Abstract
Experimental measurements of cellular mechanical properties have shown large variability in whole-cell mechanical properties between cells from a single population. This heterogeneity has been observed in many cell populations and with several measurement techniques but the sources are not yet fully understood. Cell mechanical properties are directly related to the composition and organization of the cytoskeleton, which is physically coupled to neighboring cells through adherens junctions and to underlying matrix through focal adhesion complexes. This high level of heterogeneity may be attributed to varying cellular interactions throughout the sample. We tested the effect of cell-cell and cell-matrix interactions on the mechanical properties of vascular smooth muscle cells (VSMCs) in culture by using antibodies to block N-cadherin and integrin β1 interactions. VSMCs were cultured on substrates of varying stiffness with and without tension. Under each of these conditions, cellular mechanical properties were characterized by performing atomic force microscopy (AFM) and cellular structure was analyzed through immunofluorescence imaging. As expected, VSMC mechanical properties were greatly affected by the underlying culture substrate and applied tension. Interestingly, the cell-to-cell variation in mechanical properties within each sample decreased significantly in the antibody conditions. Thus, the cells grown with blocking antibodies were more homogeneous in their mechanical properties on both glass and soft substrates. This suggests that diversified adhesion binding between cells and the ECM is responsible for a significant amount of mechanical heterogeneity that is observed in 2D cell culture studies.
Keywords: Vascular Smooth Muscle Cells, Atomic Force Microscopy, AFM, Cell Mechanics
1. Introduction
All cells in the body are subjected to mechanical stimuli in their surrounding environment. These stimuli are transmitted through the cell’s intracellular signal transduction pathways to the nucleus, ultimately resulting in altered growth, differentiation, migration, contractility, and apoptosis (Boudreau and Bissell, 1998; Huang and Ingber, 1999; Maniotis et al., 1997; Schwartz and Ginsberg, 2002; Tang and Gerlach, 2017). Deviation in cell structural and mechanical properties will alter the way cells respond to forces, which may result in changes in cell function and which may possibly lead to disease (Costa, 2004; Ingber, 2003; Kai et al., 2016; Zhang et al., 2018). Therefore, the study of cellular mechanics is important for understanding cell behavior and disease progression. Studies so far have noted high level of heterogeneity in whole-cell mechanical properties between cells from a single population (Charras and Horton, 2002; Dulinska-Molak et al., 2014; Hemmer et al., 2008; Jaasma et al., 2006b; Jones et al., 1999; Mahaffy et al., 2000). This heterogeneity has been observed in many cell populations and with several measurement techniques. In particular, the coefficient of variation (COV = standard deviation/mean) values for cellular mechanical parameters within a single population are in the range of 43–103% in atomic force microscopy (AFM) studies and 30–128% in micropipette aspiration studies (Jaasma et al., 2006a). This variability cannot be credited solely to the measurement techniques, as their repeatability has been statistically confirmed (Jaasma et al., 2006b).
Vascular Smooth Muscle Cells (VSMCs) are one of the main constituents of medial layer of blood vessels, embedded in a network of collagen (types I, III, & V), elastin, and proteoglycans. During vascular development, VSMCs differentiate from a synthetic phenotype to a contractile phenotype. Synthetic VSMCs are highly migratory, undergo rapid cell proliferation, exhibit very high rates of extracellular matrix (ECM) synthesis, and cannot contract (Owens et al., 2004). Contractile VSMCs assist in contraction; they are largely non-migratory, have a low proliferation rate, and exhibit very low rates of ECM synthesis (Owens et al., 2004). VSMCs are capable of major changes in phenotype in response to changes in local environmental cues including growth factors/inhibitors, mechanical influences, cell-cell and cell-matrix interaction, and various inflammatory mediators. The phenotype depends on the complex interaction of the environmental cues and may range from completely synthetic to completely contractile. In turn, mechanical properties of VSMCs depend on their phenotype, which can change dramatically in development and with varying environmental cues (Hemmer et al., 2009; Na et al., 2007; Sun et al., 2008). Several studies have reported that VSMCs stiffen in response to stretching (Na et al., 2007). Sun et al. applied forces to specific ECM adhesion sites on VSMCs and discovered that, mechanical force, when applies to integrin-fibronectin binding sites, induced an actin-dependent myogenic like event (contraction) (Sun et al., 2008).
It is important to understand the effect of cell-cell and cell-matrix interactions on cell mechanics. In addition, elucidating the sources of heterogeneity and variability in cell mechanics in vitro could help us better understand how these in vitro results can (or cannot) be interpreted in the context of cells in more in vivo-like conditions. Therefore, to assess directly how cell-cell and cell-matrix interactions affect VSMC mechanical properties and mechanical heterogeneity in vitro, we investigated the effect of blocking specific cell-cell and cell-matrix interactions of VSMCs in a variety of cell culture conditions. Cell anchor the underlying extracellular matrix scaffolds through focal adhesion complexes and to neighboring cells through adherens junctions (Boudreau and Bissell, 1998). Through these interactions, the tensed cytoskeletal filaments within the cell are physically coupled to the matrix and cells in their environment. Focal adhesion complexes are comprised of clusters of transmembrane integrins. Integrin β1 mediates cellular interactions with collagen through α2β1 and fibronectin through α5β1 (Huang and Ingber, 1999). N-cadherin based junctions’ help to physically couple to cytoskeleton of one cell to that of its neighbor. The main objective of this study was to determine if culturing cells under N-cadherin and integrin β1 blocking conditions affected the cells’ mechanical properties and if those conditions also resulted in a more mechanically homogeneous population. In addition, to understand how these interactions may change during different culture conditions, the cells were cultured on soft gel substrates and under cyclic physiological-like tension conditions as well as standard glass substrates. The results from these studies provide insights from a basic science perspective about the impact of the cellular microenvironment on cell behavior.
2. Experimental Materials and Methods
2.1. Cell Isolation and Sample Preparation:
Aortic Vascular Smooth Muscle Cells (VSMC) were isolated from 12 week Sprague-Dawley rats following the protocol by Winn.et.al (Winn, 2009), approved by the Clemson University Animal Care and Use Committee. Cells were cultured in T75 flasks under standard conditions (37°C, 5% CO2) in T75 cell culture flasks with standard media containing Dulbecco’s Modified Eagle’s Medium (Fisher) supplemented with 10% Fetal Bovine Serum (Sigma-Aldrich) and 1% antibiotic-antimycotic solution (Sigma-Aldrich). Cells used in the experiments were from passages 5 through 8.
2.2. Preparation of substrates:
Glass coverslips (12mm) were coated with a thin layer of 0.25 mg/ml Rat-tail collagen I (BD Biosciences, Bedford, MA, USA) or 5-mg/ml fibronectin from human plasma (BD Bioscience) overnight and cells were platted at the density of 20,000 cells/cm2. For cells on hydrogels, soft collagen-coated polyacrylamide gels were made with elastic moduli (E) of 10 kPa, 25 kPa, and 75 kPa by varying the concentration of bisacrylamide following the protocol by Tse.et.al. and Wang.et.al (Tse and Engler, 2010; Wang and Pelham, 1998). Gel moduli were confirmed with AFM indentation (see supplemental information). The stiffness range mentioned was used as it approximately replicates the range VSMCs would encounter in vivo (Oie et al., 2009).
2.3. Applied cyclic strain culture
Cells were cultured on collagen-coated silicone elastomer membrane (BioFlex culture plates, Flexcell International, Hillsborough, NC, USA) and then exposed to physiologically relevant cyclic strain of 0–4% with the frequency of 1 Hz (Acampora et al., 2007; Acampora, 2008). Cells were kept under static conditions for 48 hours, followed by cyclic strain for 3 days using Flexcell FX-3000 tension system bioreactor. Cells were subjected to 0–4% cyclic strains at 0.1 Hz for 30 minutes, followed by 0.5 Hz for 30 minutes, and finally at 1 Hz for 3 days. This strain range was selected since it is physiologically relevant level for VSMCs in a healthy blood vessel (Giddens et al., 1993).
2.2. Antibody-blocking culture
To perform blocking studies, 5 different media conditions were used: (i) regular VSMC media, (ii) VSMC media with 50 μg/ml anti-integrin β1 antibody (Fisher), (iii) VSMC media with 50 μg/ml anti-N-cadherin antibody (Sigma-Aldrich), (iv) VSMC media with 50 μg/ml of anti-integrin β1 antibody and 50 μg/ml of anti-N-cadherin antibody (both antibodies), and (v) VSMC media with 50 μg/ml of non-immune IgG (Sigma-Aldrich). Non-immune IgG was used as a negative control as it was not expected to affect cellular interactions and mechanical properties. The antibody concentration of 50 μg/ml was chosen as it has been shown to be effective blocking concentration in other studies (Mendrick and Kelly, 1993; Sun et al., 2005; Yiin et al., 2009). Cells were exposed to different media conditions as soon as they were seeded on the substrate and were maintained in culture for a period of 5 days after which AFM nanoindentation studies were performed to study mechanical properties. This culture period was selected as VSMCs have been shown previously to stiffen in the first 3–5 days of culture (Hemmer et al., 2008, Hemmer et al., 2009). After this initial stiffening period, cell mechanical properties remain stable for 7–10 days (Hemmer et al. 2009, Deitch et al. 2012).
2.3. AFM Nanoindentation:
VSMCs from each sample were mechanically probed using AFM under contact mode in liquid (cell culture media) using Asylum Research MFP-3D AFM (Asylum Research, Santa Barbara, CA, USA)(Thomas et al., 2013). A 5 μm diameter borosilicate spherical tipped probe on a silicon-nitride cantilever with a spring constant of ~0.12 N/m (Novascan, Ames, IA, USA) was used to mechanically probe individual cells. Cells remained on the substrates throughout the study and warm (37°C) media was exchanged every thirty minutes to maintain the culture temperature. The AFM optical microscope was used to position tip of the cantilever over the center of a cell (avoiding nucleus) before data was collected. Each cell was indented five times to approximately 1 μm depth at the speed of 1 μm/sec (5 force curves/cell). Each cell was also subjected to two-1 μm step indentation and 60 second hold experiments (2 stress relaxation curves). For each condition, 20 cells were tested.
2.4. Immunofluorescence
Day five VSMCs were fixed and stained for nuclei, filamentous actin, and microtubules. Anti-N-cadherin and anti-integrin β1 samples were also stained to confirm antibody blocking. For these stains, nuclei and filamentous actin for N-cadherin samples or nuclei and plasma membrane for integrin β1 samples were also stained as reference of cellular structure. For polyacrylamide gel samples, since the cells were on softer substrates, they were not mounted on microscope slides. Instead they were kept in their well plates, covered in PBS, and stored in the dark after staining and during imaging. All the samples were viewed using an Olympus IX81 spinning disk confocal microscope (Olympus, Tokyo, Japan) and digital images were collected and processed using MetaMorph Image Analysis software (Molecular Devices, Sunnyvale, CA, USA).
2.5. Data and image analysis
AFM data was analyzed using custom MATLAB scripts (MathWorks, Natick, MA, USA). The apparent elastic modulus (E) of each cell was calculated by fitting Hertz model to first 300 nm of data (Dimitriadis et al., 2002; Guz et al., 2014; Hertz, 1882; Q. Li et al., 2008; Liu et al., 2013; Rebelo et al., 2013). The indentation depth was chosen as approximately 10% of the average cell height as Hertz model only remains accurate within 10% strain range. The stress relaxation (SR) data were normalized to the peak force and fit to the Quasilinear Viscoelastic (QLV) model reduced relaxation function (Deitch et al., 2012; Radmacher et al., 1993; Tripathy and Berger, 2012). The percent relaxation during 60-second hold was calculated for each stress relaxation curve (E. Darling et al., 2006; E. M. Darling et al., 2007; Deitch et al., 2012). The Immunofluorescence images were analyzed to calculate average cell area and perimeter using ImageJ (C. A Schneider. et al., 2012). To determine how round the cells were, the circularity of each cell was calculated. The circularity is given by the following equation:
Thus, the circularity of a perfect circle is 1.
Student’s t-test was used to determine statistical significance (p<0.05). Cell-to-cell and repeated point coefficients of variation (COV) were calculated to access the variation within each sample as a measure of cell-to-cell variation and within each cell-loading session as a measure of data repeatability on a single point of indentation.
3. Results
3.1. Antibody-blocking studies on glass
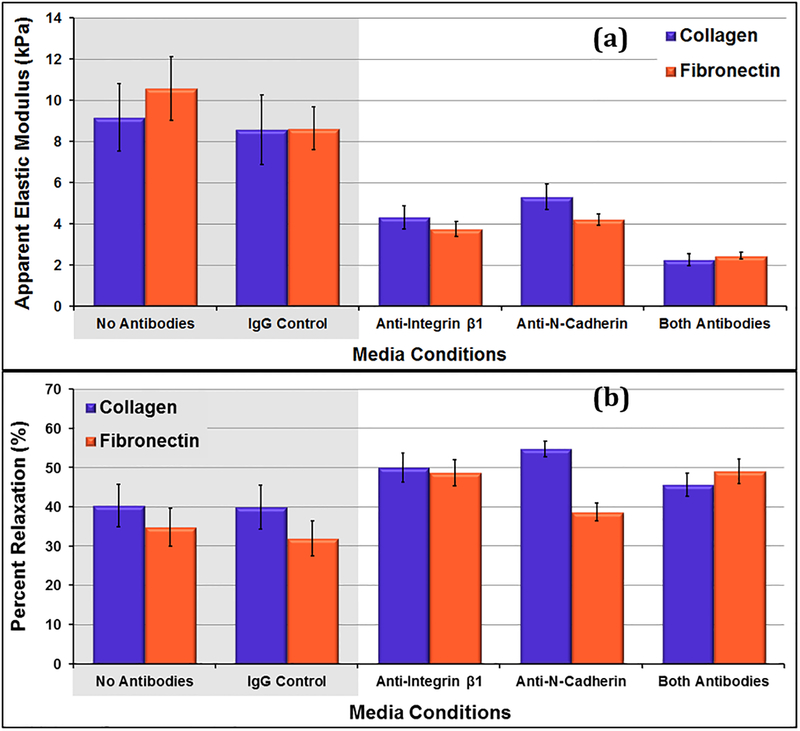
Cells under control conditions (no antibodies, IgG control) had elastic moduli (E) ranging from 8.6kPa to 10.6kPa. For both the collagen and fibronectin samples, there was no significant difference between the cells cultured in standard media or standard media with non-immune IgG antibodies (p ≥ 0.39). The cells under test conditions had E ranging from 2.3kPa to 5.3kPa (figure 1 (a)). Within the antibody samples, the cells cultured with both antibodies were the softest. This difference was considered statistically significant for cells on both collagen and fibronectin (p≤0.003). Within each sample type, there was no significant difference between the elastic moduli measures on collagen and those on fibronectin. The repeated point COV measures (Table 1) were much lower than the cell-to-cell COV measures, indicating that variations in the measurement technique could not account for the cell-to-cell variations that were observed.
Figure 1:
(a) Apparent elastic moduli of day 5 VSMCs on collagen and fibronectin with different media conditions. All test samples exhibit significantly lower elastic modulus compared to controls (p ≤ 0.025) except for anti-N-cadherin samples on collagen (p = 0.070). (b) Percent relaxations during 60 second hold for day 5 VSMCs on collagen and fibronectin with different media conditions. The Anti-N-Cadherin cells grown on collagen relaxed more than the control cells grown on collage (p = 0.012) Data presented as mean ± standard error.
Table 1:
VSMC cell-to-cell repeated point COV for elastic modulus and percent relaxation measures (average of measures for cells on collagen and fibronectin matrices).
| Sample | Average Cell-to-Cell COV (%) | Average Repeated Point COV (%) | ||
|---|---|---|---|---|
| Elastic Modulus | No Antibodies | 72.7 | Mean = 71.7 | 17.5 |
| IgG Control | 70.7 | 7.0 | ||
| Anti-Integrin β1 | 49.7 | Mean = 44.8 | 12.5 | |
| Anti-N-Cadherin | 41.6 | 13.6 | ||
| Both Antibodies | 43.2 | 13.4 | ||
| Percent Relax | No Antibodies | 60.9 | Mean = 61.9 | 6.0 |
| IgG Control | 62.8 | 11.9 | ||
| Anti-Integrin β1 | 31.7 | Mean = 27.2 | 5.3 | |
| Anti-N-Cadherin | 21.3 | 6.8 | ||
| Both Antibodies | 28.5 | 8.3 | ||
For both the collagen and fibronectin samples, there was no significant difference in the percent relaxation measures (PRM) of control samples (p≥0.66) (figure 1 (b)). Overall, the antibody media conditions promoted cells that relaxed more during the 60 second hold, although this difference was only statistically significant between the cells grown in the presence of N-cadherin antibodies and the control cells grown on collagen (p=0.012).
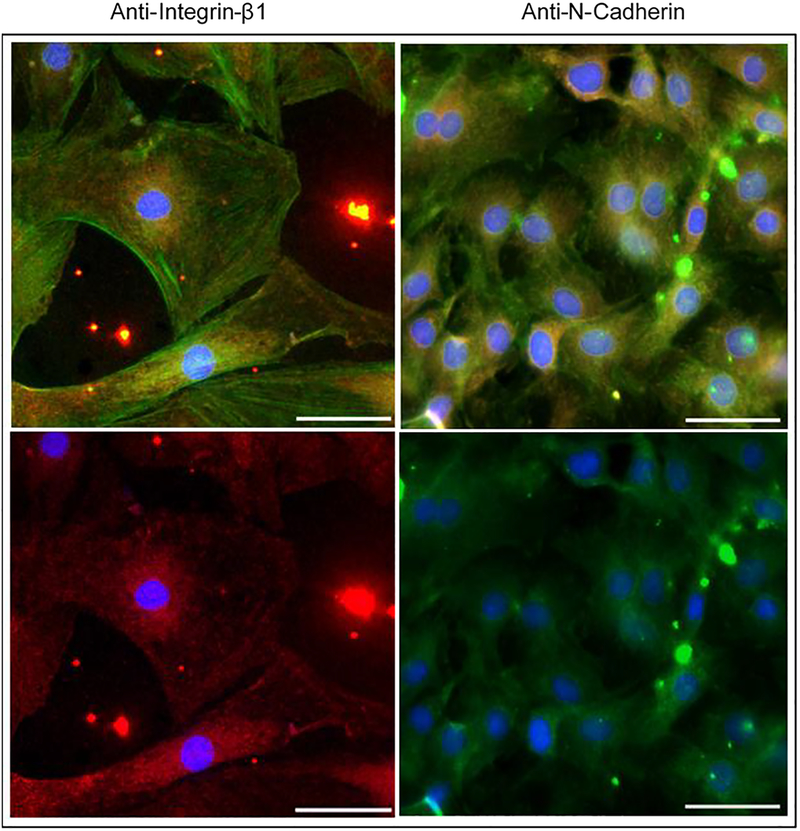
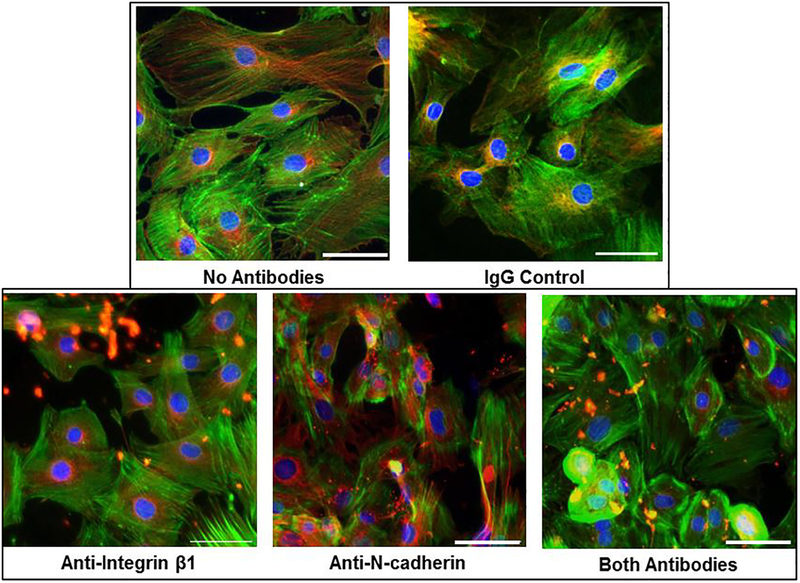
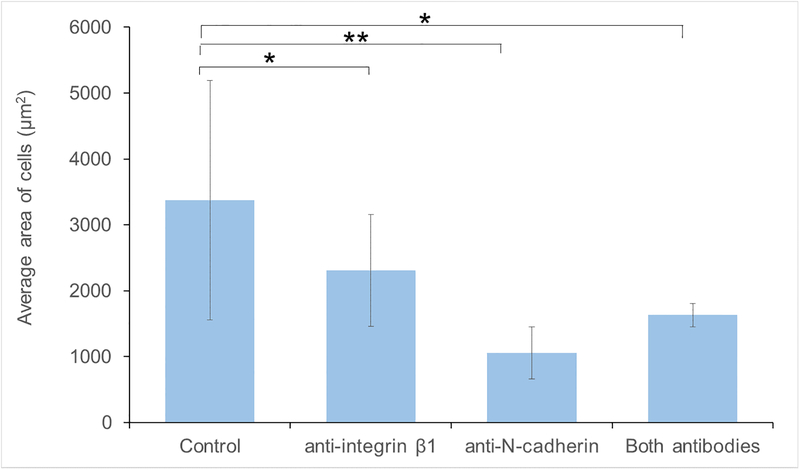
Immunofluorescence microscopy was used to confirm N-cadherin and integrin β1 blocking using antibodies present in the media during the experiments (figure 2). Stains for nuclei, microtubules, and actin highlighted differences in cell morphology among the samples with different media conditions in this study (figure 3). The VSMCs in the control samples were highly variable in size and generally exhibited more cell extensions and cell-cell connections. A continuum of phenotypes, from contractile to synthetic, was present. VSMCs in the blocking antibody conditions generally took on a more rounded. The area and perimeter of the cells were calculated from the images (N = 7–13 per group). Cells in test antibody conditions were found to be statistically smaller in area (figure 4) than those in the control media (p<0.05). The cells in the anti-N-cadherin media were also statistically smaller (p = 0.003) than the cells in the anti-integrin-β1 conditions. While the circularity of the cells in the blocking antibody conditions were on average closer to 1 than those in the control, these differences were not statistically significant (p = 0.07).
Figure 2:
Confirmation of antibody blocking in day 5 VSMCs. Cells with anti-integrin β1 media conditions (left) are stained for nuclei (blue), filamentous actin (red), and the integrin β1 primary antibody (red). Cells with anti-N-cadherin media conditions (right) are stained for nuclei (blue), plasma membrane (red), and the N-cadherin primary antibody (green). Top images show all stains and bottom images leave out the actin/plasma membrane stains to highlight N-cadherin and integrin β1 staining (Scale bar=50μm)
Figure 3:
Representative immunofluorescence images of day 5 VSMCs with different media conditions. Cells are stained for nuclei (blue), filamentous actin (green), and microtubules (red). Scale bar = 50μm).
Figure 4:
Average area of the cells grown on collagen in different media conditions. The cells grown in the presences of the blocking antibody conditions were significantly smaller than those in control media conditions (* p<0.05, ** p<0.01)
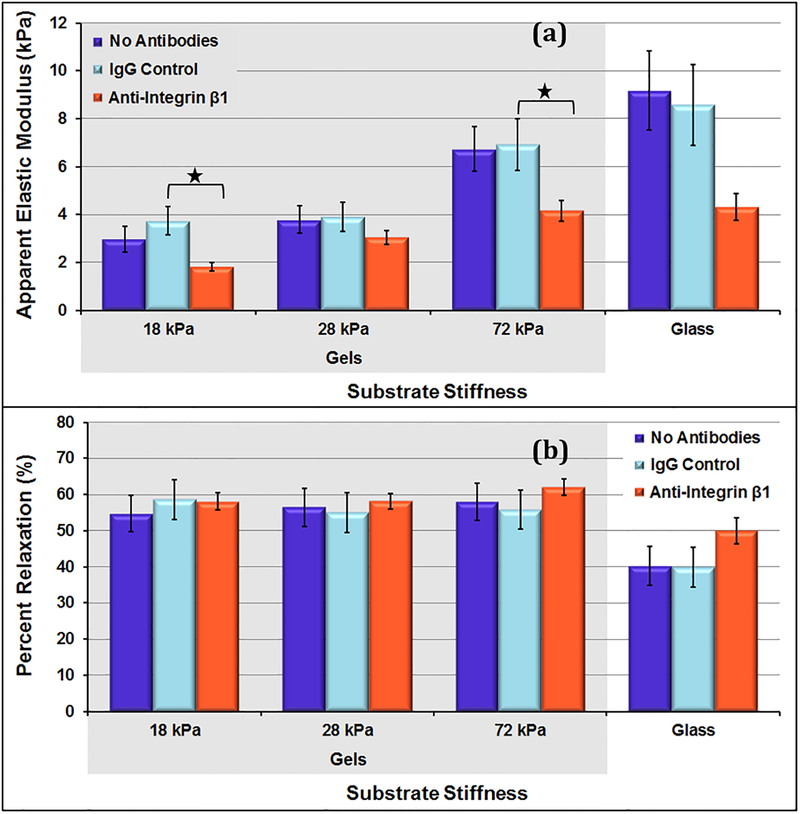
3.2. Antibody-blocking studies on polyacrylamide gels of varying stiffness
Overall, the VSMCs on the gels had E in the range of 1.8 kPa – 6.9 kPa, while the VSMCs on the stiffer glass substrates had E in the range of 4.3 kPa – 9.2 kPa (figure 5). For all sample types, cellular elastic moduli measures increased with increasing substrate stiffness. Overall, the VSMCs on gels tend to relax more during the 60-second hold compared to ones on stiffer glass slides. For cells grown on hydrogels, there were no significant differences in relaxation behavior among different gels or across different media conditions. The average cell-to-cell percent relaxation COV for the control conditions was 42.0%.
Figure 5:
(a) Apparent elastic moduli of day 5 VSMCs on substrates of varying stiffness. For control (no antibody) conditions, cellular stiffness increased significantly from the 18 kPa and 28 kPa gel samples to the 72 kPa gel and glass slide samples. (b) Percent relaxation during 60 second hold for day 5 VSMCs on substrates of varying stiffness. On each gel, there were no significant differences among different gel stiffness’s or across different media conditions. Data presented as mean ± standard error; * p<0.05.
This amount of variation is lower than the variation that was found in percent relaxation measures for control samples on glass slides. This suggests that there might be less variation among the cellular viscoelastic properties on a softer gel substrate.
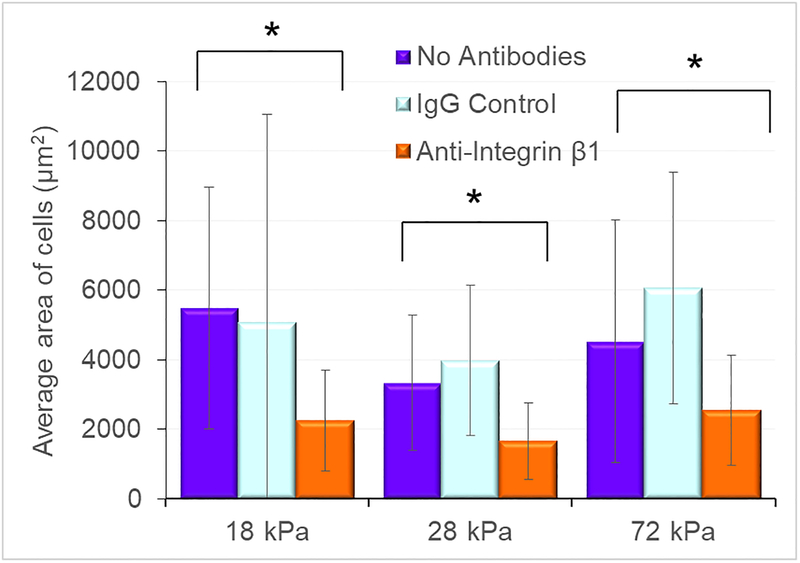
For all the hydrogel samples, nuclei, actin, and microtubules were stained to compare VSMC structure and samples (figure 6). No clear differences were observed between cells on each of the gels. The cells on all gels with anti-integrin β1 conditions were uniformly smaller (figure 7, p < 0.01, N = 20–25 for each condition) and did not exhibit the extensive cellular extensions of the cells grown on glass substrates. The circularity parameter was not statistically different between the anti-integrin-β1 condition and the matching control substrate condition (p >0.06).
Figure 6:
Representative immunofluorescence images of day 5 VSMCs on each gel with different media conditions (scale bar = 200 μm). Cells are stained for nuclei (blue), filamentous actin (green), and microtubules (red).
Figure 7:
Average area of the cells grown on soft substrates in different media conditions. The cells grown in the presence of anti-integrin β1 antibodies were significantly smaller than those in cultured in the control conditions (* p < 0.05)
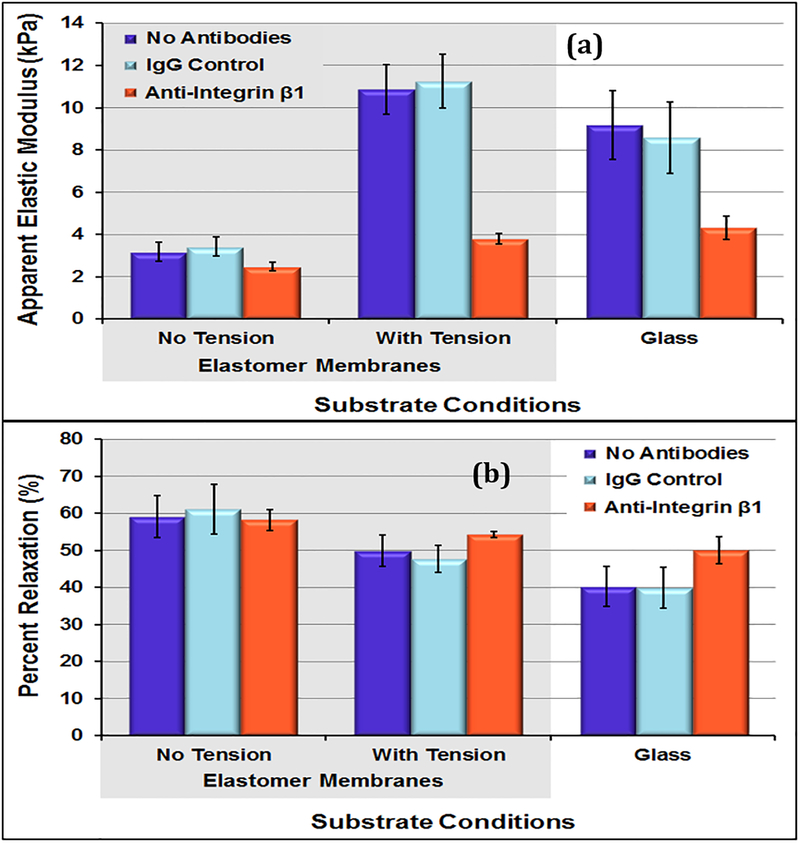
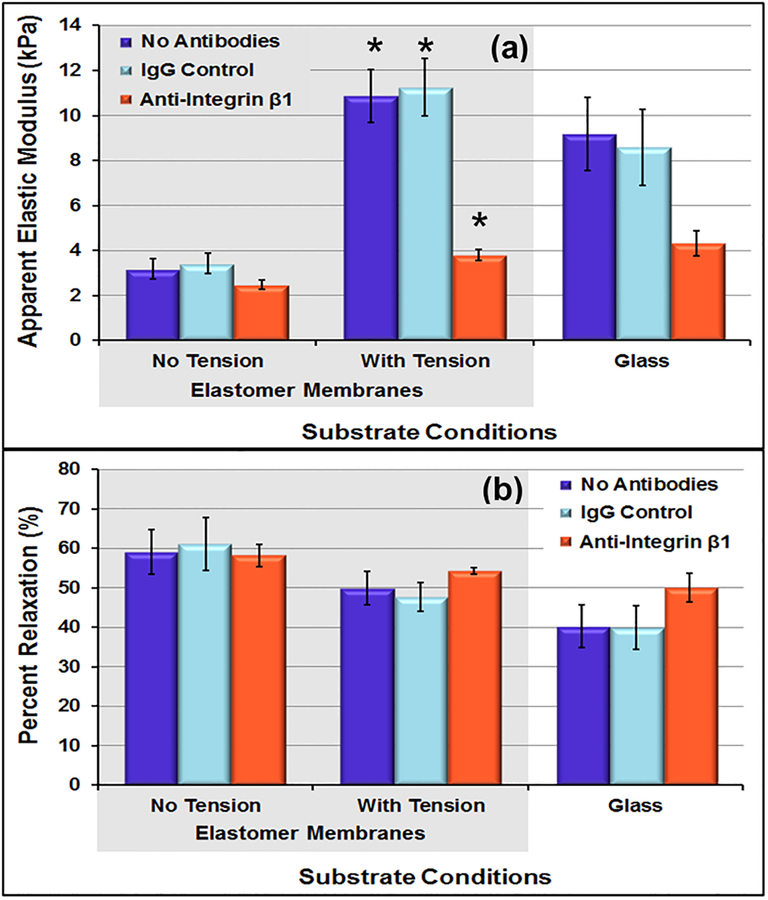
3.3. Antibody-blocking on cells under tension conditions.
For the cells on elastomer membrane, the cells without tension conditions were significantly softer than those that underwent cyclic tension and the ones on glass from previous study (p = 0.07). The average cell-to-cell elastic modulus COV for the control conditions was 70.2%, almost identical to the variations found in previous studies (70.3%, 71.7%). As observed on glass, even on hydrogel samples, anti-integrin β1 samples showed decrease in the level of variation. For control samples under tension conditions, the level of variation decreased to 49.5%. Within the tension samples, the cell-to-cell elastic moduli COV measure decreased to 28.8% for cells with anti-integrin β1 conditions. The repeated point COV measures were low and consistent with the experiments on glass substrates.
For samples on elastomer membrane, the mean apparent elastic moduli measures of the cells with tension conditions remained low for the anti-integrin β1 sample but increased dramatically for the control samples (figure 6). The cells without tension conditions exhibited the largest percent relaxation during 60-second hold (~60%). Also, the cells subjected to cyclic tension conditions relaxed less during hold (~50%) (p<0.01).
4. Discussion and Conclusion
In this study, VSMC’s interactions with its microenvironment were limited by interrupting cell-cell and cell-matrix interactions through antibody blocking. Cell-cell and cell-matrix interactions have been blocked or inhibited for various purposes in previous research. Specifically, N-cadherin and integrin β1 interactions have been studied for their role in cellular proliferation, migration, differentiation, adhesion, disease progression, and apoptosis (Cheng et al., 2000; Hermiston and Gordon, 1995; Koutsouki et al., 2005; G. Li et al., 2001; McNally and Anderson, 2002; Steinberg and McNutt, 1999; Uglow et al., 2003). This study investigates the effects of N-cadherin and integrin β1 mediated interactions on vascular smooth muscle cell mechanical properties and heterogeneity.
Our results showed that both measures of cellular mechanical properties (elastic modulus and percent relaxation) changed under the antibody blocking conditions. The values obtained on glass were fairly uniform for antibody conditions, suggesting that integrin β1 and N-cadherin play an equally important role in determining cellular mechanical properties. This finding is similar to findings in another study where the resistance to apoptosis provided by cell-cell contacts was found to be of a similar magnitude to that provided by cell-matrix contacts (Koutsouki et al., 2005). Significant differences were observed in both elastic moduli and percent relaxation measures for VSMCs cultured on soft polyacrylamide hydrogels, in comparison to the stiffer glass slides. For all gel samples, cellular elastic moduli increased with increasing stiffness. On the substrates: 18 kPa gel, 28 kPa gel, 72 kPa gel, and glass, the average cellular elastic moduli for control samples were 3.4 kPa, 3.8 kPa, 6.8 kPa, and 8.9 kPa respectively. This is consistent with prior studies with other cell types. In a study on fibroblasts, Solon et al. found that cellular elastic moduli were 4.5 kPa on a 10 kPa, 5 kPa on a 20 kPa gel, and 7 kPa on glass (Solon et al., 2007). They found that elastic moduli measurements did not increase significantly on gels with stiffness’s between 20 kPa and glass.
Immunofluorescence staining provided a measure of cellular phenotype within each of the samples. Revealing that the cells in the presence of blocking antibodies were significantly smaller. They appeared somewhat more rounded and less contractile in appearance. However, it should be noted that the changes in circularity parameter were not statistically significant; this may be in part because of the high variability in cell circularity observed in the control condition cells. This is consistent with previous studies that showed that, on day 5, synthetic VSMCs are softer than contractile VSMCs (Hemmer et al., 2009). Additionally, decreased cell spreading has been correlated to reduced cellular stiffness (Domke et al., 2000). A less complex actin network (characteristic of synthetic phenotypes) has also been shown to cause increase in relaxation percentage in stress relaxation experiments (Hemmer et al., 2009). The lack of changes across gel samples with different stiffness was observed previously, as Solon at al. did not see any changes in cell morphology/spreading on substrates over 10 kPa (Solon et al., 2007). The cellular extensions that we observed are typical of cells grown on gels before confluency is reached (Yeung et al., 2005). To better analyze the correlation between cell shape and modulus, it would be ideal to compare each individual cell’s size and morphology to its own modulus. Unfortunately, we were not able to get this type of single cell correlation with our experimental setup. Thus, this study was limited by analyzing the average modulus and cell shape in each sample.
The level of cell-to-cell variation in mechanical properties within each sample decreased from the control conditions to the antibody conditions. Thus, the cells with blocking antibodies were more homogeneous in their mechanical properties on both glass and gels. The cell size and morphology analysis also indicated that the cells in the blocking conditions were more homogeneous in size and shape than the control. This suggests that diversified adhesion binding between neighboring cells and ECM components throughout a cellular population is responsible for a significant amount of heterogeneity that is observed in 2D cell culture studies.
Elastic moduli of VSMCs grown on elastomeric membranes without tension were significantly lower than the elastic moduli of VSMCs grown on glass slides but were similar to those grown on the 28kPa gels. This is to be expected as the elastomeric membranes are softer substrate have properties closer to the polyacrylamide gels than the glass. The tension conditions resulted in a significant increase in cellular elastic moduli measures in control samples; cells grown under tension were stiffer than those grown without tension on elastomeric membranes or glass. Within the tension sample, the average elastic modulus for anti-integrin β1 remained very low (3.8 kPa), indicating that these cells were limited in their mechanotransduction abilities. During stress-relaxation testing, the cells relaxed the most on the static sample, followed by the “with tension” sample, and finally the glass sample. Immunofluorescence imaging showed reduction in size of the cells from static to cyclic tension samples (p< 0.05). In a previous study on VSMCs under the same level of cyclic, cells from the cyclic tension group exhibited significantly lower cell area compared to controls (Acampora et al., 2007) similar to what was observed in this study. The cell-to-cell measures decreased from the static controls to the tension conditions, indicating that the cyclic tension conditions promoted a more mechanically homogenous sample. Within the tension samples, the cellular mechanics heterogeneity further decreased under anti-integrin β1 conditions, indicating that blocking cell-matrix interactions resulted in a more mechanically homogeneous cell population under tension conditions.
Integrin-β1 was the focus of this study as other studies have indicated that this had significant effects on the cell mechanical properties (Sun et al., 2005). Our cells were still able to attach and form stress fibers when in the presence of the antibodies. This could be because the antibody concentration was not high enough to completely block all the β1. Another possibility is that the cells were still able to use the integrin to bind to the matrix despite the presence of antibodies. Sun et al. showed that this concentration of antibody does destabilize α5β1-integrin binding to fibronectin. However, in their experiments the VSMCs were still able to bind to fibronectin but the probability of binding was greatly reduced and had reduced rupture force as measured with AFM (Sun et al., 2005). If the binding probability and binding forces of the integrin-fibronectin interaction were similarly reduced here, this may explain why the cells in our tension experiments stayed much softer in the presence of the integrin antibody than in the control conditions. With less effective cell to matrix binding, the coupling of the matrix and stress fibers, and thus the mechanotransduction mechanisms, would also be reduced.
The influence of N-cadherin mediated cell-cell interactions and integrin β1 mediated cell-matrix interactions on cellular mechanics was assessed for the first time in this study. Blocking these interactions individually and in combination resulted in reduced cellular elastic moduli measures (less stiff) and increased cellular percent relaxation measures (more viscous). Both softer polyacrylamide gel substrates and application of cyclic tension resulted in changes to cellular phenotype and mechanical properties. The results from studies done on physiologically relevant conditions suggest that VSMCs most likely exhibit heterogeneous mechanical properties. These results provide researchers with a greater understanding of the role of cellular adhesions in regulating whole cell mechanical properties. Within these measurements, it was found that cells exhibited more homogeneous mechanical properties when their cell-cell and cell-matrix interactions were limited. This suggests that varying cellular adhesions throughout a sample is responsible, at least in part, for the high level of heterogeneity that is commonly observed in cellular mechanical properties.
This study is the first to report a decrease in cellular mechanical heterogeneity within a sample population by limiting cellular interactions in culture. Our results suggest that varying cell-cell and cell-matrix interactions are partly responsible for high level of heterogeneity that is commonly observed in 2D cell culture studies.
Figure 8:
(a) Apparent elastic moduli of day 5 VSMCs with and without tension conditions. (b) Percent relaxation during 60-second hold for day 5 VSMCs with and without tension. Cells in the tension conditions were significantly stiffer (* p < 0.01) than the cells without tension on the elastomer membranes. In addition, the cells in the anti-Integrin β1 conditions were significantly (p < 0.05) softer than the cells in the matching control conditions. Data presented as mean ± standard error.
Table 2:
Day 5 VSMC cell-to-cell and repeated point coefficients of variation for elastic modulus and percent relaxation measures (averages for all measures on gels).
| Sample | Average Cell-to-Cell COV (%) | Average Repeated Point COV (%) | ||
|---|---|---|---|---|
| Elastic Modulus | No Antibodies | 69.9 | Mean = 70.2 | 13.9 |
| IgG Control | 70.4 | 11.6 | ||
| Anti-Integrin β1 | 43.4 | 14.9 | ||
| Percent Relax | No Antibodies | 40.7 | Mean = 42.0 | 6.3 |
| IgG Control | 43.3 | 7.1 | ||
| Anti-Integrin β1 | 17.2 | 5.7 | ||
Table 3:
VSMC cell-to-cell and repeated point coefficients of variation for elastic modulus and percent relaxation measures with and without tension conditions.
| Sample | Average Cell-to-Cell COV (%) | Average Repeated Point COV (%) | |||
|---|---|---|---|---|---|
| Elastic Modulus | Static | No Antibodies | 61.6 | Mean = 61.2 | 5.6 |
| IgG Control | 60.7 | 5.5 | |||
| Anti-Integrin β1 | 38.4 | 8.0 | |||
| Tension | No Antibodies | 48.0 | Mean = 49.5 | 7.9 | |
| IgG Control | 50.9 | 9.8 | |||
| Anti-Integrin β1 | 28.8 | 8.3 | |||
| Percent Relax | Static | No Antibodies | 42.4 | Mean = 45.7 | 4.4 |
| IgG Control | 49.0 | 5.6 | |||
| Anti-Integrin β1 | 21.6 | 7.9 | |||
| Tension | No Antibodies | 37.5 | Mean = 35.5 | 5.8 | |
| IgG Control | 33.4 | 4.9 | |||
| Anti-Integrin β1 | 7.1 | 3.9 | |||
Acknowledgements:
Funding by grants from the National Institutes of Health National Heart Lung Blood Institute (NIH K25 HL092228) and the National Science Foundation (NSF) (CAREER CBET 1254609 and EPS-0903795).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have no conflict of interests related to this work.
REFERENCES
- Acampora KB, Langan EM, Miller RS, LaBerge M, 2007. Development of a novel vascular simulator and injury model to evaluate smooth muscle cell response following balloon angioplasty. Annals of Vascular Surgery 21, 734–741. [DOI] [PubMed] [Google Scholar]
- Acampora KB, 2008. Effect of Clinically Relevant Mechanical Forces on Smooth Muscle Cell Response in Model of Balloon Angioplasty.
- Boudreau N, Bissell MJ, 1998. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Current Opinion in Cell Biology 10, 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras GT, Horton MA, 2002. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophysical Journal 82, 2970–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Shin CS, Towler DA, Civitelli R, 2000. A dominant negative cadherin inhibits osteoblast differentiation. Journal of Bone and Mineral Research 15, 2362–2370. [DOI] [PubMed] [Google Scholar]
- Costa KD, 2004. Single-cell elastography: probing for disease with the atomic force microscope. Disease Markers 19, 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling E, Zauscher S, Guilak F, 2006. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis and Cartilage 14, 571–579. [DOI] [PubMed] [Google Scholar]
- Darling EM, Zauscher S, Block JA, Guilak F, 2007. A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: do cell properties reflect metastatic potential? Biophysical Journal 92, 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch S, Gao BZ, Dean D, 2012. Effect of matrix on cardiomyocyte viscoelastic properties in 2D culture. Molecular & Cellular Biomechanics: MCB 9, 227–249. [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS, 2002. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophysical Journal 82, 2798–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domke J, Dannöhl S, Parak WJ, Müller O, Aicher WK, Radmacher M, 2000. Substrate dependent differences in morphology and elasticity of living osteoblasts investigated by atomic force microscopy. Colloids and Surfaces B: Biointerfaces 19, 367–379. [DOI] [PubMed] [Google Scholar]
- Dulinska-Molak I, Mao H, Kawazoe N, Chen G, 2014. Variation of mechanical property of single-walled carbon nanotubes-treated cells explored by atomic force microscopy. Journal of Biomedical Nanotechnology 10, 651–659. [DOI] [PubMed] [Google Scholar]
- Giddens D, Zarins C, Glagov S, 1993. The role of fluid mechanics in the localization and detection of atherosclerosis. Journal of Biomechanical Engineering 115, 588–594. [DOI] [PubMed] [Google Scholar]
- Guz N, Dokukin M, Kalaparthi V, Sokolov I, 2014. If cell mechanics can be described by elastic modulus: study of different models and probes used in indentation experiments. Biophysical Journal 107, 564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmer JD, Nagatomi J, Wood ST, Vertegel AA, Dean D, LaBerge M, 2009. Role of cytoskeletal components in stress-relaxation behavior of adherent vascular smooth muscle cells. Journal of Biomechanical Engineering 131, 041001. [DOI] [PubMed] [Google Scholar]
- Hemmer JD, Dean D, Vertegel A, Langan E 3rd, LaBerge M, 2008. Effects of serum deprivation on the mechanical properties of adherent vascular smooth muscle cells. Proceedings of the Institution of Mechanical Engineers.Part H, Journal of Engineering in Medicine 222, 761–772. [DOI] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI, 1995. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. The Journal of Cell Biology 129, 489–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz H, 1882. Über die Berührung fester elastischer Körper. Journal Für Die Reine Und Angewandte Mathematik 92, 156–171. [Google Scholar]
- Huang S, Ingber DE, 1999. The structural and mechanical complexity of cell-growth control. Nature Cell Biology 1, E131–E138. [DOI] [PubMed] [Google Scholar]
- Ingber D, 2003. Mechanobiology and diseases of mechanotransduction. Annals of Medicine 35, 564–577. [DOI] [PubMed] [Google Scholar]
- Jaasma MJ, Jackson WM, Keaveny TM, 2006a. The effects of morphology, confluency, and phenotype on whole-cell mechanical behavior. Annals of Biomedical Engineering 34, 759–768. [DOI] [PubMed] [Google Scholar]
- Jaasma MJ, Jackson WM, Keaveny TM, 2006b. Measurement and characterization of whole-cell mechanical behavior. Annals of Biomedical Engineering 34, 748–758. [DOI] [PubMed] [Google Scholar]
- Jones WR, Ping Ting-Beall H, Lee GM, Kelley SS, Hochmuth RM, Guilak F, 1999. Alterations in the Young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. Journal of Biomechanics 32, 119–127. [DOI] [PubMed] [Google Scholar]
- Kai F, Laklai H, Weaver VM, 2016. Force matters: biomechanical regulation of cell invasion and migration in disease. Trends in Cell Biology 26, 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouki E, Beeching CA, Slater SC, Blaschuk OW, Sala-Newby GB, George SJ, 2005. N-cadherin-dependent cell-cell contacts promote human saphenous vein smooth muscle cell survival. Arteriosclerosis, Thrombosis, and Vascular Biology 25, 982–988. [DOI] [PubMed] [Google Scholar]
- Li Q, Lee G, Ong C, Lim C, 2008. AFM indentation study of breast cancer cells. Biochemical and Biophysical Research Communications 374, 609–613. [DOI] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Herlyn M, 2001. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Research 61, 3819–3825. [PubMed] [Google Scholar]
- Liu H, Sun Y, Simmons CA, 2013. Determination of local and global elastic moduli of valve interstitial cells cultured on soft substrates. Journal of Biomechanics 46, 1967–1971. [DOI] [PubMed] [Google Scholar]
- Mahaffy R, Shih C, MacKintosh F, Käs J, 2000. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Physical Review Letters 85, 880. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE, 1997. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America 94, 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally AK, Anderson JM, 2002. β1 and β2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. The American Journal of Pathology 160, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrick DL, Kelly DM, 1993. Temporal expression of VLA-2 and modulation of its ligand specificity by rat glomerular epithelial cells in vitro. Laboratory Investigation; a Journal of Technical Methods and Pathology 69, 690–702. [PubMed] [Google Scholar]
- Na S, Meininger G, Humphrey J, 2007. A theoretical model for F-actin remodeling in vascular smooth muscle cells subjected to cyclic stretch. Journal of Theoretical Biology 246, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie T, Murayama Y, Fukuda T, Nagai C, Omata S, Kanda K, Yaku H, Nakayama Y, 2009. Local elasticity imaging of vascular tissues using a tactile mapping system. Journal of Artificial Organs 12, 40–46. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR, 2004. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews 84, 767–801. [DOI] [PubMed] [Google Scholar]
- Radmacher M, Tillmann RW, Gaub HE, 1993. Imaging viscoelasticity by force modulation with the atomic force microscope. Biophysical Journal 64, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo L, De Sousa J, Mendes Filho J, Radmacher M, 2013. Comparison of the viscoelastic properties of cells from different kidney cancer phenotypes measured with atomic force microscopy. Nanotechnology 24, 055102. [DOI] [PubMed] [Google Scholar]
- Schneider CA; Rasband WS, Eliceiri KW, 2012. NIH Image to ImageJ: 25 years of image analysis, Nature Methods 9(7): 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH, 2002. Networks and crosstalk: integrin signalling spreads. Nature Cell Biology 4, E65–E68. [DOI] [PubMed] [Google Scholar]
- Solon J, Levental I, Sengupta K, Georges PC, Janmey PA, 2007. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophysical Journal 93, 4453–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS, McNutt PM, 1999. Cadherins and their connections: adhesion junctions have broader functions. Current Opinion in Cell Biology 11, 554–560. [DOI] [PubMed] [Google Scholar]
- Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA, 2008. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. American Journal of Physiology.Cell Physiology 295, C268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Martinez-Lemus LA, Trache A, Trzeciakowski JP, Davis GE, Pohl U, Meininger GA, 2005. Mechanical properties of the interaction between fibronectin and alpha5beta1-integrin on vascular smooth muscle cells studied using atomic force microscopy. American Journal of Physiology.Heart and Circulatory Physiology 289, H2526–35. [DOI] [PubMed] [Google Scholar]
- Tang DD, Gerlach BD, 2017. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respiratory Research 18, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Burnham NA, Camesano TA, Wen Q, 2013. Measuring the mechanical properties of living cells using atomic force microscopy. JoVE (Journal of Visualized Experiments), e50497–e50497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S, Berger E, 2012. Quasi-linear viscoelastic properties of costal cartilage using atomic force microscopy. Computer Methods in Biomechanics and Biomedical Engineering 15, 475–486. [DOI] [PubMed] [Google Scholar]
- Tse JR, Engler AJ, 2010. Preparation of hydrogel substrates with tunable mechanical properties. Current Protocols in Cell Biology, 10.16. 1–10.16. 16. [DOI] [PubMed] [Google Scholar]
- Uglow EB, Slater S, Sala-Newby GB, Aguilera-Garcia CM, Angelini GD, Newby AC, George SJ, 2003. Dismantling of cadherin-mediated cell-cell contacts modulates smooth muscle cell proliferation. Circulation Research 92, 1314–1321. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pelham RJ, 1998. [39] Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods in Enzymology 298, 489–496. [DOI] [PubMed] [Google Scholar]
- Winn B, et al. , 2009. Rat aortic smooth muscle cell primary isolation protocol., 1–2.
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA, 2005. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motility and the Cytoskeleton 60, 24–34. [DOI] [PubMed] [Google Scholar]
- Yiin JJ, Hu B, Jarzynka MJ, Feng H, Liu KW, Wu JY, Ma HI, Cheng SY, 2009. Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro-Oncology 11, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wen Y, Du J, Yu Y, Liu S, Wu X, Zhao H, 2018. Effects of mechanical stretch on the functions of BK and L-type Ca(2+) channels in vascular smooth muscle cells. Journal of Biomechanics 67, 18–23. [DOI] [PubMed] [Google Scholar]