Abstract
Purpose:
We have previously shown that stress prior to induction worsens clinical presentation and inflammatory parameters in a rat model of endometriosis. This study was designed to examine whether stress during the development of endometriosis can affect the growth of endometriotic implants through nerve growth and immune alterations.
Methods:
Endometriosis was surgically induced in female Sprague-Dawley rats by suturing uterine horn implants onto the small intestine mesentery. Two weeks later, one group of rats (endo-stress) was subjected to a 10-day swim stress protocol. Controls had no stress (endo-no stress) or sutures only and stress (sham-stress). On day 60, all rats were killed and examined for the presence of endometriotic vesicles. The size of each vesicle was measured. The uterus and colon were removed and assessed for damage, cell infiltration, and expression of nerve growth factor (NGF), its receptors (p75 and Tropomyosin receptor kinase A (Trk-A)/pTrk-A), and calcitonin gene–related peptide, a sensory fiber marker. A differential analysis of peritoneal fluid white blood cell count was performed.
Results:
Stress significantly increased endometriotic vesicle size but not colonic damage and increased infiltration of mast cells. Significantly increased expression of NGF and its receptors was found in the uterus of animals with endometriosis receiving stress.
Conclusions:
Stress stimulates the development of ectopic endometrial vesicles in an animal model of endometriosis and increases inflammatory cell recruitment to the peritoneum. In addition, stress promotes nerve fiber growth in the uterus.
Keywords: endometriosis, nerve growth factor, p75, Trk, stress
Introduction
Endometriosis is a common gynecological disease associated with incapacitating pelvic pain and infertility. Symptoms of this disease are often so severe and chronic that they are considered stressful events that negatively impact the daily life of these patients.1–3 Women with endometriosis report more stress, pain, and mental health issues (depression, anxiety) as well as poorer quality of life than women who have other pain syndromes, and often have the added stress of infertility.4–5 Although the relationship between stress and endometriosis is still not completely clear, current knowledge in reproductive biology suggests that stress may impact the mechanisms responsible for endometriosis. For instance, we know that growth in the size of fibroid tumors is often seen during times of stress,6 and women with fibroids, similar to those with endometriosis, often have specific stress due to this condition, including concerns about their general health, the painful symptoms, and possible infertility. Our laboratory has provided evidence for the deleterious effects of stress in the development of endometriosis and its associated inflammatory parameters.7 We showed that rats receiving stress prior to the surgical induction of endometriosis had vesicles of a greater severity and number and increased cellular infiltration in peritoneal fluid and colonic tissues. This suggests that stress prior to endometriosis most likely contributed to the development of vesicles and symptomatology in this animal model through mechanisms involving inflammatory cell recruitment and release of cell mediators. Recently, we demonstrated that stress during the disease process, perhaps a situation more akin to that found in patients, can worsen the condition.8 Importantly, we also showed that stress management could prevent the outgrowth of endometriotic vesicles and inflammation in this animal model, providing a causal link between stress and endometriosis.8
Retrograde flow of menses through fallopian tubes is one of the mechanisms by which endometrial cells travel outside the uterus, and aberrant responses to reproductive hormones contribute to the proliferation of endometrial cells at ectopic sites, causing activation of inflammatory mechanisms.9–11 Stress research has recognized the impact of acute and prolonged stressful events on these mechanisms.12 Chronic activation of stress responses, which include the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic–adrenal–medullary axis, results in sustained/prolonged expression of glucocorticoid receptors on a variety of immune cells. These can bind cortisol and interfere with the function of nuclear factor-kappa beta, which regulates the activity of cytokine-producing immune cells. Among these immune cells are mast cells, which have previously been reported to be increased at sites of peritoneal endometriosis.13 Anaf et al demonstrated that mast cells are significantly more abundant in the endometriotic lesions of patients than in nonaffected tissues, and in addition deep infiltrating lesions have more mast cells located close to nerves.14
Based on these observations, we hypothesize that since stress can affect the immune system and thus recruitment of inflammatory cells, such as mast cells, to the areas of damage, these cells may contribute to the pathogenesis of the chronic pain and inflammation that characterize endometriosis. There is cross talk between mast cells and nerves, with mast cell-derived mediators increasing the excitability of neurons, and release of mediators such as substance P and the neurotrophin nerve growth factor (NGF) inducing mast cell degranulation.15–17 There is also evidence that endometrial tissues of patients show increased levels of nerve fibers compared to controls, and that new nerve growth around endometriotic lesions might help the abnormally located tissue to survive. It has been speculated that the high-density nerve fibers observed in both eutopic and ectopic endometrium from patients may play an important role in the pathogenesis of pain and tenderness.18,19 The aim of the present study was to examine the impact of stress during the disease process on the development of the lesions and determine whether nerve fiber development could be enhanced in this model, via increased secretion of NGF, and increased expression/activation of its receptors, in peritoneal organs. Elucidating the importance of stress on nerve growth during endometriosis will provide further justification for the possible use of complementary therapies, such as stress management techniques, as an important part of the multidisciplinary management of endometriosis signs and symptoms, primarily inflammation and pain.20
Methods
The experiments reported herein were performed in accordance with the principles described in the “Guide for the Care and Use of Laboratory Animals,” publication no. DHMS (NIH) 86-23.
Animal Model
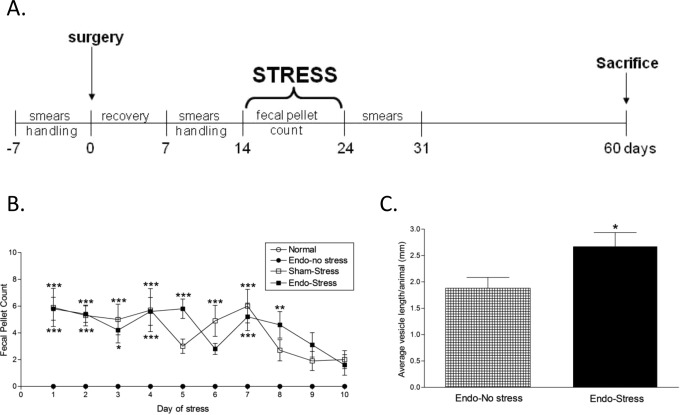
Studies were performed with female Sprague-Dawley rats weighing 200 to 250 g (Southern Veterinary Service, Ponce, Puerto Rico), with 10 to 11 animals per treatment group. All animals were maintained in a restricted-access room with controlled temperature (23oC) and light–dark cycle. Standard laboratory chow and tap water were provided ad libitum. All experimental procedures involving animals were approved by the Animal Care and Use Committee at Ponce Health Sciences University. All animals were handled for 7 days (5 min/d/rat) prior to beginning the experimental protocol in order to reduce experimenter-induced stress on the animal, and daily vaginal cytological smears were carried out for all rats to check their reproductive cyclicity (Figure 1A). The experiments were carried out every day at the same time (early afternoon) to minimize the influence of circadian rhythms, and all of the animals were randomly assigned to their treatment groups before the start of the overall experimental protocol.
Figure 1.
A, Experimental design. Animals were subjected to swim stress for 10 consecutive days. All animals were checked for regular cycling by analysis of smears during the protocol and killed 60 days after surgery, with a smear at the time of killing. B, Stress increases anxiety levels. Both the sham-stress and endo-stress groups had increased fecal pellet counts compared to endo-no stress or normal indicating increased anxiety levels (n = 10-11 [SEM]; *P <.05, **P <.01, ***P <.001 vs endo-no stress or normal). C, Stress increases implant severity. None of the normal or sham-stress animals developed vesicles. All of the endo animals developed vesicles in at least one of the implant sites. The endo-stress animals had significantly bigger vesicles (n = 10-11 [SEM]; *P <.05). SEM indicates standard error of the mean.
Induction of Endometriosis
Endometriosis was induced surgically in mature female rats under pentobarbital anesthesia, based on the method by Vernon and Wilson (N = 21).7,8,21 Briefly, the distal 2 cm of the right uterine horn were removed and immersed in warm (37oC) sterile culture medium. The endometrium was exposed by opening the uterine horn lengthwise with a pair of sterile scissors. Four pieces of uterine horn measuring 2 × 2 mm were cut. These implants were sutured with the endometrial surface next to the mesenteric vessels of the small intestine. The peritoneal cavity was kept moist with copious amounts of saline solution throughout the surgery to reduce adhesions. In the sham operated group (N = 10), 4 sutures were attached to the mesentery of the intestine without implants, and the right uterine horn was massaged with fingertips for 2 minutes to minimize any effects resulting from the mechanical handling of the uterine horn. All animals were killed 60 days after surgery.
Swim Stress Protocols
Seven days after the surgical induction of endometriosis, the rats were handled again for 7 days (5 min/d/rat) prior to exposure to a swim stress protocol (Figure 1A). This consisted of a plastic pool (150 cm diameter, 60 cm deep) filled with water (37oC [1oC]) and made opaque with nontoxic water-soluble paint. The position of the animals during the task was monitored and recorded using a ceiling-mounted video camera (HVS-Image, Hampton, United Kingdom) connected to a computerized tracking/imaging analyzer system (Watermaze Software, Edinburgh, United Kingdom). Each animal received 10 exposures to swim stress per day, for 10 days. The duration of each exposure was 60 seconds, separated by a 1-minute interval, to allow the animal to rest. The water temperature in the pool was closely monitored and maintained at approximately 37oC. The colonic propulsive activity was assessed by counting the number of fecal pellets expelled during each stress exposure.22,23 The average from the 10 exposures was calculated for each day of the stress protocol per animal, then expressed as the average number of fecal pellets/minute (standard error of the mean) per group. The normal and endo-no stress animals were maintained undisturbed in their respective home cages and fecal pellets expelled were counted during a time period similar to that of the animals undergoing the stress protocol.
Collection of Tissues
At the time of killing all animals had a cytologic smear taken so as to allow for interpretation of any effects of the stage of estrous cycle on the experimental results. A laparotomy was performed and peritoneal fluid was aseptically aspirated using a sterile micropipette, taking care not to contaminate with blood. A Wright’s stained smear was prepared from a drop of peritoneal fluid to enable quantification of inflammatory cells. The number of white blood cells (WBCs) in the peritoneal fluid was determined microscopically by examining 5 high-power fields (×400) and obtaining an average number. To perform the WBC differential, we counted 100 cells/slide at high-power field and classified them as neutrophils, lymphocytes, monocytes, basophils, and eosinophils based on the shape of their nucleus and the presence of cytoplasmic granules. The percentage for each cell type was calculated.
The peritoneal cavity was systematically examined for the presence of the implants and the original sutures. The classifications of implants in terms of grades of growth were carried out as previously described.7,24 In brief, the site of the implants was examined for the presence/development of vesicles or cysts and their diameter measured and weight noted. Vesicles <2 mm received a grade of 2, vesicles with fluid ≥2 mm to <4.5 mm received a grade of 3, and vesicles ≥ 4.5 mm received a grade of 4. If the implant had disappeared, it received a grade of 1.
Macroscopic and Microscopic Damage
The whole colon was removed and examined for macroscopic damage (ulceration, adhesions, colon thickness, and diarrhea) using an established, previously well-defined scoring system.7 Tissue segments were fixed in 10% formalin. After routine processing, sections were stained with hematoxylin–eosin to determine the extent of inflammatory infiltration and the appearance of the underlying muscle layers. Histological assessment of damage was performed using previously published criteria.7,8 Briefly, we evaluated the loss of mucosal architecture, muscle thickness, neutrophil infiltration, crypt abscess formation, and goblet cell depletion. Samples of colon were taken for myeloperoxidase (MPO) measurement and histology, and peritoneal fluid was stored for later analysis.
Microscopic Analysis for Mast Cells
Segments of rat colon (randomly selected from the midregion) and sections of uterus from the left uterine horn were fixed in Carnoy’s fixative, processed by routine techniques and mounted on glass slides. The sections were stained with Alcian blue and counterstained with Safranine O to permit visualization of the mast cells. The numbers of mast cells in 10 randomly selected high-power fields were counted and the mean number of mast cells per field were calculated.
Measurement of Neutrophil Infiltration
Tissue MPO activity was determined in colonic tissues as an index of granulocyte infiltration. Myeloperoxidase is an enzyme found within the azurophilic granules of neutrophils and other cells of myeloid origin. It has been demonstrated previously that these levels reflect the state of inflammation in the mucosa of the intestine.25 Approximately 100 mg of flash-frozen tissues that were collected from both the distal region and most proximal region of the colon were analyzed. A modified technique described by Bradley et al was used.26 In this assay, hydrogen peroxide is broken down by MPO released from tissue samples by the addition of a detergent. The resultant oxygen radical combines with a hydrogen donor and the colorimetric change is measured on a plate reader.
Immunodetection of Nerves and Receptors
Formalin-fixed 4-μm tissue sections of the uterus and colon were deparaffinized with xylene, then hydrated through descending grades of ethanol to distilled water. Endogenous peroxidase was blocked with 3% aqueous hydrogen peroxide for 15 minutes. Following antigen retrieval on a hot plate with the appropriate buffer (0.01 M citrate buffer, pH 6.0, 95°C-99°C for 40 minutes for NGF, Trk-A-p75, and calcitonin gene–related peptide [CGRP]; EDTA—high to boiling, then 15 minutes at sub-boiling temperature for pTrk-A), slides were cooled at room temperature for 20 minutes, rinsed with distilled water (2 changes, 2 minutes each), and placed in phosphate buffered saline or tris buffered saline/Tween for 5 minutes. Slides were dried and blocked with normal serum for 15 minutes, followed by overnight incubation with the primary antibody, NGF (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), Trk-A (1:200 dilution; Sigma, St. Louis, MO), pTrK-A (1:10 dilution; Sigma, St. Louis, MO), p75 (1:50 dilution; Millipore, Burlington, MA), and CGRP (1:100 dilution; Abcam). Super-sensitive link-label immunohistochemistry (IHC) detection system and liquid 3,3′-diaminobenzidine solution (BioGenex) were used. Pictures were taken of representative areas of the slides and were scored blinded in triplicate by 3 observers. A score was assigned depending on the strength of staining, where 0 indicated the lightest, or absence of, staining; while 3 represented the strongest staining.
Statistical Analysis
The data were analyzed by calculating the mean or the median difference. These differences were expressed as mean difference (± standard error), mean difference (± standard deviation), or median difference (± standard deviation). The statistical significance of differences before and after was assessed by the paired Wilcoxon test, and the independent samples Wilcoxon test was used for the differences among groups. The weight, damage scores, and IHC data were analyzed using an analysis of variance (ANOVA) followed by a Tukey-Kramer multiple comparisons test. Fecal pellet counts were analyzed using ANOVA followed by a Bonferroni corrected multiple comparisons procedure. Statistical analyses were performed using GraphPad Instat version 3.0 (GraphPad Software, San Diego, California). In all cases, P <.05 was considered to represent a significant difference.
Results
Stress Biomarkers
The number of fecal pellets produced during the stress protocol was used as an indirect index of anxiety related to stress.27 During the 10 days of the stress protocol, the fecal output of the endo-no stress or normal animals (placed in a new clean cage) during the time of the stress procedure remained around zero (Figure 1B). In contrast both groups receiving stress had significantly higher defecation than those not receiving stress throughout the 10-day period, indicating higher levels of anxiety regardless of whether the animals had endometriosis or not. Post hoc analysis showed no significant difference in fecal output between the 2 groups receiving stress (endo-stress and sham-stress) indicating that both groups were equally anxious.
Ectopic Implant Size
There was no difference in the body weight between the groups at the time of killing (276.02 [2.13] g). After the animals were killed, classification of the implant’s growth into cystic vesicles was performed as described.7,24 As expected, none of the normal or sham-stress animals developed vesicles. All of the experimental (implanted) rats developed vesicles in at least one of the implant sites. While we did not observe any significant difference in the total number of implants between the groups, the severity of the lesions in rats undergoing stress was greater with larger vesicles. The endo (no stress) animals had an average length per vesicle of 1.88 [0.20] mm. This was significantly increased to 2.68 [0.26] mm in the endo-stress group (P < .05; Figure 1C). Moreover, 40% of the vesicles in the group receiving stress was larger than 2 mm (grade 3 or 4). No grade 4 vesicles were found in the endo-no stress group.
All rats were surgically induced when in estrous and proestrous, however, at the time of killing there was a spread among proestrous, estrous, and anestrous phase, with the majority (about 80%) being in either proestrous or estrous. There was no significant difference in the cycle stage between the different groups at the time of killing.
Colonic Damage and Cell Infiltration
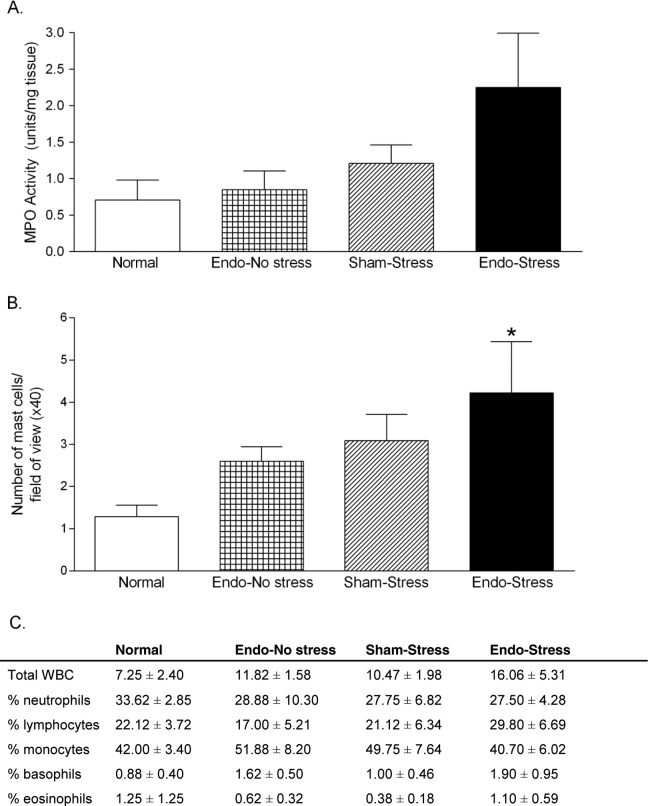
Segments of colons were analyzed for measurement of MPO activity, to give an indication of neutrophil infiltration. Although twice the amount of MPO was measured in the endo-stress animals than in the endo-no stress animals (2.25 [0.75] units/mg tissue vs 0.84 [0.25] units/mg of tissue), this difference failed to reach statistical significance (P = .08; Figure 2A).
Figure 2.
Effect of stress on inflammatory parameters. The endo-stress animals had (A) the highest MPO levels although this did not reach statistical significance and (B) increased numbers of mast cells compared to normal (*P< .05). (C) No significant differences were observed in numbers of white blood cells in the peritoneal fluid (n = 8-11 [SEM]). MPO indicates myeloperoxidase; SEM, standard error of the mean.
Segments of colons were analyzed macroscopically for damage and stained with hematoxylin and eosin for microscopic analysis. The parameters taken into consideration to produce a microscopic damage score included epithelium integrity, cellular infiltration, goblet cell depletion, crypts assess formation, and muscle thickness. No significant differences were found in the histology scores for the colon, being equally high across all groups (5.70 [0.63] for endo-no stress, 6.60 [0.37] for sham-stress, 6.82 [0.46] for endo-stress, and 6.11 [0.45] in normal). No significant differences were observed in the macroscopic damage in the colon between groups.
There were twice as many mast cells observed in those endometriosis animals which were exposed to stress (4.22 [1.21] mast cells/high-power field of view) compared to nonstressed controls (2.60 [0.34] mast cells/high-power field of view) but this did not reach significance. Colonic tissue from normal rats had significantly fewer mast cells (1.28 [0.77] mast cells/high-power field of view; P <.05) than in endo-stress rats (Figure 2B).
The number of WBC in the peritoneal fluid was measured to determine the presence of peritoneal inflammation in the experimental and control rats (Figure 2C). The endo-stress rats had 16.06 (5.31) WBC/high-power field of view compared to 11.82 (1.98) WBC/high-power field of view in the endo no-stress rats, which was not significantly different. A differential analysis revealed that the majority of the cells in the peritoneal fluid were monocytes regardless of treatment group, and the rest were neutrophils and lymphocytes. Very few basophils or eosinophils were found, and the cellular distribution appeared to be similar between the groups.
Expression of NGF and Its Receptors
Uterine and colon tissue from normal, endo-no stress, sham-stress, and endo-stress animals were stained by immunohistochemistry for the presence of NGF and its receptors, Trk-A/pTrk-A and the p75 protein, in endometrial glands and stroma, and in colon muscle and mucosa, respectively. We also examined uterine and colon tissue sections for the expression of CGRP, a sensory nerve fiber marker.
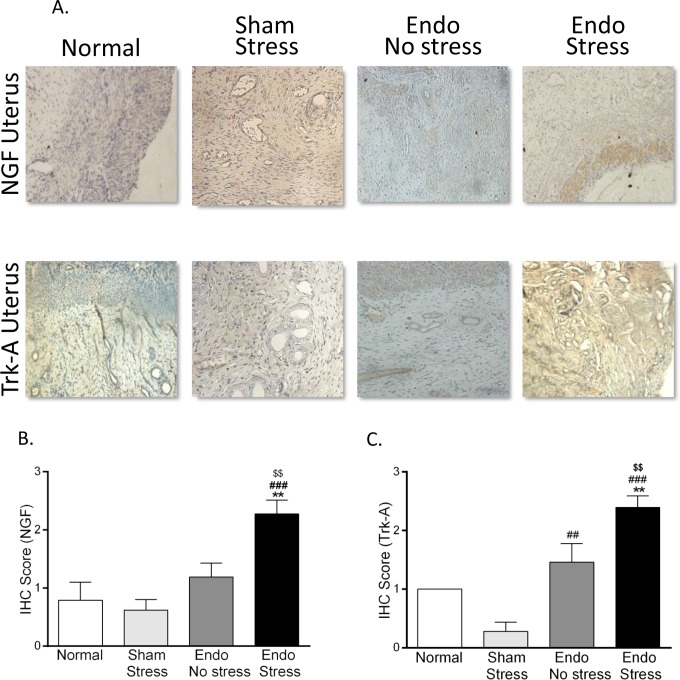
In the uterus, both NGF and Trk-A expression was significantly increased by exposure of the endometriosis animals to stress, reaching significance in the uterine stroma (P < .01 vs endo-no stress; Figure 3). No significant differences were found in the expression of the phosphorylated form of Trk-A or the p75 receptor, in either uterine stroma or glands. Trk-A expression was increased in endo-no stress animals compared to sham-stress (P < .01). There were no significant differences in the expression of NGF and the NGF high-affinity receptor (Trk-A) between normal and sham-stress in either the glands and stroma. Staining for CGRP was found to be significantly higher in the uterine stroma of all animals with endometriosis, regardless of exposure to stress (1.00 [0.00] and 1.00 [0.36] for endo-no stress and endo-stress vs 0.27 [0.06] for normal; P <.05). No significant differences were found between the groups in the glandular compartment.
Figure 3.
Immunohistochemical staining in rat uterus. (A) Representative pictures of NGF and Trk-A expression in uterus (×40). Stress significantly increased the expression of (B) NGF and (C) its receptor Trk-A in uterus (n = 6-8 animals per group [SEM], **P <.01 vs normal, ## P <.01, ### P <.001 vs sham-stress, $$ P <.01 vs endo). NGF indicates nerve growth factor; SEM, standard error of the mean.
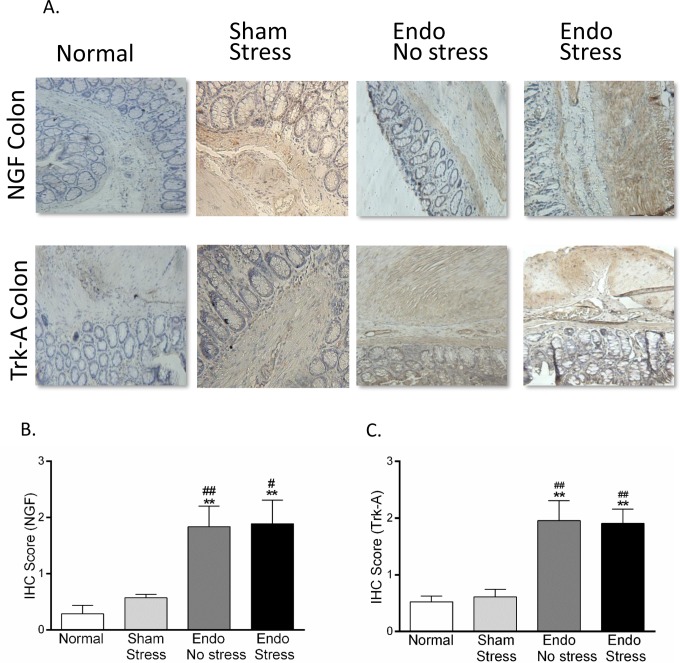
In the colon, there was a greater than 3-fold increase in expression of NGF and Trk-A in colon muscle in both the endo-no stress and endo-stress groups versus normal and sham (Figure 4; P < .01). Similar results were observed in the mucosal layer, but they did not quite reach statistical significance. The highest expression of pTrk-A was found in both layers of the colon in the endo-stress animals although this difference did not reach significance. In contrast, expression of the low-affinity receptor, p75, was not found to be different between the groups in either muscle or mucosa (data not shown). No significant differences were found in the expression of CGRP in either colon muscle or mucosa.
Figure 4.
Immunohistochemical staining in rat colon. (A) Representative pictures of NGF and Trk-A expression in colon (×40). Stress significantly increased the expression of (B) NGF and (C) its receptor Trk-A in colon (n = 6-8 animals per group [SEM], **P <.01 vs normal; # P <.05, ## P <.01 vs sham-stress). NGF indicates nerve growth factor; SEM, standard error of the mean.
Discussion
It is becoming recognized that stress is a significant component of many recurrent and chronic health problems. Our team has been one of the first to examine the involvement of stress in the progression of endometriosis, a multifactorial and chronic disease, and its symptoms.7,8,28 We have previously shown that stress during the disease process promoted the growth of endometriotic lesions.8 Importantly, we also showed that stress management could prevent the outgrowth of the lesions and the inflammation in this animal model, providing a causal link between stress and endometriosis. The present study provides further evidence for an important role of stress in enhancing inflammatory and pain mechanisms that may result in a higher morbidity of patients with endometriosis who are exposed to chronic stress. In patients, studies on the role of stress are still limited for example, one showing that women in stressful jobs had a doubled risk for short cycle length (a known risk factor for endometriosis) compared with women who did not consider their jobs stressful, and a case report measuring physiologic and neural reactivity in a patient, which correlated with stress due to family issues.6,29,30 The results presented here provide further direct evidence for the apparent negative effects of stress on nerve growth during the progression of endometriosis, showing an exacerbation of the condition in animals exposed to chronic stress.
The immune system is known to play a key role in the onset and development of endometriosis.11 This disease is associated with increased secretion of pro inflammatory cytokines and impaired function of cell-mediated natural (macrophages, natural killer cells) and specific (T and B cells) immunity. This network of locally produced cytokines modulates the inflammatory process induced by ectopic growth of endometrial implants. Pro inflammatory proteins from endometriotic lesions and associated immune cells contribute to the augmented and sustained inflammatory reactions that are associated with endometriosis symptomatology. The development of endometriosis may also be influenced by the quantity and quality of endometrial cells in the peritoneal fluid, and by immune factors, including increased numbers and heightened activation of inflammatory cells, impaired immune recognition and clearance of ectopic endometrial cells, and formation of autoantibodies.31 Because of the known associations between stress, aberrant HPA axis, and inflammatory process, our present study was conducted to identify the possible role of stress not only in promoting lesion growth but also in causing physiological changes in other peritoneal organs.
In endometriosis, the ectopic endometrial tissue is surrounded by abundant fibrotic tissue and inflammatory infiltrate, but the triggering factors for these processes are not yet clearly understood. It has been previously reported that mast cells are significantly increased at sites of peritoneal endometriosis,13 and diffuse infiltration of numerous mast cells throughout the stromal lesions has also been found in cases of endometriosis. These mast cells exhibited degranulation and scattered granules, suggesting that an abnormal immune response, specifically a hypersensitivity reaction, is strongly related to endometriosis.32 Moreover, proteases secreted from mast cells play an important role in fibrogenesis.33 Anaf et al14 demonstrated that in patients with endometriosis, there was an increase in both activated and degranulating mast cells in deeply infiltrating lesions, which are the most painful type. The close histological relationship observed between the mast cells and nerves suggests that these cells can contribute to the development of pain and the pathophysiology of this disease. Our study supports these findings with the endometriosis rats which received stress having higher mast cell infiltration and a trend toward an increased number of WBCs. It is widely accepted that in other inflammatory diseases of the intestine, such as inflammatory bowel disease and irritable bowel syndrome (IBS), the involvement of stress and the immune system with its mediators and cells, including mast cells, are crucial to the disease progression.34–36 Increased numbers of mucosal mast cells and T cells have been found in patients with IBS,37 with activated mast cells lying in close anatomical association with axonal fibers of the enteric nervous system.38 This suggests that these cells when activated may trigger visceral hypersensitivity and contribute toward the pain and motility complaints often associated with gastrointestinal disorders. In this study, those endometriosis animals receiving stress had increased fecal pellet counts (indicative of increased motility) compared to controls.
It is known that the endometriotic implants in rats and women are able to develop their own sensory and sympathetic innervation, which is thought to contribute to the symptoms observed in the disease.19,39 In patients, increased density of nerve fibers in deep infiltrating endometriosis compared to superficial peritoneal lesions, and strong expression of NGF and its receptors, including TrkA has been observed.40 Increased levels of NGF in tissues with inflammation can lead to hyperalgesia, and elevated levels of NGF has been more frequently found in the peritoneal fluid of patients with more severe pain.41 In rats, Berkley and colleagues have found the presence of TrkA on CGRP-positive sensory nerve fibers and an increase in NGF in the vesicles during the time of the cycle associated with the greatest hyperalgesia.42 To determine whether endometriosis exacerbation by stress involves nerve fiber development in eutopic endometrium and colon, contributing to the morbidity of this condition, immunohistochemical staining for NGF and its receptors were performed on colon and uterine tissues. Nerve growth factor is a small secreted protein that induces the differentiation and survival of particular target neurons, critical for the survival and maintenance of sympathetic and sensory neurons. It can also stimulate the release of natural chemicals that increase the sensitivity of nociceptors to pain.14,40 Trk-A is the high-affinity catalytic receptor for NGF, and it has been shown to have both acute and long-term effects on nociceptor functions.40 Nerve growth factor binding to TrkA results in TrkA autophosphorylation. This active form of TrkA subsequently induces a series of intracellular signaling pathways43 that may be important for the development of inflammation and hyperalgesia characteristic of endometriosis.
Positive immunostaining for NGF and Trk-A was found in colon muscle and mucosa in the endometriosis rats, which was not altered further by stress. In contrast, in the uterus, this increased expression was most evident in the endometriosis rats receiving stress, being significantly higher in the uterine stroma. These findings demonstrate for the first time the presence of new nerve growth in eutopic uterine tissue in this animal model. It is well-known that NGF levels increase in inflamed tissues, which can lead to hyperalgesia by both peripheral and central mechanisms42 Peripherally, NGF directly activates and sensitizes primary afferent nociceptors.44 In addition, previous studies have shown that NGF induces sprouting of sympathetic fibers in lesions.45 Interestingly, recent studies targeting inhibition of the TrkA receptor have found inhibition of neurite outgrowth under in vitro conditions as well as attenuation of mechanical hyperalgesia in a rat model.46,47 These results concur with other findings showing that neurotrophin growth factors and receptors are important regulators of development and maintenance of the nervous system, particularly NGF/TrkA signaling.48 It has been postulated that the new nerve growth around endometriotic lesions might help the abnormally located tissue to survive, and that increased density of nerve fibers may play an important role in the pathogenesis of pain and tenderness.47,49,50 We have extended this work by providing evidence for increased levels of NGF which has been shown by others to play a role in the hyperalgesia of organs in the peritoneum such as colon and uterus. It will be of interest to research the possibility that the higher NGF levels could lead to increased uterine excitability and secretion of prostaglandins and heightened pain symptoms. Intriguingly, such a finding could offer an additional potential explanation for the infertility associated with endometriosis: increased nervous excitation of the uterus may not be conducive toward implantation.
Limitations of our study include the fact that we examined one type of stressor during a defined time period. In the human condition, patients are subjected to many different types of stress, which can be present in a chronic or acute fashion or may actually result from dealing with the symptoms of the disease. Also, our measurement of fecal pellet count, while considered an indirect measure of increased intestinal motility that is often linked with a high-anxiety state, is not directly comparable to a similar measurement in patients. In this set of experiments, we also did not include a sham-no stress group since we have previously shown that rats with experimental endometriosis have higher levels of inflammatory factors (such as inflammatory cells in the peritoneal fluid) than rats with a sham-surgery (equivalent to sham-no stress). The present studies were therefore designed to test the hypothesis that stress will make these parameters even worse. Experimental limitations also included the small amount of tissue that could be collected from the vesicles in many cases which precluded the concomitant extraction of nucleic acids and proteins for our studies. We chose to analyze protein since they are the final product of gene expression.
In summary, in this animal model of endometriosis, exposure to an uncontrollable stressor increased the number and size of the endometriotic vesicles that developed, the local inflammatory parameters, and nerve fiber development in the uterus. It is likely that exposure to stress modulates the immune and nervous systems which then contributes to the symptomatology of endometriosis. Given the omnipresent nature of psychological stress in today’s society, these results suggest that stress-management interventions may be a useful avenue for future therapeutic alternatives for the painful signs and symptoms of endometriosis.
Acknowledgments
The authors would like to acknowledge the technical support of Olga Santiago for the surgeries, Alcira Benitez for histological preparation and analysis, and Angel A. Isidro and Victor A. Rodriguez for help with analysis and staining. In addition, we will like to acknowledge the epidemiological and statistical support to this project by the Epidemiology and Biostatistics CORE Program of Ponce School of Medicine, especially Dr Carolina Alvarez. This CORE Program was federally funded by NIH/NCRR/RCMI Grant Number 2G12RR03050.
Authors’ Note: Institution where work was done was Ponce Health Sciences University, Ponce, PR.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were supported in part by F31 GM082281 (MC), R01HD050559 (IF), S06-GM08239 (KJT and IF), and R15AT006373 (CBA) from the National Institutes of Health (NIH); and by G12-RR03050 (CBA) from the National Center of Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
References
- 1. Tariverdian N, Rucke M, Szekeres-Bartho J, et al. Neuroendocrine circuitry and endometriosis: progesterone derivative dampens corticotropin-releasing hormone-induced inflammation by peritoneal cells in vitro. J Mol Med. 2010;88(3):267–278. [DOI] [PubMed] [Google Scholar]
- 2. Toth B. Stress, inflammation and endometriosis: are patients stuck between a rock and a hard place? J Mol Med. 2010;88(3):223–225. [DOI] [PubMed] [Google Scholar]
- 3. Huntington A, Gilmour JA. A life shaped by pain: women and endometriosis. J Clin Nurs. 2005;14(9):1124–1132. [DOI] [PubMed] [Google Scholar]
- 4. Barnack JL, Chrisler JC. The experience of chronic illness in women: a comparison between women with endometriosis and women with chronic migraine headaches. Women Health. 2007;46(1):115–133. [DOI] [PubMed] [Google Scholar]
- 5. Cousineau TM, Green TC, Corsini E, Seibring A, et al. Online psychoeducational support for infertile women: a randomized controlled trial. Hum Reprod. 2008;23(3):554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrison V, Rowan K, Mathias J. Stress reactivity and family relationships in the development and treatment of endometriosis. Fertil Steril. 2005;83(4):857–864. [DOI] [PubMed] [Google Scholar]
- 7. Cuevas M, Flores I, Thompson KJ, Ramos-Ortolaza DL, Torres-Reveron A, Appleyard CB. Stress exacerbates endometriosis manifestations and inflammatory parameters in an animal model Reprod Sci. 2012;19(8):851–862. Epub 2012 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Appleyard CB, Cruz ML, Hernández S, Thompson KJ, Bayona M, Flores I. Stress management affects outcomes in the pathophysiology of an endometriosis model. Reprod Sci. 2015;22(4):431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 10. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertile Steril. 2001;75(1):1–10. [DOI] [PubMed] [Google Scholar]
- 12. Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24(8):444–448. [DOI] [PubMed] [Google Scholar]
- 13. Matsuzaki S, Canis M, Darcha C, Fukaya T, Yajima A, Bruhat MA. Increased mast cell density in peritoneal endometriosis compared with eutopic endometrium with endometriosis. Am J Reprod Immunol. 1998;40(4):291–294. [DOI] [PubMed] [Google Scholar]
- 14. Anaf V, Chapron C, Nakadi IE, De Moor V, Simonart T, Noel J-C. Pain, mast cells, and nerves in peritoneal, ovarian and deep infiltrating endometriosis. Fertil Steril. 2006;86(5):1336–1343. [DOI] [PubMed] [Google Scholar]
- 15. Bienenstock J, Tomioka M, Matsuda H, et al. The role of mast cells in inflammatory processes: evidence for nerve/mast cell interactions. Int Arch Allergy Appl Immunol. 1987;82(3-4):238–243. [DOI] [PubMed] [Google Scholar]
- 16. Dines KC, Powell HC. Mast cell interactions with the nervous system: relationship to mechanisms of disease. J Neuropathol Exp Neurol. 1997;56(6):627–640. [PubMed] [Google Scholar]
- 17. Marshall JS, Gomi K, Blennerhassett MG, Bienenstock J. Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism. J Immunol. 1999;162(7):4271–4276. [PubMed] [Google Scholar]
- 18. Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101(30):11094–11098. Epub 2004 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PLoS One. 2012;7(2):e31758 Epub 2012 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D’Hooghe T, Hummelshoj L. Multi-disciplinary centres/networks of excellence for endometriosis management and research: a proposal. Hum Reprod. 2006;21(11):2743–2748. Epub 2006 Sep 18. [DOI] [PubMed] [Google Scholar]
- 21. Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44(5):684–694. [PubMed] [Google Scholar]
- 22. Appleyard CB, Cruz ML, Rivera E, Hernandez GA, Flores I. Experimental endometriosis in the rat is correlated with colonic motor function alterations but not with bacterial load. Reprod Sci. 2007;14(8):815–824. [DOI] [PubMed] [Google Scholar]
- 23. Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119(6):1569–1579. [DOI] [PubMed] [Google Scholar]
- 24. Ingelmo MR, Quereda F, Acién P. Intraperitoneal and subcutaneous treatment of experimental endometriosis with recombinant human interferon-α-2b in a murine model. Fertil Steril. 1999;71(5):907–911. [DOI] [PubMed] [Google Scholar]
- 25. Boughton-Smith NK, Wallace JL, Whittle BJ. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1998;25(1-2):115–123. [DOI] [PubMed] [Google Scholar]
- 26. Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Derm. 1982;78(3):206–209. [DOI] [PubMed] [Google Scholar]
- 27. Venkova K, Johnson AC, Myers B, Greenwood-Van Meerveld B. Exposure of the amygdala to elevated levels of corticosterone alters colonic motility in response to acute psychological stress. Neuropharmacology. 2010;58(7):1161–1167. [DOI] [PubMed] [Google Scholar]
- 28. Hernandez S, Cruz ML, Seguinot II, Torres-Reveron A, Appleyard CB. Impact of psychological stress on pain perception in an animal model of endometriosis. Reprod Sci. 2017;24(10):1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11–22; discussion 34–6, 396–406. [DOI] [PubMed] [Google Scholar]
- 30. Fenster L, Waller K, Chen J, et al. Psychological stress in the workplace and menstrual function. Am J Epidemiol. 1999;149(2):127–134. [DOI] [PubMed] [Google Scholar]
- 31. Kyama CM, Debrock S, Mwenda JM, D’Hooghe TM. Potential involvement of immune system in the development of endometriosis. Reprod Biol Endocrinol. 2003;1:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sugamata M, Ihara T, Uchiide I. Increase of activated mast cells in human endometriosis. Am J Reprod Immunol. 2005;53(3):120–125. [DOI] [PubMed] [Google Scholar]
- 33. Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type 1 collagen in human lung fibroblasts. J Clin Invest. 1997;99(6):1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hart A, Kamm MA. Review article: mechanisms of initiation and perpetuation of gut inflammation by stress. Aliment Pharmacol Therap. 2002;16(12):2017–2028. [DOI] [PubMed] [Google Scholar]
- 35. Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tache Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(suppl 1):137–142. Review. [DOI] [PubMed] [Google Scholar]
- 37. O’Sullivan M, Clayton N, Breslin NP, et al. Increased mast cell in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12(5):449–457. [DOI] [PubMed] [Google Scholar]
- 38. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cell in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. [DOI] [PubMed] [Google Scholar]
- 39. Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587–1589. [DOI] [PubMed] [Google Scholar]
- 40. Anaf V, Simon P, El Nakadi I, et al. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17(7):1895–1900. [DOI] [PubMed] [Google Scholar]
- 41. Kajitani T, Maruyama T, Asada H, et al. Possible involvement of nerve growth factor in dysmenorrhea and dyspareunia associated with endometriosis. Endocr J. 2013;60(10):1155–1564. [DOI] [PubMed] [Google Scholar]
- 42. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. [DOI] [PubMed] [Google Scholar]
- 43. Kaplan DR, Martin-Zanaca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the Trk proto-oncogene product induced by NGF. Nature. 1991;350(6314):158- 160. [DOI] [PubMed] [Google Scholar]
- 44. Rueff A, Mendell LM. Nerve growth factor NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J Neurophysiol. 1996;76(5):3593–3596. [DOI] [PubMed] [Google Scholar]
- 45. Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R162–R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barcena de Arellano ML, Arnold J, Lang H, et al. Evidence of neurotropic events due to peritoneal endometriotic lesions. Cytokine. 2013;62(2):253–261. [DOI] [PubMed] [Google Scholar]
- 47. Alvarez P, Levine JD. Screening the role of pronociceptive molecules in a rodent model of endometriosis pain. J Pain. 2014;15(7):726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaplan DR, Miller FD. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol 1997;9(2):213–221. [DOI] [PubMed] [Google Scholar]
- 49. Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, Fraser IS. Hyperinnervation in intestinal deep infiltrating endometriosis. J Minim Invasive Gynecol. 2009;16(6):713–719. [DOI] [PubMed] [Google Scholar]
- 50. Anaf V, El Nakadi I, De Moor V, Chapron C, Pistofidis G, Noel JC. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112–117. [DOI] [PubMed] [Google Scholar]