Abstract
Background:
Carcinoma cervix of uterus (CaCx) is the most common malignancy affecting women worldwide. It is an established fact that infection of specific types of human papilloma virus (HPV) is essential for the development of cervical cancer. The present study reports the high-risk viruses (HPV 16 and 18) type distribution in rural central India, which has unique climatic condition. To our knowledge, no molecular study on HPV prevalence has been done in this region of rural population, this intended us do such study.
Materials and Methods:
Sexually active women reporting to the Gynecology were divided in three groups, first being asymptomatic women with normal cervix (52 cases), second group with benign cervical lesion (52 cases), and third group of women with frank cervical malignancy (40 cases). Cervical swabs were collected for HPV DNA sampling. The incidence of HPV positivity was recorded in each group.
Results:
Fifty-two women with asymptomatic normal cervix showed 44.23% positivity for HPV 16 and 5.76% positivity for HPV 18. Fifty-two women with benign cervical lesion showed 38.46% positivity for HPV 16 and 3.84% positivity for HPV 18. Forty women with frank cervical malignancy were with prevalence of 62.5% for HPV 16 and 22.5% for HPV 18.
Conclusion:
The results of the study are definitely helpful to know the prevalence of HPV in this region of rural population and will enrich the national epidemiological data related to HPV infection in cervical cancer.
Keywords: Carcinoma cervix, human papilloma virus, molecular genetic study
INTRODUCTION
In developing countries the cancer is a growing health problem. In past 30 years, the global cancer burden has doubled, and by 2020 the cancer burden of 2000 is expected to double again. Worldwide, carcinoma cervix of uterus (CaCx) is the second most common malignancy affecting women aged between 15 to 44 years, in terms of both incidence and mortality rates. In India, >1,30,000 women develop cervical cancer every year and about 70,000 deaths occur due to cervical cancer. The most of the cervical cancer cases can be prevented by timely screening.[1] The countries that have high coverage of cervical cancer screening have reduced invasive cervical cancer incidence by about 70–90%. Almost 40–90% of women in the developed countries are screened for cervical cancer.[2] On the contrary < 5% of women in developing countries undergo cervical screening due to lack of effective, organized, and opportunistic cervical cytology screening programs anywhere in the country.[3] For this reason the incidence of invasive cervical cancer remains high, especially in rural India.[4] For over a century, it was a belief that cancer of cervix is in association with 'sexual behavior' of an individual which indicate the involvement of a sexually transmissible infectious agent. Initially, herpes simplex virus type 2 (HSV-2) was considered as the possible candidate, but the absence of HSV-2 DNA in most cervical tumors together with the results of several epidemiological studies clearly demonstrated that HSV-2 was not directly involved in cervical cancer development. It was only in the early 1980s that the prevailing controversy over the involvement of human papilloma viruses (HPVs) was settled after cloning of several HPV genomes, including the most prevalent HPV 16 from cervical carcinomas and genital warts. In 1974, Professor Harald zur Hausen published his first report at attempting to find HPV DNA in cervical cancer and genital wart biopsies by hybridizing tumor DNA with cRNA obtained from purified plantar wart HPV DNA.[5] Thus, it was established that infection of specific types of HPV was essential for the development of cervical cancer.
HPVs are small double-stranded DNA tumor viruses with a genome size of approximately 8000 base pairs (bp) belonging to family papillomaviridae.[6] The genome is divided into three regions: the long control region (LCR) or upper regulatory region (URR), region of early proteins (E1–E7), and the region of late proteins (L1 and L2). The early regions constitute 45–50% of the viral genome lying downstream of URR and consist of open reading frames i.e., E1, E2, E4, E5, E6, and E7. They encode regulatory proteins [Figure 1].
Figure 1.
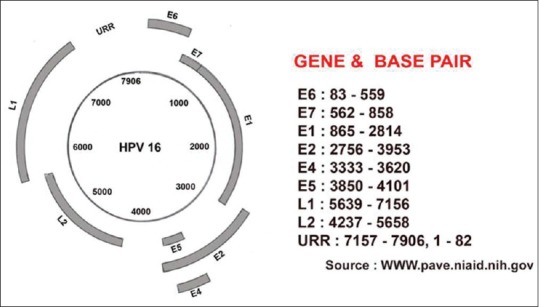
Structure of HPV 16 genome
Different regions of HPV genome: E1: maintains viral genome as episome, E2 and E4: regulatory role in viral genome amplification, E5: complex with epidermal-growth factor, platelet derived-growth factor, and colony-stimulating factor-1 receptors promoting growth, E6: binds to and degrades p53, E7: binds to and degrades Rb protein, L1: it is a major capsid protein and is the basis of a successful group of prophylactic HPV vaccines designed to elicit virus-neutralizing antibodies that protect against initial HPV infection, L2: less abundant minor capsid protein, facilitates the packaging of the viral genome into nascent virions and functions in the infectious entry of the virus into new host cells, LCR/URR: has no protein-coding function, but bears the origin of replication. Approximately 140 genotypes of HPVs have been described till date. Out of these 15 types are high-risk viruses (HR-HPVs) types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82). Others are low-risk (LR-HPVs) types (HPV 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81, and CP6108).[7,8] The causative role of high-risk types of HPV in the development of cervical intraepithelial neoplasia (CIN) 2, 3, and cervical cancer is well established. But out of high-risk HPV, the types 16 and 18 are considered to be the most prevalent types which cause approximately 70% of invasive cervical cancers. In India, the infection of HPV type 16 is found to be the exclusively high and followed by HPV type 18 in cervical cancer cases.[7] Vaccine of HPV 16 and 18 have been shown to prevent persistent HPV infection in clinical trials.[9] The occurrence and frequency of HPV type in cervical lesion depends on the geographical and racial variation.[10] So the efficacy of vaccine with few HPV types may be doubtful in specific area. The knowledge about the distribution of HPV types in cervical cancers and HPV types circulating in the communities in different regions of India would be useful in planning the optimum strategy for vaccination in India as well as for application in the National Cancer Prevention Strategies. The information concerning genotype distribution in invasive cervical cancers is definitely important as it is the key to estimate the protection that will be offered against cervical cancers by currently available vaccines.[10]
The present study reports the high-risk viruses (HPV 16 and 18) type distribution in the rural population of central India, which has unique climatic condition. To our knowledge, no molecular study on HPV prevalence has been done in this region of rural population. So, we ventured into the unexplored and virgin geographical area to see the prevalence of HPV types.
MATERIALS AND METHODS
One hundred forty-four sexually active women reporting to the Gynecology out-patient department were the subjects. They were divided in three groups, first being asymptomatic women with normal cervix (52 cases), second group with benign cervical lesion (52 cases), and third group of women with frank cervical malignancy (40 cases) based on clinical and colposcopic findings. Benign cervical lesion refers to cervical erosion and cervical polyp. Cervical swabs were collected for Pap smear and HPV DNA sampling by the trained clinicians after obtaining the written consent. The incidence of HPV positivity was recorded in each group and comparison was done. The patients with history of infections like-HIV and TORCH were excluded. The study was approved by the Institutional Ethical Committee.
HPV sampling: Taking the Pap smear with the spatula and the scraped cell material was transferred to a 15 ml collection vial containing 5 ml of phosphate buffer saline (PBS) on ice and then was stored at 4°C till transportation to the laboratory. In the laboratory, HPV DNA sample was extracted from cervical scrapes using the standard proteinase-K digestion and phenol-chloroform extraction method. Extracted DNA was dissolved in 10 × TE buffer. These DNA sample was used for the polymerase chain reaction (PCR) detection of HPV strains. PCR reaction was set up using 10 × PCR assay buffer and 2.5 mM MgCl2, 10 pm of each forward and reverse primers, 1U Taq DNA polymerase, 2.5 mM each dNTPs, 200 ng template DNA, and added the water up to reaction solution volume to make up 50 μl reaction mixture. Amplification was performed in a 50 ul reaction mix in a DNA thermal cycler (Veriti, Applied Biosystem). After PCR reaction, amplified product was separated on 1% agarose gel electrophoresis containing ethidium bromide and TAE buffer. PCR product bands were compared with 100 bp DNA marker lane. When amplified DNA band was going to correspond with desired bp marker this confirm desired HPV strain.
RESULTS
Fifty-two (52) women with asymptomatic normal cervix showed 44.23% positivity for HPV 16 and 5.76% positivity for HPV 18. Fifty-two women with benign cervical lesion showed 38.46% positivity for HPV 16 and 3.84% positivity for HPV 18. Forty women with frank cervical malignancy were with prevalence of 62.5% for HPV 16 and 22.5% for HPV 18.
We found high percentage of HPV 16 in frank cervical malignancy (62.5%) than controls (44.23%) and benign cervical lesion (38.46%). Our results showed high percentage of HPV 18 in frank cervical malignancy (22.5%) than controls (5.76%) and benign cervical lesion (3.84%). We also noted high percentage of HPV 16 in frank cervical malignancy (62.5%) than HPV 18 (22.5%) [Figures 2, 3 and Tables 1–3].
Figure 2.
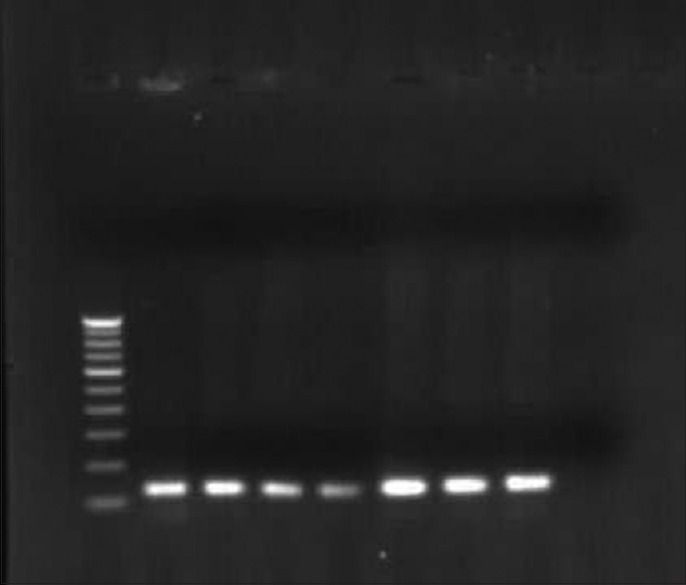
Agarose gel electrophoresis of HPV 16 E6 PCR product of 120 bp and in first lane 100 bp marker
Figure 3.
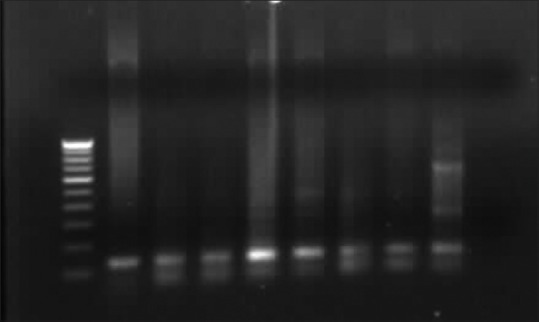
Agarose gel electrophoresis of HPV 18 E7 PCR product of 137 bp and in first lane 100 bp marker
Table 1.
Prevalence of human papilloma virus 16 and 18 in controls and cervical lesion

Table 3.
Comparison of Indian studies on human papilloma virus in cervical cancers and healthy controls
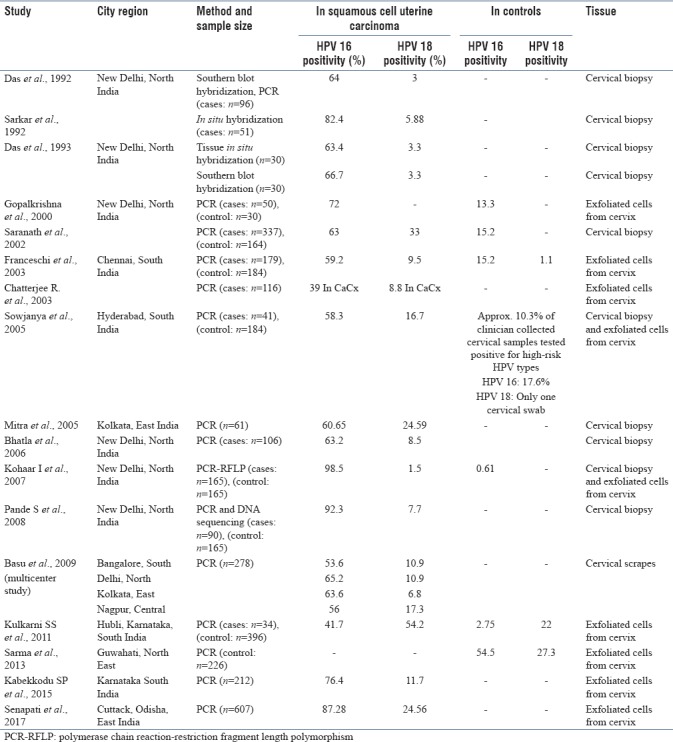
Table 2.
Details of primer sequences of β-globin, L1 consensus, human papilloma virus 16 and human papilloma virus 18 with their respective amplification size used in human papilloma virus detection study

Significant difference is observed between the proportions of HPV 16 positivity among the three groups. The F value (F = 29.58) is suggestive of statistically significant (P < 0.01) difference among the proportion of HPV 16 positivity in the groups.
DISCUSSION
According to the 2012 world cancer statistics, cervical cancer is the fourth most common cause of cancer deaths among women worldwide and accounted for 5,28,000 new cases every year and 2,66,000 deaths out of 8.2 million cancer deaths. It is the fourth most common cancer affecting women after breast, colorectal, and lung cancer. HPV variant data are important in developing HPV diagnostics, vaccines, and other therapeutic approaches to control virus-induced diseases and will definitely enrich the national epidemiological data related to HPV infection in cervical cancer. Availability of specific targeted HPV vaccine in developing countries like India will be a great achievement not only for to reduce the cancer burden but also for to reduce the cost burden for cervical cancer screening programs. We therefore evaluated the prevalence of major high-risk group HPV types in three different groups of women in rural setting.
Global scenario: In a study of HPV-prevalence rates of cervical cancer were compared between a high-risk area (Greenland) and a low-risk area (Denmark) and found 1.5 times higher HPV 16/18 prevalence rates in Denmark compared to Greenland.[11] Another interesting comparative study was done to determine the prevalence and type-specific distribution of HPVs from cervical specimens from the women undergoing hysterectomy for benign (non-neoplastic) diseases. The reports showed positivity for HPV DNA in 46% of the cases from Japan and 33% from Pakistan. But with the results of prevalence of high risk HPVs in cancer cases, no significant difference was observed between these countries.[12] In a case-control study, the prevalence of HPVs among controls in California and Spain was found to be 3.4% versus 4.3% by Munoz et al. But with PCR technique they observed a higher prevalence of 13.3% in Californian women than 4.6% in Spanish women. Lehtinen et al. conducted a case-control study of carcinoma in situ and invasive cervical carcinoma from a cohort of 18,814 Finnish women who were followed up to 23 years. After the initial screening they showed the only significant association of carcinoma of cervix was with the presence of antibodies to HPV type 16. The study revealed that 76% of the CIN lesions could be attributed to HPV infection, mainly with oncogenic type of HPV.[13] Chimeddorj et al. (2008) analyzed samples from 374 randomly selected women who attended the National Cancer Center of Mongolia. HPV genotyping detected 101 HPV 16 positive samples. Among these samples 92 were available for subsequent variant analysis, including 66 invasive cervical cancer samples, 25 CIN samples, and 1 cytologically normal sample. A total of 14 different variants were identified.[14] In 2009, Chopjitt et al. did study in Northeast Thailand. HPV infections were found in 33.8% of normal cervical cells, 97.3% of cervical intraepithelial neoplasia II and III, and 100% of squamous cell carcinomas. They found that the prevalence of HPV 16 increased significantly with histological grade.[15] Tsiodras et al. in 2010 found that out of 1270 women evaluated 241 (18.5%) had abnormal cytology. Cytology diagnosed high-grade squamous intraepithelial lesion (HSIL) or invasive carcinoma in 21 (1.7%) cases, whereas 26 (2%) women had CIN2+ or greater histology. PCR detected HPV in 397/1270 samples. All CIN 3+ lesions harbored high-risk oncogenic HPV type infections.[16] The PCR-based method detected six HPV 16 positive, three HPV 18 positive, and two HPV 33 positive out of 70 samples collected in Tehran.[17] Nunes et al. in 2014 found the HPV prevalence as 80.4%, with 17 virus types detected, including HPV 16, 18, 58, 6, and 11.[18] Globally, distribution pattern of HPV appears to be similar in different countries: 60–65% positivity for HPV 16; 4–20% for HPV 18; and a low prevalence of other HPV types.
Indian scenario: Cervical cancer is the most common cancer in women in India after breast cancer in women living in rural area. In India, approximately 1,32,000 new cases are diagnosed and approximately 74,000 deaths are noted every year, which contribute to the one-third of the global cervical cancer deaths.[19,20] After a 12-year follow-up study, a significant association of HPV 16/18 as a risk factor for developing cervical cancer has been demonstrated. Detection of HPV in cervical cancer specimens from different parts of India showed that HPV 16 is the most predominant type.[21,22,23,24,25] In 2009, Sankaranarayanan et al. did HPV screening in India. Out of the 34,126 women in the HPV-testing group, 27,192 (79.7%) were screened and 2812 (10.3%) had positive results; out of the 32,058 women in the cytological-testing group, 25,549 (79.7%) were screened and 1787 (7.0%) had positive results.[3] Cervical cancer was diagnosed in 127 subjects (of whom 39 had Stage II or higher), as compared with 118 subjects (of whom 82 had advanced disease) in the control group HPV genotyping was done in 41 cervical cancer specimens obtained from women attending a regional cancer hospital in Hyderabad. HPV DNA testing was also done in 185 cervicovaginal samples collected from women enrolled in the cervical cancer screening pilot study conducted in the rural community of Medchal Mandal (20 km away from Hyderabad). High-risk HPV types were found in 87.8% of the squamous cell carcinomas using a PCR-based line blot assay. Among the HPV positive cancers, the overall type distribution of the major high-risk HPV types was as follows: HPV 16 (66.7%), HPV 18 (19.4%), HPV 33 (5.6%), HPV 35 (5.6%), HPV 45 (5.6%), HPV 52 (2.8%), HPV 58 (2.8%), HPV 59 (2.8%), and HPV 73 (2.8%).[9]
Till now, there is no report of HPV prevalence from central region of India except a multi-center study done by Basu et al. in 2009.[10] In our study the prevalence of HPV 16 in women with frank cervical malignancy is 62.5% which is quite similar to study done by Das et al. 1992 (64%), Das et al. 1993 (63.4%), Saranath et al. 2002 (63%), Bhatla et al. 2006 (63.2%), and Basu et al. 2009 (63.6%).[26,27] Prevalence of HPV 18 in women with frank cervical malignancy is 22.5% which is quite similar to the results of study done by Mitra et al. 2005 and Senapati et al. 2017.[28,29] There are very few HPV prevalence studies done in controls population. Our findings of HPV 16 prevalence (44.23%) in controls are quite similar with study done by Sarma et al. 2013 (54.5%)[30] [Table 3]. We noticed high percentage of HPV 16 in frank cervical malignancy (62.5%) than controls (44.23%) and benign cervical lesion (38.46%) indicating that it has a role in carcinogenesis.
Analyzing the results of the different prevalence studies, it is definitely clear that there exist regional variations in prevalence of high-risk types of HPV 16 and 18 infections in India. The persistent high-risk HPV infection has been considered to be the principal etiological factor in causation of cervical malignancy in spite of an established fact that several other factors are involved including early age at marriage, multiple sexual partners, early coitarche, and parity. The results of the study are definitely helpful to know the prevalence of HPV in central region of India. Our study will enrich the national epidemiological data related to HPV infection in cervical cancer. Results can be utilized for prevention of HPV infection-derived cervical cancer through orientation programs for women which include sex education and information regarding screening tests. Such prevalence studies are really necessary to increase assess of health care facilities by the rural population of India.[31]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mishra GA, Pimple SA, Shastri SS. An overview of prevention and early detection of cervical cancers. Indian J Med Paediatr Oncol. 2011;32:125–32. doi: 10.4103/0971-5851.92808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirovich BE, Welch HG. The frequency of Pap smear screening in the United States. J Gen Intern Med. 2004;19:243–50. doi: 10.1111/j.1525-1497.2004.21107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–94. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 4.Sur D, Chakravorty R. Present status of cervical neoplasia control and human papilloma virus epidemiology in India: The wind is blowing; unfolding the truth. J Cancer Sci Ther. 2015;7:363–6. [Google Scholar]
- 5.zur Hausen H, Schulte-Holthausen H, Wolf H, Dörries K, Egger H. Attempts to detect virus. specific DNA in human tumors. II. Nucleic acid hybridizations with complementary RNA of human herpes group viruses. Int J Cancer. 1974;13:657–64. doi: 10.1002/ijc.2910130510. [DOI] [PubMed] [Google Scholar]
- 6.Duensing S, Münger K. Human papillomaviruses and centrosome duplication errors: Modeling the origins of genomic instability. Oncogene. 2002;21:6241–8. doi: 10.1038/sj.onc.1205709. [DOI] [PubMed] [Google Scholar]
- 7.Das BC, Hussain S, Nasare V, Bharadwaj M. Prospects and prejudices of human papillomavirus vaccines in India. Vaccine. 2008;26:2669–79. doi: 10.1016/j.vaccine.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 9.Sowjanya AP, Jain M, Poli UR, Padma S, Das M, Shah KV, et al. Prevalence and distribution of high-risk human papilloma virus (HPV) types in invasive squamous cell carcinoma of the cervix and in normal women in Andhra Pradesh, India. BMC Infect Dis. 2005;5:116. doi: 10.1186/1471-2334-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu P, Roychowdhury S, Bafna UD, Chaudhury S, Kothari S, Sekhon R, et al. Human papillomavirus genotype distribution in cervical cancer in India: Results from a multi-center study. Asian Pac J Cancer Prev. 2009;10:27–34. [PubMed] [Google Scholar]
- 11.Kjaer SK, de Villiers EM, Haugaard BJ, Christensen RB, Teisen C, Møller KA, et al. Human papillomavirus, herpes simplex virus and cervical cancer incidence in Greenland and Denmark. A population-based cross-sectional study. Int J Cancer. 1988;41:518–24. doi: 10.1002/ijc.2910410408. [DOI] [PubMed] [Google Scholar]
- 12.Anwar K, Inuzuka M, Shiraishi T, Nakakuki K. Detection of HPV DNA in neoplastic and non-neoplastic cervical specimens from Pakistan and Japan by non-isotopic in situ hybridization. Int J Cancer. 1991;47:675–80. doi: 10.1002/ijc.2910470508. [DOI] [PubMed] [Google Scholar]
- 13.Lehtinen M, Dillner J, Knekt P, Luostarinen T, Aromaa A, Kirnbauer R, et al. Serologically diagnosed infection with human papillomavirus type 16 and risk for subsequent development of cervical carcinoma: Nested case-control study. BMJ. 1996;312:537–9. doi: 10.1136/bmj.312.7030.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chimeddorj B, Pak CY, Damdin A, Okamoto N, Miyagi Y. Distribution of HPV-16 intratypic variants among women with cervical intraepithelial neoplasia and invasive cervical cancer in Mongolia. Asian Pac J Cancer Prev. 2008;9:563–8. [PubMed] [Google Scholar]
- 15.Chopjitt P, Ekalaksananan T, Pientong C, Kongyingyoes B, Kleebkaow P, Charoensri N. Prevalence of human papillomavirus type 16 and its variants in abnormal squamous cervical cells in Northeast Thailand. Int J Infect Dis. 2009;13:212–9. doi: 10.1016/j.ijid.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Tsiodras S, Georgoulakis J, Chranioti A, Voulgaris Z, Psyrri A, Tsivilika A, et al. Hybrid capture vs. PCR screening of cervical human papilloma virus infections. Cytological and histological associations in 1270 women. BMC Cancer. 2010;10:53. doi: 10.1186/1471-2407-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raji N, Sadeghizadeh M, Tafreshi KN, Jahanzad E. Detection of human papillomavirus 18 in cervical cancer samples using PCR-ELISA (DIAPOPS) Iran J Microbiol. 2011;3:177–82. [PMC free article] [PubMed] [Google Scholar]
- 18.Nunes JD, Vidal FC, Ferraro CT, Chein MB, Brito LM, Monteiro SC, et al. Molecular detection of human papillomavirus in Brazilian women with cervical intraepithelial neoplasia in a Northeast Brazilian city. Genet Mol Res. 2014;13:9077–85. doi: 10.4238/2014.October.31.23. [DOI] [PubMed] [Google Scholar]
- 19.Kaarthigeyan K. Cervical cancer in India and HPV vaccination. Indian J Med Paediatr Oncol. 2012;33:7–12. doi: 10.4103/0971-5851.96961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Das BC, Sharma JK, Gopalkrishna V, Das DK, Singh V, Gissmann L, et al. A high frequency of human papillomavirus DNA sequences in cervical carcinomas of Indian women as revealed by southern blot hybridization and polymerase chain reaction. J Med Virol. 1992;36:239–45. doi: 10.1002/jmv.1890360402. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar S, Verma K, Kaur H, Seth P. Detection of human papilloma virus types 16 and 18 DNA in cervical lesions of Indian women using in situ hybridization. Indian J Med Res. 1992;96:356–60. [PubMed] [Google Scholar]
- 23.Thankamani V, Kumari TV, Vasudevan DM. Detection of herpes simplex virus type-2 DNA and human papilloma virus DNA sequences in cervical carcinoma tissue by molecular hybridization. J Exp Pathol. 1992;6:55–64. [PubMed] [Google Scholar]
- 24.Pillai MR, Lakshmi S, Sreekala S, Devi TG, Jayaprakash PG, Rajalakshmi TN, et al. High-risk human papillomavirus infection and E6 protein expression in lesions of the uterine cervix. Pathobiology. 1998;66:240–6. doi: 10.1159/000028029. [DOI] [PubMed] [Google Scholar]
- 25.Munirajan AK, Kannan K, Bhuvarahamurthy V, Ishida I, Fujinaga K, Tsuchida N, et al. The status of human papillomavirus and tumor suppressor genes p53 and p16 in carcinomas of uterine cervix from India. Gynecol Oncol. 1998;69:205–9. doi: 10.1006/gyno.1998.4991. [DOI] [PubMed] [Google Scholar]
- 26.Saranath D, Khan Z, Tandle AT, Dedhia P, Sharma B, Contractor R, et al. HPV16/18 prevalence in cervical lesions/cancers and p53 genotypes in cervical cancer patients from India. Gynecol Oncol. 2002;86:157–62. doi: 10.1006/gyno.2002.6735. [DOI] [PubMed] [Google Scholar]
- 27.Bhatla N, Dar L, Patro AR, Kriplani A, Gulati A, Verma K, et al. Human papillomavirus type distribution in cervical cancer in Delhi, India. Int J Gynecol Pathol. 2006;25:398–402. doi: 10.1097/01.pgp.0000209574.62081.e4. [DOI] [PubMed] [Google Scholar]
- 28.Mitra S, Misra C, Singh RK, Panda CK, Roychoudhury S. Association of specific genotype and haplotype of p53 gene with cervical cancer in India. J Clin Pathol. 2005;58:26–31. doi: 10.1136/jcp.2004.019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senapati R, Nayak B, Kar SK, Dwibedi B. HPV genotypes distribution in Indian women with and without cervical carcinoma: Implication for HPV vaccination program in Odisha, Eastern India. BMC Infect Dis. 2017;17:30. doi: 10.1186/s12879-016-2136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarma U, Mahanta J, Borkakoty BJ, Talukdar KL, Gogoi R, Yadav K. Demographic characteristics of HPV infection in women – A hospital based study from Guwahati, India. Natl J Med Res. 2013;3:1–4. [Google Scholar]
- 31.Jayant K, Nene BM, Badwe RA, Panse NS, Thorat RV, Khan FY. Rural cancer registry at Barshi, Maharashtra and its impact on cancer control. Natl Med J India. 2010;23:274–7. [PubMed] [Google Scholar]


