Abstract
Objective:
The objective of this study is to evaluate the neutrophil-to-lymphocyte ratio (NLR) and platelet–lymphocyte ratio, from the hemograms obtained from children and adolescents with dilated cardiomyopathy (DCM), and to correlate them with the levels of B-type natriuretic peptide (BNP) and with the clinical evolution of these patients in the long term.
Materials and Methods:
Follow-up of 57 patients with DCM was made retrospectively, with hemogram and BNP level determination being performed after optimized therapy for heart failure. We compared the findings of the patients' examinations that progressed with stability in relation to the occurrence of transplant listing, cardiac transplantation, or evolution to death.
Results:
The average age was 48 months, and the follow-up was 64 months. The average of the levels of neutrophils was greater in poor evolution group (7026 vs. 3903; P = 0.011) as well as the average of NLR (5.5 vs. 1.9; P = 0.034). The averages of hemoglobin, total leukocytes, lymphocytes, and platelets were similar in the groups. The area under the receiver operating characteristic curve for NLR in relation to the poor evolution was of 72.9%, being the best cutoff point of NLR ≥5.2 (sensitivity: 93.8% and specificity: 87.8%). Kaplan–Meier curves demonstrate that patients with NLR ≤5.2 (P = 0.001) and BNP <1000 pg/dl (P < 0.0001) presented greater survival.
Conclusions:
NLR (≥5.2) and lymphopenia (≤1000 lymphocyte/μL) were associated with a poor prognosis and a higher chance of evolution to death or cardiac transplant, similar to the findings for BNP.
Keywords: Dilated cardiomyopathy, heart failure, lymphocyte count, neutrophil count, prognosis
INTRODUCTION
Heart failure (HF) is a chronic inflammatory process, with hormonal and immunological mechanisms not yet well elucidated. The dilated cardiomyopathy (DCM) is the most common type of cardiomyopathy in childhood and progresses to systolic and/or diastolic dysfunction, resulting in different degrees of HF.[1] Despite the treatment, prognosis remains poor, with high risks of death, hospital readmission, or need for cardiac transplant. Some factors related to prognosis involve age, degree of HF, arrhythmias, and the degree of impairment of ventricular function.[2,3,4,5] The use of biomarkers has been proposed to complement the prognostic evaluation and to provide risk stratification in those patients. Biomarkers such as B-type natriuretic peptide (BNP), ultrasensitive troponins, and soluble protein ST2 emerge as useful to estimate the seriousness of the HF and the risk of death in the short and the long-term.[6,7,8] These still have a high cost and little availability. On the other hand, the hemogram is easily available and generally requested during follow-up of these patients. The possibility of a low-cost biomarker drawn from the hemogram data is very useful in clinical practice. The neutrophil-to-lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) are being considered as new inflammatory biomarkers and used in the staging prognosis of several chronic diseases.[9,10,11] As HF is an inflammatory process, these ratios may be much valid in the prognostic evaluation of these patients.
The objective of this study is to evaluate the NLR and PLR, from the hemograms obtained from children and adolescents with DCM, and to correlate them with the levels of BNP and with the clinical evolution of these patients in the long term.
MATERIALS AND METHODS
This is a retrospective, observational study performed in specialized outpatient clinic in the period from January 2010 to December 2015. Patients were included with age lower than 18 years, with DCM diagnosis and under treatment for chronic HF, with regular follow-up and correct use of medications, and with informed consent to participate in the study. The hemograms analyzed were those collected after at least 6 months of optimized HF therapy. Complete therapy was considered as adequate dose of diuretics in the presence of congestive symptoms, angiotensin-converting enzyme inhibitors, beta-blockers, spironolactone for those with persistently <30% left ventricular ejection fraction (LVEF), and digoxin in cases of refractory symptoms. All the hemograms were done in the same laboratory and by the same test methods (Neubauer chamber hemocytometer and manual evaluation cytometry). The quantitative levels of BNP were determined at the same time, using the immunofluorescence method (Triage test, Biosite Diagnostics). The NLR and PLR were calculated for each patient as well as the ratios between the total number of neutrophils and the total number of lymphocytes and between total platelet number and total lymphocyte number, respectively. We compared the findings of the examinations in relation to the occurrence of listing for transplantation, performing a heart transplant, or progression to obit.
Statistical analysis
For the analysis, the statistical package for the social sciences (SPSS Inc., Chicago, IL, United States of America, version 14.0) for Windows, was used. The categorical variables were expressed as frequencies or percentages and compared with Chi-square or Fisher tests, whenever appropriate. The quantitative variables were described as mean ± standard deviation, and the Mann-Whitney test was used to compare the variables between the two groups. Spearman's coefficient was used to evaluate the correlation including nonparametric categorical variable and Pearson's coefficient to evaluate the degree of correlation between the values of NLR and BNP. The findings were interpreted as directly proportional correlation (if +) or inversely proportional (if −) and weak (if 0.1–0.29), moderate (if 0.3–0.59), strong (if 0.6–0.79), very strong (if from 0.8 to 0.99), or perfect (if 1).[12]
The receiver operating characteristic (ROC) curve was drafted to define the best cutoff point of the significant variables relative to the poor evolution (death or transplant). From that selected cutoff point, a Kaplan–Meier curve was drafted to evaluate the survival free from death or transplant and applied the log-rank test for comparison of the curves. The significance level adopted was of 5%.
RESULTS
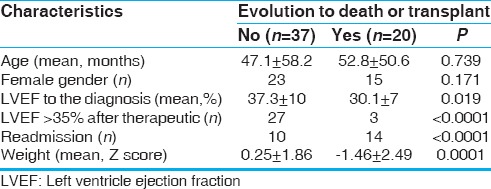
Follow-up was made on 57 patients, 60% of the female gender, with age varying from 1 month to 16.7 years (mean of 48 ± 55.9 months; median of 16 months), with higher incidence in infants of <12 months of age. The period of follow-up ranged from 6 to 60 months (mean of 46 ± 14). The mean LVEF at the diagnosis of cardiomyopathy was 35.5% ± 9, 8%. The general characteristics of the population are shown in Tables 1 and 2.
Table 1.
General characteristics of the children and adolescents with dilated cardiomyopathy

Table 2.
General characteristics of each group
After optimized therapy for HF, it was observed that 37/57 (64.9%) of the patients had a good evolution, remaining in class Functional I–II New York Heart Association (NYHA), but 4 (7%) remained in serious condition (Class III–IV NYHA) waiting list for transplant and 16 (28.1%) had evolved to death or were submitted to cardiac transplant. The results of laboratory analysis are shown in Table 3. The evolutive groups were represented in Figure 1.
Table 3.
Average laboratory result comparison between groups
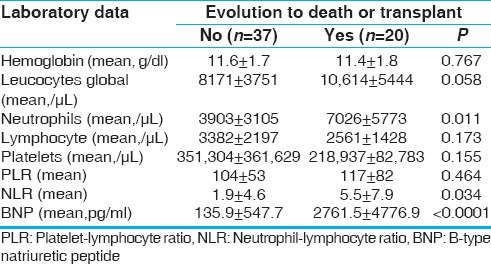
Figure 1.
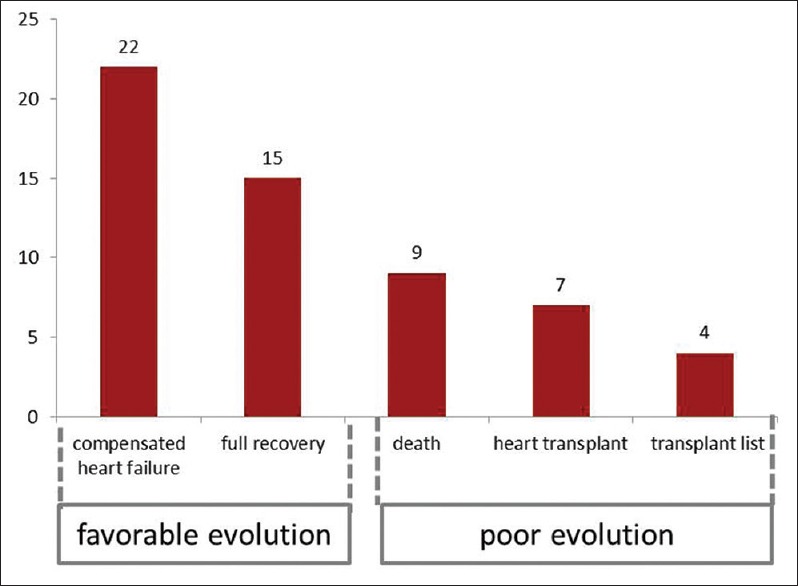
Distribution of patients according to progression after complete treatment of heart failure
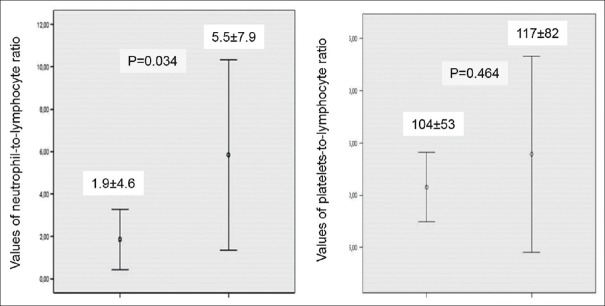
There were no observed differences between the evolutive groups in relation to the results of hemoglobin, global count of leukocytes, lymphocytes, and platelets. In the same way, the PLR appeared similar on the two groups (P = 0.464).
In unfavorable evolution, group presented a higher mean of neutrophils (P = 0.011) and a lower mean of lymphocytes although it did not reach statistical significance (P = 0.173). These results influenced the increase in NLR in this group (P = 0.034), Figure 2. The Pearson coefficient for NLR values relative to an unfavorable evolution of the DCM showed a moderate degree of positive correlation (r = 0.34. P = 0.010).
Figure 2.
Comparison of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio between the two groups
The ROC curve [Figure 3] drawn for the NLR values showed the area under the curve (AUC) of 72.9% (HF 95%: 0.540–0.873; P = 0.016), so that the best cutoff point for predicting death or cardiac transplant was a value equal or >5.2 (sensitivity: 93.8% and specificity: 87.8%). For this range of results, the positive predictive value was 85.7%, the negative predictive value was 80%, positive likelihood ratio being 15%, negative likelihood ratio being 64.1%, and the accuracy being 80.7%. The AUC when drawn for BNP values showed an excellent value (AUC: 94.8%, HF 95%: 0.849–1.030; P < 0.0001); it was noted that all the patients with NLR ≥5.2 had BNP values >1000 pg/ml (P < 0.0001). The result of Pearson coefficient for the NLR values in relation to the BNP values showed a moderate degree of positive correlation (r = 0.49. P < 0.0001).
Figure 3.
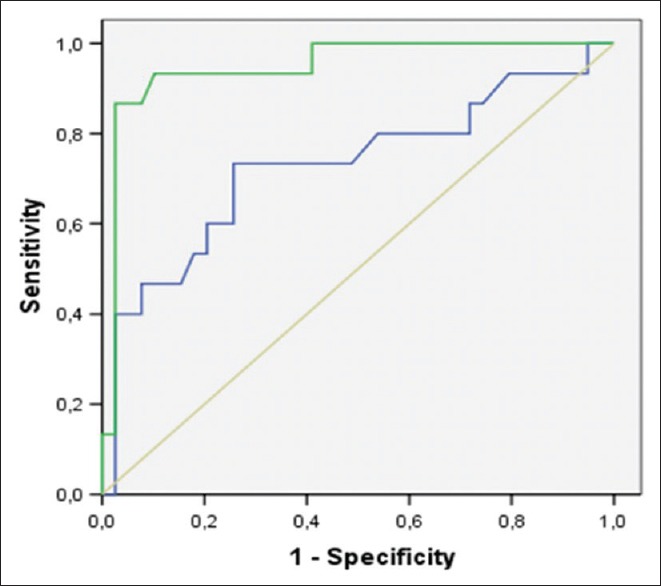
Receiver operating characteristic curves based on a univariate model examining the power of brain natriuretic peptide and neutrophil-to-lymphocyte ratio to predict cardiac death or heart transplantation (green line=brain natriuretic peptide; blue line=neutrophil-to-lymphocyte ratio)
In addition, it was observed that a lymphocyte count below 1000/μL in these patients was related to a poor prognosis, that is, evolution to death or cardiac transplant (sensitivity of 81% and specificity of 93%).
When the LVEF was analyzed in relation to the values of NLR [Figure 4], a statistically significant difference was observed, so that the patients with LVEF lower than 35% presented a higher frequency of NLR ≥5.2 (P = 0.03).
Figure 4.
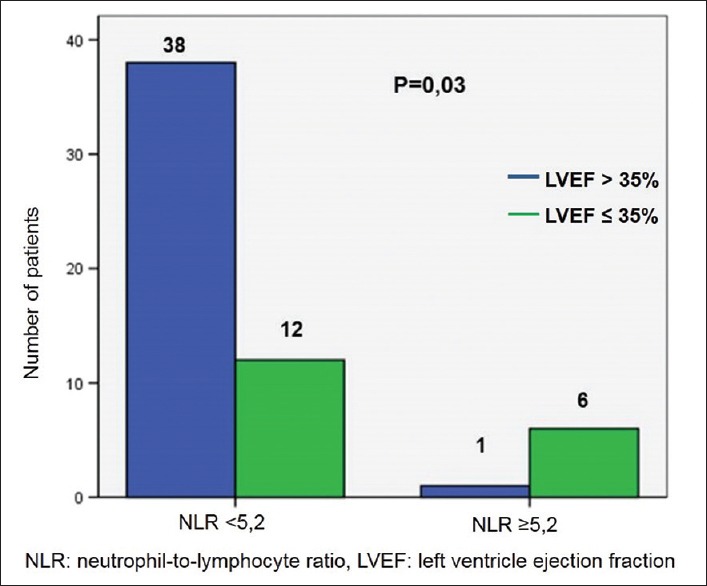
Patient distribution according to the neutrophil-to-lymphocyte ratio and left ventricle ejection fraction
The Kaplan–Meier curve [Figure 5] showed a greater survival for patients with NLR <5.2 (P = 0.001). The biggest mortality index for patients with NLR ≥5.2 occurred between 24 and 36 months of the follow-up. Rates of survival free of events (death or transplant) after 80 months of follow-up were of 81.9% and 16.7% for patients with NLR <5.2 and NLR ≥5.2, respectively.
Figure 5.
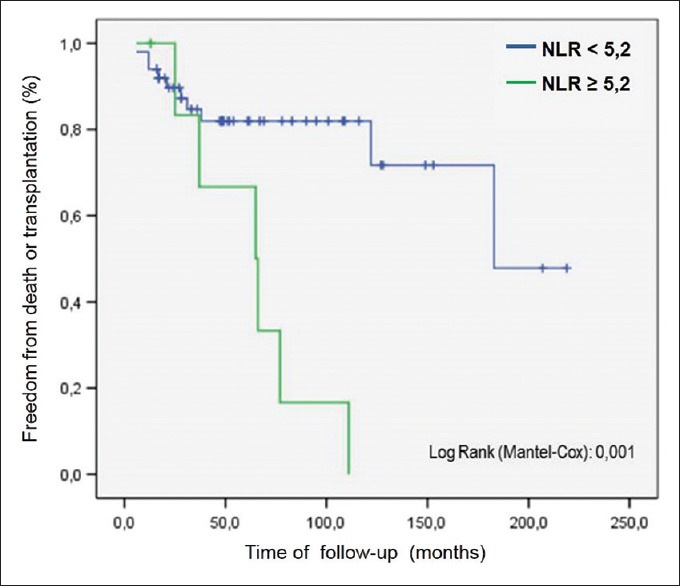
Survival analysis by Kaplan–Meier method and log-rank test for neutrophil-to-lymphocyte ratio
In a similar way, the Kaplan–Meier curve drawn for the survival of the patients relative to the range of values of BNP, having as cutoff point value of 1000 pg/ml [Figure 6], showed a greater survival for the patients with BNP <1000 pg/ml (P < 0.0001).
Figure 6.
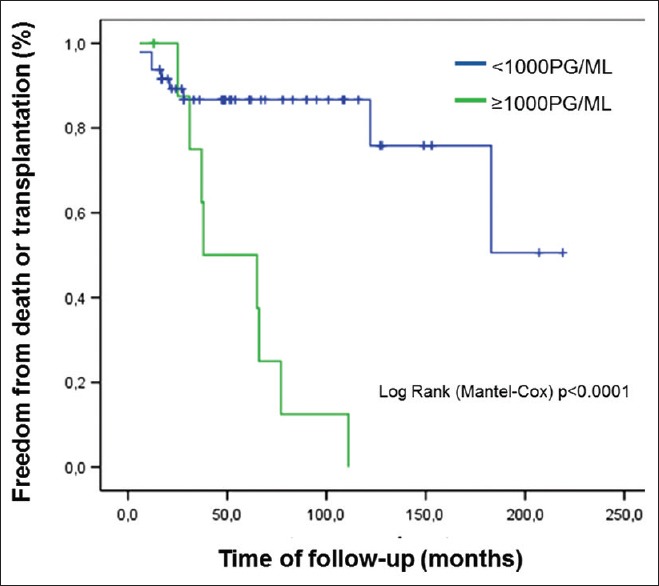
Survival analysis by Kaplan–Meier method and log-rank test for B-type natriuretic peptide
DISCUSSION
The inflammatory process is observed in chronic diseases, such as cancer, diabetes, systemic arterial hypertension, connective tissue diseases, and chronic kidney disease, among others. The HF also develops as a chronic inflammatory process.[13,14] Immunological and hormonal mechanisms are activated, although this chain has not been completely elucidated. The reflexes of these alterations may be noticed peripherally, through the analysis of blood cells.
Neutrophils are recruited very early after the myocardial injury, followed by pro-inflammatory monocytes and lymphocytes, increasing the total numbers of circulating leukocytes.[15] In this inflammatory context, the platelets interact with the endothelium and with the leukocytes, with the occurrence of the expression of cellular adhesion molecules, such as the E-selectin, on the surface of blood vessels walls and in leukocytes present in the blood flow. They can release a variety of cytokines and chemokines, supplying fast mechanisms in the acute inflammatory response as well as also synthetizing interleukin-1β which, when activated, may induce inflammatory response in endothelial human cells.[16] Not only the increase in the number of neutrophils but also the number of platelets represents nonspecific inflammatory reactions. However, the reduction in the number of lymphocytes is justified by situations of great stress. Patients who are significantly sick suffer physiological stress, with increase in cortisol production by activation of the hypothalamic-pituitary-adrenal axis, which may result in a shift in the leukocytes differential count, reducing the relative concentration of lymphocytes.[16,17]
It has already been demonstrated that lymphopenia is an independent prognostic factor, being associated with lower survival rates in patients with HF.[17,18,19,20] Similar results were demonstrated by the present study, being observed that patients with lymphocyte count equal or <1000 lymphocyte/μL presented a poor prognostic.
NLR and PLR are considered new inflammatory biomarkers used as prognostic factors in several diseases and associated with higher mortality of adult patients with HF.[10,11] The study, in thesis, demonstrated that NLR was increased in the group of patients with poor functional class and was related with a death outcome or cardiac transplant. Similar findings were found in a recent study comparing NLR results of patients with functional Class III–IV NYHA and cardiomyopathy hypertrophy with those of the control group.[21] Uthamalingam et al. also showed that higher levels of NLR were associated with a higher mortality rate of adults with decompensated HF, being its capacity for predicting the mortality superior to the neutrophils count, the total count of leukocytes, and the relatively low lymphocyte[11] count.
Durmus et al. concluded that NLR and PLR were higher in adult patients with HF than with the controls matched by sex and age (P < 0.01), and that NLR was, just in itself, an independent predictor of death. They determined too that the best cutoff point of NLR to predict HF was 3.0 (sensitivity 86.3% and specificity 77.5%) and to predict death it was 5.1 (sensitivity 75% and specificity 62%).[18] Our results were in agreement with those shown by these authors. We verified that there was no association of PLR with the prognosis and the cutoff point of NLR to predict death or the need for cardiac transplant was also similar, of 5.2.
In the same way, studies were conducted in situations of pulmonary hypertension (PH), with similar conclusions. Patients with PH may present an increase in the total number of neutrophils and increased NLR, related to a more severe pulmonary disease, worse functional class, and poor prognosis, with high mortality rates. Harbaum et al. provided evidence in their study with adult population that patients with PH and high values of NLR presented a worse functional class (III and IV) (P = 0.0281) and progressed to death or pulmonary transplant (P = 0.0394).[22] The present study excluded patients with PH that was not secondary to DCM.
Among other chronic noncardiac conditions where this ratio has been used, one is obesity. Aydin et al. evaluated hematological data, including NLR, in obese adolescents, compared to a healthy control group. The authors found significant differences between the groups with a rise in the NLR and the mean value of the platelet count in obese patients. This fact appears to be related to the physiopathology of chronic physiological and inflammatory stress, directly influencing the increase of NLR.[23] The patients of the present study were not in the obesity range.
It is not only the studies conducted with patients with chronic diseases that have correlated NLR with factors of poor prognosis. Many studies are being made relative to acute diseases, such as urinary tract infection (UTI). Han et al. studied the usefulness of NLR in the analysis of febrile UTI in children of <3 years. Their results showed that NLR has a better predictive factor for vesicoureteral reflux when compared to other factors such as the absolute value of leukocytes, C-reactive protein, and erythrocyte sedimentation rate, with P < 0.001.[24] The patients of the present study did not present acute infections concomitant with the symptoms of DCM.
Several authors have noted that persistently low LVEF was associated with a worse prognosis.[25,26,27] The present study also showed that LVEF was related to high values of NLR and in this way to prognosis. Durmus et al. also evaluated the ratios of the hemogram and the LVEF, in a study of multivariate logistic regression and determined that the only independent predictor of mortality in adults with HF was the NLR (P = 0.045) and not the LVEF (P = 0.179) or the PLR (P = 0.464).[18]
The determination of the level of BNP is presently available but still expensive for routine use in many countries. A study by Kim et al. demonstrated that children with levels of BNP higher than 600 pg/ml presented poor evolution for DCM.[28] A study by Yan et al. compared values of NLR with values of BNP in an elderly population with chronic HF and related them to cardiovascular events, concluding that NLR was a risk marker with equivalent power to BNP.[29] The present study demonstrated similar results, being noted that levels of BNP higher than 1000 pg/ml were associated with high NLR and also to unfavorable prognosis. It should be stressed that the follow-up by hemogram analysis is more accessible in clinical practice, so that the determination of BNP may be reserved for selected cases.
It should be stressed that the greater part of the studies related to this theme was conducted in adult populations. The present study is a pioneering one by evaluating the prognosis of HF in children and adolescents with DCM through the variables of the hemogram. Therefore, there are no data available to compare the obtained results with a population of similar age group. Subsequent studies may elucidate these facts.
CONCLUSIONS
The hemogram is useful to evaluate children and adolescents with DCM and in the treatment of HF. High NLR (≥5.2) and lymphopenia (≤1000 lymphocyte/μL) were associated with a poor prognosis and a higher chance of evolution to death or cardiac transplant.
Limitations of the study
This study presents limitations, among them the small size of the sample, justified by the low prevalence of the disease in that population and by the long-term follow-up. We also stress the lack of a control group, with matching by gender and age. Since it was a retrospective analysis, the examinations were evaluated in a single sample of each patient, at different stages of the disease, in a nonstandardized way.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank “Alere™ Brazil” for collaboration in providing some BNP dosing supplies.
We are grateful to Mr. Geoge Borten for his generous assistance in text revision.
REFERENCES
- 1.Rossano JW. Clinical management of patients with acute heart failure. Cardiol Young. 2015;25(Suppl 2):67–73. doi: 10.1017/S1047951115000852. [DOI] [PubMed] [Google Scholar]
- 2.Dipchand AI, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Levvey BJ, et al. The registry of the International Society for Heart and Lung Transplantation: Seventeenth official pediatric heart transplantation report-2014; focus theme: Retransplantation. J Heart Lung Transplant. 2014;33:985–95. doi: 10.1016/j.healun.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Azevedo VM, Santos MA, Albanesi Filho FM, Castier MB, Tura BR, Amino JG, et al. Outcome factors of idiopathic dilated cardiomyopathy in children – A long-term follow-up review. Cardiol Young. 2007;17:175–84. doi: 10.1017/S1047951107000170. [DOI] [PubMed] [Google Scholar]
- 4.Hollander SA, Bernstein D, Yeh J, Dao D, Sun HY, Rosenthal D, et al. Outcomes of children following a first hospitalization for dilated cardiomyopathy. Circ Heart Fail. 2012;5:437–43. doi: 10.1161/CIRCHEARTFAILURE.111.964510. [DOI] [PubMed] [Google Scholar]
- 5.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–76. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 6.Kantor FP, Rusconi P. Biomarkers in pediatric heart failure: their role in diagnosis and evaluating disease progression. Prog Pediatr Cardiol. 2011;33:53–7. [Google Scholar]
- 7.Tan LH, Jefferies JL, Liang JF, Denfield SW, Dreyer WJ, Mott AR, et al. Concentrations of brain natriuretic peptide in the plasma predicts outcomes of treatment of children with decompensated heart failure admitted to the Intensive Care Unit. Cardiol Young. 2007;17:397–406. doi: 10.1017/S1047951107000601. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, Ramirez-Correa GA, Murphy AM. Proteomics of pediatric heart failure: From traditional biomarkers to new discovery strategies. Cardiol Young. 2015;25(Suppl 2):51–7. doi: 10.1017/S1047951115000839. [DOI] [PubMed] [Google Scholar]
- 9.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–7. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Park JJ, Jang HJ, Oh IY, Yoon CH, Suh JW, Cho YS, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–42. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433–8. doi: 10.1016/j.amjcard.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Bunchaft G, Kellner SR. Statistic without mysteries. 2th ed. Petrópolis: Vozes; 1999. [Google Scholar]
- 13.Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: An overview. Heart. 2004;90:464–70. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S, et al. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–73. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira I, Girão MJ, Sampaio MU, Oliva ML, Andrade SS. Platelets: Traditional and nontraditional roles in hemostasis, inflammation and cancer. ABCS Health Sci. 2013;38:153–61. [Google Scholar]
- 17.Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79:812–4. doi: 10.1016/s0002-9149(96)00878-8. [DOI] [PubMed] [Google Scholar]
- 18.Durmus E, Kivrak T, Gerin F, Sunbul M, Sari I, Erdogan O, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol. 2015;105:606–13. doi: 10.5935/abc.20150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maisel AS, Knowlton KU, Fowler P, Rearden A, Ziegler MG, Motulsky HJ, et al. Adrenergic control of circulating lymphocyte subpopulations. Effects of congestive heart failure, dynamic exercise, and terbutaline treatment. J Clin Invest. 1990;85:462–7. doi: 10.1172/JCI114460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ, et al. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97:19–22. doi: 10.1161/01.cir.97.1.19. [DOI] [PubMed] [Google Scholar]
- 21.Ozyilmaz S, Akgul O, Uyarel H, Pusuroglu H, Gul M, Satilmisoglu MH, et al. The importance of the neutrophil-to-lymphocyte ratio in patients with hypertrophic cardiomyopathy. Rev Port Cardiol. 2017;36:239–46. doi: 10.1016/j.repc.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Harbaum L, Baaske KM, Simon M, Oqueka T, Sinning C, Glatzel A, et al. Exploratory analysis of the neutrophil to lymphocyte ratio in patients with pulmonary arterial hypertension. BMC Pulm Med. 2017;17:72. doi: 10.1186/s12890-017-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aydin M, Yilmaz A, Donma MM, Tulubas F, Demirkol M, Erdogan M, et al. Neutrophil/lymphocyte ratio in obese adolescents. North Clin Istanb. 2015;2:87–91. doi: 10.14744/nci.2015.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han SY, Lee IR, Park SJ, Kim JH, Shin JI. Usefulness of neutrophil-lymphocyte ratio in young children with febrile urinary tract infection. Korean J Pediatr. 2016;59:139–44. doi: 10.3345/kjp.2016.59.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matitiau A, Perez-Atayde A, Sanders SP, Sluysmans T, Parness IA, Spevak PJ, et al. Infantile dilated cardiomyopathy. Relation of outcome to left ventricular mechanics, hemodynamics, and histology at the time of presentation. Circulation. 1994;90:1310–8. doi: 10.1161/01.cir.90.3.1310. [DOI] [PubMed] [Google Scholar]
- 26.Azevedo VM, Albanesi Filho FM, Santos MA, Castier MB, Tura BR. Prognostic value of chest roentgenograms in children with idiopathic dilated cardiomyopathy. J Pediatr (Rio J) 2004;80:71–6. [PubMed] [Google Scholar]
- 27.McMahon CJ, Nagueh SF, Eapen RS, Dreyer WJ, Finkelshtyn I, Cao X, et al. Echocardiographic predictors of adverse clinical events in children with dilated cardiomyopathy: A prospective clinical study. Heart. 2004;90:908–15. doi: 10.1136/hrt.2003.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim G, Lee OJ, Kang IS, Song J, Huh J. Clinical implications of serial serum N-terminal prohormone brain natriuretic peptide levels in the prediction of outcome in children with dilated cardiomyopathy. Am J Cardiol. 2013;112:1455–60. doi: 10.1016/j.amjcard.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Yan W, Li RJ, Jia Q, Mu Y, Liu CL, He KL, et al. Neutrophil-to-lymphocyte ratio compared to N-terminal pro-brain natriuretic peptide as a prognostic marker of adverse events in elderly patients with chronic heart failure. J Geriatr Cardiol. 2017;14:127–34. doi: 10.11909/j.issn.1671-5411.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]