Abstract
Ulipristal acetate (UPA) is a selective progesterone receptor modulator (PRM), which is used as an emergency contraceptive in women. Recent studies demonstrated the efficacy of an UPA contraceptive vaginal ring (UPA-CVR) as a blocker of ovulation. However, the endometrium of women exposed to UPA over a 6-month period display glandular changes, termed PRM-associated endometrial changes (PAECs). We, therefore, investigated whether UPA-induced PAECs are associated with altered expression of the transcription factor heart- and neural crest derivatives-expressed protein 2 (HAND2) whose downregulation is observed in endometrial epithelial hyperplasia and cancer. Our results showed that while exposure to mifepristone, a well-known PRM, leads to suppression of endometrial HAND2 expression, long-term exposure to UPA-CVR did not cause downregulation of this marker. Further studies, using human primary endometrial stromal cells, confirmed that whereas mifepristone-mediated suppression of HAND2 elevated the levels of its downstream target fibroblast growth factor 18, UPA did not significantly alter the expression of this growth factor. A rationale for the differential regulation of HAND2 by these PRMs was provided by our observation that mifepristone-bound progesterone receptors turn over at a faster rate than those bound to UPA. Collectively, these results support the selective effects of different PRMs and indicate that chronic exposure to UPA does not alter the HAND2 pathway whose dysregulation is linked to complex atypical endometrial hyperplasia and cancer. The results from this study involving a limited number of clinical samples should pave the way for a larger study to determine the safety of UPA for long-term use.
Keywords: ulipristal acetate, mifepristone, contraception, endometrium, HAND2
Introduction
It is estimated that 225 million women worldwide lack access to effective and acceptable contraceptive methods. Therefore, the development of novel clinically safe and effective methods of fertility control remains a necessity. The steroid hormone progesterone (P) acting through its nuclear receptor critically controls the ovulatory process as well as endometrial function in the human. Progesterone receptor modulators (PRMs) are synthetic compounds that interact with the progesterone receptor (PR) to suppress ovulation and/or induce endometrial atrophy, resulting in amenorrhea, a condition that is perceived favorably in many cultures around the world.1 Therefore, the development and use of PRMs as contraceptives is of particular interest.
Ulipristal acetate (UPA), also referred to as VA/CDB-2914, is a new and promising PRM.2–6 Ulipristal acetate has been approved as an emergency contraceptive7,8 in the United States and abroad and as a treatment for heavy menstrual bleeding due to uterine fibroids9,10 in Canada and Europe. Successful use of this PRM as an emergency contraceptive has raised the possibility that a simplified continuous delivery of UPA could improve long-term contraceptive safety and efficacy and compliance. With this goal in mind, an UPA contraceptive vaginal ring (UPA-CVR) was designed for long-term contraceptive use by the Population Council, New York. In a study conducted by the Council, healthy women with normal baseline ovulation were randomized to receive UPA-CVR for 2 consecutive 12-week treatment periods, followed by a recovery cycle.11 The results from these studies indicated that the UPA-CVR has the potential to become an effective long-acting, user-controlled contraceptive. However, endometrial biopsies taken at the end of the treatment period displayed histological glandular changes, described as PRM-associated endometrial changes (PAECs).11 While these endometrial changes are considered to be benign due to the lack of cytological atypia,12 an in-depth study is needed to confirm the absence of any endometrial abnormality, including hyperplasia, following chronic PRM use.
The endometrium, the innermost layer of the uterus, undergoes proliferation and differentiation in a cyclical manner in response to the steroid hormones, 17β-estradiol (E) and P acting via their cognate receptors.13–15 While E acting via estrogen receptor alpha (ERα) functions as a mitogen and promotes the growth and proliferation of the endometrial epithelium in a cyclical fashion during the reproductive cycle, P acting via PR inhibits E-induced epithelial proliferation and causes differentiation. Uncontrolled proliferation of the endometrial epithelium results in alterations of glandular architecture (shape and size) and an increase in endometrial gland to stroma ratio, leading to endometrial hyperplasia.15–17 The majority of cases of endometrial hyperplasia are associated with compromised P signaling that fails to oppose E signaling.17–19
We have previously shown that the transcription factor heart- and neural crest derivatives-expressed protein 2 (HAND2), which is regulated by the PR present in the endometrial stroma, is a key mediator of the well-known antiproliferative effect of P on the endometrial epithelium.20 HAND2 suppresses the production of several stromal fibroblast growth factors (FGFs), which act in a paracrine manner via the FGF receptors (FGFR) to promote epithelial proliferation. Therefore, in the absence of HAND2, the endometrial epithelium undergoes unbridled FGF-induced proliferation that leads to complex atypical hyperplasia. It is of interest to note that the HAND2 gene locus is prone to epigenetic alterations. Our recent studies revealed that the HAND2 gene is hypermethylated and silenced in endometrial hyperplasia and cancer.21 When compared to other frequent DNA-based alterations in endometrial cancers, such as p53, PTEN, and PIK3CA mutations, HAND2 hypermethylation was found to be the most common.21 Since the downregulation of HAND2 expression is linked to endometrial hyperplasia and cancer, we examined the expression of this factor in endometrial biopsies of women exposed to UPA-CVR for 24 weeks. We also compared the endometrial effects of UPA with those of mifepristone, a well-known PRM.
Materials and Methods
Endometrial Biopsies
Endometrial biopsy samples were obtained using either a Pipelle (Cooper Surgical, Trumbull, Connecticut, Santo Domigo, Dominican Republic, Santiago, Chile) or an Explora (Cooper Surgical, Portland, Oregon ) device. A portion of each sample was placed for use in 10% neutral buffered formaldehyde for histology and immunohistochemistry (IHC) studies.
In Vitro Decidualization of Human Endometrial Stromal Cells
Our studies involving primary human endometrial stromal cell (HESC) cultures follow the regulations stated for the protection of human participants participating in clinical research and are approved by the institutional review boards of Emory University, Wake Forest University (Winston-Salem, North Carolina), and the University of Illinois at Urbana–Champaign. Endometrial samples from the early proliferative stage of the menstrual cycle were obtained by Pipelle biopsy at Emory University and Wake Forest Medical Centers from fertile, regularly cycling volunteers with no sign of uterine abnormality, providing written informed consent as described previously.21,22
Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 medium (Invitrogen, Grand Island, NY) supplemented with 5% (vol/vol) fetal bovine serum (FBS; Hyclone, Logan, Utah), 50 μg/mL penicillin, and 50 μg/mL streptomycin (Invitrogen, Grand Island, NY). For in vitro differentiation, the cells were treated with differentiation cocktail composed of 10 nM E (Sigma, St. Louis, MO), 1 μM P (Sigma, St. Louis, MO), 0.5 mM 8-bromoadenosine-cAMP (Sigma, St. Louis, MO), and 10 μM UPA or mifepristone in DMEM/F-12 medium (Invitrogen, Grand Island, NY) supplemented with 2% (vol/vol) charcoal dextran-stripped FBS for 0 to 6 days. At the end of the culture (2 or 6 days), the cells were detached from the plates, counted, and stored at −80°C for RNA extraction. Additionally, some cells were fixed for immunocytochemical (ICC) analysis. In some experiments, the cells were treated with differentiation cocktail composed of 10 nM E, 1 μM P, 0.5 mM 8-bromoadenosine-cAMP, and 5 μM UPA or mifepristone in DMEM/F-12 medium supplemented with 2% (vol/vol) charcoal dextran-stripped FBS for 0 to 6 days. Cultures were terminated at days 2 to 6 for RNA extraction.
Chemicals, Reagents, and Antibodies
Progesterone, E, naphthol AS-MX phosphate, fast blue RR (4-benzoylamino-2,5-dimethoxyaniline diazonium), collagenase, pancreatin, dimethyl sulfoxide, 8-bromoadenosine 3′, 5′-cyclic monophosphate salt (cAMP), and trypan blue were purchased from Sigma (St. Louis, MO). Hanks’ balanced salt solution, dispase, DMEM/F-12 medium with (4-(2-hydroxethyl)-1-piperazinethanesulfonic acid (HEPES), no phenol red (DMEM/F-12), penicillin–streptomycin, and fungizone were purchased from Life Technologies, NY. The FBS was purchased from Hyclone, Logan, UT. Fluoromount-G with 4′, 6-diamidino-2-phenylindole (DAPI) was purchased from eBiosciences, Hatfield, PA.
Endometrial sections or endometrial stromal cells were incubated with 1 or more of the following primary antibodies: HAND2 (1:250, Santa Cruz Biotechnology, Santa cruz, CA; SC-9409), FGF18 (1:100, Santa Cruz Biotechnology, Santa Cruz, CA; SC-393471), PR (1:100, DAKO, Denmark; DAKO-A0098), and Progesterone receptor B isoform (PRB)22 (1:300, Cell Signaling, Beverly, MA; CST-31575). The fluorescent-tagged secondary antibodies and normal donkey serum were purchased from Jackson ImmunoResearch, West Grove, PA. The following secondary antibodies were used: rhodamine or Cy3 donkey anti-rabbit, 488 donkey anti-rabbit, 488 donkey anti-mouse, 488 donkey anti-goat, and Cy3 donkey anti-rat.
The IHC and ICC
Paraffin-embedded endometrial biopsy sections were subjected to IHC as described previously. Tissue sections were deparaffinized in xylene, rehydrated through a graded series of ethanol, and washed in tap water. For most of the immunostaining, antigen retrieval was performed in a pressure cooker in 10 mM sodium citrate buffer (pH 6.0) for 20 minutes and then the slides were cooled to room temperature. The sections were washed between steps (3 times for 5 minutes each) using 1× phosphate-buffered saline solution containing 0.05% Tween 20 (PBS-T). Nonspecific binding was inhibited by incubating the sections with 10% normal serum for 1 hour at room temperature. After the serum block, sections were incubated overnight at 4°C with the diluted antibody solution in PBS-T containing 1% normal serum.
Labeling was visualized by incubation with a fluorescent-tagged secondary antibody for 1 hour at room temperature. All incubations were done using a humidified chamber protected from light. Slides were mounted using a mounting solution containing DAPI. Pictures were taken using the Olympus BX51 microscope equipped for fluorescent imaging and connected to a Jenoptik ProgRes C14 digital camera with c-mount interface containing a 1.4 Megapixel CCD sensor. Fluorescent images were processed and merged using Adobe Photoshop Extended CS6 (Adobe Systems). HSCOREs were determined as described previously.23
For ICC analysis of HESC, cells were fixed in 10% (vol/vol) neutral buffered formalin solution for 10 minutes and then washed with PBS. Cells were then permeabilized using PBS containing 0.1% Triton X for 10 minutes at room temperature. Nonspecific binding was inhibited by incubating the sections with 10% normal serum for 1 hour at room temperature. After the serum block, the cells were incubated overnight at 4°C with the diluted antibody solution in PBS containing 1% normal serum. Labeling was visualized by incubation with a fluorescent-tagged secondary antibody for 1 hour at room temperature. One drop of mounting solution containing DAPI was added to each well to stain the nucleus. Pictures were taken using the Olympus Ix70 inverted microscope adapted to a Diagnostic Instrument digital camera containing a 2.0 Megapixel CCD sensor. Fluorescent images were merged and processed using Adobe Photoshop Extended CS6.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA was isolated from endometrial cells using a standard TRIzol-based protocol. The RNA concentration of each sample was determined at 260 nm using a Nanodrop ND1000 UV-vis spectrophotometer (Nanodrop, Applied Biosystems, Foster City, CA). RNA samples were reverse transcribed using the high-capacity complementary DNA reverse transcription kit (Applied Biosystems), according to the manufacturer’s instructions. Real-time quantitative polymerase chain reactions (PCRs) were carried out using SYBR Green Master Mix (Applied Biosystems, Foster City, CA) in a 7500 Applied Biosystems Real-time PCR machine (Applied Biosystems, Foster City, CA). For each sample, the mean threshold cycle (Ct) was calculated from Ct values obtained from 3 replicates. The normalized ΔCt in each sample was calculated as mean Ct of target gene subtracted by the mean Ct of the reference gene. The fold change of gene expression in each sample relative to a control was generated using the 2−ΔΔCt mathematical model for relative quantification of quantitative PCR. The mean fold induction and standard error of mean (SEM) were calculated from at least 3 or more independent experiments. The housekeeping gene RPLP0 (36B4), which encodes a ribosomal protein, was used as a reference gene.
Statistical Analyses
Experimental data for studies related to UPA-CVR were collected from 12 independent participants. For each participant, 4 endometrial biopsy samples were obtained. Biopsy 1 was an endometrial specimen obtained before administration of UPA-CVR, biopsies 2 and 3 were endometrial specimens obtained after each 12-week period in which UPA-CVR released UPA daily, and biopsy 4 was obtained following a 4-week posttreatment recovery period. Results from mifepristone studies were obtained from 6 independent clinical samples. Data related to primary HESCs were collected from 3 independent clinical samples, which were subjected to the same experimental conditions. All numerical data are expressed as mean ± SEM. When experimental samples were compared with control samples, statistical significance between the control and experimental sample was determined using the Student t test. A P value of ≤.05 was considered to be significant.
Results
Expression of HAND2 Is Unaltered in Human Endometrial Biopsies Exposed to UPA-CVR
Human endometrial biopsies were obtained from 3 different clinics located in the United States, Dominican Republic, and Chile. We have analyzed a total of 12 independent participants. For each participant, 4 endometrial biopsy samples (biopsies 1-4) were obtained. Biopsy 1 is an endometrial specimen obtained before administration of UPA-CVR during the luteal phase based on urine luteinizing hormone determinations. Biopsies 2 and 3 are endometrial specimens obtained after each 12-week period in which UPA-CVR released 1.5 or 2.5 mg UPA daily. Biopsy 4 was obtained following a 4-week posttreatment recovery period in the luteal phase, determined as above. Figure 1 shows representative endometrial samples at baseline, before administration of UPA-CVR (panel A), after exposure to UPA-CVR (panel B), and in the recovery phase (panel C). Baseline samples show normal mid-secretory phase endometrium. Upon exposure to UPA-CVR, the glands show variable cystic dilatation, mildly disordered architecture, nonphysiological secretory appearances, and coexistent mitoses and apoptotic bodies. The stroma is compact and nondecidualized and contains occasional thick-walled vessels. These features are characteristic of PAECs. In the recovery phase, the endometrium exhibits normal early secretory phase appearances.
Figure 1.
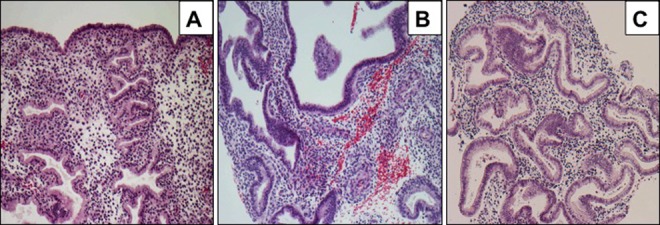
Endometria of women exposed to ulipristal acetate contraceptive vaginal ring (UPA-CVR) display progesterone receptor modulator (PRM)-associated endometrial changes (PAECs). Hematoxylin and eosin staining of endometrial sections obtained from normal mid-secretory phase endometrium (panel A), UPA-CVR releasing 2.5 mg UPA daily for 2 consecutive 12-week treatment periods (panel B), and posttreatment recovery period in the luteal phase (panel C). Note cystic dilatation, mildly disordered architecture, and nonphysiological secretory appearances in the endometria of women with UPA-CVR. These endometrial samples are part of the large clinical trial. Representative images are shown.
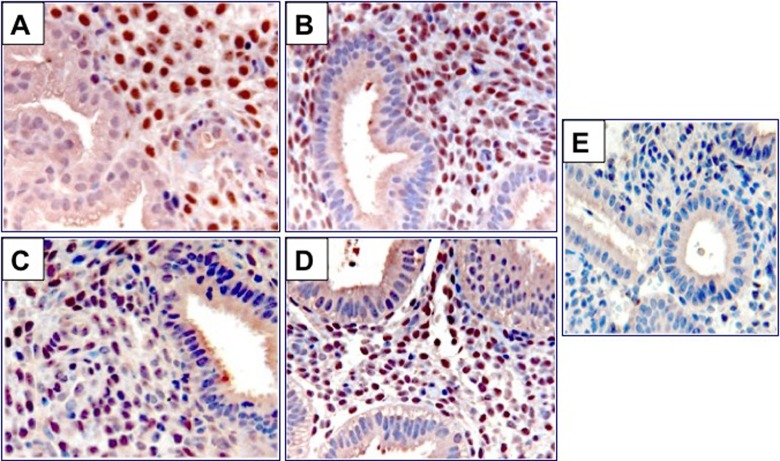
To examine the molecular changes in the endometrium following prolonged exposure to UPA-CVR, we investigated the expression of HAND2 in the biopsy specimens. An intense nuclear staining specific to HAND2 was observed in the endometrial stromal cells of pretreatment biopsy 1 specimen (Figure 2). This expression of stromal HAND2 remained unaltered in the biopsies exposed to UPA (biopsy 2 and biopsy 3; Figure 2B and C) and in the posttreatment biopsy specimen (biopsy 4; Figure 2D).To more accurately quantify the immunohistochemical findings, HSCOREs were analyzed. The HSCOREs of endometrial HAND2 immunostaining revealed no significant changes across the treatment period (Figure 3). These results indicate that a CVR releasing 1.5 or 2.5 mg/d of UPA for 24 weeks does not affect HAND2 expression.
Figure 2.
Expression of heart- and neural crest derivatives-expressed protein 2 (HAND2) in human endometrial biopsies exposed to ulipristal acetate contraceptive vaginal ring (UPA-CVR) for 24 weeks. Immunohistochemical localization of HAND2 in endometrial sections before and after exposure to UPA-CVR. A total of 12 independent participants were analyzed, and for each participant, 4 endometrial biopsy samples were obtained (N = 48). Panel A represents endometrial specimen obtained during the luteal phase before administration of UPA-CVR. Panels B and C indicate endometrial specimens obtained after each 12-week period with UPA-CVR releasing 1.5 or 2.5 mg UPA daily. Panel D represents endometrial specimen collected during the luteal phase following a posttreatment recovery period. Panel E shows endometrial sections from a biopsy sample after a 12-week exposure to UPA-CVR and subjected to immunohistochemistry (IHC) protocol omitting the primary antibody. Red staining indicates positive staining for HAND2 in endometrial sections. Representative images are shown. S and E indicate stroma and epithelium, respectively. UPA-CVR indicates ulipristal acetate contraceptive vaginal ring; IHC, immunohistochemistry.
Figure 3.
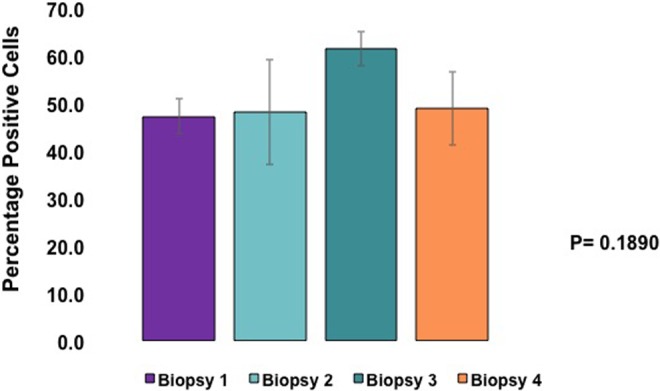
Heart- and neural crest derivatives-expressed protein 2 (HAND2) expression is unaltered in human endometrial biopsies exposed to ulipristal acetate contraceptive vaginal ring (UPA-CVR). The percentages of the immunostaining positive cells for HAND2 were analyzed by ImageJ software. The values represent mean ± SEM of 12 independent samples (N = 5 for UPA-CVR releasing 1.5 mg and N = 7 for UPA-CVR releasing 2.5 mg UPA daily) with a total of N = 48 clinical samples. No obvious dose–response effects were noted between the 2 doses.
Expression of HAND2 Is Reduced in Human Endometrial Biopsies Exposed to Mifepristone
We also analyzed the expression of HAND2 in endometrial biopsies collected from women exposed to mifepristone, a well-known PRM. In this study, 50 mg of oral mifepristone was administered every other day for 12 weeks. Endometrial biopsies were taken in the secretory phase of the last week of mifepristone treatment. Luteal phase biopsies from unexposed women demonstrated robust expression of HAND2 in the nuclei of stromal cells, as expected. In contrast, endometrial biopsies of women treated with mifepristone showed a significant decline in the expression of HAND2 (Figure 4). Quantification of HAND2 immunopositive cells in the stroma revealed greater than 80% reduction in HAND2 expression in mifepristone-exposed biopsies when compared to unexposed controls. Collectively, these results suggest that UPA-CVR and mifepristone have differential effects on endometrial HAND2 expression. It is possible that the differences are due to the pharmacology of the PRM compounds, their doses, duration, or route of administration.
Figure 4.
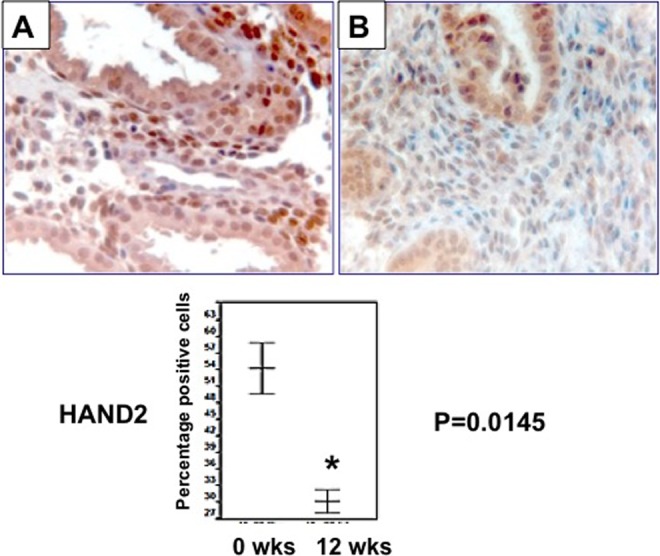
Expression of heart- and neural crest derivatives-expressed protein 2 (HAND2) is reduced in human endometrial biopsies exposed to mifepristone. Upper: Immunohistochemical analysis of HAND2 in human endometrium before (panel A) and after administration of 50 mg of oral mifepristone every other day for 12 weeks of the menstrual cycle (panel B). Representative images are shown. Lower: HSCOREs of HAND2-positive cells in the endometrium revealed a significant reduction in HAND2 expression in mifepristone-exposed biopsies when compared to unexposed controls (N = 6). B indicates baseline (0 wks) and after 12 weeks (12 wks) indicates end of treatment, and they show a significant decrease (P < .02).
Both UPA and Mifepristone Differentially Regulate HAND2 and FGF18 Expression in Cultured HESCs
To directly examine the pharmacological effects of UPA and mifepristone on HAND2 expression in the endometrial stroma under identical study conditions, we utilized a well-established HESC culture system. In this system, undifferentiated stromal cells isolated from human endometrial biopsies (HESC) obtained from normal women in the proliferative stage of the menstrual cycle were placed in culture and subjected to decidualization in response to a hormonal mixture containing 10 nM E, 1 µM P, and 0.5 mM 8-bromo-cAMP.24,25 Under the treatment conditions, cells were treated with the hormonal mixture with or without 10 µM UPA or mifepristone. Human endometrial stromal cells were cultured in the presence of hormones with or without PRMs for up to 6 days. The HAND2 messenger RNA (mRNA) expression was reduced when endometrial stromal cells were exposed to UPA or mifepristone for 2 days, compared to cells not treated with PRMs (Figure 5). While UPA exposure reduced HAND2 expression by 20%, treatment with mifepristone resulted in almost 40% reduction in HAND2 expression (P < .05). Further, the inhibitory effect of mifepristone on HAND2 expression increased in severity with longer duration of treatment. Stromal cells exposed to mifepristone for 6 days displayed more than 80% reduction in HAND2 expression when compared to untreated control cells. In contrast, treatment with UPA for 6 days had milder effects, resulting in a 30% reduction in HAND2 expression (P < .05). Consistent with the RNA profile, ICC analysis revealed similar reductions in HAND2 in the UPA-exposed (Figure 6B) and mifepristone-exposed (Figure 6C) endometrial stromal cells compared to UPA- or vehicle-treated stromal cells (Figure 6A).
Figure 5.
Ulipristal acetate (UPA) and mifepristone differentially regulate heart- and neural crest derivatives-expressed protein 2 (HAND2) messenger RNA (mRNA) expression in human endometrial stromal cells. Primary cultures of human stromal cells were grown in Dulbecco’s modified Eagle’s medium/F-12 medium containing 5% charcoal-stripped fetal bovine serum. The cells were treated with a hormone mixture containing 10 nM 17β-estradiol (E), 1 μM progesterone (P), 0.5 mM 8-bromo-cAMP, and 10 μM UPA, mifepristone, or vehicle for 6 days. Cells were harvested 2 days (left panel) or 6 days (right panel) after addition of hormone mixture. Total RNA was isolated and subjected to quantitative polymerase chain reaction (qPCR) using primers for HAND2. The level of Rplp0 was used as an internal control to normalize gene expression. The values are presented as the mean fold induction ± SEM, P < .05.
Figure 6.
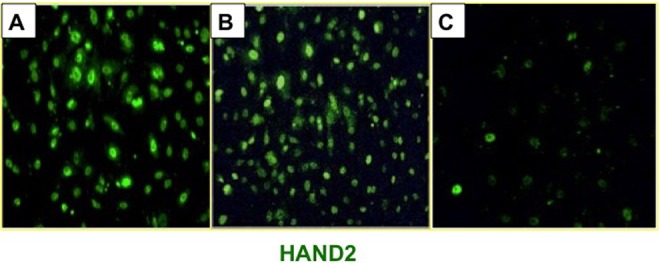
Ulipristal acetate (UPA) and mifepristone differentially regulate heart- and neural crest derivatives-expressed protein 2 (HAND2) protein expression in human endometrial stromal cells. Immunocytochemical analysis of HAND2 in stromal cells during in vitro decidualization. Panels represent primary cultures of human endometrial stromal cells cultured in the absence of UPA or mifepristone (A), in the presence of UPA (B), and in the presence of mifepristone (C) for 6 days. Representative images from 3 independent experiments are shown.
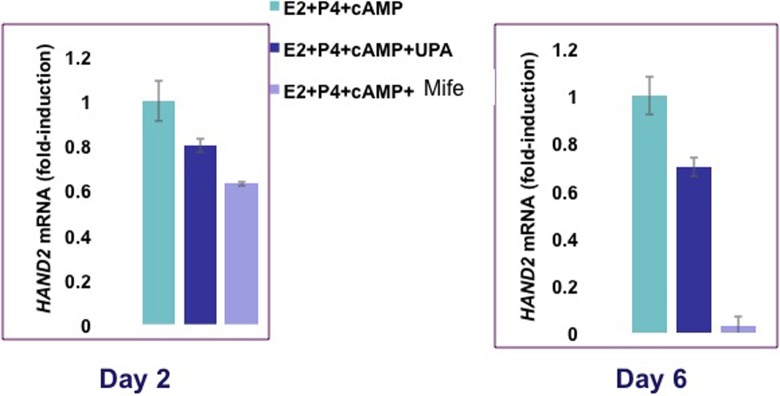
To further investigate the differential effects of UPA and mifepristone on HAND2 expression, we reduced the levels of PRMs from 10-fold to 5-fold molar excess of P. Human endometrial stromal cells were cultured in the presence of hormones with or without 5 µM UPA or mifepristone for up to 6 days. HAND2 mRNA expression was monitored on days 2 to 6 after initiation of the culture. As shown in Figure 7, treatment of HESC with 5 µM UPA did not affect the expression of HAND2 on days 2 to 6 upon initiation of the culture. By contrast, administration of 5 µM mifepristone led to a significant downregulation of HAND2 expression in HESCs. The decline in HAND2 expression was evident on day 2 and continued up to day 6 of culture.
Figure 7.
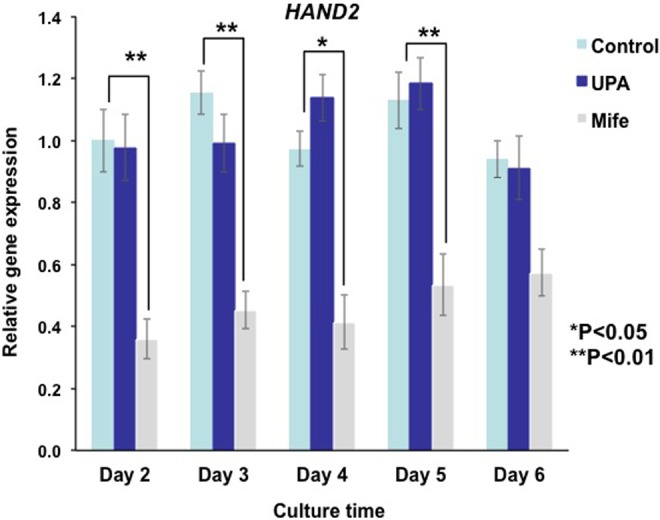
Ulipristal acetate (UPA) and mifepristone differentially regulate HAND2 messenger RNA (mRNA) expression in human endometrial stromal cells. Primary cultures of human stromal cells were treated with a hormone mixture containing 10 nM 17β-estradiol (E), 1 μM progesterone (P), 0.5 mM 8-bromo-cAMP, and 5 μM UPA, mifepristone, or vehicle for 6 days. Cells were harvested 2, 3, 4, 5, and 6 days after addition of hormone mixture. Total RNA was isolated and subjected to quantitative polymerase chain reaction (qPCR) using primers for HAND2. The level of Rplp0 was used as an internal control to normalize gene expression. The values are presented as the mean fold induction ± SEM, *P < .05, **P < .01.
Our previous studies have shown that Hand2 expression in the stroma suppresses the production of FGFs and inhibits cell proliferation.20 In the absence of Hand2, continued induction of FGFs in the stroma activates FGFR signaling in the epithelium to promote cell proliferation.20 Consistent with this observation, a recent study reported a decrease in HAND2 expression and a marked increase in the levels of FGF18 in human endometrial adenocarcinoma.26 Mifepristone-treated endometrial stromal cells demonstrated a marked increase in the levels of FGF18 mRNA (Figure 8, upper panel) and protein (Figure 8, lower panel) compared to vehicle-treated controls. In contrast, endometrial stromal cells exposed to UPA did not exhibit alterations in FGF18 expression (Figure 8). Collectively, these results support our in vivo findings and indicate that endometrial stromal cells cultured with mifepristone or UPA under identical in vitro conditions exhibit differential effects on HAND2 and FGF18 expression.
Figure 8.
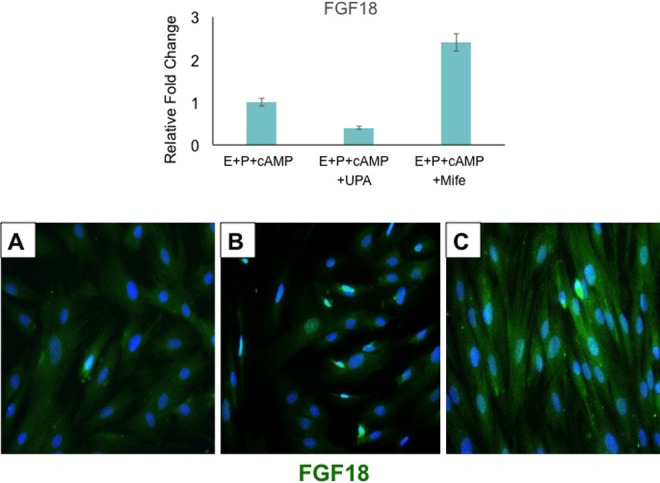
Downregulation of fibroblast growth factor (FGF) 18 expression in response to mifepristone in human endometrial stromal cells. Human endometrial stromal cells were subjected to differentiation in response to 0.5 mM 8-bromo-cAMP, 1 μM progesterone (P), 10 nM 17β-estradiol (E), and 10 μM ulipristal acetate (UPA) or mifepristone for 6 days. Upper: Total RNA was isolated and subjected to quantitative polymerase chain reaction (qPCR) using primer for FGF18. Y axis indicates fold induction. The level of Rplp0 was used as an internal control to normalize gene expression. The data are represented as the mean fold induction ± SEM from 3 separate samples. Lower: Immunocytochemical analysis of FGF18 expression in endometrial stromal in the absence of UPA or mifepristone (left panel), in the presence of UPA (middle panel), and in the presence of mifepristone (right panel). Representative images are shown.
Mifepristone and UPA Differentially Affect PR Stability in HESCs
Both UPA and mifepristone are known to regulate the function of a tissue by modulating the activity of PR, so it is interesting that endometrial stromal cells display differential gene expression when exposed to the same concentrations of these 2 PRMs. We considered the possibility that the stability of endometrial PR might be regulated differentially by mifepristone and UPA. To investigate this possibility, we determined the expression of total PR protein in P-, UPA-, or mifepristone-treated HESC by ICC. Cells exposed to P or UPA for 6 days displayed prominent nuclear PR staining, while those treated with mifepristone showed markedly reduced levels of PR (Figure 9).
Figure 9.
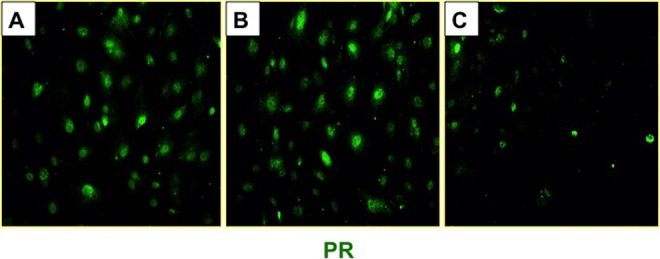
Progesterone receptor (PR) stability in response to ulipristal acetate (UPA) or mifepristone in human endometrial stromal cells. Primary cultures of human stromal cells were treated with a hormone mixture containing 10 nM 17β-estradiol (E), 1 μM progesterone (P), 0.5 mM 8-bromo-cAMP, and 10 μM UPA, mifepristone, or vehicle for 6 days. Immunocytochemical analysis of PR in endometrial stromal in the absence of UPA or mifepristone (left panel), in the presence of UPA (middle panel), and in the presence of mifepristone (right panel) are shown. Representative images are shown.
Our recent studies revealed that the PR isoform PR-B plays a predominant functional role during human endometrial stromal differentiation by controlling the expression of a large number of target genes, including HAND2.21 We noted distinct expression of PR-B22 in the nuclei of P- or UPA-treated stromal cells (Figure 10). In contrast, nuclei of stromal cells exposed to mifepristone were mostly devoid of PR-B expression. Taken together, these results are consistent with our view that UPA and mifepristone differentially affect PR stability in HESCs and this is reflected in altered expression of PR target genes, such as HAND2, in response to these ligands in the endometrial stroma.
Figure 10.
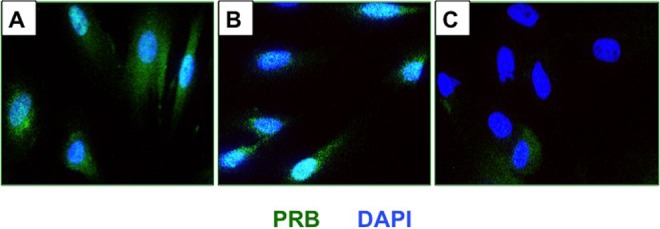
Progesterone receptor B (PR-B) stability in response to ulipristal acetate (UPA) or mifepristone in human endometrial stromal cells. Primary cultures of human stromal cells were treated with a hormone mixture containing 10 nM 17β-estradiol (E), 1 μM progesterone (P), 0.5 mM 8-bromo-cAMP, and 10 μM UPA, mifepristone, or vehicle for 6 days. The PR-B expression in endometrial stromal in the absence of UPA or mifepristone (left panel), in the presence of UPA (middle panel), and in the presence of mifepristone (right panel) are shown. Representative images are shown.
Discussion
Acritical balance of E and P drives proper endometrial stromal–epithelial cross talk and maintains normal uterine physiology. Disruption of PR function results in unopposed E action, causing epithelial hyperplasia and potentially carcinoma.17–19 The HAND2, a PR-regulated gene in the stromal cells, mediates the antiproliferative action of P to regulate endometrial epithelial function. Loss of the antiproliferative actions of P in the uterus has been linked to E-dependent endometrial cancer.27 Indeed, our recent study showed that the HAND2 gene is hypermethylated in premalignant endometrial lesions compared to normal endometrium and its expression is suppressed in endometrial hyperplasia and cancer.21 Therefore, HAND2 has emerged as a key molecular alteration in endometrial cancer that could potentially be employed as a biomarker for early detection of endometrial cancer.
To determine the clinical utility of UPA as a long-term contraceptive, it is critical to assess whether this compound, which effectively blocks PR action and ovulation, also alters the critical balance of E and P in the endometrium. Evaluation of endometrial histology following chronic UPA treatment revealed the presence glandular changes, known as PAECs, which did not show any cytological atypia, a characteristic feature of hyperplasia and cancer. However, routine histological examination of the endometrium may not provide molecular information related to a subtle imbalance of E- and P-dependent signaling that may arise due to PRM exposure. In this study, we show that the expression of HAND2, which critically regulates the balance of P- and E-dependent signaling in the endometrium, is unaffected in women exposed to UPA-CVR continuously for 24 weeks. Since downregulation of endometrial HAND2 has been linked to complex atypical hyperplasia and cancer, unaltered expression of this factor gives us confidence that exposure to the studied dose of UPA by the vaginal route of administration does not disrupt the critical balance of E- and P-dependent signaling necessary for normal endometrial physiology.
In contrast, we found that endometrial biopsies from women treated with mifepristone for 12 weeks displayed a dramatic downregulation of HAND2. Differential effects of UPA and mifepristone on HAND2 expression were confirmed in endometrial stromal cells cultured under identical conditions, suggesting that distinct mechanisms underlie the actions of these PRMs. Analysis of PR in endometrial stromal cells following in vitro exposure to PRMs demonstrated that mifepristone downregulates the PR levels, whereas equivalent molar concentrations of UPA did not have these effects. This suppression of cellular PR levels by mifepristone is consistent with previous reports that addition of mifepristone to a progestogen-only regimen of contraception leads to downregulation of PR-B.28 Additionally, recent studies have demonstrated that administration of mifepristone to an endometrial coculture system causes suppression of PR expression compared to vehicle-treated controls.29 While the mechanism by which mifepristone causes PR turnover remains unclear, we believe that this downregulation of PR is in part responsible for the dramatic suppression of HAND2 expression observed in response to mifepristone compared to UPA.
We have previously shown that HAND2 mediates the antiproliferative effects of P by suppressing the production of the FGF growth factors that mediate the growth-inducing effects of E on the endometrial epithelium. In the E-dominant proliferative endometrium, FGFs secreted from the stroma act on the FGFR(s) in the epithelium to promote proliferation.20 Following ovulation and in response to P production and signaling, HAND2 is induced in stromal cells, causing inhibition of FGF synthesis and attenuation of epithelial proliferation. Disruption of PR function in the endometrium therefore runs the risk of increasing FGF signaling, leading to inappropriate uterine epithelial growth, hyperplasia, and cancer. Similar findings were noted in the epithelial glands of rhesus macaques treated with mifepristone.30 Indeed, a recent study has shown downregulation of HAND2 and upregulation of FGF18 in human endometrial adenocarcinoma.26 We demonstrate that administration of mifepristone to cultured endometrial stromal cells caused inhibition of HAND2 expression and a concomitant enhancement of FGF18 expression. However, treatment of endometrial stromal cells with UPA did not significantly affect the expression of either HAND2 or FGF18, further confirming that UPA does not significantly alter the P-dependent antiproliferative pathways in the endometrium.
In summary, this study shows that UPA and mifepristone exhibit differential effects on endometrial gene expression in vivo and in vitro, apparently due to differences in stability of PRs in response to these PRMs. It also confirms that chronic exposure to UPA-CVR over a 24-week period does not lead to adverse effects, such as suppression of the expression of HAND2, which is reported to occur in endometrial hyperplasia and cancer. The results from this study involving a limited number of clinical samples should pave the way for a larger study to determine the safety of UPA for long-term use.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported in part by a grant from the NICHD/NIH U54 HD 29990.
References
- 1. Wagenfeld A, Saunders PT, Whitaker L, Critchley HO. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets. 2016;20(9):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glasier A: The rationale for use of ulipristal acetate as first line in emergency contraception: biological and clinical evidence. Gynecol Endocrinol. 2014;30(10):688–690. [DOI] [PubMed] [Google Scholar]
- 3. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375(9714):555–562. [DOI] [PubMed] [Google Scholar]
- 4. Gainer EE, Ulmann A. Pharmacologic properties of CDB(VA)-2914. Steroids. 2003;68(10-13):1005–1011. [DOI] [PubMed] [Google Scholar]
- 5. Nichols MI. Ulipristal acetate: a novel molecule and 5-day emergency contraceptive. Obstet Gynecol. 2010;116(6):1252–1253. [DOI] [PubMed] [Google Scholar]
- 6. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25(9):2256–2263. [DOI] [PubMed] [Google Scholar]
- 7. Blithe DL, Nieman LK, Blye RP, Stratton P, Passaro M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68(10-13):1013–1017. [DOI] [PubMed] [Google Scholar]
- 8. Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol. 2006;108(5):1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donnez J, Tomaszewski J, Vazquez F, et al. PEARL II study group. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432. [DOI] [PubMed] [Google Scholar]
- 10. Fernandez H, Schmidt T, Powell M, Costa AP, Arriagada P, Thaler C. Real world data of 1473 patients treated with ulipristal acetate for uterine fibroids: Premya study results. Eur J Obstet Gynecol Reprod Biol. 2017;208:91–96. [DOI] [PubMed] [Google Scholar]
- 11. Huang Y, Jensen JT, Brache V, et al. A randomized study on pharmacodynamic effects of vaginal rings delivering the progesterone receptor modulator ulipristal acetate: research for a novel estrogen-free, method of contraception. Contraception. 2014;90(6):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21(5):591–598. [DOI] [PubMed] [Google Scholar]
- 13. Pawar S, Hantak AM, Bagchi IC, Bagchi MK. Minireview: steroid-regulated paracrine mechanisms controlling implantation. Mol Endocrinol. 2014;28(9):1408–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hantak AM, Bagchi IC, Bagchi MK. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int J Dev Biol. 2014;58(2-4):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chandra V, Kim JJ, Benbrook DM, Dwivedi A, Rai R. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol. 2016;27(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horn LC, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia: clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer. 2004;14(2):348–353. [DOI] [PubMed] [Google Scholar]
- 17. Daud S, Jalil SS, Griffin M, Ewies AA. Endometrial hyperplasia—the dilemma of management remains: a retrospective observational study of 280 women. Eur J Obstet Gynecol. Reprod Biol. 2011;159(1):172–175. [DOI] [PubMed] [Google Scholar]
- 18. Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22(4):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. [DOI] [PubMed] [Google Scholar]
- 20. Li Q, Kannan A, DeMayo FJ, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones A, Teschendorff AE, Li Q, et al. Role of DNA methylation and epigenetic silencing of HAND2 in endometrial cancer development. PLoS Med. 2013;10(11):e1001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitaker LH, Murray AA, Matthews R, et al. Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium. Hum Reprod. 2017;32(3):531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pritts EA, Ryan IP, Mueller MD, et al. Angiogenic effects of norplant contraception on endometrial histology and uterine bleeding. J Clin Endocrinol Metab. 2005;90(4):2142–2147. [DOI] [PubMed] [Google Scholar]
- 24. Kaya HS, Hantak AM, Stubbs LJ, Taylor RN, Bagchi IC, Bagchi MK. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol. 2015;29(6):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Q, Kannan A, Das A, Demayo FJ, et al. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154(1):446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flannery CA, Fleming AG, Choe GH, et al. Endometrial cancer-associated FGF18 expression is reduced by bazedoxifene in human endometrial stromal cells in vitro and in murine endometrium. Endocrinology. 2016;157(10):3699–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glasier AF, Wang H, Davie JE, Kelly RW, Critchley HO. Administration of an antiprogesterone up-regulates estrogen receptors in the endometrium of women using norplant: a pilot study. Fertil Steril. 2002;77(2):366–372. [DOI] [PubMed] [Google Scholar]
- 29. Boggavarapu NR, Berger C, von Grothusen C, Menezes J, Gemzell-Danielsson K, Lalitkumar PG. Effects of low doses of mifepristone on human embryo implantation process in a three-dimensional human endometrial in vitro co-culture system. Contraception. 2016;94(2):143–151. [DOI] [PubMed] [Google Scholar]
- 30. Greb RR, Kiesel L, Selbmann AK, et al. Disparate actions of mifepristone (RU 486) on glands and stroma in the primate endometrium. Hum Reprod. 1999;14(1):198–206. [DOI] [PubMed] [Google Scholar]