Abstract
Transforming growth factor β (TGFβ) induces migration of lung cancer cells (A549, H460 and H1299), dependent on activation of c-Jun N-terminal kinase (JNK1), and is inhibited by the JNK1 inhibitor SP600125. Moreover, TGFβ-induced migration of the cells is also blocked by the nuclear export inhibitor leptomycin B (LMB) and the orphan nuclear receptor 4A1 (NR4A1) ligand 1,1-bis(3’-indolyl)-1-(p-hydroxyphenyl)methane (CDIM8) which retains NR4A1 in the nucleus. Subsequent analysis showed that the TGFβ/TGFβ receptor/PKA/MKK4 and −7/JNK pathway cascade phosphorylates and induces nuclear export of NR4A1 which in turn forms an active complex with Axin2, Arkadia (RNF111) and RNF12 (RLIM) to induce proteasome-dependent degradation of SMAD7 and enhance lung cancer cell migration. Thus, NR4A1 also plays an integral role in mediating TGFβ-induced lung cancer invasion, and the NR4A1-ligand CDIM8 which binds nuclear NR4A1 represents a novel therapeutic approach for TGFβ-induced blocking of lung cancer migration/invasion.
Implications:
Effective treatment of TGFβ-induced lung cancer progression could involve a number of agents including the CDIM/NR4A1 antagonists which block not only TGFβ-induced migration but several other NR4A1-regulated pro-oncogenic genes/pathways in lung cancer cell lines.
Keywords: TGFβ, lung cancer, progression, NR4A1, antagonists
INTRODUCTION
Lung cancer is the leading cause of cancer deaths in the United States and it is estimated that in 2017, 222,500 new cases of lung cancer will be diagnosed in this country and 155,780 patient deaths will be observed (1). Greater than 85% of lung cancers are classified as non-small cell lung cancer (NSCLC) and despite significant advances in treatment regimens, the overall survival rate of NSCLC patients is 15.9% and this rate has not significantly improved over the past decade (2). Smoking is the major risk factor for lung cancer and exposure to secondary smoke, various occupational exposures, air pollution, and genetic factors also contribute to the high incidence of this disease. NSCLC is a highly complex disease with multiple subtypes and histologies that are accompanied by mutations of oncogenes (i.e. EGFR, KRAS, EML4-ALK) and tumor suppressor genes (i.e. p53) (3–6) and lung cancer therapy is driven, in part, by the tumor type and its pathological and molecular characteristics and traditional surgery, radiation and combinations of cytotoxic and mechanism-based drugs are extensively used (3–7). Targeted therapies for treating lung cancer have had limited success, and the more recent development and applications of immunotherapeutics that target programmed cell death ligand 1 (PD-L1) and programmed cell death 1 (PD-1) are promising new approaches (8,9). Despite the advances in lung cancer chemotherapy, the improvement in patient survival remains low and most therapies are accompanied by unwanted side-effects and drug resistance. Thus, it is critical to develop new therapeutics which target multiple pro-oncogenic pathways and can be used in combination therapies.
The transforming growth factor β family of ligands and receptors play an important and somewhat paradoxical role in cancer in which TGFβ act as an inhibitor of early stage cancers but acts as a tumor promoter for later stage cancers. Several studies report that TGFβ induces lung cancer cell migration/invasion and EMT, and this involves multiple kinases and downstream targets (10–19). A recent study showed that TGFβ-induced migration/invasion of triple negative breast cancer cells was also NR4A1-dependent where NR4A1 interacts with Arkadia, AXIN2 and RNF12 to induce proteasome-dependent degradation of SMAD7, resulting in TGFβR1/TGFβR2 homodimerization and activation (20). We have also confirmed that NR4A1 plays a key role in breast cancer invasion where TGFβ induces nuclear export of NR4A1 which interacts with E3 ligase complex proteins to induce SMAD7 ubiquitination and degradation (20,21). We previously reported that NR4A1 was a negative prognostic factor for lung cancer patient survival and NR4A1 was a pro-oncogenic factor regulating lung cancer cell proliferation and survival (22), and this has also been observed in cell lines derived from other solid tumors (23–30). Structure activity studies among a series of 1,1-bis(3’-indolyl)-1-(substituted phenyl)methane compounds showed that some of these analogs bound NR4A1 and in cancer cell lines, acted as NR4A1 antagonists (22–30). The most active compound 1,1-bis(3’-indolyl)-1-)p-hydroxyhenyl)methane (CDIM8; DIM-C-pPhOH) which acts as a nuclear NR4A1 antagonist (29) in lung and other cancer cell lines inhibited NR4A1-dependent pro-oncogenic genes/pathways (22–30). We hypothesized that DIM-C-pPhOH would also inhibit TGFβ-induced lung cancer cell migration/invasion, and our results show for the first time that TGFβ-induced invasion of lung cancer cells is due to JNK1-dependent phosphorylation and nuclear export of NR4A1 that is inhibited by NR4A1 antagonists.
MATERIALS AND METHODS
Cell lines, reagents and plasmids.
Lung cancer cell lines (A549, H460, and H1299) were purchased from American Type Culture Collection (Manassas, VA). A549 cells were maintained 37°C in the presence of 5% CO2 in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium with 10% fetal bovine serum with antibiotic H460, and H1299 lung cancer cells were maintained in RPMI-1640 medium with 10% fetal bovine serum and antibiotic. Alexa Fluor 488 and 455, Hoechst 33342, leptomycin B, SP600125, SB202190, LY294002 and PD98059 were obtained from Cell Signaling Technologies (Manassas, VA), and TGFβ was purchased from BD Biosystems (Bedford, MA). Dulbecco’s Modified Eagle’s Medium, 14–22 Amide PKA inhibitor, ALK5i inhibitor (LY-364947) and 36% formaldehyde were purchased from Sigma-Aldrich (St. Louis, MO), and hematoxylin was purchased from Vector Laboratories (Burlingame, CA). The antibodies and their sources are summarized in Supplemental Table 1. FLAG-NR4A1, FLAG-NR4A1-(A-B), and FLAG-NR4A1-(C-F) were synthesized in the lab using site directed mutagenesis (31); pcDNA3-FLAG-MKK4WT, pcDNA3-FLAG-MKK7-JNK1A1WT [MKK7(CA)] and pcDNA3-FLAG-MKK7-JNK1A1APF [(MKK7(DN)] were purchased from Origene Technologies (Rockville, MD). pCMV5-FLAG-SMAD7 was a gift from Lin SC et. al. (Department of Biochemistry, Hong Kong University of Science and Technology, Kowloon, Hong Kong, China).
Boyden Chamber Assay.
A549, H460, and H1299 lung cancer cells (3.0 × 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 hr. After various treatments including knockdown of various genes (48 hr), cells were allowed to migrate for 24 hr, fixed with formaldehyde, and then stained with hematoxylin, and cells migrating through the pores were then counted as described (31).
RT PCR.
RNA was isolated using Zymo Research Quick-RNA MiniPrep kit (Irvine, CA). Quantification of mRNA (slug, snail, and NR4A1) was performed using Bio-Rad iTaq Universal SYBER Green 1-Step Kit (Richmond, CA) using the manufacturer’s protocol with real-time PCR. TATA Binding Protein (TBP) mRNA was used as a control to determine relative mRNA expression.
Immunoprecipitation and chromatin immunoprecipitation.
A549 cells were transfected with various constructs and, 6 hr after transfection, cells were treated with DMSO or various agents and immunoprecipitation experiments and subsequent analysis were carried out previously described (31).
The chromatin immunoprecipitation (ChIP) assay was performed using the ChIP-IT Express magnetic chromatin immunoprecipitation kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. The treatment conditions and analysis were performed as described (31). The primers for detection of the NR4A1 promoter region were 5’- CCTGCCCTCGGGAAGG −3’ (forward) and 5’- CAGGCCGCGGGCTGAGG −3’ (reverse). PCR products were resolved on a 2% agarose gel in the presence of RGB-4103 GelRed Nucleic Acid Stain.
Nuclear/cytosolic extraction and western blots.
Lung cancer cells were treated with various agents/constructs, and nuclear and cytosolic fractions were isolated using Thermo Scientific NE-PER Nuclear and Cytoplasmic Extraction Kit (Rockford, IL) according to manufacturer’s protocol. Fractions were analyzed by western blots as described (31). GAPDH and p84 were used as cytoplasmic and nuclear positive controls, respectively.
Immunofluorescence.
A549 cells (1.0 × 105 per well) were treated with either DMSO or TGFβ (5 ng/ml) was added for 4 hr after ± pretreatment with various agents or transfection for 48 hr. Cells were then fixed with 37% formalin, blocked, treated with fluorescent NR4A1 primary antibody [Nur77 (D63C5)] XP®) for 24 hrs. Cells were then washed with PBS and treated with Anti-rabbit IgG Fab2 Alexa Fluor® 488 secondary antibody for 3 hrs. Cells were then treated with Hoechst (Hoechst 33342) stain and phalloidin (Alexa Fluor® 555 Phalloidin) for 15 min in the dark following manufacturer’s protocol and visualized by confocal microscopy (Zeiss LSM 780 confocal microscope, Peabody, MA) as previously described (31) Cells were analyzed by western blot as described previously (24–28).
Small interfering RNA interference assay.
SiRNA experiments were conducted as described previously (21). The siRNA complexes used in the study that were purchased from Sigma-Aldrich are as follows: siGL2–5’: CGU ACG CGG AAU ACU UCG A; siNR4A1(1): SASI_Hs02_00333289; siNR4A1(2): SASI_Hs02_00333290; siAxin2(1): SASI_Hs01_00110148; siAxin2(2): SASI_Hs01_00110149; siArkadia(1): SASI_Hs01_00064840; siArkadia(2): SASI_Hs01_00064841; siRNF12 (1): SASI_Hs01_00238255; siRNF12(2): SASI_Hs02_00348888; siTAK1(1): SASI_Hs02_00335227; siTAK1(2): SASI_Hs01_00234777; siTAB1(1): SASI_Hs01_00094398; siTAB1(2): SASI_Hs02_00340933; siTRAF6(1): SASI_Hs01_00116391; siTRAF6(2): SASI_Hs01_00116390; siMKK4(1): SASI_Hs02_00334897; siMKK4(2): SASI_Hs02_00334898; siMKK7(1): SASI_Hs01_00059905; siMKK7(2): SASI_Hs01_00059906; siJNK1(1): SASI_Hs01_00010441; siJNK1(2): SASI_Hs01_00010442; sic-fos (1): SASI_Hs01_00184572; sic-fos(2): SASI_Hs01_00184573; siATF2(1): SASI_Hs01_00147372; siATF2(2): SASI_Hs01_00147373; siElk1(1): SASI_Hs02_00326325; siElk1(2): SASI_Hs02_00326324. The following siRNA complexes that were used in this study were purchased from Santa Cruz Biotechnology: c-jun siRNA(h): sc29223; c-jun siRNA(h2): sc-44201 SRF siRNA: sc-36563; PKACα siRNA: sc-36240.
PKA activity assay.
A549 lung cancer cells (3.0 × 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 hr. Cells were then treated with above described treatments as used in other assays, then lysed with PKA lysis buffer (made in the laboratory using the manufacturer’s recipe). PKA activity assay (Promega, Madison, WI) was performed following manufacturer’s protocol, then lysates were resolved on a 2% agarose gel.
Statistical analysis.
Statistical significance of differences between the treatment groups was determined as previously described (31).
RESULTS
1. TGFβ-induced nuclear export of NR4A1 is JNK-dependent
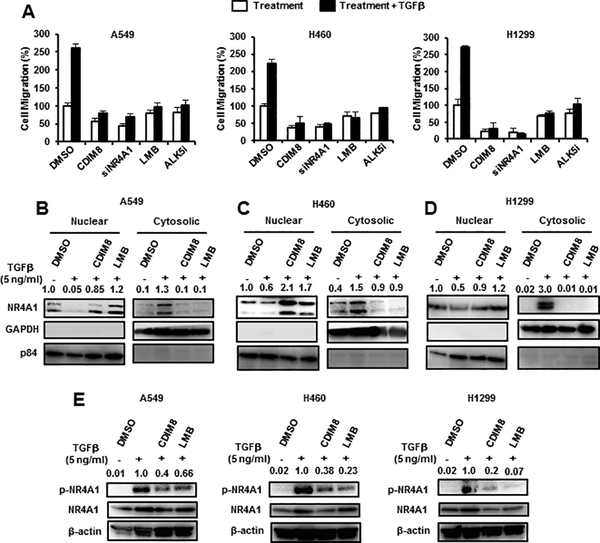
In this study, we initially used three NSCLC cell lines (A549, H460 and H1299) to investigate the role of NR4A1 in TGFβ-induced migration/invasion using a Boyden Chamber assay. TGFβ induced migration of the three cell lines (Fig. 1A) and cotreatment with the NR4A1 antagonist CDIM8, the nuclear export inhibitor leptomycin B (LMB), the TGFβ receptor inhibitor ALK5i, or knockdown of NR4A1 by RNA interference (RNAi) (siNR4A1) significantly inhibited the TGFβ-induced cell migration. The results also showed that CDIM8 and siNR4A1 also inhibited basal migration of the lung cancer cell lines. TGFβ also induced nuclear export of NR4A1 in A549, H460 and H1299 lung cancer cells (Figs. 1B-1D, respectively) which was inhibited by LMB and CDIM8. We also observed that TGFβ induced both expression and phosphorylation (S351) of NR4A1 and this was inhibited by cotreatment with CDIM8 and LMB (Fig. 1E). The intracellular location of NR4A1 in these experiments was determined by western blots of nuclear and cytosolic extracts using GAPDH (cytosolic) and P84 (nuclear) as subcellular controls.
Figure 1.
Role of NR4A1/CDIM8 in TGFβ-induced lung cancer cell migration. (A) A549, H460 and H1299 lung cancer cells were treated 5 ng/ml TGFβ (for 5 hr) and various reagents including siNR4A1 oligonucleotide (for NR4A1 knockdown), and cell migration was determined in a Boyden chamber assay. A549 (B), H460 (C) and H1229 (D) cells were treated with 5 ng/ml alone and in combination with LMB (20 nM) or CDIM8 (20 μM), and cytosolic and nuclear (B-D) or whole cell lysates (E) were analyzed by western blots. Results (A) are expressed as means ± SE for 3 separate determinations, and significant (p<0.05) induction of migration compared to solvent control (DMSO/CTL) is indicated (*). Bands in western blots (B-E) were quantitated relative to β-actin, and control values of NR4A1 were 1.0. The LMB and CDIM8 concentrations indicated above were used in subsequent experiments.
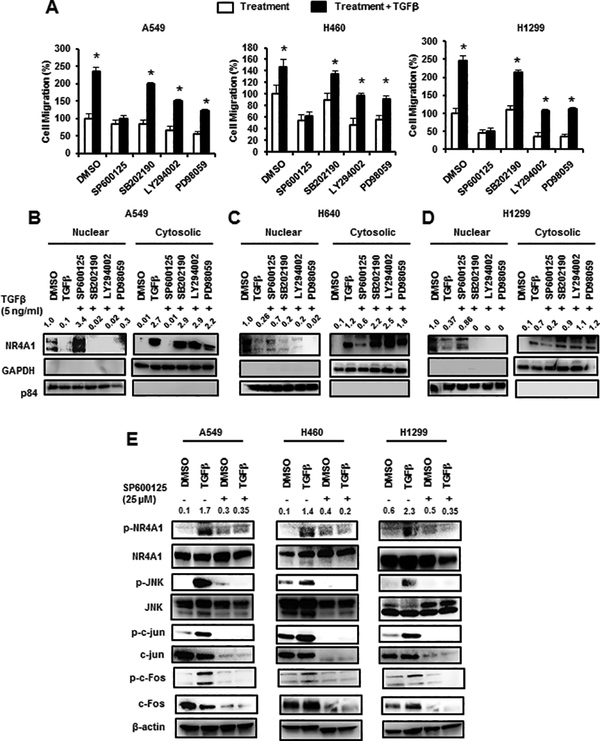
We also examined the effects of kinase inhibitors on TGFβ-induced cell migration and the JNK inhibitor SP600125 but not p38MAPK (SP202190), p42/44MAPK (PD98059) or PI3K (LY294002) inhibitors blocked TGFβ-induced migration of A549, H460 and H1299 cells (Fig. 2A). SP600125 also inhibited TGFβ-mediated nuclear export of NR4A1 in A549 (Fig. 2B), H460 (Fig. 2C) and H1299 (Fig. 2D) cells, whereas cotreatment with SB202190, LY294002 or PD98059 did not inhibit nuclear export of NR4A1 in cells treated with TGFβ, indicating that TGFβ-induced nuclear export of NR4A1 was JNK-dependent in lung cancer cells. TGFβ also induced phosphorylation of (S351) NR4A1, JNK1, c-jun and c-fos which was also inhibited by SP600125, demonstrating that TGFβ induces JNK and genes downstream from JNK.
Figure 2.
Effects of kinase inhibitors on TGFβ-induced migration and nuclear export of NR4A1. (A) Cells were treated with TGFβ alone or in combination with kinase inhibitors SP600125 (30 μM), SB202190 (30 μM), LY294002 (30 μM) and PD98059 (30 μM), and effects on cell migration were determined. A549 (B), (H460 (C) and H1299 (D) cells were treated with TGFβ alone and in combination with kinase inhibitors, and nuclear and cytosolic extracts were analyzed for NR4A1 expression by western blots. (E) Lung cancer cells were treated with TGFβ and SP600125 alone or in combination, and whole cell lysates were analyzed by western blots. Significant (p<0.05) induction of TGFβ-induced cell migration is indicated (*). Bands in western blots (B-D) were quantitated relative to β-actin, and DMSO control values of NR4A1 were 1.0. Relative intensities of p-NR4A1 are given in (E). The kinase concentrations indicated above were used in subsequent experiments.
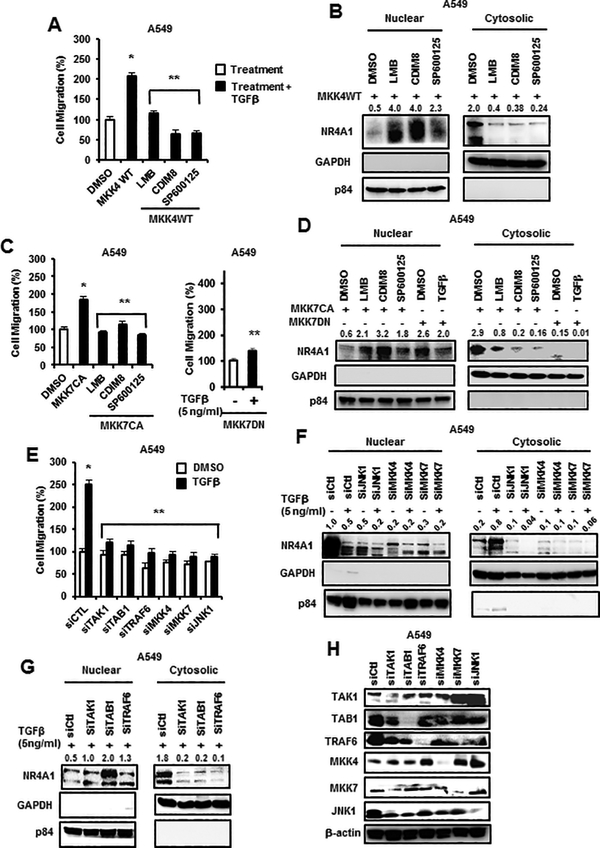
Since the TGFβ-JNK-NR4A1 (nuclear export) pathway is critical for enhanced migration of lung cancer cells, we used A549 cells as a model to further investigate the role of upstream kinases in this pathway. MKK4 and MKK7 are upstream from JNK1, and overexpression of FLAG-MKK4 (wild-type) enhanced invasion of A549 cells and this was inhibited by LMB, CDIM8 and SP600125 (Fig. 3A). Overexpression of MKK4 also induced nuclear export of NR4A1 and this was inhibited by LMB, CDIM8 and SP600125 (Fig. 3B). Overexpression of FLAG-MKK7(CA) also induced A549 cell migration which was inhibited by LMB, CDIM8 and SP60012, and TGFβ-induced migration was inhibited by a dominant negative FLAG-MKK7(DN) (Fig. 3C). MKK7 overexpression also induced nuclear export of NR4A1 which was inhibited by LMB, CDIM8, SP600125, and dominant negative MKK7 (Fig. 3D). We also observed that TGFβ-induced migration in A549 cells was inhibited by transfecting a construct expressing MKK7(DN) and also by knockdown of JNK1 (siJNK1) and upstream kinases including MKK4 (siMKK4), and MKK7 (siMKK7), TRAF6 (siTRAF6), TAK1 (siTAK1), and TAB1 (siTAB1) (Fig. 3E). TGFβ-induced nuclear export of NR4A1 was also inhibited in A549 cells transfected with siJNK, siMKK4 and siMKK7, confirming that the intact MKK4/7-JNK pathway is required for NR4A1 nuclear export (Fig. 3F). We also observed that knockdown of the upstream kinases TAB1, TAK1 and TRAF6 inhibited TGFβ-induced nuclear export of NR4A1 and the loss of TAB1 increased levels of nuclear NR4A1 (Fig. 3G). TRAF6 potentially plays a role in activation of PKA, such as recruitment of PKA to the plasma membrane or enhance dissociation of regulatory subunits of PKA. TRAF6 is K63 polyubiquitinated and forms signaling cascades that activate MAPK (like JNK1). Knockdown efficiencies are indicated in Figure 3H. Knockdown studies were performed using at least two different oligonucleotides (see Materials and Methods).
Figure 3.
Role of upstream kinases and CDIM8 and other inhibitors on TGFβ/kinase-induced responses in lung cancer cells. Overexpression of FLAG-MKK4 on A549 cell migration (A) and nuclear export of NR4A1 (B) and effects of LMB, CDIM8 and SP600125 were determined in a Boyden chamber assay and by western blot analysis of nuclear and cytosolic extracts, respectively. Overexpression of FLAG-MKK7(CA) alone or in combination with various inhibitors or expression of FLAG-MKK7(DN) (± TGFβ) on cell migration (C) and nuclear export of NR4A1 (D) were determined in a Boyden chamber assay or by western blot analysis of nuclear and cytosolic extracts, respectively. Kinase knockdown by RNA interference on TGFβ-induced migration (E) and nuclear export of NR4A1 (F and G) were determined in a Boyden chamber assay and by western blot analysis of nuclear and cytosolic extracts, respectively. (H) Various oligonucleotides targeting kinases were transfected into A549 cells and, after 72 hr, whole cell lysates were analyzed by western blots. Results in A, C and E are means ± SE for 3 separate determinations, and significantly (p<0.05) enhanced migration (*) and inhibition of this response (**) are indicated. Relative concentrations of NR4A1 (B, D, F, G) were relative to β-actin were determined.
We also investigated the effects of TGFβ, MKK4, and MKK7(CA) alone and in various combinations with TGFβ, LMB, SP600125 and CDIM8, and also MKK7(DN) alone and in combination with TGFβ by immunostaining and confocal microscopy. In DMSO treated cells, NR4A1 was primarily nuclear and this was significantly decreased after treatment with TGFβ, whereas TGFβ-mediated nuclear export of NR4A1 was inhibited after cotreatment with LMB, CDIM8 and SP600125 or transfected with MKK7(DN) (Suppl. Figs. 1 and 2). Overexpression of FLAG-MKK4 and FLAG-MKK7(CA) also induced nuclear export of NR4A1 as determined by confocal microscopy and this response was inhibited after cotreatment with LMB, CDIM8 and SP600125 (Suppl. Fig. 2).
2. Mechanism of TGFβ-induced expression of NR4A1
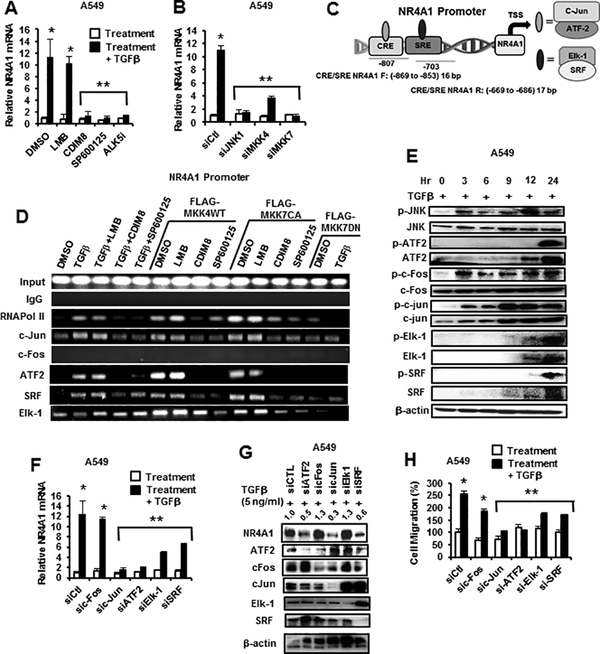
TGFβ induces NR4A1 in breast cancer cells, (31) and this was also observed in lung cancer cells (Fig. 1E) and the mechanism of this response was further investigated. Treatment of A549 cells with TGFβ for 5 hr induced a >10-fold increase in NR4A1 mRNA levels and these effects were inhibited after cotreatment with CDIM8, SP600125 or ALK5i (TGFβ receptor inhibitor) but not LMB (Fig. 4A) or after transfection with siJNK1, siMKK4 and siMMK7 (Fig. 4B). The effects of TGFβ alone on induction of NR4A1 protein were minimal (Figs. 3B and 3F) as were the effects of LMB on this response, and this was in contrast to the induction of NR4A1 gene expression by TGFβ (Fig. 4A). Downstream targets of JNK, such as a c-jun, c-fos, ATF2, Elk-1 and SRF, bind AP1 (c-jun, c-fos), CRE (c-jun, ATF2) and SRE (Elk-1, SRF) promoter elements, and CRE and SRE sites were identified within the NR4A1 promoter (Fig. 4C). Therefore, we used a ChIP assay to investigate association of c-Jun/ATF2 and Elk-1 and SRF with the CRE/SRE motifs using primers that cover the −807 to −703 region of the NR4A1 promoter. A549 cells were treated with TGFβ, transfected with FLAG-MKK4 or FLAK-MKK7(CA) alone and this resulted in recruitment of c-jun, ATF2 and SRF and also Pol II to the promoter and Elk-1 was constitutively bound in control (DMSO) cells (Fig. 4D). CDIM8 and SP600125 but not LMB blocked recruitment of c-jun, ATF2 and SRF to the NR4A1 promoter, and the result for LMB correlated with its effect (or lack thereof) on TGFβ-induced levels of NR4A1 mRNA (Fig. 4A). ChIP assay results in cells transfected with FLAG-MKK7(DN) showed that TGFβ-induced recruitment of c-Jun, ATF2 and SRF to the promoter was blocked by the DN plasmid (Fig. 4D). We did not detect any c-fos bound to the NR4A1 promoter, which is consistent with the fact that no putative AP1 promoter elements were identified within the promoter. The time-dependent activation of JNK phosphorylation by TGFβ was also accompanied by activation and/or induction of phosphorylated ATF2, SRF, c-jun and Elk-1 (Fig. 4E) and these results are consistent with recruitment of these factors to the NR4A1 promoter as results of the ChIP assay (Fig. 4D). TGFβ-induced NR4A1 mRNA (Fig. 4F) and protein (Fig. 4G) expression was inhibited in A549 cells after knockdown of c-jun, ATF2, SRF and Elk-1 but not c-fos (Fig. 4F) and this complemented results of the ChIP assay. There was some off-target variability in this experiment; for example, knockdown of c-jun also resulted in decreased c-fos expression and, loss of Elk-1 and SRF increased levels of c-jun and this may indicate some interactions and crosstalk between of these transcription factors. Since c-jun, ATF2 and SRF are recruited to the NR4A1 promoter and regulate expression of NR4A1, we also observed that their loss (by RNAi) also resulted in decreased TGFβ-induced migration (Fig. 4H).
Figure 4.
NR4A1 regulation in A549 cells. A549 cells were treated with TGFβ alone or in combination with various inhibitors (A) or after knockdown of JNK1 pathway kinases (B), and NR4A1 mRNA levels were determined by real time PCR. (C) Identification of cis-elements on the NR4A1 gene promoter. (D) Cells were treated with TGFβ or transfected with MKK4(WT), MKK7(CA) and MKK7(DN) alone or in combination with various agents for 6 hr and then analyzed in a ChIP assay using multiple antibodies and primers targeting the −869 to −853 (F) and −669 to −86 (R) regions of the NR4A1 promoter. (E) A549 cells were treated with TGFβ (5 ng/ml) for 0, 3, 6, 9, 12 and 24 hr, and whole cell lysates were analyzed by western blots. A549 cells were transfected with oligonucleotides targeted to factors that regulate NR4A1 expression, and their effects on NR4A1 mRNA levels (F) and protein knockdown efficiencies (G) were determined by real time PCR and western blot analysis of whole cell lysates, respectively. The effects of knockdown of these same factors on cell migration (H) and intracellular location (nucleus vs. cytosol) of NR4A1 (I) was determined in Boyden chamber assays and western blots, respectively. The NR4A1 band relative to β-actin in western blot (G) was quantitated, and control values of NR4A1 were set at 1.0.
3. TGFβ-induced PKA is also involved in nuclear export of NR4A1
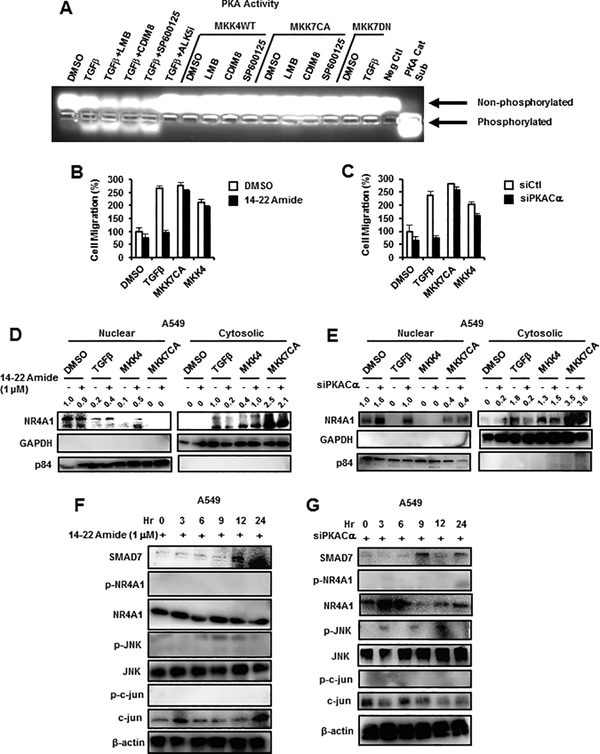
TGFβ-induced activation of CRE and CRE binding factors suggests that protein kinase A (PKA) may also be activated by TGFβ, and we therefore used a fluorescent peptide (kempTide) with multiple PKA phosphorylation sites and show that TGFβ alone or in combination with LMB, CDIM8 and SP600125 induced phosphorylation (activity), whereas this response was not observed in cells cotreated with TGFβ plus ALK5i, the TGFβ receptor inhibitor (Fig. 5A). In cells transfected with FLAG-MKK4-WT or FLAG-MKK7(CA) alone or in combination with CDIM8, LMB and SP600125 or FLAG-MKK7(DN) ± TGFβ, phosphorylation of PKA was not observed. As a positive control, we also observed increased phosphorylation of PKA in cells overexpressing the PKA catalytic subunit (Fig. 5A). Treatment of cells with the PKA inhibitor 14–22 Amide or knockdown of PKA-Cα (siPKA-Cα) by RNAi inhibited TGFβ-induced A549 cell migration but did not affect MKK4/7-induced migration which are downstream from PKA (Figs. 5B and 5C). We also investigated the effects of 14–22 Amide and siPKA-Cα (Figs. 5D and 5E) on TGFβ/MKK4/7-induced nuclear export of NR4A; only the TGFβ-induced effect was inhibited and MKK4/7 differentially enhanced nuclear export of NR4A1 independent of PKA inhibition. Results obtained for MKK4 ± 14–22 Amide (Fig. 5D) were somewhat inconsistent; however, results in Supplemental Figure 3 confirm these observations using confocal microscopic analysis. We also examined the effects of 14–22 Amide and siPKA-Cα (Figs. 5F and 5G) on NR4A1 and the JNK1 pathway in A549 cells treated with TGFβ and show that phosphorylation of NR4A1, JNK1 and jun were inhibited and SMAD7 levels were increased compared to that observed in cells treated with TGFβ alone.
Figure 5.
Role of PKA in TGFβ-NR4A1 interactions. (A) The Promega PKA activity assay kit was used to investigate PKA activation by TGFβ, MKK4(WT) and MKK7(CA) alone and in combination with various agents and by TGFβ plus MKK7(DN). A549 cells were treated with TGFβ or transfected with MKK7(CA) and MKK4(WT) alone or in combination with 14–22 Amide or cotransfected with siPKA-Cα (knockdown) and effects on cell migration (B and C) and intracellular location (nucleus vs. cytosol) of NR4A1 (D and E) were determined by Boyden chamber and western blot assays, respectively. The effects of 14–22 Amide (F) and siPKA-Cα (G) on the time-dependent expression of TGFβ-induced proteins was determined by western blot analysis of whole cell lysates. Significant (p<0.05) inhibition of induced migration (determined in triplicate) by 14–22 Amide or siPKA-Cα is indicated (*). The NR4A1 band relative to β-actin in western blots (D, E) was quantitated, and control values of NR4A1 were set to 1.0.
4. TGFβ induces proteasome-dependent degradation of SMAD7
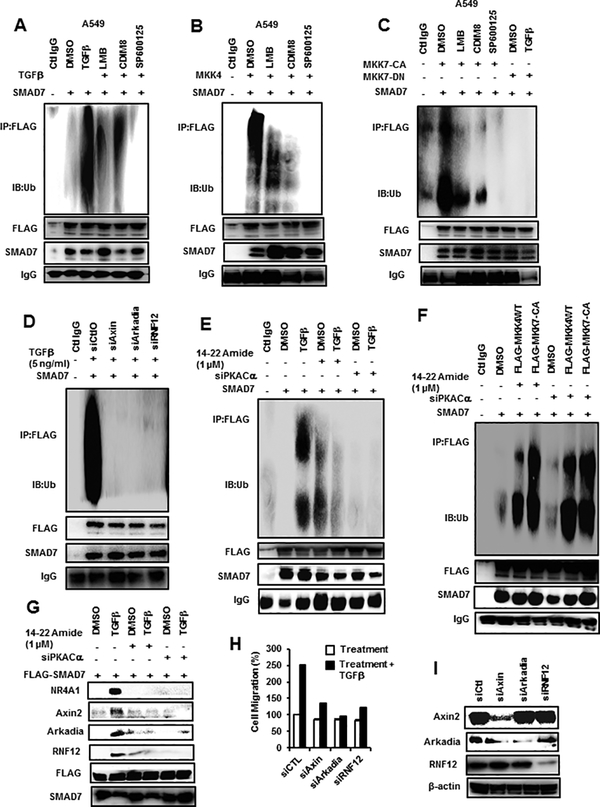
Previous studies show that TGFβ induces proteasome-dependent SMAD7 degradation via an NR4A1/RNF12/Arkadia/Axin2 complex (21,31,32) and treatment of A549 cells with TGFβ or transfection with FLAG-MKK4 and FLAG-MKK7(CA) followed by immunoprecipitation with NR4A1 antibodies showed that NR4A1 interacts with Axin2 and SMAD7 but not RNF12 or Arkadia (Suppl. Figs. 4A-4C). Cotreatment with LMB, CDIM8 or SP600125 significantly decreased these interactions and transfection with FLAG-MKK7(DN) blocked TGFβ-induced interactions of NR4A1 with Axin2 and SMAD7 (Suppl. Fig. 4C). The same treatment groups were used and A549 cells were also transfected with FLAG-NR4A1-LBD (containing the LBD region of NR4A1) and immunoprecipitated with FLAG antibodies, and results showed that SMAD7 interacted with the ligand binding domain of TGFβ/MKK4/MKK7(CA)-activated NR4A1 (Suppl. Figs. 4D-4F). Using a SMAD7-FLAG construct in 549 cells treated with TGFβ, we also showed that SMAD7 interacts with Axin2, RNF12, Arkadia and NR4A1, and these interactions are blocked by LMB, CDIM8 and SP600125. Treatment of A549 cells with TGFβ or transfection with MKK4 or MKK7(CA) followed by immunoprecipitation by SMAD7 antibodies showed that a broad band of ubiquitinated SMAD7 proteins were formed (Figs. 6A-6C). Moreover, the intensity of the TGFβ-induced ubiquitinated SMAD7 was inhibited by LMB, CDIM8 and SP600125. In addition, TGFβ-induced ubiquitination was blocked after transfection with FLAG-MKK7(DN) and minimal ubiquitinated SMAD7 was observed in the control IgG lane (Fig. 6C). TGFβ-induced ubiquitination of SMAD7 was inhibited after knockdown of Axin2, Arkadia and RNF12 (Fig. 6D) and TGFβ-induced ubiquitination of SMAD7 was also inhibited by 14–22 Amide or after transfection with siPKA-Cα (Fig. 6E). We also observed that MKK4/7 enhanced ubiquitination of SMAD7 (Fig. 6F) and these responses were not blocked by inhibition of PKA since both kinases are downstream from PKA. In contrast, both 14–22 Amide and siPKA-Cα inhibited TGFβ-induced interactions of NR4A1, Axin2, Arkadia and RNF12 with SMAD7 (Fig. 6G). The importance of the ubiquitin ligase complex members in mediating TGFβ-induced migration of A549 cells is consistent with results in Figure 6H showing that knockdown of Axin2, Arkadia or RNF12 inhibits the TGFβ-induced response. Figure 6I illustrates the specificity of the ubiquitin ligase complex proteins after knockdown by RNA interference. These results demonstrate the critical role of NR4A1, Axin2, Arkadia and RNF12 in mediating degradation of SMAD7 and TGFβ-induced cell migration and identify several inhibitors of this pathway including the NR4A1 antagonist CDIM8.
Figure 6.
Role of kinase pathways and proteasome complex proteins on SMAD7 ubiquitination and cell migration. A549 cells were transfected with FLAG-SMAD7 and treated with TGFβ (A), transfected with MKK4(WT) (B), transfected with MKK7(CA) or MKK7(DN) (± TGFβ) (C), and treated with various agents. Whole cell lysates were immunoprecipitated with FLAG antibodies and analyzed for ubiquitinated FLAG-SMAD7 by ubiquitin antibodies. A549 cells were transfected with FLAG-SMAD7, treated with TGFβ alone or in combination with oligonucleotides that knockdown Axin2, arkadia and RNF12 (D), treated with TGFβ alone or in combination with 14–22 Amide or siPKA-Cα (transfected) (E) or MKK4(WT)/MKK7(CA) (transfected) alone or in combination with 14–22 Amide or siPKA-Cα (transfected) (F), and whole cell lysates were immunoprecipitated FLAG antibodies and analyzed for ubiquitinated FLAG-SMAD7 using ubiquitin antibodies. (G) A549 cells were transfected with FLAG-SMAD7 and treated with TGFβ alone or in combination with 14–22 Amide or siPKA-Cα (transfected), immunoprecipitated with FLAG antibodies, and the immunoprecipitate was analyzed by a western blot. (H) A549 cells were treated with TGFβ and transfected with siAxin2, siArkadia and siRNF12 oligonucleotides and effects on cell migration were determined in a Boyden chamber assay. (I) The efficiency of siAxin2, siArkadia and siRNF12 on protein knockdown was determined by western blot analysis of whole cell lysates.
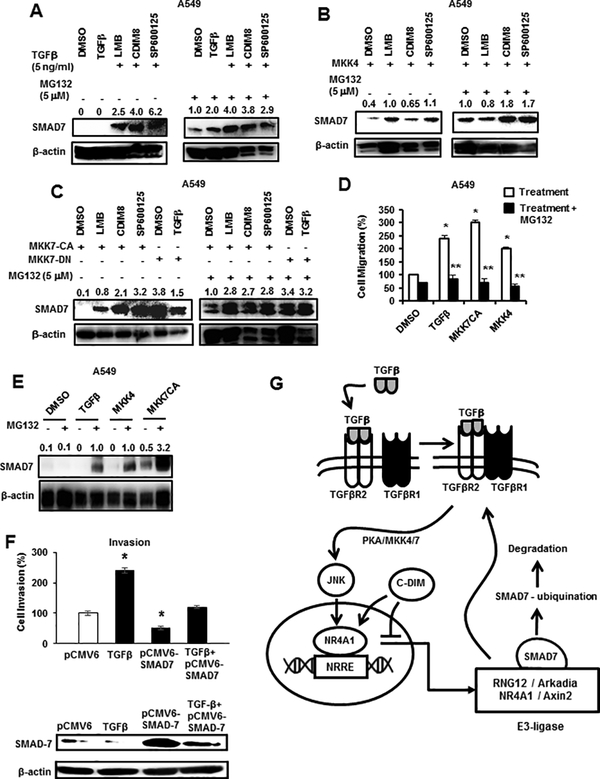
Since TGFβ and elements of the TGFβ signaling pathway and the ubiquitin ligase complex proteins (including NR4A1) play a role in SMAD7 expression and ubiquitination, we further examined their role in SMAD7 degradation. A549 cells were treated with TGFβ (Fig. 7A) or transfected with FLAG-MKK4 (Fig. 7B), FLAG-MKK7(CA) or FLAG-MKK7(DN) ± TGFβ (Fig. 7C) plus or minus the proteasome inhibitor MG132. Kinase activation alone decreased expression of SMAD7 which is consistent with activation of TGFβ signaling; however, cotreatment with LMB, CDIM8 or SP600125 ± MG132 prevented SMAD7 degradation and this was consistent with their resulting blockade of TGFβ-induced signaling and cell migration (Figs. 1B and 2B). The critical effects of SMAD7 degradation on TGFβ-induced migration are illustrated in Figure 7D in which TGFβ-, MKK7(CA)- and MKK4-induced migration of A549 cells is blocked by cotreatment with MG132 which increases SMAD7 levels due to inhibition of proteasome-dependent degradation of SMAD7 (Fig. 7E). We also confirmed the critical role of TGFβ-induced SMAD7 degradation by showing that TGFβ-induced invasion can be inhibited by overexpression of SMAD7 (Fig. 7F). Figure 7G illustrates the unique TGFβ-NR4A1-SMAD7 interactions in lung cancer cells and the role of PKA-MKK4/7-JNK in mediating the phosphorylation of NR4A1 and its nuclear export. Although TGFβ-induced nuclear export of NR4A1 and its role in degradation of SMAD7 are common in breast and lung cancer cells, there are significant cell context-dependent differences in TGFβ-induced kinase pathways and PKA-dependent induction of NR4A1 in lung vs. β-catenin/TCF/LEF-mediated induction of NR4A1 in breast cancer cells (31).
Figure 7.
TGFβ induces proteasome-dependent degradation of SMAD7 that is inhibited by NR4A1 ligand CDIM8 (DIM-C-pPhOH). A549 cells were treated with TGFβ (A) and MKK4(WT) (alone, transfected) (B) alone or in combination with MG132 and various agents. Whole cell lysates were analyzed for SMAD7 expression by western blots. (C) A549 cells were transfected with MKK7(CA) alone or in combination with MG132 and various agents and transfected with MKK7(DN) ± TGFβ, and whole cell lysates were analyzed for SMAD7 expression by western blots. A549 cells were treated with DMSO, TGFβ, transfected with MKK7(CA) and MKK4(WT) alone or in combination with MG132 and effects on cell migration (D) and SMAD7 expression (E) were determined in Boyden chamber and western blot assays, respectively. (F) Cells were treated with TGFβ alone or transfected with pCMV6 (empty vector), pCMV6-SMAD7 alone or in combination with TGFβ, and effects on A549 cell migration were determined; cell lysates from these treatment groups were also analyzed for SMAD7 expression by western blots. Results (D and F) are means ± SE for 3 separate determinations, and significantly (p<0.05) enhanced migration (*) and inhibition of this response (**) are indicated. (G) Summary of TGFβ-PKA-MKK4/7-JNK phosphorylation and nuclear export of NR4A1 and inhibition by CDIM8/NR4A1 antagonist. The NR4A1 band intensities relative to β-actin in the western blots (A-C, E) were determined.
DISCUSSION
In lung cancer cells, several reports demonstrate that TGFβ induces cell migration, invasion and EMT through modulation of multiple genes/pathways (10–19) and these pro-oncogenic functions of TGFβ have been observed in many other tumor types (32–36). Recent studies in breast cancer cells show that TGFβ-induced migration involves the orphan nuclear receptor NR4A1 which is part of an ubiquitin ligase complex required for proteasome-dependent degradation of SMAD7, an inhibitor of TGFβ-activated signaling (20,31). Studies in this laboratory previously showed the pro-oncogenic functions of NR4A1 in lung cancer cells and NR4A1 was overexpressed in tumors from lung cancer patients and inversely correlated with their survival (22). Based on these observations, we hypothesized that NR4A1 may also play a role in TGFβ-induced lung cancer migration/invasion and that this pathway can be inhibited by DIM-C-pPhOH/CDIM8, a compound that binds nuclear NR4A1 and acts as an NR4A1 antagonist in cancer cells (24).
We initially used three lung cancer cell lines as models and show that TGFβ-induced migration was blocked by knockdown of NR4A1 or treatment with CDIM8, LMB or the TGFβ receptor inhibitor Alk5i (Fig. 1). These data confirm that TGFβ-dependent activation of the TGFβ receptor is important for cell migration, and western blot analysis confirmed that the TGFβ-induced response requires nuclear export of NR4A1 which is blocked by LMB and CDIM8. Results of kinase inhibitor studies show that the JNK1 inhibitor SP600125 also blocked TGFβ-induced cell migration, nuclear export of NR4A1 (Fig. 2) and inhibitors of nuclear export (LMB and CDIM8), and JNK also inhibited phosphorylation of NR4A1 (Figs. 1B-1D and Fig. 2). These results are consistent with previous studies showing that selected apoptosis-inducing agents also induce phosphorylation-dependent nuclear export of NR4A1 through activation of JNK1 or other kinases (37–40). In addition, we also investigated both MKK4 and MKK7 which are upstream from JNK and demonstrate that overexpression of MKK4 or MKK7 recapitulated the effects observed with TGFβ in terms of enhanced cell migration and nuclear export of NR4A1 and inhibition of these responses by LMB, CDIM8 and SP600125 (Fig. 3 and Suppl. Figs. 1 and 2). Moreover, MKK7(DN) also inhibited MKK7 and TGFβ-induced responses and thus, linking the upstream effects of TGFβ with MKK4/7-mediated activation of JNK.
Our previous studies in breast cancer cells (31) demonstrated that TGFβ-dependent phosphorylation and nuclear export of NR4A1 was due to activation of MKK3/MKK6 and p38 but not MKK4/7 and JNK. Moreover in breast cancer cells, we observed that TGFβ induced expression of both β-catenin and NR4A1, and the mechanism of NR4A1 expression involved β-catenin/TGF/LEF interactions with the NR4A1 promoter (31). In contrast, we did not observe induction of β-catenin in lung cancer cells treated with TGFβ, whereas TGFβ-induced expression of NR4A1 protein (1.5- to 2-fold) (Figs. 1B-1D) and RNA (> 10-fold) (Fig. 4A). We identified upstream CRE and SRE sites in the NR4A1 promoter at −807 and −703 (Fig. 4C) that bind c-jun/ATF2 and Elk1/SRF which are among some of the genes induced by JNK1 and these were induced by TGFβ in A549 cells (Fig. 4E). Moreover, like the upstream kinases, knockdown of c-jun, ATF2, Elk1 and SRF inhibited induction and nuclear export of NR4A1 and migration of A549 cells treated with TGFβ (Fig. 4).
Previous studies report that induction of NR4A1 expression in multiple cell types is associated with PKA, cAMP or cAMP inducers (41–46) and cadmium induction of NR4A1 in A549 cells is both PKA- and MAPK-dependent (41). These reports, coupled with the identification of PKA-activated transcription factors interacting with the NR4A1 promoter (Fig. 4D), suggested that PKA may be a potential kinase downstream from TGFβ/TGFβ receptor in lung cancer cells. Moreover, there is prior evidence demonstrating that TGFβ/TGFβ receptor induces PKA (47–49). Data illustrated in Figure 5 confirm that TGFβ induces PKA activity and the PKA inhibitor 14–22 Amide or transfection with siPKA-Cα inhibits TGFβ-induced migration and NR4A1 expression, nuclear export of NR4A1 and transcription factors associated with induction of NR4A1. TGFβ also induces NR4A1 in fibroblasts through activation of a SMAD3/SMAD4/Sp1 complex bound to GC-rich sites in the NR4A1 promoter (50), thus illustrating cell context-dependent differences in regulating NR4A1 expression.
Thus, TGFβ activates the TGFβ receptor–PKA–MKK4/7–JNK1 pathway which in turn phosphorylates NR4A1 and subsequently undergoes nuclear export (Fig. 7G). Although TGFβ/kinase-dependent nuclear export of NR4A1 is necessary for A549 cell migration, this process also involves subsequent responses associated with extranuclear NR4A1 since TGFβ-induced A549 cell migration is also inhibited by LMB and CDIM8 (Fig. 1). Previous studies in breast cancer cells showed that phosphorylated NR4A1 was a necessary component of a RNF12/Arkadia/Axin2/SMAD7 complex that induced ubiquitination and proteasome-dependent degradation of SMAD7 which in turn activated TGFβ/TGFβ receptor signaling (31,36). Results illustrated in Figure 6 and Supplemental Figure 4 confirm that this same complex is also functional in A549 cells and is necessary for ubiquitination and subsequent degradation of SMAD7. Thus, another major difference between breast and lung cancer cells is TGFβ-dependent activation of p38 (breast) vs. PKA/JNK (lung) which is required for nuclear export of NR4A1 and subsequent activation of proteasome-dependent degradation of SMAD7. The importance of SMAD7 degradation in mediating TGFβ-induced A549 cell migration is also supported by results showing that overexpression of SMAD7 inhibits the TGFβ-induced effect (Fig. 7F). Figure 7G illustrates that the mechanism of TGFβ-induced migration is a cyclic rather than a linear process since inhibition is observed by TGFβ receptor inhibitors (Alk5i), kinase inhibitors, NR4A1 antagonists, nuclear export and proteasome inhibitors. Thus, effective treatment of TGFβ-induced lung cancer progression could involve a number of agents including the CDIM/NR4A1 antagonists which block not only TGFβ-induced migration but several other NR4A1-regulated pro-oncogenic genes/pathways in lung cancer cell lines (22).
Supplementary Material
Acknowledgements:
The financial assistance of the National Institutes of Health (P30-ES023512, S. Safe), Texas AgriLife Research (S. Safe) and Sid Kyle Chair endowment (S. Safe) is gratefully acknowledged.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of Potential Conflicts of Interest: There are no conflicts of interest to declare.
LITERATURE CITED
- 1.American Cancer Society. Cancer Facts and Figures 2017. Atlanta, GA: American Cancer Society 2017. [Google Scholar]
- 2.Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013; 11:645–53; quiz 53. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008; 359:1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014; 14:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson MR, Gazdar AF, Clarke BE. The pivotal role of pathology in the management of lung cancer. J Thorac Dis 2013; 5 Suppl 5:S463–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol 2010; 28:5311–20. [DOI] [PubMed] [Google Scholar]
- 7.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015; 4:36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giroux Leprieur E, Dumenil C, Julie C, Giraud V, Dumoulin J, Labrune S, et al. Immunotherapy revolutionises non-small-cell lung cancer therapy: Results, perspectives and new challenges. Eur J Cancer 2017; 78:16–23. [DOI] [PubMed] [Google Scholar]
- 10.Zu L, Xue Y, Wang J, Fu Y, Wang X, Xiao G, et al. The feedback loop between miR-124 and TGF-beta pathway plays a significant role in non-small cell lung cancer metastasis. Carcinogenesis 2016; 37:333–43. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura K, Seike M, Okano T, Matsuda K, Miyanaga A, Mizutani H, et al. MiR-134/487b/655 cluster regulates TGF-beta-induced epithelial-mesenchymal transition and drug resistance to gefitinib by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer Ther 2014; 13:444–53. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Yang T, Lei Z, Wang L, Yang H, Tong X, et al. RNF111/Arkadia is regulated by DNA methylation and affects TGF-beta/Smad signaling associated invasion in NSCLC cells. Lung Cancer 2015; 90:32–40. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Wang L, Zhao J, Chen Y, Lei Z, Liu X, et al. TGF-beta-activated SMAD3/4 complex transcriptionally upregulates N-cadherin expression in non-small cell lung cancer. Lung Cancer 2015; 87:249–57. [DOI] [PubMed] [Google Scholar]
- 14.Miao L, Wang Y, Xia H, Yao C, Cai H, Song Y. SPOCK1 is a novel transforming growth factor-beta target gene that regulates lung cancer cell epithelial-mesenchymal transition. Biochem Biophys Res Commun 2013; 440:792–7. [DOI] [PubMed] [Google Scholar]
- 15.Lin LC, Hsu SL, Wu CL, Hsueh CM. TGFbeta can stimulate the p(38)/beta-catenin/PPARgamma signaling pathway to promote the EMT, invasion and migration of non-small cell lung cancer (H460 cells). Clinical and Experimental Metastasis 2014; 31:881–95. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Chen X, Wang Y, Qu Z, Lu Q, Zhao J, et al. Notch3 is important for TGF-beta-induced epithelial-mesenchymal transition in non-small cell lung cancer bone metastasis by regulating ZEB-1. Cancer Gene Ther 2014; 21:364–72. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Lorton B, Gupta V, Shechter D. A TGFbeta-PRMT5-MEP50 axis regulates cancer cell invasion through histone H3 and H4 arginine methylation coupled transcriptional activation and repression. Oncogene 2017; 36:373–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mise N, Savai R, Yu H, Schwarz J, Kaminski N, Eickelberg O. Zyxin is a transforming growth factor-beta (TGF-beta)/Smad3 target gene that regulates lung cancer cell motility via integrin alpha5beta1. J Biol Chem 2012; 287:31393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong YC, Hsu SF, Wu CL, Li TM, Kao ST, Tsai FJ, et al. Transforming growth factor-beta1 increases cell migration and beta1 integrin up-regulation in human lung cancer cells. Lung Cancer 2009; 64:13–21. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F, Drabsch Y, Dekker TJ, de Vinuesa AG, Li Y, Hawinkels LJ, et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling. Nat Commun 2014; 5:3388. [DOI] [PubMed] [Google Scholar]
- 21.Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. NR4A1 antagonists inhibit β1-integrin-dependent breast cancer cell migration. Molecular and Cellular Biology 2016; 36:1383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SO, Andey T, Jin UH, Kim K, Singh M, Safe S. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene 2012; 31:3265–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SO, Jin UH, Kang JH, Kim SB, Guthrie AS, Sreevalsan S, et al. The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Molecular Cancer Research 2014; 12:527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SO, Li X, Hedrick E, Jin UH, Tjalkens RB, Backos DS, et al. Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Molecular Endocrinology 2014; 28:1729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy. Endocrine-Related Cancer 2015; 22:831–40. [DOI] [PubMed] [Google Scholar]
- 26.Hedrick E, Lee SO, Kim G, Abdelrahim M, Jin UH, Safe S, et al. Nuclear receptor 4A1 (NR4A1) as a drug target for renal cell adenocarcinoma. PloS One 2015; 10:e0128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacey A, Hedrick E, Li X, Patel K, Doddapaneni R, Singh M, et al. Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS). Oncotarget 2016; 7:31257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacey A, Rodrigues-Hoffman A, Safe S. PAX3-FOXO1A Expression in Rhabdomyosarcoma Is Driven by the Targetable Nuclear Receptor NR4A1. Cancer Research 2017; 77:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedrick E, Li X, Safe S. Penfluridol represses integrin expression in breast cancer through induction of reactive oxygen species and downregulation of Sp transcription factors. Molecular Cancer Therapeutics 2017; 16:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedrick E, Lee SO, Safe S. The nuclear orphan receptor NR4A1 regulates beta1-integrin expression in pancreatic and colon cancer cells and can be targeted by NR4A1 antagonists. Molecular Carcinogenesis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedrick E, Safe S. TGFbeta/NR4A1 Inducible Breast Cancer Cell Migration and Epithelial to Mesenchymal Transition is p38alpha (MAPK14) Dependent. Molecular and Cellular Biology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nature Reviews: Cancer 2010; 10:415–24. [DOI] [PubMed] [Google Scholar]
- 33.Wakefield LM, Hill CS. Beyond TGFbeta: roles of other TGFbeta superfamily members in cancer. Nature Reviews: Cancer 2013; 13:328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 2014; 7:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003; 113:685–700. [DOI] [PubMed] [Google Scholar]
- 36.Massague J TGFbeta in Cancer. Cell 2008; 134:215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Q, Liu S, Ye XF, Huang ZW, Su WJ. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis 2002; 23:1583–92. [DOI] [PubMed] [Google Scholar]
- 38.Holmes WF, Soprano DR, Soprano KJ. Early events in the induction of apoptosis in ovarian carcinoma cells by CD437: activation of the p38 MAP kinase signal pathway. Oncogene 2003; 22:6377–86. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, Zhao W, Xie G, Huang M, Hu M, Jiang X, et al. Induction of Nur77-dependent apoptotic pathway by a coumarin derivative through activation of JNK and p38 MAPK. Carcinogenesis 2014; 35:2660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han YH, Cao X, Lin B, Lin F, Kolluri SK, Stebbins J, et al. Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene 2006; 25:2974–86. [DOI] [PubMed] [Google Scholar]
- 41.Shin HJ, Lee BH, Yeo MG, Oh SH, Park JD, Park KK, et al. Induction of orphan nuclear receptor Nur77 gene expression and its role in cadmium-induced apoptosis in lung. Carcinogenesis 2004; 25:1467–75. [DOI] [PubMed] [Google Scholar]
- 42.Martin LJ, Boucher N, El-Asmar B, Tremblay JJ. cAMP-induced expression of the orphan nuclear receptor Nur77 in MA-10 Leydig cells involves a CaMKI pathway. Journal of Andrology 2009; 30:134–45. [DOI] [PubMed] [Google Scholar]
- 43.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. Journal of Biological Chemistry 2005; 280:29256–62. [DOI] [PubMed] [Google Scholar]
- 44.Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, et al. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Molecular Endocrinology 2002; 16:1638–51. [DOI] [PubMed] [Google Scholar]
- 45.Inaoka Y, Yazawa T, Uesaka M, Mizutani T, Yamada K, Miyamoto K. Regulation of NGFI-B/Nur77 gene expression in the rat ovary and in leydig tumor cells MA-10. Molecular Reproduction and Development 2008; 75:931–9. [DOI] [PubMed] [Google Scholar]
- 46.Hamid T, Malik MT, Millar RP, Kakar SS. Protein kinase A serves as a primary pathway in activation of Nur77 expression by gonadotropin-releasing hormone in the LbetaT2 mouse pituitary gonadotroph tumor cell line. International Journal of Oncology 2008; 33:1055–64. [PubMed] [Google Scholar]
- 47.Yang Y, Pan X, Lei W, Wang J, Shi J, Li F, et al. Regulation of transforming growth factor-beta 1-induced apoptosis and epithelial-to-mesenchymal transition by protein kinase A and signal transducers and activators of transcription 3. Cancer Res 2006; 66:8617–24. [DOI] [PubMed] [Google Scholar]
- 48.Yang H, Li G, Wu JJ, Wang L, Uhler M, Simeone DM. Protein kinase A modulates transforming growth factor-beta signaling through a direct interaction with Smad4 protein. J Biol Chem 2013; 288:8737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Lee CJ, Zhang L, Sans MD, Simeone DM. Regulation of transforming growth factor beta-induced responses by protein kinase A in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 2008; 295:G170–G78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palumbo-Zerr K, Zerr P, Distler A, Fliehr J, Mancuso R, Huang J, et al. Orphan nuclear receptor NR4A1 regulates transforming growth factor-beta signaling and fibrosis. Nature Medicine 2015; 21:150–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.