Abstract
Proven treatment strategies for obsessive–compulsive disorder (OCD) include pharmacotherapy with serotonin reuptake inhibitors and cognitive behavior therapy (CBT). A significant proportion of patients (25%–30%) fail to respond to these treatment options, necessitating the need for additional treatment options to improve treatment outcomes and quality of life in patients with OCD. Augmentation strategies using various glutamatergic agents have been explored, with diverse outcomes. The aim of this review is to give an overview of the glutamatergic system in the brain with a focus on glutamatergic abnormalities in OCD and to review the existing evidence for various glutamatergic agents used for augmentation.
Keywords: Augmentation, glutamate, N-methyl-D-aspartate receptors, obsessive– compulsive disorder
INTRODUCTION
Glutamatergic system in the brain
Glutamate is a ubiquitous neurotransmitter, chemically belonging to AMINE group of neurotransmitters. It is the principal excitatory neurotransmitter in the cerebral cortex of humans. Glutamate is present both intracellularly and extracellularly, each serving different purposes with respect to metabolic functions and signal transmission.[1] Glutamate in the brain is primarily synthesized by pyruvic acid and alpha-ketoglutarate which receives an amino group donated by “leucine, isoleucine, valine, glutamine, and aspartate” amino acids.[2] Metabolic studies have shown that all of the glucose which enters the brain virtually gets converted into glutamate.[3] Glutamate release and reuptake are tightly regulated by VGlut and EAAT proteins.[1] Glutamatergic neurons are found to be extensively distributed across various brain regions including basal ganglia, cerebellum, brainstem circuits, and various intracortical and cortico-subcortical connections.[4] Owing to its ubiquitous distribution and role in cell signaling and excitatory nature of neurotransmission, it likely plays a modulatory role in health and disease.
Glutamate receptors
Glutamate receptors fall into two categories – metabotropic and ionotropic. The metabotropic receptors consist of mGLU1-8. The main ionotropic receptors include N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and kainate receptors. While ionotropic receptors are responsible for the immediate effects, metabotropic receptors are responsible for longer lasting effects, including changes at the cellular and nuclear level.[4] There exists a complex relationship between glutamatergic neurotransmission and other neurotransmitter signaling.[5] Glutamate signaling abnormalities have been implicated in the pathogenesis and pathophysiology of various psychiatric disorders including schizophrenia, mood disorders, and obsessive– compulsive disorder (OCD). Specific polymorphisms in NMDA receptor subunits (NR1 and NR2a and 2b) and glutamate transporter proteins, solute carrier family 1 member 1 (SLC1A1/EAAC1andEAAT3), have been implicated in OCD risk.[6] Members of SLC1A1 family function as transporters of extracellular glutamate at the postsynaptic region where they stop glutamate action and aid in neuroprotection. Mutations in genes coding for SLC1A1 are shown to be associated with OCD in males.[7] NMDA receptors are made up of one obligatory (NR1) and two additional (NR2, NR3) subunits. Mutations in genes coding for subunit NR2 have been associated with OCD risk and presence of some specific symptoms (contamination obsessions and cleaning).[8]
Glutamatergic dysfunction in obsessive–compulsive disorder
Dysfunction of cortico-striato-thalamo-cortical (CSTC) circuits is a well-documented abnormality underlying OCD. There is emerging evidence from genetic, neurochemical, and neuroimaging studies that glutamate transmission abnormalities in CSTC may contribute to the pathophysiology of OCD.[4]
Cortico-striato-thalamo-cortical circuit and the glutamatergic system
Direct and indirect pathways, operating by means of glutamate and gamma-aminobutyric acid (GABA), exist in CSTC circuit. They work in balance in normalcy/physiological conditions (Baxter's model).[9] Glutamatergic signaling operates between key regions of CSTC, namely the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and striatal structures.
Through the direct pathway, excitatory signals originating from OFC and ACC increase inhibitory GABAergic signaling to downstream inhibitory nuclei such as globus pallidus interna (GPi) and substantia nigra (SNr).[10] Subsequently, there is a reduction in signal transmission from these nuclei, resulting in an increase in thalamic output to the cortex. The indirect pathway serves as a modulator of glutamatergic transmission by the direct pathway. Through striatum, it inhibits GPe (Globus pallidus Externa), resulting in stimulation of STN, GPi, and SNr, leading to thalamic inhibition.[11] The parallel and partially nonoverlapping CSTC circuits in OCD might have differential impairment of these pathways.[12]
Genetic studies
Dysfunction in the postsynaptic glutamate signaling represents the most likely candidate mechanism at the molecular level to explain the macrolevel changes seen in CSTC glutamate transmission. Several animal studies and human genetic studies have tried to investigate and explain the changes at a macrolevel from a molecular perspective. In this regard, a brief review of the evidence from all sources would be useful to understand the basis for glutamate dysfunction in OCD. Knockout mice studies have found abnormalities in genes controlling NMDA receptor subunit composition (DLGAP3, slitrk) to be associated with increased OCD-like behaviors.[13] In human genetic studies, genes coding for postsynaptic glutamate transport proteins (EAAC1, EAAT3), NMDA receptor subunits (GRIN2B), Kainate receptor subunits (GRIK2/3), and members of postsynaptic density units (DLGAP1) have been associated OCD and variants. Some studies have also found an association of SLC1A1 gene mutations with OCD in males.[7] A similar association has been reported in mutations affecting GRIN2B,[8] which also has an association with symptom dimension of contamination and cleaning.[8] Even though the SNPs examined have varied, there is a paucity of consistent replication studies.[4] Further, results from the OCD Collaborative Genetic Association Studies and International OCD Foundation Genetic Collaborative GWAS studies with large sample sizes have failed to identify any SNPs at genome-wide significance level.[14,15] Currently, the genetic basis of OCD is less well known, and further studies are needed for an in-depth understanding of this condition. The glutamatergic model remains an attractive way to explore the genetic basis of this condition.
Neuroimaging studies
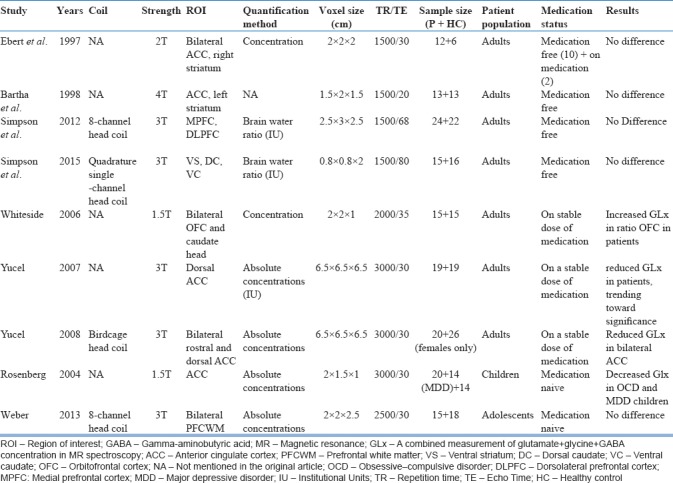
Evidence for glutamatergic dysfunction in OCD comes mainly from proton magnetic resonance spectroscopic studies (H-MRS). Research has yielded mixed results so far. Some studies have found evidence for elevated glutamate levels in the caudate nucleus in both medicated adult patients[16] and unmedicated pediatric patients.[17] These findings have not been subsequently well replicated. Majority of the studies conducted later have either found no striatal glutamatergic abnormalities[18,19,20,21] or reduced glutamate levels in different parts of ACC (rostral and dorsal).[22,23] On the other hand, a study by Gnanavel et al.[24] has reported increased GLx levels in ACC. However, one needs to interpret the findings from these studies keeping mind the variations in image acquisition and analysis factors such as coil type employed, definitions of region of interest used, the quantification method used, voxel size and placement, strength of the magnetic field, clinical characteristics of the patient population studied, and the medication status. Most of the studies[16,20,21,25] were done on patients who were either on a stable dose of medications or were free of medications, suggesting that medication exposure has happened before the time of scanning, which can influence the neuroanatomy and neurochemistry in different ways. Only a handful of studies have taken medication-naive patients.[19,26] These studies have been conducted in either children or adolescents, which limits the generalizability of their study findings. More studies with rigorous methodology on carefully chosen patient samples would be required to assess if glutamate abnormalities are due to methodological differences or they represent actual dysfunctions in glutamate signaling. Table 1 summarizes the MRS studies investigating glutamatergic abnormalities in OCD.
Table 1.
Magnetic resonance spectroscopic studies investigating glutamate abnormalities in obsessive compulsive disorder
Augmentation with glutamatergic agents
Augmentation – what do we mean by this?
Augmentation is a method for improving treatment response. It is achieved by administering medication(s) with a different mechanism of action along with primary medication to enhance its efficacy. Augmentation as a treatment strategy is commonly used for treating various psychiatric illnesses as multiple neurotransmitters are implicated in the pathophysiology of these illnesses. Multiple neurotransmitters are associated with the pathophysiology of OCD as well. Hence, there is a theoretical rationale for augmentation in the treatment of OCD.[27]
Glutamatergic medications used as augmenting agents in obsessive–compulsive disorder
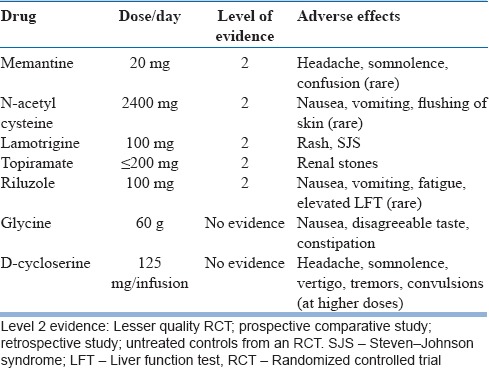
Glutamatergic medications that have been used have no single mechanism of action. Rather, they work in complex and varied ways to bring about their effects. They are best described as glutamate modulators. Chemically, they can be receptor antagonists, glutamate co-agonists, reuptake inhibitors, and ion channel modulators that act to bring about changes in glutamate transmission. In general, these medications are well tolerated. However, specific side effects have been noted with some of the agents. Table 2 summarizes the various medications used, their recommended optimal doses, and specific adverse effects.
Table 2.
Summary of Glutamatergic medications used in treatment of obsessive-compulsive disorder
Memantine
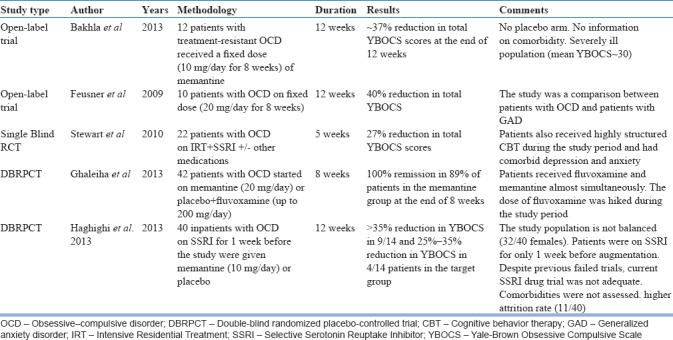
Memantine acts as a noncompetitive NMDA receptor antagonist that preferentially targets extrasynaptic NMDA receptors, blocking ion channel pores, and reducing calcium influx. Research suggests that it may reduce the activity of the direct pathway of CSTC, modulate connectivity between ACC, OFC, and aberrant activity between amygdala and hippocampus[11] which are implicated in the pathophysiology of OCD. The important studies examining the utility of memantine in OCD are summarized in Table 3. Whereas most of the open-label studies have reported a modest reduction in YBOCS score (27%–40% at the end of study period), two randomized controlled trials (RCTs)[28,29] have reported significantly greater rates of response, reaching up to 100% in one of the RCTs.[28] In a single-blind study by Stewart et al., it was shown that memantine was efficacious as an augmenting agent. However, the sample consisted of patients who also received cognitive behavior therapy (CBT) and other standard OCD medications, which may limit the specificity of the impact; memantine might have had on the overall outcome. Two recent RCTs (both from Iran) have demonstrated the efficacy of memantine as an augmenting agent for treatment of OCD. Both studies recruited patients who may not have been necessarily treatment resistant. In addition, RCT of Ghaleiha et al., fluvoxamine was almost simultaneously started along with memantine and was hiked up to 200 mg/d which was continued for the rest of the study period. Hence, it remains to be seen if the improvement reported, actually represents the effect of add-on memantine, or it is due to serotonin reuptake inhibitor alone. Memantine was generally well tolerated in all the studies, and overall body of evidence indicates the potential clinical utility of this medication as an augmentation method in OCD.
Table 3.
The summary of studies examining the role of memantine as an augmentation in obsessive–compulsive disorder
N-acetyl cysteine
N-acetyl cysteine (NAC) is a drug conventionally used to treat paracetamol (acetaminophen) poisoning. In addition, it is used as a mucolytic in the treatment of airway diseases. Recently, studies have investigated the utility of this relatively safe molecule across various psychiatric conditions including OCD. By virtue of its antioxidant properties, it is believed to exert its effects on psychiatric conditions in which oxidative stress plays a role. Cysteine component of NAC is central to its antioxidant properties and glutamate modulation. Availability of cysteine is a rate-limiting step in the production of glutathione, the major cellular antioxidant. Cysteine is also a substrate in the metabolism of the glutamatergic system. By providing cysteine, NAC is believed to exert a modulatory influence on glutamatergic transmission.[30] There are a few studies, which have looked into the efficacy of NAC as an augmentation strategy in the treatment of OCD and related disorders. Earlier trials from Iran have reported a significant benefit of adding NAC to ongoing Selective Serotonin Re-uptake Inhibitor (SSRI) treatment.[31,32] However, the same has not been replicated in two recently published, 16-week, placebo-controlled RCTs.[33,34] Both studies did not find a positive impact of NAC addition on YBOCS scores. The first of these double-blind randomized placebo-controlled trials (DBRPCTs) by Sarris et al. (n = 44) found a temporary, albeit significant, “time*treatment” interaction in favor of NAC in the form of reduction in “compulsion” subscale of YBOCS at the end of week 12, which disappeared at the end of the study period (week 16). In another DBRPCT by Costa et al. (n = 40), no significant difference was observed at the end of the study period (week16) between participants on NAC and those on placebo. Therefore, the evidence favoring the use of NAC at this stage is preliminary and needs further substantiation. However, given its safety profile and possible significant effects in a subset of patients, it remains an attractive option for glutamatergic augmentation.
Lamotrigine
Lamotrigine is an antiepileptic agent, which has also been used as a mood stabilizer. It inhibits voltage-gated calcium channels on presynaptic neurons, thereby reducing glutamate outflow. Lamotrigine is usually well tolerated except for a rare possibility of causing Steven–Johnson syndrome, subject to various factors.[35] A handful of studies have examined the role of lamotrigine as an augmenting agent to SSRI treatment in treatment-resistant OCD. Benefits of lamotrigine augmentation in OCD were reported initially in case reports and case series.[36,37] Such benefits were also reported in patients with schizophrenia/schizoaffective disorder and bipolar disorder who had comorbid OCD.[38,39] Subsequently, two DBRPCTs have examined the efficacy of lamotrigine augmentation in OCD. In a study by Bruno et al. (n = 33, 16 weeks), it was shown to reduce YBOCS compulsion scores together with improvement in affective symptoms and semantic fluency in the treatment group.[40] A more recent RCT (L = 26; P = 27, 12 weeks) which compared addition of lamotrigine (L) to SSRI with a placebo (P) arm, a reduction in YBOCS total scores (Mean reduction = 8.73) as well as obsessions (mean reduction = 4.15) and compulsions (mean reduction = 4.5) was reported. The reduction was statistically significant when compared to the placebo group (P = 0.04, 0.03, and 0.01 for obsessions, compulsions, and total score, respectively).[41] Hence, at this point, there is emerging evidence to support the use of lamotrigine as an augmenting agent. However, these results need to be replicated in larger samples with a longer period of follow-up before any conclusive statement can be made about the use of lamotrigine.
Topiramate
Topiramate was originally designed as a hypoglycemic drug that was subsequently known to have antiepileptic properties. It interacts with voltage-gated calcium channels and modulates glutamate release from axon terminals. It is known to inhibit glutamate release and enhance GABA release.[42] Evidence from two out of three double-blind randomized placebo-controlled trials[43,44,45] conducted suggest that topiramate augmentation may confer modest benefit in the treatment of OCD in a subset of the population. The study by Berlin et al. (T = 18; P = 18, 12 weeks) reported a significant reduction in compulsions subscale only. Poor tolerability to topiramate was noted, with 28% of study participants discontinuing due to side effects. Mowla et al.[45] studied the effects of topiramate augmentation in a DBRPCT study design among 41 treatment-refractory OCD patients with comorbid depression. Nearly 32% reduction in YBOCS scores was reported in the topiramate arm, which also recorded lower HAM-D scores at the end of 12 weeks. It is debatable if the reduction in YBOCS is attributable to depression getting better or to augmentation effects of topiramate on OCD itself. In the RCT (12 weeks) by Afshar et al., which was conducted in a treatment-refractory OCD sample of 36 patients, the reduction in YBOCS scores was similar across both arms. None of the studies had a long-term follow-up arm to objectively assess the long-term sustainability of the beneficial effects observed. Thus, poor tolerability, comorbid depression, and small sample sizes with a lack of data on long-term efficacy limit generalizability of findings.
Ketamine
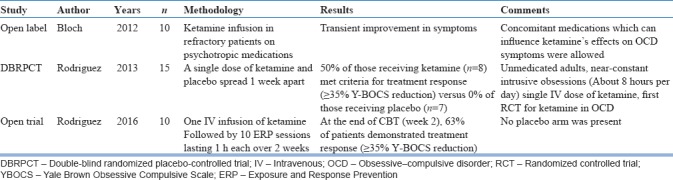
Ketamine is an open-channel nonselective antagonist at NMDA receptor. At low doses, It increases glutamatergic activity in the prefrontal cortex by complex mechanisms, despite being a receptor antagonist.[46] Ketamine infusion has been noted to result in rapid resolution of depressive symptoms and suicidality across various studies.[47,48,49] Encouraged by the results of studies in depression, the efficacy of ketamine augmentation in OCD has been examined by a handful of studies. In an open-label study, Bloch et al. reported transient improvement which did not persist beyond a week.[50] Subsequently, a cross-over RCT examined the effect of single-dose ketamine compared to saline infusion in medication-naive patients with OCD.[51] Fifty percent of the participants receiving ketamine infusion met criteria for treatment response as per YBOCS reduction, which was maintained at 1 week postinfusion. No participant, who received saline infusion, reported improvement. Another open-label trial reported additional benefit of adding exposure-based CBT in extending the effects of ketamine infusion.[52] Recently, in an open-label observation from our center, 2 out of 11 patients satisfied response criteria of 25%.[53] These patients have also failed trials of multiple SSRIs, exposure-based CBT, and pharmacological augmentation strategies. Hence, it can be seen that in a “refractory” sample, the response rate is not comparable to that in the other studies. So far, evidence suggests that ketamine has some benefit in less severely affected, unmedicated patients[51] and needs to be investigated more systematically in treatment-resistant OCD. The rapidity of onset of effects makes ketamine an attractive augmentation strategy which requires more DBRPCTs to explore the evidence. The summary of the studies examining the efficacy of ketamine in OCD is shown in Table 4.
Table 4.
Studies on ketamine augmentation in obsessive–compulsive disorder
Glycine
Glycine is a nonessential amino acid, which acts as an obligatory co-agonist at the NMDA receptor, thereby regulating the physiological actions at the NMDA receptor. Glycine on its own cannot modulate the NMDA receptor. However, it is essential for normal glutamate signaling. Based on this hypothesis, glycine was investigated as an augmenting agent for the treatment of OCD in one controlled trial (Greenberg et al., 2009).[54] However, the results showed that both groups did not have a statistically significant difference in terms of reduction in YBOCS score. The requirement of large doses (~60 gm/d) and poor tolerability leading to high dropout rates were a concern noted in this trial. Subsequently, there have been no further studies on glycine. However, recent trials using sarcosine – a glycine re-uptake inhibitor – and rapastinel – a putative glycine site co-agonist at the NMDA receptor – have shown benefit in medication-naive patients with OCD.[55] At this stage, there is no convincing evidence to recommend the use of glycine as a glutamatergic augmentation strategy in treatment-refractory OCD. Further studies are required to establish glycine and glycine modulators as efficacious augmenting strategies.
Riluzole
Riluzole acts through various mechanisms to reduce glutamate outflow in cortical neurons and potentiates reuptake of extracellular glutamate by the glial cells.[56] Case reports and open-label trials[57,58,59] reported modest benefits of riluzole augmentation in patients with refractory OCD. In an open trial in a pediatric population with treatment-resistant OCD,[60] riluzole was not found to be superior to placebo in reducing YBOCS scores. Results from controlled trials[56,61] have been less convincing. Three DBRPCTs have been conducted so far. In the first trial by Pittenger et al. (n = 39; 12 weeks), riluzole augmentation was studied in outpatients and inpatients. Only a subset of patients seemed to benefit from riluzole augmentation, which failed to reach statistical significance in the trial. In the trial by Sahra et al.,[61] all patients receiving riluzole seemed to benefit. Hence, the results with riluzole so far have been mixed. Other factors, including differences in the patient population, clinical characteristics, treatment setting, sample size, and methodological differences may explain the inconsistent results.
D-cycloserine
D-cycloserine is a D-enantiomer of cycloserine, which is used as a second-line bacteriostatic agent in the treatment of tuberculosis. By various mechanisms of actions, it is believed to modulate glutamate signaling and facilitate fear extinction.[62] Randomized trials[63,64,65,66,67] have examined the role of D-cycloserine in facilitating fear extinction by preadministering D-cycloserine as an intravenous infusion (125-mg mean dose)[64]1–2 h before CBT sessions. Results have been largely unconvincing, with no study showing substantial benefit which can be clearly attributed to D-cycloserine pretreatment. Perhaps, it may accelerate the effect of CBT initially, even though evidence suggests that it may not influence the outcome.
SUMMARY
The glutamatergic hypothesis of OCD is an attractive proposition especially for patients who become resistant to SSRI treatment. A number of glutamatergic agents have been investigated for their efficacy in OCD. However, only a few have demonstrated benefit as augmenting agents.
These agents represent a viable alternative in treatment refractory patients, where better-proven strategies have been exhausted. Among these, memantine appears to be more promising in terms of the number of good quality positive trials. Ketamine for OCD is still in experimental stages, unlike in treatment of depression where encouraging results are obtained. However, there is a need to further evaluate its efficacy, given the rapid onset of action. It may lend itself as a molecule to examine the glutamatergic hypothesis of the disorder. Anticonvulsant medications – topiramate and lamotrigine – have demonstrated only a modest benefit. However, they may work in a small subset of patients. NAC has large RCT with positive results and some recent RCTs with negative findings. NAC may be considered as a potential option for augmentation even though further studies are required. At this point, the Indian Psychiatric Society guidelines recommend the use of memantine and lamotrigine with a strength of recommendation-level B (one high-quality study, several studies with some limitations).[68] Future research aimed at identifying a subset who may benefit from it may help us understand the predictors of response and is likely to have an important impact on pharmacological management of treatment-resistant OCD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100:656–64. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pellerin L, Magistretti PJ. Neuroenergetics: Calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–40. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: Neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132:314–32. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meldrum BS. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J Nutr. 2000;130:1007S–15S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- 6.Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA, et al. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: A preliminary study. Psychopharmacology (Berl) 2004;174:530–8. doi: 10.1007/s00213-004-1847-1. [DOI] [PubMed] [Google Scholar]
- 7.Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, et al. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1027–33. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- 8.Alonso P, Gratacós M, Segalàs C, Escaramís G, Real E, Bayés M, et al. Association between the NMDA glutamate receptor GRIN2B gene and obsessive-compulsive disorder. J Psychiatry Neurosci. 2012;37:273–81. doi: 10.1503/jpn.110109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–86. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 10.Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: An integrative genetic and neurobiological perspective. Nat Rev Neurosci. 2014;15:410–24. doi: 10.1038/nrn3746. [DOI] [PubMed] [Google Scholar]
- 11.Vlček P, Polák J, Brunovský M, Horáček J. Role of glutamatergic system in obsessive-compulsive disorder with possible therapeutic implications. Pharmacopsychiatry. 2018;51:229–42. doi: 10.1055/s-0043-118665. [DOI] [PubMed] [Google Scholar]
- 12.van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. 2016;26:810–27. doi: 10.1016/j.euroneuro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Wu K, Hanna GL, Rosenberg DR, Arnold PD. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol Biochem Behav. 2012;100:726–35. doi: 10.1016/j.pbb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattheisen M, Samuels JF, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, et al. Genome-wide association study in obsessive-compulsive disorder: Results from the OCGAS. Mol Psychiatry. 2015;20:337–44. doi: 10.1038/mp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold PD, Askland KD, Barlassina C, Bellodi L, Bienvenu OJ, Black D, et al. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018;23:1181–8. doi: 10.1038/mp.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starck G, Ljungberg M, Nilsson M, Jönsson L, Lundberg S, Ivarsson T, et al. A1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: Relationship between metabolite concentrations and symptom severity. J Neural Transm (Vienna) 2008;115:1051–62. doi: 10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ, et al. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39:1096–103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Gershkovich M, Wheaton MG, Simpson HB. Management of treatment-resistant obsessive-compulsive disorder. Curr Treat Options Psychiatry. 2017;4:357–70. [Google Scholar]
- 19.Strawn JR, Chu WJ, Whitsel RM, Weber WA, Norris MM, Adler CM, et al. Apilot study of anterior cingulate cortex neurochemistry in adolescents with generalized anxiety disorder. Neuropsychobiology. 2013;67:224–9. doi: 10.1159/000347090. [DOI] [PubMed] [Google Scholar]
- 20.Lázaro L, Bargalló N, Andrés S, Falcón C, Morer A, Junqué C, et al. Proton magnetic resonance spectroscopy in pediatric obsessive-compulsive disorder: Longitudinal study before and after treatment. Psychiatry Res. 2012;201:17–24. doi: 10.1016/j.pscychresns.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Bédard MJ, Chantal S. Brain magnetic resonance spectroscopy in obsessive-compulsive disorder: The importance of considering subclinical symptoms of anxiety and depression. Psychiatry Res. 2011;192:45–54. doi: 10.1016/j.pscychresns.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, et al. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry. 2008;42:467–77. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- 23.Yücel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:946–55. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- 24.Gnanavel S, Sharan P, Khandelwal S, Sharma U, Jagannathan NR. Neurochemicals measured by (1)H-MR spectroscopy: Putative vulnerability biomarkers for obsessive compulsive disorder. MAGMA. 2014;27:407–17. doi: 10.1007/s10334-013-0427-y. [DOI] [PubMed] [Google Scholar]
- 25.Simpson HB, Shungu DC, Bender J, Jr, Mao X, Xu X, Slifstein M, et al. Investigation of cortical glutamate-glutamine and γ-aminobutyric acid in obsessive-compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology. 2012;37:2684–92. doi: 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald KD, Moore GJ, Paulson LA, Stewart CM, Rosenberg DR. Proton spectroscopic imaging of the thalamus in treatment-naive pediatric obsessive-compulsive disorder. Biol Psychiatry. 2000;47:174–82. doi: 10.1016/s0006-3223(99)00286-3. [DOI] [PubMed] [Google Scholar]
- 27.Arumugham SS, Reddy JY. Augmentation strategies in obsessive-compulsive disorder. Expert Rev Neurother. 2013;13:187–202. doi: 10.1586/ern.12.160. [DOI] [PubMed] [Google Scholar]
- 28.Ghaleiha A, Entezari N, Modabbernia A, Najand B, Askari N, Tabrizi M, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: Randomized double-blind placebo-controlled study. J Psychiatr Res. 2013;47:175–80. doi: 10.1016/j.jpsychires.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Haghighi M, Jahangard L, Mohammad-Beigi H, Bajoghli H, Hafezian H, Rahimi A, et al. In a double-blind, randomized and placebo-controlled trial, adjuvant memantine improved symptoms in inpatients suffering from refractory obsessive-compulsive disorders (OCD) Psychopharmacology (Berl) 2013;228:633–40. doi: 10.1007/s00213-013-3067-z. [DOI] [PubMed] [Google Scholar]
- 30.Sansone RA, Sansone LA. Getting a knack for NAC: N-acetyl-cysteine. Innov Clin Neurosci. 2011;8:10–4. [PMC free article] [PubMed] [Google Scholar]
- 31.Afshar H, Roohafza H, Mohammad-Beigi H, Haghighi M, Jahangard L, Shokouh P, et al. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2012;32:797–803. doi: 10.1097/JCP.0b013e318272677d. [DOI] [PubMed] [Google Scholar]
- 32.Paydary K, Akamaloo A, Ahmadipour A, Pishgar F, Emamzadehfard S, Akhondzadeh S, et al. N-acetylcysteine augmentation therapy for moderate-to-severe obsessive-compulsive disorder: Randomized, double-blind, placebo-controlled trial. J Clin Pharm Ther. 2016;41:214–9. doi: 10.1111/jcpt.12370. [DOI] [PubMed] [Google Scholar]
- 33.Sarris J, Oliver G, Camfield DA, Dean OM, Dowling N, Smith DJ, et al. N-acetyl cysteine (NAC) in the treatment of obsessive-compulsive disorder: A 16-week, double-blind, randomised, placebo-controlled study. CNS Drugs. 2015;29:801–9. doi: 10.1007/s40263-015-0272-9. [DOI] [PubMed] [Google Scholar]
- 34.Costa DL, Diniz JB, Requena G, Joaquim MA, Pittenger C, Bloch MH, et al. Randomized, double-blind, placebo-controlled trial of N-acetylcysteine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2017;78:e766–73. doi: 10.4088/JCP.16m11101. [DOI] [PubMed] [Google Scholar]
- 35.Garcia G, Logan GE, Gonzalez-Heydrich J. Management of psychotropic medication side effects in children and adolescents. In: Child and Adolescent Psychiatric Clinics of North America. 2012;21:713–38. doi: 10.1016/j.chc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Uzun O. Lamotrigine as an augmentation agent in treatment-resistant obsessive-compulsive disorder: A case report. J Psychopharmacol. 2010;24:425–7. doi: 10.1177/0269881108098809. [DOI] [PubMed] [Google Scholar]
- 37.Arrojo-Romero M, Tajes Alonso M, de Leon J. Lamotrigine augmentation of serotonin reuptake inhibitors in severe and long-term treatment-resistant obsessive-compulsive disorder. Case Rep Psychiatry 2013. 2013 doi: 10.1155/2013/612459. 612459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poyurovsky M, Glick I, Koran LM. Lamotrigine augmentation in schizophrenia and schizoaffective patients with obsessive-compulsive symptoms. J Psychopharmacol. 2010;24:861–6. doi: 10.1177/0269881108099215. [DOI] [PubMed] [Google Scholar]
- 39.Bisol LW, Lara DR. Improvement of obsessive-compulsive disorder with divalproex and lamotrigine in two patients with bipolar II disorder. Pharmacopsychiatry. 2009;42:37–9. doi: 10.1055/s-0028-1085439. [DOI] [PubMed] [Google Scholar]
- 40.Bruno A, Micò U, Pandolfo G, Mallamace D, Abenavoli E, Di Nardo F, et al. Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: A double-blind, placebo-controlled study. J Psychopharmacol. 2012;26:1456–62. doi: 10.1177/0269881111431751. [DOI] [PubMed] [Google Scholar]
- 41.Khalkhali M, Aram S, Zarrabi H, Kafie M, Heidarzadeh A. Lamotrigine augmentation versus placebo in serotonin reuptake inhibitors-resistant obsessive-compulsive disorder: A randomized controlled trial. Iran J Psychiatry. 2016;11:104–14. [PMC free article] [PubMed] [Google Scholar]
- 42.Suppes T. Review of the use of topiramate for treatment of bipolar disorders. J Clin Psychopharmacol. 2002;22:599–609. doi: 10.1097/00004714-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Berlin HA, Koran LM, Jenike MA, Shapira NA, Chaplin W, Pallanti S, et al. Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72:716–21. doi: 10.4088/JCP.09m05266gre. [DOI] [PubMed] [Google Scholar]
- 44.Afshar H, Akuchekian S, Mahaky B, Zarean E. Topiramate augmentation in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19:976–81. [PMC free article] [PubMed] [Google Scholar]
- 45.Mowla A, Khajeian AM, Sahraian A, Chohedri AH, Kashkoli F. Topiramate augmentation in resistant OCD: A Double-blind placebo-controlled clinical trial. CNS Spectr. 2010;15:613–7. doi: 10.1017/S1092852912000065. [DOI] [PubMed] [Google Scholar]
- 46.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: A meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46:1459–72. doi: 10.1017/S0033291716000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW, et al. Asystematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45:693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- 50.Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, Leckman JF, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72:964–70. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: Proof-of-concept. Neuropsychopharmacology. 2013;38:2475–83. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez CI, Wheaton M, Zwerling J, Steinman SA, Sonnenfeld D, Galfalvy H, et al. Can exposure-based CBT extend the effects of intravenous ketamine in obsessive-compulsive disorder? An open-label trial. J Clin Psychiatry. 2016;77:408–9. doi: 10.4088/JCP.15l10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma L, Arumugam SS, Narayanaswamy JC, Reddy YC. Ketamine in Treatment Resistant OCD – A Case Series; (unpublished) [Google Scholar]
- 54.Greenberg WM, Benedict MM, Doerfer J, Perrin M, Panek L, Cleveland WL, et al. Adjunctive glycine in the treatment of obsessive-compulsive disorder in adults. J Psychiatr Res. 2009;43:664–70. doi: 10.1016/j.jpsychires.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Köse S, Çetin M. Ketamine and rapastinel: NMDA receptor modulators in the rapid treatment of obsessivecompulsive disorder. Psychiatry Clin Psychopharmacol. 2017;27:213–4. [Google Scholar]
- 56.Pittenger C, Bloch MH, Wasylink S, Billingslea E, Simpson R, Jakubovski E, et al. Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: A pilot randomized placebo-controlled trial. J Clin Psychiatry. 2015;76:1075–84. doi: 10.4088/JCP.14m09123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grant P, Lougee L, Hirschtritt M, Swedo SE. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2007;17:761–7. doi: 10.1089/cap.2007.0021. [DOI] [PubMed] [Google Scholar]
- 58.Coric V, Milanovic S, Wasylink S, Patel P, Malison R, Krystal JH, et al. Beneficial effects of the antiglutamatergic agent riluzole in a patient diagnosed with obsessive-compulsive disorder and major depressive disorder. Psychopharmacology (Berl) 2003;167:219–20. doi: 10.1007/s00213-003-1396-z. [DOI] [PubMed] [Google Scholar]
- 59.Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: An open-label trial. Biol Psychiatry. 2005;58:424–8. doi: 10.1016/j.biopsych.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 60.Grant PJ, Joseph LA, Farmer CA, Luckenbaugh DA, Lougee LC, Zarate CA, Jr, et al. 12-week, placebo-controlled trial of add-on riluzole in the treatment of childhood-onset obsessive-compulsive disorder. Neuropsychopharmacology. 2014;39:1453–9. doi: 10.1038/npp.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emamzadehfard S, Kamaloo A, Paydary K, Ahmadipour A, Zeinoddini A, Ghaleiha A, et al. Riluzole in augmentation of fluvoxamine for moderate to severe obsessive-compulsive disorder: Randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2016;70:332–41. doi: 10.1111/pcn.12394. [DOI] [PubMed] [Google Scholar]
- 62.Bürkner PC, Bittner N, Holling H, Buhlmann U. D-cycloserine augmentation of behavior therapy for anxiety and obsessive-compulsive disorders: A meta-analysis. PLoS One. 2017;12:e0173660. doi: 10.1371/journal.pone.0173660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storch EA, Geffken GR, Merlo LJ, Mann G, Duke D, Munson M, et al. Family-based cognitive-behavioral therapy for pediatric obsessive-compulsive disorder: Comparison of intensive and weekly approaches. J Am Acad Child Adolesc Psychiatry. 2007;46:469–78. doi: 10.1097/chi.0b013e31803062e7. [DOI] [PubMed] [Google Scholar]
- 64.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–8. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–41. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 66.Farrell LJ, Waters AM, Boschen MJ, Hattingh L, McConnell H, Milliner EL, et al. Difficult-to-treat pediatric obsessive-compulsive disorder: Feasibility and preliminary results of a randomized pilot trial of D-cycloserine-augmented behavior therapy. Depress Anxiety. 2013;30:723–31. doi: 10.1002/da.22132. [DOI] [PubMed] [Google Scholar]
- 67.Storch EA, Wilhelm S, Sprich S, Henin A, Micco J, Small BJ, et al. Efficacy of augmentation of cognitive behavior therapy with weight-adjusted d-cycloserine vs placebo in pediatric obsessive-compulsive disorder: A randomized clinical trial. JAMA Psychiatry. 2016;73:779–88. doi: 10.1001/jamapsychiatry.2016.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janardhan Reddy YC, Sundar AS, Narayanaswamy JC, Math SB. Clinical practice guidelines for obsessive-compulsive disorder. Indian J Psychiatry. 2017;59:S74–90. doi: 10.4103/0019-5545.196976. [DOI] [PMC free article] [PubMed] [Google Scholar]


