Abstract
Childhood adversity has been associated with poor adult health. However, it is unclear whether timing of adversity matters in this association and whether adversity is related to poorer age-related physical health status. A representative sample of the adult Dutch population (N = 3,586, age M = 54.94, age range = 18–92) completed surveys on health and diagnoses of age-related diseases. Information about weight and fat percentage was collected using weighing scales and childhood experiences were assessed retrospectively. Adversity was associated with higher body mass index and fat percentage, more physical problems, and high cholesterol, and this association was most pronounced in individuals with experiences of adversity during early adolescence. In addition, individuals with adversity more often reported physical problems or a medical diagnosis at a younger age. This study indicates that (1) timing of exposure to adversity matters in the relationship between experienced childhood adversity and health and (2) adversity is associated with a higher prevalence of age-related diseases at earlier ages.
Keywords: physical health, adverse childhood experiences, child abuse, age-related diseases
Adverse childhood experiences (ACEs), including sexual, physical, and emotional maltreatment; domestic violence; or parental loss and separation, have profound negative effects on health. Multiple ACE studies have shown an association between childhood adversity and elevated mortality rates from chronic diseases, such as a 1.5–2.0 greater incidence of autoimmune disorders, cardiovascular disease, and premature mortality (Anda et al., 2009; Dube et al., 2009; Halonen et al., 2015). Several studies have shown a dose–response relationship between the number of ACEs and many psychological and medical conditions (Anda et al., 2008; Felitti, 2002; Felitti et al., 1998). For example, Felliti et al. (1998) found a graded relationship between the number of ACEs and the presence of adult diseases including cardiovascular disease or chronic lung disease. Other studies point to an increased risk of obesity (Burke, Hellman, Scott, Weems, & Carrion, 2011; Luecken, Jewell, & MacKinnon, 2016; Thomas, Hyppönen, & Power, 2008) and unhealthy behaviors such as smoking and lack of physical activity (Felitti, 2002) in adults with ACEs. Thus, ACEs seem to increase the risk of disease by setting an individual on a risk pathway leading to an unhealthy lifestyle, resulting in poor physical health later in life.
The association between ACEs and health persists even when controlling for the role of health behaviors and socioeconomic conditions (Danese & Tan, 2014). This may indicate that stressful events during childhood are associated with health by direct mechanisms that exert a long-lasting biological imprint (Friedman, Montez, Sheehan, Guenewald, & Seeman, 2015). For example, it has been shown that early adversity increases inflammatory reactivity (Giletta et al., 2018), which in turn increases risk of cardiovascular disease (Slopen, Koenen, & Kubzansky, 2012). Another possible direct mechanism underlying the association between childhood experiences and morbidity and mortality in adulthood is telomere shortening (Puterman et al., 2016; Ridout et al., 2018). Telomere length is considered a marker of cellular aging and has been related to normative aging and age-related diseases that are more prevalent in individuals with ACEs, including cardiovascular disease and diabetes (Fitzpatrick et al., 2007). A shorter telomere length has also been found in maltreated adults (Tyrka et al., 2010) and children with experiences of social deprivation (Drury et al., 2011). Accelerated biological aging may therefore represent one biological mechanism by which early adversity is translated into increased risk of age-related diseases and mortality (Drury et al., 2011). However, an interesting question that remains unanswered is whether experienced adversity is related to a higher prevalence of age-related diseases at earlier ages.
Moreover, the role of developmental timing of exposure to adversity remains unclear. There is some evidence that certain periods during development are more sensitive to effects of adversity and that exposure to stressors during these periods has greater effects on health later in life. Bosch et al. (2012) showed that exposure to childhood adversity during middle childhood (6–11 years) but not early childhood (0–5 years) or early adolescence (12–15 years) was associated with increased cortisol levels. Similarly, Krause, Shaw, and Cairney (2004) showed that ACEs during age 6–11 were more strongly associated with health in adulthood compared to ACEs during other childhood age categories. This is consistent with a meta-analysis in which we found that maltreatment in middle childhood was more strongly associated with neurobiological changes of the hippocampus, a stress-sensitive brain region, than maltreatment in early childhood (Riem, Alink, Out, Van Ijzendoorn, & Bakermans-Kranenburg, 2015).
However, findings resulting from studies examining the association between adversity and mental health show a slightly different pattern. Ogle, Rubin, and Siegler (2013) showed that adults with a history of ACEs during childhood (3–12 years) show most severe symptoms of post-traumatic stress disorder and lower subjective happiness, whereas other studies indicate that exposure during adolescence is most strongly related to poor mental health outcomes. For example, maltreatment during adolescence is more strongly associated with problem behaviors, including internalizing and externalizing problems, delinquency, and drug use, than maltreatment experienced earlier in childhood (Ireland, Smith, & Thornberry, 2002; Thornberry, Ireland, & Smith, 2001). Adolescence is a period that is associated with onset and exacerbation of mental health problems (Kessler et al., 2007) and is a unique phase with respect to its extreme social, cognitive, and hormonal changes. For example, adolescence is characterized by transformations in relationships with caregivers and a changing balance between dependence on caregivers and exploratory behaviors (Allen & Tan, 2016). Because of this changing balance, traumatic events that occur within a family context during adolescence may be particularly disruptive.
In the current study, we examine the relation between retrospectively reported ACEs and physical health in adulthood, taking into account the role of timing of adversity. We will examine the association between multiple types of ACEs, including maltreatment and family loss and dissolution, and various aspects of physical health in adults, including physical problems, body mass index (BMI) and fat percentage, diagnoses of high blood pressure, high cholesterol, and diabetes. Consistent with previous studies, we predict that timing of exposure to adversity in childhood matters for adults’ physical functioning. Since traumatic experiences may be particularly disruptive during early adolescence (Allen & Tan, 2016; Marshall, 2016), we expect that in particular exposure to adversity in middle childhood and adolescence is related to poor health in adulthood. In addition, we expect that the association between childhood adversity and physical health is dependent on the quantity of exposures and is most pronounced in individuals with experiences of multiple types of adversity. Lastly, this study examines the hypothesis that ACEs relate to a higher prevalence of age-related diseases at earlier ages.
Method
Participants and Procedure
Data for the current study were part of the Longitudinal Internet Studies for the Social sciences (LISS) panel, operated by CentERdata at Tilburg University, the Netherlands. The LISS panel (http://www.lissdata.nl) is based on a representative random sample drawn from the Dutch population register by Central Bureau of Statistics and consists of more than 5,000 households and, in total, over 8,000 participants (see Online Supplemental Material for more information about the LISS panel). Data for the current study were extracted from five surveys conducted in 2011–2014. Participants were asked monthly to fill out several online questionnaires, all lasting 15–30 min, and received a monetary reward (15 euros per hour). Households without access to internet were equipped with a computer/and or an internet connection if needed in order to increase the representativeness of the sample.
Only participants older than 18 years, who were not living with their parents, and who participated in two surveys measuring childhood experiences were selected. The total sample of participants with complete data on childhood adversity and health was n = 3,586 (52.6% female, 88% born in the Netherlands). BMI data were collected in a subsample consisting of a random selection of LISS panel members who were willing to participate in a weighing project survey of CentERdata. A subsample of 951 participants with complete ACE data participated in the survey on BMI. The mean ACEs did not differ significantly between the samples and the total sample (p = .34, see Online Supplemental Material for comparisons regarding background variables). Mean age of the total sample was M = 54.94 (SD = 14.71, range 18–92). Mean educational level of the total sample was 3.52 (SD = 1.51) on a scale ranging from 1 (elementary school) to 6 (university). The distribution across educational level in the total sample was a good representation of the Dutch population (elementary school 8.6%, intermediate secondary education 27.0%, higher secondary education 8.9%, intermediate vocational education 23.0%, higher educational education 23.9%, and university 8.3%).
Measures
ACEs
In the present study, ACEs consisted of the following six categories of trauma: (1) family dissolution and loss, (2) severe parental conflict, (3) poor-quality relationship with parents, (4) emotional abuse, (5) physical abuse, and (6) sexual abuse. These categories partly correspond to the conventional ACE categories identified in previous studies (Burke et al., 2011; Chapman et al., 2004; Felitti, 2002), although three additional categories (an alcohol and/or drug abuser in the household; an incarcerated household member; someone who is chronically depressed, mentally ill, institutionalized, or suicidal) were missing because these experiences were not assessed in LISS panel surveys. However, it should be noted that the conventional selection of ACEs in previous studies was not based on a systematic process of conceptualizing and measuring ACEs, and there is no consensus of what constitutes an ACE (Mersky, Janczewski, & Topitzes, 2017).
Participants were asked to indicate whether or not they had experienced emotional abuse, physical abuse, or sexual abuse during childhood with either “yes” or “no” and to indicate the age of exposure to the abuse. In addition, participants reported on their living arrangement at birth and what changes they experienced in their living arrangement before leaving home and living independently. Detailed information was gathered about living arrangements after changes, and participants were asked to indicate how old they were when the change in living arrangement took place (Kalmijn, 2013; Oudejans & Kalmijn, 2013). We considered several family situations that were characterized by loss and family dissolution as one ACE category (see Mersky et al., 2017). This classification included living with a single parent, living in foster care, living with family other than the parents, living in an institution, divorce of parents, and death of one or both parents, and was assigned to participants who had experienced one or more types of these living arrangements. Severe parental conflict was measured with the following four items: “How often did it happen that your parents had fierce discussions?,” “How often did it happen that one parent strongly reproached the other?,” “How often did it happen that your parents refused to talk to each other for a while?,” and “How often did it happen that arguments got out of hand?.” These questions were answered on a Likert-type scale ranging from 1 to 3 (never, once or twice, several times) or I don’t know (see Online Supplemental Material). The classification severe parental conflict was assigned to participants who rated at least 2 items with a score of three (several times). There was a significant association between the classification severe parental conflict and divorce, χ2(1) = 400.95, p < .001, which supports the validity of our classification procedure. The quality of a participant’s relationship with his or her parents was assessed with the following 4 items: “I could always turn to my mother/father with my problems,” “My mother/father and I had a close bond,” “I always felt supported by my mother/father,” and “My mother/father could well understand my preoccupations.” The items were rated twice on a 5-point Likert-type scale ranging from disagree entirely to agree entirely in order to assess the quality of the relationship with father and mother. Mean scores of quality of the relationship with mother and father were calculated. The classification poor-quality relationship parent was assigned to participants with a mean score of quality relationship mother lower than two or a mean score of quality relationship father lower than two. There was a significant association between the classification poor-quality relationship parent and reported emotional abuse, χ2(1) = 386.79, p < .001, which supports the validity of our classification procedure.
Similar to Burke, Hellman, Scott, Weems, and Carrion (2011), we examined mental and physical health of individuals in three ACE groups: individuals without ACEs, individuals with few ACEs, and individuals with multiple ACEs. A sum score of the six categories of trauma was calculated and recoded into the following categories: no adversity, one or two adversities, ≥ three adversities. There were 2,532 participants without experiences of adversity, 911 participants with experiences of one or two adverse experiences, and 143 participants with experiences of three or more adversities. See Table 1 for the prevalence of the specific types of adversities.
Table 1.
Prevalence of Individual Types of Adverse Childhood Experiences in the Current Sample.
| Type of Adversity | Prevalence (%) |
|---|---|
| Emotional abuse | 5.8 |
| Sexual abuse | 2.6 |
| Physical abuse | 3.2 |
| Household dysfunction | 12.5 |
| Parental conflict | 15.3 |
| Poor-quality relation parent | 5.7 |
In order to examine the effect of developmental timing of ACEs on health, participants were assigned to specific age stages, based on the age of exposure to adversity. The designated stages compared were similar to Andersen et al. (2008): infancy (0–2 years, n = 160) preschool (3–5 years, n = 111), latency (6–8 years, n = 130), prepubertal (9–10 years, n = 76), pubertal (11–13 years, n = 104), and adolescent (14–18 years, n = 147). Mean age at exposure to adversity was M = 8.13 (SD = 5.29). When participants reported multiple adversities at different ages or continuing exposure to adversity, they were assigned to the age stage corresponding to the onset of the adversity. For family violence and poor-quality relationships with parents, no data concerning age were present because these experiences often continue over a larger time span.
Physical health
Participants were asked to indicate whether they suffered from the following physical problems: (1) back-, knee-, hip-pain or pain in any other joint, (2) heart complaints or angina, pain in the chest due to exertion, (3) short of breath or problems with breathing, (4) coughing or a stuffy nose and/or flu-related complaints, (5) stomach or intestinal problems, (6) headache, (7) fatigue, (8) sleeping problems, and (9) other recurrent complaints. Physical problems were recoded into a categorical variable (no physical problems, one or more physical problems) because the distribution was skewed. In addition, participants were asked to indicate whether they were told by a physician that they suffered from one of the following diseases or problems during the last year: diabetes or a too high blood sugar level, high blood pressure or hypertension, and high cholesterol content in blood. Analyses with physical problems and more chronic diseases (diabetes, high blood pressure, hypertension, and high cholesterol) were performed separately.
BMI and fat percentage
Data on BMI and fat percentage were extracted from the weighing project survey of CentERdata. A random selection of LISS panel members who were willing to participate in the weighing project received a weighing scale that was connected to an Internet gateway via a radio signal (Kooreman & Scherpenzeel, 2014). With instruction videos, manual, and e-mails, participants were instructed to weigh barefooted. When a participant stepped on the weighing scale, body weight and fat percentage were measured, and measurements were sent to the panel’s central server. Before the first measurement, participants were asked to answer several questions (e.g., indicate their height, gender) and were registered in order to link measurements to the right person. The scale used the body impedance analysis method to calculate body composition, a procedure to calculate the body composition and monitor its trends over time. Body fat was derived from impedance and estimated using a time-independent equation with the following variables: waist measurement, weight, impedance, gender, birth year, and activity level. The parameters were calibrated by Youw8 (see http://www.youw8.com). See Online Supplemental Material for information regarding the weighing procedure and calculation of fat percentage.
Statistical Analysis
Prior to statistical analysis, data were inspected for outliers and distributions (see Online Supplemental Material). We performed multiple hierarchical regression analyses with BMI, fat percentage as outcome variables and ACEs as predictor variables to examine the association between quantity of adversity and BMI, fat percentage, and physical health. Similar to Burke et al. (2011), exposure to ACEs was dummy-coded as no adversity (baseline category), one or two adversities, and more than three ACEs in order to examine the effect of quantity of adversity (ACEs = 1 or 2 and ≥3 compared to ACE score = 0). Age, educational level, sex, living with a partner or not, occupational status, country of origin (the Netherlands vs. other), and medication use were entered as covariates in all analyses. Logistic regression analyses were performed in order to examine whether exposure to ACEs was related to physical problems, and diagnoses of diabetes, high blood pressure, and high cholesterol. In order to examine whether exposure to ACEs was associated with accelerated age-related decline in physical health status, the interaction between ACEs (no ACEs, one or two ACEs, and more than three ACEs) and age was added in the last step of the logistic regression analysis with physical problems as dependent variable. Another logistic regression analysis was performed with diagnoses of diabetes, high blood pressure, and high cholesterol recoded into the dependent categorical variable medical diagnosis, consisting of the categories no diagnosis and one or more diagnoses. BMI and fat percentage were not included in this analysis, since age is not linearly related to BMI and fat (Meeuwsen, Horgan, & Elia, 2010).
In addition, we examined the effect of developmental timing of ACEs on physical health. Analysis of variance (ANOVA) with BMI and fat percentage as dependent variables was conducted to examine effects of age at exposure to adversity. Age at exposure to ACEs was entered as between-subject variable. Logistic regression analysis was performed in order to examine whether age at exposure to ACEs (continuous) was related to physical problems; obesity; and diagnoses of diabetes, high blood pressure, and high cholesterol.
Results
Quantity of ACE
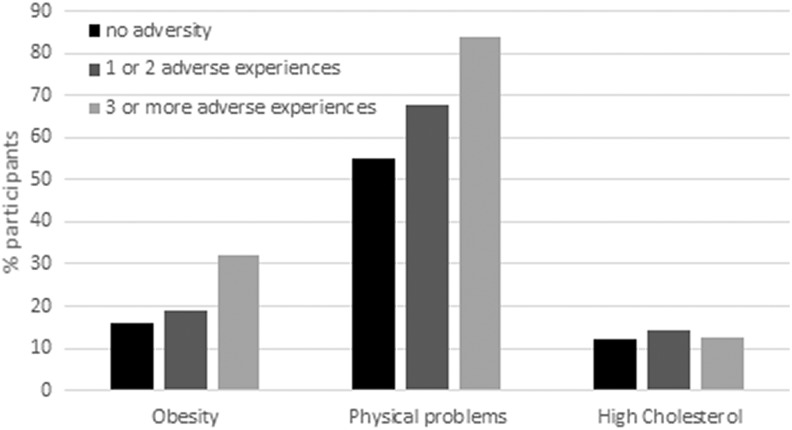
There was a significant effect of ACEs on BMI and fat percentage, fat: F(8, 935) = 141.49, p < .001, R2 overall model = .55, BMI: F(8, 935) = 8.33, p < .001, R2 overall model = .07. Individuals with three or more ACEs showed a significantly higher BMI and fat percentage than individuals without adverse experiences (see Tables 2 and 3). There was no significant difference in BMI and fat percentage between individuals with one or two ACEs and individuals without ACEs. Logistic regression analysis was performed in order to examine whether exposure to ACEs was associated with increased likelihood of being obese (defined as BMI > 30). The experience of three or more ACEs was significantly associated with being obese, model χ2(9) = 41.91, p < .001, Nagelkerke R2 = .07. The experience of one or two ACEs was not significantly associated with being obese (see Table 4). As shown in Figure 1, 31.91% of participants with experiences of three or more ACEs were obese, whereas only 19.09% of participants with one or two ACEs and 15.85% of participants without ACEs were obese.
Table 2.
Summary of Hierarchical Regression Analysis With Fat Percentage, and Body Mass Index (BMI) as Dependent Variable and Childhood Adversity (1 or 2 Adversities, n = 241, ≥3 Adversities, n = 47) as Predictor.
| Outcome Variable | 1 or 2 Adversities | ≥3 Adversities | ||||||
|---|---|---|---|---|---|---|---|---|
| B | SE (B) | β | p | B | SE B | β | p | |
| BMI | .10 | .32 | .01 | .74 | 1.58 | .64 | .08 | .01 |
| % Fat | .32 | .39 | .02 | .41 | 2.42 | .78 | .07 | .002 |
Table 3.
Means and Standard Deviations of Body Mass Index (BMI) and Fat Percentage for Individuals Without Adverse Childhood Experience (ACE), With One or Two ACE and Three or More ACE.
| No. of ACE | BMI | Fat | |||
|---|---|---|---|---|---|
| N | M | SD | M | SD | |
| 0 | 656 | 26.22 | 4.04 | 28.06 | 7.18 |
| 1 or 2 | 241 | 26.18 | 4.62 | 28.76 | 7.90 |
| ≥3 | 47 | 27.54 | 5.71 | 32.79 | 9.09 |
Table 4.
Estimates of Risks of Obesity, Physical Problems, High Blood Pressure, High Cholesterol, and Diabetes Based on Childhood Adversity, Corrected for the Covariates Age, Educational Level, Sex, Living With a Partner, Medication Use, Occupational Status, and Country of Origin.
| Outcome Variable | 1 or 2 ACE | ≥3 ACE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | Wald | p | OR | B | SE | Wald | p | OR | |
| Obesity | .26 | .20 | 1.64 | .25 | 1.29 | 0.83 | .35 | 5.77 | .02 | 2.30 |
| Physical problems | .46 | .09 | 28.23 | <.001 | 1.58 | 1.31 | .24 | 29.35 | <.001 | 3.69 |
| High blood pressure | .01 | .11 | 0.01 | .92 | 1.01 | 0.03 | .23 | 0.02 | .90 | 1.03 |
| High cholesterol | .25 | .12 | 4.12 | 0.04 | 1.28 | 0.04 | .27 | 0.02 | .90 | 1.04 |
| Diabetes | .21 | .16 | 1.68 | .20 | 1.23 | −0.12 | .38 | 0.10 | .76 | 0.89 |
Note. ACE = adverse childhood experience; OR = odds ratio.
Figure 1.
Percentage of participants with obesity, physical problems, or diagnoses of high cholesterol in the three adverse childhood experience (ACE) groups (no ACEs, one or two ACEs, three or more ACEs).
Logistic regression analysis was performed in order to examine whether exposure to ACEs was related to physical problems and diagnoses of diabetes, high blood pressure, and high cholesterol. We found that exposure to one or two ACEs and three or more ACEs was significantly associated with increased likelihood of reporting physical problems, model χ2(9) = 487.54, p < .001, Nagelkerke R2 = .17; see Table 4). Of the participants, 67.90% with one or two ACEs and 84.03% of participants with three or more ACEs reported physical problems, whereas 55.21% of participants without ACEs reported physical problems (see Figure 1). In addition, exposure to one or two ACEs, but not three or more ACEs, was significantly associated with increased likelihood of high cholesterol, model χ2(9) = 456.62, p < .001, Nagelkerke R2 = .23. As shown in Figure 1, 14.05% of participants with one or two ACEs had high cholesterol compared to 12.01% of participants without ACEs. No significant associations between ACEs and high blood pressure and diabetes were found (see Table 4).
ACE and Accelerated Aging
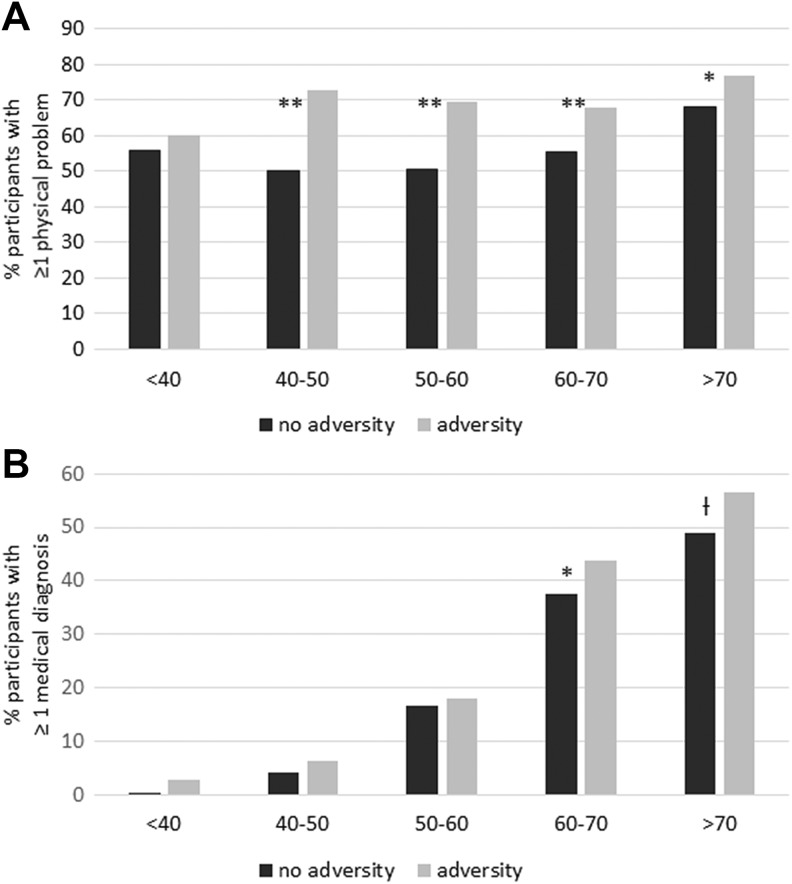
Logistic regression analysis with physical problems as outcome variable showed that there was a significant interaction between ACEs and age (one or two ACEs: B = −.01, SE = .01, Wald = 4.48, p = .03, three or more ACEs: B =.00, SE = .02, Wald = 0.01, p = .91). The experience of one or two ACEs interacted significantly with the effect of age. A significant interaction between one or two ACEs and three or more ACEs and age was found in the analysis with medical diagnosis (high blood pressure, diabetes, or high cholesterol) as dependent variable (one or two ACEs: B = .02, SE = .01, Wald = 4.43, p = .04, three or more ACEs: B = .05, SE = .02, Wald = 4.38, p = .04). Age was recoded into five categories (<40, 40–50, 50–60, 60–70, and >70 years) in order to interpret interaction effects on physical health. Figure 2 presents the percentages of participants reporting one or more physical problems or one or more medical diagnoses for each age category separately for individuals without ACEs or one or more ACEs. Individuals with ACEs more often report physical problems or a medical diagnosis at younger ages than individuals without ACEs, indicating that ACEs accelerate the effect of age on physical health.
Figure 2.
Percentage of participants with and without adverse childhood experiences (ACEs; 0 vs. ≥1 ACEs) reporting one or more medical diagnoses (high blood pressure, high cholesterol, or diabetes) and one or more physical problems for the age categories <40, 40–50, 50–60, 60–70, and >70 years.
Age of Exposure to ACE
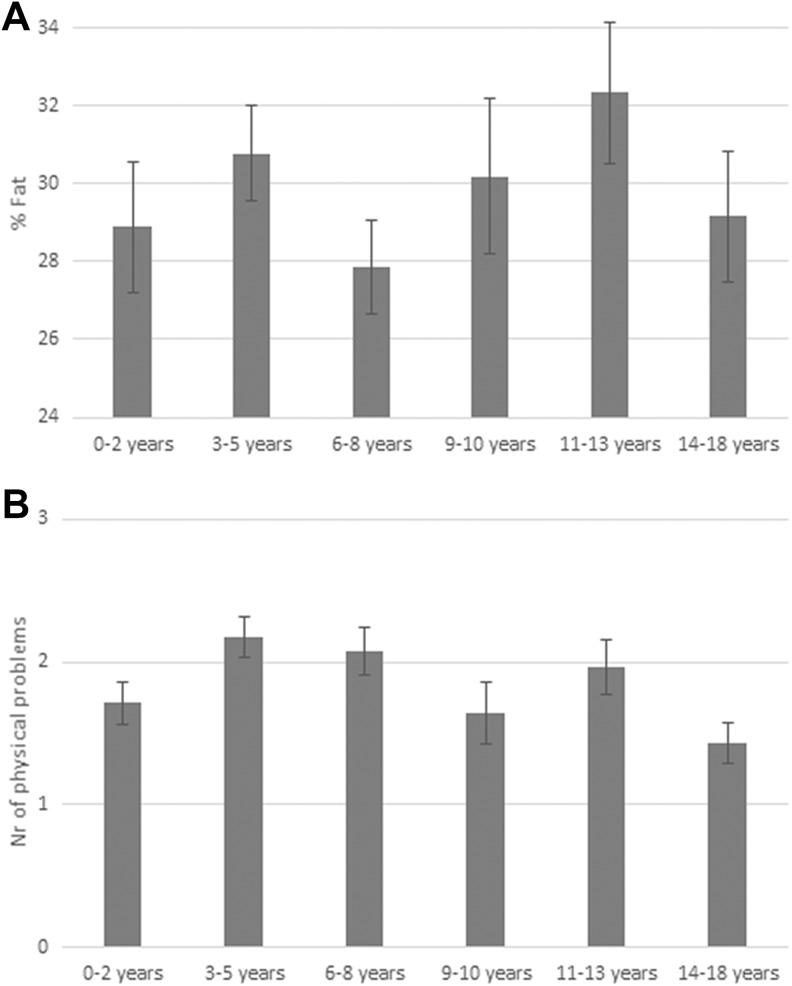
The role of developmental timing of ACEs during childhood was examined. ANOVAs with BMI and fat as dependent variables showed that age at exposure to ACEs was significantly related to fat percentage, F(5, 159) = 3.04, p < .05, partial η2 = .09, but not to BMI, F(5, 159) = 1.44, p = .21, partial η2 = .04. Individuals with ACE during puberty (aged 11–13 years) had the highest fat percentage (see Figure 3).
Figure 3.
(A) Fat percentage for individuals with adverse childhood experiences (ACEs) during different age stages infancy (0–2 years, n = 27): preschool (3–5 years, n = 41), latency (6–8 years, n = 36), prepubertal (9–10 years, n = 19), pubertal (11–13 years, n = 24), and adolescent (14–18 years, n = 25). (B) Mean number of physical problems reported by individuals with ACEs during different age stages infancy (0–2 years, n = 104), preschool (3–5 years, n = 153), latency (6–8 years, n = 121), prepubertal (9–10 years, n = 67), pubertal (11–13 years, n = 96), and adolescent (14–18 years, n = 130).
Logistic regression was performed in order to examine whether age at exposure to ACEs was associated with likelihood of having physical problems, diabetes, high cholesterol, or being overweight or obese. Age at exposure was significantly associated with having physical problems, Wald = 4.46, OR = 0.96, p < .05, model χ2(9) = 70.60, p < .001, Nagelkerke R2 = .15. Individuals who were exposed to ACEs at ages 3–5 years, 6–8 years, and 11–13 years reported the highest number of physical problems (see Figure 3). No significant association was found between age at exposure and diabetes (Wald = 1.53, OR = 1.04, p = .22), high cholesterol (Wald = 0.67, OR = 1.02, p = .41), and obesity (Wald = 1.74, OR = 1.06, p = .19).
Discussion
In the current study, we examined the relation between retrospectively reported ACEs and physical health in adulthood. We found that individuals with ACEs reported more physical problems, showed a higher BMI and fat percentage, were at increased risk of obesity, and were more likely to be diagnosed with high cholesterol. Consistent with previous ACE studies (Felitti et al., 1998; Friedman et al., 2015), we found that quantity of ACE matters for adults’ physical functioning. A graded relationship between the number of adversities and physical health was found, with a higher prevalence of being overweight or having physical problems when the number of ACEs increased. Individuals with three or more types of ACEs, but not individuals with only one or two ACEs, showed a significantly higher BMI and fat percentage and were at increased risk of obesity compared to individuals without ACEs. This is consistent with previous research showing a strong dose–response relationship between the quantity of ACEs and multiple health risk factors, disease, and even leading causes of death in adults (Felitti et al., 1998).
In addition, our findings indicate that the association between ACEs and health is dependent on the timing of exposure to ACEs. We found that in particular exposure to ACEs at ages 11–13 years was associated with a higher fat percentage. Exposure to ACEs at ages 11–13 years and 3–8 years was strongly associated with the experience of physical problems in adulthood. Thus, middle childhood and early adolescence may be sensitive periods, such that exposures to ACEs during these periods may be particularly related to poorer adult health. Previous research indicates that adolescents are more likely to engage in problematic behaviors in response to adverse experiences than younger children (Thornberry et al., 2001). In addition, they have more access to maladaptive coping strategies like unhealthy eating behaviors or drug or alcohol abuse (Ireland et al., 2002), which may explain increased risk of poor health in adulthood. However, timing of exposure to ACEs was not related to diagnoses of high cholesterol, diabetes, or obesity. This is consistent with a previous study showing that timing of ACEs mattered only for risk of heart disease, with increased risk of adults with exposures at ages 6–10 and 15–17 years, but not for risk of diabetes and obesity, possibly because the physiological systems related to these conditions may be more malleable throughout life (Friedman et al., 2015). Thus, the role of timing of retrospectively reported ACEs may not be the same for different aspects of physical health.
Our findings extend previous research examining the association between ACEs and increased risk of age-related diseases by showing that age-related diseases and physical problems are not only more prevalent in individuals with ACEs but also appear at earlier ages. To our knowledge, only one previous study showed some tentative support for an association between early adversity and earlier age of onset of diseases. McCrory, Dooley, Layte, and Kenny (2015) reported a trend toward earlier onset of physical and psychiatric diseases in individuals with experiences of early adversity. Our finding that age-related diseases are more prevalent in individuals with ACEs is in line with a study showing that ACE is associated with a stronger age-related decline in general markers of health, that is, increased disability, and reduced quality of life and cognitive functioning (Shrira, 2014).
One limitation of the current study is that ACEs were measured retrospectively with self-report questionnaires. This limits inferences about causality and may have led to underreport of ACE, in particular in the category 0–3 years, because adults might be less likely to recall events during their infancy. Moreover, we did not control for duration of adverse experience in the present study. Since adverse experience that span a longer period of time may be more likely to be remembered, in particular in early infancy, we cannot rule out the possibility that commencement was confounded with duration of adversity. Future studies on the effects of adversity should either use structured interviews (Davis et al., 2014), data from child protective services records, or longitudinal designs. Data on high blood pressure, cholesterol, and diabetes were also based on self-report. This may explain the unexpected finding that exposure to one or two ACEs, but not three or more ACEs, was related to high cholesterol. Participants were asked to indicate whether they were diagnosed with high cholesterol by a physician, but no cholesterol levels were measured. Studies comparing self-reported and objectively measured weight find that BMI is often underreported (Gorber, Tremblay, Moher, & Gorber, 2007). Similarly, individuals may underreport other aspects of poor health or may be unaware of abnormal cholesterol levels if they do not seek medical care. Self-reports on diagnoses by a physician may be influenced by medical care seeking behavior or avoidance, which depends on individual characteristics (Kannan & Veazie, 2014) and may also be related to childhood experiences. It is, therefore, important to use direct measurements of physical health. In the current study, direct measurements of body mass were sent to the panel’s central server using a wireless weighing scale, thereby giving reliable and objective information of participants’ BMI and fat percentage. Another limitation is that the number of medical conditions was short and was assessed only in reference to last year. Lastly, we did not control for influences of experiences of more recent trauma in adulthood. This can be considered a limitation, since a previous study showed that adverse experiences in adulthood may be a more important predictor for adult health than adverse experiences during childhood (Krause, Shaw, & Cairney, 2004).
In conclusion, in the current study, we examined the relation between ACEs and various aspects of physical health, with a particular focus on the role of timing of exposure to ACEs, quantity of ACEs, and associations with age-related diseases. Consistent with previous research, our findings confirm that there is a dose–response relationship between the number of ACEs during childhood and medical conditions and that timing of exposure to ACEs matters for some health outcomes. Future studies should examine influences of timing and quantity of ACEs and underlying mechanisms because these factors may also matter in interventions to reduce or prevent the protracted consequences of adversity. Our findings seem to indicate that experiences of childhood adversity during middle childhood and early adolescence are particularly disruptive, possibly due to transformations in relationships with attachment relationships with caregivers (Allen & Tan, 2016). Future studies should therefore examine the consequences of ACEs in adolescents, for example, by studying underlying neurobiology. Moreover, our study extends previous ACE studies by showing that age-related diseases are not only more prevalent in individuals with ACEs but also appear at earlier ages. This may have implications for prevention and intervention programs as higher prevalence and earlier disease onset motivates the identification of individuals with ACEs as risk groups for poor health. Given the profound associations between ACEs and adult health, policies targeted at reducing exposure to ACEs could have substantial health benefits.
Supplemental Material
Supplementary_material for Childhood Adversity and Adult Health: The Role of Developmental Timing and Associations With Accelerated Aging by Madelon M. E. Riem, and Annemiek Karreman in Child Maltreatment
Footnotes
Authors’ Note: In this article, we make use of data of the Longitudinal Internet Studies for the Social Science (LISS) panel administered by CentERdata (Tilburg University, the Netherlands).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- Allen J. P., Tan J. S. (2016). The multiple facets of attachment in adolescence In Cassidy J., Shaver P. R. (Eds.), Handbook of attachment (pp. 399–415). New York, NY: Guilford Press. [Google Scholar]
- Anda R. F., Brown D. W., Dube S. R., Bremner J. D., Felitti V. J., Giles W. H. (2008). Adverse childhood experiences and chronic obstructive pulmonary disease in adults. American Journal of Preventive Medicine, 34, 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda R. F., Dong M., Brown D. W., Felitti V. J., Giles W. H., Perry G. S.…Dube S. R. (2009). The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health, 9, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. L., Tomada A., Vincow E. S., Valente E., Polcari A., Teicher M. H. (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. Journal of Neuropsychiatry and Clinical Neuroscience, 20, 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch N. M., Riese H., Reijneveld S. A., Bakker M. P., Verhulst F. C., Ormel J., Oldehinkel A. J. (2012). Timing matters: Long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study. Psychoneuroendocrinology, 37, 1439–1447. [DOI] [PubMed] [Google Scholar]
- Burke N. J., Hellman J. L., Scott B. G., Weems C. F., Carrion V. G. (2011). The impact of adverse childhood experiences on an urban pediatric population. Child Abuse & Neglect, 35, 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D. P., Whitfield C. L., Felitti V. J., Dube S. R., Edwards V. J., Anda R. F. (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82, 217–225. [DOI] [PubMed] [Google Scholar]
- Danese A., Tan M. (2014). Childhood maltreatment and obesity: Systematic review and meta-analysis. Molecular Psychiatry, 19, 544–554. [DOI] [PubMed] [Google Scholar]
- Davis C. R., Dearing E., Usher N., Trifiletti S., Zaichenko L., Ollen E.…Crowell J. A. (2014). Detailed assessments of childhood adversity enhance prediction of central obesity independent of gender, race, adult psychosocial risk and health behaviors. Metabolism: Clinical and Experimental, 63, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S. S., Theall K., Gleason M. M., Smyke A. T., De Vivo I., Wong J. Y. Y.…Nelson C. A. (2011). Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Molecular Psychiatry, 17, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S. R., Fairweather D., Pearson W. S., Felitti V. J., Anda R. F., Croft J. B. (2009). Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine, 71, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V. J. (2002). The relationship of adverse childhood experiences to adult health: Turning gold into lead. Zeitschrift fur Psychosomomatische Medizin und Psychotherapie, 48, 359–369. [DOI] [PubMed] [Google Scholar]
- Felitti V. J., Anda R. F., Nordenberg D., Williamson D. F., Spitz A. M., Edwards V.…Marks J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick A. L., Kronmal R. A., Gardner J. P., Psaty B. M., Jenny N. S., Tracy R. P.…Aviv A. (2007). Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology, 165, 14–21. [DOI] [PubMed] [Google Scholar]
- Friedman E. M., Montez J. K., Sheehan C. M., Guenewald T. L., Seeman T. E. (2015). Childhood adversities and adult cardiometabolic health: Does the quantity, timing, and type of adversity matter? Journal of Aging and Health, 27, 1311–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M., Slavich G. M., Rudolph K. D., Hastings P. D., Nock M. K., Prinstein M. J. (2018). Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. Journal of Child Psychology and Psychiatry, 59, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorber S. C., Tremblay M., Moher D., Gorber B. (2007). A comparison of direct vs. self-report measures for assessing height, weight and body mass index: A systematic review. Obesity Reviews, 8, 307–326. [DOI] [PubMed] [Google Scholar]
- Halonen J. I., Stenholm S., Pentti J., Kawachi I., Subramanian S., Kivimäki M., Vahtera J. (2015). Childhood psychosocial adversity and adult neighborhood disadvantage as predictors of cardiovascular disease: A cohort study. Circulation, 132, 371–379. [DOI] [PubMed] [Google Scholar]
- Ireland T. O., Smith C. A., Thornberry T. P. (2002). Developmental issues in the impact of child maltreatment on later delinquency and drug use. Criminology, 40, 359–400. [Google Scholar]
- Kalmijn M. (2013). Adult children’s relationships with married parents, divorced parents, and stepparents: Biology, marriage, or residence? Journal of Marriage and Family, 75, 1181–1193. [Google Scholar]
- Kannan V. D., Veazie P. J. (2014). Predictors of avoiding medical care and reasons for avoidance behavior. Medical Care, 52, 336–345. [DOI] [PubMed] [Google Scholar]
- Kessler R. C., Amminger G. P., Aguilar-Gaxiola S., Alonso J., Lee S., Ustun T. B. (2007). Age of onset of mental disorders: A review of recent literature. Current Opinion in Psychiatry, 20, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooreman P., Scherpenzeel A. (2014). High frequency body mass measurement, feedback, and health behaviors. Economics & Human Biology, 14, 141–153. [DOI] [PubMed] [Google Scholar]
- Krause N., Shaw B. A., Cairney J. (2004). A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychology and Aging, 19, 637–648. [DOI] [PubMed] [Google Scholar]
- Luecken L. J., Jewell S. L., MacKinnon D. P. (2016). Prediction of postpartum weight in low-income Mexican-origin women from childhood experiences of abuse and family conflict. Psychosomatic Medicine, 78, 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A. D. (2016). Developmental timing of trauma exposure relative to puberty and the nature of psychopathology among adolescent girls. Journal of the American Academy of Child & Adolescent Psychiatry, 55, 25–32.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersky J. P., Janczewski C. E., Topitzes J. (2017). Rethinking the measurement of adversity: Moving toward second-generation research on adverse childhood experiences. Child Maltreatment, 22, 58–68. [DOI] [PubMed] [Google Scholar]
- McCrory C., Dooley C., Layte R., Kenny R. A. (2015). The lasting legacy of childhood adversity for disease risk in later life. Health Psychology, 34, 687–696. [DOI] [PubMed] [Google Scholar]
- Meeuwsen S., Horgan G. W., Elia M. (2010). The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clinical Nutrition, 29, 560–566. [DOI] [PubMed] [Google Scholar]
- Ogle C. M., Rubin D. C., Siegler I. C. (2013). The impact of the developmental timing of trauma exposure on PTSD symptoms and psychosocial functioning among older adults. Developmental Psychology, 49, 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudejans M., Kalmijn M. (2013). Life History Questionnaire. Tilburg, the Netherlands: CentERdata. [Google Scholar]
- Puterman E., Gemmill A., Karasek D., Weir D., Adler N. E., Prather A. A., Epel E. S. (2016). Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study. Proceedings of the National Academy of Sciences, 113, E6335–E6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout K. K., Levandowski M., Ridout S. J., Gantz L., Goonan K., Palermo D.…Tyrka A. R. (2018). Early life adversity and telomere length: A meta-analysis. Molecular Psychiatry, 23, 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem M. M., Alink L. R., Out D., Van Ijzendoorn M. H., Bakermans-Kranenburg M. J. (2015). Beating the brain about abuse: Empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Development and Psychopathology, 27, 507–520. [DOI] [PubMed] [Google Scholar]
- Shrira A. (2014). Greater age-related decline in markers of physical, mental and cognitive health among Israeli older adults exposed to lifetime cumulative adversity. Aging & Mental Health, 18, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N., Koenen K. C., Kubzansky L. D. (2012). Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: A systematic review. Brain, Behavior, and Immunity, 26, 239–250. [DOI] [PubMed] [Google Scholar]
- Thomas C., Hyppönen E., Power C. (2008). Obesity and type 2 diabetes risk in midadult life: The role of childhood adversity. Pediatrics, 121, e1240–e1249. [DOI] [PubMed] [Google Scholar]
- Thornberry T. P., Ireland T. O., Smith C. A. (2001). The importance of timing: The varying impact of childhood and adolescent maltreatment on multiple problem outcomes. Development and Psychopathology, 13, 957–979. [PubMed] [Google Scholar]
- Tyrka A. R., Price L. H., Kao H.-T., Porton B., Marsella S. A., Carpenter L. L. (2010). Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biological Psychiatry, 67, 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_material for Childhood Adversity and Adult Health: The Role of Developmental Timing and Associations With Accelerated Aging by Madelon M. E. Riem, and Annemiek Karreman in Child Maltreatment