Abstract
The pathogenesis of SpA is multifactorial and involves a range of immune cell types and cytokines, many of which utilize Janus kinase (JAK) pathways for signaling. In this review, we summarize the animal and pre-clinical data that have demonstrated the effects of JAK blockade on the underlying molecular mechanisms of SpA and provide a rationale for JAK inhibition for the treatment of SpA. We also review the available clinical trial data evaluating JAK inhibitors tofacitinib, baricitinib, peficitinib, filgotinib and upadacitinib in PsA, AS and related inflammatory diseases, which have demonstrated the efficacy of these agents across a range of SpA-associated disease manifestations. The available clinical trial data, supported by pre-clinical animal model studies demonstrate that JAK inhibition is a promising therapeutic strategy for the treatment of SpA and may offer the potential for improvements in multiple articular and extra-articular disease manifestations of PsA and AS.
Keywords: spondyloarthropathies (including psoriatic arthritis), DMARDs, cytokines and inflammatory mediators, bone, gastrointestinal, ligaments and tendons, skin, synovium
Rheumatology key messages
Janus kinases mediate cytokine signaling for many immune cell responses underlying the pathogenesis of spondyloarthritis.
Janus kinase inhibition offers the potential for improvements in articular and extra-articular spondyloarthritis disease manifestations.
Tofacitinib and other Janus kinase inhibitors may provide clinically meaningful benefits for patients with spondyloarthritis.
Introduction
SpA encompass PsA and AS, and a wider spectrum of inflammatory diseases. In addition to skeletal involvement encompassing peripheral arthritis, axial disease, isolated enthesitis and dactylitis, PsA and AS are associated with a range of extra-articular manifestations, including uveitis, psoriasis and IBD [1]. SpA currently has fewer therapeutic options than RA, and sometimes exhibits heterogeneous therapeutic responses between skeletal, eye and gut involvement. Given the complexity of SpA and the need for new therapeutic options, this review considers the entire disease spectrum with respect to Janus kinase (JAK) inhibition and was undertaken after a meeting by a group of experts active in SpA research.
Unmet treatment need in SpA
Treatment recommendations recognize that appropriate choice of therapy for SpA depends upon multiple factors and should be optimized based on the presenting symptoms, involvement of other diseases and comorbidities, disease activity and prior therapies. TNF inhibitors (TNFi) feature in recent treatment recommendations for PsA in all key domains, including peripheral arthritis, axial disease, enthesitis, dactylitis, plaque psoriasis and nail psoriasis [2]. Similarly, TNFi are approved in AS and have demonstrated efficacy in improving axial and peripheral arthritis as well as other articular and entheseal disease manifestations. While TNFi demonstrate efficacy across key disease domains, a significant proportion of patients have inadequate or poor response and others may not tolerate these therapies [3]. Consequently, treatments with alternative mechanisms of action (MoA) may be welcomed for patients with SpA.
As our understanding of SpA pathogenesis has increased, the importance of innate immunity and cytokine signaling pathways rather than classical adaptive immunity has fully emerged. This is evidenced through the emergence of novel agents that target IL-12/23, IL-17 A and IL-23 [4], which have been developed and have often shown better efficacy in PsA compared with in RA [5].
JAK inhibitors
JAK inhibitors are an emerging class of therapies that have demonstrated efficacy for the treatment of inflammatory diseases, in which they have broad effects on cytokine production [6]. There are several excellent recent papers on the JAK pathway itself, which will not be discussed further [7, 8]. In this paper, we review and interpret the available basic and clinical evidence to provide context and rationale for the use of JAK inhibitors for the treatment of PsA and AS. We discuss why it is that, at the population level, neither TNF nor IL-17 directly signal via JAK pathways, and relate this to the efficacy of JAK inhibition in experimental SpA and emergent clinical data.
Immunopathogenesis of SpA
The aetiology of SpA is complex, with interacting environmental and genetic factors combining to elicit a chronic inflammatory response involving the innate and adaptive immune systems (Fig. 1). At the micro-anatomical level, there is increasing evidence—especially from animal models—that the earliest disease manifestations in arthritis emanate from entheses, with inflammation subsequently involving immediately adjacent tissues, including synovio–entheseal complexes [9]. Genome-wide association studies have identified a number of genetic risk factor variants common to PsA and AS, including HLA-B27, IL23R, IL1A and IL12B [10]. Several of these genetic risk factors are also associated with psoriasis and IBD [11].
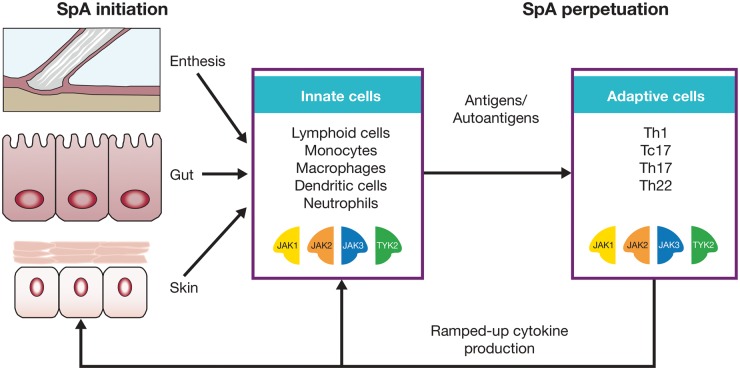
Fig. 1.
Innate and adaptive immune responses in the initiation and perpetuation of SpA
The JAK pathway sits at the crossroads of both key innate and adaptive immune cell populations that are thought to be important in SpA pathogenesis. The tissue-specific targets of SpA-related disease, including the skeleton, skin and gut, interact with diverse innate immune cells to maintain tissue homeostasis. Although SpA is immunologically heterogeneous, there is strong evidence for adaptive immune responses that could be due to autoantigens or to other antigens that breech tissue barriers. JAK: Janus kinase; Tc: cytotoxic T cell; Th: T helper; TYK: tyrosine kinase.
A key mechanism in the immunopathogenesis of psoriasis is thought to centre on the cluster of differentiation (CD)8+ T cell response against melanocyte peptides, but thus far, direct proof in the case of PsA or AS has been lacking [12]. However, analogous to skin disease, a population of CD8+ T cells that are enriched for IL-17 production is evident compared with RA [13]. In PsA, the infiltration of macrophages and activated T cells into articular locations leads to the production of effector cytokines, including IL-1β, IL-2, IL-10, IFNγ and TNFα, and further recruitment and proliferation of immune cells associated with tissue destruction [14]. However, in PsA and AS, there is strong experimental evidence that disease localization and the initial inflammation occurs at entheses and other sites of high mechanical stress, including the sacroiliac (SI) joints [15].
The IL-23/IL-17 axis is strongly implicated in the pathogenesis of PsA and AS [14] and in the psoriatic skin phenotype [16]. IL-23 contributes to differentiation of innate and adaptive cognate T cell-expressing lymphocytes which, in turn, secrete IL-17 A, IL-22 and TNFα [14]. These effector cytokines are linked to keratinocyte production associated with skin manifestations of psoriatic disease and to erosions and new bone formation, although the exact mechanisms underlying the altered phenotypes in the skin and joint are not well understood [14].
There is increased interest in the potential role that the human intestinal microbiome plays in the pathogenesis of diseases such as PsA and AS [17–19]. When the normal homeostasis that exists between the gut microbiota and immune cells in the gut lining is disrupted, the ensuing dysbiosis may contribute to systemic inflammation. Similar to the pathogenesis of IBD, in SpA the inflammatory response is likely typified by IL-23–mediated activation of innate and adaptive intestinal lymphocytes, providing further support for therapeutic strategies targeting the IL-22 and IL-23/IL-17 axis [20].
JAK–STAT signaling
A large number of cytokines, including many of those implicated in the pathogenesis of SpA, signal through JAK pathways (Fig. 2). The JAK family of intracellular protein tyrosine kinases consists of JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2) [21]. In conjunction with Signal Transducer and Activator of Transcription (STAT) intracellular transcription factors, JAKs mediate signaling for a range of extracellular cytokines and growth factors and ultimately influence a variety of cellular functions [21]. Cytokine binding to receptors at the cell surface activates JAKs bound to the intracellular domains of these cytokine receptors via autophosphorylation events. Subsequently, activated JAKs phosphorylate sites on intracellular domains of cytokine receptors that become docking sites for STAT molecules from the cell cytoplasm. STAT molecules are then phosphorylated by the activated JAKs. Phosphorylated STATs dissociate from the intracellular domain of the receptor and form dimers that regulate gene expression and DNA transcription in the cell nucleus [21].
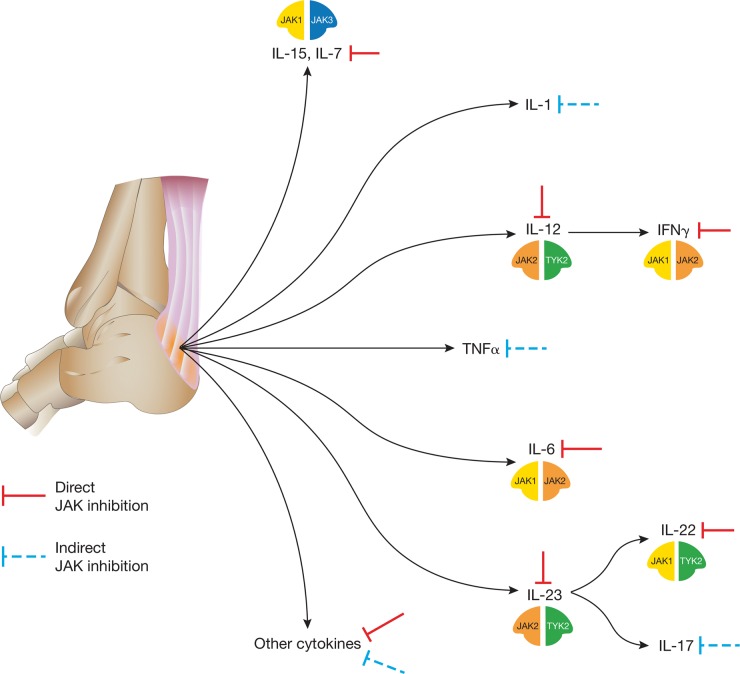
Fig. 2.
JAK inhibition of cytokine pathways involved in the pathogenesis of SpA
Cytokine signaling and production at the enthesis: signaling for a number of key cytokine pathways implicated in the pathogenesis of SpA is blocked through direct inhibition of JAKs, including IFNγ, IL-7, IL-12, IL-15, IL-22 and IL-23. Other important cytokines, such as TNFα, IL-1 and IL-17, signal independently of JAKs, but their expression is regulated by JAK-dependent cytokines and, therefore, may be blocked indirectly via JAK inhibition. These cytokines influence cellular function for a broad range of innate and adaptive cell types, including many of those shown in Fig. 1. JAK, Janus kinase; TYK, tyrosine kinase.
Different cytokines signal using different pairings of individual JAKs. The six γ-common cytokines (IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21) signal through the JAK1/JAK3 combination, modulating adaptive immune functions, including Th cell differentiation and function [21]. Innate lymphoid cells (ILCs), present in psoriatic skin lesions and implicated in the pathophysiology of SpA, are also strongly dependent upon IL-7 for signaling [22]. IFNγ and IL-12 signaling via JAK1/JAK2 and JAK2/TYK2 combinations, respectively, are critical for Th1 cell response and ultimately for production of TNFα by macrophages [23].
Importantly, given the role of the IL-23/IL-17 axis in SpA [24], JAKs influence signaling for several key cytokines involved in this pathway. IL-23, which is produced by activated dendritic cells, signals using the JAK2/TYK2 combination. As well as direct blockade of IL-23 signaling, an indirect consequence of JAK inhibition is downstream blockade of IL-17 production [23]. IL-6 is also involved in ILC type 3 and Th17 cell activation and functions using the JAK1/JAK2 combination [21]. IL6R single-nucleotide polymorphisms have been associated with AS, but thus far, IL-6 blockade has failed in phase 2 clinical trials [25], and the precise role of IL-6 in SpA remains to be defined.
Signaling for IL-22, and type I IFNs—which have been strongly implicated in psoriasis—is mediated by the JAK1/TYK2 pairing [23]. The cellular and molecular mechanisms underlying JAK inhibition in psoriasis were elucidated in a phase 2 randomized trial of tofacitinib, and serve as a benchmark for MoA studies of JAK inhibitors in SpA [26]. Analysis of skin lesion biopsies in this trial demonstrated that tofacitinib attenuated JAK–STAT signaling in psoriatic keratinocytes (likely mediated by pathogenic cytokines that use JAK1 to signal, i.e. IL-19, IL-20, IL-22 and IFN) [26]. A further example of the effect of JAK inhibition on non-immune cell types is provided by studies of human primary PsA synovial fibroblasts and ex vivo PsA synovial explants, in which tofacitinib inhibited STAT1 and STAT3 phosphorylation [27].
Given the range of cytokines that utilize JAK–STATs for signaling, JAK inhibition offers the potential to modulate multiple inflammatory pathways implicated in the pathogenesis of SpA (Fig. 2). Ultimately, activation of these pathways brings about the proliferation of inflammatory cells in articular and extra-articular locations, and of cell types associated with bone loss, joint destruction and psoriatic skin changes—the hallmarks of SpA [24, 28]. In this context, therefore, therapeutic agents targeting JAKs could suppress articular as well as extra-articular symptoms of PsA and AS.
Animal and pre-clinical data evaluating JAK inhibition in SpA
In the absence of clinical trial data evaluating the MoA for JAK inhibition in SpA indications, experimental models provide an opportunity for studying the effects of JAK blockade on the underlying molecular mechanisms of the disease. A number of animal models have been employed to probe mechanistic aspects of SpA, though none are able to fully replicate human disease [29]. A common denominator in these animal models is that the effector mechanisms are mediated by inflammatory cytokines, including TNF, IL-17, IL-22, IL-23 and several others. HLA-B27 overexpression in rats results in an SpA phenotype featuring colitis, arthritis and spondylitis [30]. TNF blockade in this model prevented intestinal and joint disease manifestations [31], and IL-17 blockade reduced structural damage, including new bone formation [32]. Deregulated TNF expression in the TNFΔARE model induces SpA with Crohn’s, spine and enthesitis manifestations [33], and β-glucan–induced SpA in the SKG mouse model results in a colitis disease phenotype [34]. IL-23–induced inflammation has been studied in a murine model in which innate-like T cells (most likely γδ T cells) triggered inflammation of the gut, skin, joint and spine [35]. TNFα-induced protein 3 (also known as A20) negatively regulates inflammation by blocking nuclear factor-κB, and entheseal inflammation is a feature of myeloid-specific A20-deficient mice in which disease commences in the synovio–entheseal complex of the Achilles tendon [36]. Many of the cytokines driving inflammation in these models are under the control of JAK–STAT signaling. The proof of this principle is provided by the A20 deficiency model, in which JAK inhibition with tofacitinib demonstrated significant reductions in enthesitis and a direct link between STAT1-dependent inflammation and A20 deficiency [36]. Importantly, joint inflammation in the A20 model is independent of TNF, providing pre-clinical suggestions that JAK inhibition may offer value in TNF-resistant SpA [36]. The effect of JAK inhibition has been studied in murine osteoclast-like cells, in which tofacitinib was found to inhibit bone destruction mediated by TNFα and IL-6 [37]. Pre-clinical data have also suggested a link between JAK–STAT pathways and osteoblast differentiation, with JAK–STAT signaling implicated in alkaline phosphatase regulation [38].
Clinical data evaluating JAK inhibitors
Several JAK inhibitors with various reported selectivity are being investigated for use in autoimmune diseases. To date, the only JAK inhibitor to have been investigated in SpA clinical trials is the oral JAK inhibitor tofacitinib. In cellular assays, tofacitinib demonstrated preferential inhibition of JAK1 and JAK3, with 5- to 100-fold selectivity over JAK2 [39]. Its pharmacokinetics are characterized by rapid absorption (∼1 h to peak concentration) and elimination (half-life of ∼3.2 h) [40], and its pharmacodynamic effects are generally reversible following 14 days of treatment discontinuation [41].
Clinical trials of tofacitinib have demonstrated efficacy in reducing the signs and symptoms of PsA. Two phase 3, randomized controlled trials evaluated tofacitinib 5 mg twice daily (BID) and 10 mg BID in patients with active PsA and IR to conventional synthetic DMARDs (csDMARDs) [42] or TNFi [43]. In OPAL Broaden (NCT01877668), significant improvements vs placebo in ACR20 response rates (20% improvement in ACR core set measures) and improvements from baseline in HAQ-Disability Index (HAQ-DI) scores were observed with tofacitinib 5 and 10 mg BID at month 3 and maintained (relative to baseline) to month 12. Improvements were also seen in enthesitis, dactylitis and skin psoriasis.
In the 6-month trial in TNFi-IR patients (OPAL Beyond [NCT01882439]), significant improvements vs placebo in ACR20 response rates and HAQ-DI scores were observed at month 3 with tofacitinib 5 and 10 mg BID and were maintained (relative to baseline) to month 6. Enthesitis, dactylitis and skin psoriasis were also improved. Both doses showed generally similar clinical efficacy, though tofacitinib 10 mg BID demonstrated greater efficacy vs placebo in skin psoriasis compared with 5 mg BID [43].
In a 16-week (12-week treatment, 4-week washout), phase 2, dose-ranging trial (NCT01786668) in TNFi-naïve patients with active AS and IR or intolerance to NSAIDs [44], the ASAS20 response rate was significantly higher vs placebo with tofacitinib 5 mg BID. Both tofacitinib 5 and 10 mg BID improved objective measures of disease, including Spondyloarthritis Research Consortium of Canada MRI scores of SI and spine joints at week 12 [44]. In this study, greater response to tofacitinib was correlated with the magnitude of the CRP elevation and the degree of spinal MRI positivity (Spondyloarthritis Research Consortium of Canada SI joint cut-off ⩾2) at baseline. This study demonstrated that JAK inhibitors may be effective treatment options for axial disease, but their efficacy has not been fully established and further studies are required in order to assess their efficacy over longer follow-up periods.
As outlined previously, IBD (ulcerative colitis [UC] and Crohn’s disease) and SpA share a number of genetic and immunopathogenic aspects, with the role of the gut microbiota implicated in their pathophysiology [45]. Cytokines implicated in the pathogenesis of IBD that signal using JAKs include IL-2, IL-7, IL-15 and IL-21, which utilize the JAK1/JAK3 pairing, IFNγ (JAK1/JAK2), IL-22 (JAK1/TYK2) and IL-12 and IL-23 (JAK2/TYK2) [46]. Randomized controlled trials of tofacitinib demonstrated a significant effect of treatment for UC [47] but only modest efficacy for Crohn’s disease [48]. The JAK1 inhibitor filgotinib is also being investigated for Crohn’s disease and has shown efficacy in inducing clinical remission in a phase 2 study [49]. With respect to the disparate efficacy observed with tofacitinib in UC and Crohn’s diseases, there is a consensus that, at the population level, there is a greater role for adaptive immunity in UC compared with Crohn’s disease [50]. The recently reported efficacy of tofacitinib in phase 3 UC studies [47] was substantially greater than that reported in phase 2 studies of tofacitinib for Crohn’s disease [48]. Given that a greater role for innate immunity is ascribed to Crohn’s disease and a greater role for adaptive immunity in UC, these findings might suggest a greater magnitude of effect of JAK inhibitors on the adaptive arm of immunity implicated in the pathogenesis of SpA (as set out in Figs 1 and 2).
In patients with moderate to severe plaque psoriasis, phase 2 and 3 studies have demonstrated the efficacy of oral tofacitinib [26, 51–53], and a topical formulation has been assessed for psoriasis [54] and atopic dermatitis [55]. The MoA of tofacitinib for psoriasis was evaluated in a phase 2 randomized trial [26]. Marked reduction of cellular immune infiltrates in skin lesions was observed—with the earliest changes observed in CD11c+ dendritic cells—as well as a reduction in IL-23/IL-17 axis cytokines that followed the cellular reductions. These cellular reductions may be mediated by changes in signaling of cytokines such as IL-7 (which generally promotes survival of immune cells) and IL-2. Observed reduction in IL-17 was likely due to an indirect effect of tofacitinib through modulation of other cytokines that support growth and survival of the immune cell infiltrates in skin lesions. As the only study to evaluate tofacitinib MoA in human disease, results from this study provide important information regarding immune cell signaling pathways that may be generalizable to SpA.
In atopic dermatitis, inhibition of IL-4 via JAK1/JAK3 blockade is thought to modulate Th2-mediated inflammation in the disease. In addition, improvements in pruritus noted in the atopic dermatitis trial were linked to inhibition of IL-31 signaling achieved via JAK1/JAK2 blockade [55]. The oral JAK1/JAK2 inhibitor baricitinib is also being investigated for psoriasis, and demonstrated a significant treatment effect in patients with moderate to severe psoriasis in a 12-week phase 2b dose-ranging trial [56].
Cytokines and activation of Th1 and Th17 cells are also involved in the pathogenesis of dry eye disease, in which topical tofacitinib has demonstrated efficacy in improving signs and symptoms of the disease in a phase 1/2 study [57]. Although the IL-23/IL-17 pathway is implicated in the pathogenesis of uveitis, and JAK inhibition thus represents a promising approach for its treatment, clinical trials evaluating the efficacy of JAK inhibitors in uveitis have not been conducted [58]. In general, there is a sense that anti–IL-17 therapies may be more effective for the treatment of skin symptoms, whereas TNFi may be more effective against joint manifestations, depending on the dose.
In common with SpA, the inflammatory response in RA is mediated by a range of effector cytokines, a number of which signal using JAKs, including IL-6, IL-7, IL-10, IL-12, IL-15, IL-21, IL-23, IFNα and IFNβ [41]. The efficacy of tofacitinib either as monotherapy, or in combination with csDMARDs, for the treatment of RA has been established in a variety of patient populations (including DMARD-IR and TNFi-IR patients) in randomized controlled phase 3 trials and in open-label long-term extension studies [59–65]. Baricitinib has demonstrated clinical efficacy in a phase 3 randomized controlled trial of patients with RA refractory to treatment with csDMARDs and biologic DMARDs (bDMARDs) [66], and is approved in Europe for the treatment of patients with moderately to severely active RA. A selective inhibitor of JAK1, upadacitinib (ABT-494), has demonstrated efficacy in TNFi-IR patients with RA [67]. Pharmacokinetic/pharmacodynamic analyses of filgotinib have been conducted and dose-ranging studies in RA are planned [68]. The JAK1/JAK3 inhibitor peficitinib demonstrated efficacy vs placebo in patients with RA when dosed at 50 mg in combination with MTX, but did not otherwise show a dose-dependent effect over 12 weeks [69].
The efficacy of JAK inhibitors with varying potencies against the JAK family of tyrosine kinases demonstrated across the range of inflammatory diseases described above is likely due to a pan-JAK inhibitory effect, whereby each of the JAK protein kinases is inhibited to some degree at the clinical dose. Beyond the direct effects of tofacitinib and other JAK inhibitors on cytokine signaling, there is a downstream effect on biologic processes—that is, inhibition of Th1, Th2 and Th17 functions through JAK1/JAK3 inhibition of the γ-common cytokines—that contributes to the efficacy of JAK inhibitors. In addition, cells expressing the IL-7 and IL-15 receptors, including ILCs, could also be affected via the same pathway [22, 70]. The clinical efficacy of tofacitinib observed in SpA indications correlates with the immunomodulatory effects of tofacitinib characterized in other diseases, but more data are required to better understand the specific underlying mechanisms in PsA and AS that are affected by JAK inhibitors.
Agents that inhibit TYK2 may be advantageous for the treatment of peripheral arthritis in SpA due to the prominent role of IL-23 signaling (as suggested by genetic studies), particularly since SpA family diseases are linked to IL-23 pathway single nucleotide polymorphisms. Thus, an inhibitor with JAK1/TYK2 specificity may be expected to deliver greater efficacy than JAK1/JAK3 inhibition with tofacitinib or JAK1-selective inhibitors, through targeting the IL-23 genetic signature (through TYK2 inhibition) in addition to type I IFN (through JAK1 inhibition). Although JAK2-selective inhibition could inhibit IL-23 signaling in a similar fashion to a TYK2-selective agent, it would also inhibit signaling of other hematopoietic factors, such as erythropoietin and thrombopoietin, potentially leading to undesirable side effects such as anemia and thrombocytopenia. Though more studies are required in different disease populations to establish how JAK inhibitors with different putative JAK selectivity may be differentiated from one another in the clinic, available data evaluating cytokine inhibition profiles of different JAK inhibitor agents currently suggest limited differentiation between agents at clinically relevant doses [71]. Given the immunological heterogeneity in the SpAs, and given that some drugs, including TNF fusion proteins and anti–IL-17 therapies, do not work in IBD and uveitis, the inhibition of multiple cytokines may auger well for JAK inhibition across the full spectrum of SpA disease manifestations, and there is a strong potential for JAK inhibition strategies that target the SpA spectrum of disease.
Owing to the pleiotropic nature of JAK–STAT signaling, including its role in hematopoiesis and host defence, monitoring the safety profile of JAK inhibitors is an important aspect of clinical studies evaluating their use. Data from the tofacitinib RA clinical trials, which include up to 96 months of observation, currently provide perhaps the best indication of the long-term safety of JAK inhibitors [72]. In this patient population, incidence rates for adverse events of special interest (including serious infections, cardiovascular events, malignancies and mortality) have not increased with longer tofacitinib exposure. Changes in laboratory parameters observed with tofacitinib treatment (including decreases in lymphocyte, neutrophil and platelet counts, and increases in low- and high-density lipoprotein cholesterol and in serum creatinine) were generally stable with long-term therapy and reversible with treatment discontinuation or medical management (e.g. use of lipid-lowering agents) [72]. The safety profile of tofacitinib in patients with AS [44], PsA [42, 43], psoriasis [51–54], atopic dermatitis [55] and IBD [47, 48] has been generally consistent with that observed in RA, with no new or unexpected safety findings. However, exposure and sample size in these patient populations are not as large as those for RA.
In general, reported safety events for other JAK inhibitors have been consistent with those reported with tofacitinib, with infections and small changes in clinical laboratory parameters a feature of trials evaluating baricitinib, upadacitinib and filgotinib [49, 56, 66, 67]. Again, sample size and drug exposure were relatively low in these trials, and continued evaluation of the safety profiles of the different JAK inhibitors in different patient populations is required.
Conclusions
The SpAs include several chronic inflammatory autoimmune conditions with a multifactorial pathogenesis involving a range of immune cell types and cytokine signaling pathways. Therapeutic disease management for PsA and AS is complicated by the variety of musculoskeletal and extra-articular manifestations with which the diseases may present. While bDMARDs, including TNFi, IL-12p40 and IL-17 inhibitors, have demonstrated efficacy in treating the spectrum of clinical manifestations of SpA, additional agents with novel MoAs could provide useful alternatives for patients who do not respond or lose initial response to therapy with bDMARDs across many SpA manifestations, including those in the gut, skin and joint.
JAK–STAT pathways mediate cytokine signaling for many innate and adaptive immune responses underlying the pathogenesis of SpA as well as other associated inflammatory diseases. Consequently, JAK inhibition is a promising therapeutic strategy for the treatment of SpA as it offers the potential for improvements in multiple manifestations of PsA and AS.
A number of JAK inhibitors—including tofacitinib, baricitinib, peficitinib, filgotinib and upadacitinib—have demonstrated efficacy in other autoimmune conditions of relevance. To date, tofacitinib is the only JAK inhibitor that has been investigated in PsA and AS clinical trials and was recently approved for the treatment of PsA by the US Food and Drug Administration. Studies evaluating other agents within this MoA class are required in order to confirm JAK inhibition as an additional treatment option and to expand upon the available mechanistic information regarding their use in SpA. Thus far, the efficacy of JAK inhibitors demonstrated across a variety of SpA-associated disease manifestations indicates that they may be an important new oral therapy option for SpA. As further research is conducted and additional JAK inhibitors are evaluated in SpA, tofacitinib and other emerging JAK inhibitors may add to the available treatment options and provide clinically meaningful benefits for patients with SpA.
Acknowledgements
Medical writing support, under the guidance of the authors, was provided by Daniel Binks, PhD, at CMC Connect, a division of Complete Medical Communications Ltd, Macclesfield, UK and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461–4). All authors contributed to the conception and design of the review article, searched and reviewed the literature for relevant articles, developed content for the figures in the article, contributed to the writing of the first draft of the manuscript and critically reviewed subsequent drafts of the manuscript for intellectual content. The authors also met in 2016 and discussed the potential of JAK inhibitors in the context of the SpA concept. All authors read and approved the final draft for submission.
Funding: This work was funded by Pfizer Inc.
Disclosure statement: D.J.V. has received honoraria from, been a member of the speaker bureau for, and/or received research funding from AbbVie, Actelion, BMS, Janssen, MSD, Novartis, Pfizer Inc, Roche and UCB. D.M. has received honoraria from AbbVie, BMS, Celgene, Janssen, Novartis, Pfizer Inc and UCB. I.B.M. has received honoraria and/or research funding from AbbVie, AstraZeneca, BMS, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc and UCB. J.G.K. has received research funding from AbbVie, Amgen, Boehringer Ingelheim, BMS, EMD Serono, Innovaderm, Kineta, Leo Pharma, Novartis, Parexel, Pfizer Inc, Regeneron and Vitae; and been a consultant for AbbVie, Acros, Biogen Idec, Boehringer Ingelheim, Escalier, Janssen, Lilly, Novartis, Pfizer Inc, Roche and Valeant. C.T.R. has received research funding from AbbVie, Amgen and UCB; and been a consultant for AbbVie, Amgen, Celgene, Janssen, Novartis, Pfizer Inc, Sun and UCB. D.E. has received honoraria and/or research funding from AbbVie, Boehringer Ingelheim, BMS, Janssen, MSD, Novartis, Pfizer Inc, Roche and UCB. K.S.K, T.H, G.B, J.H and J-B.T are employees and stockholders of Pfizer Inc.
References
- 1. van der Horst-Bruinsma IE, Nurmohamed MT.. Management and evaluation of extra-articular manifestations in spondyloarthritis. Ther Adv Musculoskelet Dis 2012;4:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coates LC, Kavanaugh A, Mease PJ. et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71. [DOI] [PubMed] [Google Scholar]
- 3. Orr C, Veale DJ.. Is there a need for new agents with novel mechanisms of action in psoriatic arthritis? Ann Rheum Dis 2014;73:951–3. [DOI] [PubMed] [Google Scholar]
- 4. Fragoulis GE, Siebert S, McInnes IB.. Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu Rev Med 2016;67:337–53. [DOI] [PubMed] [Google Scholar]
- 5. McGonagle D, Aydin SZ, Gul A, Mahr A, Direskeneli H.. ’MHC-I-opathy’-unified concept for spondyloarthritis and Behcet disease. Nat Rev Rheumatol 2015;11:731–40. [DOI] [PubMed] [Google Scholar]
- 6. Ghoreschi K, Gadina M.. Jakpot! New small molecules in autoimmune and inflammatory diseases. Exp Dermatol 2014;23:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villarino AV, Kanno Y, O’Shea JJ.. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat Immunol 2017;18:374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A.. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 2013;72 (Suppl 2):ii111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benjamin M, McGonagle D.. Histopathologic changes at “synovio–entheseal complexes” suggesting a novel mechanism for synovitis in osteoarthritis and spondylarthritis. Arthritis Rheum 2007;56:3601–9. [DOI] [PubMed] [Google Scholar]
- 10. Baeten D, Breban M, Lories R, Schett G, Sieper J.. Are spondylarthritides related but distinct conditions or a single disease with a heterogeneous phenotype? Arthritis Rheum 2013;65:12–20. [DOI] [PubMed] [Google Scholar]
- 11. Ellinghaus D, Jostins L, Spain SL. et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet 2016;48:510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arakawa A, Siewert K, Stöhr J. et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med 2015;212:2203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menon B, Gullick NJ, Walter GJ. et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol 2014;66:1272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sherlock JP, Joyce-Shaikh B, Turner SP. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4–CD8– entheseal resident T cells. Nat Med 2012;18:1069–76. [DOI] [PubMed] [Google Scholar]
- 15. Jacques P, Lambrecht S, Verheugen E. et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis 2014;73:437–45. [DOI] [PubMed] [Google Scholar]
- 16. Nestle FO, Kaplan DH, Barker J.. Psoriasis. N Engl J Med 2009;361:496–509. [DOI] [PubMed] [Google Scholar]
- 17. Costello ME, Ciccia F, Willner D. et al. Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol 2015;67:686–91. [DOI] [PubMed] [Google Scholar]
- 18. Tito RY, Cypers H, Joossens M. et al. Brief report: dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol 2017;69:114–21. [DOI] [PubMed] [Google Scholar]
- 19. Van de Wiele T, Van Praet JT, Marzorati M, Drennan MB, Elewaut D.. How the microbiota shapes rheumatic diseases. Nat Rev Rheumatol 2016;12:398–411. [DOI] [PubMed] [Google Scholar]
- 20. Scher JU, Littman DR, Abramson SB.. Microbiome in inflammatory arthritis and human rheumatic diseases. Arthritis Rheumatol 2016;68:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghoreschi K, Laurence A, O’Shea JJ.. Janus kinases in immune cell signaling. Immunol Rev 2009;228:273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spits H, Bernink JH, Lanier L.. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol 2016;17:758–64. [DOI] [PubMed] [Google Scholar]
- 23. Langrish CL, McKenzie BS, Wilson NJ. et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 2004;202:96–105. [DOI] [PubMed] [Google Scholar]
- 24. Smith JA, Colbert RA.. The IL-23/IL-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol 2014;66:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sieper J, Braun J, Kay J. et al. Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann Rheum Dis 2015;74:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krueger J, Clark JD, Suárez-Fariñas M. et al. Tofacitinib attenuates pathologic immune pathways in patients with psoriasis: a randomized phase 2 study. J Allergy Clin Immunol 2016;137:1079–90. [DOI] [PubMed] [Google Scholar]
- 27. Gao W, McGarry T, Orr C. et al. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis 2016;75:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paine A, Ritchlin C.. Bone remodeling in psoriasis and psoriatic arthritis: an update. Curr Opin Rheumatol 2016;28:66–75. [DOI] [PubMed] [Google Scholar]
- 29. Braem K, Lories RJ.. Insights into the pathophysiology of ankylosing spondylitis: contributions from animal models. Joint Bone Spine 2012;79:243–8. [DOI] [PubMed] [Google Scholar]
- 30. Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD.. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27–associated human disorders. Cell 1990;63:1099–112. [DOI] [PubMed] [Google Scholar]
- 31. Milia AF, Ibba-Manneschi L, Manetti M. et al. Evidence for the prevention of enthesitis in HLA-B27/hβ(2)m transgenic rats treated with a monoclonal antibody against TNF-α. J Cell Mol Med 2011;15:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Tok M, van Duivenvoorde LM, Kramer I. et al. Anti-IL-17A treatment blocks inflammation, destruction and new bone formation in experimental spondyloarthritis in HLA-B27 transgenic rats. Arthritis Rheumatol 2015;67 (suppl 10) http://acrabstracts.org/abstract/anti-il-17a-treatment-blocks-inflammation-destruction-and-new-bone-formation-in-experimental-spondyloarthritis-in-hla-b27-transgenic-rats/ (20 March 2018, date last accessed). [Google Scholar]
- 33. Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G.. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 1999;10:387–98. [DOI] [PubMed] [Google Scholar]
- 34. Ruutu M, Thomas G, Steck R. et al. ß-glucan triggers spondylarthritis and Crohn’s disease–like ileitis in SKG mice. Arthritis Rheum 2012;64:2211–22. [DOI] [PubMed] [Google Scholar]
- 35. Reinhardt A, Yevsa T, Worbs T. et al. Interleukin-23–dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol 2016;68:2476–86. [DOI] [PubMed] [Google Scholar]
- 36. De Wilde K, Martens A, Lambrecht S. et al. A20 inhibition of STAT1 expression in myeloid cells: a novel endogenous regulatory mechanism preventing development of enthesitis. Ann Rheum Dis 2017;76:585–92. [DOI] [PubMed] [Google Scholar]
- 37. Yokota K, Sato K, Miyazaki T. et al. Combination of tumor necrosis factor α and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol 2014;66:121–9. [DOI] [PubMed] [Google Scholar]
- 38. Mikami Y, Asano M, Honda MJ, Takagi M.. Bone morphogenetic protein 2 and dexamethasone synergistically increase alkaline phosphatase levels through JAK/STAT signaling in C3H10T1/2 cells. J Cell Physiol 2010;223:123–33. [DOI] [PubMed] [Google Scholar]
- 39. Meyer DM, Jesson MI, Li X. et al. Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond) 2010;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dowty ME, Lin J, Ryder TF. et al. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a Janus kinase inhibitor, in humans. Drug Metab Dispos 2014;42:759–73. [DOI] [PubMed] [Google Scholar]
- 41. Hodge JA, Kawabata TT, Krishnaswami S. et al. The mechanism of action of tofacitinib – an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol 2016;34:318–28. [PubMed] [Google Scholar]
- 42. Mease P, Hall S, Fitzgerald O. et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017;377:1537–50. [DOI] [PubMed] [Google Scholar]
- 43. Gladman D, Rigby W, Azevedo VF. et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017;377:1525–36. [DOI] [PubMed] [Google Scholar]
- 44. van der Heijde D, Deodhar A, Wei JC. et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017;76:1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asquith M, Elewaut D, Lin P, Rosenbaum JT.. The role of the gut and microbes in the pathogenesis of spondyloarthritis. Best Pract Res Clin Rheumatol 2014;28:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Danese S, Grisham MB, Hodge J, Telliez JB.. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol 2016;310:G155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sandborn WJ, Su C, Sands BE. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 48. Panés J, Sandborn WJ, Schreiber S. et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vermeire S, Schreiber S, Petryka R. et al. Filgotinib, a selective JAK1 inhibitor, induces clinical remission in patients with moderate-to-severe Crohn’s disease: interim analysis from the Phase 2 FITZROY study. J Crohns Colitis 2016;10:S15. [Google Scholar]
- 50. Marks DJ. Defective innate immunity in inflammatory bowel disease: a Crohn’s disease exclusivity? Curr Opin Gastroenterol 2011;27:328–34. [DOI] [PubMed] [Google Scholar]
- 51. Bachelez H, van de Kerkof PC, Strohal R. et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386:552–61. [DOI] [PubMed] [Google Scholar]
- 52. Bissonnette R, Iversen L, Sofen H. et al. Tofacitinib withdrawal and retreatment in moderate-to-severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol 2015;172:1395–406. [DOI] [PubMed] [Google Scholar]
- 53. Papp KA, Menter A, Abe M. et al. Two Phase 3 studies of oral tofacitinib in patients with moderate to severe plaque psoriasis: 16-week efficacy and safety results. J Am Acad Dermatol 2015;72 (Suppl 1):AB66. [Google Scholar]
- 54. Ports WC, Khan S, Lan S. et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol 2013;169:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bissonnette R, Papp KA, Poulin Y. et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol 2016;175:902–11. [DOI] [PubMed] [Google Scholar]
- 56. Papp KA, Menter MA, Raman M. et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol 2016;174:1266–76. [DOI] [PubMed] [Google Scholar]
- 57. Liew SH, Nichols KK, Klamerus KJ. et al. Tofacitinib (CP-690, 550), a Janus kinase inhibitor for dry eye disease: results from a Phase 1/2 trial. Ophthalmology 2012;119:1328–35. [DOI] [PubMed] [Google Scholar]
- 58. Lin P, Suhler EB, Rosenbaum JT.. The future of uveitis treatment. Ophthalmology 2014;121:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Burmester GR, Blanco R, Charles-Schoeman C. et al. Tofacitinib (CP-690, 550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. [DOI] [PubMed] [Google Scholar]
- 60. Fleischmann R, Kremer J, Cush J. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. [DOI] [PubMed] [Google Scholar]
- 61. Kremer J, Li Z-G, Hall S. et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. [DOI] [PubMed] [Google Scholar]
- 62. Lee EB, Fleischmann R, Hall S. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. [DOI] [PubMed] [Google Scholar]
- 63. van der Heijde D, Tanaka Y, Fleischmann R. et al. Tofacitinib (CP-690, 550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 64. van Vollenhoven RF, Fleischmann R, Cohen S. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. [DOI] [PubMed] [Google Scholar]
- 65. Wollenhaupt J, Silverfield J, Lee EB. et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. [DOI] [PubMed] [Google Scholar]
- 66. Genovese MC, Kremer J, Zamani O. et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. [DOI] [PubMed] [Google Scholar]
- 67. Kremer JM, Emery P, Camp HS. et al. A Phase IIb study of ABT-494, a selective JAK-1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti-tumor necrosis factor therapy. Arthritis Rheumatol 2016;68:2867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Namour F, Diderichsen PM, Cox E. et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic modeling of filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of Phase IIb dose selection. Clin Pharmacokinet 2015;54:859–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kivitz AJ, Gutierrez-Ureña SR, Poiley J. et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol 2017;69:709–19. [DOI] [PubMed] [Google Scholar]
- 70. Ghoreschi K, Jesson MI, Li X. et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690, 550). J Immunol 2011;186:4234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dowty M, Lin T, Wang L. et al. Lack of differentiation of Janus kinase inhibitors in rheumatoid arthritis based on Janus kinase pharmacology and clinically meaningful concentrations. Ann Rheum Dis 2014;73 (Suppl 2):116. [Google Scholar]
- 72. Wollenhaupt J, Silverfield J, Lee EB. et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and clinical and radiographic efficacy in open-label, long-term extension studies over 7 years. Arthritis Rheumatol 2015;67:1645 (abstract).26013245 [Google Scholar]