous placental tissues were snap-frozen in liquid nitrogen and stored at −80°C until used. Total RNA was extracted from placenta using Trizol reagent (Invitrogen, Carlsbad, California). Complementary DNA (cDNAs) were synthesized from 1 μg of total RNA using SuperScript II reverse transcriptase (Invitrogen). The qRT-PCR was performed to quantify mRNA levels of TPBG and 18S rRNA. Primers were designed using Primer Express 2.0 (Applied Biosystems/Life Technologies, Grand Island, New York), sequences are as follows: 5T4-forward: GGAAGTCGTCGCTACTCTGG; 5T4-Reverse: CACCTCTTCGCCTCTTGTTG; 18S-forward: GCAATTATTCCCCATGAACG; 18S reverse-GGCCTCACTAAACCATCCA. Real-time PCR amplification of cDNAs was carried out in a 10 μL reaction mixture containing SYBR GREEN PCR Master Mix (Applied Biosystems) and primers (600 nM each). Amplification and fluorescence detection were carried out using the ABI Prism 7500 Real-Time PCR System (Applied Biosystems). Cycling conditions included an initial hold step (95°C for 10 minutes) and 40 cycles of a 2-step PCR (92°C for 15 seconds, then 60°C for 1 minute), followed by a dissociation step (95°C for 15 seconds, 60°C for 15 seconds, and then 95°C for 15 seconds). The comparative cycle threshold (CT) method was used for relative quantification of the amount of mRNA for each sample normalized to 18S RNA.
Western Blot Analysis
Protein from cultured cells or snap-frozen tissues was collected by lysis in RIPA buffer containing protease inhibitors (aprotinin, 10 μg/mL; leupeptin, 10 μg/mL; PMSF, 100 μg/mL). Protein concentrations were determined for each sample using the DC protein assay (Bio-Rad). Proteins (10 μg) from total cell lysate or microvesicle/exosome preparations were electrophoresed under denaturing or nondenaturing conditions and transferred to a nitrocellulose membrane (Optitran BA-S 83, Whatman GmbH, Germany). The membranes were blocked in 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 2 hours at room temperature and probed overnight at 4°C with gentle rocking with the following antibodies: sheep anti-human 5T4 (AF4975; R & D Systems, Minneapolis, Minnesota), mouse anti-human CD63 (sc-5275; Santa Cruz Biotechnology, Dallas, Texas) and rabbit anti-α/β-tubulin (2148; Cell Signaling, Danvers, Massachusetts). Membranes were then washed in TBS-T and incubated with species-specific horseradish peroxidase-labeled secondary antibody for 1 hour at room temperature. After washing with TBS-T, membranes were developed to detect bound antibodies using the ECL chemiluminescence detection system (Pierce/Life Technologies). Additional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analyses of 5T4 in 10 000g and 150 000g microvesicle and exosomes fractions, respectively, were performed under reducing conditions as described.28
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded placental sections (5 μm). Placental tissues were fixed in 4% paraformaldehyde overnight, dehydrated through a series of increasing concentrations of ethanol, and embedded in paraffin as previously described.29 Tissue sections were subjected to antigen retrieval using Reveal buffer (BioCare Medical, Walnut Creek, California) according to the manufacturer’s protocol, and nonspecific antibody binding was blocked in 10% donkey serum (Sigma-Aldrich). Sheep anti-human 5T4 antibody (5 μg/mL; R&D Systems; Cat# AF4975) or sheep IgG (Sigma-Aldrich) was added to tissue sections overnight at 4°C. After washing, biotinylated donkey anti-sheep antibody (Sigma-Aldrich) was added to sections followed by depletion of endogenous peroxidase with 0.5% hydrogen peroxide in methanol. Positive staining was detected using aminoethyl carbazole kit (Invitrogen). To screen 5T4 expression in other normal tissues, a universal control tissue microarray was purchased from US Biomax, Inc. (Cat # UNC241; Rockville, MD). These slides have 5 μm thick, formalin fixed, paraffin embedded tissue sections representing 12 different organs including some malignant tissues. Immunohistochemistry staining for 5T4 was performed as mentioned above.
Immunofluorescence
Paraffin embedded decidual sections (Mount Sinai Hospital, Toronto, Ontario, Canada) at 5 μm thickness were deparaffinized and rehydrated as described above. Antigen retrieval was performed using Reveal buffer and blocked in PBS containing 50% super block (Thermo Scientific/Life Technologies) and 2% donkey serum (Sigma-Aldrich). Slides were incubated overnight at 4°C with sheep antihuman 5T4 (5 μg/mL). After several washes, Alexa Fluor 594-conjugated donkey anti-sheep secondary antibody (1:1000; Invitrogen) was added. For dual staining, this was followed by washing and incubating with a second primary antibody, mouse antihuman cytokeratin (clone OV-TL 12/30; 5 μg/mL, DAKO) for 1 hour at room temperature. Slides were then washed and Alexa Fluor 488-conjugated anti-mouse secondary antibody (1:2000; Invitrogen) was added. As controls, sheep IgG and mouse IgG1 were substituted for primary antibodies, and slides were counterstained with DAPI (Prolong Gold Antifade, Invitrogen) for nuclear localization. Sections were viewed using a Zeiss Pascal confocal microscope.
Statistical Analysis
Statistical analyses were conducted using SigmaStat software (Systat, Chicago, Illinois). Differences between gestational age were calculated by 1-way analysis of variance followed by Tukey post hoc multiple comparisons test. Differences between EGF-treated and untreated trophoblast cells, ambient and low oxygen culture conditions, and control and PE samples were compared by 2-tailed, paired Student t test. Results with a P value of ≤.05 were considered significantly different.
Results
Clinical Characteristics of Patient Samples
Detailed clinical characteristics of PE and matched control placental samples have been described by Linscheid et al30 Patients did not differ in maternal age, body mass index (BMI), or gestational age at delivery; infant birth weight was significantly lower for patients with PE (1930 ± 210 g vs 2514 ± 141 g; mean ± SEM for PE and controls, respectively; P < .001). Other samples were collected at term (>37 weeks gestation) from uncomplicated Cesarean deliveries without labor; clinical information for 5 specimens is available; average gestation age was 39.23 ± 0.04 weeks; maternal BMI, 32.1 ± 0.99, and infant birth weight 3664 ± 80.5 g.
Expression of 5T4 Is Localized to Syncytiotrophoblast and Extravillous Cytotrophoblast Cells
Expression of 5T4 was investigated in first-trimester (n = 11), second-trimester (n = 2), and term (n = 13) placenta by immunohistochemistry using a commercially available antibody. In first-trimester placenta, 5T4 localized to both chorionic villous and extravillous trophoblast cells (Figure 1A and B). Expression was prominent at the apical membrane of the syncytiotrophoblast, although a few villi had either weak or no staining. In contrast, 5T4 immunoreactivity was minimally expressed or absent on underlying precursor cytotrophoblast cells; expression also appeared to be absent on mesenchymal cells, endothelial cells, and Hofbauer cells. Second-trimester placenta (not shown) and term placenta (Figure 1C) displayed a similar expression pattern to first-trimester placenta, with abundant expression of 5T4 on the syncytiotrophoblast microvillus surface.
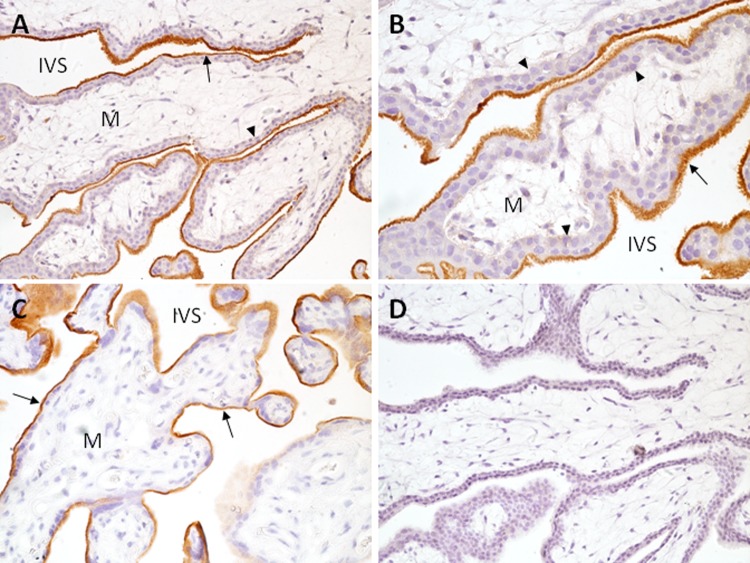
Figure 1.
Expression of 5T4 in first trimester and term chorionic villi. First trimester (A, B, D) and term (C) tissue sections were incubated with antihuman 5T4 antibody (A-C) or IgG (D) for immunohistochemistry as described in Methods. A, B, Villous placenta showing prominent 5T4 expression (red-brown staining) on microvillus surface of syncytiotrophoblast (arrows); underlying cytotrophoblast cells (arrowheads) and villous mesenchyme (M) lack obvious 5T4 expression. C, Term villous placenta showing strong immunoreactivity at the apical surface of the syncytiotrophoblast (arrow). A, C, and D, 200× magnification; B, 400× magnification; IVS indicates intervillous space; 5T4, trophoblast glycoprotein.
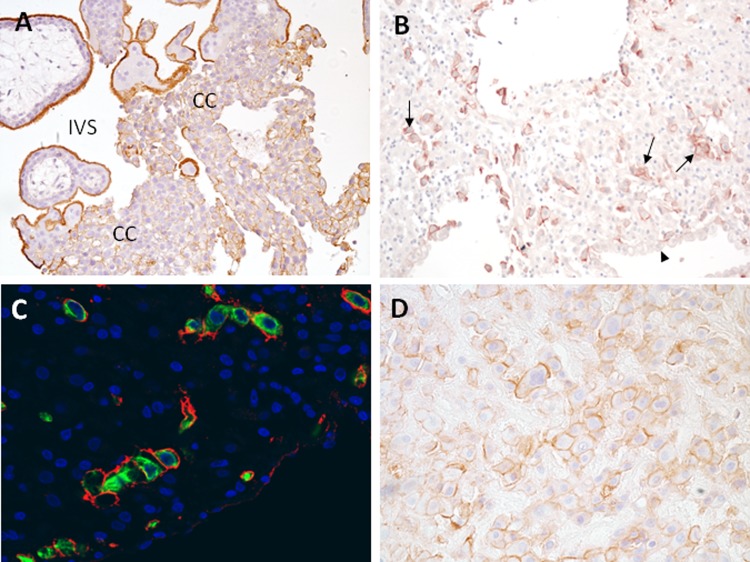
Trophoblast glycoprotein was also observed in a manner consistent with cell-surface expression on extravillous trophoblast cells. On trophoblast cell columns, 5T4 expression was low in cells proximal to villi, but became higher as cells progressed away from the villi, suggesting its expression was increased in association with epithelial-mesenchymal transition of these cells (Figure 2A). Prominent expression could also be seen on interstitial trophoblast cells in the decidua of first trimester, and basal plate trophoblast cells of term placenta (Figure 2B-D). Identity of interstitial trophoblast cells was confirmed by immunofluorescent colocalization of 5T4 with cytokeratin-7 (Figure 2C). Although low expression was occasionally observed on glandular epithelium of the uterus, no other cell type within the decidua exhibited positive staining.
Figure 2.
Expression of 5T4 in first trimester and term extravillous trophoblast cells. A. Villous placental tissue showing extravillous trophoblast cell columns (CC). B, First trimester decidual tissue containing prominently stained interstitial trophoblast cells (arrows); decidual cells and luminal epithelial cells (arrowhead) are unstained. C, interstitial trophoblast cells are costained for 5T4 (red) and cytokeratin 7 (green), demonstrating surface-associated 5T4 on these cells. D, Term basal plate placenta; extravillous trophoblast cells express 5T4; 5T4 indicates trophoblast glycoprotein.
Placental expression of 5T4 was further confirmed by Western blot analysis (Figure 3). Trophoblast glycoprotein protein was readily detectable in whole placental lysate from all gestational ages, which revealed 5T4 as a sharp, ∼75 kDa band; some samples additionally showed a more diffuse band that migrated slightly faster than 75 kDa. Trophoblast glycoprotein abundance increased as gestation advanced, with approximately 2-fold higher levels at term than in the first trimester (P = .009). In addition, primary trophoblast cells from term placenta as well as the trophoblast and choriocarcinoma cell lines Swan 71, BeWo, JEG3, and HTR8-SVneo were positive for 5T4 expression by Western blot analysis (Figure 3C).
Figure 3.

Placental expression of 5T4 across gestation. A, Whole placenta lysate was examined by Western blot analysis using anti-5T4 antibody, or anti-α/β-tubulin as a loading control. B, Semiquantitative analysis of 5T4 in first trimester (n = 3), second trimester (n = 4), and term (n = 6) placenta by scanning densitometry. Samples were normalized to tubulin and analyzed by 1-way analysis of variance (ANOVA). C, Western blot analysis of purified term cytotrophoblast cells (CTB), first trimester trophoblast cell lines (SW71 and HTR8/SVneo), and choriocarcinoma cells (BeWo and JEG-3). HEK-293 cells were used as a negative control. 5T4 indicates trophoblast glycoprotein.
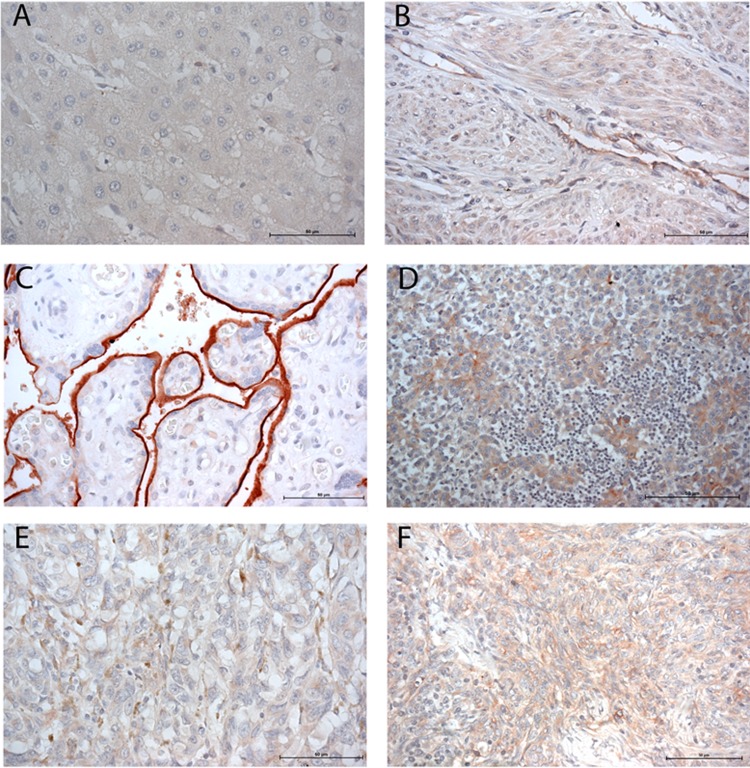
To determine the expression pattern of 5T4 on normal tissues outside the placenta, immunohistochemistry was performed on a tissue microarray. Trophoblast glycoprotein was either absent (liver, tonsil, brain, and lung) or low in expression (colon epithelium, thyroid endothelium, prostate smooth muscle, and uterine smooth muscle and endothelium) in normal healthy tissues (Figure 4A and B and data not shown). Malignant breast carcinoma, thymoma, and melanoma, on the other hand, exhibited consistent 5T4 immunoreactivity (Figure 4D-F). Notably, placental syncytiotrophoblast exhibited more intense immunoreactivity than any of the normal or malignant tissues (Figure 4C).
Figure 4.
Immunoreactive 5T4 in normal and malignant tissues. A commercially obtained tissue microarray was screened for expression of 5T4 across normal and malignant tissues. Tissues shown are liver (A), uterus (B), placenta (C), breast carcinoma (D), thymoma (E), and melanoma (F).
Expression of 5T4 in Trophoblast Cells Increases With EGF-Induced Differentiation
Because our initial immunohistochemical analysis showed 5T4 to be localized to the syncytiotrophoblast, we reasoned that differentiation of trophoblast cells into syncytiotrophoblast would be associated with elevation of 5T4 expression. Epidermal growth factor potently induces syncytialization of villous cytotrophoblast cells in vitro and likely plays a similar role in vivo.31,32 Primary term villous cytotrophoblast cells were treated with medium alone or 5 ng/mL EGF for 24 or 48 hours. By 48 hours of culture, EGF increased 5T4 expression by 4-fold as compared to untreated controls (Figure 5). At this time, EGF had also promoted syncytialization by approximately 2-fold (17.6% ± 2.4% vs 33.5% ± 6.6% syncytialization in untreated and EGF-treated trophoblast cells, respectively; P = .023; n = 6); CGB secretion at this time appeared to increase, although differences were not significant (19.8 ± 4.4 IU/mL vs 42.3 ± 11.3 IU/mL; P = .09; n = 3). Interestingly, we also observed a significant 2-fold induction of 5T4 expression at 24 hours following EGF treatment (Figure 5), prior to statistically significant changes in either syncytialization (18.05% ± 7.8% vs 28.7% ± 5.1%; P = .19) or CGB production (2.5 ± 1.2 IU/mL vs 9.6 ± 3.6 IU/mL; P = .1). Thus EGF promotes expression of 5T4 prior to its effects on morphological and biochemical differentiation of trophoblast cells.
Figure 5.
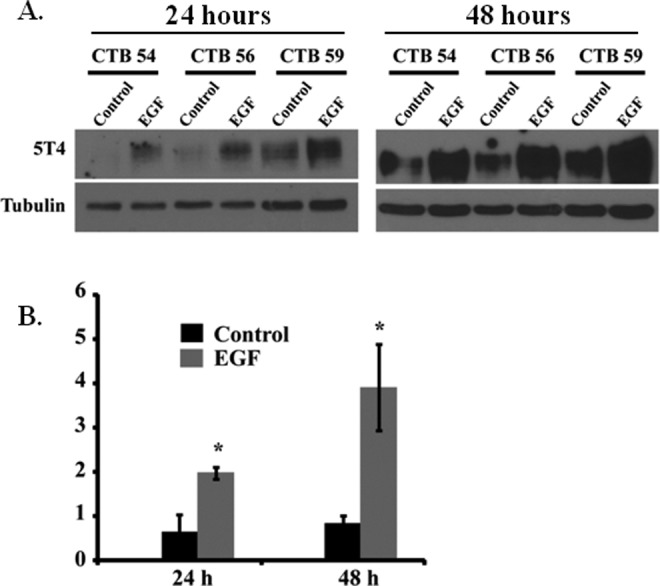
EGF on 5T4 expression in primary trophoblast cells. A, Western blot analysis of 3 different samples of purified trophoblast cells cultured in the presence or absence of EGF for 24 or 48 hours. CTB (number) represents the deidentification code used to denote individual samples. Tubulin was used as a loading control. B, Semiquantitative analysis of 5T4 protein expression. Asterisks denote significant differences as compared to controls within time points (P < .05). Data were analyzed by 2-tailed t test; CTB indicates cytotrophoblast; EGF, epidermal growth factor.
Hypoxia Increases 5T4 Transcripts in Primary Trophoblast Cells
In addition to elevated expression of 5T4 on the syncytiotrophoblast, we noted that 5T4 expression appeared to be elevated on invasive cytotrophoblast cells. During early pregnancy, invasive trophoblast cells obstruct maternal blood flow to the placenta, resulting in a relatively hypoxic fetal-placental environment.33 These observations, together with the proposed role of 5T4 in tumor invasion34 prompted us to investigate the influence of low oxygen on expression of 5T4 by trophoblast cells. Preliminary studies suggested that 5T4 mRNA expression is elevated at 2% oxygen (hypoxia) in comparison to both 8% (P = .026) and 21% (P = .016) oxygen, which were similar to each other (P > .05). Thus, term purified cytotrophoblast cells were exposed to 2% and 21% oxygen for 24 hours to mimic hypoxic and ambient conditions, respectively. Although 5T4 protein levels between the treatment groups were not significantly different, 5T4 mRNA was elevated in low, relative to ambient, oxygen (P < .05, n = 6) (Figure 6A-C).
Figure 6.
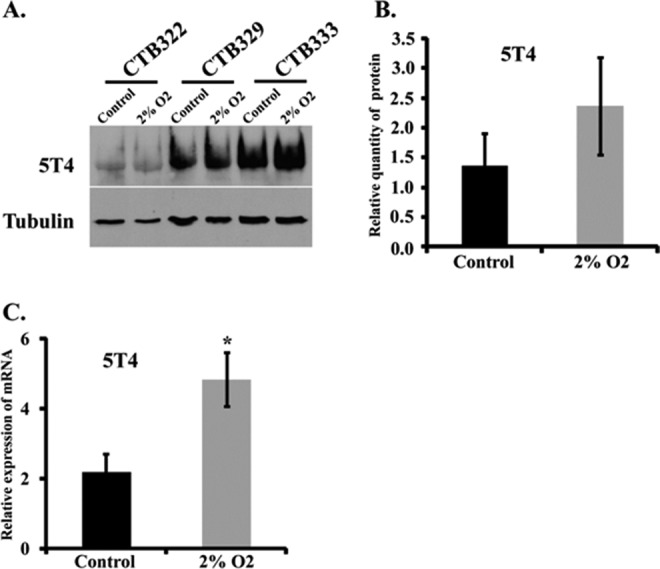
Low oxygen increases 5T4 messenger RNA (mRNA) expression. A. Western blot analysis of 3 different samples of purified trophoblast cells cultured under ambient (control) or low-oxygen (2% O2) conditions. CTB (number) represents the deidentification code used to denote individual samples. B and C, Semiquantitative analysis of the effect of low oxygen on 5T4 expression on protein (B) and mRNA (C) expression; CTB indicates cytotrophoblast.
Messenger RNA Levels of 5T4 Are Elevated in Preeclamptic (PE) Placentas
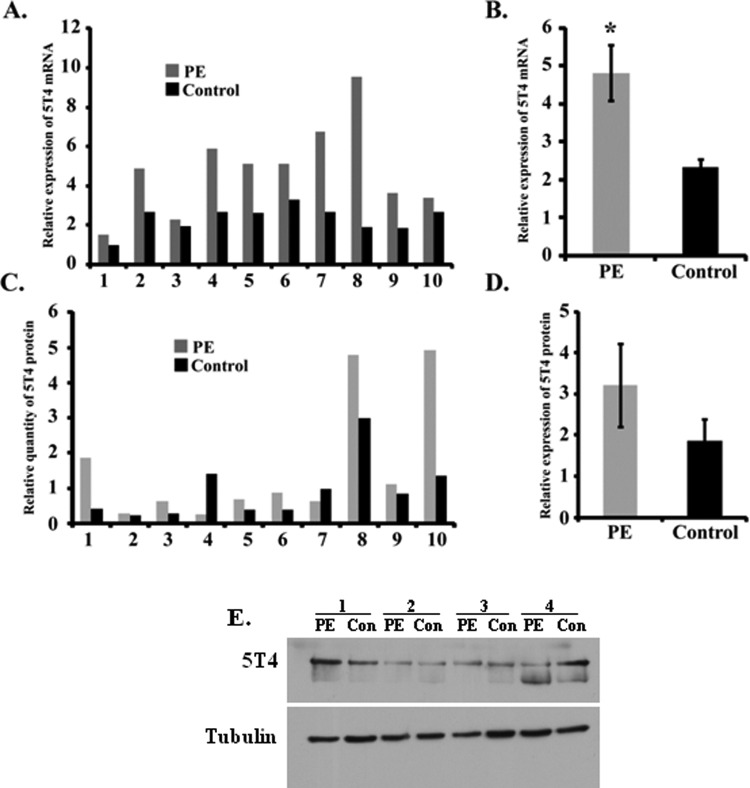
Preeclampsia is a maternal disease of pregnancy that is associated with placental oxidative stress, possibly due to intermittent oxygenation of the placenta.35 The observation that low oxygen in vitro augmented 5T4 mRNA in trophoblast cells led us to ask whether 5T4 expression changes in placentas obtained after delivery from PE women. Trophoblast glycoprotein transcripts and protein levels from 10 PE and gestational age- and delivery-matched controls were analyzed. Relative expression of 5T4 mRNA was similar to (n = 3) or increased (n = 7) in the 10 PE placentas as compared to their respective controls; on average, this difference was statistically significant (Figure 7A and B, P < .005). However, protein levels varied between groups, with PE placentas exhibiting increased, decreased, or unchanged 5T4 expression in comparison to controls (Figure 7C), with no obvious correlation to mRNA expression patterns levels. Collectively, 5T4 protein tended to be elevated in PE placentas, but the difference was not statistically significant (Figure 7D, E; P = .062). The cellular distribution, as evaluated by immunohistochemistry, was also similar: as in controls, 5T4 protein was restricted to syncytiotrophoblast and extravillous trophoblast cells (data not shown). Finally, there was no obvious correlation between 5T4 expression and fetal sex, weight, presence or absence of labor, route of delivery, gestation age, or maternal BMI.
Figure 7.
5T4 messenger RNA (mRNA) transcripts are elevated in preeclampsia. 5T4 protein and mRNA expression were examined in 10 preeclamptic (PE) and 10 gestational age- and delivery-matched healthy control placentas by reverse transcription-polymerase chain reaction (RT-PCR) and Western blot, respectively. Individual (A and C) and mean (B and D) normalized values of mRNA (A and B) and protein (C and D) are shown among samples. Asterisk indicates statistically significant difference as determined by 2-tailed t test. E, Representative Western blot; 5T4 indicates trophoblast glycoprotein.
Trophoblast Glycoprotein Is Released From First-Trimester and Term Placenta via Syncytial Microvesicles and Exosomes
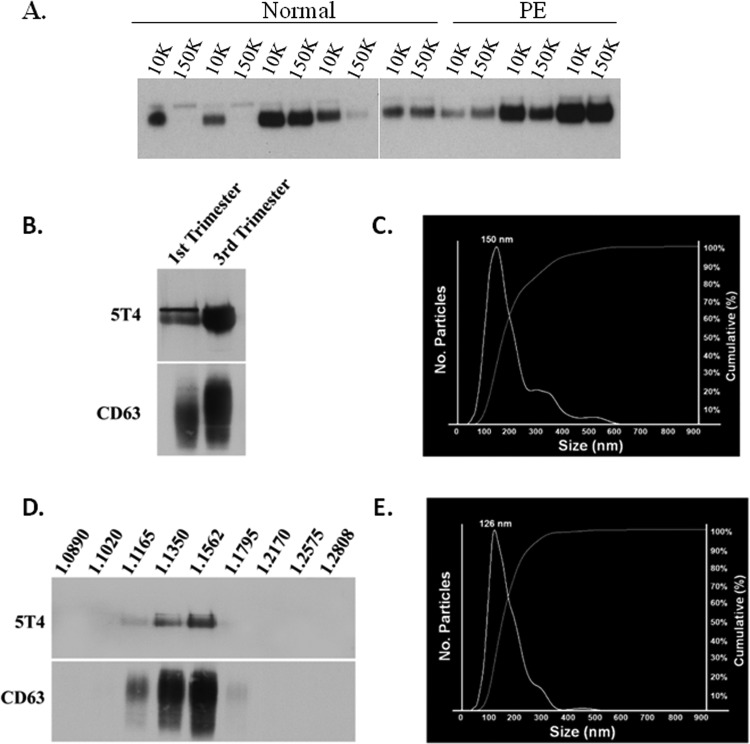
We recently showed that the immunomodulatory molecules HLA-G5, B7-H1, and B7-H3 are released from first-trimester and term placenta via exosomes.26 To determine whether the placenta releases 5T4 in association with syncytiotrophoblast extracellular vesicles, placentas from normal healthy pregnancies (n = 5) and from pregnancies complicated by PE (n = 3) were perfused ex vivo. To isolate vesicles, pellets were collected from perfusates subjected to centrifugation at either 10 000g (10K pellet) followed by 150 000g (150K pellet). Western blot analysis of resulting pellets revealed strongly immunoreactive bands of the appropriate size for 5T4 in all but 2 of the 150 000g pellets, both derived from normal placentas, and in all of the 10 000g pellets (Figure 8A). After correcting for loading error using the reactive brown-stained blots to reveal total protein, using densitometric analysis, no significant differences when either normal and PE placentas, or when 10 000 and 150 000g pellets were compared (P > .05).
Figure 8.
Trophoblast glycoprotein (5T4) is present within exosomes isolated from first trimester and term placental explant culture. A, Placentas from normal healthy pregnancies or pregnancies complicated by preeclampsia (PE) were perfused ex vivo, and perfusates were subjected to centrifugation at 10 000g (10K) or 150 000g (150K). Resulting pellets were analyzed by Western blot for 5T4 expression. B-D, Placental explants were cultured for 24 hours in exosome-free medium, and supernatant subjected to sequentially increasing centrifugation. Pellets were examined by Western blot analysis (B) and nanoparticle tracking analysis (C). D and E, Pellets were further subjected to sucrose gradient centrifugation and similarly examined. Numbers along top of image in D represent densities (g/mL) of each collected fraction.
To further query whether 5T4 may be released from trophoblast cells specifically in association with exosomes, we cultured first-trimester and term placental explants in exosome-free medium and isolated exosomes by sequential centrifugation. Pellets resulting from the final ultracentrifugation step (100 000g) were reconstituted and analyzed for 5T4 and the exosome marker CD63. Pellets recovered from both first-trimester and term placental explants showed strong positive signals for 5T4 by Western blot (Figure 8B). In addition, nanoparticle tracking analysis (NTA) of pellets demonstrated the presence of a major peak of 150 nm (Figure 8C). Additional material clustered around the 300 to 400 nm size, and the 500 to 600 nm size, which is consistent with our previous observations by electron microscopy that 100 000g pellets contain larger vesicles.26 Therefore, we subjected the same pellets to further centrifugation over continuous 2.0 to 0.25 M sucrose gradients, followed by Western blot analysis of the resultant fractionated vesicles. Signals for both 5T4 and CD63 were most prominently associated with densities corresponding to 1.13 to 1.15 g/L sucrose (Figure 8D), consistent with the range at which exosomes are known to float.36 The size of vesicles collected from the 1.1562 g/L fraction was determined by NTA to range from 70 to 350 nm with a predominant peak of 126 nm (Figure 8E). These results are in agreement with the idea that 5T4 is secreted from the placenta via exosomes.
Discussion
Trophoblast glycoprotein is among a class of tumor antigens that are expressed at low levels in normal adult tissues, but at high levels in both tumor cells and fetal tissues including the placenta. Here, we characterized in detail the expression pattern of 5T4 in the human placenta across gestation. Trophoblast glycoprotein is abundantly expressed at the apical microvillus surface of the syncytiotrophoblast throughout gestation, and lower but not absent in villous cytotrophoblast cells. The in vivo induction of 5T4 in trophoblast cells during the process of differentiation into syncytiotrophoblast could be recapitulated in vitro upon treatment with EGF, a well-known inducer of trophoblast syncytialization. Trophoblast glycoprotein is also expressed by extravillous trophoblast cells, most prominently within distal cell columns and decidua. In the normal tissues examined, 5T4 is either undetectable or minimally expressed at the protein level. Finally, 5T4 protein was found to be released in vitro, both from placental explants and from perfused placenta, in association with microvesicles and exosomes.
Trophoblast glycoprotein is a glycoprotein containing a 42 kDa core protein and 7 predicted N-linked glycosylation sites.37 In some experiments, immunoblot analysis revealed more than 1 band in freshly isolated tissues (Figures 3A and 7E), cell lines (Figure 3C), and vesicles (Figure 8B). Differential glycosylation may also explain differences in “sharpness” of the bands between freshly isolated tissues (Figures 3A and 7E) and cultured cells and tissues/vesicles (Figures 3C, 5A, 6A, and 8). Culturing trophoblast cells and placental tissues may lead to alterations in glycosylation of 5T4, resulting in these changes in comparison to freshly isolated tissues.
Trophoblast cells exhibit features that are in common with cancer cells, including invasion into host tissues and evasion from immune surveillance. These commonalities have prompted investigations to search for antigens shared by the placenta and tumors in efforts to find targets for cancer immunotherapy. Hole and Stern first identified 5T4 in 1988, reporting its expression on trophoblast cells using a mouse monoclonal antibody.13 Immunohistological analysis of 5T4 revealed its expression on the membrane and cytoplasm of syncytiotrophoblasts from term placenta, and a negative staining of villous cytotrophoblast cells.38 Our results are similar, showing precise and prominent expression of 5T4 at the microvilli of syncytiotrophoblasts cells and surface of extravillous cytotrophoblast cells. The limited expression of 5T4 on normal nonmalignant tissues is also in agreement with earlier studies.13,38
Trophoblast glycoprotein is increasingly expressed in cell columns as trophoblast cells progress away from chorionic villi and remains expressed on interstitial trophoblasts in the basal plate decidua. During normal placentation, column trophoblast cells lose their epithelial morphology as they advance toward the uterine tissue, undergoing a developmental EMT event as they acquire an invasive phenotype.7,39 Intriguingly, 5T4 has been implicated in cellular motility and EMT: its overexpression in fibroblasts and epithelial cells induces loss of E-cadherin, cell contact, and adherence, as well as disruption of the cytoskeleton and increased motility.18 In murine embryonic fibroblasts, 5T4 expression is required for surface expression of the G-protein coupled chemokine receptor, CXCR4, and for chemotaxis induced by the CXCR4 ligand, CXCL12.40 In the absence of 5T4, surface CXCR4 drops and the preferred receptor for CXCL12 switches to CXCR7, which shifts the response to the ligand from chemotaxis to proliferation.41 Additional insights into possible 5T4 function were gained with the observation that the zebrafish paralog of 5T4, WAIF1, inhibits the canonical Wnt signaling pathway via interference with the Wnt co-receptor, LRP6.19,42 Collectively, these observations raise intriguing possibilities for regulation of proliferation and invasion of extravillous cytotrophoblast cells, which express CXCL12 and its receptors,43,44 and use Wnt signaling during placentation.45 In vitro experiments together with in vivo studies of placentation in 5T4-deficient mice could yield insight into whether a 5T4-regulated system might control trophoblast invasion and proliferation.
The placenta develops in a hypoxic environment as the blood flow to the intervillous space commences only at gestation weeks 10 to 12.46 In our experiments, under low oxygen tension, 5T4 transcripts as well as the protein levels were elevated, albeit the latter not significantly. Although the physiologic level of oxygen in normal placenta after the first trimester is around 8%, we found that 5T4 expression in trophoblast cells cultured at 8% and 21% oxygen were similar (unpublished observation). Elevated mRNA expression was mirrored in experiments conducted with PE placentas, suggesting that fluctuating oxygen concentrations and/or inflammatory signals in PE may upregulate 5T4 mRNA in these placentas. Detailed examination of 5T4 promoter sequence15 fails to reveal canonical HIF-1 alpha responsive elements, suggesting that other regulatory mechanisms are responsible for regulating 5T4 under low oxygen conditions. Further, our studies also suggest that trophoblast differentiation promotes 5T4 expression (Figure 5); because hypoxia inhibits differentiation in trophoblast cells,47 the results together suggest that low oxygen promotes 5T4 mRNA expression independently of its effects on trophoblast differentiation. The apparent lack of low oxygen and PE condition on protein expression suggests differential regulation of 5T4 mRNA by oxygen and/or other pathologic factors.
Interestingly, statistically significant increases in 5T4 expression were observed prior to significant induction of morphological and biochemical (CGB) induction of cytotrophoblast differentiation into syncytiotrophoblast, indicating a possible role in differentiation. The functional significance of its expression at the apical surface of syncytiotrophoblast is less clear, although it may explain the gestational-age associated increase in expression, as the abundance of syncytiotrophoblast per unit tissue increases as the gestation advances. Its presence on the apical syncytiotrophoblast surface is also consistent with its presence in syncytiotrophoblast-derived vesicles and exosomes. Further supporting our data, 5T4 was identified in syncytiotrophoblast microvesicles from perfused placentas by mass spectrometry (Tannetta et al, unpublished observation, 2010). Similarly, 5T4 has also been documented in exosomes purified from cultured bladder cancer cells by proteomic analysis and showed elevated expression in exosomes of prostate patients with cancer as compared to healthy patients.20,48 Thus, trophoblast cells share commonality with tumor cells in their ability to specifically release tumor-associated antigens via exosomes.
The in vivo mechanism by which placental exosomes exert their biological response remains under scrutiny, one strong possibility being that exosomes are involved in the modulation of maternal immune cells during pregnancy to support fetal survival.26,49,50 Although there is no evidence that 5T4 is an immunomodulator in its own right, its association with microvesicles and exosomes raises other intriguing possibilities. For example, tumor cell antigens can be cross-presented by professional antigen presenting cells to T cells.51 Likewise, paternally inherited antigens from the fetus are cross-presented by maternal antigen presenting cells, also in the context of major histocompatibility complex (MHC).52,53 Uptake of human placental microvesicles and exosomes by antigen presenting cells has been demonstrated54 (Petroff et al, unpublished material, 2016); these vesicles and their cargo likely enter the antigen processing pathway for ultimate presentation of fetal antigens, possibly including shared placenta-tumor antigens. We hypothesize that extracellular vesicle-associated shared placenta-tumor antigens could prime the maternal immune system based not on their exclusive expression from the paternally inherited chromosomes, but rather based on their unique and abundant expression by the placenta; indeed, placental expression may serve as an initial exposure of the maternal immune system to these antigens.
In conclusion, the present study characterizes placental 5T4 expression throughout gestation and describes for the first time the placental secretion of shared placenta-tumor antigens via exosomes. This finding is of substantial interest due to potential immunomodulatory effects of this protein on the maternal immune system. Elucidating the pathways involved in 5T4 regulation and function may further help reveal the underlying mechanisms involved in placental pathologies.
Acknowledgments
The authors thank the administrative and nursing staff at the University of Kansas Medical Center Department of Obstetrics and Gynecology and at the Center for Women’s Health for assistance in collection of placental specimens. The authors thank David Albertini for assistance with fluorescence microscopy.
Authors’ Note: S. M. K. Alam and S. Jasti contributed equally to this article. S. M. K. Alam is currently affiliated with the Department of Biochemistry and Molecular Biology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by NIH grant R01HD045611 (MGP) and a clinical translational grant to MGP and TAF from the Kansas IDeA Network for Biomedical Research Excellence (P20GM103418). Funding for studies performed at the University of Oxford was provided by the Medical Research Council Programme Grant MR/J003360/1, United Kingdom. Additional support was provided by Michigan State University and AgBioResearch.
References
- 1. Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblast invasion of human decidua from 8-18 weeks of pregnancy. Placenta. 1980;1(1):3–19. [DOI] [PubMed] [Google Scholar]
- 2. Harris LK, Aplin JD. Vascular remodeling and extracellular matrix breakdown in the uterine spiral arteries during pregnancy. Reprod Sci. 2007;14(suppl 8):28–34. [DOI] [PubMed] [Google Scholar]
- 3. Bulmer JN, Innes BA, Levey J, Robson SC, Lash GE. The role of vascular smooth muscle cell apoptosis and migration during uterine spiral artery remodeling in normal human pregnancy. FASEB J. 2012;26(7):2975–2985. [DOI] [PubMed] [Google Scholar]
- 4. Pijnenborg R, Robertson WB, Brosnens I, Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981;2(1):71–91. [DOI] [PubMed] [Google Scholar]
- 5. Murray MJ, Lessey BA. Embryo implantation and tumor metastasis: common pathways of invasion and angiogenesis. Semin Reprod Endocrinol. 1999;17(3):275–290. [DOI] [PubMed] [Google Scholar]
- 6. Strickland S, Richards WG. Invasion of the trophoblasts. Cell. 1992;71(3):355–357. [DOI] [PubMed] [Google Scholar]
- 7. Perry JK, Lins RJ, Lobie PE, Mitchell MD. Regulation of invasive growth: similar epigenetic mechanisms underpin tumor progression and implantation in human pregnancy. Clin Sci (Lond). 2010;118(7):451–457. [DOI] [PubMed] [Google Scholar]
- 8. Jordan NV, Johnson GL, Abell AN. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle. 2011;10(17):2865–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilczynski JR. Cancer and pregnancy share similar mechanisms of immunological escape. Chemotherapy. 2006;52(3):107–110. [DOI] [PubMed] [Google Scholar]
- 10. Jungbluth AA, Silva WA, Jr, Iversen K, et al. Expression of cancer-testis (CT) antigens in placenta. Cancer Immun. 2007;7:15. [PMC free article] [PubMed] [Google Scholar]
- 11. Koslowski M, Sahin U, Mitnacht-Kraus R, Seitz G, Huber C, Türeci O. A placenta-specific gene ectopically activated in many human cancers is essentially involved in malignant cell processes. Cancer Res. 2007;67(19):9528–9534. [DOI] [PubMed] [Google Scholar]
- 12. Butterfield LH. Cancer vaccines. BMJ. 2015;350:h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57(3):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. King KW, Sheppard FC, Westwater C, Stern PL, Myers KA. Organisation of the mouse and human 5T4 oncofoetal leucine-rich glycoprotein genes and expression in foetal and adult murine tissues. Biochim Biophys Acta. 1999;1445(3):257–270. [DOI] [PubMed] [Google Scholar]
- 15. Myers KA, Rahi-Saund V, Davison MD, Young JA, Cheater AJ, Stern PL. Isolation of a cDNA encoding 5T4 oncofetal trophoblast glycoprotein. An antigen associated with metastasis contains leucine-rich repeats. J Biol Chem. 1994;269(12):9319–9324. [PubMed] [Google Scholar]
- 16. Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11(6):725–732. [DOI] [PubMed] [Google Scholar]
- 17. Awan A, Lucic MR, Shaw DM, et al. 5T4 interacts with TIP-2/GIPC, a PDZ protein, with implications for metastasis. Biochem Biophys Res Commun. 2002;290(3):1030–1036. [DOI] [PubMed] [Google Scholar]
- 18. Carsberg CJ, Myers KA, Stern PL. Metastasis-associated 5T4 antigen disrupts cell-cell contacts and induces cellular motility in epithelial cells. Int J Cancer. 1996;68(1):84–92. [DOI] [PubMed] [Google Scholar]
- 19. Kagermeier-Schenk B, Wehner D, Özhan-Kizil G, et al. Waif1/5T4 inhibits Wnt/β-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Devel Cell. 2011;21(6):1129–1143. [DOI] [PubMed] [Google Scholar]
- 20. Mitchell PJ, Welton J, Staffurth J, et al. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med. 2009;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. Methods Mol Med. 2006;121:203–217. [DOI] [PubMed] [Google Scholar]
- 22. Holets LM, Carletti MZ, Kshirsagar SK, Christenson LK, Petroff MG. Differentiation-induced post-transcriptional control of B7-H1 in human trophoblast cells. Placenta. 2009;30(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graham CH, Hawley TS, Hawley RG, et al. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206(2):204–211. [DOI] [PubMed] [Google Scholar]
- 24. Straszewski-Chavez SL, Abrahams VM, Alvero AB, et al. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30(11):939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 26. Kshirsagar SK, Alam SM, Jasti S, et al. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33(12):982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dragovic RA, Gardiner C, Brooks AS, et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine. 2011;7(6):780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dragovic RA, Collett GP, Hole P, et al. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. Methods. 2015;87:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holets LM, Hunt JS, Petroff MG. Trophoblast CD274 (B7-H1) is differentially expressed across gestation: influence of oxygen concentration. Biol Reprod. 2006;74(2):352–358. [DOI] [PubMed] [Google Scholar]
- 30. Linscheid C, Heitmann E, Singh P, et al. Trophoblast expression of the minor histocompatibility antigen HA-1 is regulated by oxygen and is increased in placentas from preeclamptic women. Placenta. 2015;36(8):832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morrish DW, Bhardwaj D, Dabbagh LK, Marusyk H, Siy O. Epidermal growth factor induces differentiation and secretion of human chorionic gonadotropin and placental lactogen in normal human placenta. J Clin Endocrinol Metab. 1987;65(6):1282–1290. [DOI] [PubMed] [Google Scholar]
- 32. Morrish DW, Dakour J, Li H. Functional regulation of human trophoblast differentiation. J Reprod Immunol. 1998;39(1-2):179–195. [DOI] [PubMed] [Google Scholar]
- 33. Jaffe R, Jauniaux E, Hustin J. Maternal circulation in the first-trimester human placenta—myth or reality? Am J Obstet Gynecol. 1997;176(3):695–705. [DOI] [PubMed] [Google Scholar]
- 34. Carsberg CJ, Myers KA, Evans GS, Allen TD, Stern PL. Metastasis-associated 5T4 oncofoetal antigen is concentrated at microvillus projections of the plasma membrane. J Cell Sci. 1995;108(pt 8):2905–2916. [DOI] [PubMed] [Google Scholar]
- 35. Roberts JM, Escudero C. The placenta and preeclampsia. Pregnancy Hypertens. 2012;2(2):72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hole N, Stern PL. Isolation and characterization of 5T4, a tumor-associated antigen. Int J Cancer. 1990;45(1):179–184. [DOI] [PubMed] [Google Scholar]
- 38. Southall PJ, Boxer GM, Bagshawe KD, Hole N, Bromley M, Stern PL. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer. 1990;61(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta—epithelial–mesenchymal transition (EMT) and placental development. Placenta. 2010;31(9):747–755. [DOI] [PubMed] [Google Scholar]
- 40. Southgate TD, McGinn OJ, Castro FV, et al. CXCR4 mediated chemotaxis is regulated by 5T4 oncofetal glycoprotein in mouse embryonic cells. PLoS One. 2010;5(4):e9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McGinn OJ, Marinov G, Sawan S, Stern PL. CXCL12 receptor preference, signal transduction, biological response and the expression of 5T4 oncofoetal glycoprotein. J Cell Sci. 2012;125(pt 22):5467–5478. [DOI] [PubMed] [Google Scholar]
- 42. Zhao Y, Malinauskas T, Harlos K, Jones EY. Structural insights into the inhibition of Wnt signaling by cancer antigen 5T4/Wnt-activated inhibitory factor 1. Structure. 2014;22(4):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schanz A, Baston-Bust D, Krussel JS, Heiss C, Janni W, Hess AP. CXCR7 and syndecan-4 are potential receptors for CXCL12 in human cytotrophoblasts. J Reprod Immunol. 2011;89(1):18–25. [DOI] [PubMed] [Google Scholar]
- 44. Schanz A, Red-Horse K, Hess AP, Baston-Büst DM, Heiss C, Krüssel JS. Oxygen regulates human cytotrophoblast migration by controlling chemokine and receptor expression. Placenta. 2014;35(12):1089–1094. [DOI] [PubMed] [Google Scholar]
- 45. Pollheimer J, Loregger T, Sonderegger S, et al. Activation of the canonical wingless/T-Cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 2006;168(4):1134–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nelson DM, Johnson RD, Smith SD, Anteby EY, Sadovsky Y. Hypoxia limits differentiation and up-regulates expression and activity of prostaglandin H synthase 2 in cultured trophoblast from term human placenta. Am J Obstet Gynecol. 1999;180(4):896–902. [DOI] [PubMed] [Google Scholar]
- 48. Welton JL, Khanna S, Giles PJ, et al. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9(6):1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191(11):5515–5523. [DOI] [PubMed] [Google Scholar]
- 50. Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176(3):1534–1542. [DOI] [PubMed] [Google Scholar]
- 51. Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. [DOI] [PubMed] [Google Scholar]
- 52. Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117(5):1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. 2009;182(12):8080–8093. [DOI] [PubMed] [Google Scholar]
- 54. Abumaree MH, Stone PR, Chamley LW. The effects of apoptotic, deported human placental trophoblast on macrophages: Possible consequences for pregnancy. J Reprod Immunol. 2006;72(1-2):33–45. [DOI] [PubMed] [Google Scholar]