Abstract
Obsessive–compulsive disorder (OCD) is a complex neuropsychiatric disorder with a chronic course, contributing to significant socio-occupational dysfunction. Forty percent of patients remain treatment refractive despite mainstream treatment options such as serotonin-reuptake inhibitors and cognitive behavior therapy. Noninvasive brain stimulation approaches such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have piqued interest as add-on treatment options in OCD. This review focuses on summarizing the TMS and tDCS studies in OCD with respect to their study design and stimulation parameters and key findings. We also briefly discuss the limitations and future directions noninvasive brain stimulation in OCD.
Keywords: Neuromodulation, noninvasive brain stimulation, obsessive compulsive disorder, repetitive transcranial magnetic stimulation, transcranial direct current stimulation
INTRODUCTION
Obsessive–compulsive disorder (OCD) is characterized by repetitive, irrational, and intrusive thoughts and compulsions, resulting in significant socio-occupational dysfunction. While there are several treatment options available, the mainstay treatment has been psychopharmacological approach in the form of serotonin-reuptake inhibitors or psychotherapy in the form of cognitive behavior therapy or both.[1] Despite adequate treatment, nearly 40% of patients do not respond to either treatment, warranting need for developing newer treatment strategies.[2] Furthermore, invasive treatment strategies such as deep brain stimulation or surgical procedures have shown partial success.
In this scenario, noninvasive brain stimulation approaches such as transcranial magnetic stimulation (TMS) and transcranial electric current stimulation (tECS) hold promise in alleviating symptoms in OCD and other neuropsychiatric disorders.[3,4] These noninvasive techniques involve application of either magnetic field or electric current transcranially over the scalp to modulate specific brain regions to alleviate the symptoms. The noninvasive neuromodulation in OCD focuses on specific brain regions considered to be functioning abnormally. The rationale for choosing the target region is based on their involvement in the neurobiological basis of OCD. Converging evidence indicates the involvement of cortico-striato-thalamo-cortical (CSTC) circuits in the pathophysiology of OCD.[5] Neuroimaging studies have shown the involvement of prefrontal-cortical regions in the neurobiological basis of OCD. Indeed, structural and functional changes corresponding to the regions subserving the CSTC circuits explain the neurobiological alterations seen in OCD.[6] In this review, we describe and discuss various studies that have examined the effects of repetitive TMS (rTMS) and transcranial direct current stimulation (tDCS) in alleviating the symptoms in OCD patients.
REPETITIVE TRANSCRANIAL MAGNETIC STIMULATION IN OBSESSIVE–COMPULSIVE DISORDER
rTMS is a noninvasive brain stimulation technique used in various neuropsychiatric conditions. rTMS uses low-intensity magnetic field to stimulate specific brain areas by inducing neurophysiologic changes. Low-frequency stimulation, i.e., <5 Hz, leads to decreased neuronal activity while high-frequency stimulation, i.e., >10 Hz, increases neuronal activity.[7] A recent modification to this technique in the form of deep TMS seems to allow stimulation of deeper and larger brain structures as compared to conventional rTMS. In this section, we review the evidence of rTMS utilization in OCD around specific areas of stimulation.
SUPPLEMENTARY MOTOR AREA
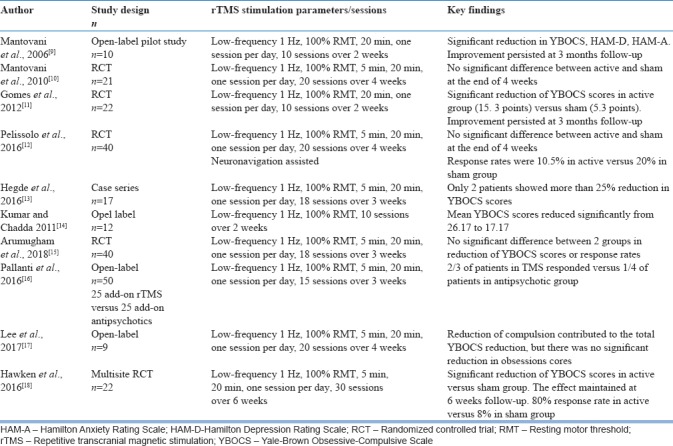
There is evidence that suggests reduced cortico-subcortical inhibition and cortical hyperexcitability result in the repetitive behavior associated with OCD.[8] An open-label study by Mantovani et al., 2006[9] had shown initially that low-frequency rTMS over supplementary motor area (SMA) had led to significant improvement of OC symptoms in 10 patients with comorbid Tourette syndrome. This improvement in clinical symptoms had correlated with improved resting-state cortical motor threshold and also sustained up to 3 months follow-up. Later, a double-blind, randomized controlled trial (RCT) from the same study group in 18 patients with resistant OCD showed greater reduction of symptoms in active stimulation compared to sham stimulation.[10] However, this was not statistically significant (P = 0.154). In another RCT with 22 patients with resistant OCD, low-frequency active rTMS treatment significantly reduced the symptoms score and this improvement remained stable at 12 weeks of follow-up compared to sham rTMS.[11] However, an RCT from France, in 40 patients with resistant OCD, failed to demonstrate significant difference between active and sham low-frequency stimulation of SMA.[12] An open-label case series observation, from India, in 17 patients with resistant OCD, had shown that low-frequency rTMS is less likely to produce improvement in severely ill patients.[13] Another open-label study from India had shown significant improvement in 12 patients with treatment-refractory OCD over 15 sessions of low-frequency rTMS.[14] However, an RCT from our center involving 36 patients with refractory OCD failed to demonstrate any significant difference between active and sham low-frequency rTMS over bilateral pre-SMA.[15]
A pilot open-label trial had shown significant reduction of OCD in two-third of patients treated with rTMS for 3 weeks who had not previously responded to antidepressant trial.[16] In a recent open-label study from Korea, 4 weeks of low-frequency TMS over SMA did not produce any significant change in OCD symptoms.[17] A 6-week, multisite RCT had shown clinically significant reduction in symptoms in patients with refractory OCD.[18] The symptom reduction achieved at the end of 6 weeks remained stable for further 6 weeks. The studies conducted using rTMS over pre-SMA/SMA is shown in Table 1.
Table 1.
Studies using repetitive transcranial magnetic stimulation over presupplementary motor area/supplementary motor area
DORSOLATERAL PREFRONTAL CORTEX
Dorsolateral prefrontal cortex (DLPFC) exerts control over multiple subcortical structures that are clinically relevant for attention, concentration, and executive functions. Dysfunction in DLPFC and related circuits had been implicated in various symptom dimensions of OCD.
Right dorsolateral prefrontal cortex
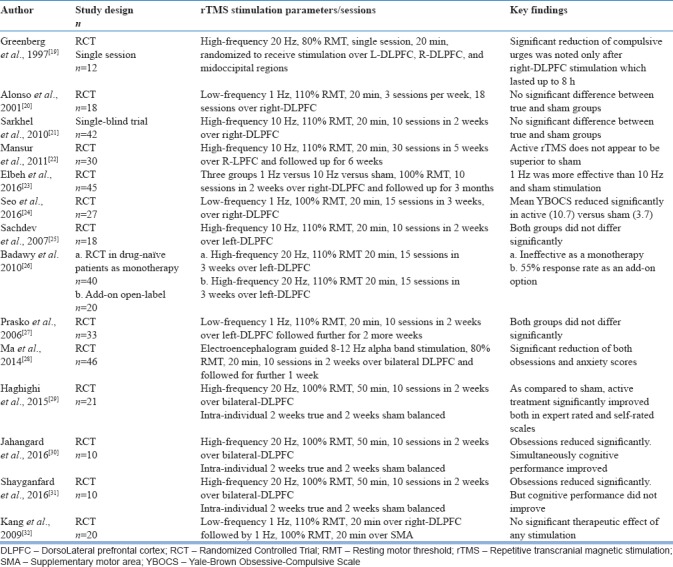
High-frequency 20 Hz single-session stimulation in 12 patients, with each patient randomized to receive over right/left DLPFC or mid-occipital regions, had shown that only after right DLPFC stimulation, compulsive urges reduced significantly and the effect lasted about 8 h after stimulation.[19] One of the earliest sham-controlled RCT reported that low-frequency 1 Hz stimulation over right DLPFC, three sessions per week for 6 weeks, was ineffective in producing any significant change in patients with OCD.[20] In an open-label study, adjunctive high-frequency rTMS to the right DLPFC did not show any significant change of obstetric cholestasis (OC) symptoms in 42 patients. However, both depression and anxiety scores reduced significantly.[21] In 30 treatment-resistant OCD patients, high-frequency 10 Hz stimulation over right DLPFC consisting of 30 sessions failed to show any significant difference between active and sham condition.[22] Forty-five patients randomized to three arms to receive low-frequency 1 Hz versus high-frequency 10 Hz versus sham stimulation over right DLPFC had concluded that only 1 Hz stimulation had significantly reduced symptoms.[23] A 3-week RCT, comparing active versus sham stimulations of low-frequency 1 Hz stimulation over right DLPFC reported superiority of active simulation over sham in relieving OCD symptoms.[24]
Left dorsolateral prefrontal cortex
A sham-controlled double-blind study suggested that 2 weeks of high-frequency rTMS over left DLPFC was ineffective in bringing any change in Yale-Brown OC scale (YBOCS) scores. However, when extended for 4 weeks, active stimulation reduced OC symptoms significantly but not after controlling for change in depression scores.[25] A novel study from Egypt had attempted application of high-frequency 20 Hz over left DLPFC with 15 sessions as monotherapy in 20 drug-free patients with OCD. However, there was no significant change between active and sham stimulation. In the same study, when high-frequency stimulation was applied as an add-on to antidepressant medication over left DLPFC, it led to significant reduction in symptoms.[26] Low-frequency 1 Hz rTMS applied over left DLPFC as an add-on to medications in 30 patients did not show any significant change between active and sham stimulation.[27]
Bilateral dorsolateral prefrontal cortex
Electroencephalography-guided, frequency-balanced, bilateral DLPFC stimulation over 10 sessions using a sham-controlled design in 46 OCD patients had shown significant reduction of obsessions and anxiety symptoms in active stimulation compared to sham stimulation.[28] A single-blind RCT with intra-individual cross-over design using bilateral high-frequency 20 Hz DLPFC stimulation reported significant reduction of symptoms with active stimulation. Both responders and partial responders were observed only in true condition in this study.[29] Another intra-individual sham-controlled study, involving high frequency over bilateral DLPFC had reported significant reduction in symptoms as well as cognitive performance after true stimulation condition.[30] This same study group also replicated the finding on efficacy of bilateral DLPFC high-frequency 20 Hz stimulation in improving symptoms but not cognitive function.[31] Further, sequential administration of low-frequency 1 Hz stimulation over DLPFC and SMA did not produce any significant change in symptoms.[32] The key rTMS studies with DLPFC as the target region are summarized in Table 2.
Table 2.
Studies using repetitive transcranial magnetic stimulation over dorsolateral prefrontal cortex
ORBITOFRONTAL CORTEX
Neuroimaging studies suggest hyper-excitability of orbitofrontal cortex (OFC) along with other cortical/subcortical structures in the pathophysiology of OCD. A retrospective study from India had suggested that there is a role of applying low-frequency 1 Hz to inhibit this hyper-excitable state of left OFC and ameliorating symptoms.[33] A double-blind RCT study that involved the administration of low-frequency rTMS over right OFC demonstrated significant reduction of symptoms in addition to reduction of metabolism in bilateral OFC in positron emission tomography.[34] Another study conducted on 23 medication-resistant OCD patients that applied low-frequency rTMS over left OFC for 15 days reported significant reduction in symptoms in true condition that lasted till 10 weeks but not 12 weeks.[35] The key rTMS studies with OFC as the target region are summarized in Table 3.
Table 3.
Studies using repetitive transcranial magnetic stimulation over orbitofrontal cortex

Summary
Only studies that administered inhibitory rTMS over OFC had reported consistently positive effects on symptom reduction. However, those studies are few in number and sample size, also proven negative when compared against other areas in a meta-analysis.[36] Studies involving SMA and DLPFC areas had reported mixed results and all the findings from those studies are limited by small sample sizes. Various researchers had attempted pooling data from the above studies to overcome this limitation by conducting meta-analyses. All five meta-analyses[36,37,38,39,40] had uniformly suggested that there is definite benefit of add-on true rTMS in patients with resistant OCD. Zhou et al., 2017[36] had suggested that low-frequency/high-frequency stimulation of SMA area and DLPFC area is beneficial with overall largest effect size of g = 0.71 (SMA g = 0.56; left DLPFC g = 0.47; right DLPFC g = 0.93). Most of the studies had been conducted on resistant OCD patients and for a shorter duration of time ranging from 1 to maximum 12 weeks. Large, multicenter trials in both resistant and nonresistant OCD patients are the need of the hour. Along with application of electroencephalograph/perfusion imaging in guiding specific target areas and stimulation parameters might advance our understanding about variations in efficacy and eventually to overcome the limitations.
TRANSCRANIAL ELECTRIC CURRENT STIMULATION
tECS is a technique involving application of small-intensity electric current on the scalp using specialized devices. tECS is mainly of two types, transcranial alternating current stimulation (tACS) and tDCS. tACS involves application of alternating current at various frequencies, whereas tDCS involves application of direct current which is constant over time. tDCS has been widely studied and used more often in the treatment of psychiatric disorders.
TRANSCRANIAL DIRECT CURRENT STIMULATION IN OBSESSIVE–COMPULSIVE DISORDER
tDCS is a re-emergent noninvasive brain stimulation technique that has shown promise in alleviating symptoms in various neuropsychiatric disorders. Most of the studies pertaining to application of tDCS in psychiatric disorders have been done in patients with depression and schizophrenia. Only a handful of studies have been done in OCD and related disorders. Volpato et al., 2013[41] probably described the first application of tDCS in an OCD patient. This was a single case study with active and sham tDCS cross-over. The stimulation montage involved cathodal stimulation of left DLPFC, with a primary aim of comparing the effect of tDCS and rTMS on resting-state brain activity. The study reported no change observed in OC symptoms, whereas improvement in depression was noted after true tDCS. Following this, there have been 11 studies that are published looking at the beneficial effects of tDCS in OCD and one study employing tACS. The current review focuses on studies involving transcranial electric current stimulation in OCD with a detailed discussion on tDCS.
The primary focus of all the studies has been symptom improvement as measured by changes in YBOCS scores and improvement in affective symptoms such as anxiety and depression. Only one study has looked at cognitive improvement following tDCS.[42] All the studies published so far have reported improvement in OC symptoms to a varying extent. However, all these studies are either single case study or case series or open-label study designs. There has not been a single study employing randomized sham-controlled double-blind design. Only one study by D’Urso et al., 2016[42] employed a randomized, controlled double-blind partial cross-over design but without a sham arm. A randomized double-blind sham-controlled study (Gowda et al., unpublished) from our center examined the efficacy of anodal tDCS to preSMA in OCD. Twenty five patients were randomly allocated into “verum” and “sham” arms and 2 mA of current was applied for 5 days (10 sessions). The response rate was significantly greater in the verum tDCS (4 out of 12) compared to sham tDCS. Given the limitations in the study design and sample size, these results are to be interpreted cautiously. Moreover, positive reports of a new treatment approach could be due to publication bias. The details of these studies are summarized in Table 4.
Table 4.
Summary of transcranial direct current stimulation studies in obsessive-compulsive disorder
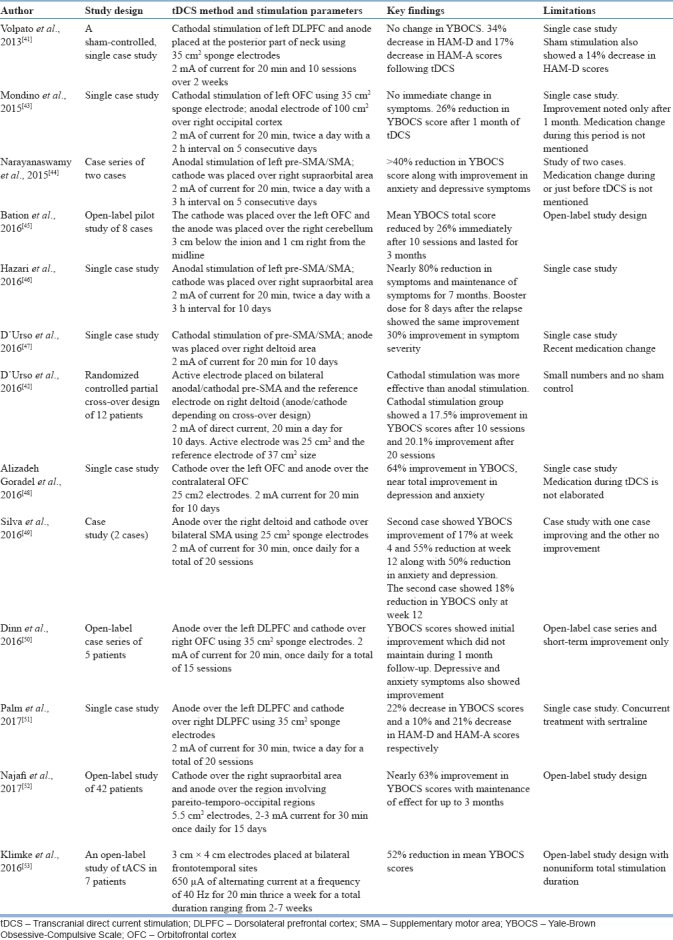
Despite the limitations of these studies, there are certain factors that make tDCS treatment unique and attractive. One of the most important factors among them is the magnitude of response. Studies described in Table 4 report varying levels of improvement ranging from 15% reduction in YBOCS scores to as high as 80%. The magnitude of response is important here mainly because most of the studies reported have applied tDCS in treatment-refractory patients. Next important factor is the persistence of treatment response after tDCS treatment. Longitudinal studies report a minimum of 1 month to a maximum of 7 months sustenance of effects. One of the most interesting observations of tDCS treatment in OCD is the rapidity with which tDCS brings about changes in OCD. All the studies reported have applied tDCS for 5 days (10 sessions) to a maximum of few weeks with a good clinical improvement. In addition, tDCS has demonstrated a favorable adverse effect profile and is one of the most common observations in most tDCS studies, irrespective of the disorder. The minimal side effects reported in any study are mild tingling and burning sensation which was well tolerated by all the participants. However, long-term safety is yet to be established. Despite all the unique features mentioned above, one has to remember that these features require systematic testing and reproducibility in larger samples.
TRANSCRANIAL DIRECT CURRENT STIMULATION PROTOCOLS IN OBSESSIVE–COMPULSIVE DISORDER
Almost all the studies in OCD have utilized conventional tECS device which delivers current using a pair of conductive rubber electrodes wrapped in sponge pouches soaked in saline. tECS involves the application of weak electric current in the range of 1–2 mA over specified brain regions in an attempt to modulate them.[54] The stimulus is delivered using electrodes of various sizes placed over designated areas of interest on the scalp, which is one of the crucial factors determining the outcomes of tDCS. Depending on the polarity of the stimulus, the brain region underneath the electrode gets stimulated or inhibited (anode stimulates and cathode inhibits the underlying area). However, the stimulation/inhibition is not site limited. In addition to site of stimulation, the other important parameters that can affect the outcome of tDCS are the current density, duration of stimulation, and size of the electrodes the details of which are beyond the scope of this review.
Site of stimulation can be considered as one of the most crucial parameters determining the outcome of tDCS. This is entirely dependent on the target disorder or target symptom. The neural substrates of psychiatric disorders are still inconclusive. Various studies aiming at elucidating the same have implicated many neural systems in the pathophysiology of OCD. However, given the complexity of the disorder as well as the brain and its circuitry, there is no one single neural substrate that can act as a switch for OCD symptom generation. Several multimodal neuroimaging studies have demonstrated aberrant activities (hypo- or hyper-activity) in brain regions comprising the orbitofrontal-striato-pallido-thalamic network, including orbitofrontal cortex (OFC), DLPFC, cingulate cortex, SMA, caudate and thalamus, and limbic system.[55,56] All the studies of noninvasive neuromodulation involving both tDCS and TMS have mainly focused on modulating the orbitofronto-striato-pallido-thalamic network, which has been critically implicated in the neurobiology of OCD.[4]
MECHANISM OF ACTION OF TRANSCRANIAL DIRECT CURRENT STIMULATION IN OBSESSIVE–COMPULSIVE DISORDER
Given the heterogeneity associated with the networks as well as symptoms in OCD, several rTMS studies have targeted mostly SMA, OFC, or medial prefrontal cortex for ameliorating OC symptoms, whereas few studies have targeted DLPFC with the hope of alleviating comorbid affective symptoms in OCD.[4] Taking cues from these rTMS studies and the evidence from the circuit dysfunction theories in OCD, tDCS studies have mainly focused on OFC and SMA/pre-SMA and two studies focusing on DLPFC modulation [Table 4]. We discuss the mechanism of action of tDCS along the lines of site of stimulation.
DORSOLATERAL PREFRONTAL CORTEX
Three studies in OCD have targeted DLPFC with varying electrode montages. In the first study by Volpato et al., 2013,[41] the cathode was placed on the left DLPFC and anode was placed extracephalically at the posterior part of the neck using 35 cm2 sponge electrodes. This study reported no change in OC symptoms, while there was a significant decrease in anxiety and depression scores following tDCS. This was based on the earlier evidence of decreased functional connectivity in the default-mode network, anterior cingulate gyrus (ACC), middle frontal gyrus, and putamen in OCD patients.[57] The authors concluded that the cathodal stimulation probably increased the functional activity in the rostral part of ACC and the midline OFC with simultaneous reduction in the asymmetry of activation between right and left DLPFC. Palm et al., 2017[51] targeted bilateral DLPFC with anode over the left DLPFC and cathode over the right DLPFC using 35 cm2 sponge electrodes. The authors based their strategy on the evidence from studies on the effect of tDCS on cognition in healthy controls and patients with depression. DLPFC being the major hub of cognitive, behavioral, control might also have a significant influence on other connected networks. The authors hypothesized that the DLPFC stimulation using tDCS might modulate the cognitive control over obsessions and compulsions, simultaneously targeting the comorbid affective symptoms. Yet, another study by Dinn et al., 2016[50] also placed anode over left DLPFC, while placing cathode over right OFC. However, cathodal OFC was the active electrode and left DLPFC anodal stimulation in this study was mainly targeting the executive dysfunction and affective symptoms than the OC symptoms.
ORBITOFRONTAL CORTEX
Functional neuroanatomical studies have assigned a critical role to the OFC in manifestation of OC symptoms. Studies have also proposed that specific areas of the OFC contribute to reward-guided learning and decision-making. Hyperactivity of these areas within the OFC has been proposed to attribute anticipatory affective value to future events, resulting in rumination about future aversive events.[58,59] Several neuroimaging studies have reported hyperactivity in OFC during symptom provocation tasks and also during resting state.[60,61] In fact, the hyperactivity was one of the predictors of treatment response since it showed a direct correlation with symptom severity.[62]
Overall five studies have used OFC as the target site of stimulation in tDCS for OCD. The first study using this montage was by Mondino et al., 2015,[43] wherein cathodal stimulation of left OFC was done using 35 cm2 sponge electrode and anodal electrode of 100 cm2 was placed over the occipital cortex. This was based on the earlier reports of improvement in OC symptoms following low-frequency rTMS applied over OFC with a subsequent observation of decrease in OFC activity.[34,35] In their study, Mondino et al., 2015 found a 26% reduction in OC symptoms after 1 month of tDCS, attributing the effects to decrease in OFC activity following cathodal stimulation. Along the same lines, Alizadeh Goradel et al., 2016[48] in their single case study reported 64% improvement in OC symptoms and near-total improvement in anxiety and depression following cathodal stimulation of OFC.
Bation et al., 2016[45] in an open-label pilot study design of eight patients placed cathode over left OFC and anode over right cerebellum. This was based on the evidence of hypoactivity of cerebellum and its role in OCD as reported by meta-analysis of several functional and structural neuroimaging studies.[63,64] Further, neuroimaging studies have also demonstrated aberrant interconnections between orbitofronto-striato-pallido-thalamic loop and cerebellum in OCD patients, which could be critical in the generation of OC symptoms or the cognitive correlates of OCD.[6,65,66] Bation et al., 2016 reported a 26% reduction in OC symptoms which persisted for nearly 3 months, attributing the improvement to tDCS-induced modulation of a large interconnected corticosubcortical network involved in the pathophysiology of OCD.
Dinn et al., 2016[50] in their study aimed at simultaneous modulation of OFC and DLPFC, targeting OC symptoms and neurocognitive functions, such as executive function and working memory in OCD, respectively. In this study, the cathode was placed on right OFC and anode was placed on left DLPFC. This 3-week open-label tDCS on five patients reported a significant reduction in OC symptoms and affective symptoms, with a considerable improvement in executive function. In addition, there was a correlation between improvement in OC symptoms and improvement in executive function, suggesting a critical role of DLPFC in alleviation of OC symptoms.
The largest open-label study of tDCS in OCD comprising 42 patients by Najafi et al., 2017[52] also used cathodal stimulation of OFC (right) with anode placed over the area covering left pareito-temporo-occipital regions. This study reported nearly 63% improvement in OC symptoms following 3 weeks of tDCS treatment, along with the persistence of improvement for 3 months.
PRE-SUPPLEMENTARY MOTOR AREA/SUPPLEMENTARY MOTOR AREA
Pre-SMA/SMA has been implicated in OCD pathogenesis as much as the OFC. As discussed earlier in the TMS section, there is evidence that suggests reduced cortico-subcortical inhibition and cortical hyperexcitability resulting in the repetitive behavior associated with OCD. However, there is contradicting evidence of hypoactivity at pre-SMA, contributing to lack of inhibition of striatal function resulting in hyperactivity of striatum, giving rise to OC symptoms.[67] Based on this evidence, one of the first studies to target pre-SMA/SMA was a case report by[44] The authors based their hypothesis on the theory of deficient response inhibition in OCD, the function attributed to pre-SMA/SMA.[68,69] In view of this theory, the authors placed anode over left pre-SMA/SMA and cathode over the right supraorbital area. The study results reported >40% reduction in YBOCS score along with improvement in anxiety and depressive symptoms. The authors hypothesized that the clinical improvement could be due to increase in the left SMA activity after anodal tDCS improving the response inhibition. Subsequently, the same group reported a significant improvement (80%) in episodic OCD following anodal tDCS to pre-SMA. This study also reported long-term sustenance of improvement and significant benefits following booster tDCS, 7 months after relapse.[46]
Subsequent to these, there were two studies targeting pre-SMA/SMA in OCD. Both studies from the same group applied cathodal tDCS to pre-SMA/SMA in a single case report study with a significant improvement following tDCS.[47] The second study involved placing either the anode/cathode electrode on bilateral pre-SMA depending on the cross-over design in 12 patients. The results of this study demonstrated superiority of cathodal stimulation of pre-SMA over anode. Another study by Silva et al.[49] followed the same montage of cathodal stimulation of bilateral pre-SMA with good improvement in clinical symptoms.
Summary
TMS studies that administered inhibitory rTMS over OFC had reported consistently positive effects on symptom reduction. However, those studies are few in number and sample size was less when compared against other areas in a meta-analysis.[36] Studies involving SMA and DLPFC areas had reported mixed results, and all the findings from those studies are limited by small sample sizes. Various researchers had attempted pooling data from the above studies to overcome this limitation by conducting meta-analyses. All five meta-analyses[36,37,38,39,40] had uniformly suggested that there is definite benefit of add-on true rTMS in patients with resistant OCD. Recent studies[36,39] had suggested that low-frequency/high-frequency stimulation of SMA area and DLPFC area are beneficial. Most of the studies had been conducted on resistant OCD patients and for a shorter duration of time ranging from 1 to maximum 12 weeks.
In case of tDCS, the studies are very few when compared to TMS and the studies published so far are very preliminary. Most of these studies are case reports/case series and open-label study designs with lesser evidential value compared to RCTs. In addition, similar to TMS, tDCS studies have also tried to explore their way into modulating the best neural correlates of OC symptoms. As a result, studies have mainly explored the beneficial effects of tDCS-induced modulation of either OFC or pre-SMA/SMA. All these studies have reported significant benefit suggesting OFC and pre-SMA/SMA as the ideal targets. DLPFC stimulation as explored by two studies mainly contributes to improvement in comorbid affective symptoms rather than OC symptoms. In addition to lack of systematic study design, these studies have employed varying stimulation parameters such as duration of stimulation, intensity of current, inter-stimulus interval, and electrode sizes, suggesting prematurity of these findings.
CONCLUSIONS
Pressing need of the hour is to establish further support with large trials in both resistant and nonresistant OCD patients, more so in case of tDCS where there is a serious lack of systematic studies. In addition to evaluating the persistence of improvement induced by both rTMS and tDCS, longitudinal studies are essential to establish long term safety of these techniques. In addition, systematic study designs coupled with multimodal investigative tools exploring the neuroimaging measures and electrophysiological measures might advance our understanding about variations in treatment response and mechanism of action of noninvasive brain stimulation techniques.
Financial support and sponsorship
Dr. Venkatasubramanian acknowledges the support of the SwarnaJayanti Fellowship by the Department of Science and Technology, Government of India (DST/SJF/LSA-02/2014-15). Dr. Narayanaswamy acknowledges the support of the Wellcome Trust / DBT India Alliance Intermediate Fellowship (IA/CPHI/16/1/502662).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This topic was presented by Prof. Ganesan Venkatasubramanian during the 2nd Symposium on OCD and related disorders held at NIMHANS on October 27–28, 2017. “The tDCS studies from NIMHANS mentioned in the manuscript are supported through Wellcome trust DBT India Alliance intermediate fellowship grant to Dr. Janardhanan C. Narayanaswamy (IA/CPHI/16/1/502662) and the Department of Science and Technology (DST) (Government of India) Research Grant (DST/SJF/LSA-02/2014-15) to Dr. Ganesan Venkatasubramanian. Dr Shivakumar is supported by the Indian Council of Medical Research (DHR/HRD/Young Scientist/Type-VI-(2)/2015).
REFERENCES
- 1.Jenike MA. Clinical practice. Obsessive-compulsive disorder. N Engl J Med. 2004;350:259–65. doi: 10.1056/NEJMcp031002. [DOI] [PubMed] [Google Scholar]
- 2.Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-compulsive disorder: Advances in diagnosis and treatment. JAMA. 2017;317:1358–67. doi: 10.1001/jama.2017.2200. [DOI] [PubMed] [Google Scholar]
- 3.Brunelin J, Mondino M, Bation R, Palm U, Saoud M, Poulet E, et al. Transcranial direct current stimulation for obsessive-compulsive disorder: A systematic review. Brain Sci. 2018;8:pii: E37. doi: 10.3390/brainsci8020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocchi L, Zalesky A, Nott Z, Whybird G, Fitzgerald PB, Breakspear M, et al. Transcranial magnetic stimulation in obsessive-compulsive disorder: A focus on network mechanisms and state dependence. Neuroimage Clin. 2018;19:661–74. doi: 10.1016/j.nicl.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. 2016;26:810–27. doi: 10.1016/j.euroneuro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Nakao T, Okada K, Kanba S. Neurobiological model of obsessive-compulsive disorder: Evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin Neurosci. 2014;68:587–605. doi: 10.1111/pcn.12195. [DOI] [PubMed] [Google Scholar]
- 7.Houdayer E, Degardin A, Cassim F, Bocquillon P, Derambure P, Devanne H, et al. The effects of low- and high-frequency repetitive TMS on the input/output properties of the human corticospinal pathway. Exp Brain Res. 2008;187:207–17. doi: 10.1007/s00221-008-1294-z. [DOI] [PubMed] [Google Scholar]
- 8.Rossi S, Bartalini S, Ulivelli M, Mantovani A, Di Muro A, Goracci A, et al. Hypofunctioning of sensory gating mechanisms in patients with obsessive-compulsive disorder. Biol Psychiatry. 2005;57:16–20. doi: 10.1016/j.biopsych.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette's syndrome (TS) Int J Neuropsychopharmacol. 2006;9:95–100. doi: 10.1017/S1461145705005729. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Simpson HB, Fallon BA, Rossi S, Lisanby SH. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2010;13:217–27. doi: 10.1017/S1461145709990435. [DOI] [PubMed] [Google Scholar]
- 11.Gomes PV, Brasil-Neto JP, Allam N, Rodrigues de Souza E. A randomized, double-blind trial of repetitive transcranial magnetic stimulation in obsessive-compulsive disorder with three-month follow-up. J Neuropsychiatry Clin Neurosci. 2012;24:437–43. doi: 10.1176/appi.neuropsych.11100242. [DOI] [PubMed] [Google Scholar]
- 12.Pelissolo A, Harika-Germaneau G, Rachid F, Gaudeau-Bosma C, Tanguy ML, BenAdhira R, et al. Repetitive transcranial magnetic stimulation to supplementary motor area in refractory obsessive-compulsive disorder treatment: A sham-controlled trial. Int J Neuropsychopharmacol. 2016;19:pii: pyw025. doi: 10.1093/ijnp/pyw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde A, Ravi M, V S S, Arumugham SS, Thirthalli J, Janardhan Reddy YC, et al. Repetitive transcranial magnetic stimulation over presupplementary motor area may not be helpful in treatment-refractory obsessive-compulsive disorder: A case series. J ECT. 2016;32:139–42. doi: 10.1097/YCT.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 14.Kumar N, Chadda RK. Augmentation effect of repetitive transcranial magnetic stimulation over the supplementary motor cortex in treatment refractory patients with obsessive compulsive disorder. Indian J Psychiatry. 2011;53:340–2. doi: 10.4103/0019-5545.91909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumugham SS, Vs S, Hn M, B V, Ravi M, Sharma E, et al. Augmentation effect of low-frequency repetitive transcranial magnetic stimulation over presupplementary motor area in obsessive-compulsive disorder: A randomized controlled trial. J ECT. 2018;34:253–7. doi: 10.1097/YCT.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 16.Pallanti S, Marras A, Salerno L, Makris N, Hollander E. Better than treated as usual: Transcranial magnetic stimulation augmentation in selective serotonin reuptake inhibitor-refractory obsessive-compulsive disorder, mini-review and pilot open-label trial. J Psychopharmacol. 2016;30:568–78. doi: 10.1177/0269881116628427. [DOI] [PubMed] [Google Scholar]
- 17.Lee YJ, Koo BH, Seo WS, Kim HG, Kim JY, Cheon EJ, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in treatment-resistant obsessive-compulsive disorder: An open-label pilot study. J Clin Neurosci. 2017;44:264–8. doi: 10.1016/j.jocn.2017.06.057. [DOI] [PubMed] [Google Scholar]
- 18.Hawken ER, Dilkov D, Kaludiev E, Simek S, Zhang F, Milev R, et al. Transcranial magnetic stimulation of the supplementary motor area in the treatment of obsessive-compulsive disorder: A multi-site study. Int J Mol Sci. 2016;17:420. doi: 10.3390/ijms17030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg BD, George MS, Martin JD, Benjamin J, Schlaepfer TE, Altemus M, et al. Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: A preliminary study. Am J Psychiatry. 1997;154:867–9. doi: 10.1176/ajp.154.6.867. [DOI] [PubMed] [Google Scholar]
- 20.Alonso P, Pujol J, Cardoner N, Benlloch L, Deus J, Menchón JM, et al. Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: A double-blind, placebo-controlled study. Am J Psychiatry. 2001;158:1143–5. doi: 10.1176/appi.ajp.158.7.1143. [DOI] [PubMed] [Google Scholar]
- 21.Sarkhel S, Sinha VK, Praharaj SK. Adjunctive high-frequency right prefrontal repetitive transcranial magnetic stimulation (rTMS) was not effective in obsessive-compulsive disorder but improved secondary depression. J Anxiety Disord. 2010;24:535–9. doi: 10.1016/j.janxdis.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Mansur CG, Myczkowki ML, de Barros Cabral S, Sartorelli Mdo C, Bellini BB, Dias AM, et al. Placebo effect after prefrontal magnetic stimulation in the treatment of resistant obsessive-compulsive disorder: A randomized controlled trial. Int J Neuropsychopharmacol. 2011;14:1389–97. doi: 10.1017/S1461145711000575. [DOI] [PubMed] [Google Scholar]
- 23.Elbeh KA, Elserogy YM, Khalifa HE, Ahmed MA, Hafez MH, Khedr EM, et al. Repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorders: Double blind randomized clinical trial. Psychiatry Res. 2016;238:264–9. doi: 10.1016/j.psychres.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Seo HJ, Jung YE, Lim HK, Um YH, Lee CU, Chae JH, et al. Adjunctive low-frequency repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex in patients with treatment-resistant obsessive-compulsive disorder: A randomized controlled trial. Clin Psychopharmacol Neurosci. 2016;14:153–60. doi: 10.9758/cpn.2016.14.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachdev PS, Loo CK, Mitchell PB, McFarquhar TF, Malhi GS. Repetitive transcranial magnetic stimulation for the treatment of obsessive compulsive disorder: A double-blind controlled investigation. Psychol Med. 2007;37:1645–9. doi: 10.1017/S0033291707001092. [DOI] [PubMed] [Google Scholar]
- 26.Badawy AA, El Sawy H, Abd El Hay M. Efficacy of repetitive transcranial magnetic stimulation in the management of obsessive compulsive disorder. Egypt J Neurol, Psychiatr Neurosurg. 2010;47:393–8. [Google Scholar]
- 27.Prasko J, Pasková B, Záleský R, Novák T, Kopecek M, Bares M, et al. The effect of repetitive transcranial magnetic stimulation (rTMS) on symptoms in obsessive compulsive disorder. A randomized, double blind, sham controlled study. Neuro Endocrinol Lett. 2006;27:327–32. [PubMed] [Google Scholar]
- 28.Ma X, Huang Y, Liao L, Jin Y. A randomized double-blinded sham-controlled trial of α electroencephalogram-guided transcranial magnetic stimulation for obsessive-compulsive disorder. Chin Med J (Engl) 2014;127:601–6. [PubMed] [Google Scholar]
- 29.Haghighi M, Shayganfard M, Jahangard L, Ahmadpanah M, Bajoghli H, Pirdehghan A, et al. Repetitive transcranial magnetic stimulation (rTMS) improves symptoms and reduces clinical illness in patients suffering from OCD – results from a single-blind, randomized clinical trial with sham cross-over condition. J Psychiatr Res. 2015;68:238–44. doi: 10.1016/j.jpsychires.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Jahangard L, Haghighi M, Shyayganfard M, Ahmadpanah M, Sadeghi Bahmani D, Bajoghli H, et al. Repetitive transcranial magnetic stimulation improved symptoms of obsessive-compulsive disorder, but also cognitive performance: Results from a randomized clinical trial with a cross-over design and sham condition. Neuropsychobiology. 2016;73:224–32. doi: 10.1159/000446287. [DOI] [PubMed] [Google Scholar]
- 31.Shayganfard M, Jahangard L, Nazaribadie M, Haghighi M, Ahmadpanah M, Sadeghi Bahmani D, et al. Repetitive transcranial magnetic stimulation improved symptoms of obsessive-compulsive disorders but not executive functions: Results from a randomized clinical trial with crossover design and sham condition. Neuropsychobiology. 2016;74:115–24. doi: 10.1159/000457128. [DOI] [PubMed] [Google Scholar]
- 32.Kang JI, Kim CH, Namkoong K, Lee CI, Kim SJ. A randomized controlled study of sequentially applied repetitive transcranial magnetic stimulation in obsessive-compulsive disorder. J Clin Psychiatry. 2009;70:1645–51. doi: 10.4088/JCP.08m04500. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Singh S, Chadda RK, Verma R, Kumar N. The effect of low-frequency repetitive transcranial magnetic stimulation at orbitofrontal cortex in the treatment of patients with medication-refractory obsessive-compulsive disorder: A retrospective open study. J ECT. 2018;34:e16–e19. doi: 10.1097/YCT.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 34.Nauczyciel C, Le Jeune F, Naudet F, Douabin S, Esquevin A, Vérin M, et al. Repetitive transcranial magnetic stimulation over the orbitofrontal cortex for obsessive-compulsive disorder: A double-blind, crossover study. Transl Psychiatry. 2014;4:e436. doi: 10.1038/tp.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruffini C, Locatelli M, Lucca A, Benedetti F, Insacco C, Smeraldi E, et al. Augmentation effect of repetitive transcranial magnetic stimulation over the orbitofrontal cortex in drug-resistant obsessive-compulsive disorder patients: A controlled investigation. Prim Care Companion J Clin Psychiatry. 2009;11:226–30. doi: 10.4088/PCC.08m00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou DD, Wang W, Wang GM, Li DQ, Kuang L. An updated meta-analysis: Short-term therapeutic effects of repeated transcranial magnetic stimulation in treating obsessive-compulsive disorder. J Affect Disord. 2017;215:187–96. doi: 10.1016/j.jad.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Berlim MT, Neufeld NH, Van den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): An exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res. 2013;47:999–1006. doi: 10.1016/j.jpsychires.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Ma ZR, Shi LJ. Repetitive transcranial magnetic stimulation (rTMS) augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant obsessive-compulsive disorder (OCD): A meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2014;7:4897–905. [PMC free article] [PubMed] [Google Scholar]
- 39.Rehn S, Eslick GD, Brakoulias V. A meta-analysis of the effectiveness of different cortical targets used in repetitive transcranial magnetic stimulation (rTMS) for the treatment of obsessive-compulsive disorder (OCD) Psychiatr Q. 2018;89:645–65. doi: 10.1007/s11126-018-9566-7. [DOI] [PubMed] [Google Scholar]
- 40.Trevizol AP, Shiozawa P, Cook IA, Sato IA, Kaku CB, Guimarães FB, et al. Transcranial magnetic stimulation for obsessive-compulsive disorder: An updated systematic review and meta-analysis. J ECT. 2016;32:262–6. doi: 10.1097/YCT.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 41.Volpato C, Piccione F, Cavinato M, Duzzi D, Schiff S, Foscolo L, et al. Modulation of affective symptoms and resting state activity by brain stimulation in a treatment-resistant case of obsessive-compulsive disorder. Neurocase. 2013;19:360–70. doi: 10.1080/13554794.2012.667131. [DOI] [PubMed] [Google Scholar]
- 42.D’Urso G, Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, Mantovani A, et al. Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depress Anxiety. 2016;33:1132–40. doi: 10.1002/da.22578. [DOI] [PubMed] [Google Scholar]
- 43.Mondino M, Haesebaert F, Poulet E, Saoud M, Brunelin J. Efficacy of cathodal transcranial direct current stimulation over the left orbitofrontal cortex in a patient with treatment-resistant obsessive-compulsive disorder. J ECT. 2015;31:271–2. doi: 10.1097/YCT.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 44.Narayanaswamy JC, Jose D, Chhabra H, Agarwal SM, Shrinivasa B, Hegde A, et al. Successful application of add-on transcranial direct current stimulation (tDCS) for treatment of SSRI resistant OCD. Brain Stimul. 2015;8:655–7. doi: 10.1016/j.brs.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Bation R, Poulet E, Haesebaert F, Saoud M, Brunelin J. Transcranial direct current stimulation in treatment-resistant obsessive-compulsive disorder: An open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:153–7. doi: 10.1016/j.pnpbp.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Hazari N, Narayanaswamy JC, Chhabra H, Bose A, Venkatasubramanian G, Reddy YC, et al. Response to transcranial direct current stimulation in a case of episodic obsessive compulsive disorder. J ECT. 2016;32:144–6. doi: 10.1097/YCT.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 47.D’Urso G, Brunoni AR, Anastasia A, Micillo M, de Bartolomeis A, Mantovani A, et al. Polarity-dependent effects of transcranial direct current stimulation in obsessive-compulsive disorder. Neurocase. 2016;22:60–4. doi: 10.1080/13554794.2015.1045522. [DOI] [PubMed] [Google Scholar]
- 48.Alizadeh Goradel J, Pouresmali A, Mowlaie M, Sadeghi Movahed F. The effects of transcranial direct current stimulation on obsession-compulsion, anxiety, and depression of a patient suffering from obsessive-compulsive disorder. Pract Clin Psychol. 2016;4:75–80. [Google Scholar]
- 49.Silva RM, Brunoni AR, Miguel EC, Shavitt RG. Transcranial direct current stimulation for treatment-resistant obsessive-compulsive disorder: Report on two cases and proposal for a randomized, sham-controlled trial. Sao Paulo Med J. 2016;134:446–50. doi: 10.1590/1516-3180.2016.0155010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinn WM, Aycicegi-Dinn A, Göral F, Karamursel S, Yildirim EA, Hacioglu-Yildirim M, et al. Treatment-resistant obsessive-compulsive disorder: Insights from an open trial of transcranial direct current stimulation (tDCS) to design a RCT. Neurol Psychiatry Brain Res. 2016;22:146–54. [Google Scholar]
- 51.Palm U, Leitner B, Kirsch B, Behler N, Kumpf U, Wulf L, et al. Prefrontal tDCS and sertraline in obsessive compulsive disorder: A case report and review of the literature. Neurocase. 2017;23:173–7. doi: 10.1080/13554794.2017.1319492. [DOI] [PubMed] [Google Scholar]
- 52.Najafi K, Fakour Y, Zarrabi H, Heidarzadeh A, Khalkhali M, Yeganeh T, et al. Efficacy of transcranial direct current stimulation in the treatment: Resistant patients who suffer from severe obsessive-compulsive disorder. Indian J Psychol Med. 2017;39:573–8. doi: 10.4103/IJPSYM.IJPSYM_388_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klimke A, Nitsche MA, Maurer K, Voss U. Case report: Successful treatment of therapy-resistant OCD with application of transcranial alternating current stimulation (tACS) Brain Stimul. 2016;9:463–5. doi: 10.1016/j.brs.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Hou J, Wu W, Lin Y, Wang J, Zhou D, Guo J, et al. Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: A resting-state fMRI study. J Affect Disord. 2012;138:313–21. doi: 10.1016/j.jad.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Li B, Mody M. Cortico-striato-thalamo-cortical circuitry, working memory, and obsessive-compulsive disorder. Front Psychiatry. 2016;7:78. doi: 10.3389/fpsyt.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang JH, Kim JH, Jung WH, Choi JS, Jung MH, Lee JM, et al. Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder. Neurosci Lett. 2010;474:158–62. doi: 10.1016/j.neulet.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 58.Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: Implications for DBS in psychiatry. Neurosci Biobehav Rev. 2008;32:408–22. doi: 10.1016/j.neubiorev.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;35:26–37. [PubMed] [Google Scholar]
- 60.Simon D, Kaufmann C, Müsch K, Kischkel E, Kathmann N. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology. 2010;47:728–38. doi: 10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 61.Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. 2008;20:1251–83. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eng GK, Sim K, Chen SH. Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: An integrative review. Neurosci Biobehav Rev. 2015;52:233–57. doi: 10.1016/j.neubiorev.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 64.de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171:340–9. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 65.Gillan CM, Robbins TW. Goal-directed learning and obsessive-compulsive disorder. Philos Trans R Soc Lond B Biol Sci. 2014;369:pii: 20130475. doi: 10.1098/rstb.2013.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–54. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 68.de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, et al. Presupplementary motor area hyperactivity during response inhibition: A candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 2012;169:1100–8. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- 69.Hsu TY, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJ, et al. Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage. 2011;56:2249–57. doi: 10.1016/j.neuroimage.2011.03.059. [DOI] [PubMed] [Google Scholar]


