Abstract
T cells are known as the most potent killer cells of the immune system, designed by nature to prevent unwanted challenges. The first class of therapeutic products harnessing the power of T cells for target-specific treatment of oncological diseases was bispecific antibodies. The first T-cell engaging bispecific antibodies that obtained approval were catumaxomab and blinatumomab1,2. Eight years later, the first chimeric antigen receptor (CAR)-T cells received regulatory approval3. CAR-T cells are the cellular interpretation of T-cell engaging therapies and have shown remarkable clinical results. CAR-T cells belong to the regulatory group of advanced therapy medicinal products (ATMPs). Due to the cell-/gene-based complex nature, ATMPs are far more challenging to develop than other, more defined, medicinal products. Despite very encouraging clinical results, there have been many set-backs in the development of ATMPs during the past 20 years. Therefore, the approval of the first two CAR-Ts KYMRIAH and YESCARTA is highly encouraging for the field. In this article we review the current landscape of CAR-Ts as a special class of ATMPs. This comprises the pathway to approval including the use of dedicated regulatory tools and challenges that were faced during the procedure. Furthermore, we highlight important future trends in the field.
Keywords: CAR-T, PRIME, ATMP, RMAT, genome editing, regulatory strategy
Current Landscape
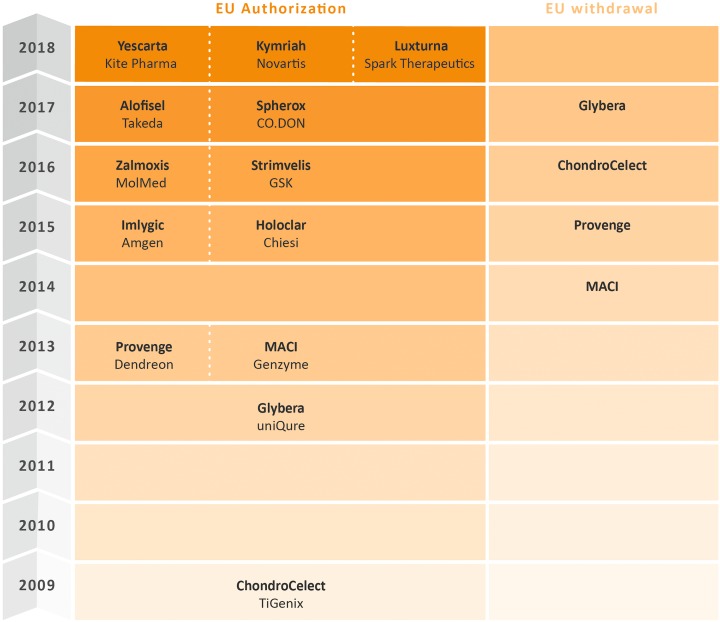
Advanced therapy medicinal products (ATMPs) or cell and gene therapy products (CGTPs), as they are called in the US, bear game-changing potential for the treatment of severe conditions for which we have no appropriate therapies today. Several ATMPs have been licensed over the last decade in the European Union (EU) (as shown in Figure 1) as well as in the United States (US), and the number of ATMPs reaching the market is expected to grow over the next decade1. Table 1 provides an overview of all products shown in Figure 1 including their indications. A corresponding overview of approved cell- and gene therapy products in the US is provided in Table 2.
Figure 1.
Overview of the status of ATMP and CAR-T products approved in the EU, as of October 2018.
Table 1.
Licensed or Withdrawn ATMP and CAR-T Products Including their Indications in Europe, Sorted by Product Name.
| Product Name | Marketing Authorization Holder | Indication |
|---|---|---|
| Alofisel | Takeda | Treatment of perianal fistulas in Crohn’s disease |
| ChondroCelect | TiGenix | Ex-vivo expanded, autologous chondrocytes |
| Glybera | uniQure | Treatment of lipoprotein lipase deficiency |
| Holoclar | Chiesi | Regeneration of cornea stem cells |
| Imlygic | Amgen | Treatment of malignant melanoma |
| Kymriah | Novartis | Treatment of ALL and DLBCL |
| Luxturna | Spark Therapeutics | Treatment of retinal dystrophies caused by RPE65 mutations |
| MACI | Genzyme | Autologous cultured chondrocytes |
| Provenge | Dendreon | Autologous immunotherapy for prostate cancer |
| Spherox | CO.DON | Treatment of articular cartilage defects |
| Strimvelis | GSK | ADA-SCID |
| Yescarta | Kite Pharma | Treatment of DLBCL and PMBCL |
| Zalmoxis | MolMed | T-Cell modification |
Table 2.
Overview of Approved Cell- and Gene Therapy Products in the US, as of July 2018.
| Product | Company | Description | Indication / Use | Date and special approval type as applicable |
|---|---|---|---|---|
| HPC, Cord Blood | MD Anderson Cord Blood Bank | Allogeneic cord blood HPC therapy | Unrelated donor HCT | June 21, 2018 |
| KYMRIAH | Novartis Pharmaceuticals Corporation | tisagenlecleucel, CD-19-directed chimeric antigen receptor (CAR) T cell therapy | Treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma and DLBCL arising from follicular lymphoma | May 1, 2018 |
| KYMRIAH | Novartis Pharmaceuticals Corporation | tisagenlecleucel, CD-19-directed chimeric antigen receptor (CAR) T-cell therapy | Treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse | Aug 30, 2017 Accelerated Approval |
| LUXTURNA | Spark Therapeutics, Inc. | voretigene neparvovec-rzyl, adeno-associated virus vector-based gene therapy | Treatment of patients with confirmed biallelic RPE65 mutation-associated retinal dystrophy | Dec 19, 2017 |
| YESCARTA | Kite Pharma, Inc. | axicabtagene ciloleucel, CD-19-directed CAR-T cell therapy | Treatment of diffuse large B-cell lymphoma | Oct 18, 2017 |
| MACI | Vericel Corporation | Autologous cultured chondrocytes on a porcine collagen Membrane | Cartilage defects | Dec 13, 2016 |
| CLEVELORD | Cleveland Cord Blood Center | Allogeneic cord blood HPC therapy | Unrelated donor HCT | Sep 1, 2016 |
| HPC, Cord Blood- Bloodworks | Bloodworks | Allogeneic cord blood HPC therapy | Unrelated donor HCT | Jan 28, 2016 |
| IMLYGIC | BioVex, Inc. | Genetically modified oncolytic viral therapy | Local treatment of nodal lesions in melanoma patients | Oct 27, 2015 |
| HPC, Cord Blood- Life South | LifeSouth Community Blood Centers, Inc. | Allogeneic cord blood HPC therapy | Unrelated donor HCT | Jun 13, 2013 |
| ALLOCORD | SSM Cardinal Glennon Children’s Medical Center | Allogeneic cord blood hematopoietic progenitor cell (HPC) therapy | Unrelated donor hematopoietic progenitor cell transplantation (HPCT) | May 30, 2013 |
| DUCORD | Duke University School of Medicine | Allogeneic cord blood HPC therapy | Unrelated donor HCT | Oct 4, 2012 |
| HPC, Cord Blood | Clinimmune Labs, University of Colorado Cord Blood Bank | Allogeneic cord blood HPC therapy | Unrelated donor HCT | May 24, 2012 |
| GINTUIT | Organogenesis Inc. | Allogeneic cultured keratinocytes and fibroblasts in bovine collagen | Treatment of mucogingival conditions | Mar 9, 2012 |
| HEMACORD | New York Blood Center, Inc. | Allogeneic cord blood HPC therapy | Unrelated donor HCT | Nov 10, 2011 |
| LAVIV | Fibrocell Technologies, Inc. | Azficel-T, autologous cellular product | Improvement of severe nasolabial fold wrinkles | Jun 21, 2011 |
| PROVENGE | Dendreon Corporation | Sipuleucel-T, autologous cellular immunotherapy | Treatment of prostate cancer | Apr 29, 2010 |
HPC: Hematopoietic progenitor cell; HPCT: Hematopoietic progenitor cell transplantation; HCT: Hematopoietic cell transplantation; ALL: Acute lymphoblastic leukemia; CAR: Chimeric antigen receptor.
Adoptive T-cell transfer (ACT), a subclass of ATMPs, is a new chapter of transfusion medicine. Antitumor, antiviral, or anti-inflammatory effects are mediated by infusion of lymphocytes. It has been a rapid development from a promising form of immuno-oncology in preclinical models to the recent marketing approvals of chimeric antigen receptor (CAR)-T cells for treatment of leukemia and lymphoma4. CAR-T cells have been shown to be one of the most promising therapeutic approaches for treatment of pediatric and young adult refractory hematologic B-cell malignancies as well as adult relapsed or refractory large B-cell lymphomas. The design of CAR-Ts has evolved substantially over the years. Features such as the co-expression of costimulatory molecules, cytokines, and suicide genes are incorporated to further improve efficacy and safety. The tumor targets for CAR-T cells have expanded from CD19 to a great range of further targets, including but not limited to CD22, CD30, CD33, CD138, CD171, CEA, epidermal growth factor receptor, EFGRvIII, ErbB, FAP, GD2, Glypican 3, Her 2, Mesothelin, and NKG2D5. Adoptive CAR-T cell therapy will hopefully prove to be as effective in solid tumors as in onco-hematological indications. This field is booming with significant investments. The ability to reprogram our own immune system to fight cancer has certainly created huge expectations. In 2017, the first two CAR-T therapies have been approved in the US—KYMRIAH, from Novartis and YESCARTA from Gilead / Kite Pharma6. In 2018 both products received also EU approval7.
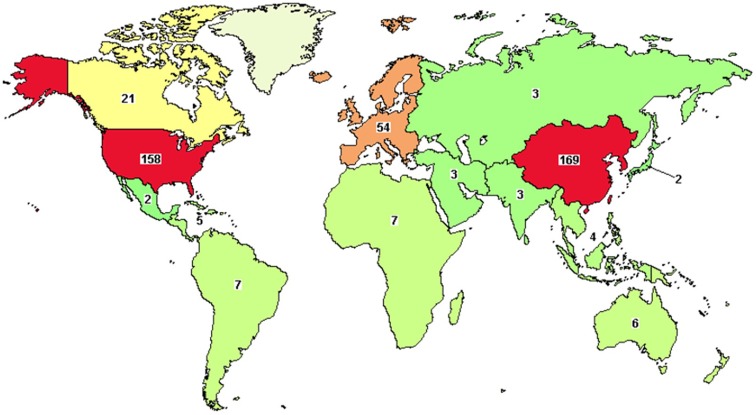
This will, of course, further fuel the already booming scene, in which many CAR-T based products are already in advanced stages of development. There are currently over 400 CAR-T clinical trials in various stages ongoing, many of them targeting the CD19 antigen8. Figure 2 shows an overview of clinical trials involving CAR-T products worldwide.
Figure 2.
Overview of clinical trials with CAR-T products worldwide, as of September 2018, based on data from clinicaltrials.gov.
CAR-Ts have shown huge remission rates in clinical trials, up to 94%, in severe types of pediatric and young adult refractory hematologic B-cell malignancies. This is remarkable, as most CAR-T clinical trials recruit cancer patients that have not responded to various other available treatments6.
Although CAR-Ts have a great potential, there are also some downsides to this approach. In fact, CAR-T cells have been linked to severe side effects, such as neurotoxicity and cytokine release syndrome9. In 2016, several companies reported multiple death cases in late-stage clinical trials with CAR-T therapy10. This emphasized that this technology still needs improvements.
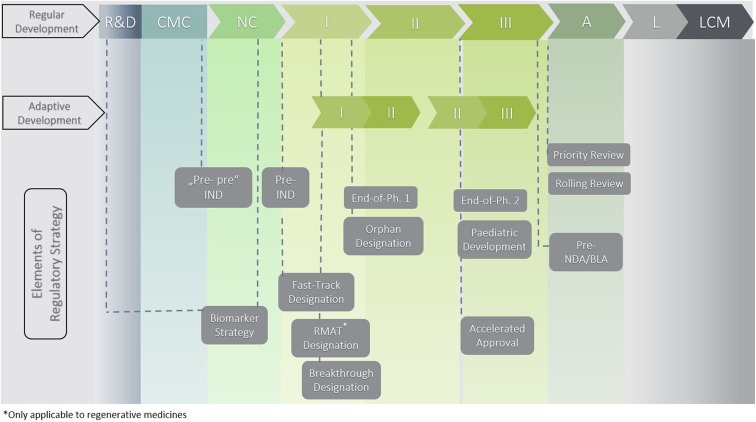
However, not only clinical challenges but also issues in development, manufacturing, and commercialization must be overcome in order to further enhance this technology. Compared with other product classes where issues are, in the majority of cases, in the clinical part of the dossier, ATMPs show shortcomings in all parts of the dossier, from product quality to the non-clinical program up to the clinical program and data. This leads to a high number of objections, including major objections during the evaluation of the Marketing Authorization Application. In order to respond to regulators’ questions and to address major objections, long clock stops are often needed during the evaluation procedure. For some products and programs, the issues are so severe that they cannot be sufficiently addressed during the procedure. As a consequence, applicants either withdraw their application or regulators issue a negative opinion11. An overview on the existing Food and Drug Administration (FDA) regulatory pathways and tools including the strategic use thereof is presented in Figure 3.
Figure 3.
Regulatory tools to consider and integrate into overall US development strategy. Abbreviations: A, approval/authorization; CMC, chemistry manufacturing and control development; I, Phase I clinical development; II, Phase II clinical development; III, Phase III clinical development; L, launch; LCM, life cycle management; NC, non-clinical development; R&D, research and development.
The Development Program of the Approved CAR-T Products
Though Novartis and Kite pursued similar strategies in the development of their CAR-T products, there were also substantial differences. In the following we outline and compare the labeled indications and the development programs including encountered challenges for the two licensed CAR-T products, KYMRIAH and YESCARTA. Table 3 compares the approved labels for both products in the EU and the US.
Table 3.
| Approved Labels | |||
|---|---|---|---|
| KYMRIAH | YESCARTA | ||
| Indication | EU | Pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant or in second or later relapse. | -- |
| Adult patients with relapsed/ refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy | Adult patients with relapsed/ refractory DLBCL | ||
| Primary mediastinal B-cell lymphoma (PMBCL) | |||
| US | Patients up to 25 years of age with B-cell precursor ALL that is refractory or in second or later relapse | ||
| Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high-grade B-cell lymphoma and DLBCL arising from follicular lymphoma | Adult patients with relapsed or refractory large DLBCL after two or more lines of systemic therapy, including DLBCL not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma | ||
| Dosage | EU |
Pediatric and young adult
B-cell ALL patients 50 kg and below: 0.2 to 5 × 106 CAR-positive viable T cells/kg body weight above 50 kg: 0.1 to 2.5 × 108 CAR-positive viable T cells (non-weight based) |
A single dose contains 2 × 106 CAR-positive viable T cells per kg of body weight (or maximum of 2 × 108 CAR-positive viable T cells for patients 100 kg and above) |
|
Pediatric and Young Adult B-cell ALL patients
0.6 to 6 × 108 CAR-positive viable T cells (non-weight based). |
|||
| US |
Pediatric and Young Adult B-cell ALL (up to 25 years of age) Patients 50 kg or less: 0.2 to 5.0 × 106 CAR-positive viable T cells per kg body weight Patients above 50 kg: 0.1 to 2.5 × 108 CAR-positive viable T cells |
||
|
Adult r/r DLBCL
0.6 to 6.0 × 108 CAR-positive viable T cells |
Adult r/r DLBCL
2 × 106 CAR-positive viable T cells per kg body weight, 2 × 108 CAR-positive viable T cells (total maximum) |
||
ALL: Acute lymphoblastic leukemia; DLBCL: Diffuse large B-Cell lymphoma; TFL: Transformed follicular lymphoma; ASCT: Autologous stem cell transplant; LBCL: Large B-cell lymphoma; CAR: Chimeric antigen receptor.
In the following we compare the main steps in development of both products, including challenges that were faced. Information on both products was obtained from their Summary Basis for Regulatory Action and Summary of Product Characteristics—KYMRIAH12,13, YESCARTA14,15.
KYMRIAH
Chemistry, Manufacturing and Controls (CMC)
KYMRIAH (tisagenlecleucel) is composed of autologous T cells that are genetically modified with a lentiviral vector encoding a CAR. The CAR specifically recognizes the CD19 protein present on CD19+ B lineage tumor cells as well as normal B cells13.The manufacturing process comprises receipt of the patient’s white blood cells, stimulation of enriched T cells, and transduction with the lentiviral vector. After expansion of the CAR-expressing autologous T cells, they are washed and formulated with infusion media for cryopreservation. The chain of identity of the entire process from leukapheresis to infusion and throughout all manufacturing steps is controlled by a computer-based system to ensure the product’s identity and product traceability. KYMRIAH manufacturing has experienced an approximately 9% failure rate so far.
Topics of concern for the FDA during review were replication competent lentivirus (RCL)—but to date, no RCL has been detected in any clinical trials using a lentiviral vector-transduced cell product—and insertional mutagenesis, which has been addressed through vector design and a limited copy number per cell13.
After a pre-license inspection (PLI) at Novartis Pharmaceuticals Corporation in April 2017 for the manufacture of KYMRIAH, the FDA issued a Form 483, which summarizes critical findings during an inspection.
After a PLI at a contract manufacturing organization (CMO) involved in the manufacturing of the lentiviral vector, the FDA issued a Form 483. Also, for the CMO involved in the sterilization, concentration, and filling of the lentiviral vector, the FDA issued a Form 483.
All three companies responded to the observations, and the corrective actions were reviewed and found to be acceptable. All inspectional issues were considered to be satisfactorily resolved13.
Non-Clinical
Key non-clinical studies conducted for KYMRIAH included:
evaluation of the specificity of the CD19-binding domain using a human plasma membrane protein array,
assessment of in vivo antitumor activity of KYMRIAH in mouse xenograft tumor models,
evaluation of selected toxicology parameters, cell distribution, and persistence of KYMRIAH in tumor-bearing mice, and
genomic insertion site analysis of lentiviral integration into the human13.
Clinical
In summary, Novartis to date has conducted four Phase II trials and further planned one Phase I trial, four Phase II trials, including long-term follow-up and managed access program, as well as two Phase III trials, including event-free survival8. The Clinical Program study B2202 (ELIANA, NCT02228096)16 provided the basis for the biologics license application (BLA) submission for a regular approval for KYMRIAH17. B2202 evaluated the safety and effectiveness for the treatment of pediatric and young adult patients with second or later relapse or primary refractory B-cell precursor acute lymphoblastic leukemia (ALL). This study was conducted under a Special Protocol Assessment. Primary efficacy endpoint was the overall remission rate (ORR), which has been assessed during the 3 months after administration; ORR included complete remission (CR) and complete remission with incomplete blood count recovery (CRi), as determined by independent review committee (IRC) assessment from all manufacturing sites15.The efficacy of KYMRIAH in pediatric and young adults with r/r B-cell precursor ALL was evaluated in an open-label, multicenter single-arm trial (ELIANA, NCT02228096) and established with 63 evaluable patients on the basis of CR within 3 months after infusion, the duration of CR, and proportion of patients with CR and minimal residual disease (MRD). Among the 63 infused patients, 83% achieved CR/CRi, all of which were MRD-negative13. In the DLBCL JULIET study (NCT02445248)18 90% of the patients had a survival > 1 year; 43% showed a complete response, 33% a partial response, and 22% a stable disease8.
YESCARTA
CMC
YESCARTA (axicabtagene ciloleucel) comprises human autologous T cells transduced with a retroviral vector containing a CAR directed against human CD19. The manufacturing process comprises receipt of the patient’s white blood cells, stimulation of enriched T cells, and transduction with the retroviral vector. After expansion of the CAR-expressing autologous T cells they are washed and formulated with infusion media for cryopreservation.
A major objection from the European Medicines Agency (EMA) was that the consistency of transduction of the autologous cells had not been fully demonstrated. On the basis of the comprehensive responses and clarification provided by the applicant, together with various commitments, the issue was considered resolved14.
A topic of concern for the FDA was loss of chain of custody (COC)/chain of identity (COI). COC/COI checks were incorporated throughout the manufacturing process and before final product administration. Testing of the integrated systems used to create, control, and trace COI and COC for YESCARTA commercial manufacturing processes was included as a separate component of process validation, and it was concluded that this system was suitable for its intended purpose.
The FDA also addressed replication competent retrovirus (RCR), but to date no RCR has been detected in any clinical trials using YESCARTA, as well as insertional mutagenesis which has been addressed through vector design and a limited copy number per cell.
After a PLI at Kite Pharma in June 2017 for the manufacture of YESCARTA and at a CMO responsible for manufacturing of the retrovirus vector, the FDA issued a Form 483.
Both companies responded to the observations, and the corrective actions were reviewed and found to be acceptable. All inspectional issues were considered to be satisfactorily resolved15.
Non-Clinical
Kite Pharma performed for
Pharmacodynamics (PD): Primary PD studies, which comprised (1) comparability between NCI and Kite products, (2) CD19 expression profile summary, (3) in vitro characterization of human Anti-CD19 CAR-T Cells, and (4) in vivo studies using a murine model of lymphoma and anti-murine CD19 CAR-T Cells
Pharmacokinetics: (1) Distribution, (2) Metabolism, (3) Excretion, and (4) Pharmacokinetic drug interactions
Toxicology: (1) On-target/off-tumor toxicity of CD19 CAR-T cells and (2) an ecotoxicity/environmental risk assessment
Clinical
Clinical Program
The ZUMA-1 study formed the basis for the FDA’s review team recommendation for regular approval of YESCARTA. ZUMA-1 was a single-arm, open-label, multicenter Phase I/II study for refractory aggressive B-cell non-Hodgkin lymphoma (NHL), with a primary endpoint of ORR per investigator after a single infusion of YESCARTA preceded by cyclophosphamide/fludarabine lymphodepleting chemotherapy. In total, Kite initiated or completed four Phase I, six Phase II, and one Phase III study, and has one Phase I, one Phase II, and one Phase III studies planned.
Of 111 patients who underwent leukapheresis, 101 received YESCARTA. Efficacy was established on the basis of CR rate and duration of response, as determined by an IRC. The median time to response was 0.9 months (range: 0.8–6.2 months). The ORR was 72%, the CR rate 51% and the partial remission rate 21%15.
Regulatory Tools used for Approved CAR-T Products
The development of CAR-T cells and other cell and tissue-engineered products is frequently associated with challenges due to their complex and unique nature. Both KYMRIAH and YESCARTA have used a number of regulatory tools in order to reach the market as quickly as possible. For instance, both are the first medicines supported through EMA’s PRIority MEdicines (PRIME) scheme to receive positive opinions from the Committee for Medicinal Products for Human Use (CHMP). The voluntary scheme provides early and enhanced scientific and regulatory support to medicines that have the potential to address, to a significant extent, patients’ unmet medical needs. The early appointment of the CHMP/CAT rapporteur and a multidisciplinary group of experts allows enhanced interaction and early dialog with developers of promising medicines, to optimize development plans and speed up evaluation so these medicines can reach patients earlier. KYMRIAH was granted eligibility to PRIME on June 23, 2016, for the treatment of ALL12. YESCARTA was granted eligibility to PRIME on May 26, 2016 for the treatment of diffuse large B-cell lymphoma (DLBCL)14. According to EMA’s PRIME page there are 16 further ATMPs developed under a PRIME designation (status as of 1 August)19. KYMRIAH in addition benefited from the Pilot EMA-HTA Parallel Scientific Advice procedure (now called “Parallel Consultation with Regulators and Health Technology Assessment Bodies” (EMA-HTA Parallel Consultation) which facilitates the initiation of early dialog between medicines developers, regulators, and health technology assessment bodies to discuss and agree on a development plan that generates data that both parties can use to determine a medicine’s benefit–risk balance and value. A strong interaction between regulators and health technology assessment bodies is critical to enable innovation to reach patients, and ultimately for the benefit of public health. The inclusion of patient representatives in such EMA-HTA Parallel Consultation meetings is encouraged, and has been proven to be highly beneficial to support the approval and reimbursement process.
Both medicines were also granted “orphan designation” in the US and the EU. In the US they both also received “breakthrough therapy designation,” and KYMRIAH additionally received “rare pediatric disease” designation to potentially qualify for a Rare Pediatric Disease Priority Review Voucher. In the following Tables 4 and 5 we provide an overview of regulatory history and regulatory tools and measures applied for both products in the EU and the US (based on publicly available information).
Table 4.
Regulatory Tools and Measures Applied Pre- and Post-Authorization in the EU for KYMRIAH12 and YESCARTA14.
| KYMRIAH | YESCARTA |
|---|---|
| Pre-authorization | |
| Orphan designation | Orphan designation(s) |
| 2014 (ALL) | 2014 (DLBCL) |
| 2016 (DLBCL) | 2015 (ALL, CLL / SLL, PMBCL, FL) |
| EMA and national Scientific Advice pertained to quality, non-clinical and clinical aspects | Scientific Advice pertained to quality, non-clinical and clinical aspects |
| 2014 | 2015 (twice) |
| 2016 (twice), incl. EMA-HTA Parallel SA | 2017 (twice) |
| 2017 (twice) | |
| Pediatric Investigation Plan (PIP) | Pediatric Investigation Plan |
| 2015 (ALL) | |
| 2017 (DLBCL) | 2017 |
| PRIME designation: June 23, 2016 | PRIME designation: May 26, 2016 |
| MAA: November 02, 2017 | MAA submission: July 29, 2017 |
| positive CHMP opinion for granting a MA on June 28, 2018 | positive CHMP opinion for granting a MA on June 29, 2018 |
| MA issued August 23, 2018 | MA issued August 23, 2018 |
| Post-authorization | |
| Educational program for patients and healthcare professionals | Educational program for patients and healthcare professionals |
| Hospitals and associated centers qualified to dispense | Hospitals and associated centers qualified to dispense |
| Periodic safety update reports (PSURs) | Periodic safety update reports (PSURs) |
| 0–2 years every 6 months | 0–2 years every 6 months |
| 2–4 years annually | 2–4 years annually |
| 4 years + every 3 years | 4 years + every 3 years |
| Post-authorization efficacy study (PAES) | Post-authorization efficacy study (PAES) |
| Post-authorization safety study (PASS) | Post-authorization safety study (PASS) |
| Non-interventional regular follow-up until December 2038 | Non-interventional regular follow-up until December 2038 |
| In patients below 3 years of age annual reporting required until December 2023 | -- |
| In patients with relapsed/ refractory DLBCL by June 2022 | -- |
| Qualification of patient registry | Qualification of patient registry |
| Workshop on patient registries for CAR-T cell therapies | Workshop on patient registries for CAR-T cell therapies |
CLL: chronic lymphocytic leukemia/ SLL: small lymphocytic lymphoma; PMBCL: primary mediastinal B-cell lymphoma; FL: follicular lymphoma; MAA: Marketing authorization application; CHMP: Committee for Medicinal Products for Human Use; MA: Marketing authorization; PSURS: Periodic safety update reports; PAES: Post-authorization efficacy study; PASS: Post-authorization safety study; CAR: Chimeric antigen receptor.
Table 5.
| KYMRIAH | YESCARTA |
|---|---|
| Indication: Pediatric and young adult ALL | Indication: Adult DLBCL |
| PreIND Meeting April 2013 & March 2014 | |
| Special Protocol Assessment (SPA) March 2014 | |
| IND submission September 2014 | IND submission December 2014 |
| Rare Disease Designation September 2014 | |
| Orphan Designation ALL January 2014 | Orphan designation for |
| DLBCL March 2014 | |
| PMBCL April 2016 | |
| FL April 2016 | |
| Breakthrough Therapy Designation February 2016 | Breakthrough Therapy Designation in December 2015 for refractory, aggressive NHL |
| Pre-BLA Meeting November 2016 | Type B pre-BLA meeting October 2016 |
| BLA submission February 2017 | BLA submission (rolling submission) |
| first module December 2016 | |
| final modules March 2017 | |
| Rare Pediatric Disease Designation March 2017 | |
| BLA filed August 2017 | BLA filed October 2017 |
| PDUFA Action Due Date October 2017 | PDUFA action due date November 2017 |
| Indication: DLBCL | |
| Breakthrough Therapy Designation April 2017 | |
| Pre-sBLA meeting August 2017 | |
| Orphan Designation for DLBCL August 2017 | |
| sBLA submission for DLBCL October 2017 | |
| sBLA submission for changes in manufacture November 2017 | |
| sBLA approval for DLBCL May 2018 |
DLBCL: Diffuse large B-cell lymphoma; IND: Investigational new drug; PMBCL: primary mediastinal B-cell lymphoma; FL: follicular lymphoma; NHL: non-Hodgkin lymphoma; BLA: biologics license application; PDUFA: Prescription Drug User Fee Act.
During the regulatory process several challenges were faced. For example, after a Type B pre-BLA meeting for YESCARTA, the FDA indicated that it was premature to submit a BLA on 12/30/2016 due to <6 months follow-up for efficacy in the ZUMA-1 study and fewer than the prespecified number of subjects in the primary analysis. The FDA requested data on response and response duration after 6 months of follow-up for all subjects. The Agency agreed to a rolling submission, with a late component on the COC/COI process validation to be received within 30 days. And after BLA submission in March 2017, the FDA held a teleconference in May 2017 with Kite Pharma due to inadequate follow-up for efficacy with the pre-BLA meeting (IRC) and 1/2017 (investigator) data cuts. Alignment was reached to submit updated efficacy data by 6/30/2017, using a 4/26/2017 cut-off date for both investigator and IRC assessments15.
In March 2017, the US FDA introduced the new Regenerative Medicine Advanced Therapy (RMAT) designation, thus recognizing the enormous potential of these medicines and the need for efficient regulatory tools to accelerate their development and their commercial availability. The development of regenerative medicines is very challenging because of their complex and unique nature, especially to the rather inexperienced small- and medium-sized developing enterprises. With the new RMAT designation, the FDA aims at providing intensive support to companies developing cell- and tissue-based therapies, tissue-engineering products, and combination treatments20. The RMAT program is open to companies developing cell- and tissue-based therapies, tissue-engineering products, and combination treatments including genetically modified cells that lead to a durable modification of cells or tissues. Since the program’s introduction in March 2017, the FDA announced in July that they have already received two dozen applications for RMAT designation. As of April 2018, a total of 11 products have been granted RMAT designation, including Juno’s CAR-T cell therapy JCAR017 (recently acquired by Celgene)20. Regulatory tools, like RMAT, are precious as timely engagement with regulators can be crucial for the development process and thus provides the potential to shorten the development time. The RMAT designation should be considered as part of the integrated development and regulatory strategy for advanced therapies in the US.
RMAT has neither been applied in the development process for KYMRIAH nor for YESCARTA, most likely due to the fact that both programs were at an advanced stage of development already at the time.
In general, it is recommended to establish a robust system of data collection for the post-authorization phase that would suit the specificities of these medicines. Other important tools for the agencies are monitoring and mitigation strategies for the side effects, which are described in the product information and in the risk management plan, an integral part of the authorization, as well as the utilization of a patient registry to monitor the long-term safety and efficacy of these therapies, as a condition for the marketing authorisation7.
Challenges in the Development of CAR-T Products
In the following sections we want to give an overview about the current status, some of the challenges and also the possibilities to optimize this product class.
Manufacturing
As CAR-T cell therapy moves into later-phase clinical trials and becomes an option for more patients, compliance of the CAR-T cell manufacturing process with global regulatory requirements becomes a topic for extensive discussion. In addition, the challenges of taking a CAR-T cell manufacturing process from a single institution to a large-scale multi-site manufacturing center must be addressed. A major challenge with scaling out the production of CAR-T cell therapies is the transition from a flexible process at a single academic institution to a highly controlled process that can be implemented across many collection, manufacturing, and treatment sites. Therefore, effective coordination among the collection, manufacturing, and treatment sites involved is crucial to ensure that the material is handled correctly, and patients are appropriately scheduled throughout the therapeutic process. Success in developing a global manufacturing process of CAR-T cells will be driven by a robust understanding of both the product and the process in order to establish the target product profile and critical quality attributes5.Therefore, early process improvements are key to enable proper upscaling in order to lower cost of goods (COG) to ultimately lower drug prices, or to increase margins, and to reduce the amount of comparability testing.
Safety
Although some degree of immune stimulation and inflammation was expected with T cell activation after ACT, severe cytokine release syndrome (CRS) has been observed with CD19-specific, BCMA-specific, and CD22-specific CAR-T cells. Unexpected neurologic complications ranging in severity from mild to life-threatening have also been reported across different clinical studies with CD19- and BCMA-specific CAR-T cells. The neurologic toxicities described with CD19-specific CAR-T cells have been largely reversible4. In 2016 patients died in a clinical trial testing a CAR-T therapy from Juno Therapeutics, known as JCAR015. After the patient deaths in July 2016, when the FDA halted testing, the Agency soon allowed the clinical trial to resume under a revised protocol. But two more patients died later that year, and Juno pivoted to other products in its pipeline. The Juno trial deaths represented a serious setback for the CAR-T field at the time10. Overall, 34 deaths were reported from the time of informed consent to the data cut-off for the YESCARTA study (January 27, 2017). Thirty patients died of progressive disease and four deaths were attributed to the product as per FDA analysis. Three deaths occurred within 30 days of YESCARTA infusion. Fatal cases of CRS and neurologic toxicity have occurred after receiving YESCARTA15. Overall, 29 deaths have been reported from time of informed consent to the data cut-off of the KYMRIAH study (November 23, 2016) as submitted for the BLA. Six patients died in the failed screened population, 12 pre-infusion, awaiting manufacture of KYMRIAH (six from ALL, five from infection, and one respiratory failure), and 11 patients died post-infusion. Of the 29 deaths, two were attributable to the product and were considered by the FDA as related to CRS13. The severity of CRS seems to be proportional to the tumor burden. Although CRS is an adverse effect of CAR-T cell therapy, there may be a correlation between the development of CRS and response to therapy. Patients who are not developing CRS may be less likely to benefit from CAR-T cells, whereas those who are developing CRS often respond to the therapy. Even if there may be some correlation between developing CRS and efficacy, there does not appear to be a strong correlation between the degree of CRS and response to therapy21.
Despite the outstanding achievement of CAR-T therapy in B-cell neoplasms, a significant subset of patients (mainly chronic lymphocytic leukemia (CLL) and some NHL) does not respond initially and about 40–50% of the responding ALL patients will eventually relapse. Based on the disease behavior and phenotype, two main patterns of relapse after CART19 therapy can be recognized. The first includes relapses that occur in the presence of a weak or absent CART19 in vivo expansion and persistence: typically, these relapses are characterized by a CD19+ leukemia. In these patients, a retreatment with CART19 can be explored: Maude et al. reported that out of 50 pediatric patients, 14 were re-treated with repeated doses of CART19 and some of these patients responded22. The second form of relapse that accounts for about two-third of relapse is characterized by a novel escape mechanism where the wild-type CD19 protein is absent in leukemic blasts23. The pathogenesis is unclear, but one explanation may be pre-existing splicing variants of CD19 where cells that express CD19 isoforms lacking the CART-triggering domain are selected24. A variant of CD19-negative relapses is the myeloid lineage switch, mainly observed in MLL1 mutated ALL25. Another pattern of relapse after CART19 therapy has been reported by Ruella et al.26—a patient relapsing 9 months after CD19-targeted CAR T cell (CTL019) infusion with CD19—leukemia that aberrantly expressed the anti-CD19 CAR26. The CAR gene was unintentionally introduced into a single leukemic B cell during T-cell manufacturing, and its product bound in cis to the CD19 epitope on the surface of leukemic cells, masking it from recognition by and conferring resistance to CTL01926. The patient was in complete remission at day 28 post-CTL019 infusion. However, qPCR for routine monitoring of peripheral blood for CAR-specific sequences identified the emergence of a second expansion phase of CAR cells starting at day 252, which did not correlate with re-expansion of CAR+ T cells by flow cytometry. At day 261, the patient experienced frank relapse, as noted by abundant infiltration (>90%) of CD10+CD19– leukemic cells in the bone marrow and the presence of circulating blasts. Further immunophenotyping of this population revealed that these CAR19-expressing cells were CD3–CD10+CD22+CD45dim, indicating that they were, in fact, CAR-transduced B-cell leukemia (CARB) cells. The patient’s CARB cells continued to expand, and the patient ultimately died of complications related to progressive leukemia. Ruella et al.26 demonstrated that the transduction of a single leukemic cell with an anti-CD19 CAR lentivirus during CTL019 manufacturing is sufficient to mediate resistance through masking of the CD19 epitope. This is a rare event, as this is the only case out of 369 patients reported worldwide at the time of publication. Other possible causes of CD19-negativity, including CD19 mutations, CD19 splicing variants, and structural alterations of the B-cell receptor complex have been excluded. These findings illustrate the need for improved manufacturing technologies that can purge residual contaminating tumor cells from engineered T cells. From a regulatory perspective this means that an even closer collaboration (e.g., more frequent updates/meetings) between the agencies and the sponsors would be beneficial in order to proactively manage such events and apply learning to other development candidates of the same product category as appropriate.
COG and Pricing Considerations
The financial burdens imposed by effective but non-curative therapies that are encountered by patients with hematologic malignancies, particularly CLL and multiple myeloma, also present challenges. CLL is the most common form of leukemia in the US; about 100,000 patients were living with the disease in 2000 and, because of improved but non-curative targeted therapies such as Ibrutinib and Idelalisib, an increase to ∼200,000 cases in the US is projected. However, targeted therapies for CLL present a substantial economic burden for both patients and the economy, now estimated at a lifetime cost of $604,000 per patient, and the total cost of CLL management in the US alone is estimated to exceed $5 billion per year by 2025. It is likely that CAR-T cell therapies are more cost-effective than current standard-of-care therapies for leukemia and lymphoma4. On September 5, 2018, the National Health Service (NHS) England announced that the ground-breaking CAR-T therapy KYMRIAH for the treatment of pediatric and young adult patients up to 25 years of age with relapsed or refractory B-cell ALL is available for UK NHS patients under the first full access deal on breakthrough CAR-T therapies, at a list price of £282,000. At that time, the NHS England’s commercial deal with the manufacturer Novartis was the first in Europe, and came less than 10 days after the treatment was granted its European marketing authorization. It represents one of the fastest funding approvals in the 70-year history of the NHS. The National Institute for Health and Care Excellence (NICE) also green-lighted the treatment for entry into the reformed NHS Cancer Drugs Fund. This fast and successful outcome was based on the ELIANA study data, which showed an 83% ORR in the relapsed or refractory B-cell ALL patient population with limited treatment options and historically poor outcomes. This constructive fast-track negotiation also shows how responsible and flexible life sciences companies can succeed—in partnership with the NHS—to make revolutionary treatments available to patients27.
However, and of note, right after YESCARTA was approved in the EU for the treatment of adult patients with relapsed or refractory DLBCL, NICE on the one hand recognized that YESCARTA is a step-change in treatment for patients who have no other treatment options, but on the other hand that the drug’s price is too high to be considered a cost-effective use of the NHS resources28, given a lack of data on the long-term benefits of CAR-T treatments. In the US, YESCARTA sells for $373,000. But then in early October Gilead reached a deal with NHS England on YESCARTA, surpassing competitor KYMRIAH to become the first CAR-T therapy available to adult blood cancer patients on England’s public health system29.
Regulatory
CAR-T cell and ATMP developers should have a thorough understanding of the diverse regulatory landscape. Until harmonized regulations are established (especially around genetically modified organism (GMO)-related aspects) and there is common experience among regions, a significant level of uncertainty in product development and a greater reliance on case-by-case regulatory assessments can be expected. As an example, there are the different requirements associated with manufacturing in different regions. For instance, donor screening and testing, traceability and labeling, patient confidentiality, and apheresis requirements can vary widely among countries. This is particularly challenging if the donor starting material and final product are shipped across international borders. Another example is that the definitions of the materials used (i.e., the starting or raw material) and the requirements for quality control of these materials vary across regions. Balancing different country requirements with starting material quality requirements can be challenging, but it is crucial for multi-national trials. Therefore, the origin, traceability, composition, and certification of each reagent must be readily accessible. Lastly, as each region has unique documents and recommendations related to materials used in cell and gene therapy manufacturing, the requirements for the human or animal serum used for cell culture differ among global regions30.
Comprehensive regulatory guidance documents related to all development and regulatory aspects of ATMPs are available in the EU on EMA’s webpage31. Of note, the FDA has recently issued six new draft guidance documents related to the manufacture of ATMPs, clinical follow-up time, and orphan- and indication-specific guidance32.
Future Trends
While building on the success seen with CAR-Ts, further developments are ongoing to address issues related to manufacturability and COG, improve safety and efficacy as well as exploring additional targets and further technologies for genetic modification. Fourth-generation CARs as well as smart CARs have already entered clinical trials. These may overcome the obstacles encountered in the current trials, such as loss of targeted antigen, off-target toxicity, or low persistence.
Allogeneic CAR-Ts
One way to enhance the technology is allogeneic CAR-T or “universal” CAR-T therapy; that is, sourcing T cells from a healthy donor so that they are ready to go when the patient needs it, as opposed to engineering each patient’s T cells individually. Thereby several issues could be overcome, for example quality and quantity of patient cells for genetic modification and reduction of precious time for the patients between cell collection and administration of modified cells. Furthermore, manufacturing following an off-the-shelf rather than an individualized approach is suggested to reduce logistical complexity and COG. The challenge with allogeneic CAR-T cells is, however, how to best overcome major histocombatibility complex (MHC) barriers. In this regard, genome editing technologies offer promising strategies33.
Genome Editing
Allogeneic CAR-T cell developments are underway using different types of genome editing to either inactivate target genes or specifically insert gene sequences using a donor template. For example, Cellectis is applying editing by TALENs with the intention to inactivate the TCRalpha constant gene to eliminate the T cell receptors (TCRs) of the T-cell donor and minimize the risk of graft versus host disease34. Crispr Therapeutics is using their proprietary Crispr/Cas9 genome editing technology for generation of allogeneic CAR-Ts against various tumor targets34.
Optimized CARs and TRUCKs
While CAR-T cells have shown exciting results in leukemia, there are numerous challenges in solid tumors. One is to overcome the microenvironment and to increase the migration and infiltration of CAR-T cells into solid tumors, bone metastases, or the core of solid tumors. Another one is to overcome the immunosuppressive microenvironment encountered in solid tumors to avoid on-target-off-tumor toxicity, since most of the tumor-associated antigens are not tumor specific and might also be expressed on normal cells. It is also important to avoid tumor escape mechanism by antigen loss. While many preclinical studies for the generation of anti-sarcoma CAR-T cells are currently underway, only few clinical studies have been reported in pediatric sarcomas. An interesting approach to overcome the immunosuppressive effect of the tumor microenvironment is the use of TRUCKs. TRUCK stands for T Cell Redirected Universal Cytokine Killing, and describes the use of CAR-T cells as a vehicle to produce and release large amounts of cytokines that accumulate in the solid tumor microenvironment and induce an inflammatory immune response35. An exciting future avenue to increase long-term remission is the use of novel strategies where two or more antigens on tumor cells are targeted for preventing antigen-loss relapses36. CAR-T cells targeting additional antigens, such as CD22, CD20, or CD123, can be combined with anti-CD19 CAR in order to prevent relapse36. Lastly, also as a strategy to overcome relapse and resistance mechanisms, CAR-T therapy can also be combined with other immune enhancers, such as checkpoint inhibitors37.
Solid Tumors and Increase of Specificity
CAR-T cells fail to be as effective as in liquid tumors for the inability to reach and survive in the microenvironment surrounding the neoplastic foci. The intricate net of cross-interactions occurring between tumor components, stromal and immune cells leads to an ineffective anergic status favoring the evasion from the host’s defences38. CAR-T technology has now been shown to have broader applications beyond CD19, and early phase clinical trials of CAR-T cells targeting BCMA and CD22 have reported similarly potent antitumor activity in multiple myeloma and ALL, respectively. However, BCMA and CD22, like CD19, are highly restricted to the B-cell lineage, which resides in tissue that can be targeted with manageable toxicity. Unfortunately, attempts to target tumor-associated antigens in solid tumors have achieved limited success so far4.
A potential approach targeting solid tumors and increasing safety could be combinatorial antigen recognition strategy, as solid tumors often do not have antigens uniquely expressed on the tumor. A preclinical study showed that CAR-T cells could be used to target tumors expressing two antigens, PSCA and PSMA; the CAR-T cells did not kill tumor cells expressing only one of these antigens. Combinatorial antigen recognition strategies may, therefore, be important to consider when designing CARs for the treatment of solid tumors30.
Safety Switches/Self-Inactivation Systems
Other areas of consideration for the future of manufacturing a CAR-T cell therapy include mitigating possible adverse events, both short and long term. For example, one adverse effect related to CAR-T cell therapy is severe CRS, which occurs in a minority of patients. CRS is caused by the release of pro inflammatory cytokines directly from the CAR-T cells and dying tumor cells. Although most cases of CRS are manageable with established treatment algorithms, it has been speculated that mechanisms to inactivate CAR-T cells in patients experiencing adverse effects could be useful to improve the safety of CAR-T cell therapy. Using a suicide switch incorporated into the CAR construct is a strategy that is currently being investigated as a potential way to specifically deplete CAR-T cells in a controlled manner, and it may become important as CAR-T cell therapy is investigated in an ever-growing clinical population30.
Future Aspects for CAR-T Cell Manufacturing
The manufacturing processes now used for highly personalized engineered T cell therapies incur high costs that need to be decreased to be sustainable in the long run. The initiation of manufacturing with defined subpopulations of T cells that can be derived from a blood draw instead of a leukapheresis product would reduce the scale and therefore the cost of manufacturing. New sources of T cells that could alleviate the need to obtain autologous T cells are also being investigated. The intensified interest from biotech and pharmaceutical companies will surely accelerate the development of improved manufacturing platforms. An increasing number of tools are available for clinical CAR-T cell manufacturing and consortia such as the Centre for Commercialization of Regenerative Medicine in Canada and the National Network for Manufacturing Innovation in the US are forming. The optimization of appropriate quality control release testing and tracking of products will need to be drastically improved in terms of efficiency and cost effectiveness. The simplification of workflows, the increase in process robustness, and the implementation of automated closed systems should enable scalability and reduce the COG and human error during the many complex manufacturing steps while maintaining the efficiency of the CAR-T cell products5.
Exploitation of the Intracellular Tumor-Antigen Repertoire by Engineered TCRs
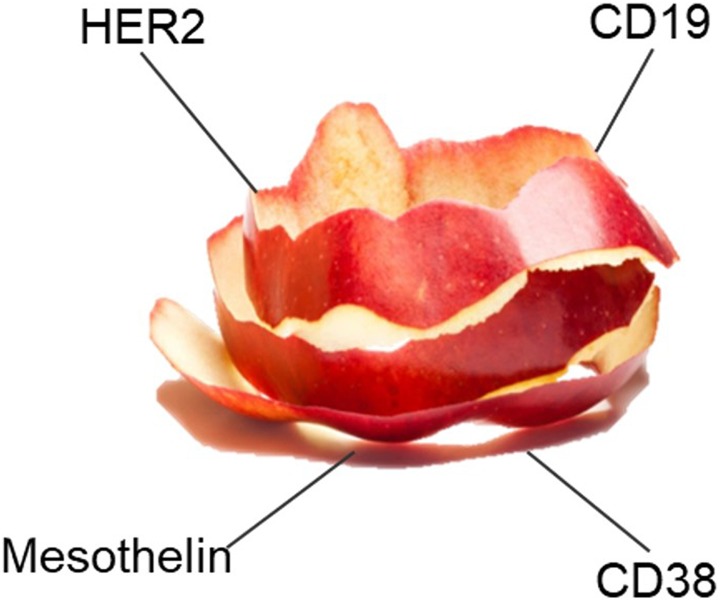
While CAR-T cells typically target surface tumor-associated antigens, engineered TCRs offer the exploitation of the intracellular tumor-associated antigen repertoire that is presented by the MHC. As the intracellular antigen repertoire accounts for approximately 70% of the human proteome compared with 30% for surface proteins, targeting tumors by engineered TCRs offers a significant potential. To provide an example, Medigene is focusing on the development of genetically engineered TCRs. Figures 4 and 5 exemplify the cellular targets of CAR-Ts and TCRs.
Figure 4.
CAR-Ts target cell surface antigens [Figure was kindly provided by Medigene].
Figure 5.
TCRs target the intracellular antigen repertoire [Figure was kindly provided by Medigene].
A detailed comparison of CAR-Ts and TCRs technology features is provided in Table 6.
Table 6.
Characteristics of CAR- and TCR-engineered T Cells, Modified Based On June et al4.
| CAR-Ts | TCRs |
|---|---|
| Signal amplification from synthetic biology: 200 targets can trigger CAR-T cells | Sensitive signal amplification derived by evolution of the TCR |
| Avidity more easily to control | Low avidity, unless engineered or selected for high avidity |
| CAR targets surface structure proteins and glycans | TCR targets intracellular proteome |
| MHC-independent recognition of tumor targets | Requires MHC class I expression and HLA matching on tumor |
| At least decade-long persistence | Lifelong persistence |
| Serial killers of tumor cells | Serial killers of tumor cells |
| Cytokine release syndrome more severe than with TCR-based therapy | Off-tumor toxicity potentially more difficult to predict |
CAR: Chimeric antigen receptor; TCR: T-cell receptor.
Beyond cell-based approaches, research is ongoing to develop bispecific molecule formats harnessing the potential of TCRs to exploit the intracellular tumor-associated antigen repertoire and building on the power of bispecific T-cell engaging antibodies. Such approaches are followed, for example, by Immatics.
On-Target/Off-Tumor Toxicity
The absence of cancer-restricted surface markers is a major impediment to antigen-specific immunotherapy using CAR-T cells. Kim et al.39 generated CD33-deficient human hematopoietic stem and progenitor cells (HSPCs) and demonstrated normal engraftment and differentiation in immunodeficient mice. Autologous CD33 KO HSPC transplantation in rhesus macaques demonstrated long-term multilineage engraftment of gene-edited cells with normal myeloid function. CD33-deficient cells were impervious to CD33-targeting CAR-T cells, allowing for efficient elimination of leukemia without myelotoxicity. These studies illuminate a novel approach to antigen-specific immunotherapy by genetically engineering the host to avoid on-target, off-tumor toxicity. This approach has the potential to serve as a next-generation hematopoietic stem cell transplant, delivering both anti-leukemic T cells as well as HSPCs resistant to CART33, followed by definitive therapy with CART33 to ensure disease eradication and potent targeted immunosurveillance39.
Future Applications beyond Oncology
Ongoing advances in T-cell engineering, gene editing, and cell manufacturing have the potential to broaden T-cell-based therapies to other cell types such as induced pluripotent stem cells, hematopoietic stem cells, and natural killer cells, and to foster new applications beyond oncology in infectious diseases, organ transplantation, and autoimmunity4.CAR-T products can also be used in combination with checkpoint inhibitors and other immune-oncology treatments, for example targeting follicular viral-producing cells using antiviral (CAR) T cells co-expressing the follicular homing chemokine receptor CXCR5. This might be able to suppress viral replication, and lead to long-term durable remission of SIV and HIV40.
Conclusions
In many ways, the marketing approvals for KYMRIAH and YESCARTA will pave the way for further ATMPs to come in the near future. Both products have proven the feasibility and the potential of CAR-T cell technology. Although several ATMPs received EU approval, four of them are no longer approved, mainly due to commercial reasons. Though questions regarding affordability of KYMRIAH and YESCARTA as first-in-class products also remain in certain indications, the effect size that the products have demonstrated in certain patient populations is unprecedented. Smart pricing and reimbursement strategies will be needed to allow access to a larger group of patients.
As far as the approval strategy and time to market is concerned, it needs to be acknowledged that both products were approved about 1 year earlier in the US compared with the EU. This is not uncommon for ground-breaking technologies in oncology. It needs to be acknowledged that the FDA is a single country with harmonized provisions throughout, while the EU is a union of multiple countries, where differences still exist, despite all harmonization efforts. This is particularly true for ATMPs belonging to the group of GMOs, which is applicable for both approved CAR-Ts. A higher degree of harmonization is clearly needed to facilitate development and approval in the EU.
The sponsors of KYMRIAH and YESCARTA were proactively using important regulatory tools such as the breakthrough designation in the US or PRIME designation in the EU as part of their overall development and approval strategy. Though RMAT designation would have been a valuable option for both products in the US, it is assumed that the development was already too far advanced at the time the RMAT designation was put in place.
As discussed in our article, there are still numerous challenges throughout all aspects of CAR-T development. However, academia and industry are already crafting smart strategies for the future based on the learning from earlier developments and approvals. These strategies comprise, for example, the design of the CAR-T cells to make the technology safer and even more effective, or streamlining the manufacturing activities toward more competitive COG up to smarter regulatory strategies in close interaction with the authorities. As shown with the examples of YESCARTA and KYMRIAH, utilization of dedicated regulatory tools can help to speed up the time to market for ATMPs. Important regulatory tools are, for instance, orphan designation, rare disease and rare pediatric disease designation, breakthrough designation, and RMAT designation in the US, and orphan designation as well as PRIME designation in the EU. Building on the success of CAR-Ts, engineering of T cells with TCRs or using T-cell engaging bispecific-TCR recombinant protein formats offer a huge potential because the intracellular antigen repertoire can be targeted in the future.
In conclusion, with the recent approvals of the two CAR-Ts KYMRIAH and YESCARTA, ATMPs have now come from infancy to the adolescent stage—with the adult stage yet to come.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM×anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010;36(6):458–67. DOI: 10.1016/j.ctrv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 2. Le Jeune C, Thomas X. Potential for bispecific T-cell engagers: Role of blinatumomab in acute lymphoblastic leukemia. Drug Des Devel Ther. 2016;10:757–65. DOI: 10.2147/DDDT.S83848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. FDA. Press Announcements - FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm (2017, accessed 4 October 2018).
- 4. June CH, Connor RSO, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;1365(March):1361–365. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015 DOI: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sadelain M. CD19 CAR T Cells. Cell. 2017;171(7):1471 DOI: 10.1016/j.cell.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 7. EMA. First two CAR-T cell medicines recommended for approval in the European Union. https://www.ema.europa.eu/news/first-two-car-t-cell-medicines-recommended-approval-european-union (2018, accessed 10 October 2018).
- 8. ClinicalTrials.gov. https://clinicaltrials.gov/ (2018, accessed 4 October 2018).
- 9. Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, Halton E, Wang X, Senechal B, Purdon T, Cross JR, Liu H, Vachha B, Chen X, DeAngelis LM, Li D, Bernal Y, Gonen M, Wendel HG, Sadelain M, Brentjens RJ. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018. DOI: 10.1158/2159-8290.CD-17-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartmann J, Schüßler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells—challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9(9):e201607485 DOI: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seimetz D. ATMPs: How to successfully master challenges and foster the regulatory success rate? Pharmazeutische Medizin. 2016;18(3):132–39. https://static1.squarespace.com/static/57d16156414fb5307d454a1d/t/59b107ef03596ea2cfbaa206/1504774134784/ATMP_Seimetz.pdf (accessed 1 October 2018). [Google Scholar]
- 12. EMA. Kymriah EPAR Annex 1 Summary of Product Characteristics SmPC. https://www.ema.europa.eu/documents/product-information/kymriah-epar-product-information_en.pdf (2018, accessed 1 October 2018).
- 13. FDA. SBRA - KYMRIAH. https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM577221.pdf (2017, accessed 1 October 2018).
- 14. EMA. Yescarta EPAR Annex 1 Summary of Product Characteristics SmPC. (2018).
- 15. FDA. SBRA - YESCARTA. http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm134048.htm (2017, accessed 1 October 2018).
- 16. Novartis. Determine Efficacy and Safety of CTL019 in Pediatric Patients With Relapsed and Refractory B-cell ALL (ELIANA). https://clinicaltrials.gov/ct2/show/NCT02435849?%20term=Tisagenlecleucel&recrs=de&rank=3 (2018, accessed 1 October 2018).
- 17. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448. DOI: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novartis. Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients (JULIET). https://clinicaltrials.gov/ct2/show/NCT02445248?%20term=Tisagenlecleucel&recrs=de&rank=5 (2018, accessed 1 October 2018).
- 19. EMA. PRIME: priority medicines. https://www.ema.europa.eu/human-regulatory/research-development/prime-priority-medicines (2018, accessed 4 October 2018).
- 20. Vaggelas A, Seimetz D. Expediting drug development. Ther Innov Regul Sci. 2018;216847901877937 DOI: 10.1177/2168479018779373. [DOI] [PubMed] [Google Scholar]
- 21. Hettle R, Corbett M, Hinde S, Hodgson R, Jones-Diette J, Woolacott N, Palmer S. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21(7):1–204. DOI: 10.3310/hta21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maude SL, Barrett DM, Ambrose DE, Rheingold SR, Aplenc R, Teachey DT, Callahan C, Barker CS, Mudambi M, Shaw PA, et al. Efficacy and safety of humanized Chimeric Antigen Receptor (CAR)-Modified T Cells targeting CD19 in children with relapsed/refractory ALL. Blood. 2015;126(23):683 LP–683. http://www.bloodjournal.org/content/126/23/683.abstract (accessed 1 October 2018). [Google Scholar]
- 23. Maude S, Barrett DM. Current status of chimeric antigen receptor therapy for haematological malignancies. Br J Haematol. 2016;172(1):11–22. DOI: 10.1111/bjh.13792. [DOI] [PubMed] [Google Scholar]
- 24. Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, Sussman R, Lanauze C, Ruella M, Gazzara MR, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discovery. 2015;5(12):1282–295. DOI: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gardner R, Wu D, Cherian S, Fang M, Hanafi L-A, Finney O, Smithers H, Jensen MC, Riddell SR, Maloney DG, Turtle CJ. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406–410. DOI: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, Klichinsky M, Shestova O, Patel PR, Kulikovskaya I. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24(10):1499–503. DOI: 10.1038/s41591-018-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weintraub A. After snubbing Gilead, British cost watchdogs give thumbs-up to Novartis’ CAR-T Kymriah. https://www.fiercepharma.com/pharma/after-snubbing-gilead-uk-cost-watchdog-nice-gives-a-thumbs-up-to-novartis-car-t-kymriah?%20mkt_tok=eyJpIjoiWWpReE5qbGlZV1V5WlRZMCIsInQiOiJTXC9IVTZMZzVTODg1VUdrYTU3TWpTOVFBbWdrS3RWMEZhWW5qSFk1M0dBNzdjRGxHU0dTV1NGYUVlXC9MTm5 (2018, accessed 4 October 2018).
- 28. Sagonowsky E. Gilead CAR-T drug Yescarta turned away by NICE cost-effectiveness watchdogs. https://www.fiercepharma.com/pharma/gilead-s-yescarta-turned-away-at-england-s-nice-over-cost?%20mkt_tok=eyJpIjoiTmpjMU1qRTJNMlJqWlRRMyIsInQiOiJvWE1HaGpQTnVtd1l4QzQxVWhFd0lLcnkwTEMwMWJwaVoyTnFuQ3d3VzZ3XC93UWh5dzhiUnhGVUt5d3hIbXBLZG1GV1NGUDVCV2Z2V1RVbk1pcHBuY (2018, accessed 4 October 2018).
- 29. Liu A. Beat you to it, Kymriah: Gilead strikes discount Yescarta deal with NHS in adults. https://www.fiercepharma.com/pharma/beat-you-to-it-kymriah-gilead-strikes-yescarta-deal-nhs-england-at-discount?%20mkt_tok=eyJpIjoiTURWbU1XRTFOalE1TVRaayIsInQiOiJoK3ljd296K0hTV2dmSFRJRnZnemdFODN1K2xBM2pRRjNKWUNPZG4ydlFKMmpNTUZBMGxtbHFqdXlPbHRGc2VXK3ZuQXlxWXg (2018, accessed 11 October 2018).
- 30. Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4(March):92–101. DOI: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. EMA. Guidelines relevant for advanced therapy medicinal products. https://www.ema.europa.eu/en/human-regulatory/research-development/advanced-therapies/guidelines-relevant-advanced-therapy-medicinal-products (2018, 4 October 2018).
- 32. FDA. Cellular & Gene Therapy Guidances. https://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/cellularandgenetherapy/default.htm (2018, accessed 1 October 2018).
- 33. Tasian SK. Acute myeloid leukemia chimeric antigen receptor T-cell immunotherapy: how far up the road have we traveled? Ther Adv Hematol. 2018;9(6):135–48. DOI: 10.1177/2040620718774268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Cancer Institute. CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. https://www.cancer.gov/about-cancer/treatment/research/car-t-cells (2018, accessed 1 October 2018).
- 35. Handgretinger R, Schlegel P. Emerging role of immunotherapy for childhood cancers. Chin Clin Oncol. 2018;7(2):14–14. DOI: 10.21037/cco.2018.04.06. [DOI] [PubMed] [Google Scholar]
- 36. Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Scholler J, Lacey SF, Melenhorst JJ, Nazimuddin F, Perazzelli J, Christian DA, Hunter CA, Porter DL, June CH, Grupp SA, Gill SI. Combination of Anti-CD123 and Anti-CD19 chimeric antigen receptor T Cells for the treatment and prevention of antigen-loss relapses occurring after CD19-targeted immunotherapies. Blood. 2015;126(23):2523 LP–2523. http://www.bloodjournal.org/content/126/23/2523.abstract (accessed 1 October 2018). [Google Scholar]
- 37. Peng W, Lizée G, Hwu P. Blockade of the PD-1 pathway enhances the efficacy of adoptive cell therapy against cancer. OncoImmunology. 2013;2(2): e22691 DOI: 10.4161/onci.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: The long and winding road to solid tumors review-article. Cell Death and Disease. 2018;9(3). DOI: 10.1038/s41419–018-0278–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim MY, Yu KR, Kenderian SS, Ruella M, Chen S, Shin TH, Aljanahi AA, Schreeder D, Klichinsky M, Shestova O. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T Cell immunotherapy for Acute Myeloid Leukemia. Cell. 2018;173(6):1439–453.e19. DOI: 10.1016/j.cell.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haran KP, Hajduczki A, Pampusch MS, Mwakalundwa G, Vargas-Inchaustegui DA, Rakasz EG, Connick E, Berger EA, Skinner PJ. Simian immunodeficiency virus (SIV)-specific chimeric antigen receptor-T cells engineered to target B cell follicles and suppress SIV replication. Front Immunol. 2018;9(March):1–12. DOI: 10.3389/fimmu.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]