Abstract
Due to the worldwide prevalence of multidrug-resistant pathogens and high incidence of diseases such as cancer, there is an urgent need for the discovery and development of new drugs. Nearly half of the FDA-approved drugs are derived from natural products that are produced by living organisms, mainly bacteria, fungi, and plants. Commercial development is often limited by the low yield of the desired compounds expressed by the native producers. In addition, recent advances in whole genome sequencing and bioinformatics have revealed an abundance of cryptic biosynthetic gene clusters within microbial genomes. Genetic manipulation of clusters in the native host is commonly used to awaken poorly expressed or silent gene clusters, however, the lack of feasible genetic manipulation systems in many strains often hinders our ability to engineer the native producers. The transfer of gene clusters into heterologous hosts for expression of partial or entire biosynthetic pathways is an approach that can be used to overcome this limitation. Heterologous expression also facilitates the chimeric fusion of different biosynthetic pathways, leading to the generation of “unnatural” natural products. The genus Streptomyces is especially known to be a prolific source of drugs/antibiotics, its members are often used as heterologous expression hosts. In this review, we summarize recent applications of Streptomyces species, S. coelicolor, S. lividans, S. albus, S. venezuelae and S. avermitilis, as heterologous expression systems.
Keywords: Streptomyces, Natural Products, Heterologous Expression, Biosynthetic Gene Clusters, Combinatorial Biosynthesis
1. Introduction
Natural products (NPs) have played incredibly important roles in human medicine. Nearly half of all approved therapeutics are derived directly from or can find roots in NPs. Since the discovery of penicillin and streptomycin, drugs derived from NPs have been used for almost all human diseases: infectious, cardiovascular, neurological, oncological, and more recently, depressive disorders (Dias et al., 2012; Newman and Cragg, 2016). They are equally important in agriculture, veterinary medicine, and the food industry (Zhou et al., 2008; Bekiesch et al., 2016). NPs are mainly produced by microorganisms and plants. Among the microorganisms, the genus Streptomyces is of particular interest as it has been the source of more than half of currently used antibiotics (Watve et al., 2001; Berdy, 2012; Bibb, 2013; Bekiesch et al., 2016). Since the report of the S. coelicolor A3(2) genome in 2002 (Bentley et al., 2002), more than 5,200 actinomycete genomes have been registered in the Genome OnLine Database (Reddy et al., 2015). Under current laboratory cultivation conditions, only a few major NPs are usually detected from a microbial source, whereas its genome typically shows the presence of 20~40 biosynthetic gene clusters (BGCs). Over 90% BGCs thus remain unexamined. The uncultivated microorganisms, estimated to represent ~99% of the microbial life on this planet, are an equally important source of NPs. Advances in metagenomics have made it possible to access these vast unexploited regions of “dark matter”. New tools and technologies for analyzing and mining genomes/metagenomes are allowing NPs to re-emerge as an attractive resource for drug discovery and are revolutionizing natural product and drug discovery research (Blin et al., 2017a; Douglas et al., 2015; Ziemert et al., 2016).
Heterologous expression plays an indispensable role in the study of NPs and drug discovery (Rappe and Giovannoni, 2003; Craig et al., 2009). Normally, there are several limitations to produce NPs and to study or manipulate their biosynthesis in native strains: (1) low yield; (2) slow-growth; and (3) difficulties in genetic manipulation. In addition, as revealed by recent genomics advances, the majority of BGCs for NPs remain cryptic/unexpressed in native producers. The expression of genes or BGCs in a genetically well-studied, and robustly growing host, i.e., heterologous expression, provides an efficient alternative to overcome these limitations. In addition, heterologous expression has also been widely used to determine the boundaries of a BGC, to create unnatural NPs via combinatorial biosynthesis, and to study the functions of individual genes (Du et al., 2013; Komatsu et al., 2013; Liu et al., 2018; Park et al., 2011a; Waldman et al., 2015). Modifying the architecture, even with a minor structural change, can significantly alter/improve the biological activity of a compound, an important stage in drug discovery (Cummings et al., 2014; O’Connor, 2015). Heterologous expression is an indispensable tool in studying uncultivable microorganisms. It has been estimated that only about 1% of microorganisms can be cultured. With significant advances in next-generation sequencing (NGS), bio informatics tools to assemble and analyze genomes and to predict BGCs, and cloning vectors and techniques for large DNA fragments, it has become possible to access this vast unexplored resource for the discovery of new NPs through heterologous expression (Brady et al., 2009; Zhang et al., 2017a).
Escherichia coli, Pseudomonas putida, Saccharomyces cerevisiae, Streptomyces spp., and Myxococcus xanthus are often used as heterologous hosts for the production of exogenous NPs as they are easily cultivable, come with highly developed and easily reachable genetic tools, and are genetically and physiologically well studied. E. coli is the most common, fast-growing, and easily manipulated host with comprehensive knowledge of the native metabolic networks; it has been used to produce a large number of metabolites and recombinant proteins (Kim et al., 2015b; Zhang et al., 2016b). The main limitation to using E. coli as a versatile heterologous host is the lack of specific biosynthetic machineries such as phosphopantetheinyl transferase (PPTase) and precursors such as methylmalonyl-CoA (Kim et al., 2015b), and the need for extensive genetic manipulations for actinomycete derived NPs (Zhang et al., 2016b). P. putida is a Gram-negative bacterium characterized by fast growth, well developed genetic tools, xenobiotic tolerance, a high NADPH generation rate and the presence of diverse enzymes; however, it has a low yield for polyketides and non-ribosomal peptides (NRPs) and the limited knowledge of its native metabolic networks largely restrict its applications (Zhang et al., 2016b). S. cerevisiae is the best host to produce eukaryotic NPs such as those from fungi and plants. Generally, a host that is phylogenetically close to native producers is advantageous because they may share similar traits including transcriptional and translational machineries, regulatory networks, codon usage, and precursor/substrate availability (Ongley et al., 2013). However, this general assumption has been recently challenged: Moore’s group found that the violacein gene cluster from the marine bacterium Pseudoalteromonas luteoviolacea 2tal6 was readily expressed, with robust production of violacein in the γ-proteobacterium P. putida KT2440 and the α-proteobacterium Agrobacterium tumefaciens LBA4404, however, very little was produced in E. coli strains (γ-proteobacterium) despite their closer phylogenetic relationship to the native producer (Zhang et al., 2017b).
In addition to host selection, there are many other factors which need to be considered for a successful heterologous production. Unfortunately, the efficacy of heterologous expression of a gene cluster in different hosts varies greatly, and there are no established rules for prediction of the most appropriate system: one can only test various hosts to determine the best one. Representative strategies and factors to be considered for an optimized heterologous production of NPs or unnatural derivatives are summarized in Figure 1.
Figure 1.
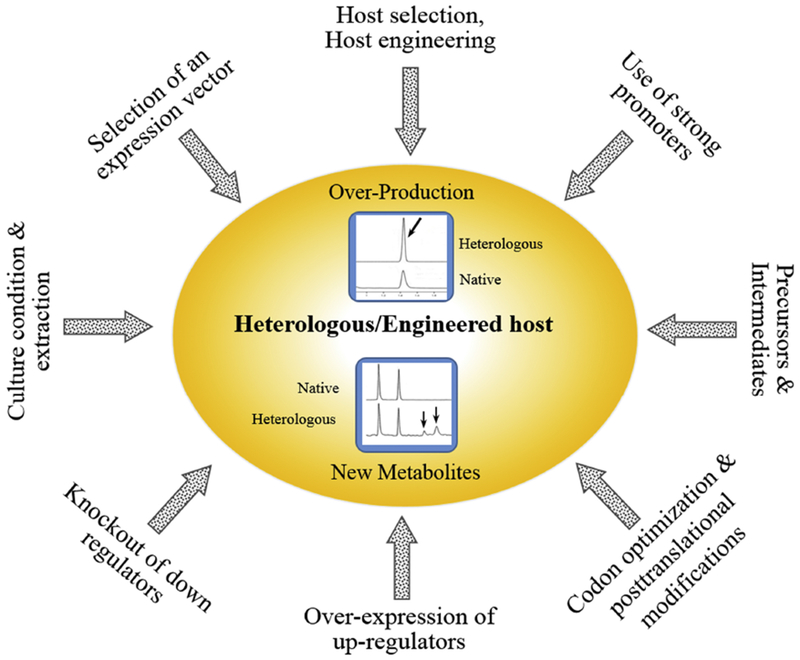
Major strategies and factors to be considered for better heterologous production of natural products or unnatural natural products via combinatorial biosynthesis.
As the most prolific source of NPs, Streptomyces strains have several advantages as heterologous expression hosts: (1) a rich pool of precursors/substrates; (2) available toolkits for genetic manipulation; (3) relatively easy cultivation; and (4) biosafety. With the exception of plant pathogen (Leiminger et al., 2013), streptomycetes are considered to have extremely low health and environmental risks (Shepherd et al., 2010). However, Streptomyces do have limitations when compared to E. coli, including relatively slow growth rates (usually hours of doubling time), less well-developed genetic engineering tools, and the presence of competing endogenous secondary metabolic pathways (Zhang et al., 2016b). Various Streptomyces species including S. coelicolor, S. lividans, S. venezuelae, S. avermitilis, S. albus, S. griseofuscus, S. ambofaciens, S. fradiae (tylosin producer), S. roseosporus, and S. toyocaensis have been used frequently for heterologous expression of foreign BGCs (Baltz, 2010; Thanapipatsiri et al., 2015). They are suitable for the production of NPs derived from both closely related species and distantly related actinomycetes and plants.
In this review, we will focus on recent heterologous expression trials in five strains, S. coelicolor A3(2), S. lividans, S. albus, S. venezuelae and S. avermitilis. Construction of a ‘clean’ host, in which endogenous BGCs have been removed, is generally preferred because more precursors, intermediates and energy can be directed towards the production of the heterologous metabolites (Kim et al., 2015b; Komatsu et al., 2010). Recent work on host engineering is also included. For a better illustration, we have classified trials/examples into different categories based on purposes or outcomes: CA, Cryptic pathway Activation/awakening; GC, Gene Characterization (individual function); GI, Gene cluster Identification; MG, MetaGenomics; NM, New Metabolite; OP, Over-Production; and P, Plant-derived NPs.
2. Streptomyces coelicolor as a heterologous host
S. coelicolor A3(2) is the most genetically well-studied strain among streptomycetes and is currently one of the best hosts for heterologous production of NPs (Gomez-Escribano and Bibb, 2012; 2014). The major metabolites produced by S. coelicolor A3(2) include an aromatic polyketide antibiotic actinorhodin (ACT), the tripyrolle antibiotic undecylprodigiosin (RED), and a non-ribosomal peptide calcium-dependent antibiotic (CDA). In 2002, the 8,667,507 bp genome of S. coelicolor A3(2) was reported (see more details in Table 1) (Bentley et al., 2002), the first genome sequence in this group of prolific NP producers. Besides ACT, RED and CDA, many more metabolites were predicted from bioinformatic analysis of potential BGCs, including type I, II and III polyketides, NRPs, terpenes and alkaloids. In addition to the wild-type S. coelicolor A3(2), mutants including CH999, M512, M1146, M1152, M1154, and M1317 (as summarized in Table 2) have been generated to better express foreign BGCs. CH999 was engineered to better produce polyketides by diminishing the production of both ACT and RED (McDaniel et al., 1993). For a better production of type III polyketides, a new host M1317 (Thanapipatsiri et al., 2015) was developed by deleting type III polyketide gene clusters (gcs, srsA, rppA) in the strain M1152 (Gomez-Escribano and Bibb, 2011).
Table 1.
General chromosomal features of S. coelicolor A3(2) (accession No: AL645882), S. lividans TK24 (accession No: NZ_CP009124), S. albus J1074 (accession No: NC_020990), S. venezuelae ATCC 15439 (accession No: NZ_CP013129), and S. avermitilis MA-4680 (accession No: AP002021-AP005050, BA000030 and AP005645).
| SN | Component |
S. coelicolor
A3(2) |
S. lividans
TK24 |
S. albus
J1074 |
S. venezuelae
ATCC15439 |
S.avermitilis
MA-4680 |
|---|---|---|---|---|---|---|
| 1 | Total Size (bp) | 8,667,507 | 8,345,283 | 6,841,649 | 9,054831 | 9,025,608 |
| 2 | G+C content (%) | 72.12 | 72.24 | 73.3 | 71.74 | 70.7 |
| 3 | Average gene length (bp) | 991 | - | 1011 | - | 1034 |
| 4 | Ribosomal RNAs (16S-23S-5S) | 6× | 6× | 7× | 7× | 6× |
| 5 | Transfer RNAs | 63 | 64 | 66 | 72 | 68 |
| 6 | Protein coding sequences | 7825 | 7360 | 5832 | 8080 | 7346 |
| 7 | Coding Density (%) | 88.9 | - | 86.8 | - | - |
| 8 | Terminal inverted repeats (TIRs) | 21,653 bp | 31,000 bp | 30,000 bp | 49 bp | |
| 9 | Doubling time | ~ 2.2 h (S. coelicolor M145) | ~ 4.2 h (S. lividans TK21) | - | ~ 1 h | - |
| 10 | Method of gene introduction | Conjugation/PEG-mediated transformation | Mostly PEG-mediated transformation | Conjugation/PEG-mediated transformation | PEG-mediated transformation | Conjugation/PEG-mediated transformation |
| 11 | Restriction barriers | Methyl-specific restriction | No methyl-specific restriction | Methyl-specific restriction | Methyl-specific restriction | Methyl-specific restriction |
-, information not available.
Table 2.
Major mutants of S. coelicolor used as heterologous expression hosts.
| SN | Strain | Features | References |
|---|---|---|---|
| 1 | S. coelicolor A3(2) | Wild-type strain | Gomez-Escribano and Bibb, 2012; 2014 |
| 2 | S. coelicolor M512 | Deletion of redD and actII-ORF4 and without plasmids SCP1 and SCP2. | Floriano and Bibb, 1996 |
| 3 | S. coelicolor CH999 | Deletion of ACT gene cluster and redE gene. | McDaniel et al., 1993 |
| 4 | S. coelicolor M1146 | Deletion of BGCs for ACT, RED, coelimycin and CDA | Gomez-Escribano and Bibb, 2011 |
| 5 | S. coelicolor M1152 | Derived from M1146 with a mutated ropB gene (C1298T). | Gomez-Escribano and Bibb, 2011 |
| 6 | S. coelicolor M1154 | Derived from M1152 with a mutated rpsL gene (A262G). | Gomez-Escribano and Bibb, 2011 |
| 7 | S. coelicolor M1317 | Derived from M1152 by deleting type III polyketides genes and operons (gcs, srsA, rppA). | Thanapipatsiri et al., 2015 |
The examples mentioned in this section are summarized in Table 4. S. coelicolor A3(2) has proven to be an efficient recipient host for giant clusters. Salinomycin, a polyether antibiotic used to prevent Coccidioidomycosis in poultry and to alter gut flora to improve nutrient absorption in ruminants, is generated by an assembly line of nine polyketide synthases (PKSs) (Jiang et al., 2012; Yin et al., 2015). Yin et al. isolated three fragments of the salinomycin gene cluster from S. albus DSM 1398, assembled them into a single 106-kb DNA fragment using the Red/ET recombination technique, and successfully expressed the giant cluster in the wild-type S. coelicolor A3(2) and restored salinomycin production (Yin et al., 2015). The 141-kb gene cluster of vancoresmycin (Figure 2), a potent antibiotic active against vancomycin-resistant Enterococcus spp. (VRE) and methicillin-resistant Staphylococcus aureus (MRSA), was expressed in S. coelicolor M1152 yielding vancoresmycin at 2.2 mg/L (Kepplinger et al., 2018).
Table 4.
Trials and examples of heterologous expression or production of natural products in Streptomyces hosts. CA, Cryptic pathway Activation/awakening; GC, Gene Characterization (individual gene function); GI, Gene cluster Identification; MG, MetaGenomics; NM, New Metabolite; OP, Over-Production; and P, Plant-derived NPs. * stands for genetically intractable organisms.
| SN | Compound | Activity | Purpose | Producing strains/Gene cluster | Heterologous host | References |
|---|---|---|---|---|---|---|
| 1 | Abyssomicins 2 and 4 | Anti-HIV | GI/OP | Streptomyces koyangensis SCSIO5802 | S. coelicolor | Tu et al., 2018 |
| 2 | Albusnodin | - | CA | S. albus DSM 41398 | S. coelicolor | Zong et al., 2018 |
| 3 | Ammosamides A-C | - | GI/CA | S. sp. CNR-698 | S. coelicolor | Jordan and Moore, 2016 |
| 4 | Anthracimycin | Anti-MRSA | OP | S. sp. CNH365, S. sp. T676 | S. coelicolor | Alt and Wilkinson, 2015 |
| 5 | Bottromycins | Antibacterial | GC/NM/OP | S. sp. BC16019, S. scabies | S. coelicolor | Hu et al., 2012; Eyles et al., 2018 |
| 6 | Cacibiocins A and B | Antibacterial | NM/OP | Catenulispora acidiphila* | S. coelicolor | Zettler et al., 2014 |
| 7 | Caprazamycin analogues | Antibiotic | OP | S. sp. MK730-62F2 | S. coelicolor | Flinspach et al., 2010 |
| 8 | Clorobiocin | Gyrase B inhibitor | OP | S. roseochromogenes subsp. oscitans | S. coelicolor | Flinspach et al., 2010 |
| 9 | Cosmomycin | Antitumor | GI/CA | Streptomyces sp. CNT-302 | S. coelicolor | Larson et al., 2017 |
| 10 | Coumermycin A1 | Gyrase B inhibitor | OP | S. rishiriensis DSM 40489 | S. coelicolor | Wolpert et al., 2008; Flinspach et al., 2010 |
| 11 | Desotamides A and B | Antibacterial | OP/GI/NM | S. scopuliridis SCSIO ZJ46 | S. coelicolor | Song et al., 2014; Li et al., 2015 |
| 12 | Erythreapeptins | Antibacterial | OP | Saccharopolyspora erythraea NRRL 2338* | S. coelicolor | Völler et al., 2012 |
| 13 | FK506 | Immunosuppressive | OP | S. tsukubaensis NRRL18444 | S. coelicolor | Jones et al., 2013 |
| 14 | Fluostatin derivatives | - | NM/CA | Micromonospora rosaria SCSION160 | S. coelicolor | Yang et al., 2015 |
| 15 | GE2270 | Anti-MRSA | Planobispora rosea ATCC 53773* | S. coelicolor | Flinspach et al., 2014 | |
| 16 | Gougerotin | Antibiotic | GC/NM/OP | S. graminearus* | S. coelicolor | Du et al., 2013; Niu et al., 2013; Wei et al., 2016 |
| 17 | Intermediates of SPMs, IDMs and LNMs | Antifungal, Anticancer | NM | S. sp. SCSIOI 03032 | S. coelicolor | Ma et al., 2017 |
| 18 | Kocurin | Anti-MRSA | GI | Kocuria rosea* | S. coelicolor | Linares-Otoya et al., 2017 |
| 19 | Neoabyssomicins A and B | Anti-HIV | GI/OP | S. koyangensis SCSIO5802 | S. coelicolor | Tu et al., 2018 |
| 20 | Oviedomycin | Antitumor | CA | S. antibiotics ATCC 11891, S. ansochromogenes | S. coelicolor | Rico et al. 2014; Xu et al., 2017 |
| 21 | Salinomycin | Antitumor | CA/OP | S. albus DSM41398 | S. coelicolor | Yin et al., 2015 |
| 22 | Streptocollin | tyrosin phosphatase 1B inhibitor | NM/OP | S. collinus Tü 365 | S. coelicolor | Iftime et al., 2015 |
| 23 | Streptothricin | Antibiotic | GI/OP | S. sp. fd1-xmd | S. coelicolor | Yu et al., 2018 |
| 24 | Vancoresmycin | Anti-MRSA, Anti-VRE | OP/GC | Amycolatopsis sp. ST101170 | S. coelicolor | Kepplinger et al., 2018 |
| 25 | 2-DOS and Paromamine | - | GC | S. kanamyceticus | S. lividans | Nepal et al., 2009 |
| 26 | 2,4-HMBA | - | GI | S. cremeus NRRL 3241 | S. lividans | Waldman et al., 2015 |
| 27 | 8-methyl-tetracenomycin C | - | OP | S. lividans | Diaz et al., 2013 | |
| 28 | Asukamycin | Antitumor | GI/GC | S. nodosus subsp. asukanensis | S. lividans | Rui et al., 2010 |
| 29 | Avermectins-A2A, B1a and A1a | Anthelmintics | NM | S. avermitillis ATCC 31267 | S. lividans | Deng et al., 2017 |
| 30 | Blasticidin S derivatives | Fungicides | NM | S. griseochromogenes* | S. lividans | Li et al., 2013b |
| 31 | Capreomycin | Antitubercolosis | OP | Saccharothrix mutabilis* | S. lividans | Felnagle et al., 2007; Chaudhary et al., 2013 |
| 32 | Cinnamic acid | Important precursor | OP | Various plants and microorganisms | S. lividans | Xiang and Moore, 2002 |
| 33 | Cremeomycin | Antitumor | GI/GC | S. cremeus NRRL 3241 | S. lividans | Waldman et al., 2015 |
| 34 | D-cycloserine | Antituberculosis/anti-anxiety | GI | S. lavendulae ATCC 11924 | S. lividans | Kumagai et al., 2010 |
| 35 | Dehydrophos | Antibacterial | NM/GI | S. luridus | S. lividans | Circello et al., 2010 |
| 36 | Hatomarubigin | Antitumor | GI/GC/NM | S. sp. 2238-SVT4 | S. lividans | Izawa et al., 2014 |
| 37 | Labyrinthopeptins A1 and A2 | Antiviral, antiallodynic | GC/GI/NM | Actinomedura namibiensis* | S. lividans | Krawczyk et al., 2013 |
| 38 | Leptomycins | Antifungal, antitumor | GI | S. sp. ATCC 39366 | S. lividans | Hu et al., 2005 |
| 39 | Lipopeptides 8D1-1 and 8D1-2, and streptothricin and borrelidin | Antibacterial | CA/NM | S. rochei | S. lividans | Xu et al., 2016 |
| 40 | Lyngbyatoxin A and derivatives | Cyanotoxin | NM | Moorea producens* | S. lividans | Zhang et al., 2016a |
| 41 | Mediomycin A | Antifungal | GI | S. mediocidicus ATCC 23936 | S. lividans | Sun et al., 2018 |
| 42 | Meridamycin | Neuroprotective | GI/OP | S. sp. NRRL 30748 | S. lividans | Liu et al., 2009 |
| 43 | Mithramycin A | Anticancer | OP | S. argillaceus ATCC12956 | S. lividans | Novakova et al., 2018 |
| 44 | Oleandomycin intermediate | Antibiotic | GI/OP | S. antibioticus* | S. lividans | Shah et al., 2000 |
| 45 | Pacidamycins D and S | Antimicrobial | OP/NM | S. coeruleorubidus | S. lividans | Rackham et al., 2010 |
| 46 | Pactamides A-F | Antitumor | CA | S. pactum SCSIO 02999 | S. lividans | Saha et al., 2017 |
| 47 | Platensismycin/Platensin | Antibacterial | GC/GI/NM | S. platensis* | S. lividans | Smanski et al., 2012 |
| 48 | Ravidomycin | Antitumor | GI | S. ravidus | S. lividans | Kharel et al., 2010 |
| 49 | Ribostamycin | Anti-immuno deficiency | GI | S. ribosidificus | S. lividans | Subba et al., 2007 |
| 50 | Terragines A-E | - | NM/MG | Environmental DNA | S. lividans | Wang et al., 2000 |
| 51 | Thioviridamide | Anticancer | GI | S. olivoviridis NA005001* | S. lividans | Izawa et al., 2013 |
| 52 | YM-216391 | Antitumor | GI/OP | S. nobilis | S. lividans | Jian et al., 2012 |
| 53 | A pentacyclic compound | - | MG/NM | Environmental DNA | S. albus | Feng et al., 2011 |
| 54 | Dithiolopyrrolone | Antibacterial, Antifungal, Insecticidal, Anticancer | OP | S. thioluteus DSM 40027 | S. albus | Zhai et al., 2016 |
| 55 | Elloramycins | Antitumor | GC/NM | S. olivaceus | S. albus | Patallo et al., 2001 |
| 56 | Fredericamycin | Antitumor | OP | S. griseus ATCC 49344 | S. albus | Chen et al., 2009 |
| 57 | Furaquinocins | Antitumor | GI/GC/NM | S. sp. KO-3988 | S. albus | Isogai, et al., 2012 |
| 58 | Grecocyclines | - | OP | S. sp. Acta 1362 | S. albus | Bilyk et al., 2016 |
| 59 | Herbicidins | Antibacterial | GI | S. sp. L-9-10 | S. albus | Jung et al., 2006 |
| 60 | Iso-migrastatin | Anticancer | OP | S. platensis NRRL18993* | S. albus | Yang et al., 2011 |
| 61 | K252a | GI/GC | Nocardiopisis longicantena JCM 11136 | S. albus | Kim et al., 2007; Chae et al., 2009 | |
| 62 | KB-346-5 derivative | Anti-MRSA, Anti-VRE | MG | Environmental DNA | S. albus | Feng et al., 2011 |
| 63 | Kinamycin | Antibiotic | GI/GC | S. galtieri sgt26 | S. albus | Liu et al., 2018 |
| 64 | Landomycin E | Antibiotic | MG | Environmental DNA | S. albus | Feng et al., 2011 |
| 65 | Leprotene and derivatives | - | NM/CA | S. argillaceus | S. albus | Becerril et al., 2018 |
| 66 | Lyngbyatoxin A and derivatives | Neurotoxin | OP | Moorea producens * | S. albus | Zhang et al., 2016a |
| 67 | Malacidins | Antibacterial, Anti-MRSA | GI/NM/MG | Environmental DNA | S. albus | Hover et al., 2018 |
| 68 | Moenomycin A | Antibacterial | OP | S. ghanaensis ATCC 14676 | S. albus | Makitrynskyy et al., 2010 |
| 69 | Myxochelin A | Iron-chelating | NM/MG | Environmental DNA | S. albus | Bitok et al., 2017 |
| 70 | Pseudoribostamycin | - | NM | S. ribosidificus | S. albus | Subba et al., 2007; Kurumbang et al., 2011 |
| 71 | Rebeccamycin | Antitumor | OP/NM | Saccharothrix aerocolonigenes ATCC39243 | S. albus | Sánchez et al., 2005 |
| 72 | Spinosyn | Insecticides | OP | Saccharopolyspora spinose | S. albus | Tan et al., 2017 |
| 73 | Staurosporine | Antitumor | OP/NM | S. staurosporeus | S. albus | Sánchez et al., 2005 |
| 74 | Steffimycin and derivatives | Antitumor | OP/NM | S. steffisburgensis NRRL 3193 | S. albus | Gullon et al., 2006; Olano et al., 2008 |
| 75 | Tetarimycin A | Anti-MRSA | NM | Environmental DNA | S. albus | Bauer et al., 2010; Kallifidas et al., 2012 |
| 76 | Thiocoraline | Antitumor | NM/OP | Micromonospora sp. ACM2 and M. sp. ML1 | S. albus | Lombó et al., 2006 |
| 77 | Utahmycins A and B | Anti-MRSA | NM/MG | Environmental DNA | S. albus | Bauer et al., 2010; Kallifidas et al., 2012 |
| 78 | 4-O-dimethylbarbamide | - | NM/GI | Moorea producens* | S. venezuelae | Kim et al., 2012 |
| 79 | Gentamicin A2 | Antibiotic | GC | Micromonospora echinospora | S. venezuelae | Park et al., 2008 |
| 80 | Apigenin | Flavonoid | P | Various plants | S. venezuelae | Park et al., 2010 |
| 81 | Chrycin | Flavonoid | P | Plant passionflower | S. venezuelae | Park et al., 2010 |
| 82 | Hopene | - | NM/CA | S. peucetius | S. venezuelae | Ghimire et al., 2015 |
| 83 | Kanamycins A-D and derivatives | Antibiotic | GI/GC/NM | S. kanamyceticus* | S. venezuelae | Park et al., 2011a |
| 84 | Naringenin | Flavanone | P | Grapefuit | S. venezuelae | Park et al., 2011b |
| 85 | Oxykanamycin C | - | NM | S. kanamyceticus/S. spectabilis | S. venezuelae | Nepal et al., 2010 |
| 86 | Oxytetracycline | Antibacterial | OP | S. rimous M4018 | S. venezuelae | Yin et al., 2016 |
| 87 | Pinocembrin | Flavanone (antioxidant) | P | Various plants, | S. venezuelae | Park et al., 2011b |
| 88 | Pinosylvin | Stilbenoid toxin | P | Pinaceae trees | S. venezuelae | Park et al., 2009 |
| 89 | Pseudoribostamycin | - | NM | S. ribosidificus | S. venezuelae | Kurumbang et al., 2011 |
| 90 | Resveratrol | Stilbenoid (dietary supplement) | P | Grape and various berries | S. venezuelae | Park et al., 2009 |
| 91 | Spectinomycin | Antibiotic | GI/GC | S. spectabilis | S. venezuelae | Thapa et al., 2008; Lamichhane et al., 2014 |
| 92 | Tylosin derivatives | - | NM/OP | S. fradiae ATCC19609 | S. venezuelae | Jung et al., 2007; Jung et al., 2008 |
| 93 | Abietatriene | - | P | Ginkgo biloba | S. avermitilis | Komatsu et al., 2013 |
| 94 | Amorpha-1,4-diene | - | P | Artemisia annua | S. avermitilis | Komatsu et al., 2010 |
| 95 | Aureothin | Antifungal/antibacterial | OP | S. sp. MM3 | S. avermitilis | Komatsu et al., 2013 |
| 96 | Bafilomycin B1 | Antifungal/anti bacterial | OP | Kitasatospora setae KM-6054 | S. avermitilis | Komatsu et al., 2013 |
| 97 | Cephamycin C | β-lactam antibiotics | GI/OP | S. clavuligerus | S. avermitilis | Komatsu et al., 2010 |
| 98 | Chloramphenicol | Antibacterial | OP | S. venezuelae ATCC10712 | S. avermitilis | Komatsu et al., 2013 |
| 99 | Clavulanic acid | β-lactam antibiotics | GC/OP | S. clavuligerus | S. avermitilis | Komatsu et al., 2013 |
| 100 | Cyslabdan A and derivatives | Anti-MRSA | GI/NM | S. cyslabdanicus K04-0144 | S. avermitilis | Ikeda et al., 2016 |
| 101 | Erythromycin | Antibacterial | OP | Sacchropolyspora erythraea NRRL2338 | S. avermitilis | Komatsu et al., 2013 |
| 102 | Holomycin | Antitumor | OP | S. clavuligerus | S. avermitilis | Komatsu et al., 2013 |
| 103 | Kasugamycin | Antitumor | OP | S. kasugaensis MB273 | S. avermitilis | Komatsu et al., 2013 |
| 104 | Lactacystin | - | OP | S. lactacvstinaeus OM-6519 | S. avermitilis | Komatsu et al., 2013 |
| 105 | Leptomycin | Antifungal | OP | S. sp. EM52 | S. avermitilis | Komatsu et al., 2013 |
| 106 | Levopimaradine | - | P | Ginkgo biloba | S. avermitilis | Komatsu et al., 2013 |
| 107 | Lyngbyatoxin A and derivatives | Cyanotoxin | OP/NM | Moorea producens* | S. avermitilis | Zhang et al., 2016a |
| 108 | Mycosporine-glycineal-anine | Anti-UV | OP | Actinosynnema mirum DSM 43827 | S. avermitilis | Miyamoto et al., 2014 |
| 109 | Novobiocin | Antibacterial | OP | S. caeruleus NCBI11891 | S. avermitilis | Komatsu et al., 2013 |
| 110 | Oxytetracycline | Antibacterial | OP | S. rimous | S. avermitilis | Komatsu et al., 2013 |
| 111 | Pentalenolactone | Antibiotic | GC | S. exfoliatus UC5319, S. arenae TÜ469 | S. avermitilis | Komatsu et al., 2013 |
| 112 | Pholipomycin | - | OP/GI | S. clavuligerus | S. avermitilis | Komatsu et al., 2013 |
| 113 | Pladienolide B | Antitumor | GI/OP | S. platensis Mer-11107 | S. avermitilis | Komatsu et al., 2010 |
| 114 | Porphyra-334 | Anti-UV | OP | Actinosynnema mirum DSM 43827 | S. avermitilis | Miyamoto et al., 2014 |
| 115 | Raimonol | - | GI | S. anulatus GM95 | S. avermitilis | Ikeda et al., 2016 |
| 116 | Rebeccamycin | Antitumor | OP | Lechevalieria aerocolonigenes ATCC 39243 | S. avermitilis | Komatsu et al., 2013 |
| 117 | Resistomycin | Antibiotic | OP/GI | S. sp. NA 97 | S. avermitilis | Komatsu et al., 2013 |
| 118 | Ribostamycin | Antitumor | OP | S. ribosidificus ATCC21294 | S. avermitilis | Komatsu et al., 2013 |
| 119 | Shinorine | Anti-UV | OP | Actinosynnema mirum DSM 43827, Pseudonocarida sp. strain P1 | S. avermitilis | Miyamoto et al., 2014 |
| 120 | Streptomycin | Antibiotic | GI/OP | S. griseus IFO13350 | S. avermitilis | Komatsu et al., 2010 |
| 121 | Taxa-1,4-diene | - | P | Taxus brevifolia | S. avermitilis | Komatsu et al., 2013 |
| 122 | Telomestatin and derivatives | Telomerase inhibitor | OP/NM | S. anulatus 3533-SV4 | S. avermitilis | Amagai et al., 2017 |
-, information not available.
Figure 2.
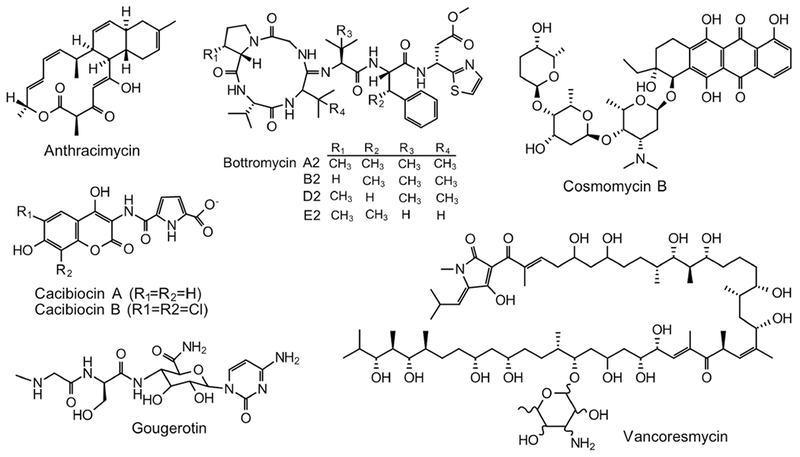
Chemical structures of representative natural products generated in the heterologous host S. coelicolor. Anthracimycin; Bottromycins; Cacibiocins; Cosmomycin B; Gougerotin; and Vancoresmycin.
Because of the well-understood genetic background and readily available molecular cloning tools, S. coelicolor is often used as an alternative host for the expression of genes or gene clusters derived from genetically intractable actinomycetes. Gougerotin (Figure 2), a peptidyl nucleoside antibiotic with antitumor, antiviral, antibacterial, antimycoplasma, anthelmintric and acaricidal activities, was originally isolated from S. graminearus (Jiang et al., 2013). The Tan group identified a fosmid D6-4H from a DNA library of S. graminearus, which contains a complete gougerotin gene cluster (Du et al., 2013). Expression of D6-4H in S. coelicolor M1146 enabled the functional characterization of two genes gouC and gouD, as well as the heterologous production of gougerotin (Wei et al., 2016). By replacing native promoters of key structural genes by the hrdB promoter, the same group increased the yield of gougerotin in M1146 by 10-fold as compared to its native producer (Du et al., 2013; Niu et al., 2013). Streptothricin (ST), an antibiotic active against both Gram-positive and Gram-negative bacteria and eukaryotes, was isolated from a soil-derived S. sp. fdl-xmd (Yu et al., 2018). The verification of ST BGC was accomplished through the expression of the gene cluster in M1146, and the yield was enhanced to 0.5 g/L after an optimization of culture conditions. Though the same level of production of ST is observed in the native producer, production takes 7-12 days, whereas in M1146 it takes only 2 days (Yu et al., 2018). Desotamides A and B, potent antibacterial cyclohexapeptides, were originally isolated from S. scopuliridis SCSIO ZJ46 (Song et al., 2014). Expression of the 39-kb dsa gene cluster in S. coelicolor M1152 afforded desotamides A and B; interestingly, a new structure desotamide G was later identified (Li et al., 2015).
As productivity has often been found to be low in native producers, heterologous expression is frequently used to enhance yield. This approach often involves trials with different hosts and promoter exchange. Two novel aminocoumarins, cacibiocins A and B (Figure 2), were isolated from the very rare actinomycete Catenulispora acidiphila DSM 44928. Their production was dramatically increased from 4.9 mg/L in the native strain to 60 mg/L in M1152 (Zettler et al., 2014). A ΦC31-based integration of a newly assembled cosmid containing the 38.6-kb gene cluster of another aminocoumarin antibiotic coumermycin Al into the M512 genome led to a production of coumermycin Al at ~7 mg/L, slightly higher than the native producer S. rishiriensis DSM 40489 (5 mg/L) (Wolpert et al., 2008). Production was increased to 52.5 mg/L by simply changing the host to M1146 (Flinspach et al., 2010). In the same set of experiments, production of clorobiocin and its derivatives exceeded 100 mg/L in S. coelicolor M512, M1146 and M1154; an optimal production of caprazamycin aglycones reached 152 mg/L in the host M1154 when 0.6 % Q-5247 and 0.2 mg/L CoCl2 were present (Flinspach et al., 2010). Similarly, kocurin, a new thiopeptide antibiotic isolated from Kocuria rosea (Schinke et al., 2017), was produced in M1146 by expressing the kocurin BGC (Linares-Otoya et al., 2017).
This issue of low yields is particularly profound with marine NPs, which have recently become an attractive source of drug discovery (Gerwick and Moore, 2012). Anthracimycin (Figure 2) is an unusual 14-membered macrolide produced by a trans-acyltransferase (trans-AT) PKS system in two marine streptomycetes S. sp. CNH365 and S. sp. T676 (Jang et al., 2013; Alt and Wilkinson, 2015). It exhibits antibacterial activity (≤0.06 μg/mL) against MRSA and VRE. The heterologous expression of the 53-kb anthracimycin gene cluster in S. coelicolor strains M1154, M1152, and M1146 yielded anthracimycin at approximately 9, 10, and 14 μg/mL, respectively (Alt and Wilkinson, 2015). The intact gene cluster of fluostatin, an atypical angucycline isolated from the marine actinomycete Micromonospora rosaria SCSIO N160, was heterologously expressed in S. coelicolor YF11; when cultured with 3% sea salts, the recombinant S. coelicolor strain produced two new fluostatin derivatives, fluo statin L and an unusual heterodimer difluostatin A which exhibit antibacterial activities (Yang et al., 2015).
Tryptophan dimers (TDs) are an important class of NPs with diverse biological activities including antibacterial, antifungal and antiproliferative. The combined use of bio informatics, targeted gene disruption and heterologous expression in S. coelicolor YF11 of the Spm gene cluster which was isolated from the deep-sea bacterium S. sp. SCSIO 03032 confirmed its indispensable role in the biosynthesis of indimicins (IDMs), lynamicins (LNMs) and spiroindimicins (SPMs) (Ma et al., 2017). Although the heterologous expression of the Spm gene cluster failed to produce final products, three compounds 6’,6”-dichloro-chromopyrrolic acid, demethyl-LNM A and 6’,6”-dichloro-bisindolylmaleimide were produced. The latter two compounds, previously known either as a “non-natural” hydrolysis product or a synthetic product, respectively, were produced for the first time in a bacterium (Ma et al., 2017).
S. coelicolor is rich in two-component systems (TCSs), which often play important roles in the physiological differentiation (Bentley et al., 2002). Yepes et al. identified arbA1/A2 encoding a pleiotropic repressor of antibiotic production in S. coelicolor (Yepes et al., 2011). The deletion of arbA1/A2 in M145 (ΔabrA) not only enhanced the production of endogenous antibiotics such as ACT, RED and CDA, but also foreign NPs; the expression of the entire oviedomycin pathway derived from S. antibiotics ATCC 11891 in the ΔabrA mutant duplicated oviedomycin production as compared to the parent host M145 (Rico et al., 2014). Interestingly, expression of a pSET152 construct containing only 12 structural genes controlled by a single hrdB promoter in S. coelicolor M1146 also produced oviedomycin (Xu et al., 2017b).
Ribosomally synthesized and post-translationally modified peptides (RiPPs) are an emerging group of NPs that are prevalent throughout nature and exhibit potent bioactivities. The elucidation of biosynthetic pathways of RiPPs is challenging as the biosynthesis takes place on a single precursor and intermediates may be rapidly proteolyzed. Bottromycin (BTM) A2 (Figure 2), a potent antibiotic against MRSA and VRE, is a typical RiPP, in which the N-terminus of the precursor peptide BtmD undergoes post-translational modifications to decorate a mature BTM scaffold (Crone et al., 2016). The genetic manipulation of the BTM producer S. sp. BC16019 has been so far restricted to the single crossover method with very low efficiency, so the BTM gene cluster has been expressed in two different hosts S. coelicolor A3(2) and S. albus J1074. The native strain produced 100 μg/L of BTM. Heterologous expression of the BTM cluster in S. coelicolor and S. albus produced very low levels of BTM, 1 and 4 μg/L, respectively, which was increased 20-fold by replacing a ~16-kb DNA fragment of the 5’-end of the BTM gene cluster with a kanamycin resistance gene and by substituting native promoters with the strong ermE* promoter (Huo et al., 2012). Further engineering of the BTM gene cluster in S. coelicolor A3(2) enabled the discovery of 3 novel BTM derivatives B2, D2, and E2 (Figure 2), and functional characterization of an O-methyltransferase and three radical SAM-dependent methyltransferases (Huo et al., 2012). Recently, the Truman group identified another BTM gene cluster in S. scabies and cloned it into the direct-capture vector pCAP (Yamanaka et al., 2014) to yield pCAPbtm. Expression of pCAPbtm in M1146 produced a very low level of BTMs; further, three constitutive promoters, PSEF14, Paac3 and PhrdB, were inserted in front of btmA, btmB and btmC, respectively, to yield pCAPbtml, expression of which in M1146 increased the productivity 20-fold as compared to pCAPbtm (Eyles et al., 2018). Further expression of pCAPbtm2, in which btmB gene was deleted, in M1146 produced 60 times more BTMs than M1146::pCAPbtm; expression of pCAPbtm2 in other hosts including S. laurentii, S. lividans and S. venezueale produced BTMs in amounts similar to or slightly higher than in M1146 (Eyles et al., 2018). However, none of the products found so far has shown the C-methylation of phenylalanine (Eyles et al., 2018). A new version of pCAPbtml and pCAPbtm2 was constructed by the insertion of theophylline-dependent riboswitches between PhrdB/PSEF14 and btmC to generate pCAPbtm6 and pCAPbtm7, respectively. While the expression of pCAPbtm6 in M1146 showed almost no production of BTMs, expression of pCAPbtm7 in M1146 increased the production of BTMs 120-fold as compared to the original construct pCAPbtm. Finally, mature BTMs were produced at a similar level as in the native strain and duplicated amounts of total BTMs (Eyles et al., 2018). By studying the heterologous production of another RiPP, Flinspach et al. found that primary metabolic genes can interfere with the expression of foreign DNA. Authors failed to express the GE2270 (ptb) gene cluster, which encodes a RiPP thiopeptide antibiotic in Planobispora rosea ATCC 53773, in M1146 and it became possible only after the deletion of ribosomal genes flanking the gene cluster. GE2270 production in M1146 was increased 2.5-fold from an initial yield of 0.7 mg/L via co-expression of the tufR resistance gene from P. rosea under the constitutive ermE* promoter (Flinspach et al., 2014). Production of albusnodin, a unique RiPP with post-translational acetylation, failed in both the native producer S. albus DSM 41398 and the heterologous host E. coli; however, expression of the gene cluster under the control of ermE* promoter in S. coelicolor M1146 or S. lividans 66 enabled the identification of albusnodin in both cell pellets and culture broth (Zong et al., 2018).
Lantibotics, a group of ribosomally synthesized and post-translationally modified peptides, are produced mainly by Gram-positive bacteria including lactococci, staphylococci and actinomycetes. Due to the lack of sufficient materials for structural characterization of erythreapeptins, a new group of class III lantibiotics, in the natural producer Saccharopolyspora erythraea NRRL 2338, Süssmuth and his group achieved the production of erythreapeptins in S. coelicolor M1146 and S. lividans TK24, which allowed the authors to manipulate the biosynthetic pathway and to elucidate the function of key genes (Völler et al., 2012). Streptocollin, representing the latest member of the venezuelin family lanthipeptides, was identified via an antiSMASH-based genome mining of S. collinus Tü 365. However, the native strain produces only trace amounts of streptocollin; by expressing streptocollin biosynthetic genes under the control of a constitutive promoter in S. collinus TU365 or heterologously in M1152, preparative amounts of streptocollin were obtained (Iftime et al., 2015). Even though no significant antibacterial or antiviral activity was detected, the compound did show moderate inhibitory activity towards protein tyrosin phosphatase 1B (PTP1B), a negative regulator of the leptin and insulin signaling pathways and its involvement in obesity and diabetes was recently demonstrated (Cho, 2013).
Recent progress in cloning tools and techniques has contributed greatly to natural product research in the post-genome era. Direct capture of BGCs and the use of transformation-associated- recombination (TAR) identified cosmomycin (Figure 2) and its analogues, and ammosamides A-C in the culture of recombinant S. coelicolor strains (Jordan and Moore, 2016; Larson et al., 2017). Following the cloning of gene cluster from S. tsukubaensis NRRL 18444 using a P1-derived artificial chromosome (PAC) expression vector, Bibb and his colleagues heterologously expressed the 83.5-kb FK506 gene cluster in a few S. coelicolor hosts (Jones et al., 2013); the yield of FK506 was increased from 1.2 mg/L to 5.5 mg/L in M1146 when the LuxR regulatory gene fkbN was over-expressed. Using the same PAC vector system, neoabyssomicin A/B and abyssomicin 2/4 were recently identified from a deep-sea isolate S. koyangensis SCSIO 5802 via expressing PAC constructs heterologously in M1152 (Tu et al., 2018). Neoabyssomicin A augments human immunodeficiency virus-1 (HIV-1) replication and abyssomicin 2 selectively reactivates latent HIV and is also active against Gram-positive pathogens including MRSA. Co-expression of the gene cluster with abmH or abmI, two potential pathway-specific regulatory genes, increased the yield of abyssomicin 2 7.6-fold (2.1 g/L) and 3-fold (0.83 g/L), respectively (Tu et al., 2018).
3. Streptomyces lividans as a heterologous host
As a close relative of S. coelicolor A3(2), S. lividans is another genetically well-studied Streptomyces, and has been used extensively as a host for heterologous expression of foreign genes or gene clusters (Ruckert et al., 2015). Notably, S. lividans accepts methylated foreign DNA, and shows low protease activity (Baltz, 2010). The genome sequence of S. lividans TK24 has been determined, indicating a size of approximately 8.3-Mb, slightly smaller than that of S. coelicolor A3(2) (details listed in Table 1) (Jayapal et al., 2007; Ruckert et al., 2015). S. lividans has gene clusters encoding for the synthesis of ACT, RED and CDA but their production is very rare and depends on the specific conditions (Kim et al., 2001; Meschke et al., 2012).
The examples discussed in this section are summarized in Table 4. Paromamine and 2-deoxystreptamine (2-DOS), key intermediates in kanamycin biosynthesis, were produced by expressing two constructs pSK-2 and pSK-7 in S. lividans TK24, thus confirming the involvement of genes in kanamycin production (Nepal et al., 2009). Ribostamycin (Figure 3) was produced in S. lividans TK24 through the expression of a cosmid pRBM4 which harbors a 31.8-kb DNA fragment isolated from the ribostamycin producer S. ribosidificus ATCC 21294 (Subba et al., 2007).
Figure 3.
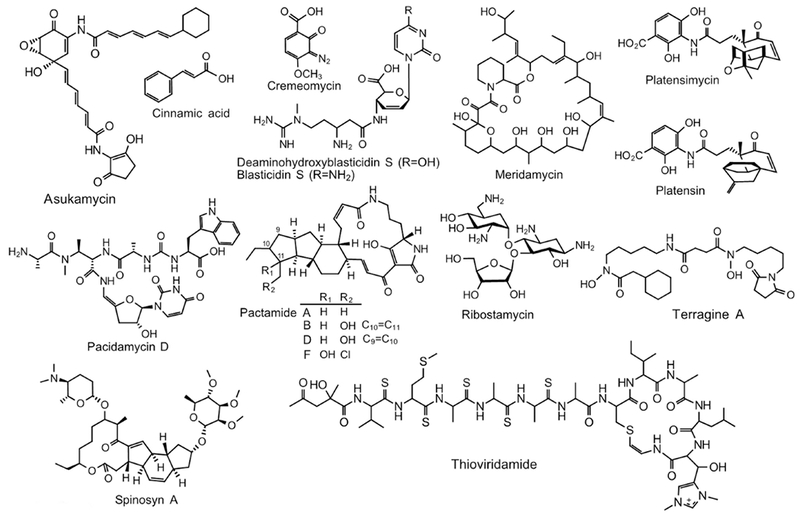
Chemical structures of representative natural products generated in the heterologous host S. lividans. Asukamycin; Cinnamic acid; Cremeomycin; Deaminohydroxyblasticidin S (blasticidin S is shown as the native compound); Meridamycin; Pacidamycin D; Pactamides; Platensimycin and Platensin; Ribostamycin; Spinosyn A; Terragine A; and Thioviridamide. C9=C10, C10=C11: double bond between the carbons.
Using a similar strategy with S. lividans TK24, a DNA locus of 33.3-kb cloned in a cosmid cosRav32 is proven sufficient to produce a antitumor angucycline ravidomycin (Kharel et al., 2010). Likewise, a recombinant S. lividans strain carrying 25 biosynthetic genes of hatomarubigins, hrbR1-hrbX and hrbY, was able to produce all known hatomarubigins including the dimer hatomarubigin D (Izawa et al., 2014). A new compound, 5-hydroxyhatomarubigin E, was detected when the hrbF gene was removed from the expressing cassette; the hrbF gene has no homology to any known angucycline biosynthetic genes (Izawa et al., 2014). Asukamycin (Figure 3), a potent antimicrobial and antitumor polyketide, was produced in S. lividans through heterologously expressing the entire gene cluster isolated from S. nodosus subsp. asukaensis (Rui et al., 2010). Leptomycin, isolated from S. sp. ATCC 39366, exerts its antifungal and antitumor activity via the inhibition of nucleo-cytoplasmic translocation in eukaryotic cells. The completeness of the 90-kb leptomycin gene cluster was confirmed by a successful production of leptomycins A and B in S. lividans (Hu et al., 2005). The BGC of mithramycin A, a potent antitumor polyketide from S. argillaceus, was heterologously expressed in S. lividans TK24 using the TAR cloning. Mithramycin A was produced at 0.86 g/L in a standard fermentation system; but after a successive engineering of S. lividans TK24 and an optimization of fermentation conditions, the yield was elevated to 3 g/L (Novakova et al., 2018). Clethramycin and mediomycin A, two linear polyene polyketide (LPP) family antibiotics with potent antifungal activity, were reported from S. mediocidicus ATCC 23936. The later contains an amino moiety substituted for the guanidino moiety. The draft genome sequence and bioinformatic analysis revealed only one gene cluster (cle) for clethramycin. Further, genome mining analysis found a remotely located amidinohydrolase MedX. The co-expression of the cle gene cluster and medX in S. lividans indeed restored the production of mediomycin (Sun et al., 2018).
Deng et al. recently constructed a BAC library of S. avermitilis ATCC 31267 with an average insert size of 100~130-kb. Heterologous expression of five clones in S. lividans produced three novel avermectin analogs A2a, B1a and A1a (Deng et al., 2017). Using the similar BAC vector, streptothricin, borrelidin, and two novel linear lipopeptides 8D1-1 and 8D1-2 were produced in S. lividans by expressing cryptic gene clusters which were isolated from S. rochei (Xu et al., 2016).
Polycyclic tetramate macrolactams (PTMs) are hybrid PKS/NRPS compounds. The PTM gene clusters are conserved and widely distributed among bacterial genomes but normally remain silent. The activation of six new PTM congeners, pactamides A-F (Figure 3), was achieved through the expression of PTM cluster isolated from a deep-sea S. pactum strain SCSIO 02999 (Saha et al., 2017). A hybrid PKS/NRPS gene cluster of approximately 90-kb was captured in a single pSBAC clone through a straightforward restriction enzyme digestion and cloning approach; expression of the construct in S. lividans produced meridamycin (Figure 3); its productivity was enhanced by feeding precursors and using the strong constitutive ermE* promoter (Liu et al., 2009). Heterologous expression of the pacidamycin BGC in S. lividans TK24 significantly increased the production of pacidamycin D (Figure 3) than in wild-type strain S. coeruleorubidus; a new compound pacidamycin S which differs from D by substituting a C-terminal tryptophan with phenylalanine was also identified (Rackham et al., 2010).
A diazo group has been found in a wide range of NPs and is extremely important due to its unique capability to facilitate 1,3-dipolar cyclo additions, carbine insertions, and alkylations. The heterologous production of cremeomycin (Figure 3) in S. lividans TK64 was the first report of heterologous production of a diazo-containing NP (Waldman et al., 2015). This heterologous expression not only enabled mechanistic studies of genes creABDAM in the diazo formation, but also identified a diazo metabolite, 2-hydroxy-4-methoxybenzoic acid (2,4-HMBA) (Waldman et al., 2015). A unique and unusual phosphonotripeptide antibacterial compound, dehyrophos, was identified in S. lividans 66 through the expression of fosmid clones isolated from the wild-type producer S. luridus NRRL 15101 (Circello et al., 2010). The heterologous expression system further determined minimal genes required for dehydrophos production. A d-cycloserine (DCS) non-producing S. lividans 66 could produce DCS through the expression of a 21-kb DNA fragment encoding 10 ORFs, DcsA-DcsJ (Kumagai et al., 2010).
Thioviridamide (Figure 3) contains five thioamide bonds and was originally isolated from S. olivoviridis NA 05001 (Hayakawa et al., 2006). Introduction of a 14.5-kb EcoRI fragment containing genes tvaB-tvaO together with an additional gene tvaA into S. lividans TK23 produced thioviridamide; further, the authors also confirmed the compound as a RiPP (Izawa et al., 2013). The antitumor agent YM-216391 is a new cyclic peptide containing a polyoxazole-thiazole moiety, originally isolated from S. nobilis (Sohda et al., 2005). Gene cluster analysis suggested it is a RiPP involving multistep posttranslational modifications. Since the strain is hard to manipulate, the entire gene cluster was expressed in S. lividans, producing ~0.2 mg/L of YM-216391, and if ymR3 gene encoding a pathway-specific repressor was deleted, the yield increased to 3.84 mg/L (Jian et al., 2012).
Low yield, particularly in the less-studied actinomycetes, is a major bottleneck to access the biosynthesis of new NPs in their native producers. In addition, these strains are often not amenable for genetic manipulation. A desert isolate Actinomadura namibiensis produces type III lantibiotics labyrinthopeptin A1 and A2. In addition to its unique structure, A2 possesses remarkable antiviral and antiallodynic activities. Due to the genetic intractability of A. namibiensis, the BGC was heterologously expressed in S. lividans enabling the identification of novel labyrinthopeptins and harnessing the flexibility of the biosynthetic machinery (Krawczyk et al., 2013). The anti-tuberculosis antibiotic capreomycin was produced at 50 mg/L when simply expressing the gene cluster in S. lividans 1326 without any modification, in comparison to a trace level production in the native strain Saccharothrix mntabilis (Fenlnagle et al., 2017). Platensimycin and platensin (Figure 3) exhibit antibacterial activity against Gram-positive bacteria by selectively inhibiting cellular lipid biosynthesis through the selective targeting of β-keto-acyl (ACP) synthase I/II (FabF/FabB) in the fatty acid pathway (Wang et al., 2006; Wang et al., 2007). PtmRl and PtnRl have been identified as pathway-specific repressors of platensimycin and platensin production in S. platensis MA7327 and S. platensis MA7339, respectively (Smanski et al., 2009). Since the strain is difficult to manipulate genetically, heterologous expression of the platensin gene cluster without the ptnR1 gene in S. lividans K4-114 enabled the production of platensin with 6 congeners, which were not detected in the native producer (Smanski et al., 2012). Similarly, due to the lack of a genetic manipulation system in S. antibioticus, heterologous production of 8,8a-dihydroxy-6-deoxyerythronolide B in S. lividans provided a useful platform to study and engineer the biosynthesis of oleandomycin, a group of important macrolide antibiotics (Shah et al., 2000).
Expression of BGCs in heterologous hosts sometimes modifies the final products due to an unexpected involvement of host catalysts. When the gene cluster of blasticidin S, a strong fungicidal agent isolated from S. griseochromogenes, was engrafted into the chromosome of S. lividans 66, an inactive deaminohydroxylblasticidin S (Figure 3) was isolated instead of blasticidin S, likely due to the action of a host deaminase in S. lividans 66 (Li et al., 2013b). Another intriguing example is the heterologous production of a cyanotoxin lyngbyatoxin A (Figure 4). Genes ltxABC were identified to direct the biosynthesis of lyngbyatoxin A in cyanobacterium Moorea producens (Edwards and Gerwick, 2004); a similar gene cluster tleABC has been recently found in S. blastmyceticus NBRC 12747 (Awakawa et al., 2014). Expression of tleABC in three different hosts S. albus G153, S. lividans TK21 and S. avermitilis SUKA22 revealed unexpected host-pathway interactions. Although two intermediates valindolmycin and indolactam V and the final product lyngbyatoxin A were detected in all hosts, new analogues that are absent in both native producer and hosts were produced (Zhang et al., 2016a). Interestingly, these analogues were produced in a host-specific manner; different host preferably recognizes different compounds in the lyngbyatoxin pathway. For example, S. lividans used indolactam V to generate analogues; while S. albus and S. avermitilis interacted with the early intermediate valindolmycin and the final product lyngbyatoxin A, respectively. The host specific generation of lyngbyatoxin A analogues was summarized in Figure 4 (Zhang et al., 2016a).
Figure 4.
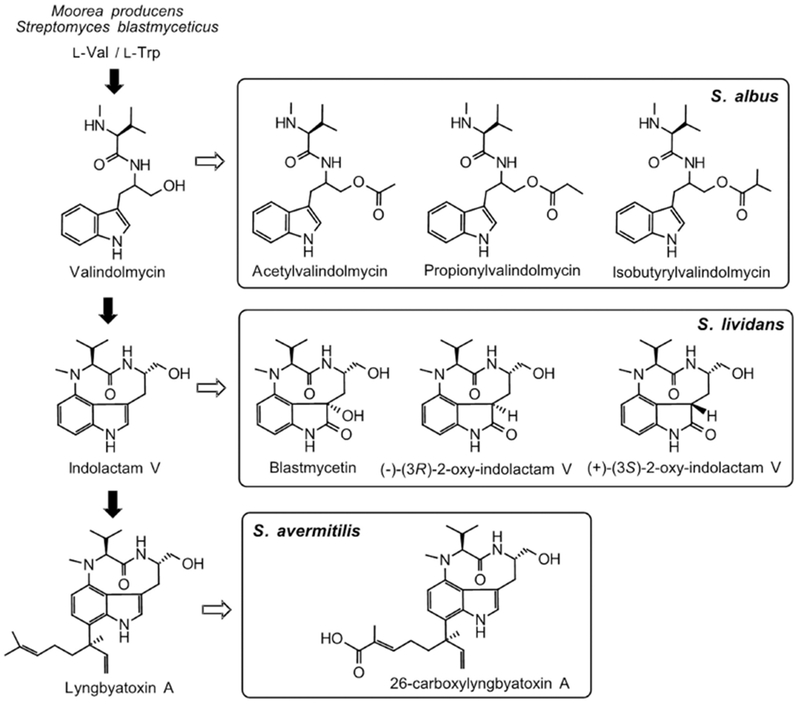
Heterologous generation of lyngbyatoxin A and its derivatives via host-specific interactions. The figure is adapted from Zhang et al., 2016a.
It has been widely recognized that secondary metabolism in Streptomyces is controlled by a complex regulatory network, which includes pathway-specific regulators (activators or repressors), global regulators (controlling multiple pathways), and small signaling molecules (van Wezel and McDowall, 2011). Overexpression of activators and knock-out of repressors are effective strategies to achieve a better production of NPs. The ppk and pstS genes encode a polyphosphate kinase that down-regulates antibiotic production and a high-affinity phosphate binding protein in S. lividans, respectively. The ppk/pstS double knock-out mutant of S. lividans showed a higher level of ACT production (Chouayekh and Virolle, 2002; Ramos et al., 2008). The expression of the cosmid cos16F4 in the mutant enhanced the production of 8-methyl-tetracenomycin C by 10-fold (Diaz et al., 2013).
The aromatic compound cinnamic acid (Figure 3) is a precursor of various phenylpropanoids including lignins, flavonoids, and coumarins, and is of industrial importance in diverse areas such as perfume production, the food industry, and pharmaceutical production. The precursor can be produced either through chemical synthesis or through microbial fermentation using E. coli or P. putida, however, the former is energy intensive and the latter usually ends with a low-yield (Nijkamp et al., 2005; Vannelli et al., 2007). Alternatively, the ubiquitous enzyme phenylalamine ammonia lyse (PAL) catalyzes the conversion of l-phenylalanine to ammonia and cinnamic acid (Cui et al., 2014). A PAL was recently identified in S. maritimus (PALSm), which is much more robust and flexible with substrates than those found in plant such as Petroselinum crispum or yeast such as Rhodosporidium toruloides. The overexpression of PALsm in S. coelicolor led to the production of cinnamic acid at 0.2~0.5 mg per 100 mL; notably, expressing the same PAL in S. lividans enhanced the production to 210 mg per L with glucose as the carbon source and to 450 mg/L, if glucose was replaced by glycerol (Xiang and Moore, 2002). A similar yield of cinnamic acid was obtained when cheap carbon sources such as raw starch or xylose were used, indicating that S. lividans represents an economically friendly alternative to supply this industrially important precursor.
It has been estimated that only about 1% of all microbes have been cultured for conventional microbial studies (Torsvik et al., 1990). The vast majority of uncultivable microorganisms are unexploited. Efficient harnessing of biosynthetic potential and discovery of new NPs have only become possible recently due to the advances in genomics and metagenomics together with heterologous expression. Five novel compounds, terragines A (Figure 3), B, C, D and E, were identified through expressing soil DNA in the surrogate host S. lividans (Wang et al., 2000). The assembly and functional analysis of individual genomes in an environmental sample is an alternative method for accessing the metabolic potential of the majority of uncultured organisms (Handelsman et al., 1998; Gillespie et al., 2002; Daniel, 2004). Most functional metagenomic studies have been limited by the poor expression of genes derived from metagenomic DNA in E. coli. To overcome this limitation, a new tool has been developed for the construction and functional screening of metagenomics libraries in S. lividans. These studies demonstrated that functionally screening metagenomic libraries in Streptomyces hosts provides access to metabolic potential different from those expressed in E. coli alone (McMahon et al., 2012).
In addition to exogenous secondary metabolic gene clusters, S. lividans is widely used for expression or overproduction of enzymes derived from diverse microbial and environmental sources. The expression of thermophilic genes (Kieser et al., 2000; Diaz et al., 2008; Li et al., 2013a), secretory enzymes (Noda et al., 2010), superoxide dismutase (SOD) genes (Halliwell and Gutteridge, 1985; Kang et al., 2006; Kang et al., 2007; Kanth et al., 2011), and glycosyltransferases (Quiros et al., 2000; Nakazawa et al., 2011) are some of the most common examples.
4. Streptomyces albus as a heterologous host
S. albus J1074, derived from S. albus G, is defective in both restriction and modification enzymes of the SalI system. The 6,823,670 bp S. albus genome (accession number NZ_DS999645.1) is the smallest among all sequenced Streptomyces genomes and has the highest G+C content (73.3%) (Table 1) (Olano et al., 2014; Zaburannyi et al., 2014). S. albus J1074 has long been known as a suitable host for the heterologous production of NPs, at least partially due to the efficient genetic manipulation systems, well-known genetic background, minimized genome, and easy culture. In addition, S. albus contains two highly active attB sites, which could also explain its excellence as a heterologous host (Bilyk and Luzhetskyy, 2014). Under normal growth conditions, S. albus J1074 produces no bioactive secondary metabolites, although genome analyses predicted 27 secondary metabolic BGCs. The Salas group applied different strategies for the activation of cryptic gene clusters and isolated a few secondary metabolites such as antimycin and 6-epi-alteramides (polyketide-NRP hybrid), candicidin (Type I polyketide), indigoidine (NRP), and glycosylated paulomycins (Olano et al., 2014).
The examples mentioned in this section are summarized in Table 4. Moenomycin A (Figure 5) was isolated from its native strain S. ghanaensis ATCC 14676 with low yield. In an attempt to express the moenomycin gene cluster in various Streptomyces hosts, the moenomycin-resistant host S. albus J1074 produced the highest level of moenomycin A; production was further increased by co-expressing the relA gene, an important component in the ppGpp signal pathway (Makitrynskyy et al., 2010). The heterologous production of furaquinocin, a polyketide-isoprenoid hybrid originally isolated from S. sp. KO-3988 (Kawasaki et al., 2006), in S. albus has been demonstrated (Isogai et al., 2012). The heterologous expression of three contiguous genes encoding a type III PKS (fur1), a monooxigenase (fur2) and an aminotransferase (fur3) in S. albus produced a novel intermediate 8-amino-2,5,7-trihydroxy naphthalene-1,4 dione. The deletion of fur3 diminished the production of furaquinocins, which can be restored by supplying 8-amino-2,5,7-trihydroxy naphthalene-1,4 dione (Isogai et al., 2012).
Figure 5.
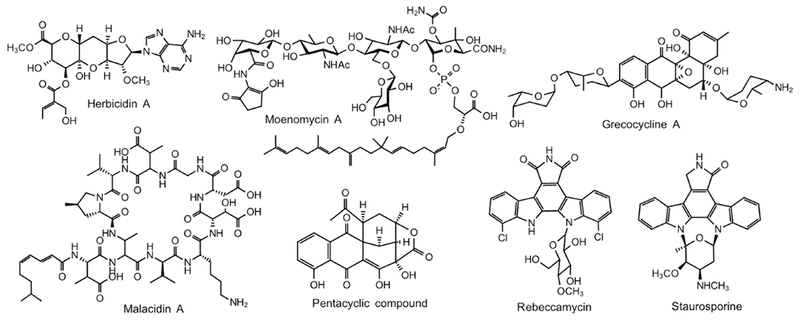
Chemical structures of representative natural products generated in the heterologous host S. albus. Grecocycline A; Herbicidin A; Malacidin A; Moenomycin A; Rebeccamycin and Staurosporine; and the pentacyclic ring compound with a new scaffold.
For the overproduction of a type I polyketide iso-migrastatin, the gene cluster was cloned from its producing strain S. platensis NRRL18993 and expressed in five different hosts, S. albus, S. lividans, S. coelicolor, and two mutants of S. avermitilis. Production was improved by 3 to 18 fold via R2YE media optimization: the highest yield of 128.6 mg/L was achieved in S. albus (Yang et al., 2011). Secondary metabolic gene clusters can sometimes span over 100-kb. To incorporate large DNA fragments, Bilyk et al. developed a new shuttle vector system which is based on the pi5a and F-factor replicons and can be maintained in E. coli, yeast and Streptomyces. Using this system, PCR amplified gene products covering several parts of the gene cluster of aromatic polyketides grecocyclines were transferred into yeast together with the newly developed vector; introduction of the assembled construct into S. albus led to the production of grecocycline A (Figure 5) and its derivatives (Bilyk et al., 2016). The fredericamycin (FDM) BGC has been expressed in S. albus yielding 120~132 mg/L of FDM, which also enabled the study of gene functions. In-frame deletion of fdmM or fdmM1, responsible for C-6 or C-8 hydroxylation, respectively, diminished the production of FDM A and a key intermediate FDM E (Chen et al., 2009; Baltz, 2010). Steffimycin is an anthracycline antibiotic isolated from S. steffisburgensis NRRL 3193 (Kunnari et al., 1997). The expression of the steffimycin BGC in S. albus J1074 produced 10 mg/L steffimycin (Gullon et al., 2006). Co-expression with neutral or branched deoxyhexose pathways has generated newly glycosylated steffimycin analogues; 3’-O-methy1steffimycin and D-digitoxyl-8-demethoxy-10-deoxy-steffimycin showed improved anti-proliferative activity with GI50 values less than 1 μM while steffimycin’s GI50 values are 2.61 ~ 6.79 μM (Olano et al., 2008). In addition, the S. albus host has been used for the in vivo characterization/verification of tailoring enzymes in the biosynthesis of specific antibiotics. For example, three methyltransferase genes elmMI, elmMII and elmMIII were expressed individually in S. albus and exhibited their consecutive methylation at different hydroxyl groups of the sugar moiety of the polyketide elloramycin (Patallo et al., 2001).
Kinamycins, diazole-containing aromatic polyketide antibiotics, were isolated from S. galtieri sgt26. A complete understanding of biosynthetic pathways was hindered by the lack of genetic manipulation systems in the native strain. Expression of a BAC clone containing kinamycin gene cluster in S. ablus J1074 restored the heterologous production of kanamycin; which also enabled the identification of boundaries of the cluster (spanning 75-kb) and the characterization of gene functions in the pathway (Liu et al., 2018).
Dithiolopyrrolone-type compounds show diverse activities including antibacterial, antifungal, insecticidal and anticancer (Li et al., 2014). Expression of the dithiolopyrrolone (aut) gene cluster, which was isolated from S. thioluteus DSM 40027, in S. albus revealed the characteristic UV spectrum of dithiolopyrrolone-type compounds; however, the yield was too low and was insufficient for structural determination. The comparison of the aut gene cluster with holomycin (hlm) gene cluster from S. clavuligerus showed the aut gene cluster lacks the hlmK gene which encodes a distinctive type II thioesterase. The production of dithiolopyrrolone in S. albus was greatly improved through the co-expression of hlmK (Zhai et al., 2016).
Rebeccamycin and staurosporine (Figure 5) are antitumor compounds which belong to the family of indolocarbazole alkaloids (Sánchez et al., 2002). Over 30 indolocarbazole derivatives have been identified through the dissection and reconstitution of the rebeccamycin biosynthetic gene cluster and co-expression of the staurosporine gene cluster in S. albus. Further, the expression of pyrH and thaI, halogenase genes from S. rugosporus and S. albogriseolus, respectively, successfully produced the halogenated derivatives (Sánchez et al., 2005). The biosynthesis of indolocarbazole K252a has remained unclear in the native producer Nocardiopisis longicantena. The co-expression of inkO and inkD genes which are responsible for the earliest steps of K252a biosynthesis in S. albus yielded chromopyrrolic acid, which may serve as a good starting point for further understanding the K252a biosynthetic pathway and the development of bioactive improved indolocarbazole analogues (Kim et al., 2007; Chae et al., 2009).
Herbicidins (Figure 5), the adenosine-based nucleoside antibiotics with a rare and unusual tricyclic undecose core decorated with a (5-hydroxy)-tiglyl moiety, have been isolated from S. sp. L-9-10 with a yield of 50~100 mg/L. It is notable that this was the first report of a bacterial pathway that uses tiglyl-CoA from the l-isoleucine catabolism to modify a secondary metabolite The completeness of the herbicidin gene cluster was confirmed by heterologous production of herbicidins in S. albus J1074 (Jung et al., 2006). Thiocoraline is an antitumor compound produced by two actinomycetes Micromonospora sp. ACM2-092 and Micromonospora sp. ML1 isolated from marine invertebrates. The thiocoraline gene cluster was cloned but was neither expressed in S. albus nor S. lividans unless the positive regulatory gene, tioA, encoding an OmpR family activator was transcribed from the ermE* promoter (Lombó et al., 2006).
Carotenoids and terpenoids are common NPs found in all photosynthetic organisms. They play vital roles as food colorants, feed supplements, nutraceuticals and pharmaceuticals. Some Streptomyces strains also contain carotenoid-type gene clusters, e.g., crta, but most of them remain silent. Mining the S. argiliaceus genome showed the presence a crta gene cluster but no carotenoids have been isolated from this strain. Becerril et al. attempted several approaches to reveal the final products of the crta gene cluster in both native and heterologous hosts. Expression of a cosmid pKC505-C25 containing the entire crta gene cluster in S. albus produced yellow-pigmented compounds which were further identified as carotenoids: leprotene, β-isorenieratene, 3,3’-dihydroxyleprotene, 3-hydroxyleprotene, and monomethylated derivatives of 3,3’-dihydroxyleprotene and 3-hydroxyleprotene (Becerril et al., 2018).
S. albus has also been very fruitful in expressing environmental DNA (eDNA) and yielding new NPs. Brady and his colleagues propagated DNA extracted directly from the environment, and produced a tetracyclic anti-MRSA antibiotic tetarimycin A , and two new azaquinones, utahmycins A and B, by expressing environmental DNA in S. albus (Bauer et al., 2010; Kallifidas et al., 2012). As a continuation of this work, the same group heterologously produced three types of antibiotics, the well-known landomycin E, a new compound with a previously uncharacterized pentacyclic ring scaffold (Figure 5), and a unique KB-3346-5 derivative which shows activity against MRSA and VRE (Feng et al., 2011). Screening for BGCs from eDNA metagenomic clones has been improved by expressing a PPTase gene in S. albus (Bitok et al., 2017), which enabled Brady’s group to identify clones containing NRPS, PKS and hybrid PKS/NRPS gene clusters and to confirm that a clone is responsible for the production of myxochelin A (Bitok et al., 2017). More recently, the same group discovered malacidins (Figure 5), a group of calcium-dependent antibiotics which are potently active against multidrug-resistant pathogens and which sterilize MRSA infections in an animal model (Hover et al., 2018). Brady argued that addressing the following points will significantly hasten the discovery of NPs from metagenomics: (1) cloning methods for large DNA fragments (100~150 kb); (2) capability of hosts to accept versatile foreign DNA; and (3) efficient screening methods (Bitok et al., 2017).
5. Streptomyces venezuelae as a heterologous host
S. venezuelae has been used as a heterologous host because of its relatively fast growth with a doubling time of ~1 h (the shortest time among streptomycetes), genetic manipulation tools, and high transformation efficiency. The genome of S. venezuleae ATCC 15439 has been sequenced recently by two independent groups (accession No. LN881739 and CP013129) revealing a genome of 9.03-9.05 Mb (Table 1) (He et al., 2016; Song et al., 2016). Three major mutants of S. venezuelae ATCC 15439 have been generated to better express foreign BGCs: DHS2001 which lacks all pik genes (Jung et al., 2006), YJ003 which lacks the des gene cluster (Hong et al., 2004), and YJ28 which lacks both pik and des genes (Jung et al., 2007).
The examples mentioned in this section are summarized in Table 4. Aminoglycoside (AMG) antibiotics including amikacin, gentamicin, kanamycin, neomycin, spectinomycin, streptomycin, and tobramycin are important antibacterial drugs, especially for serious Gram-negative infections. Heterologous production of AMGs and their derivatives in S. venezuelae have been studied rigorously and shown to be successful. The production of gentamicin A2 (Figure 6A) in S. venezuelae YJ003 was the first heterologous expression of a pseudodisaccharide biosynthetic pathway (Park et al., 2008). The pSpc8 cosmid containing the spectinomycin BGC, isolated from its producing strain S. spectabilis ATCC27741, was expressed in S. venezuelae YJ003 leading to a heterologous production of spectinomycin (Thapa et al., 2008). Interestingly, expression of only five genes spcA, spcB, spcS2, spcM and spcG in S. venezuelae ATCC 15439 also produced spectinomycin; it has been suggested that the sugar is synthesized from host catalysts (Lamichhane et al., 2014). In the biosynthesis of ribostamycin, it was generally believed that ribose is a sugar donor only for neamine. However, Sohng’s group reported the biosynthesis of 6’-deamino-6’-hydroxyribostamycin, pseudoribostamycin (Figure 6A), in S. venezuelae YJ003 as a result of ribosylation of paromamine (Kurumbang et al., 2011). Further, pseudoribostamycin was isolated from S. lividans TK24 which harbors a ribostamycin cosmid. These results demonstrated that ribosylation is possible at both paromamine and neamine (Subba et al., 2007; Kurumbang et al., 2011).
Figure 6.
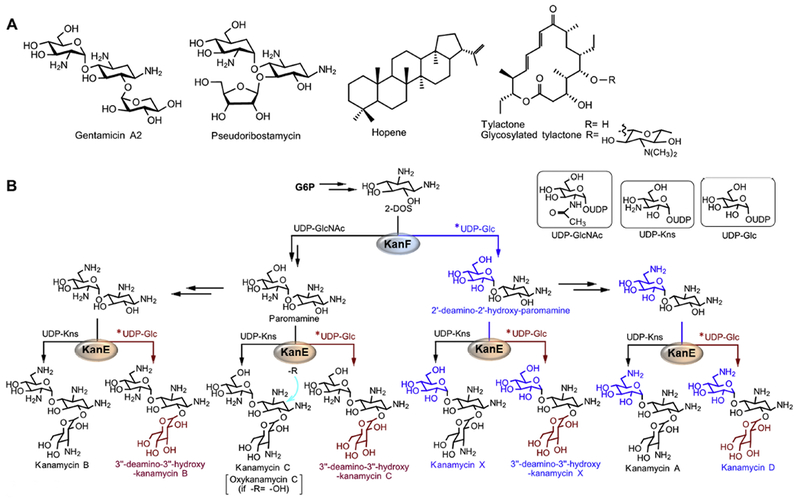
Heterologous production of natural products in the host S. venezuelae. (A) Chemical structures of representative natural products. Gentamicin A2; Hopene; Pseudoribostamycin; and Tylactone and its glycosylated derivative 5-O-mycaminosyl tylactone. (B) New kanamycins generated by the substrate flexibilities of the glycosyltranseferases KanF and KanE. New catalytic route of KanF or KanE is highlighted in blue or orange, respectively; and the corresponding new sugar donor is marked by an asterisk. 2-DOS, 2-deoxystreptamine; G6P, glucose-6-phosphate; UDP-Glc, UDP-d-glucose; UDP-GlcNAc, UDP-N-acetlyglucosamine; UDP-Kns, UDP-d-kanosamine.
Due to the difficulty of genetic manipulation in the native kanamycin producer S. kanamyceticus, the biosynthesis of kanamycins has been studied heterologously in S. venezuelae YJ003, which allows further chemical biology and combinatorial biosynthesis studies (Thapa et al., 2007). Generally, only kanamycin A, B, and C are produced in S. kanamyceticus, with kanamycin A as the major (>80%) product. By in vivo genetic engineering and combinatorial biosynthesis in S. venezuelae YJ003, i.e., expression of different sets of biosynthetic genes, and in vitro enzymatic reconstitutions, Park et al. identified a previously unexpected substrate flexibility of two glycosyltransferases KanF and KanE. Beside the known sugar donor UDP-d-N-acetylglucosamine (UDP-GlcNAc), KanF also accepts UDP-d-glucose (UDP-Glc) as the substrate; KanE accepts UDP-Glc in addition to the known donor UDP-d-kanosamine (UDP-Kns). These findings not only revealed the biosynthesis of kanamycin A, but also enabled the identification of five new kanamycins, 3”-deamino-3”-hydroxy-kanamycin B, 3”-deamino-3”-hydroxy-kanamycin C, kanamycin D, kanamycin X, and 3”-deamino-3”-hydroxy-kanamycin X (Figure 6B). Combinatorial biosynthesis further allowed the production of 1-N-AHBA-kanamycin A (also known as amikacin) (Park et al., 2011a), and a hybrid aminoglycoside oxykanamycin C (Figure 6B) through a chimeric fusion of spectinomycin and kanamycin biosynthetic genes (Nepal et al., 2010).
In addition to AMGs, various polyketides have been produced in S. venezuelae hosts. The production of oxytetracycline (oct) was increased from 75 to 431 mg/L in only 48 h by the expression of two pathway-specific regulators OctR and OtrR along with precursor supply in S. venezuelae WVR2006, a level comparable to 8 days in its native producer S. rimosus M4018. (Yin et al., 2016). The 40-kb DNA region containing five PKS genes from the tylosin pathway was cloned under the control of the strong ermE* promoter in two plasmids and introduced into S. venezuelae DHS2001, which led to the production of about 0.5 mg/L of the 16-membered tylactone and 5-O-mycaminosyl tylactone (Figure 6A) (Jung et al., 2007). Production was increased to 1.4 mg/L upon exogenous feeding of 10 mM diethyl malonate, and further improved, by 2.7- and 17.1-fold, respectively, by the co-expression of the positive regulatory gene pikD (Jung et al., 2006; Jung et al., 2008).
S. venezuelae has also been used successfully for the expression of cryptic BGCs and heterologous detection of NPs. A unique chlorinated lipopeptide 4-O-demethylbarbamide, which was originally isolated from a marine cyanobacterium Moorea praducens (Engene et al., 2012), was produced at approximately 1 μg/L in S. venezuelae DHS2001 (Kim et al., 2012). The Sohng group isolated hopene (Figure 6A) from S. venezuelae YJ28, which harbors an ermE* promoter-controlled 4-gene operon {hopA, hopB, hopD and hopE) of the hopene gene cluster from S. peucetius (Ghimire et al., 2015).
Plants are one of the richest sources of NPs; structurally diverse plant NPs have been used for drugs, cosmetics, seasonings, dyes, industrial chemicals, and nutraceuticals (Liu et al., 2017). There are major limitations for producing and purifying metabolites in plants: very low yield in plants (Chemler and Koffas, 2008; Liu et al., 2017), long time to produce a mature metabolite, seasonal production, and complex and tedious purification procedures (Chemler and Koffas, 2008). Due to the structural complexity of most plant NPs, total chemical synthesis is often challenging and usually yields large amounts of chemical wastes. Production of plant NPs in microbial hosts is an attractive alternative as it is renewable, environmentally friendly and season-independent, although there are still few challenges: (1) biosynthetic pathways are still mysterious for many plant NPs; (2) genetic background differences; (3) low or poor precursor supply or expression ofplant enzymes (Chemler and Koffas, 2008; Liu et al., 2017). Nonetheless, it is notable that S. venezuelae strains have been used to produce various plant NPs, such as naringenin (4 mg/L), pinocembrin (6.0 mg/L), resveratrol (0.4 mg/L), pinosylvin (0.6 mg/L), apigenin (15.3 mg/L) and chrycin (30.9 mg/L) through the expression of corresponding codon-optimized biosynthetic genes and exogenous feeding of precursors (Park et al., 2009; Park et al., 2010; Park et al., 2011b; Kim et al., 2015b).
6. Streptomyces avermitilis as a heterologous host
Streptomyces avermitilis is an important industrial microorganism, which produces the anthelmintic macrocyclic lactones avermectins (AVMs) (Burg et al., 1979; Nett et al., 2009; Thuan et al., 2014). In addition to AVMs, S. avermitilis also produces oligomycin and filipins (Ikeda et al., 2003; Komatsu et al., 2010); all of them are polyketides. The 9.03-Mb S. avermitilis genome (see more details in Table 1) was the second report of Streptomyces genomes (Ōmura et al., 2001; Ikeda et al., 2003). Genomic analysis indicated two unusual chromosomal features of S. avermitilis: (1) unlike terminal inverted repeats (TIRs) in other streptomycetes (generally tens to hundreds of kilobases), the TIRs in S. avermitilis is only 49-bp, which makes the S. avermitilis chromosome much more stable than others; (2) unlike the nearly even distribution of subtelomeric regions (~ 1 Mb) at both ends of a Streptomyces chromosome, S. avermitilis has 2 Mb and 0.5 Mb subtelomeric regions at the left and right end of its chromosome, respectively (Ikeda et al., 2003). In addition to the aforementioned polyketide secondary metabolites, the S. avermitilis genome indicates the presence of 34 extra secondary metabolic BGCs (mainly for NRPs, terpenoids, siderophores and bacteriocins), mostly at the left subtelomeric region (Ōmura et al., 2001; Ikeda et al., 2003; Nett et al., 2009).
Due to its unique chromosomal features, S. avermitilis is considered to be a good heterologous host; the Ikeda group systemically deleted the left subtelomeric region together with traditional gene knock-out to generate a series of large DNA deletion mutants SUKA1-SUKA17 and SUKA22 in an aim to engineer S. avermitilis as a versatile heterologous host (Komatsu et al., 2010). Of them, SUKA5 is defective in the production of AVMs, filipins and oligomycins by deleting a 1.5-Mb DNA region; SUKA17 was constructed by further deleting the BGCs of terpene compounds carotenoid, geosmin and neopentalenolactone in SUKA5 (Komatsu et al., 2010). The genome size of SUKA5 and SUKA17 is 83.2% and 81.46% of the wild-type S. avermitilis, respectively (more details listed in Table 3) (Komatsu et al., 2010). SUKA22 is isogenic to SUKA17, except the right side of the deletion region uses a mutant loxP in order to prevent unexpected recombination. Notably, all large-deletion mutants grew well on various media indicating that no essential genes were deleted (Komatsu et al., 2013). Since the production of all major endogenous metabolites, such as AVMs, filipin and oligomycin, were removed in the genome-minimized hosts, these large DNA deletions not only release metabolic precursors for exogenous biosynthetic pathways, but also facilitate the downstream purification of final products (Komatsu et al., 2013).
Table 3.
Major S. avermitilis mutants used as heterologous expression hosts.
| SN | Strain | Features | Genome size | References |
|---|---|---|---|---|
| 1 | S. avermitilis | Wild-type strain producing avermictins, oligomycins and filipins as major secondary metabolites. | 100% | Ikeda et al., 2003 |
| 2 | SUKA2 | Deletion of ~ 1.4 Mb chromosomal region (using general homologous recombination) from the left subtelomeric region of wild S. avermitilis ΔolmA (deletion of 1,487,159 bp spanning the region from sav6 to sav1205). It does not produce avermictins and filipins. | 83.12% | Komatsu et al., 2010 |
| 3 | SUKA3 | Deletion of ≥1.5 Mb chromosomal region (using Cre-loxP site specific recombination) from the left subtelomeric region of wild S. avermitilis (deletion of 1,516,020 bp spanning the region from sav17 to sav1287. It does not produce avermictins and filipins. | 83.2% | Komatsu et al., 2010 |
| 4 | SUKA4 | Deletion of oligomycin gene cluster from SUKA2 using Cre-loxP site specific recbombination. It does not produce avermictins, filipins and oligomycins. | 82.44% | Komatsu et al., 2010 |
| 5 | SUKA5 | Deletion of oligomycin gene cluster from SUKA3 using Cre-loxP site specific recbombination. It does not produce avermictins, filipins and oligomycins. | 82.11% | Komatsu et al., 2010 |
| 6 | SUKA17 | Additional deletion of gene clusters encoding the biosynthesis of the terpene compounds geosmin, neopentalenolactone, and carotenoid from SUKA5. | 81.46% | Komatsu et al., 2010 |
| 7 | SUKA22 | SUKA22 differs from SUKA17 only by carrying a mutant type loxP sequence at the right side of deletion region to prevent undesired recombination. | 81.46% | Komatsu et al., 2010 |
In his 2010 and 2013 reports, Komatsu et al. expressed a total of 25 foreign BGCs in the genome-minimized hosts (Komatsu et al., 2010; Komatsu et al., 2013). Based on the origin and generation of precursors, these pathways were grouped into 5 categories: sugar pathway, polyketide pathway, amino acid pathway, shikimate pathway, and mevalonate (MVA) or methylerythritol phosphate (MEP) pathway (Komatsu et al., 2013). Notably, most BGCs were expressed well in S. avermilitis hosts with yields better than in each gene cluster’s native strain. In this review, we will present one or two examples in each category. For a unified illustration in this section, foreign pathways reported in other references were also grouped into these categories. A Hill list of examples mentioned in this section is shown in Table 4.
The genome-minimized strains were initially used for the production of streptomycin (Figure 7) which was originally isolated in S. griseus IFO13350 (Ohnishi et al., 2008). The productivity of streptomycin in SUKA5 (~180 mg/L) is much higher than that in the S. avermilitis wild-type host (~30 mg/L) and the native streptomycin producer (~50 mg/L) (Komatsu et al., 2010). It is notable that the yield was always higher in the avermectin production medium than in the streptomycin production medium that was originally used with the native strain (Komatsu et al., 2010). In addition, 3 other pathways in the “sugar” category, ribostamycin, kasugamycin, and pholipomycin (structurally similar to moenomycin A in Figure 5), were successfully expressed in SKUA17 or 22 (Table 4) (Komatsu et al., 2013).
Figure 7.
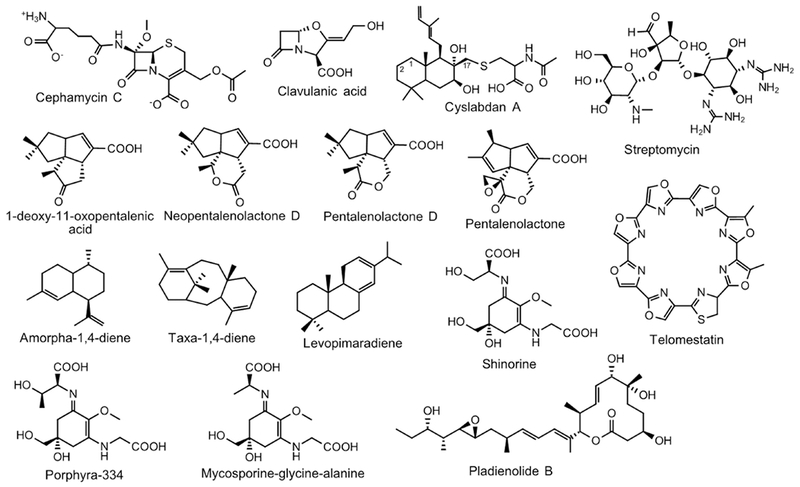
Chemical structures of representative natural products generated in the heterologous host S. avermitilis. 1-deoxy-11-oxopentalenic acid; Amorpha-1,4-diene; Cephamycin C; Clavulanic acid; Cyslabdan A; Levopimaradine; Mycosporine-glycine-alanine; Mycosporine-glycine-serine (Shinorine); Mycosporine-glycine-threonine (Porphyra-334); Neopentalenolactone D; Pentalenolactone D; Pentalenolactone; Pladienolide B; Streptomycin; Taxa-1,4-diene; and Telomestatin.
The ~35-kb cephamycin gene cluster derived from S. clavuligerus ATCC 27064 (Alexander and Jensen, 1998) was introduced into SUKA17 to check the efficacy of large deletion mutants for the expression of “amino acid” pathways. The production of cephamycin C (Figure 7) in the avermectin producing medium was ~80 mg/L, higher than that in the normally used starch-asparagine medium; further, expression of an extra copy of ccaR encoding the cephamycin pathway-specific activator further enhanced cephamycin C production to ~130 mg/L (Komatsu et al., 2010). Interestingly, adjacent to the cephamycin gene cluster, there is the gene cluster (25-kb) for the β-lactam compound clavulanic acid (Figure 7), a strong inhibitor of bacterial β-lactamases, and its expression is also controlled by CcaR (Bignell et al., 2005). Expression of the clavulanic acid gene cluster together with ccaR produced 16 mg/L clavulanic acid, whereas, if the ccaR gene is absent, no clavulanic acid was produced (Komatsu et al., 2013). In addition, holomycin and lactacystin in the “amino acid” category were also produced efficiently in SUKA22 (Table 4) (Komatsu et al., 2013).
Expression of polyketides has been attempted most frequently (8 compounds) in S. avermilitis hosts (see details in Table 4) (Komatsu et al., 2010; Komatsu et al., 2013). Pladienolides, a group of antitumor macrocyclic polyketides, were isolated in S. platensis Mer-11107 (Machida et al., 2008). Since major metabolites in S. avermitilis are polyketides (AVMs, filipins and oligomycin), the deletion of these BGCs assumes to release rich precusors for the production of foreign polyketides. However, an initial expression of the 75-kb pladienolide gene cluster in both S. avermitilis wild-type and the SUKA5 mutant hosts showed no production of pladienolides (Komatsu et al., 2010). It was demonstrated that the pathway-specific activator PldR was not expressed; the use of the constitutive ermE* promoter led to the production of pladienolide B (Figure 7) (Komatsu et al., 2010). As expected, the production of pladienolide B in SUKA5 was higher (20-fold) than that in the wild-type host. In the 2013 report, either SUKA17 or SUKA22 was used for the heterologous production of aureothin (116 mg/L), bafilomycin B1 (16 mg/L), erythromycin (4 mg/L), leptomycin (5 mg/L), oxytetracycline (20 mg/L) and resistomycin (360 mg/L) (Table 4) (Komatsu et al., 2013). The production of modular PKS-derived polyketides such as bafilomycin B1 and erythromycin indicated that the large-deletion mutants can express large gene clusters in BAC (bacterial artificial chromosome) clones with a total size of ~100-kb without detectable deletions.
Since no genes related to the shikimate pathway have been found in the S. avermitilis genome, the production of compounds using precursors or substrates, such as tryptophan and chorismite, derived from the shikimate pathway is intriguing. The indolocarbazole antibiotic rebeccamycin (Figure 5) was synthesized from two molecules of l-tryptophan in Lechevalieria aerocolonigenes ATCC 39243 (Onaka et al., 2003). The expression of the 16-kb rebeccamycin gene cluster in SUKA17 yielded 7 mg/L of rebeccamycin (Komatsu et al., 2013). Chloramphenicol and novobiocin, both derived from the important shikimate pathway intermediate chorismate, are additional examples of the “shikimate” category to be produced in SUKA22 with a titer of 250 and 1 mg/L, respectively (Table 4) (Komatsu et al., 2013).
Terpenoids, a large group of diverse natural products with more than 55,000 members, are all constructed from the common precursor isopentenyl diphosphate (IPP) (Christianson, 2017). IPP can be synthesized via the MVA pathway, also known as the 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase pathway (Buhaescu and Izzedine, 2007), or the MEP pathway (Banerjee and Sharkey, 2014). Both microbial and plant terpenoids were successfully produced in the S. avermitilis host SUKA17, in which all terpene gene clusters were deleted (Table 4) (Komatsu et al., 2010; Komatsu et al., 2013). Pentalenolactone (Figure 7) is a sesquiterpnoid antibiotic that is produced by various Streptomyces strains including S. exfoliatus UC5319 and S. arenae TÜ469 (Takeuchi et al., 1969; Martin et al., 1970; Cane et al., 1990). Interestingly, S. avermitilis prodcued a structurally-distinct new pentalenolactone, neopentalenolactone D (Figure 7) (Jiang et al., 2009). Comparing the ptl gene cluster (sav2990-sav3002, 13.4-kb) in S. avermitilis with pen (S. exfoliatus UC5319) and pnt (S. arenae TÜ469) gene clusters revealed 8 conserved and undirectionally transcribed genes (Tetzlaff et al., 2006; Jiang et al., 2009; Seo et al., 2011); a penM or pntM gene which encodes a P450 monooxygenase only exists in the pen and pnt gene clusters. It has been demonstrated that early steps are identical in all three pathways to yield a common intermediate 1-deoxy-11-oxopentalenic acid (Figure 7). In the ptl pathway (S. avermitilis), a regio-specific Baeyer-Villiger oxygenase PtlE oxidizes the intermediate to the final product neopentalenolactone D (Jiang et al., 2009); whereas in the pen or pnt pathway, PenE or PntE oxidizes the intermediate into pentalenolactone D (Figure 7), which is further catalyzed by PenD or PntD (a non-heme iron-depend dehydrogenase/oxygenase) and the cytochrome P450 PenM or PntM (for an unprecedented oxidative ring rearrangement) to yield the final product pentalenolactone (Seo et al., 2011; Zhu et al., 2011). Based on these observations, SUKA17 was transformed first with the ptl gene cluster minus ptlE/ptlD, and then stepwise with pntE/pntD genes under the control of the ermE promoter, and with penM gene together with fdxD (sav3129, encoding a ferredoxin) and fprD (sav5675, encoding a ferredoxin reductase) as an operon also under the ermE promoter, the final SUKA17 recombinant strain produced pentalenolactone, but not the S. avermitilis native product neopentalenolactone D (Komatsu et al., 2013).
A codon-optimized ads gene that encodes a plant sesquiterpene synthase, amorpha-4,11-diene synthase (ADS) in Artemisia annua and the native S. avermitilis ptlB gene (sav2997) encoding a farnesyl diphosphate synthase in the ptl pathway were co-expressed in SUKA17, yielding abundant amorp ha-1,4-diene (Figure 7) (Komatsu et al., 2010). In the 2013 report, co-expression of synthetic plant diterpene synthase genes, tds or lps gene involved in the production of taxa-4,11-diene or levopimaradine in the taxol or ginkgolide A pathway, respectively, with the S. avermitilis crtE gene (sav1022) encoding a geranylgeranyl diphosphate synthase yielded taxa-1,4-diene (Figure 7), and levopimaradine (Figure 7) and its analogue abietatriene, respectively (Komatsu et al., 2013). Notably, plant terpenoids were heterologously produced in a synthetic medium without the addition of precursors.
Cyslabdans, consisting of a unique labdan-type diterpene and an N-acetylcysteine residue, were identified in a search for compounds that could restore the anti-MRSA activity of imipenem from S. sp. K04–0144, later renamed as S. cyslabdanicus K04–0144 (Fukumoto et al., 2008a; Fukumoto et al., 2008b; Ikeda et al., 2016). Cyslabdan enhances the anti-MRSA activity by over 1,000-fold for imipenem and other β-lactams (100~1,000-fold), by inhibiting the formation of pentaglycine interpeptide bridge (Koyama et al., 2012). Genome mining of S. cyslabdanicus K04-0144 revealed the presence of four genes, cldA-cldD, putatively involved in the biosynthesis of cyslabdans. The heterologous expression of the four genes in SUKA22 (lacking all endogenous terpene pathways) produced cyslabdan A (Figure 7), and two new analogues, 2-hydroxycyslabdan A and 17-hydroxycyslabdan A (Ikeda et al., 2016). Another labdane-type diterpene raimonol, which was originally reported in the plant Nicotiana raimondii (Noma et al., 1982), was produced in SUKA22 harboring a cld-like gene cluster (rmn) isolated from S. anulatus GM95 (Ikeda et al., 2016).
The macrocyclic peptide telomestatin (Figure 7) was identified in S. anulatus 3533-SV4 as a strong telomerase inhibitor (Shin-ya et al., 2001). Low yield and difficulties in chemical synthesis of telomestatin are major bottlenecks impeding its wider applications (Linder et al., 2011; Amagai et al., 2017). The telomestatin gene cluster was identified in the native producer as a RiPP pathway and was heterologously expressed in SUKA17 under the control of a xylose-inducible promoter of a S. avermitilis gene sav7182, however, no telomestatin was produced (Amagai et al., 2017). The use of the promoter of sav2902 gene, encoding a LuxR-family transcriptional activator in oligomycin biosynthesis (Ikeda et al., 2003), produced trace amounts of telomestatin; whereas the use of sav2794 promoter efficiently yielded telomestatin at more than 5 mg/L (Amagai et al., 2017). Sav2794 is a secreted neutral metalloprotease, possibly expressed during the late logarithmic growth phase (Ikeda et al., 2003). With this system, authors generated telomestatin derivatives, 6-desmethyltelomestatin, 9-desmethyltelomestatin and 6,9-didesmethyltelomestatin by amino acid substitution in the core peptide TlsC (Amagai et al., 2017).
Mycosporines and mycosporine-like amino acids (MAAs) with an absorption maxima ranging from 310 to 360 nm are widely produced by fungi, marine bacteria, cyanobacteria, and other marine organisms to protect themselves from UV-radiation (Sinha et al., 2007; Bhatia et al., 2011). Genome mining revealed that Gram-positive bacteria Actinosynnema mirum DSM 43827 and Pseudonocardia sp. strain P1 possess a gene cluster (mysA-mysD) highly homologous to BGCs identified in cyanobacteria. However, the gene cluster was either poorly expressed in P. sp. P1 yielding a very small amount of MAA-like compounds or not transcribed at all in A. mirum DSM 43827 (Miyamoto et al., 2014). Heterologous expression of the mys gene cluster in SUKA22 using the constitutive rpsJ promoter produced shinorine (mycosporine-glycine-serine) (Figure 7), indicating clearly that the mys gene cluster is responsible for shinorine biosynthesis in A. mirum DSM 43827 and P. sp. P1, which is the first demonstration of MAA production in Gram-positive bacteria (Miyamoto et al., 2014). In addition to shinorine, porphyra-334 (mycosporine-glycine-threonine) (Figure 7) and a novel MAA compound mycosporine-glycine-alanine (Figure 7) were detected in the extracts of SUKA22 expressing the A. mirum mys gene cluster. The yield of mycosporine-glycine-alanine was increased 9.6-fold by supplementation with 400 mM NH4Cl (Miyamoto et al., 2014). It is notable that heterologous expression of the A. mirum gene cluster in SUKA22 yielded total MAAs (shinorine, porphyra-334, mycosporine-glycine and mycosporine-glycine-alanine) at 188 mg/L, equivalent to 13.9 mg MAA/g dry cell weight, which is much higher than the productivity of MAAs in native producers such as cyanobacterium (0.03 to 0.98 mg MAA/g dry cell weight) or red algae (0.2 to 7.8 mg MAA/g dry cell weight) (Karsten et al., 2009; Komatsu et al., 2008), and in heterologous producers such as E. coli expressing shinorine biosynthetic genes from cyanobacterium Anabaena variabilis ATCC 29413 under the T7 promoter (0.15 to 0.65 mg/L) (Balskus and Walsh, 2010).
7. Summary
This review shows the importance of Streptomyces hosts for the determination of boundaries or minimal gene sets for BGCs (typically seen in early studies), yield improvement, heterologous production of NPs and discovery of novel compounds via genetic engineering, or expression of cryptic BGCs and those derived from metagenome DNA. Since the report of S. coelicolor A3(2) genome in 2002, the past one and a half decades has witnessed an exponential advancement in actinomycete genomic studies, which unexpectedly revealed that secondary metabolisms in actinomycetes have been largely underestimated. Although a single digit number of NPs is usually produced by a microbe, dozens of BGCs are encoded in its genome. Equally significant advances have been made in metagenomic studies of uncultured microorganisms derived directly from environmental samples. In parallel with the advance in genomics/metagenomics, a huge number of BGCs has been predicted by bioinformatic analysis. As of this writing, there are more than 22,000 BGCs deposited in the antiSMASH platform, one of the most widely used tools for genome/gene cluster analysis (Blin et al., 2017b; Medema et al., 2011), and this number is increasing daily. How to efficiently mine the genomes, express BGCs, and detect final products are becoming the core disciplines in the study of natural products and drug discovery (Baltz, 2016; Nah et al., 2017; Walsh and Tang, 2017; Xu et al., 2017a; Ziemert et al., 2016). With this background, there is no doubt that heterologous expression will play a greater role in accessing the vast untapped chemical potential and in harnessing the unprecedented opportunity of NPs in drug discovery.
New methods such as cloning techniques for large DNA fragments as well as traditional host engineering strategies such as the removal of endogenous BGCs and the use of strong promoters, make heterologous expression an even more powerful tool for the production of new NPs (Malcolmson et al., 2013; Ochi, 2017; Sun et al., 2015; Xu et al., 2016; Walsh and Tang, 2017; Tu et al., 2018). Omics-guided pathway reconstitution and host engineering have become a very effective strategy for optimized production of targeted products in heterologous hosts. A nearly 1,000-fold increase in the yield of the insecticide spinosyn (Figure 3) in S. albus is a recent example of this approach (Tan et al., 2017). Introduction of mutations with resistance to drugs such as rifampin and streptomycin has proven an efficient strategy to activate secondary metabolic potential (Ochi, 2017). Using S. coelicolor A3(2) as a model organism, the introduction of multiple drug resistance mutations led to 1.63 g/L production of actinorhodin, i.e. ~180 fold higher than that of the wild-type strain (Wang et al., 2008). This approach has been used in engineering both native producers and heterologous hosts, such as S. coelicolor M1154 which contains a single nucleotide mutation in rpoB (rifampicin-resistance) and rpsL (streptomycin resistance), respectively (Gomez-Escribano and Bibb, 2011).
Acknowledgement
The authors thank Drs. Kozo Ochi and Peter J. McCarthy for their help and discusstion during the preparation of the manuscript. This work was supported in part by the National Institutes of Health [CA209189] to G.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DC, and Jensen SE, 1998. Investigation of the Streptomyces clavuligerus cephamycin C gene cluster and its regulation by the CcaR protein. J. Bacteriol. 180(16), 4068–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt S, and Wilkinson B, 2015. Biosynthesis of the novel macrolide antibiotic anthracimycin. ACS Chem. Biol. 10(11), 2468–2479. [DOI] [PubMed] [Google Scholar]
- Amagai K, Ikeda H, Hashimoto J, Kozone I, Izumikawa M, Kudo F, et al. , 2017. Identification of a gene cluster for telomestatin biosynthesis and heterologous expression using a specific promoter in a clean host. Sci. Rep. 7(1), 3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awakawa T, Zhang L, Wakimoto T, Hoshino S, Mori T, Ito T, Ishikawa J, Tanner ME, and Abe I, 2014. A methyltransferase initiates terpene cyclization in teleocidin B biosynthesis. J. Am. Chem. Soc, 136(28), 9910–9913. [DOI] [PubMed] [Google Scholar]
- Balskus EP, and Walsh CT, 2010. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 329(5999), 1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz RH, 2010. Streptomyces and Saccharopolyspora hosts for heterologous expression of secondary metabolite gene clusters. J. Ind. Microbiol. Biotechnol. 37(8), 759–772. [DOI] [PubMed] [Google Scholar]
- Baltz RH, 2016. Genetic manipulation of secondary metabolite biosynthesis for improved production in Streptomyces and other actinomycetes. J. Ind. Microbiol. Biotechnol. 43(2-3), 343–370. [DOI] [PubMed] [Google Scholar]
- Banerjee A, and Sharkey TD, 2014. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat. Prod. Rep. 31(8), 1043–1055. [DOI] [PubMed] [Google Scholar]
- Bauer JD, King RW, and Brady SF, 2010. Citahmycins A and B, azaquinones produced by an environmental DNA clone. J. Nat. Prod. 73(5), 976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerril A, Alvarez S, Braña AF, Rico S, Diaz M, Santamaria RI, et al. 2018. Uncovering production of specialized metabolites by Streptomyces argillaceus : activation of cryptic biosynthesis gene clusters using nutritional and genetic approaches. PLoS One 13(5), e0198145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekiesch P, Basitta P, and Apel AK, 2016. Challenges in the heterologous production of antibiotics in Streptomyces. Arch. Pharm. 349(8), 594–601. [DOI] [PubMed] [Google Scholar]
- Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, et al. , 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417(6885), 141–147. [DOI] [PubMed] [Google Scholar]
- Berdy J, 2012. Thoughts and facts about antibiotics: where we are now and where we are heading. J. Antibiot. 65(8), 385–395. [DOI] [PubMed] [Google Scholar]
- Bhatia S, Garg A, Sharma K, Kumar S, Sharma A, and Purohit AP, 2011. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Pharmacogn. Rev. 5(10), 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb MJ, 2013. Understanding and manipulating antibiotic production in actinomycetes. Biochem. Soc. Trans. 41(6), 1355–1364. [DOI] [PubMed] [Google Scholar]
- Bignell DR, Tahlan K, Colvin KR, Jensen SE, and Leskiw B,K, 2005. Expression of ccaR, encoding the positive activator of cephamycin C and clavulanic acid production in Streptomyces clavuligerus, is dependent onbldG. Antimicrob. Agents Chemother. 49(4), 1529–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyk B, and Luzhetskyy A 2014. Unusual site-specific DNA integration into the highly active pseudo-attB of the Streptomyces albus J1074 genome. Appl. Microbiol. Bio techno 1 98(11), 5095–5104. [DOI] [PubMed] [Google Scholar]
- Bilyk O, Sekurova ON, Zotchev SB, and Luzhetskyy A, 2016. Cloning and heterologous expression of the grecocycline biosynthetic gene cluster. PLoS One 11(7), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitok JK, Lemetre C, Ternei MA, and Brady SF, 2017. Identification of biosynthetic gene clusters from metagenomic libraries using PPTase complementation in a Streptomyces host. FEMS Microbiol. Lett. 364(16), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Kim HU, Medema MH, and Weber T, 2017a. Recent development of antiSMASH and other computational approaches to mine secondary metabolite biosynthetic gene clusters. Brief Bioinform, bbxl46, DOI: 10.1093/bib/bbxl46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Wolf T, Chevrette MG, Lu X, Schwalen CJ, Kautsar SA, et al. , 2017b, antiSMASH 4.0-improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 45(W1), W36–W41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SF, Simmons L, Kim JH, and Schmidt EW, 2009. Metagenomic approaches to natural products from free-living and symbiotic organisms. Nat. Prod. Rep. 26, 1488–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhaescu I, and Izzedine H, 2007. Mevalonate pathway: a review of clinical and therapeutical implications. Clin. Biochem. 40(9–10), 575–584. [DOI] [PubMed] [Google Scholar]
- Burg RW, Miller BM, Baker EE, Birnbaum J, Currie SA, Hartman R, et al. , 1979. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob. Agents Chemother. 15(3), 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane DE, Oliver JS, Harrison PHM, Abell C, Hubbard BR, Kane CT, and Lattman R, 1990. Biosynthesis of pentalenene and pentalenolactone. J. Am. Chem. Soc. 112(11), 4513–4524. [Google Scholar]
- Chae CS, Park JS, Chung SC, Kim TI, Lee SH, Yoon KM, et al. , (2009). Production of chromopyrrolic acid by coexpression of inkOD in a heterologous host Streptomyces albus. Bioorg. Med. Chem. Lett. 19(6), 1581–1583. [DOI] [PubMed] [Google Scholar]
- Chemler JA, and Koffas MA, 2008. Metabolic engineering for plant natural product biosynthesis in microbes. Curr. Opin. Biotechnol. 19(6), 597–605. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wendt-Pienkoski E, Rajski SR, and Shen B, 2009. In vivo investigation of the roles of FdmM and FdmM1 in fredericamycin biosynthesis unveiling a new family of oxygenases. J. Biol. Chem. 284(37), 24735–24743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, 2013. Protein tyrosine phosphatase IB (PTP1B) and obesity. Vitam. Horm. 91, 405–424. [DOI] [PubMed] [Google Scholar]
- Chouayekh H, and Virolle MJ, 2002. The polyphosphate kinase plays a negative role in the control of antibiotic production in Streptomyces lividans. Mol. Microbiol. 43(4), 919–930. [DOI] [PubMed] [Google Scholar]
- Christianson DW 2017. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 117(17), 11570–11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circello BT, Eliot AC, Lee JH, van der Donk WA, and Metcalf WW, 2010. Molecular cloning and heterologous expression of the dehydrophos biosynthetic gene cluster. Chem. Biol. 17(4), 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JW, Chang FY, and Brady SF, 2009. Natural products from environmental DNA hosted in Ralstonia metallidurcms. ACS Chem. Biol. 4(1), 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone WJ, Vior NM, Santos-Aberturas J, Schmitz LG, Leeper FJ, and Truman AW, 2016. Dissecting bottromycin biosynthesis using comparative untargeted metabolomics. Angew. Chem. Int. Ed. 55(33), 9639–9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JD, Qiu JQ, Fan XW, Jia SR, and Tan ZL, 2014. Biotechnological production and applications of microbial phenylalanine ammonia lyase: a recent review. Crit. Rev. Biotechnol. 34(3), 258–68. [DOI] [PubMed] [Google Scholar]
- Cummings M, Breitling R, and Takano E, 2014. Steps towards the synthetic biology of polyketide biosynthesis. FEMS Microbiol. Lett 351(2), 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, 2004. The soil metagenome-a rich resource for the discovery of novel natural products. Curr. Opin. Biotechnol. 15(3), 199–204. [DOI] [PubMed] [Google Scholar]
- Deng Q, Zhou L, Luo M, Deng Z, and Zhao C, 2017. Heterologous expression of Avermectins biosynthetic gene cluster by construction of a bacterial artificial chromosome library of the producers. Synth. Syst. Biotechnol. 2(1), 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias DA, Urban S, and Roessner U, 2012. A historical overview of natural products in drug discovery. Metabolites 2(2), 303–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M, Ferreras E, Moreno R, Yepes A, Berenguer J, and Santamaria R, 2008. High-level overproduction of thermus enzymes in Streptomyces lividans. Appl. Microbiol. Biotechnol. 79(6), 1001–1008. [DOI] [PubMed] [Google Scholar]
- Diaz M, Sevillano L, Rico S, Lombó F, Braña AF, Salas JA, et al. , 2013. High level of antibiotic production in a double polyphosphate kinase and phosphate-binding protein mutant of Streptomyces lividans. FEMS Microbiol. Lett. 342(2), 123–129. [DOI] [PubMed] [Google Scholar]
- Douglas C, Wei-Min Z, and Chao-Dong Z, 2015. A DNA barcoding system integrating multigene sequence data. Methods Ecol. Evol. 6(8), 930–937. [Google Scholar]
- Du D, Zhu Y, Wei J, Tian Y, Niu G, and Tan H, 2013. Improvement of gougerotin and nikkomycin production by engineering their biosynthetic gene clusters. Appl. Microbiol. Biotechnol. 97(14), 6383–6396. [DOI] [PubMed] [Google Scholar]
- Edwards DJ, and Gerwick WH, 2004. Lyngbyatoxin biosynthesis: sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase. J. Am. Chem. Soc, 126(37), 11432–11433. [DOI] [PubMed] [Google Scholar]
- Engene N, Rottacker EC, Kastovsky J, Byrum T, Choi H, Ellisman MH, et al. , 2012. Mooreaprodncens gen. nov., sp. nov. and Moorea bonillonii comb, nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 62, 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles TH, Vior NM, and Truman AW 2018. Rapid and robust yeast-mediated pathway refactoring generates multiple new bottromycin-related metabolites. ACS Synth. Biol. 7(5), 1211–1218. [DOI] [PubMed] [Google Scholar]
- Felnagle EA, Rondon MR, Berti AD, Crosby HA, and Thomas MG, 2007. Identification of the biosynthetic gene cluster and an additional gene for resistance to the antituberculosis drug capreomycin. Appl. Environ. Microbiol. 73(13), 4162–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Kallifidas D, and Brady SF, 2011. Functional analysis of environmental DNA-derived type II polyketide synthases reveals structurally diverse secondary metabolites. Proc. Natl. Acad. Sci. U.S.A. 108(31), 12629–12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinspach K, Kapitzke C, Tocchetti A, Sosio M, and Apel AK, 2014. Heterologous expression of the thiopeptide antibiotic GE2270 from Plcmobisporci rosea ATCC 53733 in Streptomyces coelicolor requires deletion of ribosomal genes from the expression construct. PLoS One 9(3), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinspach K, Westrich L, Kaysser L, Siebenberg S, Gomez-Escribano JP, Bibb M, et al. , 2010. Heterologous expression of the biosynthetic gene clusters of coumermycin A(l), clorobiocin and caprazamycins in genetically modified Streptomyces coelicolor strains. Biopolymers 93(9), 823–832. [DOI] [PubMed] [Google Scholar]
- Floriano B, and Bibb M, 1996. afsR is a pleiotropic but conditionally required regulatory gene for antibiotic production in Streptomyces coelicolor A3(2). Mol. Microbiol. 21(2), 385–396. [DOI] [PubMed] [Google Scholar]
- Fukumoto A, Kim YP, Hanaki H, Shiomi K, Tomoda H, and Ōmura S, 2008a. Cyslabdan, a new potentiator of imipenem activity against methicillin-resistant Staphylococcus aureus, produced by Streptomyces sp. K04–0144. II. Biological activities. J. Antibiot. 61(1), 7–10. [DOI] [PubMed] [Google Scholar]
- Fukumoto A, Kim YP, Matsumoto A, Takahashi Y, Shiomi K, Tomoda H, et al. , 2008b. Cyslabdan, a new potentiator of imipenem activity against methicillin-resistant Staphylococcus aureus, produced by Streptomyces sp. K04–0144. I. Taxonomy, fermentation, isolation and structural elucidation. J. Antibiot. 61(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Gerwick WH, and Moore BS, 2012. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 19(1), 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghimire GP, Koirala N, and Sohng JK, 2015. Activation of cryptic hop genes from Streptomyces peucetius ATCC 27952 involved in hopanoid biosynthesis. J. Microbiol. Biotechnol. 25(5), 658–661. [DOI] [PubMed] [Google Scholar]
- Gillespie DE, Brady SF, Bettermann AD, Cianciotto NP, Liles MR, Rondon MR, et al. , 2002. Isolation of antibiotics turbomycin a and B from a metagenomic library of soil microbial DNA. Appl. Environ. Microbiol. 68(9), 4301–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Escribano JP, and Bibb MJ, 2011. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb. Biotechnol. 4(2), 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Escribano JP, and Bibb MJ, 2012. Streptomyces coelicolor as an expression host for heterologous gene clusters. Method. Enzymol. 517, 279–300. [DOI] [PubMed] [Google Scholar]
- Gomez-Escribano JP, and Bibb MJ, 2014. Heterologous expression of natural product biosynthetic gene clusters in Streptomyces coelicolor: from genome mining to manipulation of biosynthetic pathways. J. Ind. Microbiol. Biotechnol. 41(2), 425–431. [DOI] [PubMed] [Google Scholar]
- Gullon S, Olano C, Abdelfattah MS, Braña AF, Rohr J, Méndez C, et al. , 2006. Isolation, characterization, and heterologous expression of the biosynthesis gene cluster for the antitumor anthracycline steffimycin. Appl. Environ. Microbiol. 72(6), 4172–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, and Gutteridge JM, 1989. Free radicals in biology and medicine. Oxford England Press. [Google Scholar]
- Handelsman J, Rondon MR, Brady SF, Clardy J, and Goodman RM, 1998. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem. Biol. 5(10), 245–249. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Sasaki K, Adachi H, Furihata K, Nagai K, and Shin-ya K, 2006. Thioviridamide, a novel apoptosis inducer in transformed cells from Streptomyces olivoviridis. J. Antibiot. 59(1), 1–5. [DOI] [PubMed] [Google Scholar]
- He J, Sundararajan A, Devitt NP, Schilkey FD, Ramaraj T, and Melancon CE, 2016. Complete genome sequence of Streptomyces venezuelae ATCC 15439, producer of the pethymycin/Pikromycin family of macrolide antibiotics, using PacBio technology. Genome. Announc. 4(3), 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JS, Park SH, Choi CY, Sohng JK, and Yoon YJ, 2004. New olivosyl derivatives of methymycin/pikromycin from an engineered strain of Streptomyces venezuelae. FEMS Microbiol. Lett. 238(2), 391–399. [DOI] [PubMed] [Google Scholar]
- Hover BM, Kim SH, Katz M, Charlop-Powers Z, Owen JG, Ternei MA, et al. , 2018. Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Nat. Microbiol. 3(4), 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Reid R, and Gramajo H, 2005. The leptomycin gene cluster and its heterologous expression in Streptomyceslividans. J. Antibiot. 58(10), 625–33. [DOI] [PubMed] [Google Scholar]
- Huo L, Rachid S, Stadler M, Wenzel SC, and Müller R, 2012. Synthetic biotechnology to study and engineer ribosomal bottromycin biosynthesis. Chem. Biol. 19(10), 1278–1287. [DOI] [PubMed] [Google Scholar]
- Iftime D, Jasyk M, Kulik A, Imhoff JF, Stegmann E, Wohlleben W, et al. , 2015. Streptocollin, a type IV lanthipeptide produced by Streptomyces collinus Tu 365. Chem Bio Chem 16(18), 2615–2623. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, et al. , 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21(5), 526–531. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Shin-Ya K, Nagamitsu T, and Tomoda H, 2016. Biosynthesis of mercapturic acid derivative of the labdane-type diterpene, cyslabdan that potentiates imipenem activity against methicillin-resistant Staphylococcus aureus: cyslabdan is generated by mycothiol-mediated xenobiotic detoxification. J. Ind. Microbiol. Biotechnol. 43(2–3), 325–342. [DOI] [PubMed] [Google Scholar]
- Isogai S, Nishiyama M, and Kuzuyama T, 2012. Identification of 8-amino-2,5,7-trihydroxynaphthalene-1,4-dione, a novel intermediate in the biosynthesis of Streptomyces meroterpenoids. Bioorg. Med. Chem. Lett. 22(18), 5823–5826. [DOI] [PubMed] [Google Scholar]
- Izawa M, Kawasaki T, and Hayakawa Y, 2013. Cloning and heterologous expression of the thioviridamide biosynthesis gene cluster from Streptomyces olivoviridis. Appl. Environ. Microbiol. 79(22), 7110–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa M, Kimata S, Maeda A, Kawasaki T, and Hayakawa Y, 2014. Functional analysis of hatomarubigin biosynthesis genes and production of a new hatomarubigin using a heterologous expression system. J. Antibiot. 7(2), 159–162. [DOI] [PubMed] [Google Scholar]
- Jang KH, Nam S-J, Locke JB, Kauffman CA, Beatty DS, Paul LA, and Fencial W, 2013. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew. Chem. Int. Ed. 52(30), 7822–7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayapal KP, Lian W, Glod F, Sherman DH, and Hu WS, 2007. Comparative genomic hybridizations reveal absence of large Streptomyces coelicolor genomic islands in Streptomyces lividans. BMC Genomics 8, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian XH, Pan HX, Ning TT, Shi YY, Chen YS, Li Y, et al. , 2012. Analysis of YM-216391 biosynthetic gene cluster and improvement of the cyclopeptide production in a heterologous host. ACS Chem. Biol. 7(4), 646–651. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wang H, Kang Q, Liu J and Baia L, 2012. Cloning and characterization of the polyether salinomycin biosynthesis gene cluster of Streptomyces albus XM211. Appl. Environ. Microbiol. 78(4), 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Tetzlaff CN, Takamatsu S, Iwatsuki M, Komatsu M, Ikeda H, et al. , 2009. Genome mining in Streptomyces avermitilis: A biochemical Baeyer-Villiger reaction and discovery of a new branch of the pentalenolactone family tree. Biochemistry 48(27), 6431–6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Wei J, Li L, Niu G, and Tan H, 2013. Combined gene cluster engineering and precursor feeding to improve gougerotin production in Streptomyces graminearus. Appl. Microbiol. Biotechnol. 97(24), 10469–77. [DOI] [PubMed] [Google Scholar]
- Jones AC, Gust B, Kulik A, Heide L, Buttner MJ, and Bibb MJ, (2013). Phage p1-derived artificial chromosomes facilitate heterologous expression of the FK506 gene cluster. PLoS One 8(7), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan PA, and Moore BS, 2016. Biosynthetic pathway connects cryptic ribosomally synthesized posttranslationally modified peptide genes with pyrroloquinoline alkaloids. Chem. Biol. 23(12), 1504–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WS, Lee SK, Hong JS, Park SR, Jeong SJ, Han AR, et al. , 2006. Heterologous expression of tylosin polyketide synthase and production of a hybrid bioactive macrolide in Streptomyces venezuelae. Appl. Microbiol. Biotechnol. 72(4), 763–769. [DOI] [PubMed] [Google Scholar]
- Jung WS, Han AR, Hong JS, Park SR, Choi CY, Park JW, et al. , 2007. Bioconversion of 12-, 14-, and 16-membered ring aglycones to glycosylated macrolides in an engineered strain of Streptomyces venezuelae. Appl. Microbiol. Biotechnol. 76(6), 1373–1381. [DOI] [PubMed] [Google Scholar]
- Jung WS, Jeong SJ, Park SR, Choi CY, Park BC, Park JW, et al. , 2008. Enhanced heterologous production of desosaminyl macrolides and their hydroxylated derivatives by overexpression of the pikD regulatory gene in Streptomyces venezuelae. Appl. Environ. Microbiol. 74(7), 1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallifidas D, Kang HS, and Brady SF, 2012. Tetarimycin A, an MRSA-active antibiotic identified through induced expression of environmental DNA gene clusters. J. Am. Chem. Soc, 134(48), 19552–19555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang IH, Kim JS, Kim EJ, and Lee JK, 2007. Cadaverine protects Vibrio vulnificus from superoxide stress. J. Microbiol. Biotechno 1. 17(1), 176–179. [PubMed] [Google Scholar]
- Kang YS, Kim YJ, Jeon CO, and Park W, 2006. Characterization of napthalene-degrading Pseudomonas species isolated from pollutant-contaminated sites: Oxidative stress during their growth on napthalene. J. Microbiol Biotechnol. 16(11), 1819–1825. [Google Scholar]
- Kanth BK, Jnawali HN, Niraula NP, and Sohng JK, 2011. Superoxide dismutase (SOD) genes in Streptomyces peucetius: effects of SODs on secondary metabolites production. Microbiol. Res. 166(5), 391–402. [DOI] [PubMed] [Google Scholar]
- Karsten El., Escoubeyrou K, and Charles F, 2009. The effect of re-dissolution, solvents and HPLC columns on the analysis of mycosporine-like amino acids in the eulittoral macroalgae Prasiola crispa and Porphyra umbilicalis. Helgol. Mar. Res. 63(3), 231–238. [Google Scholar]
- Kawasaki T, Hayashi Y, Kuzuyama T, Furihata K, Itoh N, Seto H, and Dairi T, 2006. Biosynthesis of a natural polyketide-isoprenoid hybrid compound, furaquinocin A: identification and heterologous expression of the gene cluster. J. Bacteriol. 188(4), 1236–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepplinger B, Morton-Laing S, Seistrup KH, Marrs ECL, Hopkins AP, Perry JD, et al. , 2018. Mode of action and heterologous expression of the natural product antibiotic vancoresmycin. ACS Chem. Biol. 13(1), 207–214. [DOI] [PubMed] [Google Scholar]
- Kharel MK, Nybo SE, Shepherd MD, and Rohr J, 2010. Cloning and characterization of the ravidomycin and chrysomycin biosynthetic gene clusters. ChemBioChem 11(4), 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T, Bibb M, Buttner M, Chater K, and Hopwood D, 2000. Practical Streptomyces Genetics. The John Innes Foundation, Norwich, EK. [Google Scholar]
- Kim E, Moore BS, and Yoon YJ, 2015a. Reinvigorating natural product combinatorial biosynthesis with synthetic biology. Nat. Chem. Biol. 11(9), 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Lee JH, Choi H, Pereira AR, Ban YH, Yoo YJ, et al. , 2012. Heterologous production of 4-O-demethylbarbamide, a marine cyanobacterial natural product. Org. Lett. 14(23), 5824–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Yang I, and Yoon YJ, 2015b. Developing Streptomyces venezuelae as a cell factory for the production of small molecules used in drug discovery. Arch. Pharm. Res. 38(9), 1606–1616. [DOI] [PubMed] [Google Scholar]
- Kim ES, Hong HJ, Choi CY, and Cohen SN, 2001. Modulation of actinorhodin biosynthesis in Streptomyces lividans by glucose repression of afsR2 gene transcription. J. Bacteriol. 183(7), 2198–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Park JS, Chae CS, Hyun CG, Choi BW, Shin J, et al. , 2007. Genetic organization of the biosynthetic gene cluster for the indolocarbazole K-252a in Nonomaraea longicatena JCM 11136. Appl. Microbiol Biotechnol. 75(5), 1119–1126. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Komatsu K, Koiwai H, Yamada Y, Kozone I, Izumikawa M, et al. , 2013. Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth. Biol. 2(7), 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Tsuda M, Ōmura S, Oikawa H, and Ikeda H, 2008. Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc. Natl. Acad. Sci. U.S.A. 105(21), 7422–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Uchiyama T, Ōmura S, Cane DE, and Ikeda H, 2010. Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism. Proc. Natl. Acad. Sci. U.S.A.107(6), 2646–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama N, Tokura Y, Munch D, Sahl HG, Schneider T, Shibagaki Y, et al. , 2012. The nonantibiotic small molecule cyslabdan enhances the potency of beta-lactams against MRSA by inhibiting pentaglycine interpeptide bridge synthesis. PLoS One 7(11), e48981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk JM, Völler GH, Krawczyk B, Kretz J, Bronstrup M, and Süssmuth RD, 2013. Heterologous expression and engineering studies of labyrinthopeptins, class III lantibiotics from Actinomadura namibiensis. Chem. Biol. 20(1), 111–122. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Koyama Y, Oda K, Noda M, Matoba Y, and Sugiyama M, 2010. Molecular cloning and heterologous expression of a biosynthetic gene cluster for the antitubercular agent D-cycloserine produced by Streptomyces lavendalae. Antimicrob. Agents Chemother. 54(3), 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnari T, Tuikkanen J, Hautala A, Hakala J, Ylihonko K, and Mantsala P, 1997. Isolation and characterization of 8-demethoxy steffimycins and generation of 2,8-demethoxy steffimycins in Streptomyces steffisbargensis by the nogalamycin biosynthesis genes. J. Antibiot. 50(6), 496–501. [DOI] [PubMed] [Google Scholar]
- Kurumbang NP, Liou K, and Sohng JK, 2011. Biosynthesis of ribostamycin derivatives by reconstitution and heterologous expression of required gene sets. Appl. Biochem. Biotechnol. 163(3), 373–382. [DOI] [PubMed] [Google Scholar]
- Lamichhane J, Jha AK, Singh B, Pandey RP, and Sohng JK, 2014. Heterologous production of spectinomycin in Streptomyces venezuelae by exploiting the dTDP-D-desosamine pathway. J. Biotechnol. 174,57–63. [DOI] [PubMed] [Google Scholar]
- Larson CB, Crusemann M, and Moore BS, 2017. PCR-independent method of transformation-associated recombination reveals the cosmomycin biosynthetic gene cluster in an ocean streptomycete. J. Nat. Prod. 80(4), 1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiminger J, Frank M, Wenk C, Poschenrieder G, Kellermann A, and Schwarzfischer A, 2013. Distribution and characterization of Streptomyces species causing potato common scab in Germany. Plant Pathol. 62(3), 611–623. [Google Scholar]
- Li B, Wever WJ, Walsh CT, and Bowers AA, 2014. Dithiolopyrrolones: biosynthesis, synthesis, and activity of a unique class of disulfide-containing antibiotics. Nat. Prod. Rep. 31(7), 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhao LM, Wu RJ, Zheng ZJ, and Zhang RJ, 2013a. High-level overproduction of thermobifida enzyme in Streptomyces lividans using a novel expression vector. Int. J. Mol. Sci. 14(9), 18629–18639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wu J, Deng Z, Zabriskie TM, and He X, 2013b. Streptomyces lividans blasticidin S deaminase and its application in engineering a blasticidin S-producing strain for ease of genetic manipulation. Appl. Environ. Microbiol. 79(7), 2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Song Y, Qin X, Zhang X, Sun A, and Ju J, 2015. Identification of the biosynthetic gene cluster for the anti-infective desotamides and production of a new analogue in a heterologous host. J. Nat. Prod. 78(4), 944–948. [DOI] [PubMed] [Google Scholar]
- Linares-Otoya L, Linares-Otoya V, Armas-Mantilla L, Blanco-Olano C, Crusemann M, Ganoza-Yupanqui ML, et al. , 2017. Identification and heterologous expression of the kocurin biosynthetic gene cluster. Microbiol. 163, 1409–1414. [DOI] [PubMed] [Google Scholar]
- Linder J, Garner TP, Williams HE, Searle MS, and Moody CJ, 2011. Telomestatin: formal total synthesis and cation-mediated interaction of its seco-derivatives with G-quadruplexes. J. Am. Chem. Soc. 133(4), 1044–1051. [DOI] [PubMed] [Google Scholar]
- Liu H, Jiang H, Haltli B, Kulowski K, Muszynska E, Feng X, et al. , 2009. Rapid cloning and heterologous expression of the meridamycin biosynthetic gene cluster using a versatile Escherichia coli-Streptomyces artificial chromosome vector, pSBAC. J. Nat. Prod. 72(3), 389–395. [DOI] [PubMed] [Google Scholar]
- Liu X, Ding W, and Jiang H, 2017. Engineering microbial cell factories for the production of plant natural products: from design principles to industrial-scale production. Microb. Cell Fact. 16(1), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu D, Xu M, Tao M, Bai L, Deng Z, et al. 2018. Reconstitution of kinamycin biosynthesis within the heterologous host Streptomyces albus J1074. J. Nat. Prod. 81(1), 72–77. [DOI] [PubMed] [Google Scholar]
- Lombó F, Velasco A, Castro A, de la Calle F, Braña AF, Sánchez-Puelles JM, et al. , 2006. Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two Streptomyces species. ChemBioChem 7(2), 366–376. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhang W, Zhu Y, Zhang G, Zhang H, Zhang Q, et al. , 2017. Identification and characterization of a biosynthetic gene cluster for tryptophan dimers in deep sea-derived Streptomyces sp. SCSIO 03032. Appl. Microbiol. Biotechnol. 101(15), 6123–6136. [DOI] [PubMed] [Google Scholar]
- Machida K, Arisawa A, Takeda S, Tsuchida T, Aritoku Y, Yoshida M, et al. , 2008. Organization of the biosynthetic gene cluster for the polyketide antitumor macrolide, pladienolide, in Streptomycesplatensis Mer-11107. Biosci. Biotechnol. Biochem. 72(11), 2946–2952. [DOI] [PubMed] [Google Scholar]
- Makitrynskyy R, Rebets Y, Ostash B, Zaburannyi N, Rabyk M, Walker S, et al. , 2010. Genetic factors that influence moenomycin production in Streptomycetes. J. Ind. Microbiol. Biotechnol. 37(6), 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolmson SJ, Young TS, Ruby JG, Skewes-Cox P, and Walsh CT, 2013. The posttranslational modification cascade to the thiopeptide berninamycin generates linear forms and altered macrocyclic scaffolds. Proc. Natl. Acad. Sci. U.S.A 110(21), 8483–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DG, Slomp G, Mizsak S, Duchamp DJ, and Chidester CG, 1970. The structure and absolute configuration of pentalenolactone (PA 132). Tetrahedron Lett. 11(56), 4901–4904. [DOI] [PubMed] [Google Scholar]
- McDaniel R, Ebert-Khosla S, Hopwood DA, and Khosla C, (1993). Engineered biosynthesis of novel polyketides. Science 262(5139), 1546–1550. [DOI] [PubMed] [Google Scholar]
- McMahon MD, Guan C, Handelsman J, and Thomas MG, 2012. Metagenomic analysis of Streptomyces lividans reveals host-dependent functional expression. Appl. Environ. Microbiol. 78(10), 3622–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, et al. , 2011. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 39, 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschke H, Walter S, and Schrempf H, 2012. Characterization and localization of prodiginines from Streptomyces lividans suppressing Verticillium dahliae in the absence or presence of Arabidopsis thaliana. Environ. Microbiol. 14(4), 940–952. [DOI] [PubMed] [Google Scholar]
- Miyamoto KT, Komatsu M, and Ikeda H, 2014. Discovery of gene cluster for mycosporine-like amino acid biosynthesis from Actinomycetales microorganisms and production of a novel mycosporine-like amino acid by heterologous expression. Appl. Environ. Microbiol. 80(16), 5028–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah HJ, Pyeon HR, Kang SH, Choi SS, and Kim ES, 2017. Cloning and heterologous expression of a large-sized natural product biosynthetic gene cluster in Streptomyces species. Front. Microbiol. 8, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y, Sagane Y, Sakurai S, Uchino M, Sato H, Toeda K, et al. , 2011. Large-scale production of phospholipase D from Streptomyces racemochromogenes and its application to soybean lecithin modification. Appl. Biochem. Biotechnol. 165(78), 1494–1506. [DOI] [PubMed] [Google Scholar]
- Nepal KK, Oh TJ, and Sohng JK, 2009. Heterologous production of paromamine in Streptomyces lividans TK24 using kanamycin biosynthetic genes from Streptomyces kanamyceticus ATCC12853. Mol. Cells 27(5), 601–608. [DOI] [PubMed] [Google Scholar]
- Nepal KK, Yoo JC, and Sohng JK, 2010. Biosynthetic approach for the production of new aminoglycoside derivative. J. Biosci. Bioeng. 110(1), 109–112. [DOI] [PubMed] [Google Scholar]
- Nett M, Ikeda H, and Moore BS, 2009. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 26(11), 1362–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DJ, and Cragg GM, 2016. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79(3), 629–661. [DOI] [PubMed] [Google Scholar]
- Nijkamp K, van Luijk N, de Bont JA, and Wery J, 2005. The solvent-tolerant Pseudomonas putida S12 as host for the production of cinnamic acid from glucose. Appl. Microbiol. Biotechnol. 69(2), 170–177. [DOI] [PubMed] [Google Scholar]
- Niu G, Li L, Wei J, and Tan H, 2013. Cloning, heterologous expression, and characterization of the gene cluster required for gougerotin biosynthesis. Chem. Biol. 20(1), 34–44. [DOI] [PubMed] [Google Scholar]
- Noda S, Ito Y, Shimizu N, Tanaka T, Ogino C, and Kondo A, 2010. Over-production of various secretory-form proteins in Streptomyces lividans. Protein Expr. Purif 73(2), 198–202. [DOI] [PubMed] [Google Scholar]
- Noma M, Suzuki F, Gamou K, and Kawashima N, 1982. Two labdane diterpenoids from Nicoticma raimondii. Phytochemistry 21(2), 395–397. [Google Scholar]
- Novakova R, Núñez LE, Homerova D, Knirschova R, Feckova L, Rezuchova B, et al. , 2018. Increased heterologous production of the antitumoral polyketide mithramycin A by engineered Streptomyces lividans TK24 strains. Appl. Microbiol. Biotechnol. 102(2), 857–869. [DOI] [PubMed] [Google Scholar]
- Ochi K, 2017. Insights into microbial cryptic gene activation and strain improvement: principle, application and technical aspects. J. Antibiot. 70(1), 25–40. [DOI] [PubMed] [Google Scholar]
- O’Connor SE, 2015. Engineering of secondary metabolism. Annu. Rev. Genet. 49, 71–94. [DOI] [PubMed] [Google Scholar]
- Ohnishi Y, Ishikawa J, Hara H, Suzuki H, Ikenoya M, Ikeda H, et al. , 2008. Genome sequence of the streptomycin-producing microorganism Streptomyces giiseus IFO 13350. J. Bacterid. 190(11), 4050–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano C, Abdelfattah MS, Gullon S, Braña AF, Rohr J, Méndez C, et al. , 2008. Glycosylated derivatives of steffimycin: insights into the role of the sugar moieties for the biological activity. ChemBioChem 9(4), 624–633. [DOI] [PubMed] [Google Scholar]
- Olano C, Garcia I, Gonzalez A, Rodriguez M, Rozas D, Rubio J, et al. , 2014. Activation and identification of five clusters for secondary metabolites in Streptomyces albus J1074. Microb. Biotechnol. 7(3), 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ōmura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, et al. , 2001. Genome sequence of an industrial microorganism Streptomyces avermitilis ‘. deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. U.S.A. 98(21), 12215–12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaka H, Taniguchi S, Igarashi Y, and Furumai T, 2003. Characterization of the biosynthetic gene cluster of rebeccamycin from Lechevalieria aerocolonigenes ATCC 39243. Biosci. Biotechnol. Biochem. 67(1), 127–138. [DOI] [PubMed] [Google Scholar]
- Ongley SE, Bian X, Neilan BA, and Muller R, 2013. Recent advances in the heterologous expression of microbial natural product biosynthetic pathways. Nat. Prod. Rep. 30(8), 1121–1138. [DOI] [PubMed] [Google Scholar]
- Oren A, and Gunde-Cimerman N, 2007. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 269(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu G, Yang H, Tian Y, and Tan H, 2009. The pleiotropic regulator AdpA-L directly controls the pathway-specific activator of nikkomycin biosynthesis in Streptomyces ansochromogenes. Mol. Microbiol. 72(3), 710–723. [DOI] [PubMed] [Google Scholar]
- Park JW, Hong JS, Parajuli N, Jung WS, Park SR, Lim SK, et al. , 2008. Genetic dissection of the biosynthetic route to gentamicin A2 by heterologous expression of its minimal gene set. Proc. Natl. Acad. Sci. U.S.A. 105(24), 8399–8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Park SR, Nepal KK, Han AR, Ban YH, Yoo YJ, et al. , 2011a. Discovery of parallel pathways of kanamycin biosynthesis allows antibiotic manipulation. Nat. Chem. Biol. 7(11), 843–852. [DOI] [PubMed] [Google Scholar]
- Park SR, Yoon AJ, Paik JH, Park JW, Jung WS, Ban Y-H, et al. , 2009. Engineering of plant-specific phenylpropanoids biosynthesis in Streptomyces venezuelae. J. Biotechnol. 141(3/4), 181–188. [DOI] [PubMed] [Google Scholar]
- Park SR, Paik JH, Ahn MS, Park JW, and Yoon YJ, 2010. Biosynthesis of plant-specific flavones and flavonols in Streptomyces venezuelae. J. Microbiol. Biotechnol. 20(9), 1295–1299. [DOI] [PubMed] [Google Scholar]
- Park SR, Ahn MS, Han AR, Park JW, and Yoon YJ, 2011b. Enhanced flavonoid production in Streptomyces venezuelae via metabolic engineering. J. Microbiol. Biotechnol. 21(11), 1143–1146. [DOI] [PubMed] [Google Scholar]
- Patallo EP, Blanco G, Fischer C, Braña AF, Rohr J, Méndez C, et al. , 2001. Deoxysugar methylation during biosynthesis of the antitumor polyketide elloramycin by Streptomyces olivaceus. Characterization of three methyltransferase genes. J. Biol. Chem. 276(22), 18765–18774. [DOI] [PubMed] [Google Scholar]
- Quiros LM, Carbajo RJ, Braña AF, and Salas JA, 2000. Glycosylation of macrolide antibiotics. Purification and kinetic studies of a macrolide glycosyltransferase from Streptomycesantibioticus. J. Biol. Chem. 275(16), 11713–11720. [DOI] [PubMed] [Google Scholar]
- Rackham EJ, Gruschow S, Ragab AE, Dickens S, and Goss RJ, 2010. Pacidamycin biosynthesis: identification and heterologous expression of the first uridyl peptide antibiotic gene cluster. ChemBioChem 11(12), 1700–1709. [DOI] [PubMed] [Google Scholar]
- Ramos A, Lombó F, Braña AF, Rohr J, Méndez C, and Salas JA, 2008. Biosynthesis of elloramycin in Streptomyces olivaceus requires glycosylation by enzymes encoded outside the aglycon cluster. Microbiology 154, 781–788. [DOI] [PubMed] [Google Scholar]
- Rappe MS, and Giovannoni SJ, 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57, 369–394. [DOI] [PubMed] [Google Scholar]
- Reddy TB, Thomas AD, Stamatis D, Bertsch J, Isbandi M, Jansson J, et al. , 2015. The Genomes OnLine Database (GOLD) v.5: a metadata management system based on a four level (meta)genome project classification. Nucleic Acids Res. 43(Database issue), D1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico S, Yepes A, Rodriguez H, Santamaria J, Antoraz S, Krause EM, et al. , 2014. Regulation of the AbrA1/A2 two-component system in Streptomyces coelicolor and the potential of its deletion strain as a heterologous host for antibiotic production. PLoS One 9(10), e109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckert C, Albersmeier A, Busche T, Jaenicke S, Winkler A, Friethjonsson OH, et al. , 2015. Complete genome sequence of Streptomyces lividans TK24. J. Biotechnol. 199, 21–22. [DOI] [PubMed] [Google Scholar]
- Rui Z, Petrickova K, Skanta F, Pospisil S, Yang Y, Chen CY, et al. , 2010. Biochemical and genetic insights into asukamycin biosynthesis. J. Biol. Chem. 285(32), 24915–24924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Zhang W, Zhang G, Zhu Y, Chen Y, Liu W, et al. , 2017. Activation and characterization of a cryptic gene cluster reveals a cyclization cascade for polycyclic tetramate macrolactams. Chem. Sci. 8(2), 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez C, Butovich IA, Braña AF, Rohr J, Méndez C, and Salas JA, 2002. The biosynthetic gene cluster for the antitumor rebeccamycin: characterization and generation of indolocarbazole derivatives. Chem. Biol. 9(4), 519–531. [DOI] [PubMed] [Google Scholar]
- Sánchez C, Zhu L, Braña AF, Salas AP, Rohr J, Méndez C, et al. , 2005. Combinatorial biosynthesis of antitumor indolocarbazole compounds. Proc. Natl. Acad. Sci. U.S.A. 102(2), 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinke C, Martins T, Queiroz SCN, Melo IS, and Reyes FGR, 2017. Antibacterial compounds from marine bacteria, 2010–2015. J. Nat. Prod. 80(4), 1215–1228. [DOI] [PubMed] [Google Scholar]
- Seo MJ, Zhu D, Endo S, Ikeda FL, and Cane DE, 2011. Genome mining in Streptomyces. Elucidation of the role of Baeyer-Villiger monooxygenases and non-heme iron-dependent dehydrogenase/oxygenases in the final steps of the biosynthesis of pentalenolactone and neopentalenolactone. Biochemistry 50(10), 1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Xue Q, Tang L, Carney JR, Betlach M, and McDaniel R, 2000. Cloning characterization and heterologous expression of a polyketide synthase and P-450 oxidase involved in the biosynthesis of the antibiotic oleandomycin. J. Antibiot. 53(5), 502–508. [DOI] [PubMed] [Google Scholar]
- Shepherd MD, Kharel MK, Bosserman MA, and Rohr J, 2010. Laboratory maintenance of Streptomyces species. Curr. Protoc. Microbiol. 18(1), 10E.1.1–10E.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin-ya K, Wierzba K, Matsuo K, Ohtani T, Yamada Y, Furihata K, et al. , 2001. Telomestatin, a novel telomerase inhibitor from Streptomyces amdatus. J. Am. Chem. Soc. 123(6), 1262–1263. [DOI] [PubMed] [Google Scholar]
- Sinha RP, Singh SP, and Hader DP, 2007. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. 89(1), 29–35. [DOI] [PubMed] [Google Scholar]
- Smanski MJ, Casper J, Peterson RM, Yu Z, Rajski SR, and Shen B, 2012. Expression of the platencin biosynthetic gene cluster in heterologous hosts yielding new platencin congeners. J. Nat. Prod. 75(12), 2158–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smanski MJ, Peterson RM, Rajski SR, and Shen B, 2009. Engineered Streptomyces platensis strains that overproduce antibiotics platensimycin and platencin. Antimicrob. Agents Chemother. 53(4), 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohda KY, Hiramoto M, Suzumura K, Takebayashi Y, Suzuki K, and Tanaka A, 2005. YM-216391, a novel cytotoxic cyclic peptide from Streptomyces nobilis. II. Physico-chemical properties and structure elucidation. J. Antibiot. 58(1), 32–36. [DOI] [PubMed] [Google Scholar]
- Song JY, Yoo YJ, Lim SK, Cha SH, Kim JE, Roe JH, et al. , 2016. Complete genome sequence of Streptomyces venezuelae ATCC 15439, a promising cell factory for production of secondary metabolites. J. Biotechno1. 219, 57–58. [DOI] [PubMed] [Google Scholar]
- Song Y, Li Q, Liu X, Chen Y, Zhang Y, Sun A, et al. , 2014. Cyclic hexapeptides from the deep south China sea-derived Streptomyces scopidiridis SCSIO ZJ46 active against pathogenic Gram-positive bacteria. J. Nat. Prod. 77(8), 1937–1941. [DOI] [PubMed] [Google Scholar]
- Subba B, Kurumbang NP, Jung YS, Yoon YJ, Lee HC, Liou K, et al. , 2007. Production of aminoglycosides in non-aminoglycoside producing Streptomyces lividans TK24. Bioorg. Med. Chem. Lett. 17(7), 1892–1896. [DOI] [PubMed] [Google Scholar]
- Sun F, Xu S, Jiang F, and Liu W, 2018. Genomic-driven discovery of an amidinohydrolase involved in the biosynthesis of mediomycin A. Appl. Microbiol. Biotechnol. 102(5), 2225–2234. [DOI] [PubMed] [Google Scholar]
- Sun FL, Liu Z, Zhao FL, and Ang EL, 2015. Recent advances in combinatorial biosynthesis for drug discovery. Drug Des. Devel. Ther. 9, 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Ogawa Y, and Yonehara FL, 1969. The structure of pentalenolactone (PA-132). Tetrahedron Lett. (32), 2737–2740. [DOI] [PubMed] [Google Scholar]
- Tan GY, Deng K, Liu X, Tao FL, Chang Y, Chen J, et al. , 2017. Heterologous biosynthesis of spinosad: an omics-guided large polyketide synthase gene cluster reconstitution in Streptomyces. ACS Synth. Biol. 6(6), 995–1005. [DOI] [PubMed] [Google Scholar]
- Tetzlaff CN, You Z, Cane DE, Takamatsu S, Ōmura S, and Ikeda H, 2006. A gene cluster for biosynthesis of the sesquiterpenoid antibiotic pentalenolactone in Streptomyces avermitilis. Biochemistry 45(19), 6179–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanapipatsiri A, Claesen J, Gomez-Escribano JP, Bibb M, and Thamchaipenet A, 2015. A Streptomyces coelicolor host for the heterologous expression of Type III polyketide synthase genes. Microb. Cell Fact. 14, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa LP, Oh TJ, Lee HC, Liou K, Park JW, Yoon YJ, et al. , 2007. Heterologous expression of the kanamycin biosynthetic gene cluster (pSKC2) in Streptomyces venezuelae YJ003. Appl. Microbiol. Biotechnol. 76(6), 1357–1364. [DOI] [PubMed] [Google Scholar]
- Thapa LP, Oh TJ, Liou K, and Sohng JK, 2008. Biosynthesis of spectinomycin: heterologous production of spectinomycin and spectinamine in an aminoglycoside-deficient host, Streptomyces venezuelae YJ003. J. Appl. Microbiol. 105(1), 300–308. [DOI] [PubMed] [Google Scholar]
- Thuan NH, Pandey RP, and Sohng JK, 2014. Recent advances in biochemistry and biotechnological synthesis of avermectins and their derivatives. Appl. Microbiol. Biotechnol. 98(18), 7747–7759. [DOI] [PubMed] [Google Scholar]
- Torsvik V, Goksoyr J, and Daae FL, 1990. High diversity in DNA of soil bacteria. Appl. Environ. Microbiol. 56(3), 782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J, Li S, Chen J, Song Y, Fu S, Ju J, et al. , 2018. Characterization and heterologous expression of the neoabyssomicin/abyssomicin biosynthetic gene cluster from Streptomyceskoyangensis SCSIO 5802. Microb. Cell Fact 17, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezel GP, and McDowall KJ, 2011. The regulation of the secondary metabolism of Streptomyces’. new links and experimental advances. Nat. Prod. Rep. 28(7), 1311–33. [DOI] [PubMed] [Google Scholar]
- Vannelli T, Wei Qi W, Sweigard J, Gatenby AA, and Sariaslani FS, 2007. Production of p-hydroxycinnamic acid from glucose in Saccharomyces cerevisiae and Escherichia coli by expression of heterologous genes from plants and fungi. Metab. Eng. 9(2), 142–151. [DOI] [PubMed] [Google Scholar]
- Völler GH, Krawczyk JM, Pesic A, Krawczyk B, Nachtigall J, and Süssmuth RD, 2012. Characterization of new class III lantibiotics--erythreapeptin, avermipeptin and griseopeptin from Saccharopolyspora erythraea, Streptomyces avermitilis and Streptomyces griseus demonstrates stepwise N-terminal leader processing. ChemBioChem 13(8), 1174–1183. [DOI] [PubMed] [Google Scholar]
- Waldman AJ, Pechersky Y, Wang P, Wang JX, and Balskus EP, 2015. The cremeomycin biosynthetic gene cluster encodes a pathway for diazo formation. ChemBioChem 16(15), 2172–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CT, and Tang Y, 2017. Natural Product Biosynthesis: Chemical Logic and Enzymatic Machinery. Royal Society of Chemistry (RSC) Publishing, UK. [Google Scholar]
- Wang G, Hosaka T, and Ochi K, 2008. Dramatic activation of antibiotic production in Streptomyces coelicolor by cumulative drug resistance mutations. Appl. Environ. Microbiol. 74(9), 2834–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Graziani E, Waters B, Pan W, Li X, McDermott J, et al. , 2000. Novel natural products from soil DNA libraries in a Streptomycete host. Org. Lett. 2(16), 2401–2404. [DOI] [PubMed] [Google Scholar]
- Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, et al. , 2007. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc. Natl. Acad. Sci. U.S.A. 104(18), 7612–7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, et al. , 2006. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 441(7091), 358–361. [DOI] [PubMed] [Google Scholar]
- Watve MG, Tickoo R, Jog MM, and Bhole BD, 2001. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol. 176(5), 386–390. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhang J, Jiang L, Tan H, and Niu G, 2016. Functional characterization of gouC and gouD in gougerotin biosynthesis. Acta Microbiol. Sin. 56(3), 406–417. [PubMed] [Google Scholar]
- Wolpert M, Heide L, Kammerer B, and Gust B, 2008. Assembly and heterologous expression of the coumermycin A1 gene cluster and production of new derivatives by genetic engineering. ChemBioChem 9(4), 603–612. [DOI] [PubMed] [Google Scholar]
- Xiang L, and Moore BS, 2002. Inactivation, complementation, and heterologous expression of encP, a novel bacterial phenylalanine ammonia-lyase gene. J. Biol. Chem. 277(36), 32505–32509. [DOI] [PubMed] [Google Scholar]
- Xu F, Nazari B, Moon K, Bushin LB, and Seyedsayamdost MR, 2017a. Discovery of a cryptic antifungal compound from Streptomyces albus J1074 using high-throughput elicitor screens. J. Am. Chem. Soc. 139(27), 9203–9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang J, Zhuo J, Li Y, Tian Y, and Tan H, 2017b. Activation and mechanism of a cryptic oviedomycin gene cluster via the disruption of a global regulatory gene, adpA, in Streptomyces ansochromogenes. J. Biol. Chem. 292(48), 19708–19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wang Y, Zhao Z, Gao G, Huang SX, Kang Q, et al. , 2016. Functional genome mining for metabolites encoded by large gene clusters through heterologous expression of a whole-genome bacterial artificial chromosome library in Streptomyces spp. Appl. Environ. Microbiol. 82(19), 5795–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Reynolds KA, Kersten RD, Ryan KS, Gonzalez DJ, Nizet V, Dorrestein PC, and Moore BS, 2014. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc. Natl. Acad. Sci. U.S.A. 111(5), 1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Huang C, Zhang W, Zhu Y, and Zhang C, 2015. Heterologous expression of fluostatin gene cluster leads to a bioactive heterodimer. Org. Lett. 17(21), 5324–5327. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhu X, Wu X, Feng Z, Huang L, Shen B, et al. , 2011. Titer improvement of iso-migrastatin in selected heterologous Streptomyces hosts and related analysis of mRNA expression by quantitative RT-PCR. Appl. Microbiol. Biotechnol. 89(6), 1709–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes A, Rico S, Rodriguez-Garcia A, Santamaria RI, and Diaz M, 2011. Novel two-component systems implied in antibiotic production in Streptomyces coelicolor. PLoS One 6(5), e19980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Hoffmann M, Bian X, Tu Q, Yan F, Xia L, et al. , 2015. Direct cloning and heterologous expression of the salinomycin biosynthetic gene cluster from Streptomyces albus DSM41398 in Streptomyces coelicolor A3(2). Sci. Rep 5, 15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Li Z, Wang X, Wang H, Jia X, Ai G, et al. , 2016. Heterologous expression of oxytetracycline biosynthetic gene cluster in Streptomyces venezuelae WVR2006 to improve production level and to alter fermentation process. Appl. Microbiol. Biotechnol. 100(24), 10563–10572. [DOI] [PubMed] [Google Scholar]
- Yu Y, Tang B, Dai R, Zhang B, Chen L, Yang H, et al. , 2018. Identification of the streptothricin and tunicamycin biosynthetic gene clusters by genome mining in Streptomyces sp. strain fd1-xmd. Appl. Microbiol. Biotechnol. 102(6), 2621–2633. [DOI] [PubMed] [Google Scholar]
- Zaburannyi N, Rabyk M, Ostash B, Fedorenko V, and Luzhetskyy A, 2014. Insights into naturally minimised Streptomyces albus J1074 genome. BMC Genomics 15(97), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettler J, Xia H, Burkard N, Kulik A, Grond S, Heide L, et al. , 2014. New aminocoumarins from the rare actinomycete Catenulispora acidiphila DSM 44928: identification, structure elucidation, and heterologous production. ChemBioChem 15(4), 612–621. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Bai S, Liu J, Yang L, Han L, Huang X, et al. , 2016. Identification of an unusual type II thioesterase in the dithiolopyrrolone antibiotics biosynthetic pathway. Biochem. Biophys. Res. Commun. 473(1), 329–335. [DOI] [PubMed] [Google Scholar]
- Zhang L, Hoshino S, Awakawa T, Wakimoto T, and Abe I, 2016a. Structural diversification of lyngbyatoxin A by host-dependent heterologous expression of the tleABC biosynthetic gene cluster. ChemBioChem. 17(15), 1407–1411. [DOI] [PubMed] [Google Scholar]
- Zhang MM, Wang Y, Ang EL, and Zhao H, 2016b. Engineering microbial hosts for production of bacterial natural products. Nat. Prod. Rep. 33(8), 963–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Qiao Y, Ang EL, and Zhao H, 2017a. Using natural products for drug discovery: the impact of the genomics era. Expert Opin. Drug Discov.12(5), 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Tang X, Zhang M, Nguyen D, and Moore BS, 2017b. Broad-host-range expression reveals native and host regulatory elements that influence heterologous antibiotic production in Gram-negative bacteria. mBio 8, e01291–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Xie X, and Tang Y, 2008. Engineering natural products using combinatorial biosynthesis and biocatalysis. Curr. Opin. Biotechnol. 19(6), 590–596. [DOI] [PubMed] [Google Scholar]
- Zhu D, Seo MJ, Ikeda H, and Cane DE, 2011. Genome mining in Streptomyces. Discovery of an unprecedented P450-catalyzed oxidative rearrangement that is the final step in the biosynthesis of pentalenolactone. J. Am. Chem. Soc. 133(7), 2128–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemert N, Alanjary M, and Weber T, 2016. The evolution of genome mining in microbes - a review. Nat. Prod. Rep. 33(8), 988–1005. [DOI] [PubMed] [Google Scholar]
- Zong C, Cheung-Lee WL, Elashal HE, Raj M, and Link AJ, 2018. Albusnodin: an acetylated lasso peptide from Streptomycesalbus. Chem. Commun. 54(11), 1339–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]


