Abstract
Vitamin D is known to regulate innate and adaptive immune processes at the cellular level, but the role of vitamin D status on associated inflammatory processes across pregnancy is unclear. Our primary objective was to evaluate the relationships between serum biomarkers of inflammation (interleukin [IL]-6, IL-10, tumor necrosis factor [TNF]-α), acute-phase proteins (C-reactive protein and hepcidin) and vitamin D status, 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D), measured across pregnancy and in the neonate at birth. A second objective was to identify associations between vitamin D status and clinically diagnosed infections. In this study, 158 racially and ethnically diverse pregnant adolescents were recruited from the Rochester Adolescent Maternity Program (RAMP) in Rochester, NY. Serum 1,25(OH)2D was significantly lower in adolescents and neonates with IL-6 concentrations above the 75th percentile at delivery (P = .04) and at birth (P = .004), respectively. After adjusting for other potential covariates of inflammation, maternal serum 1,25(OH)2D was significantly positively associated with TNF-α during pregnancy (P = .02), but at delivery 1,25(OH)2D and TNF-α were inversely associated with one another (P = .02). Teens with 25(OH)D concentrations <30 ng/mL were more likely to test positive for candida (P = .002) and bacterial vaginosis (P = .02) during pregnancy. African Americans exhibited significantly lower TNF-α concentrations at both mid-gestation (P = .009) and delivery (P = .001) compared to the Caucasian adolescents. These results suggest that lower maternal vitamin D status may increase risk of infection across gestation.
Keywords: vitamin D, pregnancy, adolescents, inflammation, infections
Introduction
Cytokines are known to play an important role across gestation, as they help regulate placental implantation, fetal allograft tolerance, and onset of labor.1 Research conducted over the past 30 years suggests that cytokine production during pregnancy is altered in the presence of microbial infections.2 The mother and/or fetus are thought to independently produce proinflammatory cytokines in response to systemic maternal infections and those which have reached the intra-amniotic cavity, respectively.3 Abnormally high cytokine concentrations of interleukin (IL) 6 and tumor necrosis factor α (TNF-α) measured in amniotic fluid, the amniochorionic membranes, or fetal plasma have been associated with adverse events during pregnancy, such as intra-amniotic microbial infection and spontaneous preterm labor in patients with premature rupture of membranes (PROM).4,5 The cytokine IL-6 has pleotropic functions and is known to induce both C-reactive protein (CRP)6 and hepcidin7 and promote inflammation, while cytokine IL-10 exhibits an opposing anti-inflammatory effect.8
In addition to microbial infections, various demographic, anthropometric, and biological factors may influence cytokine concentrations across pregnancy. Older maternal age, higher body mass index (BMI), and prior preterm delivery have been associated with higher plasma cytokine concentrations among pregnant European women,9 while a longer duration of labor has been shown to increase cord CRP in newborns.10 Active labor has been shown to increase maternal hepcidin concentrations11 and both cord IL-6 and hepcidin concentrations at birth.12
Calcitriol (1,25(OH)2D) is believed to have a role in maintaining a balance between inflammation and immunosuppression.13 In T helper cells (Th), in vitro studies in cultured peripheral blood mononuclear cells (PBMCs) from humans have found 1,25(OH)2D acts to inhibit Th1 cell-associated proinflammatory cytokines (IL-2, interferon [IFN]-γ, and so on),14 while other cell culture data in mice have found 1,25(OH)2D to enhance the anti-inflammatory cytokines produced by Th2 cells (IL-4, IL-5, and so on).15 In vitro studies in cultured human trophoblasts from normotensive women and women with preeclampsia have shown that 1,25(OH)2D downregulates the mRNA expression of the cytokines TNF-α, IL-6,16 and IL-10.17 However, the in vivo action of 1,25(OH)2D on cytokine production during unique human physiological states, such as pregnancy, is not well understood.
Due to the established anti-inflammatory activity of 1,25(OH)2D in vitro, it is important to understand the vital role vitamin D may play in regulating inflammatory conditions and cytokine concentrations across gestation. The present research study was undertaken in a group of racially and ethnically diverse pregnant adolescents, previously shown to be at risk for low vitamin D status.18 The primary objective of this research was to determine the relationship between serum biomarkers of vitamin D status, (25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D), and inflammation (IL-10, IL-6, TNF-α, CRP, and hepcidin) measured across pregnancy and in umbilical cord blood obtained from the neonate at birth. A second objective was to assess possible relationships between vitamin D status and clinically diagnosed infections during pregnancy.
Materials and Methods
Study Population
Pregnant adolescents (n = 158) between 13 and 18 years of age at study entry were enrolled in research studies designed to examine changes in maternal and fetal bone health, iron status, and infections across pregnancy. Adolescents were recruited from the Rochester Adolescent Maternity Program (RAMP) in Rochester, New York. Exclusion criteria consisted of pregnant adolescents with a known medical complication, such as diabetes, malabsorption disease, or HIV. Pregnant adolescents were eligible to participate if between 12 and 30 weeks of gestation at entry and carrying a single fetus and otherwise healthy. At entry into the study, self-reported demographics, height, and weight were obtained by a study health project coordinator. Prepregnancy BMI (ppBMI) was calculated and categorized as underweight (<18.5 kg/m2), normal weight (18.5 to 24.9 kg/m2), overweight (≥25.0 kg/m2), or obese (≥30.0 kg/m2) using self-reported height and weight in accordance with the 2009 Institute of Medicine guidelines.19 Mode of delivery, duration of labor, and neonatal birth data were abstracted from medical records. As previously reported, 18 clinically diagnosed infections were abstracted from the prenatal medical records by a study health project coordinator.20 Informed written consent and in those ≤14 years adolescent assent and parental consent were obtained. The study was approved by the Institutional Review Boards at Cornell University and the University of Rochester.
Biochemical Analyses
A nonfasting blood sample (10 mL) was collected at midgestation (average: 26 ± 3.4 weeks) (n = 155) and upon admission to the hospital for delivery (average: 39.8 ± 1.3 weeks; n = 134). In addition, a 10-mL cord blood sample was obtained at delivery (n = 128). Variability in sample size of biochemical markers analyzed in this study was due to missed sample collections or insufficient sample volume. Serum samples were stored at −80°C until analysis. The serum vitamin D status biomarker 25(OH)D was measured using a radioimmunoassay (RIA; Diasorin Inc.), intact parathyroid hormone (iPTH) was measured by enzyme-linked immunoabsorbent assay (ELISA; Diagnostic Systems Laboratories), and 1,25(OH)2D was measured by Dr Michael Holick (Boston, Massachusetts) using an in-house thymus receptor binding assay. Data on biomarkers of vitamin D status and iPTH at midgestation, delivery, and in cord blood have been published.18 Serum concentrations of iPTH >65 pg/mL were defined as elevated. Data were analyzed using the Endocrine Society’s 25(OH)D cutoffs for vitamin D during pregnancy: 25(OH)D 30 to 44 ng/mL (“sufficiency”), 25(OH)D <30 ng/mL (“insufficiency”), 25(OH)D <20 ng/mL (“deficiency”) and 25(OH)D <10 ng/mL (“severe deficiency”).21
Archived maternal serum samples were used to undertake analyses of cytokines (IL-6, IL-10, and TNF-α) in serum collected at mid-gestation, delivery, and in cord blood using the Magnetic Multiplex MAP kit (Millipore) in the Cornell Human Metabolic Research Unit (Ithaca, NY, USA). The limit of detection (LOD) for IL-6, IL-10, and TNF-α was 0.13 pg/mL. The acute-phase proteins, C-reactive protein (CRP) and hepcidin, were previously measured and reported.22 High-sensitivity CRP was analyzed using the Immulite 2000 immunoassay (Siemens), and hepcidin was measured by a competitive serum ELISA (Intrinsic Life Sciences). The LOD for CRP and hepcidin was 0.2 mg/L, and 5.0 ng/mL, respectively. Additionally, the adipokine leptin was measured in serum with a commercially available ELISA from Millipore as reported.18
Statistical Analyses
Concentrations of biomarkers are presented either as the mean ± SD or geometric mean (95% confidence interval [CI]), percentage of values above/below the established cutoffs or LOD (if applicable), or percentage of elevated values (>75th percentile) relative to the sample population. Undetectable values were included in all analyses and computed as ½ × LOD. Differences in mean concentrations of serum biomarkers across gestation were calculated using analysis of variance with Tukey honest significant difference test for multiple comparisons. Simple linear regression was used to identify correlations between inflammatory biomarkers. Possible determinants of maternal inflammation were explored (maternal vitamin D status, race, ethnicity, gestational age, ppBMI, serum leptin, and the presence of a clinically diagnosed infection during pregnancy) using linear regression or t tests. Likewise, potential determinants of neonatal inflammation (neonatal vitamin D status, mode of delivery, duration of labor, serum leptin, maternal gestational age, a clinically diagnosed maternal infection during pregnancy, and maternal inflammatory biomarker concentrations) were assessed. To determine the most significant predictors of inflammation, backward stepwise regression models were constructed using predictors associated with inflammation in bivariate analyses (P < .10). Natural log (Ln) transformations were applied to nonnormally distributed variables. Results were considered statistically significant at P ≤ .05. All analyses were performed using JMP 12.0 (SAS Institute, Cary, North Carolina).
Results
Patient Characteristics
Characteristics of patients are presented in Table 1. Over 60% of the teens self-identified as African American and over 30% self-identified as Caucasian which is similar to the US teen pregnancy rate which is twice as high for non-Hispanic blacks compared to non-Hispanic white teens 15 to 19 years old (43.9 vs 20.5 per 1000 females).23 While receiving age-specific care at RAMP, our study cohort had a lower rate of Cesarean (C-section) deliveries compared to US data in women <20 years (12% vs 22%), and lower rates of preterm birth (<37 weeks) (9% vs 13%), and a lower rate of babies born low birth weight (LBW <2500 g; 6.7% vs 9.3%) compared to US teens 15 to 19 years old.23 These data are consistent with the prenatal care provided at midwife-run clinics.
Table 1.
Characteristics of The Pregnant Adolescents.a
| Characteristics | Value | Range |
|---|---|---|
| Age at enrollment, years (n) | 17.1 ± 1.1 (158) | 13.6-18.7 |
| Maternal race, % | ||
| African American | 63.3 | |
| Caucasian | 35.4 | |
| Native American | 1.3 | |
| Ethnicity, % | ||
| Hispanic | 24.7 | |
| Non-Hispanic | 75.3 | |
| GA at prenatal care entry, weeks (n) | 10.5 ± 4.7 (137) | 2-24 |
| GA at delivery, weeks (n) | 39.2 ± 3.0 (154) | 21-42.1 |
| Prepregnancy BMI, kg/m2 (n) | 24.7 ± 5.4 (156) | 15.0-42.1 |
| Mode of delivery (n = 150) | ||
| C-Section, % | 12 | |
| Vaginal, % | 88 | |
| Duration of labor, h | 8.50 ± 5.4 (133) | 0.02-24.1 |
| Preterm birth <37 weeks, % (n) | 9.1 (154) | |
| Birth weight, g (n) | 3,208 ± 2,398 (150) | 1,054-4,705 |
| Diagnosed maternal infections, % (n = 158) | ||
| Bacteria vaginosis | 40 | |
| Rectovaginal GBS | 38 | |
| Candida | 42 | |
| Chlamydia | 13 |
Abbreviations: GA, gestational age; BMI, body mass index; GBS, group B streptococcus.
aValues are presented as the mean ± SD (n) or % (n).
Relevant clinically diagnosed infections in this cohort are listed in Table 1. Chorioamnionitis was documented in 14% of the placentas that were sent to pathology (n = 45). As previously described, the infection burden of this population was more than 25% higher compared to nonpregnant and pregnant female adolescents and also compared to adult women for chlamydia, bacterial vaginosis (BV), and rectovaginal group B streptococcus (GBS).20 Racial differences were evident such that African American pregnant adolescents in this cohort were more likely to test positive for BV or at least one of the following STIs: chlamydia, gonorrhea, and trichomoniasis when compared to Caucasian adolescents.20
Biomarkers of Vitamin D and Inflammation
Similar to data collected in other studies on pregnant teens (13 to 18 years of age),18,24 the prevalence of 25(OH)D <20 ng/mL was high (47% to 50%) among the adolescents and neonates studied (Table 2). No significant differences in mean mid-gestation 25(OH)D were noted between ppBMI categories. Additionally, the mean 1,25(OH)2D concentrations remained high (>100 pg/mL) across pregnancy.18
Table 2.
Markers of Vitamin D Status and Inflammation in Pregnant Adolescents and Their Neonates at Birth.
| N | Midgestation | N | Delivery | N | Cord | |
|---|---|---|---|---|---|---|
| 25(OH)D, ng/mLa | 155 | 20.2 (18.8-21.8) | 133 | 18.6 (17.0-20.5) | 121 | 18.8 (17.1-20.5) |
| <10 ng/mL, % | 6 | 11 | 13 | |||
| <20 ng/mL, % | 50 | 48 | 47 | |||
| <30 ng/mL, % | 81 | 81 | 81 | |||
| 1,25(OH)2D, pg/mLa | 100 | 117 (111-123)c | 96 | 106 (100-112)d | 74 | 44.7 (41.3-48.4)e |
| iPTH, pg/mLa | 91 | 25.0 (22.3-27.9)c | 80 | 38.4 (33.8-43.6)d | 33 | 19.0 (14.7-23.3)e |
| >65 pg/mL, % | 3.3 | 18 | 0 | |||
| IL-10, pg/mLa | 144 | 4.57 (3.18-6.57)c | 125 | 9.65 (7.22-12.9)d | 96 | 18.0 (13.6-24.0)e |
| ≤0.13 pg/mL, % | 17.4 | 5.6 | 3.1 | |||
| TNF-α, pg/mLa | 145 | 4.48 (3.92-5.14)c | 126 | 3.54 (3.17-3.97)d | 97 | 8.31 (7.40-9.33)e |
| ≤0.13 pg/mL, % | 0.7 | 0 | 0 | |||
| IL-6, pg/mLa | 145 | 1.02 (0.78-1.33)c | 126 | 3.73 (3.01-4.63)d | 97 | 8.41 (5.99-11.7)e |
| ≤0.13 pg/mL, % | 15 | 2.4 | 0 | |||
| CRP, mg/Lb | 113 | 3.78 (3.10-4.66)c | 4 | 2.19 (0.06-79.4) | 86 | 0.17 (0.13-0.23)d |
| ≤0.20 mg/L, % | 0.9 | 25 | 79 | |||
| Hepcidin, ng/mLa | 145 | 20.7 (18.0-23.7)c | 129 | 24.2 (20.2-29.1)c | 120 | 94.2 (80.4-110)d |
| ≤5.0 ng/mL, % | 4.8 | 7.8 | 0 | |||
| Leptin, µg/La | 141 | 22.3 (19.7-25.2)c | 127 | 29.4 (25.7-33.6)d | 114 | 7.47 (6.26-8.91)e |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; iPTH, intact parathyroid hormone, IL, interleukin; TNF, tumor necrosis factor; CRP, C-reactive protein; CI, confidence interval.
aValues are presented as either mean or geometric mean (95% CI).
bOnly 4 patients had CRP values at delivery, and these data were excluded from all analyses due to insufficient sample size. Letter superscripts indicate statistically significant differences between mean mid-gestation, delivery, and cord values (P ≤ .05).
Serum cytokine concentrations at mid-gestation and delivery are displayed in Table 2. At mid-gestation, positive associations were observed between hepcidin and the cytokine IL-6 (r = .16, n = 143, P = .05). Elevated CRP (>5 mg/L) at mid-gestation was evident in 42% of the teens, and the mid-gestation geometric mean leptin concentration was significantly higher in those with elevated CRP concentrations compared to those without elevated CRP (32.7 µg/l, n = 43 vs 17.5 µg/L, n = 64, P < .0001). The geometric mean mid-gestation leptin concentration was significantly lower in those with mid-gestation IL-10 above the 75th percentile compared to those below this cutoff (17.9 µg/L, n = 34 vs 24.1 µg/L, n = 104, P = .04). Maternal mid-gestation leptin was significantly positively correlated with IL-6 (r = .18, n = 139, P = .03).
Relationships between maternal and neonatal cytokines were explored. In the neonate, geometric mean cytokine concentrations were significantly higher than maternal concentrations for IL-6, IL-10, TNF-α, and hepcidin but significantly lower for CRP which may be a result of the high percentage of undetectable samples (79%) for CRP in cord blood (Table 2). Similar to patterns observed in the mother, positive linear correlations between inflammatory biomarkers were also evident in cord blood (Table 3). No significant associations were found between cord leptin and the inflammatory biomarkers in cord blood. A positive relationship between maternal mid-gestation TNF-α and umbilical cord TNF-α was observed (P = .05; Figure 1). Maternal IL-6 during pregnancy was significantly inversely associated with umbilical cord IL-10 (P = .03; Figure 2). Consistent with findings evaluating determinants of iron status in the larger cohort,12 maternal midgestation hepcidin was significantly positively correlated with cord hepcidin (P = .001; Figure 3).
Table 3.
Correlations Between Inflammation Biomarkers in Maternal Serum Collected During Pregnancy and at Delivery and in Cord Serum Obtained at Birth.a
| IL-6 | TNF-α | Hepcidin | |
|---|---|---|---|
| Pregnancy (∼26 weeks) | |||
| IL-10 | r = .47b n = 144 | r = .30b n = 144 | |
| IL-6 | r = .44b n = 145 | ||
| CRP | r = .28c n = 111 | ||
| Delivery | |||
| IL-10 | r = .49b n = 125 | r = .20d n = 125 | |
| IL-6 | r = .32b n = 126 | ||
| Neonatal Cord | |||
| IL-10 | r = .44b n = 96 | r = .38b n = 96 | r = .22d n = 95 |
| IL-6 | r = .29c n = 97 | r = .38b n = 96 | |
| TNF-α | r = .37b n = 96 | ||
| CRP | r = 0.29c n = 71 |
Abbreviations: IL, interleukin; CRP, C-reactive protein; TNF, tumor necrosis factor.
aAll biomarkers were natural log (Ln) transformed. Data presented as Correlation Coefficients (r) and sample size (n).
b P ≤ .001.
c P ≤ .01,
d P ≤ .05,
Figure 1.
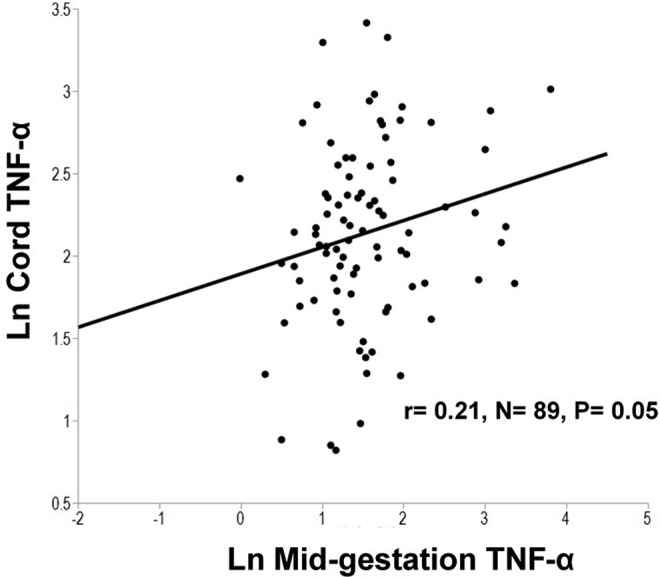
In a group of pregnant adolescents, serum concentrations of maternal tumor necrosis factor α (TNF-α) at mid-gestation were positively associated with cord concentrations of TNF-α.
Figure 2.
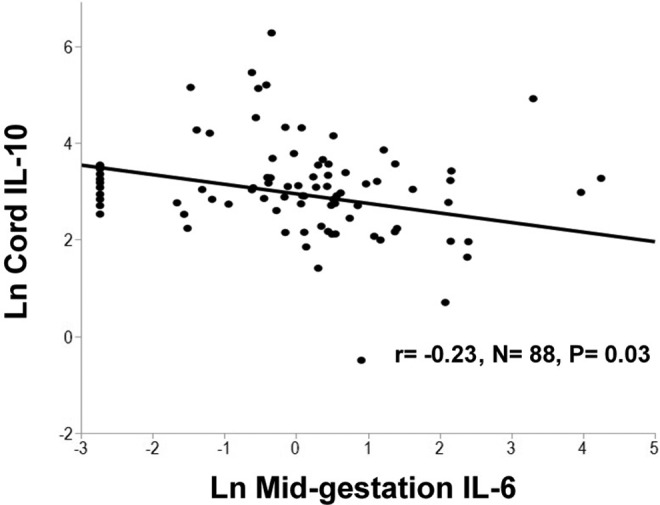
In a group of pregnant adolescents, serum concentrations of maternal interleukin (IL)-6 at mid-gestation were significantly inversely associated with cord IL-10 concentrations.
Figure 3.
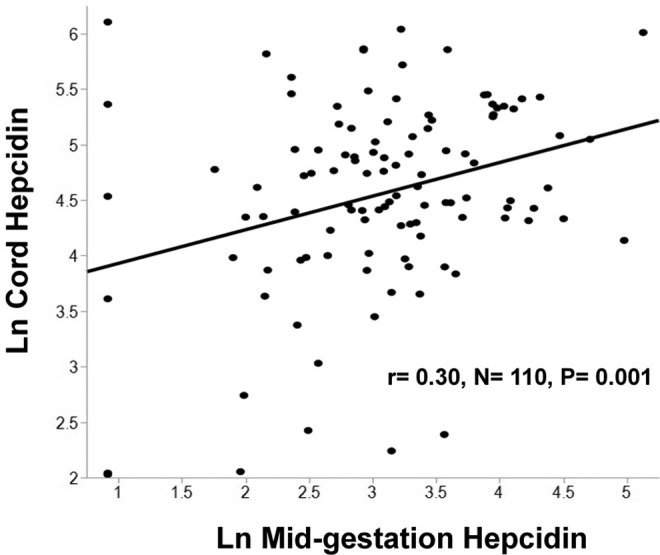
In a group of pregnant adolescents, serum concentrations of maternal hepcidin at mid-gestation were significantly positively associated with cord hepcidin concentrations.
We explored possible relationships between maternal labor and delivery variables and neonatal inflammatory biomarkers. Greater mean duration of labor was associated with cord hepcidin above the 75th percentile (P = .01) but not with CRP or any other proinflammatory cytokine measured. Of the cord inflammatory biomarkers measured, only cord TNF-α was significantly impacted by mode of delivery as evidenced by the significantly higher geometric mean concentration of cord TNF-α in neonates born via vaginal delivery compared to cesarean section (P = .0008; Table 4).
Table 4.
Impact of Delivery Mode on Serum Cytokine Concentrations in Pregnant Adolescents and Their Neonates at Birth.a
| Cytokine | Mode of Delivery | P Value | |
|---|---|---|---|
| C-section | Vaginal | ||
| Ln delivery TNF-α, pg/mL | 2.62 (1.87, 3.66), n = 14 | 3.68 (3.27, 4.14), n = 112 | P = .06 |
| Ln cord TNF-α , pg/mL | 3.95 (2.54, 6.13), n = 6 | 8.73 (7.79, 9.77), n = 91 | P = .0008 |
| Ln delivery IL-6 , pg/mL | 4.30 (2.25, 8.20), n = 14 | 3.67 (2.92, 4.61), n = 112 | P = .65 |
| Ln cord IL-6 , pg/mL | 19.7 (5.14, 75.7), n = 6 | 7.94 (5.62, 11.22), n = 91 | P = .20 |
| Ln delivery IL-10 , pg/mL | 12.8 (5.35, 30.4), n = 14 | 9.32 (6.84, 12.7), n = 111 | P = .50 |
| Ln cord IL-10 , pg/mL | 24.9 (7.16, 86.4), n = 5 | 17.7 (13.2, 23.7), n = 91 | P = .60 |
Abbreviations: C-section, cesarean section; Ln, natural log; TNF, tumor necrosis factor; IL, interleukin. Bold-face indicates statistical significance (P ≤ .05).
aValues are presented as the geometric mean and (95% confidence interval);
Demographic and Anthropometric Risk Factors
Of the demographic variables explored (race, ethnicity, and gestational age), race was the only significant predictor of inflammation. Caucasian teens (n = 46) exhibited a 2.75 times higher risk of having elevated TNF-α at delivery when compared to African American teens (n = 80; relative risk, RR: 2.75, 95% CI: 1.47-5.15). At mid-gestation, the difference in TNF-α between African American and Caucasian teens was also significant (P =.05). Compared to teens who were underweight or normal weight, teens who were overweight or obese prior to pregnancy were at a significantly higher risk of having mid-gestation IL-6 (RR: 1.75, 95% CI: 1.002-3.06) and TNF-α (RR: 1.85, (95% CI: 1.05-3.27) above the 75th percentile as well as CRP ≥5 mg/L (RR: 2.83, 95% CI: 1.79-4.47).
Associations Between Infections and Vitamin D
Differences in vitamin D biomarkers with respect to diagnosed infections are displayed in Table 5. Teens with 25(OH)D <30 ng/mL at mid-gestation were more likely to have a positive diagnosis of BV (P = .02, n = 72), and those with 25(OH)D <30 ng/mL at delivery were significantly more likely to test positive for candida (P = .002, n = 62). In addition, those who tested positive for BV at any point during gestation had significantly higher mean concentrations of serum 1,25(OH)2D at delivery (P = .007, n = 40). Because maternal race was previously shown to be associated with BV in this cohort (20), we noted that after controlling for maternal race, the association between positive BV infection and delivery 1,25(OH)2D remained significant (P = .03). Pregnant teens who tested positive for candida compared to those who tested negative for candida had significantly lower geometric mean serum 25(OH)D concentrations at mid-gestation (19.5 ng/mL, n = 67 vs 23.8 ng/mL, n = 30, P = .04) and at delivery (17.2 ng/mL, n = 56 vs 23.8 ng/mL, n = 26, P = .02).
Table 5.
Associations Between Vitamin D Concentrations and Clinically Diagnosed Infections in Pregnant Adolescents.a
| Indicator | Bacterial Vaginosis | P-value | Candida | P-value |
|---|---|---|---|---|
| Ln 25(OH)D at midgestation | Positive: 19.7 (17.6; 22.2), N = 62 Negative: 23.1 (19.6; 27.3), N = 30 |
P = .12 | Positive: 19.5 (17.5; 21.6), N = 67 Negative: 23.8 (20.3; 27.8), N = 30 |
P = .04 |
| Ln 25(OH)D at delivery | Positive: 17.5 (15.0; 20.5), N = 55 Negative: 22.0 (17.1; 28.4), N = 21 |
P = .13 | Positive: 17.2 (14.7; 20.1), N = 56 Negative: 23.8 (18.9; 29.9), N = 26 |
P = .02 |
| 1,25(OH)2D at midgestation | Positive: 121 (111; 131), N = 41 Negative: 104 (88.6; 120), N = 16 |
P = .08 | Positive: 110 (101; 120), N = 42 Negative: 119 (106; 133), N = 20 |
P = .30 |
| 1,25(OH)2D at delivery | Positive: 110 (102; 118), N = 40 Negative: 88.3 (75.2; 101), N = 16 |
P = .007 | Positive: 108 (98.0; 117), N = 39 Negative: 99.4 (86.1; 113), N = 21 |
P = .32 |
Abbreviations: Ln, natural log. Bold face indicates statistical significance (P ≤ 0.05).
aValues are presented as the mean or geometric mean and (95% confidence interval).
Relationships Between Infections and Inflammation
Diagnosis of BV at any point during pregnancy was associated with a significantly higher midgestation geometric mean hepcidin concentration (22.1 ng/mL, n = 57 vs 13.8 ng/mL, n = 29, P = .01). Higher geometric mean maternal IL-6 concentrations at delivery were weakly associated with either a diagnosis of chorioamnionitis (4.9 pg/mL, n = 17 vs 2.2 pg/mL, n = 15, P = .04) or chlamydia (6.2 pg/mL, n = 19 vs 3.4 pg/mL, n = 107, P = .049). Likewise, having a delivery geometric mean hepcidin concentration above the 75th percentile was significantly associated with a diagnosis of chorioamnionitis (n = 8, P = .004).
Significant associations between maternal infections across pregnancy and neonatal inflammation at birth were also noted. A significantly higher proportion of neonates whose mothers tested positive for chlamydia were found to have cord TNF-α concentrations above the 75th percentile (P = .005, n = 17). Also, a significantly higher geometric mean cord hepcidin concentration was observed in neonates whose mother tested positive for chlamydia at any point during pregnancy (141 ng/ml, n = 17 vs 88.1, n = 103, P = 0.04). The geometric mean IL-10 concentration in neonates whose mother tested positive for rectovaginal GBS was significantly lower compared to those testing negative for this infection (12.2 pg/mL, n = 41 vs 24.2 pg/mL, n = 53, P = .02).
Associations Between Inflammation and Vitamin D
Maternal mid-gestation 25(OH)D was significantly positively associated with delivery TNF-α (P = 0.02). Teens with midgestation 25(OH)D <12 ng/mL had a significantly higher geometric mean hepcidin concentration (31.1 ng/mL, n = 16 vs 19.6 ng/mL, n = 129, P = .04). There was a significant positive association between mid-gestation 1,25(OH)2D and mid-gestation TNF-α (P = .005, n = 100). At midgestation, the mean 1,25(OH)2D concentration was higher in teens with mid-gestation IL-6 concentrations above the 75th percentile (134 pg/mL, n = 25 vs 111 pg/mL, n = 75, P = .001) and mid-gestation CRP concentrations ≥5 mg/L (127 pg/mL, n = 34 vs 111 pg/mL, n = 52, P = .01), respectively. In contrast, at delivery the mean 1,25(OH)2D concentration was significantly lower in teens with delivery IL-6 (96.6 pg/mL, n = 26 vs 111 pg/mL, n = 69, P = .04) and delivery TNF-α (85.6 pg/mL, n = 19 vs 112 pg/mL, n = 76, P = .0005) concentrations above the 75th percentile, respectively. Using multivariate modeling, a significant interaction effect (P = .02, n = 55) was observed between BV and 1,25(OH)2D such that the inverse association between 1,25(OH)2D and log IL-6 was only significant in those that tested positive for BV.
In neonates, there was a positive correlation between cord 25(OH)D and TNF-α (P = .04, n = 94). Cord 25(OH)D was significantly positively associated with cord hepcidin (P = .01). The 1,25(OH)2D umbilical cord geometric mean concentration was significantly lower in those with cord IL-6 (37.1 pg/mL, n = 16 vs 49.0 pg/mL, n = 50, P = .004) and hepcidin (38.0 ng/mL, n = 20, vs 47.5 ng/mL, n = 54, P = .01) above the 75th percentile, respectively.
Using data obtained from the bivariate analyses described earlier, multivariate stepwise regression models were constructed to assess whether vitamin D remained a significant predictor of inflammatory biomarkers while controlling for other covariates. Predictors which best explained variability in inflammatory biomarker concentrations are displayed in Table 6.
Table 6.
Multivariate Models for Selected Inflammation Markers.a
| Variables | P value | R 2 Adj. | β |
|---|---|---|---|
| Ln midgestation IL-6 | .33 | ||
| Ln midgestation leptin | .005 | .428 | |
| Ln midgestation IL-10 | <.0001 | .259 | |
| Ln midgestation TNF-α | <.0001 | .660 | |
| Ln midgestation TNF-α | .23 | ||
| Midgestation 1,25(OH)2D | .02 | .005 | |
| Ln midgestation IL-6 | .03 | .109 | |
| Ln midgestation IL-10 | .06 | .068 | |
| African American (ref: CA) | .009 | −.365 | |
| Ln midgestation CRP | .37 | ||
| Midgestation 1,25(OH)2D | .09 | .005 | |
| Ln midgestation leptin | .003 | .454 | |
| Ln ppBMI (kg/m2) | .001 | 1.65 | |
| Ln delivery TNF-α | .24 | ||
| Delivery 1,25(OH)2D | .02 | −.005 | |
| Ln delivery IL-6 | .03 | .113 | |
| African American (ref: CA) | .001 | −.405 | |
| Ln cord hepcidin | .47 | ||
| Ln cord 25(OH)D | .09 | .320 | |
| Ln cord 1,25(OH)2D | <.0001 | −1.16 | |
| Ln midgestation hepcidin | .002 | .327 | |
| Ln cord IL-6 | .02 | .123 |
Abbreviations: Ln, natural log; IL, interleukin; TNF, tumor necrosis factor; 1,25(OH)2D, 1,25-dihydroxyvitamin D; ref, reference; CA, Caucasian.
aCovariate predictors are indented below each inflammation biomarker.
Discussion
In these pregnant adolescents, a higher proportion of vitamin D insufficiency was noted in teens who tested positive for BV and candida. Adolescents with chlamydial infections exhibited increased inflammation at delivery, a finding that was associated with decreased 1,25(OH)2D. Temporal changes in the associations between vitamin D and inflammatory outcomes from mid-gestation to delivery may be a consequence of the dynamic changes in inflammation and 1,25(OH)2D that occur across gestation. Maternal infection with chlamydia was associated with elevated hepcidin and TNF-α in cord blood. Because elevated inflammation has been associated with increased risk of adverse birth outcomes, and maternal nutritional status may attenuate inflammatory responses across gestation, more normative data on maternal vitamin D status and inflammation and its impact on the neonate at birth are needed.
The mean mid-gestation IL-6 concentration observed in this cohort at ∼26 weeks of gestation was similar to mid-gestation (22-24 weeks) values reported in a Texas study of 28 pregnant teens (<18 years) and 438 pregnant adult women (∼1.0 pg/mL),25 whereas mean mid-gestation TNF-α concentrations in our study population were significantly higher than those previously noted in adult pregnant women from Texas (∼2 pg/mL).25 The significant positive correlations we observed between IL-6 and TNF-α at mid-gestation are consistent with the positive correlations noted at 24 weeks of gestation in a study of over 100,000 pregnant European women using a multiplex flow cytometric assay.9
African American teens in our study exhibited significantly lower TNF-α at both mid-gestation and delivery when compared to the Caucasian adolescents. This finding is supported by other reports in the literature that have reported genetic differences in cytokine genes and receptors between Caucasian and African Americans. For example, unique single-nucleotide polymorphisms in TNF-α and its receptor have been reported among Caucasians and African Americans for cytokines such as TNF-α and their corresponding receptors.26,27 The significantly lower soluble TNF-α concentrations in the African American adolescents that we observed are also consistent with a previous study of fetal membranes which showed that the concentrations of soluble TNF receptors (measured using a multiplex immunoassay) were significantly decreased in culture media from African Americans but increased in culture media from Caucasians following in vitro lipopolysaccharide (LPS) stimulation.28Although we observed lower concentrations of soluble TNF-α, African Americans have been shown to have higher membrane-bound TNF-α concentrations (which we did not measure) as demonstrated in the previous study by Menon et al.28
We observed significantly higher mid-gestation IL-6, TNF-α, and CRP concentrations in teen mothers with higher ppBMIs. These findings are consistent with the positive correlation observed between ppBMI and IL-6 and CRP that we previously reported in the larger cohort.29 Similarly, other studies have demonstrated higher IL-6 in obese individuals,30 and pregnant obese mice,31 compared to lean control groups. Elevated TNF-α in nonpregnant adults has been hypothesized to play a role in obesity-induced insulin resistance.32,33
In these pregnant adolescents, vitamin D status was independently associated with clinical diagnosis of both a bacterial (BV) and a fungal infection (candida). Similar to other research findings conducted in pregnant women, we found an inverse association between serum 25(OH)D at mid-gestation and BV.34 In contrast, we observed higher serum 1,25(OH)2D at mid-gestation and at delivery in those with BV infection. These findings are consistent with previously published findings in the larger cohort which noted an inverse association between midgestation 25(OH)D and delivery 1,25(OH)2D.18 The inverse relationship between IL-6 and 1,25(OH)2D evident at delivery appeared to be moderated by the presence of BV infection suggesting that the anti-inflammatory role of 1,25(OH)2D during pregnancy may be more pronounced in those with infections. In addition, we found that lower maternal 25(OH)D status during pregnancy and at delivery was associated with a positive diagnosis of candida infection which is consistent with results from a study of hospitalized adults.35 Our results suggest that the immunomodulatory role of vitamin D may be more evident during states of infection and that lower vitamin D status may reduce one’s ability to clear infections during pregnancy.
A significant positive association between 1,25(OH)2D and TNF-α was observed during pregnancy, but an inverse association with TNF-α was observed at delivery. The shift from a positive association to an inverse association may be explained by the ability of pro-inflammatory cytokines to upregulate the vitamin D CYP27B1 enzyme in cultured human placental cells.36 Increased conversion of 25(OH)D to 1,25(OH)2D via upregulation of the CYP27B1 enzyme may decrease the circulating concentrations of proinflammatory biomarkers. Consistent with this hypothesis, prior studies have found inverse associations between 1,25(OH)2D and TNF-α in cultured trophoblasts obtained from placentas collected during uncomplicated and preeclamptic pregnancies.16,37
Temporal changes in 1,25(OH)2D but not 25(OH)D across pregnancy are important to take into account when evaluating interactions between vitamin D and cytokines. While 25(OH)D geometric mean concentrations did not significantly change between pregnancy and delivery, mean 1,25(OH)2D decreased by ∼9% from mid-gestation to delivery. Maternal 1,25(OH)2D is also dependent on 25(OH)D status, and a vitamin D supplementation study conducted in 350 pregnant women found linear relationships between 25(OH)D and 1,25(OH)2D up until 25(OH)D concentrations of 40 ng/mL were achieved,38 a concentration that most teens did not achieve in our study. A recent randomized controlled trial conducted in 57 US pregnant women found that 2,000 IU of vitamin D supplementation was more effective in increasing IL-10+ regulatory CD4+ T cells later in pregnancy (36 weeks gestation) than 400 IU of vitamin D.39 Taken together with our findings which demonstrated an inverse association between maternal 1,25(OH)2D and proinflammatory cytokines IL-6 and TNF-α at delivery, these results suggest that the anti-inflammatory action of vitamin D may be more critical later in pregnancy.
Both the pregnant adolescent and her fetus are capable of independently synthesizing 1,25(OH)2D and mounting inflammatory responses, but little is known about how maternal and fetal cytokines may interact. Although we did not observe significant correlations between maternal and fetal cytokines of the same class, data on the ability of cytokines to cross the placenta are conflicting. A previous study of 19 normal-term placentas found that TNF-α, IL-1, and IL-6 did not cross the placenta ex vivo suggesting that the inflammatory response detected in cord blood is of fetal origin.40 In contrast, an ex vivo study of 10 normal-term placentas found that IL-6 was transported across the placenta in a bidirectional manner.41 The stage of gestation may impact findings as data obtained in a rat model found that maternal IL-6 readily crossed the rat placenta at mid-gestation but transfer was reduced by late gestation.42
Chlamydia is a bacterial infection that the neonate is capable of contracting while passing through the birth canal at delivery.43 In neonates studied, maternal infection with chlamydia was associated with a significantly increased concentration of TNF-α and hepcidin. A study conducted in newborn mice demonstrated that the passive transfer of TNF-α from an immunized adult mice to the fetus in utero was associated with a significant decrease in postnatal chlamydial colonization of the newborn murine lung.44 In the present study, neonatal hepcidin was found to be positively associated with IL-10, IL-6, and TNF-α measured in cord blood. Therefore, it is likely that increased hepcidin is indicative of an inflammatory state and/or infection.
Several limitations in our study design were evident. Since no normative data on mean serum cytokine concentrations are available and there are no adjustment factors for normalizing data to account for variable analytical approaches, we could not directly compare our mean cytokine values to those reported in other similar populations. An RIA was used to measure 25(OH)D because it was the preferred approach for vitamin D analysis during the start of the study. However, liquid chromatography–tandem mass spectrometry techniques are capable of separating the biologically inactive 3-epi analogs of 25(OH)D which could improve the accuracy in our measurement of 25(OH)D and subsequently the observed associations between 25(OH)D and inflammatory biomarkers.45 Although we did detect several significant associations between maternal biomarkers (inflammation and vitamin D status) and infection status, our retrospective study design did not allow us to establish causality. In addition, there are other proinflammatory cytokines linked to preterm labor and genitourinary infections, such as IL-1β, which were not explored in the present study which could have provided additional valuable insight.
Conclusions
Despite conflicting evidence describing the impact of vitamin D status on inflammation during pregnancy, we observed significant inverse associations between 1,25(OH)2D and IL-6 and TNF-α in the mother at delivery and between 1,25(OH)2D and IL-6 and hepcidin in the neonate at birth. We also demonstrated that the presence of BV impacted the association between IL-6 and 1,25(OH)2D at delivery. Together, our results suggest that 1,25(OH)2D may influence changes in proinflammatory cytokine production during pregnancy and infections may moderate these relationships. Given that elevated inflammatory biomarkers have been linked to adverse pregnancy outcomes, more research is needed to determine whether vitamin D supplementation can modify maternal inflammatory responses and promote healthy birth outcomes in pregnant populations, particularly those at increased risk of infections and vitamin D insufficiency.
Acknowledgments
We appreciate the statistical input from Francoise Vermeylen at the Cornell Statistical Consulting Unit. We are thankful for our research study coordinator at RAMP Allison W. McIntyre and the former Cornell doctoral student who worked on the parent study, Dr Bridget Young and our health project coordinators Lauren Cowen, Sarah Caveglia, Melissa Miller, and Jessica Brunner. We would also like to thank Victoria Simon, manager of the Human Nutritional Chemistry Laboratory, and Tera Kent for technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article:: This work that was supported by the National Institute of Food and Agriculture (NIFA), U.S. Department of Agriculture [2005-35200-15218, 2009-35200-05171, 2012-67017-30216] and the National Center for Research Resources (NCRR) [UL1 RR 024160].
References
- 1. Saito S. Cytokine cross-talk between mother and the embryo/placenta. J Reprod Immunol. 2001; 52(1–2): 15–33. [DOI] [PubMed] [Google Scholar]
- 2. Romero R, Erez O, Espinoza J. Intrauterine infection, preterm labor, and cytokines. J Soc Gynecol Investig. 2005; 12(7): 463–465. [DOI] [PubMed] [Google Scholar]
- 3. Romero R, Brody DT, Oyarzun E, et al. Infection and labor. III. Interleukin-1: A signal for the onset of parturition. Am J Obstet Gynecol. 1989; 160(5 pt 1): 1117–1123. [DOI] [PubMed] [Google Scholar]
- 4. Santhanam U, Avila C, Romero R, et al. Cytokines in normal and abnormal parturition: Elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991; 3(2): 155–163. [DOI] [PubMed] [Google Scholar]
- 5. Romero R, Gomez R, Ghezzi F, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998; 179(1): 186–193. [DOI] [PubMed] [Google Scholar]
- 6. Majello B, Arcone R, Toniatti C, Ciliberto G. Constitutive and IL-6-induced nuclear factors that interact with the human C-reactive protein promoter. EMBO J. 1990; 9(2): 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004; 113(9): 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010; 10(3): 170–181. [DOI] [PubMed] [Google Scholar]
- 9. Curry AE, Vogel I, Skogstrand K, et al. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol. 2008; 77(2): 152–160. [DOI] [PubMed] [Google Scholar]
- 10. Logan CA, Thiel L, Bornemann R, et al. Delivery mode, duration of labor, and cord blood adiponectin, leptin, and C-reactive protein: Results of the population-based Ulm birth cohort studies. PLoS One. 2016; 11(2): e0149918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rehu M, Punnonen K, Ostland V, et al. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010; 85(4): 345–352. [DOI] [PubMed] [Google Scholar]
- 12. Lee S, Guillet R, Cooper EM, et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. 2016; 79(1–1): 42–48. [DOI] [PubMed] [Google Scholar]
- 13. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012; 71(1): 50–61. [DOI] [PubMed] [Google Scholar]
- 14. Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987; 79(6): 1659–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001; 167(9): 4974–4980. [DOI] [PubMed] [Google Scholar]
- 16. Noyola-Martinez N, Diaz L, Avila E, Halhali A, Larrea F, Barrera D. Calcitriol downregulates TNF-alpha and IL-6 expression in cultured placental cells from preeclamptic women. Cytokine. 2013; 61(1): 245–250. [DOI] [PubMed] [Google Scholar]
- 17. Barrera D, Noyola-Martinez N, Avila E, Halhali A, Larrea F, Diaz L. Calcitriol inhibits interleukin-10 expression in cultured human trophoblasts under normal and inflammatory conditions. Cytokine. 2012; 57(3): 316–321. [DOI] [PubMed] [Google Scholar]
- 18. Young BE, McNanley TJ, Cooper EM, et al. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with parathyroid hormone and calcitriol in pregnant adolescents. J Bone Miner Res. 2012; 27(1): 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 20. Akoh CC, Pressman EK, Cooper E, Queenan RA, Pillittere J, O’Brien KO. Prevalence and risk factors for infections in a pregnant adolescent population. J Pediatr Adolesc Gynecol. 2017; 30(1): 71–75. [DOI] [PubMed] [Google Scholar]
- 21. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96(7): 1911–1930. [DOI] [PubMed] [Google Scholar]
- 22. Lee S, Guillet R, Cooper EM, et al. Maternal inflammation at delivery affects assessment of maternal iron status. J Nutr. 2014; 144(10): 1524–1532. [DOI] [PubMed] [Google Scholar]
- 23. Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: Final data for 2012. Natl Vital Stat Rep. 2013; 62(9): 1–68. [PubMed] [Google Scholar]
- 24. Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O’Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010; 23(1): 45–52. [DOI] [PubMed] [Google Scholar]
- 25. Ruiz RJ, Jallo N, Murphey C, Marti CN, Godbold E, Pickler RH. Second trimester maternal plasma levels of cytokines IL-1Ra, Il-6 and IL-10 and preterm birth. J Perinatol. 2012; 32(7): 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Dyke AL, Cote ML, Wenzlaff AS, Land S, Schwartz AG. Cytokine SNPs: Comparison of allele frequencies by race and implications for future studies. Cytokine. 2009; 46(2): 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Menon R, Velez DR, Morgan N, Lombardi SJ, Fortunato SJ, Williams SM. Genetic regulation of amniotic fluid TNF-alpha and soluble TNF receptor concentrations affected by race and preterm birth. Hum Genet. 2008; 124(3): 243–253. [DOI] [PubMed] [Google Scholar]
- 28. Menon R, Thorsen P, Vogel I, Jacobsson B, Williams SM, Fortunato SJ. Increased bioavailability of TNF-alpha in African Americans during in vitro infection: Predisposing evidence for immune imbalance. Placenta. 2007; 28(8–9): 946–950. [DOI] [PubMed] [Google Scholar]
- 29. Cao C, Pressman EK, Cooper EM, Guillet R, Westerman M, O’Brien KO. Placental heme receptor LRP1 correlates with the heme exporter FLVCR1 and neonatal iron status. Reproduction. 2014; 148(3): 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roytblat L, Rachinsky M, Fisher A, et al. Raised interleukin-6 levels in obese patients. Obes Res. 2000; 8(9): 673–675. [DOI] [PubMed] [Google Scholar]
- 31. Sanders TR, Kim DW, Glendining KA, Jasoni CL. Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology. 2014; 155(7): 2566–2577. [DOI] [PubMed] [Google Scholar]
- 32. Nieto-Vazquez I, Fernández-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: The link TNF-alpha. Arch Physiol Biochem. 2008; 114(3): 183–194. [DOI] [PubMed] [Google Scholar]
- 33. Miyazaki Y, Pipek R, Mandarino LJ, DeFronzo RA. Tumor necrosis factor alpha and insulin resistance in obese type 2 diabetic patients. Int J Obes Relat Metab Disord. 2003; 27(1): 88–94. [DOI] [PubMed] [Google Scholar]
- 34. Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009; 139(6): 1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lim JH, Ravikumar S, Wang YM, et al. Bimodal influence of vitamin d in host response to systemic candida infection-vitamin d dose matters. J Infect Dis. 2015; 212(4): 635–644. [DOI] [PubMed] [Google Scholar]
- 36. Noyola-Martinez N, Diaz L, Zaga-Clavellina V, et al. Regulation of CYP27B1 and CYP24A1 gene expression by recombinant pro-inflammatory cytokines in cultured human trophoblasts. J Steroid Biochem Mol Biol. 2014;144 Pt A: 106–109. [DOI] [PubMed] [Google Scholar]
- 37. Diaz L, Noyola-Martinez N, Barrera D, et al. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. J Reprod Immunol. 2009; 81(1): 17–24. [DOI] [PubMed] [Google Scholar]
- 38. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011; 26(10): 2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zerofsky MS, Jacoby BN, Pedersen TL, Stephensen CB. Daily cholecalciferol supplementation during pregnancy alters markers of regulatory immunity, inflammation, and clinical outcomes in a randomized controlled trial. J Nutr. 2016; 146(11): 2388–2397. [DOI] [PubMed] [Google Scholar]
- 40. Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005; 106(4): 802–807. [DOI] [PubMed] [Google Scholar]
- 41. Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004; 103(3): 546–550. [DOI] [PubMed] [Google Scholar]
- 42. Dahlgren J, Samuelsson AM, Jansson T, Holmäng A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006; 60(2): 147–151. [DOI] [PubMed] [Google Scholar]
- 43. Much DH, Yeh SY. Prevalence of Chlamydia trachomatis infection in pregnant patients. Public Health Rep. 1991; 106(5): 490–493. [PMC free article] [PubMed] [Google Scholar]
- 44. Pal S, de la Maza LM. Mechanism of T-cell mediated protection in newborn mice against a Chlamydia infection. Microbes Infect. 2013; 15(8–9): 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu S, Cheng X, Fang H, et al. 25 OHD analogues and vacuum blood collection tubes dramatically affect the accuracy of automated immunoassays. Sci Rep. 2015; 5: 14636. [DOI] [PMC free article] [PubMed] [Google Scholar]


