Abstract
Endoscopic ultrasound-guided fine needle aspiration (EUS FNA) has made pathological diagnosis of pancreatic neoplasms, diseases involving lymph nodes at various mediastinal and abdominal sites, gastrointestinal submucosal lesions, perirectal lesions, adrenal lesions, and mediastinal masses easy. EUS-guided FNA is a multistep procedure that involves assessment of proper clinical indication, correct selection of FNA needles, and adoption of evidence-based techniques for tissue sampling. EUS FNA is done by needles that are available in different sizes, mainly 25, 22, and 19-gauge needle. The need of onsite cytopathologist, dependence on histology/core biopsy occasionally to get a diagnosis, and inability to reliably assess for molecular markers are important limitations of EUS FNA. EUS-guided fine needle biopsy (FNB) that samples the core of tissue is an exciting new development in the field of diagnostic EUS. FNB needles are expensive than FNA needles, and although the initial results are encouraging, more studies with robust evidence proving their superiority beyond any doubt are needed before they can be widely used.
Keywords: Endosonography, fine needle aspiration, fine needle biopsy, pancreas, stylet
INTRODUCTION
Endoscopic ultrasound (EUS) is an interesting diagnostic as well as interventional modality arising after amalgamation of endoscopy and ultrasound that has changed the way gastrointestinal (GI) endoscopy is practiced today. It initially started as a pure diagnostic imaging modality when, in 1980, for the first time an ultrasonography (USG) probe was attached to an endoscope and used as EUS.[1] However with subsequent advancements, EUS-guided fine needle aspiration (FNA) for tissue acquisition (TA) became its predominant role in clinical practice. In 1992, the first case of EUS-guided FNA of pancreatic lesion was reported, and in the same year EUS-guided FNA of upper GI tract as well as lower GI tract was reported.[2,3] Since then, the role EUS-guided TA has increased exponentially. In a study from USA, authors investigated various methods of TA in pancreatic diseases between 2006 and 2010 and found that TA by EUS-FNA increased by 69.3% whereas TA using surgery decreased by 41.7% whereas TA by percutaneous route remained the same.[4] Development of electronic linear ultrasound endoscopes led on to EUS-guided FNA being carried out with great precision in real time with the FNA needle being visualized throughout the procedure, and it gradually replaced the need of histological diagnosis in various GI disorders. EUS FNA has made the pathological diagnosis by means of TA in otherwise difficult anatomic locations within abdomen, retroperitoneum, mediastinum, and perirectal space easy. Pancreatic neoplasms, diseases involving lymph nodes at various mediastinal and abdominal sites, GI submucosal lesions, perirectal lesions, adrenal lesions, and mediastinal masses are routinely diagnosed by EUS FNA. Despite its role in diagnostics and superiority over other methods of TA, it is also important to recognize its limitations and imperfections. The need of onsite cytopathologist, dependence on histology/core biopsy occasionally to achieve a diagnosis, and inability to reliably assess for molecular markers are some of the important challenges that EUS FNA faces. In this review, we will discuss the various aspects of EUS-guided TA.
EUS-guided FNA is a multistep procedure that involves assessment of proper clinical indication, correct selection of FNA needles, and adoption of evidence-based techniques for tissue sampling. Before taking the patient for EUS FNA, especially in developing countries where cost and economics are important factors, appropriateness of indication and its impact on management of disease and advantages as well as disadvantages including the total cost of alternative methods to obtain the samples should be reviewed.
EUS FNA is most commonly performed to confirm the diagnosis of cancer. The most common benign conditions where EUS FNA is used for diagnosis are sarcoidosis and tuberculosis. The common sites for EUS FNA are pancreas, bile duct, suspicious GI tract wall thickening, sub mucosal lesions, adrenal glands, liver, retroperitoneal masses, lymph nodes, posterior mediastinum, and central pulmonary masses.[5] Before performing EUS FNA, it is important to exclude significant coagulopathy (INR > 1.2), thrombocytopenia (platelets <100,000), and recent use of thienopyridines (e.g., clopidogrel).
EUS-FNA devices [Figure 1]
Figure 1.
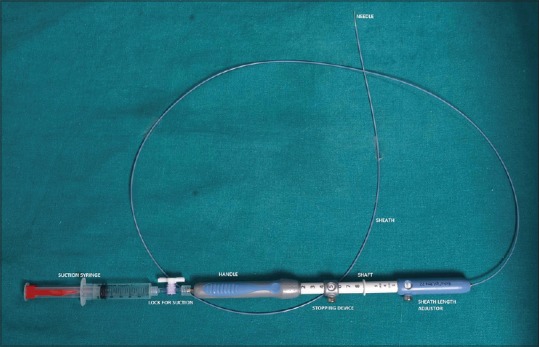
EUS FNA needle and its parts
They are available in different sizes mainly 25, 22, and 19-gauge needles. These needles vary in flexibility and size, with 25 G being smaller in diameter, highly flexible, and yielding less bloody samples.
The EUS FNA needle consists of four main components:
-
(1)
Hollow needle
-
(2)
Solid removable stylet
-
(3)
A semi-rigid protective sheath
-
(4)
A handle which has a port for insertion and removal of stylet as well as for creating negative suction pressure during FNA.
While performing FNA, the echoendoscope should be positioned as straight as possible because a straight endoscope allows easier insertion of EUS FNA needle. Before puncturing the lesion, it should be carefully assessed by EUS for the nature of lesions (solid or cystic) as cystic lesions need to be completely aspirated to prevent the risk of infection. In addition, color Doppler should be used to exclude the presence of blood vessels in the needle path. Once the protective sheath has been brought out of the instrument channel, the lesion should be positioned in the needle path. Excessive movements of tip of endoscope or the elevator should be avoided as it will increase the resistance to movements of needle leading on to its bending. FNA done by bent needle is risky as it tends to disappear from the ultrasound view. It is also important to remember to not puncture undrained, obstructed pancreatic or bile ducts as it may lead to cholangitis or pancreatitis. EUS FNA is easier from stomach or esophagus as the echoendoscope is straight in stomach and esophagus, and therefore, the needle can be easily maneuvered, whereas in the duodenum, where the scope is acutely angulated, the FNA procedure is difficult and requires advanced skills as angulated scope decreases the maneuverability of the FNA needle. Acute angulation of the endoscopic tip, large torsion through the scope shaft because of angulations, and repeated elevator use increases technical the difficulty of EUS FNA. The 25-G aspiration needle has been shown to be superior over the 22-G aspiration needle for the sampling of solid pancreatic lesions in head and uncinate process.[6,7] However, studies including meta-analysis have shown the nonsuperiority of 25-G needle over 22-G needle for lesions other than pancreas in terms of sampling adequacy as well as cytological and histological accuracy.[8,9] The 19-G needle being larger has a theoretical advantage in sampling the lesion, however, studies have found no superiority of 19-G needle over 22-G and 25-G needle.[10,11,12] American Society of Gastrointestinal Endoscopy (ASGE) technical review has recommended the use of a 25-G needle over a 22-G needle in patients undergoing EUS FNA of pancreatic masses.[13]
Role of stylet
The stylet in EUS FNA needle was designed to provide stiffness to the FNA device as well as to prevent the clogging of needle with digestive wall tissue while puncturing, and thus prevent the contamination of the sample by cells that do not originate from the intended target lesion. However, studies have shown no added advantage of stylet. Studies have shown that the routine use of stylet for these purposes did not yield better result and there was no difference in cellularity, contamination, bloodiness score, diagnostic ability, and diagnostic accuracy when FNA was performed with or without stylet. Well-designed randomized trials have shown that the sample yields were no better when stylet was used.[14,15,16] Moreover, technically, the use of stylet at every passage is labor intensive, time consuming, and prone for needle stick injury. ASGE technical review has recommended against the use of a stylet during EUS FNA in order to improve the diagnostic yield of and specimen quality.[13] We use stylet for first pass only and perform subsequent passes without stylet.
Techniques of sampling
Various FNA techniques have been described for sampling the tissue.
Standard technique: In this technique, during a pass, the needle tip is positioned at one location within the mass and then moved back and forth multiple times. For subsequent passes, a different margin of mass is targeted, with needle movement being confined to the same area
Fanning technique: In this technique, needle is positioned at different areas within the mass and then moved to and fro multiple times in each area. The needle path can be altered using either the “up/down” endoscope dial or the elevator. In a recent randomized controlled trial which compared the standard technique versus fanning technique, authors found no difference in diagnostic accuracy, technical failure, or complication rates.[17] However, fanning technique required lesser number of passes to establish the diagnosis with a higher percentage of patients achieving diagnosis on pass one (57.7% vs. 85.7%; P = 0.02).[17] We routinely use fanning technique in our clinical practice.
Suction or No suction
In technique using suction, while performing FNA, negative suction is applied using luer-lock syringe during the puncturing of lesion, and this theoretically would increase the yield of tissue. Various studies have shown that it indeed increases the tissue yield but at the cost of hemorrhagic sample.[18,19,20] In a recent randomized trial comparing 22-G and 25-G needles with or without suction, authors found that that use of suction with 22-G needles was inferior to no use of suction, and they concluded that the use of suction must be avoided in centers utilizing rapid onsite evaluation (ROSE) as it increases specimen bloodiness and number of passes needed to achieve diagnostic adequacy, particularly with 22-G needles.[21] However, suction has been shown to improve the diagnostic yield in fibrotic lesions such as pancreatic cancer.[19] ASGE technical review has recommended the use of suction during EUS FNA of pancreatic masses and against the use of suction during EUS FNA of lymph nodes because it increases the bloodiness of specimens obtained and has no impact on the overall diagnostic yield.[13] The available evidence suggests that sampling a vascular lesion/site such as lymph nodes, a nonsuction technique may result in better quality, whereas aspirating fibrotic malignant lesion of pancreas or in setting of chronic pancreatitis suction may provide a superior sample. We routinely use suction during the first pass and then tailoring the use of suction depending upon the visible quality of yield with avoiding suction in next pass if the sample is hemorrhagic.
Slow pull technique
To avoid strong suction of suction technique and thus decrease the bloodiness of the sample, an alternative low pressure technique of slow pull technique has been described. Also known as capillary technique, during the sampling of lesion, the stylet is slowly retracted along with to and fro motion of the needle, thereby creating a slow negative suction that enables increased aspiration of tissue. In a recently published prospective comparative study involving patients with pancreatic lesions, standard suction technique was compared with the slow-pull technique. Slow-pull technique was found to be having significantly superior diagnostic accuracy (88% vs 71%) with significantly less blood contamination.[22]
Handling of aspirated sample
To expel the tissue from the needle onto the slides after aspiration two techniques are used.
Use of an air-filled syringe to expel the aspirated material. However, this may lead to expelling of aspirated material in an uncontrolled manner and lead to scattering of material, which apart from loss of material is also a health hazard
Using stylet which is more controlled as material is expelled drop by drop is preferred by most endosonologists.
Fixation of slide
These are done either by air dry fixation or alcohol fixation.
Air dried fixation: The slides are dried by using air blower or hair dryer till complete drying of slides. After air fixation, slides are usually stained with Romanowsky-type stains. These slides are also suitable for IHC staining, if required later
Alcohol fixation: As soon as the slides are prepared and still wet, they are immersed in ethanol or sprayed on. These slides are stained with Papanicolaou stain.
In air dried slides, the cells spread over the glass slides as they dry expand leading to better appreciation of pleomorphism, if present, along with better visualization of intra-cytoplasmic material as well as extracellular substances such as mucin. Alcohol fixation preserves nuclear features. and thus, better highlight nuclear features and chromatin quality. In routine practice, half of the slides should be sent air dried and other half alcohol fixed as both of them are complimentary for cytological examination to reach the diagnosis.
Rapid on-site evaluation (ROSE) or on-site cytopathological evaluation (OCE)
There is a theoretical advantage that on-site evaluation of slides prepared during procedure would lead to less false negative results as well as few passes would be required to ascertain the diagnosis, leading to shortening of procedure time as well as less complication rates. The idea of ROSE came from the fact that up to 30% of FNA interpretation is nondiagnostic either because of poor cellularity and/or crush artifacts due to poor slide preparation. ROSE by allowing immediate course allocation of samples for flow cytometry and microbiology, as well as assess the need for core biopsy thus helping in quick preliminary diagnosis and improving the diagnostic accuracy of EUS FNA. Studies have shown that ROSE increases diagnostic yield by up to 20% and decreases inadequate or unsatisfactory samples and decreases number of passes required to establish diagnosis.[23,24,25,26,27] It is estimated that without ROSE there would be a 20% rise in the number of inadequate samples.[26]
However, ROSE is not as rosy as it seems. It significantly adds to the cost as it needs an on-site professional as well as extended time for procedure including anesthesia time and thus associated adverse effects. Also, equivocal onsite diagnosis or poor handling of samples may lead on to premature termination of EUS FNA, and therefore, increase the need for subsequent repeat procedures. Recently published meta-analyses which evaluated the diagnostic accuracy and the role of ROSE found conflicting results and concluded that ROSE is associated with improvement in adequacy rates at sites where the adequacy rate without ROSE is less than 90%.[28,29] ASGE has recommended that ROSE/OCE does not impact the diagnostic yield of EUS FNA; however, an on-site cytopathologist may have a role during training phase and in centers with a low diagnostic adequacy rate.[13]
Cell block
These are usually complementary to the cytological assessment of the slides. FNA with cell block is cost-effective when compared with the additional expenses of surgical biopsy and is also less invasive. In clinical situations such as pancreatic ductal malignancy, lymphoma, neuroendocrine tumor (NET), and gastrointestinal stromal tumor (GIST) where cytology sample are not adequate for diagnosis, cell blocks provide additional material for histology as well as IHC and molecular profiling. Various cell block preparation methods use different transport mediums. Cell blocks are prepared by expelling the sample from the FNA needle into a container containing a cell-preservative solution such as Roswell Park Memorial Institute Medium (RPMI-1640). Even, isotonic saline can be used as medium to transport the material but the sample should be processed quickly because of short shelf life of cells in saline. The container containing the sample is centrifuged to harvest the cells and fibrin glue is commonly used to hold the cells to form tissue fragments. After that tissue is processed for histological examination. The details of preparation, fixation, and further processing of cell blocks is beyond the scope of this review and readers are advised to consult dedicated reviews on this topic.
PITFALLS OF EUS FNA
Although EUS FNA has changed the landscape of diagnostics in gastrointestinal endoscopy by increasing the diagnostic accuracy of tissue acquisition, it is important to recognize its pitfalls. In situations such as pancreatic masses in chronic pancreatitis and autoimmune pancreatitis (AIP), EUS FNA can yield false positivity as atypical cells in CP as well as AIP may mimic malignancy. Similarly, false negativity because of technical difficulty, sampling error, or interpretative errors is also an area of concern. Moreover, diagnosing malignancy in hypocellular samples, marked desmoplastic background, as well as well-differentiated adenocarcinoma is challenging.
NEED FOR CORE BIOPSY
The basic difference between FNA specimen and fine needle biopsy (FNB) specimen is that in FNB tissue architecture is maintained. The indications for FNB would be:
When FNA is nondiagnostic or inadequate
When diagnosis is made by looking at tissue architecture such as lymphoma and AIP
When special stains (immunohistochemistry) are needed for diagnosis (e.g., gastrointestinal stromal tumors)
When there is a need to perform tissue profiling or cell culture for targeted therapies for personalized medicine
In centres with low sample adequacy of EUS FNA and nonavailability of ROSE.
ASGE has recommended that new FNB needles are highly effective for acquisition of core specimens and should be considered first-line for tissue sampling of nonpancreatic mass lesions, as a rescue technique after inadequate FNA samples, and for lesions requiring IHC.[13]
HOW TO OBTAIN TISSUE CORE USING EUS?
Tru cut biopsy
The first dedicated EUS-guided needed needle for effective core tissue acquisition was 19-G Tru Cut Needle (Cook Medical, Bloomington, IN, US) which was capable of collecting 18-mm tissue specimen. It had a spring loaded firing mechanism with good diagnostic yield, however, it was not flexible and was difficult to use.[30] The studies demonstrated that there was no clear advantage for EUS-guided Tru cut biopsy (TCB) over EUS-FNA even in patients with suspected lymphomas or subepithelial lesions.[13] Further, the technique of EUS TCB is cumbersome as compared to EUS FNA and is associated with increased complications as well as increased risk of damage to endoscope.[13]
Fine needle biopsy (FNB)
As the TCB needle was cumbersome to use with little added advantage, standard 22 and 19-G needles were used with high negative suction to sample tissue core, and this technique was termed as EUS-guided FNA. Thereafter, newer needles were designed to extract the tissue core. In these needles, the tip of needle was re-designed to have a cutting tip. Pro Core FNB (Cook Medical) was the first such needle and came in various sizes ranging from 19-G, 20-G, 22-G, and 25-G. The distal end of this needle had reverse bevel cut to acquire tissue by cutting through the lesion. Theoretically, the needle looks exciting but the studies have yielded varying results. Prospective cohort studies have shown significant improvement in diagnostic yield with ProCore needle but many retrospective series as well as randomized controlled studies have yielded varying results.[13] An RCT demonstrated the superiority of 19-G ProCore FNB over 19-G Trucut needle in terms of higher prevalence of diagnostic histology (85% vs. 57%; P = 0.006), accuracy (88% vs. 62%; P = 0.02), mean total length (19.4 vs. 4.3 mm; P = 0.001), mean complete portal triads from liver biopsies (10.4 vs. 1.3; P = 0.0004), and required fewer crossover biopsies compared to those of TruCut needle (2% vs. 65%; P = 0.0001).[31]
A multicenter study that compared the 22-G ProCore FNB and 22-G FNA needles in solid pancreatic or abdominal masses reported that FNB analysis was accurate in 91.44% cases as compared to 80% for FNA cases.[32] They concluded that EUS-guided FNB samples yielded more accurate diagnoses than EUS-guided FNA samples for pancreatic lesions, and there was no difference in diagnostic yield between EUS FNA and EUS FNB for nonpancreatic masses. In a recent meta-analysis that analyzed nine studies comparing 22-G ProCore and standard 22-G FNA needles, the authors observed no significant difference between the two needles for sample as well as diagnostic accuracy or acquisition of a core specimen, but the ProCore needle established the diagnosis with fewer passes.[33]
Various other designs of EUS FNB needles to improve upon the diagnostic yield have been developed over the last few years [Figure 2].[34] These hollow FNB needles can be either noncutting or cutting needles. The cutting needles include either side-type cutting (e.g., Tru-Cut or ProCore) or end-type cutting (such as Franseen and fork-tip). The end cutting type needles are the recent introduction to the EUS armamentarium. The two most recently introduced EUS FNB needles are Franseen needle (Acquire, Boston Scientific) and fork-tip needle (SharkCore™, Medtronic) and the initial studies with these new needles are very encouraging [Figure 3]. However, the current data comparing various EUS FNB core needles for sample adequacy, diagnostic accuracy, and acquisition of a core specimen is scanty.[34] The newly developed EUS-FNB needles are expensive than the first-generation needles and need more robust evidence of superiority than what is currently available before they can be widely used. However, still there is limited data to suggest that the use of FNB with adequate core tissue material may be performed without ROSE and therefore may replace FNA.[34]
Figure 2.

Tip of EUS FNB and FNA needle
Figure 3.
EUS FNB being performed from pancreatic head mass
Handling of core biopsy specimen
Once FNB specimen is procured, it can be processed in one of the following two ways:
Tissue sample is transferred to 10% formalin containing solution and then processed for histopathological examination
Sample is placed on a glass slide and with the help of needle is microdissected to form tissue cores.
How to identify tissue pieces
Usually the tissue pieces are pale looking whitish or discolored elongated material, and elongated red-colored material are usually blood clots. Occasionally, blood clots may contain tissue which become ascertained once tissue is paraffin embedded.[35]
Complication of EUS-guided tissue acquisition
EUS-guided TA is generally a safe procedure with very few complications and serious complications are very rare. Pain, bleeding, pancreatitis, and infection are the most frequently reported complications. In a systematic review of more than 50 studies, the overall complication rate was 0.98% with a mortality of 0.02% which were related to cholangitis and pancreatitis.[36] Usually pancreatitis is seen in patients with pancreatic cysts.[37] Extraluminal bleeding is a significant complication seen in up to 1.3% of patients undergoing FNA.[38]
Infection or bacteremia is very rare in patient undergoing EUS-guided TA of solid lesion, and therefore, antibiotic prophylaxis is not recommended. However, EUS FNA of cystic lesion are associated with an infection rate of up to 14%, and therefore, antibiotic prophylaxis is recommended while sampling cystic lesions. It is also important to remember that because of high risk of infection in mediastinal cysts, EUS FNA of mediastinal cysts is usually not recommended.[39,40] Tumor seeding and GI perforation has also been rarely reported.[41]
The future of EUS-guided tissue acquisition
The currently available FNB needles have the potential to change the way the diseases of pancreas, liver, and GI tract are diagnosed. The increased cellularity along with preserved histologic architecture of the FNB sample makes it an ideal sample for histological as well as molecular testing. With such promising advantages use of EUS FNB is going to expand and many new needles with better design than the currently available needles that will have better tissue yield as well as less adverse effects are going to be developed. The future needles would be the ones that will allow acquisition of more material with fewer passes so as to perform cytology, histology, immunocytochemistry, histochemistry, and molecular biology.
Needle-based confocal laser endomicroscopy (nCLE) probe and Microforceps are recent devices which have been used to enhance the diagnostic outcome of EUS FNA. A recent study of in-vivo nCLE Study in the Pancreas with Endosonography of Cystic Tumors (INSPECT) trial has shown sensitivity of 59% and specificity of 100% with PPV of 100% and NPV of 50% in differentiating between types of pancreatic cystic lesions.[42] A prospective study has also shown that in-vivo nCLE patterns are reproducible in ex-vivo pCLE for all major neoplastic pancreatic cystic lesions.[43]
A disposable micro-forceps 230 cm long with open jaw width of 4.5 mm which can be passed through the lumen of a 19-G FNA needle has also been developed to increase the adequacy of samples. In a pilot study, Nakai et al. demonstrated that EUS through the needle forceps biopsy was safe and technically feasible, and moreover, provided additional tissue with a single puncture of a 19-G FNA needle.[44] Reports of use of these devices in sampling pancreatic cyst wall with encouraging results are available.[45]
Furthermore, there is increased interest in telecytopathology for those centers where ROSE is not available. A recent retrospective study compared the nondiagnostic rate for EUS FNA of pancreatic lesions in patients with on-site evaluation for adequacy via telecytopathology with patients not having on-site adequacy evaluation.[46] The authors found that after adjusting for the effects of sex and lesion characteristics (solid versus cystic lesion), the odds of having a nondiagnostic specimen in group not having on-site adequacy evaluation was 6.9 times greater than the telecytopathology group (P = 0.0013).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.DiMagno EP, Buxton JL, Regan PT, Hattery RR, Wilson DA, Suarez JR, et al. Ultrasonic endoscope. Lancet. 1980;1:629–31. doi: 10.1016/s0140-6736(80)91122-8. [DOI] [PubMed] [Google Scholar]
- 2.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 3.Wiersema MJ, Hawes RH, Tao LC, Wiersema LM, Kopecky KK, Rex DK, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35–9. doi: 10.1016/s0016-5107(92)70327-7. [DOI] [PubMed] [Google Scholar]
- 4.Roy AK, Kim M, Hawes R, Varadarajulu S. 196 changing trends in tissue acquisition in pancreatic diseases. Gastrointest Endosc. 2013;77:AB134. [Google Scholar]
- 5.Cazacu IM, Luzuriaga Chavez AA, Saftoiu A, Vilmann P, Bhutani MS. A quarter century of EUS-FNA: Progress, milestones, and future directions. Endosc Ultrasound. 2018;7:141–160. doi: 10.4103/eus.eus_19_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Affolter KE, Schmidt RL, Matynia AP, Adler DG, Factor RE. Needle size has only a limited effect on outcomes in EUS guided fine needle aspiration: A systematic review and meta-analysis. Dig Dis Sci. 2013;58:1026–34. doi: 10.1007/s10620-012-2439-2. [DOI] [PubMed] [Google Scholar]
- 7.Madhoun MF, Wani SB, Rastogi A, Early D, Gaddam S, Tierney WM, et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: A meta-analysis. Endoscopy. 2013;45:86–92. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 8.Rong L, Kida M, Yamauchi H, Okuwaki K, Miyazawa S, Iwai T, et al. Factors affecting the diagnostic accuracy of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) for upper gastrointestinal submucosal or extraluminal solid mass lesions. Dig Endosc. 2012;24:358–63. doi: 10.1111/j.1443-1661.2012.01243.x. [DOI] [PubMed] [Google Scholar]
- 9.Wee E, Lakhtakia S, Gupta R, Sekaran A, Kalapala R, Monga A, et al. Endoscopic ultrasound guided fine-needle aspiration of lymph nodes and solid masses: Factors influencing the cellularity and adequacy of the aspirate. J Clin Gastroenterol. 2012;46:487–93. doi: 10.1097/MCG.0b013e31824432cb. [DOI] [PubMed] [Google Scholar]
- 10.Ramesh J, Bang JY, Hebert-Magee S, Trevino J, Hasan M, Logue AL, et al. Multi-center randomized trial comparing the 19G and 25G needles for EUS-guided FNA of solid pancreatic mass lesions [abstract] Gastrointest Endosc. 2013;77:AB1022. [Google Scholar]
- 11.Song TJ, Kim JH, Lee SS, Eum JB, Moon SH, Park DY, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 12.Itoi T, Itokawa F, Sofuni A, Nakamura K, Tsuchida A, Yamao K, et al. Puncture of solid pancreatic tumors guided by endoscopic ultrasonography: A pilot study series comparing Trucut and 19-gauge and 22-gauge aspiration needles. Endoscopy. 2005;37:362–6. doi: 10.1055/s-2004-826156. [DOI] [PubMed] [Google Scholar]
- 13.Wani S, Muthusamy VR, Komanduri S. EUS-guided tissue acquisition: An evidence-based approach (with videos) Gastrointest Endosc. 2014;80:939–59.e7. doi: 10.1016/j.gie.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 14.Wani S, Early D, Kunkel J, Leathersich A, Hovis CE, Hollander TG, et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: A prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012;76:328–35. doi: 10.1016/j.gie.2012.03.1395. [DOI] [PubMed] [Google Scholar]
- 15.Rastogi A, Wani S, Gupta N, Singh V, Gaddam S, Reddymasu S, et al. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc. 2011;74:58–64. doi: 10.1016/j.gie.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasoundguidedfine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900–3. doi: 10.1055/s-0030-1255676. [DOI] [PubMed] [Google Scholar]
- 17.Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–50. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JK, Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–51. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Puri R, Vilmann P, Saftoiu A, Skov BG, Linnemann D, Hassan H, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 20.Wallace MB, Kennedy T, Durkalski V, Eloubeidi MA, Etamad R, Matsuda K, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441–7. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]
- 21.Bang JY, Hebert-Magee S, Hasan MK, Varadarajulu S. Randomized trial comparing the 22 and 25 gauge Needles using the suction-in and no-suction (sins) techniques for EUS-guided FNA of pancreatic masses. Gastrointest Endosc. 2017;85:AB328. [Google Scholar]
- 22.Lee JM, Lee HS, Hyun JJ, Lee JM, Yoo IK, Kim SH, et al. Slow-pull using a fanning technique is more useful than the standard suction technique in EUS-guided fine needle aspiration in pancreatic masses. Gut Liver. 2018;12:360–6. doi: 10.5009/gnl17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound- guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–94. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 24.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest. Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 25.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guide fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 26.Kulesza P, Eltoum IA. Endoscopic ultrasound-guided fine needle aspiration: Sampling, pitfalls, and quality management. Clin Gastroenterol Hepatol. 2007;5:1248–54. doi: 10.1016/j.cgh.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Wani S, Mullady D, Early DS, Rastogi A, Collins B, Wang JF, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: A prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–39. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 28.Hebert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt RL, Witt BL, Matynia AP, Barraza G, Layfield LJ, Adler DG. Rapid on-site evaluation increases endoscopic ultrasound-guided fine-needle aspiration adequacy for pancreatic lesions. Dig Dis Sci. 2013;58:872–82. doi: 10.1007/s10620-012-2411-1. [DOI] [PubMed] [Google Scholar]
- 30.Gleeson FC, Clayton AC, Zhang L, Clain JE, Gores GJ, Rajan E, et al. Adequacy of endoscopic ultrasound core needle biopsy specimen of nonmalignant hepatic parenchymal disease. Clin Gastroenterol Hepatol. 2008;6:1437–40. doi: 10.1016/j.cgh.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 31.DeWitt J, Cho CM, Lin J, Al-Haddad M, Canto MI, Salamone A, et al. Comparison of EUS-guided tissue acquisition using two different19-gauge core biopsy needles: A multicenter, prospective, randomized, and blinded study. Endosc Int Open. 2015;3:E471–8. doi: 10.1055/s-0034-1392222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng B, Zhang Y, Chen Q, Sun B, Deng Z, Shan H, et al. Analysis of FNB vs FNA in diagnosis of pancreatic and abdominal masses: A prospective, multicenter, randomized controlled trial. Clin Gastroenterol Hepatol. 2018;16:1314–21. doi: 10.1016/j.cgh.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 34.James TW, Baron TH. A comprehensive review of endoscopic ultrasound core biopsy needles. Expert Rev Med Devices. 2018;15:127–35. doi: 10.1080/17434440.2018.1425137. [DOI] [PubMed] [Google Scholar]
- 35.Moller K, Papanikolaou IS, Toermer T, Delicha EM, Sarbia M, Schenck U, et al. EUS-guided FNA of solid pancreatic masses: High yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60–9. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73:283–90. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 37.Gress F, Michael H, Gelrud D, Patel P, Gottlieb K, Singh F, et al. EUS-guided fine-needle aspiration of the pancreas: Evaluation of pancreatitis as a complication. Gastrointest Endosc. 2002;56:864–7. doi: 10.1067/mge.2002.129602. [DOI] [PubMed] [Google Scholar]
- 38.Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Acute extra-luminal hemorrhage associated with EUS-guided fine needle aspiration: Frequency and clinical significance. Gastrointest Endosc. 2001;53:221–5. doi: 10.1067/mge.2001.111391. [DOI] [PubMed] [Google Scholar]
- 39.Janssen J, Konig K, Knop-Hammad V, Johanns W, Greiner L. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc. 2004;59:339–44. doi: 10.1016/s0016-5107(03)02707-x. [DOI] [PubMed] [Google Scholar]
- 40.Barawi M, Gottlieb K, Cunha B, Portis M, Gress F. A prospective evaluation of the incidence of bacteremia associated with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:189–92. doi: 10.1067/mge.2001.108966. [DOI] [PubMed] [Google Scholar]
- 41.Fujii LL, Levy MJ. Basic techniques in endoscopic ultrasound-guided fine needle aspiration for solid lesions: Adverse events and avoiding them. Endosc Ultrasound. 2014;3:35–45. doi: 10.4103/2303-9027.123006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–13. doi: 10.1055/s-0033-1344714. [DOI] [PubMed] [Google Scholar]
- 43.Krishna SG, Modi RM, Kamboj AK, Swanson BJ, Hart PA, Dillhoff ME, et al. In vivo and ex vivo confocal endomicroscopy of pancreatic cystic lesions: A prospective study. World J Gastroenterol. 2017;23:3338–48. doi: 10.3748/wjg.v23.i18.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakai Y, Isayama H, Chang KJ, Yamamoto N, Mizuno S, Mohri D, et al. A pilot study of EUS-guided through-the-needle forceps biopsy (with video) Gastrointest Endosc. 2016;84:158–62. doi: 10.1016/j.gie.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 45.Pham KD, Engjom T, Gjelberg Kollesete H, Helgeland L. Diagnosis of a mucinous pancreatic cyst and resection of an intracystic nodule using a novel through-the-needle microforceps. Endoscopy. 2016;48(Suppl 1):E125–6. doi: 10.1055/s-0042-105437. [DOI] [PubMed] [Google Scholar]
- 46.Khurana KK, Graber B, Wang D, Roy A. Telecytopathology for on-site adequacy evaluation decreases the nondiagnostic rate in endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesions. Telemed J E Health. 2014;20:822–7. doi: 10.1089/tmj.2013.0316. [DOI] [PubMed] [Google Scholar]


