ABSTRACT
The spatial regulation of cellular Rho signaling by GEF and GAP proteins and the molecular mechanisms controlling the Rho regulators themselves are still incompletely understood. We previously reported that the poorly characterized RhoGAP protein DLC3 localizes to cell-cell adhesions and Rab8-positive membrane tubules. However, it was unclear how DLC3 is targeted to these subcellular sites to execute its functions. In our recent work, protein partners of DLC3 were identified by mass spectrometry, identifying the basolateral polarity protein Scribble as a scaffold for DLC3 at cell-cell contacts. We found that the PDZ-mediated interaction of DLC3 and Scribble is essential for junctional DLC3 recruitment and its role as a local regulator of RhoA-ROCK signaling controlling adherens junction integrity and Scribble localization. Furthermore, DLC3 and Scribble depletion interfered with polarized lumen formation in a 3D model of cyst morphogenesis, emphasizing the relevance of both proteins in epithelial polarity. These findings reveal a new mechanism for spatial Rho regulation at adherens junctions in polarized epithelial cells and highlight the necessity to investigate DLC3 localization and function also in cellular contexts that require cell junction remodeling.
KEYWORDS: adherens junctions, apical-basolateral polarity, Rho GTPase-activating protein, spatial Rho regulation, PDZ domain interaction, protein scaffold, tumor suppressor
Commentary
Small Rho GTPases, including RhoA, Rac and Cdc42 as the best characterized members, play a key role in a variety of biological processes by functioning as molecular switches that coordinate the remodelling of the actin and microtubule cytoskeleton. Recent studies employing genetically encoded biosensors revealed that Rho activation is tightly controlled in time and space.1,2 However, the current knowledge about the molecular mechanisms underlying spatiotemporal Rho regulation is still limited. Guanine nucleotide exchange factors (GEFs) substitute Rho-bound GDP for GTP to activate the GTPase and promote downstream signaling. By contrast, GTPase-activating proteins (GAPs) increase the low intrinsic GTPase-activity of Rho proteins to inactivate them and terminate the signal.3 Although it is known that deregulated Rho activation, based on altered expression or function of Rho regulators, has severe consequences and contributes to tumorigenesis,4 it remains to be investigated how GEF and GAP proteins themselves are controlled in different biological contexts.
Highly coordinated Rho signaling is required for the establishment of epithelial cell polarity, including cell-cell adhesion and apical-basolateral membrane specification, to ensure normal epithelial function. To this end, Rho GTPases cooperate with polarity proteins to modulate localized cytoskeleton dynamics, polarized vesicular trafficking and the spatial organization of signaling pathways in epithelial cells.5 In general, the Par and Crumbs polarity complex define the apical membrane compartment, while the Scribble complex preserves basolateral membrane identity. It is well established that antagonistic Rac and RhoA activity gradients exist along the apical-basal axis of epithelial cells, which is a prerequisite for junction formation and maintenance.6 Rac activity, for example, is controlled by the RacGEF Tiam which itself is inhibited by apical Par3, whereas binding to the basolateral scaffold β2-syntrophin activates Tiam.7,8 Among the RhoGAP proteins, the deleted in liver cancer (DLC) family has an outstanding role, because it is deregulated in different types of cancer more frequently than any other Rho regulator.9,10 Despite the similar structural organization of the 3 DLC family members, including a conserved GAP domain regulating RhoA activity, a sterile α-motif domain (SAM) and a StAR (steroidogenic acute regulatory protein)-related lipid transfer domain (START), DLC1, DLC2 and DLC3 appear to have specific cellular functions associated with their distinct subcellular localizations.11,12 Besides the association with focal adhesions described for all DLC proteins,13,14 we previously found that DLC3 (also known as StarD8) is localized at cell-cell adhesions and Rab8-positive tubular structures.15,16 Considering that the DLC3-interactome was largely unexplored with focal adhesion-associated tensin and talin proteins being the only known DLC3 binding partners,14,17 it was elusive how DLC3 is targeted to specific subcellular sites to execute its functions.
In our recent work, we identified DLC3 protein interaction partners by performing mass spectrometry analysis and discovered the basolateral polarity protein Scribble as the first isoform-specific scaffold for DLC3 at adherens junctions (Fig. 1).18 Scribble is a multi-domain protein that contains 16 leucine-rich repeats (LRRs) and 4 PSD-95, discs large, and ZO-1 (PDZ) domains and localizes to adherens junctions and basolateral membranes in polarized epithelial cells.19,20 Moreover, Scribble has been reported to control E-cadherin function and junction integrity and acts as a potential tumor suppressor in Drosophila and mammalian cells.21-23 Biochemical analysis revealed that DLC3 binds via a unique, C-terminal PDZ ligand (PDZL) motif to the PDZ domains of Scribble, which are typically engaged in different protein interactions.24 The DLC3-Scribble interaction was further verified at the cellular level by in situ proximity ligation assay (PLA) and junctional DLC3 localization in MCF7 breast cancer cells was shown to depend on the PDZL motif and on Scribble. Additionally, we confirmed the relevance of the PDZL motif in polarized Caco-2 colorectal cancer cells in which it determined the membrane association and basolateral localization of DLC3.18 To study local RhoA activity at cell-cell contacts in dependence of DLC3 expression, we employed a location biosensor for active RhoA.25,26 By this means, DLC3 was identified as a junctional RhoA regulator and pharmacological Rho and ROCK inhibition further provided evidence for the maintenance of junction integrity and Scribble localization through DLC3-mediated Rho-ROCK regulation. Most importantly, we mechanistically demonstrated that artificial targeting of the active DLC3 GAP domain to basolateral membranes by Scribble LRR domains is sufficient to rescue the E-cadherin mislocalization and cell disaggregation observed in DLC3-knockdown cells.18 Thus, a mutual dependence of DLC3 and Scribble was elucidated involving the recruitment of DLC3 to adherens junctions by the scaffolding function of Scribble and the maintenance of cell adhesions and Scribble localization by balanced Rho-ROCK signaling through localized DLC3 GAP activity. In addition to preserving adherens junctions, DLC3 and Scribble were identified to play a role in epithelial morphogenesis. In particular, knockdown of DLC3 and Scribble in Caco-2 3-dimensional cultures impaired cyst polarization and lumen formation, emphasizing the relevance of both proteins in the establishment of polarity.18 Taken together, our work uncovered a new control mechanism for spatial Rho regulation based on junctional positioning of the RhoGAP DLC3 by the PDZ-scaffold Scribble.
Figure 1.
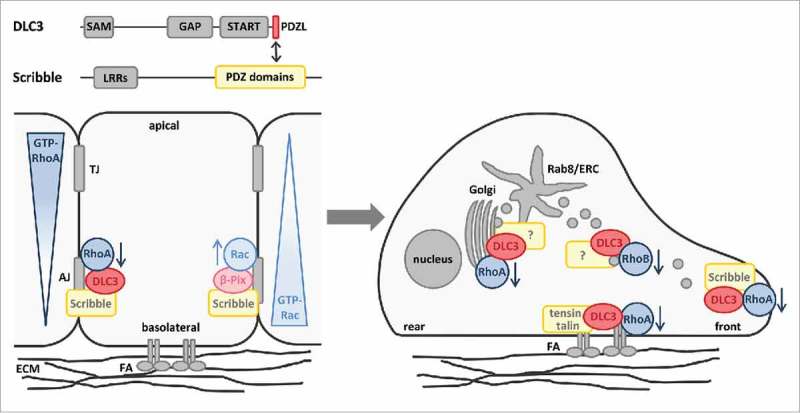
Spatial Rho regulation by DLC3 in epithelial cells with apical-basolateral and front-rear polarity. DLC3 is targeted to adherens junctions in polarized epithelial cells by the scaffold protein Scribble to exert its function as a RhoGAP. By additionally recruiting the RacGEF β-Pix, Scribble contributes to the establishment of antagonizing RhoA and Rac activity gradients along the apical-basal axis of epithelial cells. By contrast, in motile epithelial cells, DLC3 localizes to focal adhesions (FA), Rab8-positive membrane tubules of the endocytic recycling compartment (ERC) and the leading edge where it locally regulates Rho signaling. The domain organization and binding regions of DLC3 and Scribble are depicted. AJ, adherens junctions; ECM, extracellular matrix; LRR, leucine-rich repeat; PDZ, PSD-95/discs large/ZO-1; PDZL, PDZ ligand motif; SAM, sterile α motif; START, StAR-related lipid transfer; TJ, tight junctions.
Scribble is known to control Rac activity in epithelial cells by targeting the RacGEF β-Pix to adherens junctions through a PDZ-domain interaction.27,28 Its novel role in restricting RhoA activity at basolateral membranes by recruitment of DLC3 represents an analogous mechanism for spatial RhoA regulation. Thus, Scribble plays a dual role in the establishment of antagonizing Rho-Rac activity gradients along the apical-basal axis of epithelial cells (Fig. 1). Considering that the DLC family members DLC1 and DLC2 lack a C-terminal class I PDZL motif and do not interact with Scribble,18 PDZL-dependent recruitment of DLC3 serves as an isoform-specific regulatory mechanism. This supports the idea that the different functions of the DLC family members are specified by their interaction partners and result in the regulation of spatially distinct Rho pools. In DLC3, the PDZL motif was found to be essential for general membrane association.18 Thus, apart from controlling the recruitment to cell junctions, PDZL-mediated membrane association of DLC3 might also play a role at other subcellular sites, contributing to its localization at focal adhesions15 and membrane tubules of the endocytic recycling compartment14 (Fig. 1). In this context, the question arises whether there is cooperation or competition between different PDZ scaffolds to support the different subcellular localizations of DLC3. In addition to the functional characterization of further DLC3 interactors identified in our recent work,18 more sophisticated proteomic approaches will be required to shed light onto the composition of different subcellular DLC3 pools and their molecular regulation. For example, proximity-dependent labeling of DLC3 interactors based on expression of biotin ligase fusion proteins in combination with streptavidin affinity purification might be a promising strategy to identify proteins that regulate the spatially distinct DLC3 pools.29 The coordinated activation of Rho proteins by GEFs is also known to require interaction with scaffold proteins. The scaffold protein CNK1, for example, links RhoA and its GEFs Net1 and p115RhoGEF to the JNK signaling pathway,30 whereas JIP2 and spinophilin function as platforms for the RacGEF Tiam, determining its effector specificity.31,32 As a negative regulator of Rho signaling, DLC3 antagonizes the action of specific GEF proteins acting on a particular Rho pool. Considering that PDZL motifs are enriched among RhoGEFs with about 40% of the family members containing such a motif at their C-terminus,33 the GEF counterpart of DLC3 might also be recruited by a PDZ-scaffold like Scribble. It is possible that both Rho regulators compete for scaffold binding, or alternatively, they might also interact simultaneously with the scaffold forming a multi-protein complex to fine-tune Rho responses.
Front-rear polarization of cells is crucial for directed cell migration, for example during development, tissue morphogenesis and wound closure, but also in the context of pathologies like cancer. During epithelial-to-mesenchymal transition (EMT), apical-basolateral polarity and cell-cell adhesions are lost, and cells adopt a more motile phenotype promoting metastases formation and tumor progression. Given the regulatory role of DLC3 at adherens junctions and in polarized morphogenesis, it will be interesting to address DLC3 localization and function under conditions in which E-cadherin is downregulated and cell-cell contacts are lost (Fig. 1). Interestingly, several proteins involved in the ubiquitin-proteasome pathway, including the ubiquitin ligases HUWE-1 and CHIP, were isolated in our proteomic analysis,18 suggesting that DLC3 might be regulated by ubiquitylation. Similar to the PDZ-mediated complex assembly of the RhoGEF Net1 and the Scribble complex component Dlg, which protects Net1 from degradation at cell adhesions,34 DLC3 might be stabilized at adherens junctions by the PDZ-interaction with Scribble. Consequently, one might hypothesize that DLC3 is degraded by the ubiquitin-proteasome pathway when cell-cell adhesions are lost. Intriguingly, in response to HGF, the RacGEF Tiam is degraded by HUWE-1-mediated ubiquitylation, which is associated with the disruption of cell-cell contacts in MDCK cells.35 This raises the possibility that Tiam and DLC3 are not only controlled in a similar fashion at cell junctions by polarity scaffolding proteins,7,18 but also their protein turnover might be controlled by a similar molecular mechanism. Alternatively, it is also conceivable that DLC3 localization might be shifted from cell-cell contacts to endomembranes when cell-cell adhesions are downregulated, to fulfil a primary function in endocytic trafficking. Our finding that both DLC3 and Scribble are essential for the establishment of cell polarity might also be explained by an additional role in the regulation of polarized protein trafficking. This is supported by recent reports on the coordination of the vesicular transport of retromer-dependent cargos like E-cadherin by Scribble.36,37 Interestingly, cytoplasmic mislocalization of Scribble in cancer cells that have undergone EMT was associated with a gain of tumor-promoting functions,38 which strongly contrasts with its tumor-suppressive effects in polarized epithelial cells. Thus, it is tempting to speculate that DLC3 might be sequestered in the cytoplasm by mislocalized Scribble, reminiscent of a scenario described for PTEN,39 which might keep the RhoGAP in check and interfere with its potential tumor-suppressive properties. Interestingly, in migrating cells, we observed that DLC3 localized to the leading edge in a Scribble-dependent manner to locally regulate RhoA (unpublished observations) (Fig. 1). Consequently, it can be assumed that the precise cellular context determines the role of the DLC3-Scribble interaction and the way both proteins modulate each other's function. Furthermore, in the context of DLC3-coordinated endocytic transport, the RhoB isoform is discussed as a new potential substrate of DLC3.16 RhoB is typically localized on endosomes and was reported to delay EGFR trafficking in cells expressing the constitutively active GTPase.40,41 This was phenocopied in DLC3-depleted cells, in which the simultaneous knockdown of RhoA and RhoB was required to restore transferrin recycling.16 In addition, a novel function for RhoB at cell-cell contacts in the control of E-cadherin expression and cell adhesion strength was recently described,42 providing a further hint at a potential role for DLC3 in RhoB regulation.
Owing to the multi-domain nature of DLC3, it is not surprising that other domains besides the PDZ ligand motif, influence its membrane association and subcellular localization. We previously showed that the SAM domain targets DLC3 to Golgi membranes,16 whereas a polybasic region conserved in all 3 DLC isoforms mediates binding to phosphatidylinositol-4,5-bisphosphate-enriched membranes and is required for GAP activity in cells.43 Thus, together with protein interactions, lipid binding adds another layer of complexity to the molecular regulation of DLC3. In this context, the precise role of the DLC3 START domain and the identity of its potential lipid ligand remain to be determined. Moreover, there is growing evidence for the regulation of Rho GTPases and their regulators by different types of post-translational modifications.44 For example, given that the tumor suppressor PTEN is acetylated on a lysine residue within its PDZL motif45 and DLC3 contains a lysine at the same position, DLC3 might be controlled in a similar manner to modulate its binding to PDZ domain proteins. Furthermore, it has been shown that PDZ-interactions are frequently regulated by phosphorylation of serine or threonine residues within the PDZL motif or at positions in its vicinity,46 which might also apply to DLC3.
In summary, our data reveal a new control mechanism for DLC3 that involves the polarity protein Scribble as a scaffold for the RhoGAP at adherens junctions. To better understand the molecular regulation of DLC3 also at other subcellular sites, protein networks, the contributions of lipid and membrane interactions, and the impact of post-translational modifications need to be addressed. In future investigations we aim to explore in more detail the changes of DLC3 localization and function during transition of epithelial cells with apical-basolateral polarity to front-rear polarized migratory cells. High resolution microscopy and live cell imaging might help to resolve the dynamics of fluorescently-tagged DLC3 protein complexes, while genetically encoded FRET biosensors represent powerful tools to study the spatiotemporal aspects of DLC3-controlled Rho regulation. Moreover, the great potential of optogenetics to explore finely tuned spatiotemporal Rho GTPase responses was demonstrated by the manipulation of Rac activity at lamellipodia of single border cells in the Drosophila ovary47 and during de novo formation of adherens junctions in breast epithelial MCF10A cells.48 Recently, the light-mediated control of RhoA activation through an opto-engineered GEF protein was reported,49 an advance of the technique that could be transferred to GAP proteins in the future. Whereas DLC1 has an established function as a tumor suppressor in different types of human cancers,10,11 the contribution of the loss of DLC3 expression to tumor progression is less clear.50 Taking into account the importance of spatially controlled Rho signaling in epithelial tissue integrity, functional inactivation of DLC3, for example by mislocalization, rather than downregulation of its expression might be sufficient to disrupt epithelial tissue architecture and support neoplastic cell transformation. Thus, elucidating in more depth how the different subcellular DLC3 pools control downstream signaling via GAP- and perhaps even GAP-independent mechanisms, will improve our understanding of the biological and potential tumor-suppressive role of DLC3.
Abbreviations
- AJ
adherens junction
- Cdc42
cell division control protein 42 homolog
- DLC
deleted in liver cancer
- EMT
epithelial-to-mesenchymal transition
- FRET
fluorescence resonance energy transfer
- GAP
GTPase-activating protein
- GDP
guanosine diphosphate
- GEF
guanine nucleotide exchange factor
- GTP
guanosine triphosphate
- PDZ
PSD-95/discs large/ZO-1
- PDZL
PDZ ligand motif
- Pix
p21-activated kinase interacting exchange factor
- PTEN
phosphatase and tensin homolog
- Rac
Ras-related C3 botulinum toxin substrate
- Rho
Ras homology protein
- ROCK
Rho-associated, coiled-coil containing kinase
- SAM
sterile α motif
- START
StAR-related lipid transfer
- Tiam
T-cell lymphoma invasion and metastasis-inducing protein
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) Heisenberg program (grant number OL239/8–2 to M.A.O.) and the DFG grant OL239/9–2 to M.A.O.
References
- [1].Pertz O. Spatio-temporal Rho GTPase signaling – where are we now? J Cell Sci 2010; 123:1841-50; PMID:20484664; http://dx.doi.org/ 10.1242/jcs.064345 [DOI] [PubMed] [Google Scholar]
- [2].Donnelly SK, Bravo-Cordero JJ, Hodgson L. Rho GTPase isoforms in cell motility: don't fret, we have FRET. Cell Adhes Migrat 2014; 8:526-34; PMID:25482645; http://dx.doi.org/ 10.4161/cam.29712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small g proteins. Cell 2007; 130:385; http://dx.doi.org/ 10.1016/j.cell.2007.07.001 [DOI] [PubMed] [Google Scholar]
- [4].Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer 2002; 2:133-42; PMID:12635176; http://dx.doi.org/ 10.1038/nrc725 [DOI] [PubMed] [Google Scholar]
- [5].Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 2008; 9:846-59; PMID:18946474; http://dx.doi.org/ 10.1038/nrm2521 [DOI] [PubMed] [Google Scholar]
- [6].Halaoui R, McCaffrey L. Rewiring cell polarity signaling in cancer. Oncogene 2015; 34:939-50; PMID:24632617; http://dx.doi.org/ 10.1038/onc.2014.59 [DOI] [PubMed] [Google Scholar]
- [7].Mack NA, Porter AP, Whalley HJ, Schwarz JP, Jones RC, Syed AS, Bjartell A, Anderson KI, Malliri A. β2-syntrophin and Par-3 promote an apicobasal Rac activity gradient at cell-cell junctions by differentially regulating Tiam1 activity. Nat Cell Biol 2012; 14:1169-80; PMID:23103911; http://dx.doi.org/ 10.1038/ncb2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Y-i Nabeshima, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 2005; 7:270-7; PMID:15723051; http://dx.doi.org/ 10.1038/ncb1227 [DOI] [PubMed] [Google Scholar]
- [9].Kandpal RP. Rho GTPase activating proteins in cancer phenotypes. Curr Protein Pept Sci 2006; 7:355-65; PMID:16918449; http://dx.doi.org/ 10.2174/138920306778018025 [DOI] [PubMed] [Google Scholar]
- [10].Xue W, Krasnitz A, Lucito R, Sordella R, VanAelst L, Cordon-Cardo C, Singer S, Kuehnel F, Wigler M, Powers S, et al.. DLC1 is a chromosome 8p tumor suppressor whose loss promotes hepatocellular carcinoma. Gen Dev 2008; 22:1439-44; PMID:18519636; http://dx.doi.org/ 10.1101/gad.1672608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Durkin ME, Yuan B-Z, Zhou X, Zimonjic DB, Lowy DR, Thorgeirsson SS, Popescu NC. DLC-1:a Rho GTPase-activating protein and tumour suppressor. J Cell Mol Med 2007; 11:1185-207; PMID:17979893; http://dx.doi.org/ 10.1111/j.1582-4934.2007.00098.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Braun AC, Olayioye MA. Rho regulation: DLC proteins in space and time. Cell Signall 2015; 27:1643-51; PMID:25889896; http://dx.doi.org/ 10.1016/j.cellsig.2015.04.003 [DOI] [PubMed] [Google Scholar]
- [13].Kawai K, Kiyota M, Seike J, Deki Y, Yagisawa H. START-GAP3/DLC3 is a GAP for RhoA and Cdc42 and is localized in focal adhesions regulating cell morphology. Biochem Biophy Res Communicat 2007; 364:783-9; PMID:17976533; http://dx.doi.org/ 10.1016/j.bbrc.2007.10.052 [DOI] [PubMed] [Google Scholar]
- [14].Qian X, Li G, Asmussen HK, Asnaghi L, Vass WC, Braverman R, Yamada KM, Popescu NC, Papageorge AG, Lowy DR. Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci U S A 2007; 104:9012-7; PMID:17517630; http://dx.doi.org/ 10.1073/pnas.0703033104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holeiter G, Bischoff A, Braun AC, Huck B, Erlmann P, Schmid S, Herr R, Brummer T, Olayioye MA. The RhoGAP protein Deleted in Liver Cancer 3 (DLC3) is essential for adherens junctions integrity. Oncogenesis 2012; 1:e13; PMID:23552697; http://dx.doi.org/ 10.1038/oncsis.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Braun AC, Hendrick J, Eisler SA, Schmid S, Hausser A, Olayioye MA. The Rho-specific GAP protein DLC3 coordinates endocytic membrane trafficking. J Cell Sci 2015; 128:1386-99; PMID:25673874; http://dx.doi.org/ 10.1242/jcs.163857 [DOI] [PubMed] [Google Scholar]
- [17].Li G, Du X, Vass WC, Papageorge AG, Lowy DR, Qian X. Full activity of the deleted in liver cancer 1 (DLC1) tumor suppressor depends on an LD-like motif that binds talin and focal adhesion kinase (FAK). Proc Natl Acad Sci U S A 2011; 108:17129-34; PMID:21969587; http://dx.doi.org/ 10.1073/pnas.1112122108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hendrick J, Franz-Wachtel M, Moeller Y, Schmid S, Macek B, Olayioye MA. The polarity protein Scribble positions DLC3 at adherens junctions to regulate Rho signaling. J Cell Sci 2016; 129:3583-96; PMID:27505894; http://dx.doi.org/ 10.1242/jcs.190074 [DOI] [PubMed] [Google Scholar]
- [19].Albertson R, Chabu C, Sheehan A, Doe CQ. Scribble protein domain mapping reveals a multistep localization mechanism and domains necessary for establishing cortical polarity. J Cell Sci 2004; 117:6061-70; PMID:15536119; http://dx.doi.org/ 10.1242/jcs.01525 [DOI] [PubMed] [Google Scholar]
- [20].Navarro C, Nola S, Audebert S, Santoni M-J, Arsanto J-P, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, et al.. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 2005; 24:4330-9; PMID:15806148; http://dx.doi.org/ 10.1038/sj.onc.1208632 [DOI] [PubMed] [Google Scholar]
- [21].Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 2005; 171:1061-71; PMID:16344308; http://dx.doi.org/ 10.1083/jcb.200506094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 2000; 403:676-80; PMID:10688207; http://dx.doi.org/ 10.1038/35001108 [DOI] [PubMed] [Google Scholar]
- [23].Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, Russell SM, Richardson HE, Humbert PO. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene 2003; 22:9225-30; PMID:14681682; http://dx.doi.org/ 10.1038/sj.onc.1207154 [DOI] [PubMed] [Google Scholar]
- [24].Nourry C, Grant SGN, Borg J-P. PDZ domain proteins: plug and play! Sci Signal 2003; 2003:re7; http://dx.doi.org/ 10.1126/stke.2003.179.re7 [DOI] [PubMed] [Google Scholar]
- [25].Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, Actin, and Myosin during cytokinesis. Curr Biol 2008; 18:30-6; PMID:18158243; http://dx.doi.org/ 10.1016/j.cub.2007.11.068 [DOI] [PubMed] [Google Scholar]
- [26].Priya R, Gomez GA, Budnar S, Verma S, Cox HL, Hamilton NA, Yap AS. Feedback regulation through myosin II confers robustness on RhoA signalling at E-cadherin junctions. Nat Cell Biol 2015; 17:1282-93; PMID:26368311; http://dx.doi.org/ 10.1038/ncb3239 [DOI] [PubMed] [Google Scholar]
- [27].Frank SR, Bell JH, Frödin M, Hansen SH. A βPIX-PAK2 complex confers protection against Scrib-dependent and cadherin-mediated apoptosis. Curr Biol 2012; 22:1747-54; PMID:22863318; http://dx.doi.org/ 10.1016/j.cub.2012.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, Allred C, Muthuswamy SK. Deregulation of Scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell 2008; 135:865-78; PMID:19041750; http://dx.doi.org/ 10.1016/j.cell.2008.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol 2012; 196:801-10; PMID:22412018; http://dx.doi.org/ 10.1083/jcb.201112098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jaffe AB, Hall A, Schmidt A. Association of CNK1 with Rho guanine nucleotide exchange factors controls signaling specificity downstream of Rho. Curr Biol 2005; 15:405-12; PMID:15753034; http://dx.doi.org/ 10.1016/j.cub.2004.12.082 [DOI] [PubMed] [Google Scholar]
- [31].Buchsbaum RJ, Connolly BA, Feig LA. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol 2002; 22:4073-85; PMID:12024021; http://dx.doi.org/ 10.1128/MCB.22.12.4073-4085.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Buchsbaum RJ, Connolly BA, Feig LA. Regulation of p70 S6 kinase by complex formation between the Rac guanine nucleotide exchange factor (Rac-GEF) tiam1 and the scaffold spinophilin. J Biol Chem 2003; 278:18833-41; PMID:12531897; http://dx.doi.org/ 10.1074/jbc.M207876200 [DOI] [PubMed] [Google Scholar]
- [33].García-Mata R, Burridge K. Catching a GEF by its tail. Trend Cell Biol 2006; 17:36-43; http://dx.doi.org/ 10.1016/j.tcb.2006.11.004 [DOI] [PubMed] [Google Scholar]
- [34].Carr HS, Cai C, Keinänen K, Frost JA. Interaction of the RhoA exchange factor Net1 with Discs Large Homolog 1 protects it from proteasome-mediated degradation and potentiates Net1 activity. J Biol Chem 2009; 284:24269-80; PMID:19586902; http://dx.doi.org/ 10.1074/jbc.M109.029439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vaughan L, Tan C-T, Chapman A, Nonaka D, Mack NA, Smith D, Booton R, Hurlstone Adam FL, Malliri A. HUWE1 ubiquitylates and degrades the RAC activator TIAM1 promoting cell-cell adhesion disassembly, migration, and invasion. Cell Reports 2015; 10:88-102; PMID:25543140; http://dx.doi.org/ 10.1016/j.celrep.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lohia M, Qin Y, Macara IG. The scribble polarity protein stabilizes E-cadherin/p120-catenin binding and blocks retrieval of E-cadherin to the Golgi. PLoS ONE 2012; 7:e51130; PMID:23226478; http://dx.doi.org/ 10.1371/journal.pone.0051130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].de Vreede G, Schoenfeld JD, Windler SL, Morrison H, Lu H, Bilder D. The scribble module regulates retromer-dependent endocytic trafficking during epithelial polarization. Development (Cambridge, England) 2014; 141:2796-802; PMID:25005475; http://dx.doi.org/ 10.1242/dev.105403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Elsum IA, Humbert PO. Localization, not important in all tumor-suppressing properties: A lesson learnt from scribble. Cells Tissues Organs 2013; 198:1-11; PMID:23774808; http://dx.doi.org/ 10.1159/000348423 [DOI] [PubMed] [Google Scholar]
- [39].Feigin ME, Akshinthala SD, Araki K, Rosenberg AZ, Muthuswamy LB, Martin B, Lehmann BD, Berman HK, Pietenpol JA, Cardiff RD, et al.. Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer. Cancer Res 2014; 74:3180-94; PMID:24662921; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia 1. J Cell Sci 2005; 118:2661-70; PMID:15944396; http://dx.doi.org/ 10.1242/jcs.02384 [DOI] [PubMed] [Google Scholar]
- [41].Gampel A, Parker PJ, Mellor H. Regulation of epidermal growth factor receptor traffic by the small GTPase RhoB. Curr Biol 1999; 9:955-8; PMID:10508588; http://dx.doi.org/ 10.1016/S0960-9822(99)80422-9 [DOI] [PubMed] [Google Scholar]
- [42].Vega FM, Thomas M, Reymond N, Ridley AJ. The Rho GTPase RhoB regulates cadherin expression and epithelial cell-cell interaction. Cell Communicat Signal 2015; 13:1-9; PMID:25589173; http://dx.doi.org/ 10.1186/s12964-015-0085-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Erlmann P, Schmid S, Horenkamp FA, Geyer M, Pomorski TG, Olayioye MA. DLC1 activation requires lipid interaction through a polybasic region preceding the RhoGAP domain. Mole Biol Cell 2009; 20:4400-11; PMID:19710422; http://dx.doi.org/ 10.1091/mbc.E09-03-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol 2016; 17:496-510; PMID:27301673; http://dx.doi.org/ 10.1038/nrm.2016.67 [DOI] [PubMed] [Google Scholar]
- [45].Ikenoue T, Inoki K, Zhao B, Guan K-L. PTEN acetylation modulates its interaction with PDZ domain. Cancer Res 2008; 68:6908-12; PMID:18757404; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1107 [DOI] [PubMed] [Google Scholar]
- [46].Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci 2004; 5:771-81; PMID:15378037; http://dx.doi.org/ 10.1038/nrn1517 [DOI] [PubMed] [Google Scholar]
- [47].Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol 2010; 12:591-7; PMID:20473296; http://dx.doi.org/ 10.1038/ncb2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Grikscheit K, Frank T, Wang Y, Grosse R. Junctional actin assembly is mediated by Formin-like 2 downstream of Rac1. J Cell Biol 2015; 209:367-76; PMID:25963818; http://dx.doi.org/ 10.1083/jcb.201412015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wagner E, Glotzer M. Local RhoA activation induces cytokinetic furrows independent of spindle position and cell cycle stage. J Cell Biol 2016; 213:641-9; PMID:27298323; http://dx.doi.org/ 10.1083/jcb.201603025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang D, Qian X, Rajaram M, Durkin ME, Lowy DR. DLC1 is the principal biologically-relevant down-regulated DLC family member in several cancers. Oncotarget 2016; 7:45144-57. [DOI] [PMC free article] [PubMed] [Google Scholar]


