Abstract
The effects of ionizing radiation on the reproductive system have always been a matter of great interest. Both artificial and naturally occurring ionizing radiation can directly or indirectly affect the reproductive system via the introduction of DNA single-strand and double-strand breaks, the excitation of water molecules, and the generation of free radicals. In order to quantitatively investigate the effects of ionizing radiation on reproductive function, 60Co γ irradiation was applied on a model organism, Caenorhabditis elegans (C. elegans). The egg-laying and embryo-hatching activities were observed for the parent (F0) and the first 2 progeny (F1 and F2) generations. The incidence rate of ovipositor malformation was also recorded. Acridine orange was used to detect the number of apoptotic germ cells. With the above metrics, the effects of 60Co γ irradiation on the reproductive function of C. elegans were systematically evaluated. The results showed that the postirradiation egg-laying and embryo-hatching activities of the F0 generation were increasingly suppressed by increasing doses of 60Co γ irradiation. Those of the F1 generation showed a trend toward recovery although also suppressed by the radiation to the F0 generation compared with the control. Those activities were restored to normal or near-normal levels for the F2 generation. The incidence rate of ovipositor malformation was greatly increased by 60Co γ irradiation according to radiation doses. Gamma irradiation by 60Co also substantially induced germ cell apoptosis, and the apoptosis rate increased with increasing radiation doses. Therefore, 60Co γ irradiation affects the reproductive function of C. elegans. The suppression on its reproductive function increases with increasing radiation doses. The reproductive functions of progeny generations are also affected and weakened.
Keywords: 60Co, C. elegans, reproductive function
Introduction
Ionizing radiation poses certain health hazards.1 It can have health effects on the irradiated person as well as his or her descendants via genetic changes. The reproductive system is a target organ sensitive to ionizing radiation. Excessive irradiation can affect the reproductive function, causing infertility, chromosomal aberrations, and so on. Exposure during gestation may cause embryo malformations, abortion, gestation cessation, and other adverse outcomes. For males, after irradiation, sperms can have a series of biological changes including a reduction in the number of sperm, changes in shape and mobility, and chromosomal.
A model organism commonly used for reproductive radiation damage is mouse. However, because of its long reproductive cycle, it is more difficult to screen recessive mutants and the associated high material and time costs also limit the observable genetic effects. In comparison, C. elegans is another popular model organism commonly used in genetic studies due to many advantages more amenable than the mouse model. C. elegans is an unsegmented pseudocoelomate and a transparent nematode living in temperate soil environments and feeding on bacteria. Although it is a simple organism, its many advantages such as the short life cycle, the obvious developmental stage, the small size, the ease of culturing in the laboratory, and the sensitivity toward environmental changes make it a popular and useful model in genetic studies.2-6 It has been widely used for research in modern developmental biology, genetics, environmental toxicology, and so on.7-9 Studies using C. elegans for the effects of radiation on germ cell apoptosis have been reported in the literature. In our work, we report a study on the effects of 60Co γ irradiation on the reproductive function of C. elegans in terms of egg-laying and embryo-hatching activities as well as ovipositor malformation.
Methods and Materials
Caenorhabditis elegans Description and Subgrouping
The wild-type C. elegans N2 and Escherichia coli OP50 were kindly donated by Dr Huimin Zhang from School of Basic Medicine and Life Sciences, Suzhou University.10 The C. elegans samples were cultured in the Nematode Growth Media (NGM) agar medium containing E coli OP50 at 20°C. According to the different irradiation doses they received, the samples were divided into 4 groups: 0 Gy, 50 Gy, 100 Gy, and 200 Gy. Twenty worms were observed and recorded in each group.
Caenorhabditis elegans Life-Cycle Synchronization
Following the method of Shen et al,11 the C. elegans nematodes were washed with M9 and placed into the bleach solution when most of them entered the adult phase. As such, the nematodes in the synchronous L1 phase were obtained. After culturing at 20°C for 28 hours, the worms were harvested in the L4 phase. To synchronize the descendant worms, the spawning female nematodes were selected and placed in new NGM petri dishes for 2 hours. The progeny worms were selected and cultured at 20°C for 37 hours before harvesting in the L4 phase.
Irradiation
The 60Co γ irradiator in the State Key Laboratory of Radiation Medicine and Protection, School of Radiation Medicine and Protection, Soochow University, was used for the study. All irradiation was carried out as a single-dose total body irradiation with a 25 Gy/min dose rate at the 20°C room temperature. All worms were irradiated in the L4 phase. Immediately after irradiation, the worms were transferred to a new NGM agar medium containing E coli OP50 and cultured at 20°C until they became adult worms.
Assessment on Egg-Laying and Embryo-Hatching Activities
The worms synchronized in the L4 phase were individually placed into petri dishes and transferred every 24 hours until they no longer laid eggs. The total egg count was recorded for each worm to evaluate the brood size.12 The measurement method of Swain et al was followed. All egg-containing petri dishes were incubated at 20°C for 2 to 3 days until the larvae reached the L3 or L4 phase. The number of offspring larvae in each petri dish was recorded as the live egg count, and the number of unhatched eggs was recorded as the dead egg count. The hatching rate was calculated as the ratio of the live egg count to the total egg count (the sum of the live egg count and the dead egg count).
Assessment on Ovipositor Malformation Rates
Following the method of Wu et al,13 the worms were observed and photographed under a microscope. The ovipositor malformation rate was calculated as the ratio of the number of worms with an ovipositor malformation to that of all worms in each group.
Assessment on Germ Cell Apoptosis
Germ cell apoptosis was determined by generally following the method of Kelly et al with slight modifications.14 After irradiation, late-stage L4 hermaphrodites were picked and aged for 20 hours. The adults were placed in a 96-well plate. An amount of 100-μL M9 solution containing a small amount of OP50 was added, and the worms were stained by 25 μg/mL of acridine orange for 1 hour. The worms were subsequently aspirated and placed in an NGM medium without food for a 2-hour recovery. The worms were then obtained and placed at the center of a slide containing 30 μL of levamisole for observation with fluorescence microscopy. Under the microscope, the normal live cells were evenly stained with green fluorescence with normal green nucleus; whereas the apoptotic cells were dyed into densely stained green pellets of various sizes. Because the anterior arm of the C. elegans gonad is substantially affected by the intestine, only the apoptotic cells in the posterior arm of the gonad were quantified in this experiment.
Statistical Analysis
The results were analyzed using SPSS23.0 software. Data were presented as mean (standard deviation). Intergroup comparisons were conducted using the one-way analysis of variance, and the statistical significance was evaluated using the lease square difference t test. P <.05 as the difference was considered statistically significant. Each experiment was repeated at least 3 times.
Results
Effects of 60Co γ Irradiation of Varying Doses on the Egg-Laying Activities of F0, F1, and F2 Generation C. elegans
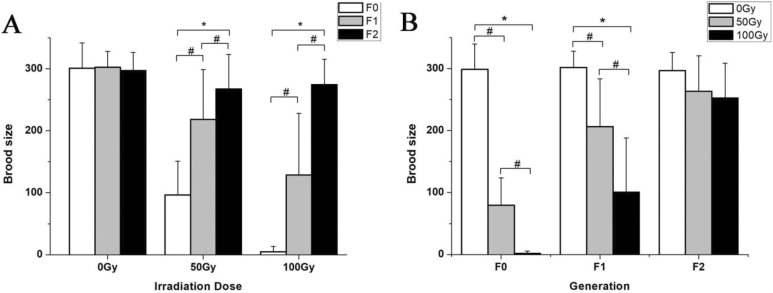
Post 60Co γ irradiation, the egg-laying capacity of C. elegans was severely inhibited (Figure 1). The irradiation significantly reduced the egg-laying capacity of adult C. elegans in the F0 generation, and the severity increased with increasing radiation doses. Compared with the control group (0 Gy), the average egg count of the 50 Gy group decreased by 67.90% (t = 11.74, P < .05), and that of the 100 Gy group decreased by 98.67% (t = 27.54, P < .05). It is noteworthy that when exposed to 200 Gy, all nematodes lost their egg-laying capacity and led to a 0 egg count in the F0 generation. The egg-laying capacity of the F1 generation worms was also affected by the F0 irradiation. Compared with the control group, the average F1 egg count of the 50 Gy group was 27.84% lower (t = 4.22, P < .05), and that of the 100 Gy group was 57.41% lower (t = 7.14, P < .05). For the F2 generation, the egg-laying capacity was restored and not affected by the dose to the F0 generation. At 50 Gy to the F0 generation, the egg-laying capacity of the F1 generation was greatly recovered, and that of the F2 generation was completely restored, with significant intergeneration differences (F = 30.45, P < .05). At 100 Gy to the F0 generation, the egg-laying capacity of the F1 generation was still greatly undermined, and the restoration was observed until the F2 generation.
Figure 1.
Effects of 60Co γ irradiation on the egg-laying activities of Caenorhabditis elegans. A, Brood sizes of C. elegans at different irradiation doses to the F0 generation; *P < .05 versus F0 group. B, Brood sizes of C. elegans for different generations; *P < .05 versus 0 Gy group. #indicates a statistically significant difference between the 2 groups (P < .05). Note: For the 200 Gy group, all F0 worms lost the egg-laying capacity, so there is no 200 Gy data set depicted in the figure.
Effects of 60Co γ Irradiation of Varying Doses on the Embryo-Hatching Activities of F0, F1, and F2 Generation C. elegans
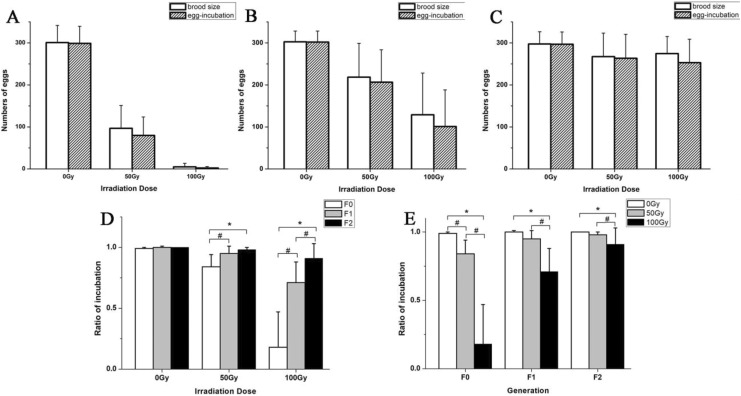
Post 60Co γ irradiation, the embryo-hatching capacity of C. elegans was also severely damaged, leading to lower survival rates for the descendants. The irradiation significantly reduced the embryo-hatching capacity of adult C. elegans in the F0 generation (Figure 2A), and the severity increased with increasing radiation doses. Compared with the control group (0 Gy), the average hatching rate of the 50 Gy group decreased by 15.17% (t = 5.78, P < .05), and that of the 100 Gy group decreased by 82.36% (t = 11.94, P < .05; Figure 2D). The embryo-hatching capacity of the F1 generation was also affected. Compared with the control group, the F1 hatching rate of the 50 Gy group was 5.10% lower (t = 3.32, P < .05), and that of the 100 Gy group was 28.70% lower (t = 5.70, P < .05; Figure 2B). In the F2 generation, the egg-hatching rate was still inhibited by the F0 irradiation. Compared with the control group, the F2 hatching rate of the 50 Gy group was 1.51% (t = 3.45, P < .05), and that of the 100 Gy group was 8.67% lower (t = 3.20, P < .05; Figure 2C). Post 60Co γ irradiation to the F0 generation, the embryo-hatching rates of the F1 and F2 generations both partially recovered but were not completely restored to normal levels (Figure 2E).
Figure 2.
Effects of 60Co γ-ray irradiation on embryo-hatching activities of Caenorhabditis elegans. A, The total number of eggs and the number of hatched eggs for the F0 generation at different doses. B, The total number of eggs and the number of hatched eggs for the F1 generation at different doses. C, The total number of eggs and the number of hatched eggs for the F2 generation at different doses. D, The ratios of incubation of C. elegans at different doses; *P < .05 versus 0 Gy group. E, The ratios of incubation of C. elegans for different generations; *P < .05 versus F0 group. #indicates a statistically significant difference between the 2 groups (P < .05). Note: For the 200 Gy group, all F0 worms lost the egg-laying capacity, so there is no 200 Gy data set depicted in the figure.
Effects on Ovipositor Malformation
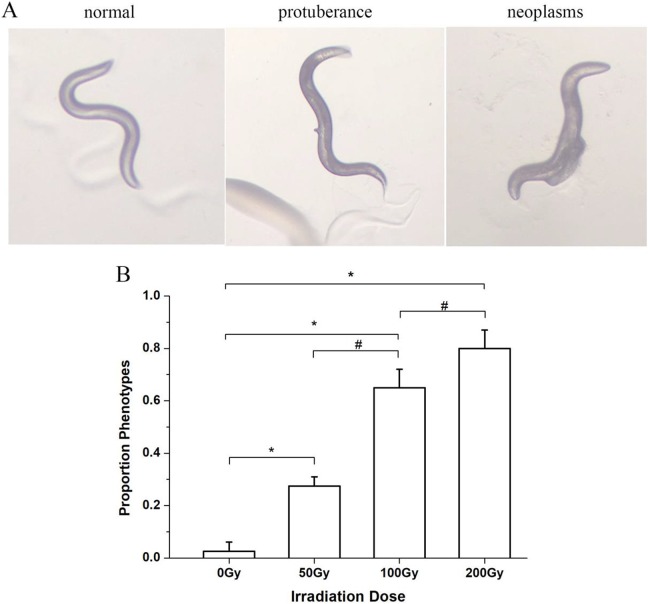
The control nematodes, unexposed to radiation, had a smooth ovipositor structure. Structural changes of the ovipositor were observed after 60Co γ-ray irradiation. Two types of ovipositor malformation were observed: ovipositor protuberance and ovipositor tumor (Figure 3A). Compared with the control group, the ovipositor malformation incidence rate significantly increased by 10 folds for the 50 Gy group (t = −7.07, P < .05), by 25 folds for the 100 Gy group (t = −11.18, P < .05), and by 31 folds for the 200 Gy group (t = −13.86, P < .05; Figure 3B). The ovipositor malformation incidence rates substantially increased with the increase of radiation dose.
Figure 3.
Effects of different doses of 60Co γ irradiation on the ovipositor formation of Caenorhabditis elegans. A, Different C. elegans morphology during the spawning period. B, Phenotype proportions under different doses of 60Co γ irradiation. *P < .05 versus 0 Gy group. #indicates a statistically significant difference between the 2 groups (P < .05).
Effects on Germ Cell Apoptosis
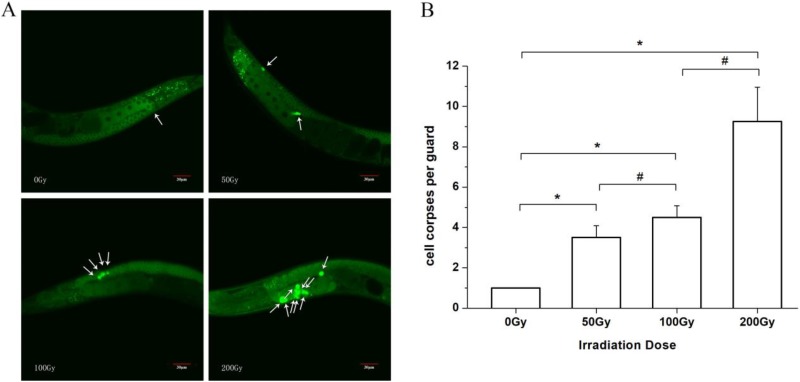
Substantial induction of germ cell apoptosis by 60Co γ irradiation was observed. Under the microscope, the normal live cells were evenly stained with green fluorescence with normal green nucleus, whereas the apoptotic cells were dyed into densely stained green pellets of various sizes (Figure 4A). Compared with the control group, the number of apoptotic cells increased by 2.50 times (t = −13.16, P < .05) for the 50 Gy group, by 3.50 times (t = −18.43, P < .05) for the 100 Gy group, and by 8.25 times (t = −13.97, P < .05) for the 200 Gy group (Figure 4B). With the increase in radiation dose, the number of apoptotic cells also increased substantially.
Figure 4.
Effects of different doses of 60Co γ irradiation on germ cell apoptosis of Caenorhabditis elegans. A, The green fluorescence staining. The arrow showed the green pellets of apoptotic cells. B, the number of apoptotic cells. *P < .05 versus 0 Gy group. #indicates a statistically significant difference between the 2 groups (P < .05).
Discussion
Caenorhabditis elegans is a model organism whose adult somatic cells remain constant but whose reproductive cells remain proliferative for the entire life cycle but are susceptible to various environmental factors such as ionizing radiation,15 ultraviolet,16 and chemicals.17 The growth pattern of C. elegans is relatively fixed. Compared with traditional rodent models, it takes only 3 days to develop from a fertilized egg to an adult and then enters the spawning period. The number of its offspring can be obtained after another 3 days of concentrated spawning. Therefore, C. elegans has unique advantages in multigeneration studies. Therefore, this experiment used C. elegans as the model organism to observe the effects of ionizing radiation on the reproductive system.
Previous studies have shown that the lethality of C. elegans was not significantly increased even when exposed to ionizing radiation doses as high as 500 Gy.18-20 They can still develop into adults even when exposed to ionizing radiation doses as high as 120 Gy.21 But the reproductive system of C. elegans is susceptible to ionizing radiation. Such kind of susceptibility was also observed in our study. As the radiation dose increased, both the egg-laying and embryo-hatching capacities of C. elegans decreased and the ovipositor malformation rate increased. Our study also found the impacts of ionizing radiation on the offspring of the exposed C. elegans. Through the quantification of the egg-laying counts and embryo-hatching rates of C. elegans in F1 and F2 generations, we found a gradual recovery trend as the generation progressed. In the F2 generation, the egg-laying capacity of C. elegans returned to its normal level, but the hatching rate was still below its normal level, indicating that the offspring survival rate gradually increased with the generation progression but was still not restored to normal levels in the F2 generation.
Apoptosis, also known as programmed cell death, is a fundamental feature of the development of multicellular animals. It plays a decisive role in the clearance of aging and excess cells as well as the maintenance of the homeostasis of normal cells. Our study demonstrated that ionizing radiation significantly increased the apoptosis of germ cells of C. elegans. With the increase in radiation dose, the number of apoptotic cells also increased significantly, and there was a dose-dependent effect.22,23 The apoptosis of C. elegans is controlled by various genes that are similar to the mammalian genes that control the apoptotic factors. Therefore, the study on the mechanism of genetic regulation of apoptosis in C. elegans could enhance and complement the researches on the biochemical and cellular mechanisms of mammalian apoptosis. In our study, the ionizing radiation-induced apoptosis of germ cells of C. elegans was observed.24,25 Current studies have shown that the mitochondrial pathway, cell death receptor pathway, endoplasmic reticulum pathway, and DNA damage response (DDR) pathway are essential for the apoptosis. While the germ cell apoptosis induced by ionizing radiation differs from apoptotic somatic cell death. The sensitive part of radiation-induced injury is in the nucleus, and DNA is a key target. We suspect that the germ cell apoptosis is due to DDR, and its underlining mechanism still awaits further investigation.
Footnotes
Authors’ Note: Fengmei Cui and Yu Tu conceived and designed the experiment. Nan Ma, Xiaojing Han, Na chen, Yue Xi, Weiye Yuan, Yufan Xu, Jianfang Han, and Xiaoyan Xu performed the experiments. Yu Tu, Fengmei Cui, and Nan Ma analyzed the data. Fengmei Cui and Nan Ma wrote the article. Feng Mei Cui, Na Ma, and Xiaojing Han contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by ITER (2014GB112006), the National Natural Science Foundation of China (grant numbers 30800922 and 81172706), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1. Mettler FJ, Voelz GL. Major radiation exposure – what to expect and how to respond. N Engl J Med. 2002;346(20):1554–1561. [DOI] [PubMed] [Google Scholar]
- 2. Singhal A, Shaham S. Infrared laser-induced gene expression for tracking development and function of single C. elegans embryonic neurons. Nat Commun. 2017;8:14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dirksen P, Marsh SA, Braker I, et al. The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC Biol. 2016;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eberhard R, Stergiou L, Hofmann ER, et al. Ribosome synthesis and MAPK activity modulate ionizing radiation-induced germ cell apoptosis in Caenorhabditis elegans. PLoS Genet. 2013;9(11):e1003943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakashita T, Hamada N, Ikeda DD, et al. Locomotion–learning behavior relationship in Caenorhabditis elegans following gamma-ray irradiation. J Radiat Res. 2008;49(3):285–291. [DOI] [PubMed] [Google Scholar]
- 6. David HE, Dawe AS, de Pomerai DI, et al. Construction and evaluation of a transgenic hsp16-GFP-lacZ Caenorhabditis elegans strain for environmental monitoring. Environ Toxicol Chem. 2003;22(1):111–118. [PubMed] [Google Scholar]
- 7. Tomazella GG, Kassahun H, Nilsen H, Thiede B. Quantitative proteome analysis reveals RNA processing factors as modulators of ionizing radiation-induced apoptosis in the C. elegans germline. J Proteome Res. 2012;11(8):4277–4288. [DOI] [PubMed] [Google Scholar]
- 8. Bukhari SI, Vasquez-Rifo A, Gagne D, et al. The microRNA pathway controls germ cell proliferation and differentiation in C. elegans. Cell Res. 2012;22(6):1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng X, Yin X, Allan R, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322(5898):110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen L, Xiao J, Ye H, Wang D. Toxicity evaluation in nematode Caenorhabditis elegans after chronic metal exposure. Environ Toxicol Pharmacol. 2009;28(1):125–132. [DOI] [PubMed] [Google Scholar]
- 12. Swain SC, Keusekotten K, Baumeister R, Sturzenbaum SR. C. elegans metallothioneins: new insights into the phenotypic effects of cadmium toxicosis. J Mol Biol. 2004;341(4):951–959. [DOI] [PubMed] [Google Scholar]
- 13. Wiener-Megnazi Z, Reznick AZ, Lahav-Baratz S, et al. Oxidation and female reproduction: the good, the bad and what’s between. Harefuah. 2011;150(3):255–259, 303. [PubMed] [Google Scholar]
- 14. Kelly KO, Dernburg AF, Stanfield GM, Villeneuve AM. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics. 2000;156(2):617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakashita T, Takanami T, Yanase S, et al. Radiation biology of Caenorhabditis elegans: germ cell response, aging and behavior. J Radiat Res. 2010;51(2):107–121. [DOI] [PubMed] [Google Scholar]
- 16. Prasanth MI, Santoshram GS, Bhaskar JP, Balamurugan K. Ultraviolet-A triggers photoaging in model nematode Caenorhabditis elegans in a DAF-16 dependent pathway. Age. 2016;38(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao Y, Wu Q, Wang D. An epigenetic signal encoded protection mechanism is activated by graphene oxide to inhibit its induced reproductive toxicity in Caenorhabditis elegans. Biomaterials. 2016;79:15–24. [DOI] [PubMed] [Google Scholar]
- 18. Weidhaas JB, Eisenmann DM, Holub JM, Nallur SV. A Caenorhabditis elegans tissue model of radiation-induced reproductive cell death. Proc Natl Acad Sci U S A. 2006;103(26):9946–9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hartman P, Goldstein P, Algarra M, Hubbard D, Mabery J. The nematode Caenorhabditis elegans is up to 39 times more sensitive to gamma radiation generated from 137Cs than from 60Co. Mutat Res. 1996;363(3):201–208. [DOI] [PubMed] [Google Scholar]
- 20. Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage – induced apoptosis and cell cycle arrest in C. elegans. Mol Cell. 2000;5(3):435–443. [DOI] [PubMed] [Google Scholar]
- 21. De Santis M, Cesari E, Nobili E, et al. Radiation effects on development. Birth Defects Res C Embryo Today. 2007;81(3):177–182. [DOI] [PubMed] [Google Scholar]
- 22. Chinnaiyan AM, O’Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275(5303):1122–1126. [DOI] [PubMed] [Google Scholar]
- 23. Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356(6369):494–499. [DOI] [PubMed] [Google Scholar]
- 24. Zhang G, Liu K, Ling X, et al. DBP-induced endoplasmic reticulum stress in male germ cells causes autophagy, which has a cytoprotective role against apoptosis in vitro and in vivo. Toxicol Lett. 2016;245:86–98. [DOI] [PubMed] [Google Scholar]
- 25. Sung M, Kawasaki I, Shim YH. Depletion of cdc-25.3, a Caenorhabditis elegans orthologue of cdc25, increases physiological germline apoptosis. Febs Lett. 2017;591(14):2131–2146. [DOI] [PubMed] [Google Scholar]