Abstract
Cell polarity, the asymmetric distribution of proteins, organelles, and cytoskeleton, plays an important role in development, homeostasis, and disease. Understanding the mechanisms that govern cell polarity is critical for creating strategies to treat developmental defects, accelerate tissue regeneration, and hinder cancer progression. This review focuses on the role of cell polarity in a number of physiological processes, including asymmetric division, cell migration, immune response mediated by T lymphocytes, and cancer progression and metastasis, and highlights microfabrication techniques to systematically parse the role of microenvironmental cues in the regulation of cell polarity.
Keywords: Cell polarity, Cell fate, Cell asymmetry, Microfabrication, Review
2. INTRODUCTION
Cell polarization, defined as the asymmetric distribution of proteins, organelles, and cytoplasm, occurs in many forms (1). The most commonly known is the apical-basal polarity of epithelial cells. However there also exists front-to-back polarity of migrating cells and planar polarity, which organizes and polarizes the cells found in one plane of the tissue (2, 3). The mechanisms by which cells polarize have been studied in a wide range of organisms and appear to be evolutionarily conserved (4). However, there are various pathways for each type of polarity that requires the interaction of multiple signaling molecules (5, 6).
Polarization of cells has been demonstrated as a critical event in evolutionary biology. For single-cell organisms, such as budding yeast, polarization is the mechanism by which reproduction occurs (1). For more complex organisms, such as Caenorhabditis elegans (C. elegans) and Drosophila melanogaster (D. melanogaster), polarization results in the development and organization of different body parts, including the nervous system and wing organization (6, 7). Without polarization, complex organisms with a multitude of cell types would not exist, migration of cells would be impossible, and cells would not be able to properly perform their functions (8).
In this review, we examine the different ways cell polarization is involved in development, homeostasis, and disease. Table 1 summarizes provides a synopsis of published work on the role of cell polarity on cell function. We proceed to discuss how microfabrication techniques have enabled systematic studies of cell polarization, and we discuss the future efforts needed to improve these techniques.
Table 1.
The impact of polarity on cell functions
| Function | Mechanism | References |
|---|---|---|
| Asymmetric division | Uneven distribution of key proteins known as fate determinants (including Numb, Brat, PAR, aPKC) and organelles during mitosis lead to asymmetric division | 16 and 17 |
| Development and differentiation | Uneven distribution of key polarity proteins results in asymmetric division and differentiation of progenitor cells. This phenomenon along with the organism’s body axes in planar polarity are required for functional formation of wings, eyes, ears and other organs | 6, 12, 16, and 27 |
| Migration | Polarity leads to upregulation and redistribution of proteins including Cdc42, Rac and PAR to the leading/front edge of the cell resulting in directional migration of cells | 11, 31, and 32 |
| Cancer | Polarity contributes to the initiation of disease by increased asymmetric divisions and progression of disease through increased migration and metastasis by the upregulation of Cdc42 | 39, 42, 47 |
| Immune Response | Polarization of proteins and fate determinants during early encounters with antigen regulate T lymphocyte differentiation to effector or central memory phenotype | 53, 54, and 55 |
3. POLARIZATION AND CELL FUNCTION
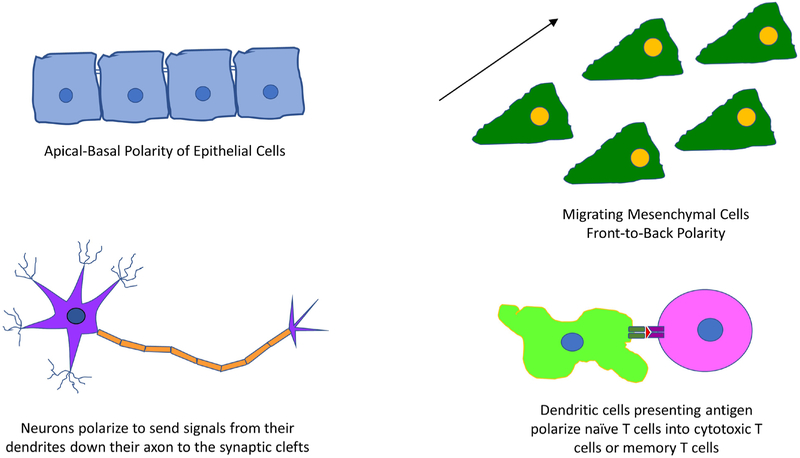
The morphology of cells is optimized to fulfill its function such as the propagation of signals through a neuron’s axon, or the squamous shape of epithelial cells that allow for selective solute transport (Figure 1) (1). The distinct shapes of cells seen in Figure 1 allow different cell types to perform their function of migrating or initiating immune responses. Cells that create compartments (e.g., epithelia) must be polarized to maintain contents in their corresponding partitions (9, 10). Epithelial cells have apical-basal polarity that allow specialized trafficking of solutes and planar polarity for organogenesis (6). The apical-basal polarity is a result of cell-cell and cell-extracellular matrix (ECM) interactions. Tight junctions between cells prevent proteins and ions from flowing freely between the apical and basal sides, effectively creating a barrier to prevent unwanted material from entering the body (Figure 1) (11). The development and maintenance of cell polarity is a result of multiple signals from polarity proteins, epithelial cadherin (E-cadherin) and focal adhesion contact with the ECM. These signals organize the cytoskeleton and aid in organelle localization. Loss of these signals results in differentiation from epithelial to mesenchymal cell types and front-to-back polarization (11).
Figure 1.
Cell polarity plays a critical role in cell function. A prime example is the epithelial cells utilizing apical-basal polarity to provide a barrier function against pathogens. Another example is cell migration which requires front-to-back polarity to allow cells to adhere to and detach from the ECM. Polarity is also required for neurons to perform their task of propagating action potentials and sending messages from the central nervous system to distant body parts.
Planar cell polarization is more complex since it is not a direct result of cell-cell contact, but rather the positioning of the cell with respect to the organism’s body axes (6). This type of polarity is responsible for the organization of feathers on a bird or the orientation of a fly’s wing. Disruption of this type of polarity can result in improper development of the eye or the inner ear, resulting in blindness or deafness,(6) demonstrating how polarity plays a large role in cell function and the lack of appropriate polarization can have dire consequences to the organism.
Neurons must also be polarized to serve their function of propagating signals to distant parts of the body (Figure 1) (1). Neuronal development in vitro begins with the spreading of filopodia that grow into small neurites. One of these neurites is selected to become the axon and begins to grow more quickly than the others. Finally, the remaining neurites become dendrites and polarize (12). The process is slightly different in vivo but all neurons require polarization to function. The polarization is a result of signaling from various secreted factors and the formation of complexes, such as Par6-Par3-aPKC (important in asymmetric division and development of D. melanogaster) (12). Other important factors for neuronal polarity include cell division control homolog 42 (Cdc42), the Ras homolog gene family, member A (RhoA), and Ras-related C3 botulinum toxin substrate 1 (Rac1) (13, 14). These factors highlight the importance of the cytoskeleton in polarization of cells.
4. POLARIZATION IN ASYMMETRIC DIVISION AND DEVELOPMENT
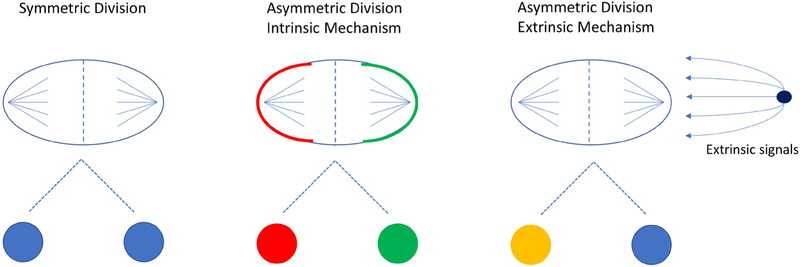
The phenomenon of asymmetric cell division was first observed and recorded by Edwin Conklin in 1905 in the developing embryo of ascidians (15). Other organisms have since been studied, in particular D. melanogaster and C. elegans, and have elucidated two mechanisms of asymmetric cell division in development (16). In the intrinsic mechanism, polarization of regulators within the cell causes an uneven distribution of proteins during mitosis, resulting in daughter cells with different internal signals that lead to differing fates (Figure 2). In D. melanogaster, the intrinsic mechanism is used in the development of the nervous system. Here, asymmetric divisions of neuroblasts give rise to one neuroblast and one ganglion mother cell (GMC), which then divides into differentiated neurons. The fate of each cell is controlled by the polarized distribution of the protein Numb and the translational inhibitor Brat (16). These fate determinants polarize the cell by localizing at the basal membrane during mitosis and can only be found in the basal cell after division. The localization of these fate determinants to the basal membrane is thought to occur because of the accumulation of PAR (partition deficient) proteins and atypical protein kinase C (aPKC) at the apical side (17). As a result of this protein polarization, the basal cell becomes a GMC while the apical cell remains a neuroblast.
Figure 2.
Cell divisions are controlled by internal and external signals. Symmetric divisions occur when there is an equal distribution of proteins, organelles, and cytoskeleton in the mother cell resulting in two daughter cells of the same fate. Asymmetric divisions through the intrinsic mechanism have an unequal distribution of proteins and fate determinants often resulting in daughter cells with different fates. Through the extrinsic mechanism, the cell divides symmetrically but daughter cells receive different signals from the microenvironment resulting in diverse cell fates.
The second mechanism of asymmetric division is known as the extrinsic mechanism. Here, cells rely on cues from the microenvironment, and asymmetric division occurs when the cell divides in a perpendicular fashion, resulting in one daughter cell that is proximal to the niche and the other that is distal (Figure 2) (17). This mechanism is more prominent in adult stem cells and best studied in D. melanogaster ovaries and testes, where direct attachment to the niche is required to maintain stemness (17–19). If a germline stem cell divides perpendicular to the niche, one of the daughter cells loses contact, stops receiving signals from the niche cells and begins to differentiate (19). The importance of polarity in asymmetric division has been confirmed in the development of C. elegans. The oocyte is not polarized before fertilization, however, the fertilization of the egg by the sperm causes a change in the cytoskeletal integrity affecting the cell contractility and polarization of the anterior and posterior PAR proteins (20–22). After the C. elegans egg is fertilized and polarized, the first division is asymmetric and results in a larger anterior body and a smaller posterior cell (23). The development of C. elegans is a continued series of asymmetric and symmetric divisions, governed by anterior-posterior polarity, resulting in the generation of the three germinal layers (ectoderm, mesoderm, and endoderm) (24, 25).
Asymmetric cell division is also critical in mammalian development but is not as well understood or studied because of the long cell cycles in mammals. Studies of the developing brain of mice show the complex development with changes from symmetric division to asymmetric division throughout the developmental process (16, 26). The symmetric divisions serve to increase the number of progenitor cells, while the asymmetric divisions generate one differentiated nerve cell and a radial glia cell (progenitor cell) (27). Neural development occurs in various stages that involve symmetric and asymmetric divisions and migration of the progenitor cells to the basal region of the neuroepithelium for terminal differentiation (17). The molecules that control asymmetric division in D. melanogaster are conserved in mammals, however, their roles as fate determinants have not been fully established—with some studies indicating that not all conserved determinants play the same role in asymmetric division in mammals (17). One determinant, Numb, has been shown to be critical in asymmetric division and subsequent fate specification in both invertebrates and vertebrates (27). Differential localization of Numb into only one of the daughter cells causes that cell to differentiate into a neuron, while the other daughter cell remains a progenitor cell. Recent findings by Jossin, et al. show how loss of the polarity protein Lethal giant larvae (Lgl) alone can result in catastrophic brain development leading to cortical heterotropia and drug resistant epilepsy (28). While this highlights the importance of polarity in brain development, further studies are still required to establish the mechanisms and the polarization of fate determinants that leads to asymmetric division in neurogenesis and mammalian development.
The importance of asymmetric division in development is clear. Organisms use symmetric divisions to clone cells and asymmetric divisions to give rise to new cells with different roles. Asymmetric division allows for the development of new cell types while maintaining a pool of progenitor cells. This physiologic process continues throughout the life of the organism and is involved in wound healing and tissue regeneration, adult stem cell differentiation, cancer, and immune responses (29, 30). All of these processes, however, would not be possible without the polarization of proteins, such as fate determinants, to induce these asymmetric cell divisions and create cellular diversity.
5. POLARIZATION AND MIGRATION
Migration of cells can occur in development, but also as a result of injury and disease progression. Microenvironmental cues cause the cell to organize its actin cytoskeleton and begin migration toward the signal. Some specialized cells, such as sperm, are always polarized and have cilia or flagella to help them migrate, while other cells polarize by growing lamellipodia or filopodia in response to stimuli (31). The stimuli cause activation of Rho family proteins, which influence the growth and attachment of actin chains (31). Cell migration can occur as a single cell or a sheet of cells, which is referred to as collective cell migration. Although the same mechanisms are required for both migration methods, collective cell migration requires synchronization among all the cells to move without disrupting cell-cell contacts (32).
Regardless of migration type, all migratory cells express mesenchymal genes and have front-rear polarity (11, 32). The front leading edge of migrating cells has proteins such as Cdc42, PAR, and activated Rac, and in some cells a microtubule organizing center. These proteins work by controlling microtubule growth and therefore where lamellipodia form. This control in lamellipodia localization results in organized migration. Since the rear of the cell lacks these proteins, protrusion extensions are prevented resulting in directional migration (31).
6. POLARIZATION AND CANCER
Cancer is caused by the abnormal over-proliferation of cells. This unchecked growth can be caused by a large variety of mutations (33–35). A contributing factor towards this dysregulation is the mutation of fate determinants or polarity proteins leading to decreased asymmetric division. These mutations prevent asymmetric division from occurring but do not stop the cell from cycling and dividing, thereby resulting in an increased number of symmetric divisions and an increase in progenitor cells (36). This increase in progenitor cells increases the amount of differentiated cells resulting in a neoplasm. This explanation agrees with the hypothesis of cancer stem cells, which are cells capable of producing all the cell types in a tumor and have stem cell markers such as those found in early progenitor cells that have not differentiated (16, 37). Although the mechanisms of asymmetric division in mammals are poorly understood, studies have focused on the loss of Numb regulation as a possible explanation for cancer propagation as a result of an imbalance of asymmetric division (38). Understanding the mechanisms by which healthy cells lose their ability to divide asymmetrically can help us determine new targets for cancer treatment, including possibly chemoand radiotherapy-resistant cancer cells.
While loss of polarization and asymmetric division is thought to play a large role in cancer, the polarization of cancer cells can help advance the disease by causing metastasis (39–41). Metastasis, the complex process of establishing a new tumor at a distant site, requires that the cells first lose their epithelial polarity from cell-cell contact and regain front-to-back polarity to migrate into the circulation and extravasate at distant sites (39). The process of epithelial to mesenchymal transition, which is also important during development, is believed to be the mechanism by which metastasis begins (39, 42, 43). As one of the key regulators of cell polarity, Cdc42 has been shown to be upregulated in cancer cells and integral in the metastatic process (31, 44, 45). Reymond et al. used siRNA to show that silencing Cdc42, by even just a transient depletion, prevents metastases to the lung by decreasing beta1 integrin levels and intercalation of cancer cells into the endothelial layers (44). These results showed that Cdc42 was required for transendothelial migration and that a reduction in Cdc42 levels was enough to reduce cancer cell migration and metastasis. Another group found similar results including a target (miR137) that can reduce Cdc42 levels and reduce colorectal cancer cell invasion (46). Furthermore, Kamai et al. showed that higher expression of Cdc42 correlated with more advanced disease, furthering evidence that Cdc42 is crucial in cancer cell invasion and metastasis (47). Together, these studies show how levels of Cdc42, a known polarity regulator, play an important role in cancer progression and suggest mechanisms by which Cdc42 levels can be controlled.
7. POLARIZATION AND THE IMMUNE SYSTEM
The effects of asymmetric cell division in the immune system have been more difficult to study because of the motile nature of these cells. Studies are further complicated by a slow differentiation rate and the subtle morphological changes that occur during differentiation. The relatively few hematopoietic cells present in the body also make it difficult to find and track individual cells undergoing asymmetric division in vivo (23, 48). Because of these hinderances, it is still unclear whether hematopoietic stem cells (HSCs) use asymmetric divisions as the mechanism to differentiate into different lineages. Studies suggest that Numb and alpha-Adaptin (an important protein in D. melanogaster asymmetric division) may play a role in the asymmetric division of HSCs (49, 50). Other proteins have been found to asymmetrically localize in HSCs, resulting in one daughter cell that is more primitive than the other, confirming that intrinsic asymmetric division occurs (51).
While studies have shown that asymmetric division does not appear to play a large role in B cell development and differentiation, it does appear to influence T cells (23, 52). The polarization and asymmetric division of polarity proteins and fate determinants is thought to occur after activation of the T cell through the T-cell receptor and antigen-presenting cell (53). The activation of the T cell results in divisions that create two types of T cells: the memory T cell and the effector T cell. Memory T cells can further asymmetrically divide to give rise to the more differentiated effector T cell phenotype and maintain the memory T cell pool, indicating a stem cell-like behavior (54).
Mechanistic studies into asymmetric division of T cells show that different determinants play a role during the primary and secondary immune response. In the primary response, CD3 and Interferon-gamma receptor (INF-gammaR) asymmetrically locate in memory cells but show little asymmetry in effector T cells (54). Further exposure to the antigen can cause the central memory T cells to mount a secondary immune response. Here, they again divide asymmetrically to produce daughter cells that are effector T cells and central memory cells. CD25 and T-bet (T cell transcription factor regulating T helper cell lineage commitment) polarize to the effector T cell, leaving low levels of both on the other daughter cell, which remains a memory T cell (54). Interestingly, the polarization of protein kinase C zeta type (PKC-zeta) differs between the primary response and the secondary response, suggesting that PKC-zeta may play a role in establishing central memory (54).
Previous studies have discovered important proteins that are polarized in T cells and regulate morphology, migration, and cell fate (55). For example, T cells exposed to antigen via dendritic cells polarized aPKC and Par3 proteins distal to the dendritic cell while Scribble and Discs large (Dlg), two important polarity proteins that form the Scribble complex, localized proximally (55). This proved that T cells use evolutionarily conserved mechanisms found in many organisms and cell types to asymmetrically divide. This study further showed that this polarity was important in memory T cell differentiation but not in effector T cell differentiation (55). Another group found that Scribble plays a major role in polarity and downregulation leading to decreased polarity and ability of T cells to migrate and present antigen (56). Thus, polarity in T cells is important for differentiation and gives the T cell the ability to perform the function of antigen presentation to mount an immune response.
8. MICROFABRICATION TECHNIQUES TO STUDY CELL POLARITY
Microfabrication techniques have been used to understand the mechanisms responsible for asymmetric cell divisions and cell polarization and their effects on cell fate and behavior, including the use of hydrogels, microfluidic devices, and cell encapsulating scaffolds (57, 58). By utilizing these systems, many biological parameters that cannot be manipulated in ordinary cell culture can now be controlled, allowing for a systematic study of individual cell properties of interest. These systems can also be utilized to better mimic biological environments in vitro, providing valuable insight into cellular behavior in vivo.
One of the simplest ways to create these environments is by using microcontact printing to produce adhesive regions of varying geometries (59). This technique has been used to study various biological phenomena such as the asymmetric division of mesenchymal stem cells (MSCs) and migration of fibroblasts and cancer cells (57, 60). Asymmetric patterns, created using microcontact printing, were able to polarize the cell by asymmetric organization of the actin and microtubule cytoskeleton and resulted in biased segregation of DNA (57). This biased segregation was found primarily in stem cells and believed to play a role in stem cell differentiation. Work by Thery et al. demonstrated that anisotropy in the ECM created via micropatterns imposed polarity in epithelial cells by examining the organization of the actin cytoskeleton as well as the localization of the nucleus and the Golgi apparatus (61). That study not only showed the importance of ECM geometry but provided a tool for controlling cell polarity to determine its effects on cell behavior.
Micropatterns have also been used to study the effects of cell polarization on cell migration. Jiang et al. was able to demonstrate that the control of cell shape asymmetry via asymmetric micropatterns could bias the direction of cell migration (62). In this study, cells were confined to various symmetric and asymmetric micropatterns, then released to examine the direction of migration. The results demonstrated that cell polarity biases the direction of migration even after the cell was no longer confined to the original geometry. Similarly, Mahmud et al. created different symmetric and asymmetric “ratchet” micropatterns and showed that they caused directional migration while the cells remained in contact with the surface features (60). Various cell types (e.g., normal and cancer cells) were used and the short-term and long-term directional biases were studied. The micropatterns polarized the Arp2/3 complex and the actin cytoskeleton leading to directional movement of different cell types and sorting cells into individual reservoirs (60).
Further studies into the mechanisms of biased cell migration using micropatterns have shown mixed results. Kushiro et al. found that lamellipodial extensions, controlled by Rac1, play a major role in migration directional bias (63). However, Kumar et al. found that directional bias was not significantly altered by changes in Rac1, RhoA, or Cdc42 expression (64). While these studies seem to contradict each other, there were various differences between the two studies that provide further clues into biased cell migration mechanisms. In the Kushiro study MCF-10A (human mammary epithelial) cells were used while Kumar used NIH3T3 fibroblasts. Furthermore, the manipulation to the proteins of interest was also different, Kushiro suppressed Rac1 expression while Kumar’s study constitutively expressed Rac1, RhoA and Cdc42. This suggests that Rac1 is important in selecting migration direction, but an overexpression does not alter this directional bias. Although not fully explained, these studies begin to unravel the complexities of regulating cell migration of polarized cells.
Several groups have employed micro-patterning to study the role of polarity in cell fate specification. A study by Peng, et al. found that cell anisotropy, induced as a result of the increased aspect ratio of the underlying micropattern, decreased adipogenic differentiation in MSCs and found that the optimal aspect ratio for osteogenesis was 2 (65). While the mechanism for this phenomenon was not fully explained, it was suggested that anisotropy alters forces from the cytoskeleton and results in modified gene expression and stem cell differentiation. A study by Harris et al. showed that MSCs on rectangles differentiated to osteoblasts in both soft and stiff matrices, while cells on squares only favored osteogenesis on the stiff matrix (66). While the aspect ratio of the rectangle was not reported, this study corroborates the results of Peng’s work that shape and polarization caused by changes in geometric aspect ratio play a role in stem cell fate (66). Recently, we created symmetric and asymmetric micropatterned hydrogels to study the role of polarity in MSC differentiation. We found that cells seeded on asymmetric micropatterns had a higher amount of osteogenesis compared to cells on symmetric micropatterns and therefore concluded that polarity signals increase osteogenic differentiation in human MSCs (67).
Micropatterning of macrophages onto elongated patterns showed that anisotropy caused M2 polarization without the presence of cytokines (68). Mechanistic studies found that the cytoskeleton played a critical role in the M2 polarization. While only a few studies of these phenomena are published, the results show the importance of cell polarity on the cytoskeleton organization and how this organization affects cell fate. Follow up studies are needed to determine the mechanisms behind polarity and cell differentiation so better tissue engineering strategies can be developed.
Asymmetric divisions depend on the orientation of the division axis, which is controlled by cortical cues including cell polarity and cell-ECM contact. Théry was able to show that asymmetric micropatterns could affect these cortical cues and change the orientation of the division axis in HeLa cells. This study shows that not all asymmetric micropatterns result in a spindle orientation that would result in asymmetric division. Instead the orientation is controlled by the torque generated by the retraction fibers and the cortical cues (58). This again demonstrates the importance of the cytoskeleton and how its organization can influence cell polarity and asymmetric division. Further investigation into these micropatterns that bias asymmetric division can provide insight into their effects on cell fate and other cellular behaviors.
Several other microfabrication techniques have also been employed to induce cell polarity, including carbon nanotubes, coated substrates and microfluidic devices. By aligning carbon nanotubes, Cheng et al. was able to control the distribution of focal adhesions, cell alignment, and polarity in both human umbilical vascular endothelial cells and human embryonic stem cells (69). Scaffolds with tailored substrates are another way to create three dimensional (3D) structures which guide cell phenotype. These scaffolds can be made of various materials, such as polymers, ceramics or metals and scaffold design should be customized for the biological environment being mimicked and the cell type used (70–72). As an example, Granziano et al. used scaffolds with tailored surface geometry to polarize dental pulp stem cells to enhance differentiation as well as proliferation (73). In this study, scaffolds with microconcavities that mimicked the architecture of bone marrow resulted in greater osteogenesis. In another recent study, Wang et al. successfully created collagen scaffolds that resembled the small intestine in guiding epithelial cells apical-basal polarity and directional migration (74). Cells in the crypt remained undifferentiated and migrated toward the villi leading to the creation of tissue architecture of small intestine. This approach has also been utilized using hydrogels of polyethylene glycol (PEG) to elucidate dynamic cell-material interaction and matrix remodeling in cell migration in the context of regenerative medicine (75).
Microfluidic devices have also been proven to be powerful tools for modeling and studying cell polarity and chemotaxis in 3D (76, 77). Because these devices are usually clear, they allow for real time measurements and visualization of cell migration that other 3D culture conditions cannot. These devices can be used to decipher the mechanisms involved in cell migration and have most often been used to characterize cell migration in healthy and cancer cells (77–79). A recent study created a microfluidic device using selective curing that incorporated electrospun fibers (80). Breast cancer cells were able to migrate through the membrane toward the chemoattractant. This new platform can control various aspects of the tumor microenvironment to determine how they affect cell behavior and polarity. By using this systematic platform, methodical testing can be performed to understand how the cell-ECM interactions lead to specific cellular behaviors, such as metastasis in cancer cells. Another group created a microfluidic device without a chemotactic gradient to monitor how cancer cells move in confined spaces in response to chemotherapeutic drugs (81). The authors found that migration still occurred when the cells were exposed to drug concentrations above those required to stop proliferation, suggesting that these cells can survive treatment, migrate to distant sites, and metastasize.
9. CONCLUSIONS
The importance of cell polarization is evident by the various functions it has in biology and by the detrimental diseases caused by its dysregulation. Our current understanding of the mechanisms used to induce cell polarity have been mainly limited to using C. elegans and D. melanogaster mutants. This knowledge is the basis for examining mammalian development and many key molecules have been found to be conserved between organisms. However, the complexities of mammals make mechanistic studies in vitro quite difficult. Microfabrication techniques provide a powerful tool to systematically study polarization in vitro to parse the role of physiological cues that govern polarization and contribute to the progression of disease states.
Further, this insight will provide a rational basis for designing stem cell culture systems and facilitate their use in regenerative medicine.
10. ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR063338.
11. REFERENCES
- 1.Nelson WJ: Adaptation of core mechanisms to generate cell polarity. Nature, 422(6933), 766–74 (2003) DOI: 10.1038/nature01602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong Y, Mo C and Fraser SE: Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature, 430, 689 (2004) DOI: 10.1038/nature02796 [DOI] [PubMed] [Google Scholar]
- 3.Vicente-Manzanares M, Webb DJ and Horwitz AR: Cell migration at a glance. J Cell Sci, 118(21), 4917–4919 (2005) DOI: 10.1242/jcs.02662 [DOI] [PubMed] [Google Scholar]
- 4.Drubin DG and Nelson WJ: Origins of Cell Polarity. Cell, 84(3), 335–344 (1996) DOI: 10.1016/S0092-8674(00)81278-7 [DOI] [PubMed] [Google Scholar]
- 5.Etienne-Manneville S and Hall A: Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol, 15(1), 67–72 (2003) DOI: 10.1016/S0955-0674(02)00005-4 [DOI] [PubMed] [Google Scholar]
- 6.Mlodzik M: Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet, 18(11), 564–71 (2002) DOI: 10.1016/S0168-9525(02)02770-1 [DOI] [PubMed] [Google Scholar]
- 7.Knoblich JA: Mechanisms of Asymmetric Stem Cell Division. Cell, 132(4), 583–597 (2008) DOI: 10.1016/j.cell.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 8.Lechler T and Fuchs E: Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature, 437(7056), 275–280 (2005) DOI: 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powell DW: Barrier function of epithelia. Am J Physiol Gastrointest Liver Physiol, 241(4), G275–G288 (1981) DOI: 10.1152/ajpgi.1981.241.4.G275 [DOI] [PubMed] [Google Scholar]
- 10.Pilot F and Lecuit T: Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn, 232(3), 685–94 (2005) DOI: 10.1002/dvdy.20334 [DOI] [PubMed] [Google Scholar]
- 11.Nelson WJ: Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol, 1(1), a000513 (2009) DOI: 10.1101/cshperspect.a000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takano T, Xu C, Funahashi Y, Namba T and Kaibuchi K: Neuronal polarization. Development, 142(12), 2088–2093 (2015) DOI: 10.1242/dev.114454 [DOI] [PubMed] [Google Scholar]
- 13.Witte H and Bradke F: The role of the cytoskeleton during neuronal polarization. Curr Opin Neurobiol, 18(5), 479–87 (2008) DOI: 10.1016/j.conb.2008.09.019 [DOI] [PubMed] [Google Scholar]
- 14.Govek EE, Wu Z, Acehan D, Molina H, Rivera K, Zhu X, Fang Y, Tessier-Lavigne M and Hatten ME: Cdc42 Regulates Neuronal Polarity during Cerebellar Axon Formation and Glial-Guided Migration. iScience, 1, 35–48 (2018) DOI: 10.1016/j.isci.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conklin EG: The Organization and Cell-lineage of the Ascidian Egg. Philadephia, PA: (1905) [Google Scholar]
- 16.Knoblich JA: Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol, 11(12), 849–60 (2010) DOI: 10.1038/nrm3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knoblich JA: Mechanisms of asymmetric stem cell division. Cell, 132(4), 583–97 (2008) DOI: 10.1016/j.cell.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 18.Morrison SJ and Spradling AC: Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell, 132(4), 598–611 (2008) DOI: 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller MT and Spradling AC: Male and Female Drosophila Germline Stem Cells: Two Versions of Immortality. Science, 316(5823), 402–404 (2007) DOI: 10.1126/science.1140861 [DOI] [PubMed] [Google Scholar]
- 20.Schneider SQ and Bowerman B: Cell polarity and the cytoskeleton in the Caenorhabditis elegans zygote. Annu Rev Genet, 37, 221–49 (2003) DOI: 10.1146/annurev.genet.37.110801.142443 [DOI] [PubMed] [Google Scholar]
- 21.Neumüller RA and Knoblich JA: Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev, 23(23), 2675–2699 (2009) DOI: 10.1101/gad.1850809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S and Kemphues KJ: par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell, 81(4), 611–620 (1995) DOI: 10.1016/0092-8674(95)90082-9 [DOI] [PubMed] [Google Scholar]
- 23.Pham K, Sacirbegovic F and Russell SM: Polarized cells, polarized views: asymmetric cell division in hematopoietic cells. Front Immunol, 5, 26 (2014) DOI: 10.3389/fimmu.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doe CQ and Bowerman B: Asymmetric cell division: fly neuroblast meets worm zygote. Curr Opin Cell Biol, 13(1), 68–75 (2001) DOI: 10.1016/S0955-0674(00)00176-9 [DOI] [PubMed] [Google Scholar]
- 25.Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha Y-H, Ali M, Priess JR and Mello CC: Wnt Signaling and an APC-Related Gene Specify Endoderm in Early C. elegans Embryos. Cell, 90(4), 707–716 (1997) DOI: 10.1016/S0092-8674(00)80531-0 [DOI] [PubMed] [Google Scholar]
- 26.Götz M and Huttner WB: The cell biology of neurogenesis. Nat Rev Mol Cell Biol, 6, 777 (2005) DOI: 10.1038/nrm1739 [DOI] [PubMed] [Google Scholar]
- 27.Shen Q, Zhong W, Jan YN and Temple S: Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development, 129(20), 4843–4853 (2002) https://www.nature.com/articles/nature11393#supplementary-informationhttps://www.nature.com/articles/ncomms14684#supplementary-information [DOI] [PubMed] [Google Scholar]
- 28.Jossin Y, Lee M, Klezovitch O, Kon E, Cossard A, Lien W-H, Fernandez TE, Cooper JA and Vasioukhin V: Llgl1 Connects Cell Polarity with Cell-Cell Adhesion in Embryonic Neural Stem Cells. Dev Cell, 41(5), 481–495.e5 (2017) DOI: 10.1016/j.devcel.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascré G, Dekoninck S, Drogat B, Youssef KK, Brohée S, Sotiropoulou PA, Simons BD and Blanpain C: Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature, 489, 257 (2012) DOI: 10.1038/nature11393 [DOI] [PubMed] [Google Scholar]
- 30.Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascré G, Simons BD and Blanpain C: Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun, 8, 14684 (2017) DOI: 10.1038/ncomms14684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell migration: integrating signals from front to back. Science, 302(5651), 1704–9 (2003) DOI: 10.1126/science.1092053 [DOI] [PubMed] [Google Scholar]
- 32.Friedl P and Gilmour D: Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol, 10(7), 445–57 (2009) DOI: 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- 33.Adams JM and Cory S: The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene, 26(9), 1324–1337 (2007) DOI: 10.1038/sj.onc.1210220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. 0P.Muller AJ and Vousden KH: p53 mutations in cancer. Nat Cell Biol, 15, 2 (2013) DOI: 10.1038/ncb2641 [DOI] [PubMed] [Google Scholar]
- 35.Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, McLaren S, Lin M-L, McBride DJ, Varela I, Nik-Zainal S, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Quail MA, Burton J, Swerdlow H, Carter NP, Morsberger LA, Iacobuzio-Donahue C, Follows GA, Green AR, Flanagan AM, Stratton MR, Futreal PA and Campbell PJ: Massive Genomic Rearrangement Acquired in a Single Catastrophic Event during Cancer Development. Cell, 144(1), 27–40 (2011) DOI: 10.1016/j.cell.2010.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison SJ and Kimble J: Asymmetric and symmetric stem-cell divisions in development and cancer. Nature, 441(7097), 1068–74 (2006) DOI: 10.1038/nature04956 [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee S, Kong J and Brat DJ: Cancer stem cell division: when the rules of asymmetry are broken. Stem Cells Dev, 24(4), 405–16 (2015) DOI: 10.1089/scd.2014.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bajaj J, Zimdahl B and Reya T: Fearful symmetry: subversion of asymmetric division in cancer development and progression. Cancer Res, 75(5), 792–7 (2015) DOI: 10.1158/0008-5472.CAN-14-2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and Weinberg RA: Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell, 117(7), 927–939 (2004) DOI: 10.1016/j.cell.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 40.Sakakibara J, Sakakibara M, Shiina N, Fujimori T, Okubo Y, Fujisaki K, Nagashima T, Sangai T, Nakatani Y and Miyazaki M: Expression of cell polarity protein scribble differently affects prognosis in primary tumor and lymph node metastasis of breast cancer patients. Breast Cancer, 24(3), 393–399 (2017) DOI: 10.1007/s12282-016-0715-2 [DOI] [PubMed] [Google Scholar]
- 41.Liu B, Xiong J, Liu G, Wu J, Wen L, Zhang Q and Zhang C: High expression of Rac1 is correlated with partial reversed cell polarity and poor prognosis in invasive ductal carcinoma of the breast. Tumor Biol, 39(7), 1010428317710908 (2017) DOI: 10.1177/1010428317710908 [DOI] [PubMed] [Google Scholar]
- 42.Voulgari A and Pintzas A: Epithelialmesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta, 1796(2), 75–90 (2009) DOI: 10.1016/j.bbcan.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 43.Kang Y and Massagué J: Epithelial-Mesenchymal Transitions: Twist in Development and Metastasis. Cell, 118(3), 277–279 (2004) DOI: 10.1016/j.cell.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 44.Reymond N, Im JH, Garg R, Vega FM, Borda d’Agua B, Riou P, Cox S, Valderrama F, Muschel RJ and Ridley AJ: Cdc42 promotes transendothelial migration of cancer cells through β1 integrin. J Cell Biol, 199(4), 653–668 (2012) DOI: 10.1083/jcb.201205169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razidlo GL, Burton KM and McNiven MA: Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42. J Biol Chem, jbc. RA118. 003276 (2018) DOI: 10.1074/jbc.ra118.003276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M, Lang N, Qiu M, Xu F, Li Q, Tang Q, Chen J, Chen X, Zhang S, Liu Z, Zhou J, Zhu Y, Deng Y, Zheng Y and Bi F: miR-137 targets Cdc42 expression, induces cell cycle G1 arrest and inhibits invasion in colorectal cancer cells. Int J Cancer, 128(6), 1269–1279 (2011) DOI: 10.1002/ijc.25452 [DOI] [PubMed] [Google Scholar]
- 47.Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y and Yoshida K-I: Overexpression of RhoA, Rac1, and Cdc42 GTPases Is Associated with Progression in Testicular Cancer. Clin Cancer Res, 10(14), 4799–4805 (2004) DOI: 10.1158/1078-0432.CCR-0436-03 [DOI] [PubMed] [Google Scholar]
- 48.Arsenio J, Metz PJ and Chang JT: Asymmetric Cell Division in T Lymphocyte Fate Diversification. Trends Immunol, 36(11), 670–683 (2015) DOI: 10.1016/j.it.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu M, Kwon HY, Rattis F, Blum J, Zhao C, Ashkenazi R, Trachette L Jackson, N. Gaiano, T. Oliver and T. Reya: Imaging Hematopoietic Precursor Division in Real Time. Cell Stem Cell, 1(5), 541–554 (2007) DOI: 10.1016/j.stem.2007.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ting SB, Deneault E, Hope K, Cellot S, Chagraoui J, Mayotte N, Dorn JF, Laverdure JP, Harvey M, Hawkins ED, Russell SM, Maddox PS, Iscove NN and Sauvageau G: Asymmetric segregation and self-renewal of hematopoietic stem and progenitor cells with endocytic Ap2a2. Blood, 119(11), 2510–22 (2012) DOI: 10.1182/blood-2011-11-393272 [DOI] [PubMed] [Google Scholar]
- 51.Beckmann J, Scheitza S, Wernet P, Fischer JC and Giebel B: Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: identification of asymmetrically segregating proteins. Blood, 109(12), 5494–5501 (2007) DOI: 10.1182/blood-2006-11-055921 [DOI] [PubMed] [Google Scholar]
- 52.Duffy KR, Wellard CJ, Markham JF, Zhou JHS, Holmberg R, Hawkins ED, Hasbold J, Dowling MR and Hodgkin PD: Activation-Induced B Cell Fates Are Selected by Intracellular Stochastic Competition. Science, 335(6066), 338–341 (2012) DOI: 10.1126/science.1213230 [DOI] [PubMed] [Google Scholar]
- 53.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W and Reiner SL: Asymmetric T Lymphocyte Division in the Initiation of Adaptive Immune Responses. Science, 315(5819), 1687–1691 (2007) DOI: 10.1126/science.1139393 [DOI] [PubMed] [Google Scholar]
- 54.Ciocca ML, Barnett BE, Burkhardt JK, Chang JT and Reiner SL: Asymmetric memory T cell division in response to re-challenge. J Immunol, 188(9), 4145–4148 (2012) DOI: 10.4049/jimmunol.1200176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliaro J, Van Ham V, Sacirbegovic F, Pasam A, Bomzon Z. e., Pham K, Ludford-Menting MJ, Waterhouse NJ, Bots M, Hawkins ED, Watt SV, Cluse LA, Clarke CJP, Izon DJ, Chang JT, Thompson N, Gu M, Johnstone RW, Smyth MJ, Humbert PO, Reiner SL and Russell SM: Asymmetric Cell Division of T Cells upon Antigen Presentation Uses Multiple Conserved Mechanisms. J Immunol, 185(1), 367–375 (2010) DOI: 10.4049/jimmunol.0903627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, Murphy A, Ellis S, Smyth MJ, Kershaw MH, Darcy PK, Humbert PO and Russell SM: A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity, 22(6), 737–48 (2005) DOI: 10.1016/j.immuni.2005.04.009 [DOI] [PubMed] [Google Scholar]
- 57.Freida D, Lecourt S, Cras A, Vanneaux V, Letort G, Gidrol X, Guyon L, Larghero J and Thery M: Human Bone Marrow Mesenchymal Stem Cells Regulate Biased DNA Segregation in Response to Cell Adhesion Asymmetry. Cell Rep, 5(3), 601–610 (2013) DOI: 10.1016/j.celrep.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 58.Théry M, Jiménez-Dalmaroni A, Racine V, Bornens M and Jülicher F: Experimental and theoretical study of mitotic spindle orientation. Nature, 447, 493 (2007) DOI: 10.1038/nature05786 [DOI] [PubMed] [Google Scholar]
- 59.Li Y and Kilian KA: Bridging the Gap: From 2D Cell Culture to 3D Microengineered Extracellular Matrices. Adv Healthc Mater, 4(18), 2780–2796 (2015) DOI: 10.1002/adhm.201500427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahmud G, Campbell CJ, Bishop KJM, Komarova YA, Chaga O, Soh S, Huda S, Kandere-Grzybowska K and Grzybowski BA: Directing cell motions on micropatterned ratchets. Nat Phys, 5, 606 (2009) DOI: 10.1038/nphys1306 [DOI] [Google Scholar]
- 61.Théry M, Racine V, Piel M, Pépin A, Dimitrov A, Chen Y, Sibarita J-B and Bornens M: Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc Natl Acad Sci U S A, 103(52), 19771–19776 (2006) DOI: 10.1073/pnas.0609267103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X, Bruzewicz DA, Wong AP, Piel M and Whitesides GM: Directing cell migration with asymmetric micropatterns. Proc Natl Acad Sci U S A, 102(4), 975–978 (2005) DOI: 10.1073/pnas.0408954102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kushiro K, Chang S and Asthagiri AR: Reprogramming directional cell motility by tuning micropattern features and cellular signals. Adv Mater, 22(40), 4516–9 (2010) DOI: 10.1002/adma.201001619 [DOI] [PubMed] [Google Scholar]
- 64.Kumar G, Co CC and Ho C-C: Steering Cell Migration Using Microarray Amplification of Natural Directional Persistence. Langmuir, 27(7), 3803–3807 (2011) DOI: 10.1021/la2000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng R, Yao X and Ding J: Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials, 32(32), 8048–57 (2011) DOI: 10.1016/j.biomaterials.2011.07.035 [DOI] [PubMed] [Google Scholar]
- 66.Harris GM, Piroli ME and Jabbarzadeh E: Deconstructing the Effects of Matrix Elasticity and Geometry in Mesenchymal Stem Cell Lineage Commitment. Adv Funct Mater, 24(16), 2396–2403 (2014) DOI: 10.1002/adfm.201303400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piroli ME and Jabbarzadeh E: Matrix Stiffness Modulates Mesenchymal Stem Cell Sensitivity to Geometric Asymmetry Signals. Ann Biomed Eng (2018) DOI: 10.1007/s10439-018-2008-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McWhorter FY, Wang T, Nguyen P, Chung T and Liu WF: Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A, 110(43), 17253–17258 (2013) DOI: 10.1073/pnas.1308887110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Q, Harris GM, Blais M-O, Rutledge K and Jabbarzadeh E: Alignment of Carbon Nanotubes: An Approach to Modulate Cell Orientation and Asymmetry. Nano LIFE, 4(1), 1450002 (2014) DOI: 10.1142/S1793984414500020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rothrauff BB, Lauro BB, Yang G, Debski RE, Musahl V and Tuan RS: Braided and Stacked Electrospun Nanofibrous Scaffolds for Tendon and Ligament Tissue Engineering. Tissue Eng Part A, 23(9–10), 378–389 (2017) DOI: 10.1089/ten.tea.2016.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shamirzaei Jeshvaghani E, Ghasemi-Mobarakeh L, Mansurnezhad R, Ajalloueian F, Kharaziha M, Dinari M, Sami Jokandan M and Chronakis Ioannis S: Fabrication, characterization, and biocompatibility assessment of a novel elastomeric nanofibrous scaffold: A potential scaffold for soft tissue engineering. J Biomed Mater Res B Appl Biomater, 0(0) (2017) DOI: 10.1002/jbm.b.34043 [DOI] [PubMed] [Google Scholar]
- 72.Nie W, Peng C, Zhou X, Chen L, Wang W, Zhang Y, Ma PX and He C: Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nanohydroxyapatite composites for bone tissue engineering. Carbon, 116, 325–337 (2017) DOI: 10.1016/j.carbon.2017.02.013 [DOI] [Google Scholar]
- 73.Graziano A, d’Aquino R, Cusella-De Angelis MG, De Francesco F, Giordano A, Laino G, Piattelli A, Traini T, De Rosa A and Papaccio G: Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J Cell Physiol, 214(1), 166–72 (2008) DOI: 10.1002/jcp.21175 [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Gunasekara DB, Reed MI, DiSalvo M, Bultman SJ, Sims CE, Magness ST and Allbritton NL: A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials, 128, 44–55 (2017) DOI: 10.1016/j.biomaterials.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schultz KM, Kyburz KA and Anseth KS: Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc Natl Acad Sci U S A, 112(29), E3757–64 (2015) DOI: 10.1073/pnas.1511304112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y, Agrawal B, Sun D, Kuo JS and Williams JC: Microfluidics-based devices: New tools for studying cancer and cancer stem cell migration. Biomicrofluidics, 5(1), 013412 (2011) DOI: 10.1063/1.3555195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shamloo A, Ma N, Poo M.-m., Sohn LL and Heilshorn SC: Endothelial cell polarization and chemotaxis in a microfluidic device. Lab on a Chip, 8(8), 1292–1299 (2008) DOI: 10.1039/b719788h [DOI] [PubMed] [Google Scholar]
- 78.Cheng S-Y, Heilman S, Wasserman M, Archer S, Shuler ML and Wu M: A hydrogel-based microfluidic device for the studies of directed cell migration. Lab on a Chip, 7(6), 763–769 (2007) DOI: 10.1039/b618463d [DOI] [PubMed] [Google Scholar]
- 79.Chung S, Sudo R, Vickerman V, Zervantonakis IK and Kamm RD: Microfluidic platforms for studies of angiogenesis, cell migration, and cell–cell interactions. Ann biomed eng, 38(3), 1164–1177 (2010) DOI: 10.1007/s10439-010-9899-3 [DOI] [PubMed] [Google Scholar]
- 80.Eslami Amirabadi H, SahebAli S, Frimat JP, Luttge R and den Toonder JMJ: A novel method to understand tumor cell invasion: integrating extracellular matrix mimicking layers in microfluidic chips by “selective curing”. Biomed Microdevices, 19(4), 92 (2017) DOI: 10.1007/s10544-017-0234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Irimia D and Toner M: Spontaneous migration of cancer cells under conditions of mechanical confinement(). Integr Biol (Camb), 1(0), 506–512 (2009) DOI: 10.1039/b908595e [DOI] [PMC free article] [PubMed] [Google Scholar]