Abstract
Background:
Mass cytometry (CyTOF) is a powerful tool for analyzing cellular networks at the single cell level. Due to the high-dimensional nature of this approach, analysis algorithms have been developed to visualize and interpret mass cytometry data. In this study, we applied these approaches to a cohort of patients with secondary acute myeloid leukemia (sAML).
Methods:
We utilized mass cytometry to interrogate localization and intensity of thrombopoietin-mediated intracellular signaling in sAML. Extracellular and intracellular phenotypes were dissected using SPADE, viSNE, and PhenoGraph.
Results:
Healthy controls exhibited highly localized signaling responses largely restricted to the hematopoietic stem/progenitor cell (HSPC) compartment. In contrast, sAML samples contained subpopulations outside the HSPC compartment exhibiting thrombopoietin (TPO) sensitivity comparable to or greater than immunophenotypically defined HSPCs. We employed unsupervised clustering by PhenoGraph to elucidate distinct subpopulations within these heterogenous samples. One metacluster composed of almost exclusively Lin− CD61+ CD34− CD38− CD45low cells was identified. This subpopulation was not readily identified by established manual gating approaches, and generally exhibited greater STAT phosphorylation in response to TPO stimulation than did Lin− CD34+ CD38− cells. Lin− CD61+ CD34− CD38− CD45low cells were identified in three additional sAML patients analyzed independently using a manual gating approach based upon the PhenoGraph results. Each patient exhibited a similar TPO hypersensitivity to the PhenoGraph metacluster.
Conclusions:
The identification of this cellular subpopulation highlights the limitations of manual gating in sAML. Our study demonstrates the potential for mass cytometry to elucidate rare subpopulations in highly heterogeneous tumors by utilizing unsupervised high dimensional analysis.
Keywords: mass cytometry, secondary AML, PhenoGraph, JAK/STAT signaling
Introduction
Secondary acute myeloid leukemia (sAML) is an acute leukemia that develops secondary to a myeloproliferative neoplasm (MPN), including myelofibrosis (MF), essential thrombocythemia (ET), or polycythemia vera (PV), as well as to myelodysplastic syndrome (MDS). Post-MPN sAML patients have an especially poor prognosis even when compared with de novo AML.(1) Furthermore, due to the low incidence of MPN, sAML is rare, leading to an insufficient number of studies characterizing its pathogenesis. In conjunction with the fact that AML is generally a disease characterized by tremendous phenotypic heterogeneity, characterization of sAML has been a great challenge.(2–5) One common feature of many sAML cases is hyperactive JAK-STAT signaling which can be driven by the JAK2 V617F mutation.(6,7) The functional role of this aberrant signaling has yet to be clearly delineated.
Mass cytometry (CyTOF) offers the potential to elucidate the signaling mechanisms driving sAML pathogenesis through comprehensive intracellular and extracellular profiling.(8) A typical experiment includes 35–40 antibodies and ~1,000,000 cells per sample.(9,10) This results in an extremely large amount of data to draw meaningful conclusions about using low-dimensional analysis by biaxials, histograms, or heatmaps. Furthermore, in cases of dysregulated hematopoiesis, it can be difficult to identify biologically distinct populations of cells solely using surface marker expression.(11,12) In this case, using methods in which only Lin− CD34+ CD38− cells, the canonical population of interest in AML,(13–15) are identified and analyzed may not be appropriate.(16) Cancer cells can have signaling characteristics typical of primitive cells while expressing incongruous surface markers.(11) Analysis of mass cytometry datasets can theoretically allow researchers to interrogate sAML patient samples on a single cell basis to identify functionally primitive populations. Mass cytometry also has the potential to pinpoint rare but relevant cell types in patient samples.(17) However, the challenge lies in determining which cells are relevant or functionally primitive, and how one can find distinct leukemic subpopulations without traditional immunophenotypic gating approaches.
In this study, a variety of data analysis tools including, viSNE,(18,19) SPADE,(19,20) and PhenoGraph(11) were applied to investigate certain biological questions in sAML. viSNE employs stochastic neighbor embedding to plot individual cells on the tSNE1 and tSNE2 axes, where their distance on the plot represents their difference in surface marker expression.(11,18) SPADE and PhenoGraph offer clustering algorithms that group cells together based upon their phenotypic similarities. This makes identifying unique cellular subpopulations possible. Phenograph’s clustering algorithm can be used in succession to first generate individual clusters from each patient and then further group all clusters into 10–20 distinct metaclusters, which represent the major cell populations found in the group of patients studied.
In this manuscript, we detail the incongruity between surface marker expression and function in sAML. We also compare our analysis to known aspects of MPN and AML pathogenesis, with regards to cytokine hypersensitivity, signaling networks, and surface marker expression.(13,17,21–23) We provide an example of how mass cytometry analysis techniques can be used to identify a subpopulation of cells with signaling characteristics of primitive cells despite surface marker expression typically assigned to mature cell types in a cohort of sAML patients. Additionally, we provide evidence that multiple such populations contribute to cytokine response and aberrant signaling in sAML. We suggest that these results demonstrate the importance of unsupervised high dimensional analysis of variegated hematologic malignancies.
Methods
Mass Cytometry Dataset
All data in these experiments was obtained utilizing peripheral blood samples collected from human patients in accordance with a protocol approved by the Washington University Human Studies Committee (WU no. 01–1014). Samples from nine sAML patients (transformed from four ET, three PV, and two PMF cases originally) and four normal volunteers were utilized for analysis. sAML data from multiple runs on the same machine with the same panel and antibody concentrations were combined. Gating was performed as described in Bendall et al.(9) and as described in Supplemental Figure S1. The panel used is detailed in Supplemental Table S1.
Mass Cytometry Data Analysis
Mass cytometry data was analyzed using Cytobank software.(24) Cytobank was used for gating and the generation of all heatmaps, biaxial plots, and histograms according to Bendall et al.(9) viSNE(18) analysis, an application of stochastic neighbor embedding (SNE), was performed as previously described, using Cytobank applications employing the Barnes-Hut implementation of t-SNE. Spanning Tree Progression of Density Normalized Events (SPADE) analysis was performed by first separating cells using viSNE and then applying the SPADE clustering algorithm on Cytobank.(19,20) Further details are provided in the Supplemental Experimental Protocols.
PhenoGraph
PhenoGraph was run using the Python-based application and as described in Levine, et al.(11) Metaclusters were generated from PhenoGraph clusters and exported to Cytobank for further analysis. See Supplemental Experimental Protocols for more details.
HSC Distance
Hematopoietic stem cell (HSC) distance was calculated using simple Euclidean distance from the viSNE map as described in Irish et al.(25) HSC distance is a metric of how similar a cell is immunophenotypically to a healthy HSC. See Supplementary Experimental Protocols for more details. HSCs were identified using manual gating for Lin− CD61− CD34+ CD38− CD90+ CD45RA− cells as described in Bendall et al.(9)
Results
sAML HSPCs are immunophenotypically and functionally distinct from normal HSCs
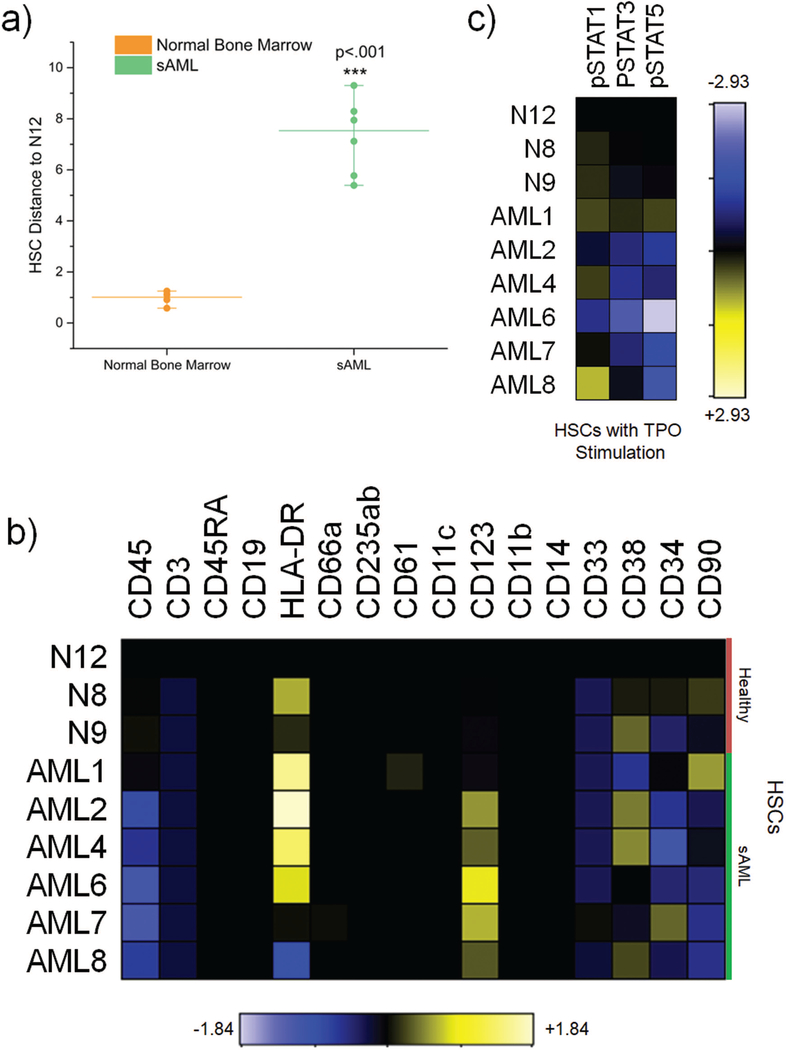
To characterize the incongruous nature of sAML surface marker expression, we aimed to use the high dimensional resolution of mass cytometry to interrogate quantitative differences between immunophenotypic HSCs in normal bone marrow and sAML. Initial analysis by HSC distance on the viSNE map revealed that sAML HSCs exhibited significant immunophenotypic differences compared to their healthy counterparts (Figure 1A). Additionally, sAML HSCs exhibited more interpatient variability than in HSCs from normal patients (Figure 1A). There was negligible difference observed between HSC distance of normal and sAML T-Cells to healthy HSCs, suggesting that the difference observed in normal and sAML HSCs was not a hematopoietic wide phenomenon (Supplemental Figure S2). The HSC distance of sAML non-lymphoid cells was significantly smaller than healthy non-lymphoid cells, suggestive that sAML myeloid cells are more phenotypically similar to HSCs and perhaps more primitive than their healthy counterparts (Supplemental Figure S2). Variability between healthy donor bone marrow was minimal. Furthermore, sAML HSCs were specifically characterized by enrichment of CD123 and diminished CD45 in five of six patients, while HLA-DR was enriched in four of six patients (Figure 1B). Additionally, in specific subsets of myeloid progenitors (CMPs, GMPs, and MEPs), CD90, a surface marker normally used to differentiate HSCs from progenitor populations, was enriched in most patients (Supplemental Figure S3). Other surface markers were variably expressed, suggesting a lack of a specific surface marker expression signature common to sAML HSCs across patients.
Figure 1.
sAML HSCs are immunophenotypically and functionally distinct from normal HSCs a) HSC Distance from HSCs in N12 from four healthy bone marrow compared and six secondary AML patients. Medians for each group are represented by the line. b) Heatmaps displaying medians normalized by arcsinh ratio to N12 HSCs for surface markers. c) Heatmaps displaying medians normalized by arcsinh ratio to N12 HSCs for signaling parameters in response to thrombopoietin (TPO) stimulation
In addition to surface marker expression, primitive cells are also characterized by their signaling response to cytokines. We stimulated normal and sAML HSCs with a number of cytokines, of which TPO proved to be the most effective in eliciting a strong signaling response through STAT proteins. HSCs from the majority of sAML patients were found to be hyposensitive to TPO stimulation compared to their healthy counterparts in terms of STAT3 and STAT5 phosphorylation (Figure1C & Supplemental Figure S4). This was somewhat contrary to expectation as TPO activates JAK-STAT signaling, which is known to be hyperactive in MPNs.(7,26,27) Together, these data demonstrate a lack of consistency between surface marker expression and corresponding healthy cell type in sAML.
viSNE analysis reveals aberrantly delocalized TPO response in sAML
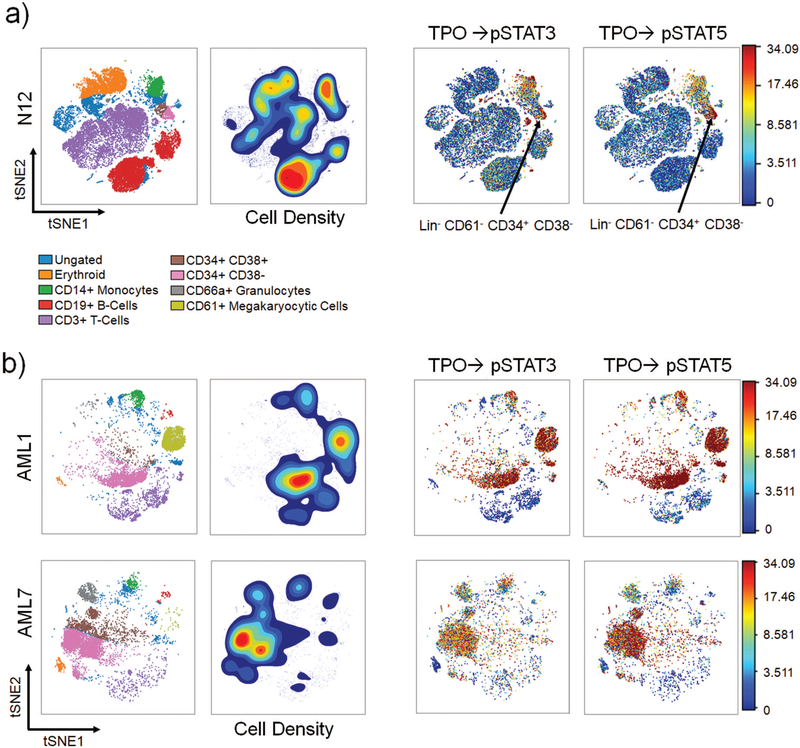
To characterize the cytokine response across the hematopoietic spectrum, viSNE analysis was employed (Figure 2, Supplemental Figure S5). In healthy donors, the pSTAT5 response to TPO primarily localized to the HSPC compartment, with the strongest response in the CD34+ CD38- cells (Figure 2A, Supplemental Figure S5A). The pSTAT3 response to TPO in healthy donors was seen in the HSPC compartment and to a lesser extent in monocytes (Figure 2A, Supplemental Figure S5A). In sAML, however, robust TPO responses were observed across multiple cell types, including CD61+ megakaryocytic cells, HSPCs, monocytes, and some cells that were not readily identified via this analysis (Figure 2B, Supplemental Figure S5B). In sAML, the viSNE maps were also characterized by an inability to resolve “islands”, or well-defined populations of cells, unlike the healthy viSNE maps (Figure 2). This was likely due to the proportion of AML blasts that were phenotypically similar, causing an aggregation of various myeloid cell populations together on the viSNE map. This type of analysis effectively showed the involvement of multiple lineages of cytokine-responsive cells in sAML but was limited in its capacity to clearly delineate specific cell populations.
Figure 2.
viSNE analysis reveal aberrantly delocalized and heterogeneous TPO response in sAML a) Far Left: Healthy viSNE map with labeled populations. Left: Density contour viSNE map where red represents the greatest density and blue represents the least. Right: viSNE map showing response to TPO colored by phospho-marker intensity where red represents the strongest signal and blue represents the least. The arrow marks the HSPC compartment in healthy bone marrow; b) Far Left: sAML viSNE map with labeled populations. Left: Density contour viSNE map where red represents the greatest density and blue represents the least. Right: viSNE map showing response to TPO colored by phospho-marker intensity
SPADE highlights heterogeneity in TPO response within canonical cellular populations
To attempt to resolve distinct TPO-hypersensitive subpopulations, hierarchical clustering was performed to generate SPADE trees from the viSNE data (Supplemental Figure S6). While the SPADE analysis revealed a near uniform pSTAT3/5 response to TPO in healthy HSPCs, substantial variability was observed in the sAML HSPCs (Supplemental Figure 7A&B). SPADE clusters were frequently heterogeneous precluding the identification of distinct subpopulations. SPADE identified two populations of CD34+ CD38+ cells, and three populations of CD61+ cells. These populations demonstrate that the density-dependent down sampling of SPADE analysis can create the challenge of determining whether very small clusters are biologically relevant populations of cells.
PhenoGraph metaclustering reveals shared distinct subpopulations in sAML
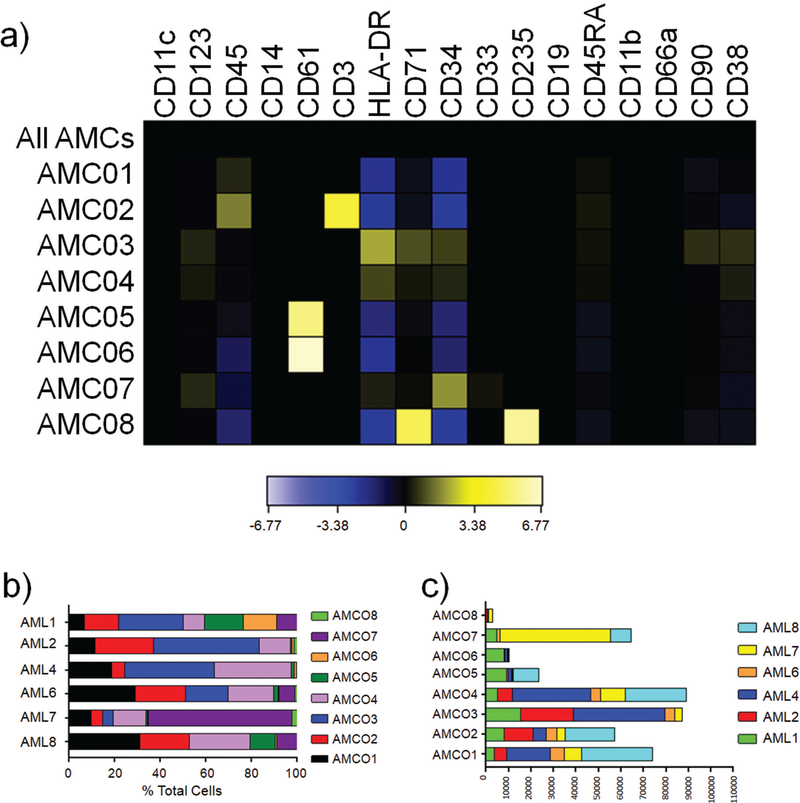
PhenoGraph employs a two-step approach that overcomes some of the limitations of SPADE.(11) PhenoGraph clustering is characterized by a high degree of reproducibility and takes a community detection approach to clustering that results in high resolution of rare populations (>1/2000 cells). This approach was chosen due to our interest in rare subpopulations, which were unable to be resolved by SPADE or viSNE. PhenoGraph clustering generated eight sAML metaclusters and ten healthy metaclusters (Figure 3A, Supplemental Figure S8A). sAML and healthy donors were analyzed separately so that healthy cell types exhibiting canonical surface marker expression would not be grouped with diseased cells displaying aberrant surface marker expression. In healthy donors, each metacluster could be characterized by known cell types or lineages (Supplemental Figure S8B). However, in sAML, the majority of clusters were subsets of CD34+ cells or CD61+ cells that were not readily distinguishable by manual gating (Figure 3A). These metaclusters were resolved by their community membership, reflecting a high number of shared phenotypic neighbors, rather than by a binomial surface marker designation (i.e. CD34 positivity, CD38 negativity, etc.). AML Metacluster 3 (AMC03), AMC04, and AMC07 represented CD34+ clusters with differential CD90 and CD38 expression (Figure 3A, Supplemental Figure S9). The relative proportion of these three CD34+ metaclusters within individual patients was highly variable, which could be suggestive of variable clonal dynamics within each patient (Figure 3B). AMC02 and AMC08 were identified as distinct populations of T-cells and erythroid lineage cells by PhenoGraph. A second viSNE analysis was performed with pooled samples representing each metacluster across all six sAML patients, which revealed that AMC07 was composed primarily of CD34+CD38−HLA-DR− cells, AMC04 of CD34+CD38+ cells, and AMC03 of CD34+HLA-DR+ cells, suggestive of a late HSPC cell type (Supplemental Figure S10). This suggests that PhenoGraph is able to recover multiple cell types in sAML. Furthermore, each metacluster contained cells from every patient, excepting AML1 and AML8 in AMC08, AML2 and AML4 in AMC07, and AML8 in AMC03 (Figure 3C). This data suggests that although sAML is characterized by obvious heterogeneity, there are important features conserved across patients as well.
Figure 3.
PhenoGraph metaclustering reveals shared distinct subpopulations in sAML a) Heatmaps showing surface marker expression of AML MCs normalized using arcsinh ratio to a concatenated file containing all AML MCs as a background. b) Bar graph showing the percentage of each AML MC that makes up the sum of the patient’s cells c) Bar graph showing the extent to which each patient’s cells are represented in each AML MC
PhenoGraph AMC06 represents a distinct cytokine hypersensitive subset of CD61 + cells
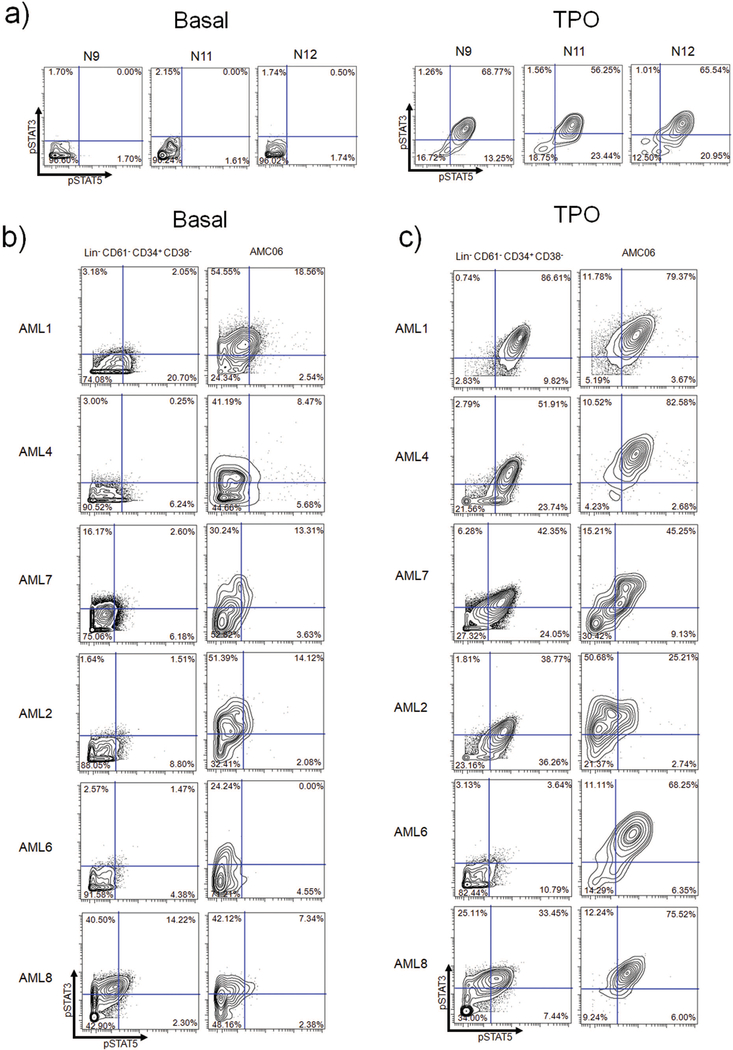
Lin− CD61+ CD34+ CD38− cells from healthy donors responded robustly to TPO stimulation in terms of STAT3/5 phosphorylation downstream of JAK2 (Figure 4A). We analyzed each metacluster to identify potentially unique populations with regards to basal and TPO-stimulated STAT3/5 phosphorylation. AMC06 stood out from the rest of the metaclusters in terms of this signaling behavior, exhibiting higher basal pSTAT3 levels (compared to Lin− CD61+ CD34+ CD38− cells) in five of six patients (Figure 4B). In four patients (AML4, AML6, AML7 and AML8), AMC06 displayed a higher percentage of pSTAT3/5 double positive cells in response to TPO compared to their Lin− CD61+ CD34+ CD38− cell counterparts (Figure 4C). All patients had a greater pSTAT3 response to TPO (Figure 4C). Notably, only AML1, AML2, and AML7 were JAK2 V617F-positive, suggesting that this phenomenon was independent of JAK2 V617F (Supplemental Table S2).
Figure 4.
PhenoGraph AMC06 represents a distinct TPO hypersensitive subset of CD61+ cells a) Normal bone marrow (N9, N11, N12) Lin− CD61− CD34+ CD38− cells basally (left) and TPO stimulated (right) b) Biaxials showing STAT3 and STAT5 basal phosphorylation in sAML Lin− CD61− CD34+ CD38− cells compared to cells from AMC06 in each patient. C) Biaxials showing STAT3 and STAT5 TPO-mediated phosphorylation in sAML Lin− CD61− CD34+ CD38− cells compared to cells from AMC06 in each patient. Quadrant gating of sAML patients based off of basal levels of corresponding normal bone marrow used in original CyTOF experiments (N9 for AML1 and AML4, N11 for AML2 and AML8, N12 for AML6 and AML7).
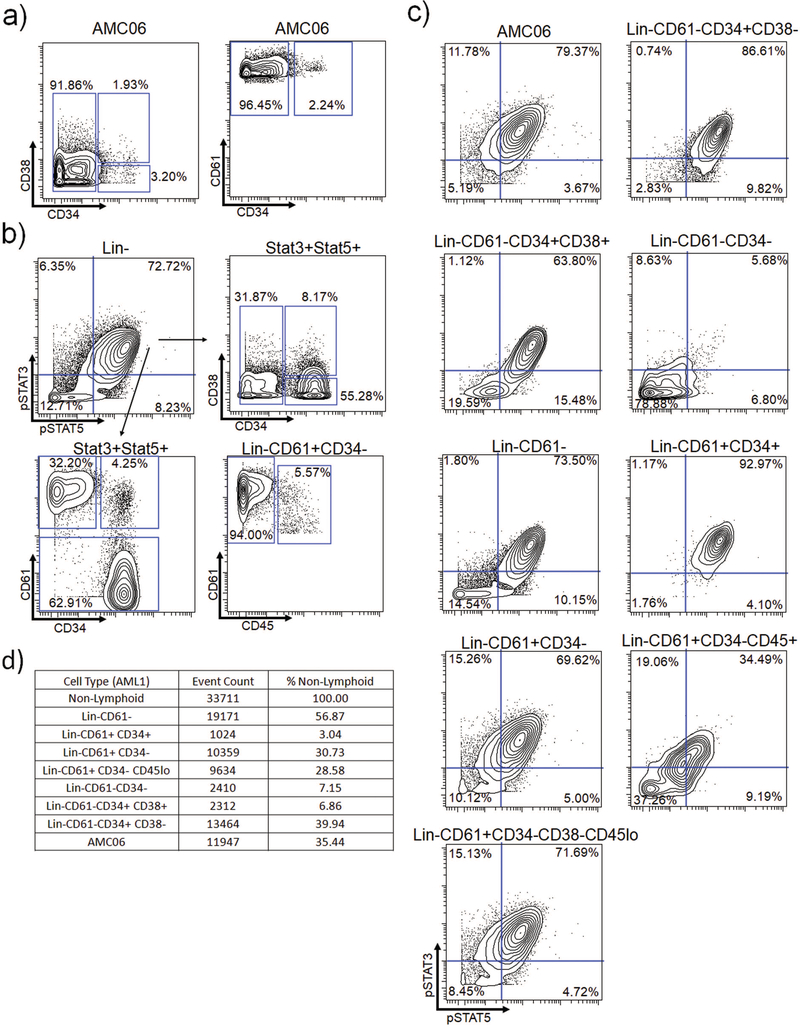
AMC06 is composed primarily of a Lin− CD61+ CD34− CD38− CD45low population
To determine if a similar population could be identified via manual gating approaches, we first attempted to delineate the characteristics of AMC06 using biaxial plots. AMC06 was composed of cells expressing relatively homogenous levels of CD45, CD61, and CD34, with varying levels of CD38 and CD90 (Supplemental Figure S11). Somewhat contrary to expectation, the majority of cells comprising AMC06 were found to be CD34 negative (Figure 5A, Supplemental Figure S12A). Notably, AMC06 exhibited uniformly high CD61 expression, with the vast majority of the cells being CD34−, although a small percentage of CD61+ CD34+ megakaryoblasts were present (Figure 5A, Supplemental Figure S12A).
Figure 5.
AMC06 is composed primarily of Lin− CD61+ CD34− CD45low cells a) AMC06 biaxials showing CD34, CD38 and CD61 expression b) Top: AMC06 biaxial showing pSTAT3 and pSTAT5 and gating on the pSTAT3/5 double positive population. Bottom: Biaxials showing surface marker expression in the pSTAT3/5 double positive population. c) Biaxial showing pSTAT3 and pSTAT5 in AMC06 and manually gated cell populations in response to TPO d) Raw number and percentages of each population of cells in AML1
In an attempt to identify the cells of interest through a back-gating approach, the surface marker characteristics of TPO stimulated Lin− pSTAT3/5 double positive cells in AML1 were examined. The majority of these cytokine-responsive cells were found to be CD34+ and CD61−, implying that they were not the same cells from AMC06 (Figure 5B, Supplemental Figure S12B). However, 32% of the pSTAT3/5 double positive subpopulation fell in the CD61+ CD34− gate, indicating a mixed population of cytokine-responsive cells comprising at least two distinct subsets.
Alternatively, cell populations defined by traditional biaxial gating were examined to identify cells similar in TPO response to AMC06 (Figure 5C, Supplemental Figure S12C). CD61+ CD34+ megakaryoblasts displayed a slightly stronger pSTAT3/5 response than AMC06. CD61+ CD34+ megakaryoblasts, an established cellular subpopulation, were less abundant than AMC06 in AML1 and of similar abundance to AMC06 in AML4, whose smaller population was more representative of the analyzed sAML patients (Figure 5D, Supplemental Figure S12D). These findings suggest that AMC06 may not be too rare to be biologically relevant.
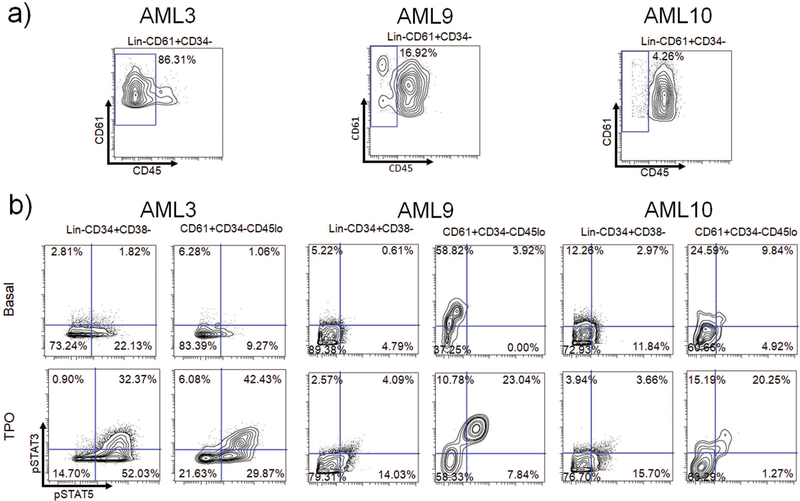
Using the surface marker expression data of metaclusters defined by PhenoGraph, we aimed to manually find a gating scheme that could lead to AMC06. Lineage negativity, CD61 expression, CD34 negativity, CD38 negativity, and importantly, diminished CD45 expression characterized AMC06. This subpopulation (Lin− CD61+ CD34− CD38− CD45low) appeared to be highly similar in pSTAT3/5 response to TPO as AMC06 (Figure 5C), which was consistent with the signaling profile identified in Figure 4. Only a few cells of a comparable population of Lin−CD61+CD38−CD45low in normal samples were identified, and the TPO response in these cells did not result in the strong STAT phosphorylation observed in the AML AMC06 population (Supplemental Figure S13). These data show that PhenoGraph metacluster analysis was able to identify a rare population that was difficult to discern intuitively via traditional gating approaches. The presence of this rare population of Lin− CD61+ CD34− CD38− CD45low cells was verified in three separate sAML patients not included in the PhenoGraph cluster analysis (Figure 6A). In all three patients, a heightened TPO-induced pSTAT3/5 response compared to Lin− CD61− CD34+ CD38− cells was observed (Figure 6B). STAT1 hypersensitivity to TPO was also noted in the post-ET sAML patients compared to healthy Lin− CD34+ CD38− cells. (Supplemental Figure S14A).
Figure 6.
Lin− CD61+ CD34− CD45low cells are present in additional sAML patients and exhibit TPO hypersensitivity a) Biaxials showing CD61 and CD45 expression in Lin− CD61+ CD34− cells in 3 separate sAML patient samples analyzed by Mass cytometry. b) upper: Biaxials showing STAT3 and STAT5 basal phosphorylation in sAML Lin− CD34+ CD38− cells compared to Lin− CD61+ CD34− CD45low cells from in each patient; lower: Biaxials showing STAT3 and STAT5 TPO-mediated phosphorylation in sAML Lin− CD34+ CD38− cells compared to Lin− CD61+ CD34− CD45low cells in each patient
AMC06 also had a more robust response than healthy Lin− CD61− CD34+ CD38− cells in the NFĸB signaling pathway as a result of TNFα stimulation, as measured by p-p65/NFĸB and total tIĸBα levels (Supplemental Figure S14B). This pathway has recently been demonstrated by our group as being commonly hyperactive in sAML(28). AMC06 also exhibited higher Ki67 levels than normal Lin− CD61− CD34+ CD38− cells in all patients examined, suggestive of a higher rate of proliferation (Supplemental Figure S15). Taken together, these results reveal a population of cytokine-hypersensitive cells in each sAML patient, defined here as displaying increased overall ability to activate JAK-STAT and/or NFkB signaling compared to normal Lin− CD61− CD34+ CD38− cells.
Discussion
Our study expands on the concept proposed in recent studies(11,16) that in AML, surface marker expression does not strictly imply function in myeloid lineage cells. It is also reasonable to expect that functionally primitive but phenotypically heterogeneous populations could arise as a result of clonal evolution, a phenomenon which has been documented extensively in secondary AML.(29–32) While a vast array of tools exist to document this heterogeneity including genomic, transcriptomic, and proteomic approaches, dissecting heterogeneous samples to identify specific rare leukemic populations has been a challenge. This study uses mass cytometry and associated analyses to illustrate approaches to computationally identify and analyze these said populations.
Using mass cytometry, we obtained a comprehensive immunophenotypic profile of sAML patient samples. In our initial studies, we observed an apparent hyposensitivity of immunophenotypic HSCs to TPO in sAML. However, an HSC-like immunophenotypic shift was observed in the non-lymphoid compartment. This suggests some amount of breakdown of surface marker designation of stem cells in sAML, which could make it difficult to study the stem cells relevant to leukemia. The shift was characterized by CD123 (IL3Rα) and HLA-DR enrichment in most of the patients, a result which was mirrored by a similar study in AML.(33) In the aforementioned study, these markers were found to be associated with patients harboring high-risk cytogenetics. These findings in conjunction with the very low observed survival (8/9 patients deceased due to AML related causes), were seemingly at odds with the data indicating that immunophenotypic HSCs were hyposensitive to cytokine stimulation. Logically, there should be some other population with hyperactive signaling driving the leukemic phenotype in these patients.
Analysis by viSNE, SPADE, and PhenoGraph in this manuscript offer an example of how high dimensional analyses can reveal these hyperactive subpopulations. Based upon the hypothesis that distinct cell subsets exhibiting hyperactive signaling existed in these sAML patients, we subjected the data to viSNE and SPADE analysis. viSNE analysis revealed substantial inter-patient heterogeneity but also conserved features shared across the cohort. Within each individual, the majority of cells were concentrated in different parts of the viSNE map, potentially representing a unique, patient-specific clonal architecture. However, each patient shared a robust and widespread pSTAT3/5 response to TPO. This analysis confirmed the existence of a hyperactive population(s) of cells but was unable to delineate the specific cell subsets given the delocalized nature of the response. SPADE analysis yielded similar results, demonstrating that hierarchical clustering or stochastic neighbor embedding alone were insufficient to identify the relevant leukemic subpopulations.
To overcome this, we employed PhenoGraph, which uses a combination of k-nearest neighbor (kNN) and social networking algorithms to group phenotypically similar cells while distinguishing rare cell populations that do not share enough connections with the more prominent communities. This method has the potential to be more robust to noise than either SPADE or viSNE, due to its ability to preserve the interconnectedness of cellular populations in clustering and dimensionality reduction. Additionally, PhenoGraph performs an unsupervised analysis that does not make assumptions regarding population representation as SPADE does, which can allow resolution of rare but distinct subpopulations.
The results of the analysis revealed a subpopulation of CD61+ cells that were functionally distinct in their strong pSTAT3/5 response to TPO but immunophenotypically heterogeneous. The cells in AMC06 responded fairly uniformly to TPO stimulation but comprised a mixed population of cells that would be primarily labeled as CD61+ CD34− cells with a small percentage of CD61+ CD34+ megakaryoblasts. The cells in AMC06 displayed higher basal and stimulated pSTAT3, a phenomenon reported in both JAK2 V617F and JAK2 WT MPNs.(34) pSTAT5, however, did not differ significantly basally or with TPO stimulation. The TPO receptor pathway is highly implicated in MPN disease pathogenesis, suggesting that these TPO-hypersensitive populations could be important in better understanding sAML pathogenesis as well the specific role of STAT3 in MPNs.(35) AMC06 also responded more robustly to TNFα than healthy primitive cells, indicating that the observed cytokine hypersensitivity was not restricted solely to TPO and the expression of megakaryocytic lineage markers.(CD61) NFĸB signaling has been shown to be a characteristic of undifferentiated cells, further indicating that AMC06 may have some stem/progenitor-like characteristics.(36,37) This is consistent with a recent study from our group reporting abnormal activation of NFĸB signaling in HSPCs in myelofibrosis and sAML.(28)
These cells were notably lacking CD34, which would typically exclude them from any supervised analysis that uses assumptions about surface marker expression to define primitive vs. mature cell types. Although lineage negative CD34− cells have been studied and shown to have bone marrow repopulating potential in some settings,(38) our study identified a specific CD34− population expressing a lineage marker that was conserved across multiple patients. Although more extensive studies are required to determine the leukemia-initiating potential of this cell population, our data shows that these cells were behaving in a fashion characteristic of classically primitive cells with regards to STAT signaling and TPO response. PhenoGraph was thus able to identify a functionally primitive but mature-labeled cellular subpopulation characterized by cytokine hypersensitivity in a situation where viSNE and SPADE were insufficient. Additionally, our results demonstrate how manual gating approaches may fall short in isolating distinct populations of functionally primitive cells obscured by a non-homogenous response to cytokine stimulation. These findings are in accordance with studies that have suggested unsupervised gating as particularly advantageous in AML, due to the vast heterogeneity of the disease.(39–41)
Taken together, our study represents a methodology using mass cytometry and high dimensional analysis to identify and characterize a novel subset of functionally primitive cells defined by cytokine hypersensitivity and a predominantly CD34− phenotype. The CD61+ CD34− population of cells could play an important role in sAML pathogenesis. Further, our data suggests that sAML immunophenotypic HSCs may frequently be characterized by cytokine hyposensitivity, while certain populations expressing mature-lineage markers could mediate a hypersensitive cytokine response in sAML. Our analysis highlights the extent of incongruity between surface defined primitive cells and perturbation-response defined primitive cells. Thus, we postulate that the combination of mass cytometry and high dimensional, unsupervised analysis is an important approach in furthering our understanding of the underlying cellular mechanics of sAML and other highly heterogeneous diseases.
Supplementary Material
Acknowledgements
This work was supported by NIH grants K08HL106576 (Oh) and T32HL007088 (Fisher, Fowles). This research was also supported by an American Cancer Society Postdoctoral Fellowship (Fisher). This work was also supported by a Doris Duke-Damon Runyon Clinical Investigator Award (Oh). Additional support was provided by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences of NIH. Support for patient sample collection and processing was provided by NIH grant P01CA101937. Technical support was provided by the Alvin J. Siteman Cancer Center Tissue Procurement Core Facility, Flow Cytometry Core, and Immunomonitoring Laboratory, which are supported by NCI Cancer Center Support Grant P30CA91842. The Immunomonitoring Laboratory is also supported by the Bursky Center for Human Immunology and Immunotherapy Programs. The authors thank O. Malkova and C. Miner for assistance with mass cytometry experiments, and K. Luber and M. Allen for assistance with clinical samples and data. None of the authors have a conflict of interest to disclose.
References
- 1.Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best Pract Res Clin Haematol 2007;20:29–37. [DOI] [PubMed] [Google Scholar]
- 2.Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood 2008;111:3941–3967. [DOI] [PubMed] [Google Scholar]
- 3.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 2012;12:323–334. [DOI] [PubMed] [Google Scholar]
- 4.Rosen JM, Jordan CT. The Increasing Complexity of the Cancer Stem Cell Paradigm. Science (New York, N.Y.) 2009;324:1670–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, Swider CR, Strzelecki AC, Cavelier C, Recher C and others. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest 2011;121:384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N and others. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61. [DOI] [PubMed] [Google Scholar]
- 7.Skoda RC, Duek A, Grisouard J. Pathogenesis of myeloproliferative neoplasms. Exp Hematol 2015;43:599–608. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy N Heterogeneity: A multidimensional overview. Nat Rev Cancer 2013;13:439. [DOI] [PubMed] [Google Scholar]
- 9.Bendall SC, Simonds EF, Qiu P, Amir E-aD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI and others. Single-Cell Mass Cytometry of Differential Immune and Drug Responses Across a Human Hematopoietic Continuum. Science 2011;332:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandyopadhyay S, Fisher DAC, Malkova O, Oh ST. Analysis of Signaling Networks at the Single-Cell Level Using Mass Cytometry. Methods Mol Biol 2017;1636:371–392. [DOI] [PubMed] [Google Scholar]
- 11.Levine Jacob H, Simonds Erin F, Bendall Sean C, Davis Kara L, Amir E-ad D, Tadmor Michelle D, Litvin O, Fienberg Harris G, Jager A, Zunder Eli R and others. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell 2015;162:184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horton SJ, Huntly BJ. Recent advances in acute myeloid leukemia stem cell biology. Haematologica 2012;97:966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730–737. [DOI] [PubMed] [Google Scholar]
- 14.Pearce DJ, Taussig D, Zibara K, Smith L-L, Ridler CM, Preudhomme C, Young BD, Rohatiner AZ, Lister TA, Bonnet D. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood 2006;107:1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A 1997;94:5320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taussig DC, Vargaftig J, Miraki-Moud F, Griessinger E, Sharrock K, Luke T, Lillington D, Oakervee H, Cavenagh J, Agrawal SG and others. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(−) fraction. Blood 2010;115:1976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irish JM, Hovland R, Krutzik PO, Perez OD, Bruserud O, Gjertsen BT, Nolan GP. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell 2004;118:217–28. [DOI] [PubMed] [Google Scholar]
- 18.Amir E-aD Davis KL, Tadmor MD Simonds EF, Levine JH Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe’er D viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotech 2013;31:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diggins KE, Ferrell PB Jr., Irish JM Methods for discovery and characterization of cell subsets in high dimensional mass cytometry data. Methods 2015;82:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu P, Simonds EF, Bendall SC, Gibbs KD Jr, Bruggner RV, Linderman MD, Sachs K, Nolan GP, Plevritis SK. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat Biotech 2011;29:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotecha N, Flores NJ, Irish JM, Simonds EF, Sakai DS, Archambeault S, Diaz-Flores E, Coram M, Shannon KM, Nolan GP and others. Single-cell profiling identifies aberrant STAT5 activation in myeloid malignancies with specific clinical and biologic correlates. Cancer Cell 2008;14:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwig L, Kessler H, Wagner M, Hoang-Vu C, Dralle H, Adler G, Bohm BO, Schmid RM. Nuclear factor-kappaB is constitutively active in C-cell carcinoma and required for RET-induced transformation. Cancer Res 2001;61:4526–35. [PubMed] [Google Scholar]
- 23.Gibbs KD Jr., Gilbert PM, Sachs K, Zhao F, Blau HM, Weissman IL, Nolan GP, Majeti R Single-cell phospho-specific flow cytometric analysis demonstrates biochemical and functional heterogeneity in human hematopoietic stem and progenitor compartments. Blood 2011;117:4226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotecha N, Krutzik PO, Irish JM. Web-based analysis and publication of flow cytometry experiments. Curr Protoc Cytom 2010;Chapter 10:Unit10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrell PB Jr., Diggins KE, Polikowsky HG, Mohan SR, Seegmiller AC, Irish JM High-Dimensional Analysis of Acute Myeloid Leukemia Reveals Phenotypic Changes in Persistent Cells during Induction Therapy. PLoS One 2016;11:e0153207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. Journal of Cell Science 2004;117:1281–1283. [DOI] [PubMed] [Google Scholar]
- 27.Drachman JG, Millett KM, Kaushansky K. Thrombopoietin signal transduction requires functional JAK2, not TYK2. J Biol Chem 1999;274:13480–4. [DOI] [PubMed] [Google Scholar]
- 28.Fisher DAC, Malkova O, Engle EK, Miner CA, Fulbright MC, Behbehani GK, Collins TB, Bandyopadhyay S, Zhou A, Nolan GP and others. Mass cytometry analysis reveals hyperactive NF Kappa B signaling in myelofibrosis and secondary acute myeloid leukemia. Leukemia 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klco Jeffery M, Spencer David H, Miller Christopher A, Griffith M, Lamprecht Tamara L, O’Laughlin M, Fronick C, Magrini V Demeter Ryan T, Fulton Robert S and others. Functional Heterogeneity of Genetically Defined Subclones in Acute Myeloid Leukemia. Cancer Cell;25:379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes AE, Magrini V, Demeter R, Miller CA, Fulton R, Fulton LL, Eades WC, Elliott K, Heath S, Westervelt P and others. Clonal architecture of secondary acute myeloid leukemia defined by single-cell sequencing. PLoS Genet 2014;10:e1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, Chen K, Larson DE, McLellan MD, Dooling D, Abbott R and others. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med 2012;366:1090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engle EK, Fisher DA, Miller CA, McLellan MD, Fulton RS, Moore DM, Wilson RK, Ley TJ, Oh ST. Clonal evolution revealed by whole genome sequencing in a case of primary myelofibrosis transformed to secondary acute myeloid leukemia. Leukemia 2015;29:869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behbehani GK, Samusik N, Bjornson ZB, Fantl WJ, Medeiros BC, Nolan GP. Mass Cytometric Functional Profiling of Acute Myeloid Leukemia Defines Cell-Cycle and Immunophenotypic Properties That Correlate with Known Responses to Therapy. Cancer Discov 2015;5:988–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anand S, Stedham F, Gudgin E, Campbell P, Beer P, Green AR, Huntly BJP. Increased basal intracellular signaling patterns do not correlate with JAK2 genotype in human myeloproliferative neoplasms. Blood 2011;118:1610–1621. [DOI] [PubMed] [Google Scholar]
- 35.Kota J, Caceres N, Constantinescu SN. Aberrant signal transduction pathways in myeloproliferative neoplasms. Leukemia 2008;22:1828–1840. [DOI] [PubMed] [Google Scholar]
- 36.Takase O, Yoshikawa M, Idei M, Hirahashi J, Fujita T, Takato T, Isagawa T, Nagae G, Suemori H, Aburatani H and others. The Role of NF-?B Signaling in the Maintenance of Pluripotency of Human Induced Pluripotent Stem Cells. PLoS ONE 2013;8:e56399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagoya Y, Yoshimi A, Kataoka K, Nakagawa M, Kumano K, Arai S, Kobayashi H, Saito T, Iwakura Y, Kurokawa M. Positive feedback between NF-κB and TNF-α promotes leukemia-initiating cell capacity. The Journal of Clinical Investigation;124:528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dooley DC, Oppenlander BK, Xiao M. Analysis of primitive CD34- and CD34+ hematopoietic cells from adults: gain and loss of CD34 antigen by undifferentiated cells are closely linked to proliferative status in culture. Stem Cells 2004;22:556–69. [DOI] [PubMed] [Google Scholar]
- 39.Aghaeepour N, Finak G, Hoos H, Mosmann TR, Gottardo R, Brinkman R, Scheuermann RH. CRITICAL ASSESSMENT OF AUTOMATED FLOW CYTOMETRY DATA ANALYSIS TECHNIQUES. Nat Methods 2013;10:228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyne S, Hu X, Wang K, Rossin E, Lin T-I, Maier LM, Baecher-Allan C, McLachlan GJ, Tamayo P, Hafler DA and others. Automated high-dimensional flow cytometric data analysis. Proceedings of the National Academy of Sciences 2009;106:8519–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruggner RV, Bodenmiller B, Dill DL, Tibshirani RJ, Nolan GP. Automated identification of stratifying signatures in cellular subpopulations. Proc Natl Acad Sci U S A 2014;111:E2770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.