ABSTRACT
The activation of Ras is common to two activities in cells of Dictyostelium discoideum: the directed movement in a gradient of chemoattractant and the autonomous generation of propagating waves of actin polymerization on the substrate-attached cell surface. We produced large cells by electric-pulse induced fusion to simultaneously study both activities in one cell. For imaging, a fluorescent label for activated Ras was combined with labels for filamentous actin, PIP3, or PTEN. Chemotactic responses were elicited in a diffusion gradient of cyclic AMP. Waves initiated at sites separate from the front of the cell propagated in all directions. Nevertheless, the wave-forming cells were capable of recognizing the attractant gradient and managed to migrate in its direction.
KEYWORDS: actin waves, cell fusion, chemotaxis, Dictyostelium, excitable systems, PIP3, PTEN, Ras
Introduction
In a recent paper we reported on the chemotactic responses of over-sized cells of Dictyostelium discoideum that we produced by electric-pulse induced fusion.1 Here we wish to address one point that was only touched in the above paper: the different actions of two systems in a single cell that both involve the activation of Ras and the synthesis of PIP3. The first system is the signal processing network that enables cells to orientate in a gradient of chemoattractant.2-5 The second system is responsible for the propagation of actin waves on the substrate-attached cell surface.6,7
The chemotactic response of a eukaryotic cell, like a neutrophil or a Dictyostelium cell, requires the control of actin-based motility according to the direction of the gradient of attractant; this means the cells form protrusions toward the source and retract a tail at the opposite pole.8 Actin waves are most profusely formed in strains of D.discoideum that lack a neurofibromin-like Ras-GTPase.9 These waves are initiated spontaneously at spots of increased PIP3 synthesis, and encircle a PIP3-enriched territory during their propagation on the substrate-attached plasma membrane. Along with the propagation of an actin wave, the excited state of increased PIP3 synthesis in the inner territory is carried with undiminished amplitude through over the entire substrate-attached plasma membrane.10 Since cells of D.discoideum that lack the Ras-GTPase are capable of responding to an external source of chemoattractant as well as forming propagating waves, the question is raised as to the relationship of the two systems that share the activation of Ras and the synthesis of PIP3. One possibility is that chemotaxis and wave propagation are separated by developmental stage.11 Indeed, waves are most profusely formed during the early starvation phase, prior to the onset of full chemotactic responsiveness to cyclic AMP. However, choosing a stage at which the two capabilities overlap enables one to study how a wave-forming cell behaves in a gradient of chemoattractant.
There are at least three possibilities if a cell is concurrently capable of responding to chemoattractant and to autonomously form propagating waves: (1) The chemoattractant suppresses autonomous wave propagation; (2) the waves interfere with the chemotactic response; (3) the two systems can co-exist independently of each other or even cooperate. The data presented here provide evidence for co-existence of the two systems.
Results
Propagating wave patterns of Ras activation
In chemotaxing Dictyostelium cells, Ras acts upstream of PIP3 in the transmission of signals from receptors of the chemoattractant cyclic-AMP to the actin system that mediates orientation of the cells.12 The Ras binding domain (RBD) of human Raf1 recognizes Dictyostelium RasG in the GTP-bound state.13 RasG is important for chemotaxis14 and localizes to the front of chemotaxing cells.12
By electric-pulse induced fusion we produced large cells,15 which provided a sufficiently expanded area of the substrate-attached cell surface for wave patterns to fully evolve.7 For chemotactic stimulation through a micropipette filled with cyclic AMP, fused cells starved for 4 hours were used, which still produced waves and were already capable of responding to the chemoattractant.
To explore the pattern of spontaneous Ras activation in giant cells, we have combined the RBD as a probe for activated Ras with PTEN, a PIP3-degrading 3-phosphatase, which is depleted from the front of chemotaxing cells.16,17 Figure 1 displays an image series from the beginning of wave formation in a giant cell, up to a fully developed pattern of moving zones of Ras activation. These data show that the formation of actin waves is associated with the activation of Ras without the need for any external signal that would activate Ras through a receptor-mediated pathway.
Figure 1.
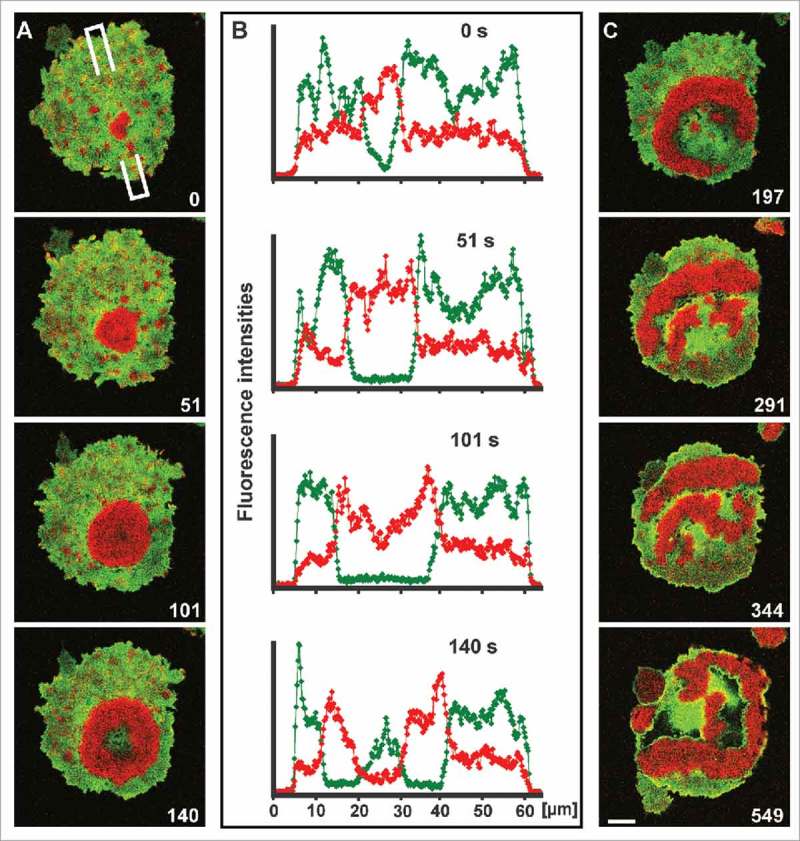
Waves of Ras activation linked to the downregulation of PTEN. (A) Initiation and propagation of a wave of activated Ras (red) in a cell that expresses mRFP-RBD and GFP-PTEN (green). At the beginning of this time series, one of the numerous actin clusters transiently formed within PTEN-depleted spots on the cell surface, is converted into a propagating wave. At the end of the series, the compact area decorated with activated Ras is turned into an expanding annulus. (B) Scans of fluorescence intensities corresponding to the images shown in (A). The wave of Ras activation is initiated in a PTEN-depleted area. This area expands while the Ras wave propagates, until the center turns back into a PTEN-rich state. (C) Continuation of the time series shown in (A), illustrating conversion of the concentric wave pattern of activated Ras into a pattern of irregular bands. Scale bar for (A) and (C), 10 μm.
Chemotactic stimulation of wave-forming cells
To examine the relationship of chemotactic responses and wave formation, we have visualized the waves with mRFP-LimEΔ, a label for filamentous actin,18 combined with either RBD-GFP for activated Ras or PHcrac-GFP for PI(3,4,5)P3 (and PI(3,4)P2).19,20 Figure 2 and Movie 1 show a cell with a wave on its substrate-attached surface. Upon the chemotactic stimulation through a micropipette, the wave split into two waves that subsequently re-united, a behavior that has previously been observed in the absence of chemotactic stimulation.7 The cell moved toward the micropipette by extending protrusions in the direction of the gradient, with activated Ras at their front (Fig. S1). When the micropipette was transposed from the bottom to the top of the frame, protrusions were redirected; the cell described an arc and finally moved toward the new pipette position. During this maneuver, the actin wave stayed intact, indicating that a wave can coexist with chemotactic responsiveness, not preventing chemotactic movement.
Figure 2.
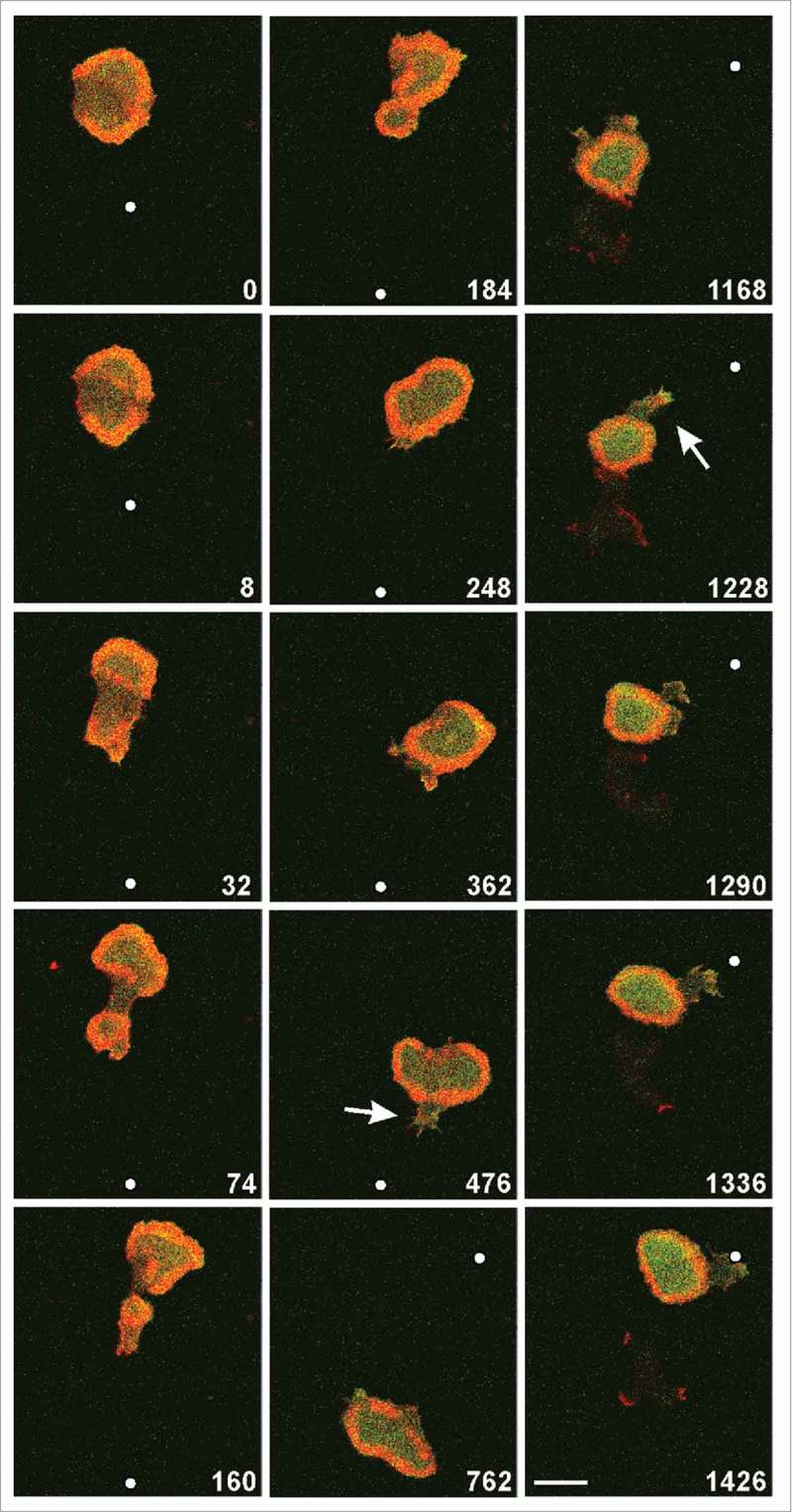
A wave-forming cell stimulated in a diffusion gradient of cyclic AMP supplied through a micropipette. The cell expressed mRFP- LimEΔ as a label for filamentous actin (red) and RBD-GFP for activated Ras (green), and was imaged by confocal fluorescence microscopy focused on the substrate-attached membrane region. Arrows indicate protrusions pointing upright the gradient. Time is indicated in seconds. The micropipette was moved at 32 s from the middle to the bottom and at 664 s from the bottom to the top of the frame; positions of its tip are indicated by white dots. Scale bar, 10 μm. The same cell is shown in Movie 1.
The example shown in Figure 2 is extraordinary in that a single wave surrounded most of the substrate-attached cell surface. In the following two figures, we show more common cases in which multiple waves propagated on the surface of large cells that were stimulated by a gradient of chemoattractant (Figs. 3, 4 and Movies 2, 3). In these cases, the cell behavior was dictated by two factors: by wave propagation into different directions and by a directed response of the cell to the gradient. The wave patterns were visualized by mRFP-LimEΔ together with PHcrac-GFP. Combination of the actin label with the label for PIP3 sharply distinguishes the inner territory from the external area,10 and thus provides an excellent tool for visualizing pattern evolution. Waves were seen to be initiated somewhere on the substrate-attached cell surface rather than specifically at the front region that pointed toward the micropipette (0-s frame of Fig. 3). Protrusions toward the source of attractant could be formed from areas outside the waves (44-s frame of Fig. 3; 78-s and 268-s frames of Fig. 4). However, when waves that propagated into the direction of the gradient reached the cell border, they dominated the movement of the whole cell along the gradient (44-s to 174-s frames of Figure 3 and 268-s to 384-s frames of Figure 4).
Figure 3.
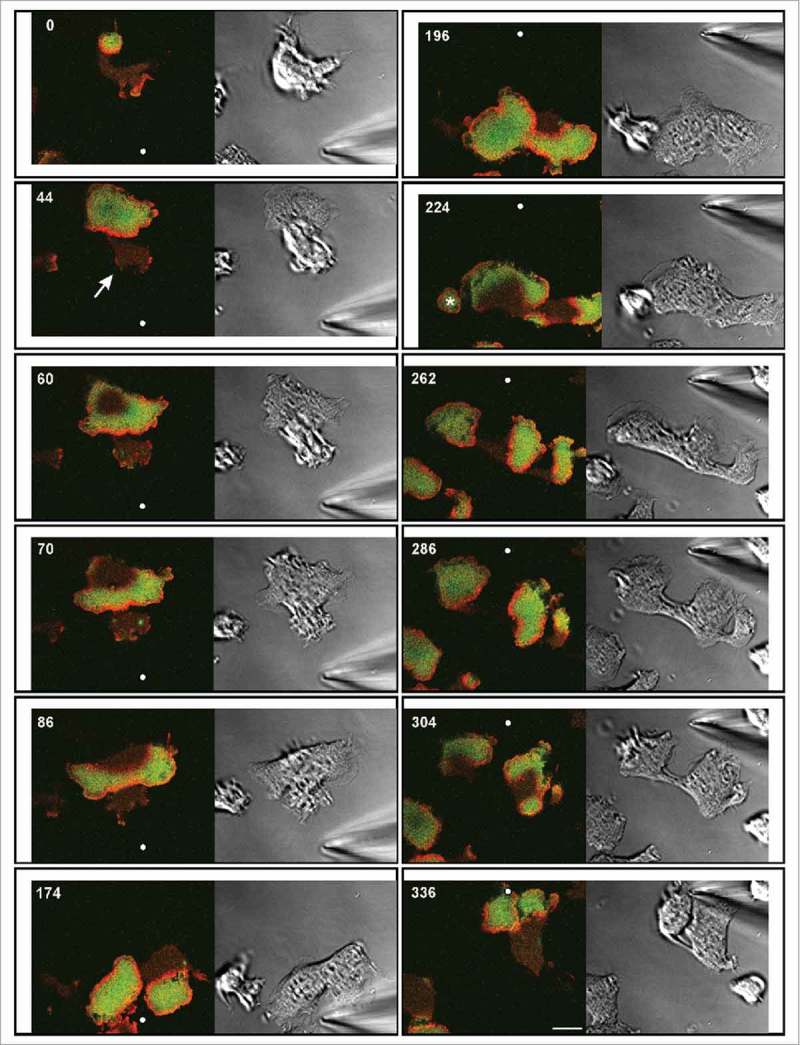
Chemotactic stimulation of a wave-forming large cell. The cell expressed mRFP-LimEΔ for filamentous actin (red) and GFP-PHcrac for PIP3 (green). The labels sharply distinguish the PIP3-rich inner territories, each one circumscribed by an actin wave, from the PIP3-depleted external areas. Time is indicated in seconds. At 190 s the micropipette is moved from the bottom to the top. Positions of its tip are indicated by white dots. Left panels: confocal fluorescence images depicting the substrate-attached membrane area. Right panels: DIC images. A protrusion formed outside a wave is indicated by arrow in the 44-s frame. The asterisk in the 224-s frame indicates a smaller cell attached to the large one. At the 286-s frame the cell responds with three protrusions, each one populated by a wave. The glass coverslip, on which the cell was moving, was slightly shifted twice during the experiment. Account of these shifts is taken by placing the images at varying positions into an invariable frame. Bar, 10 µm. The same cell is also seen in Movie 2.
Figure 4.
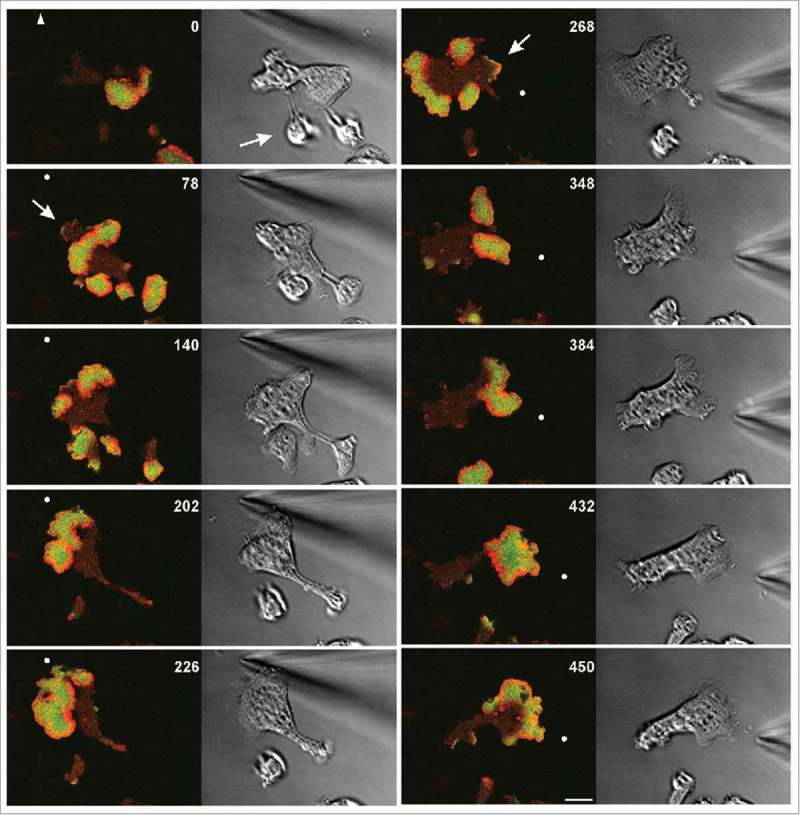
Chemotactic stimulation of a large cell expressing the same labels for actin (red) and PIP3 (green) as the cell in Figure 3. Time is indicated in seconds. At 264 s the micropipette is moved from the top left to the right. Positions of its tip are indicated by white dots, or by an arrowhead when outside the frame. Left panels: confocal fluorescence images of the substrate-attached membrane area. Right panels: DIC images. Arrows in the 78-s and 268-s frames point to protrusions formed in external areas, both of them paralyzed in consecutive frames by fast propagating waves. The arrow in the 0-s frame indicates a piece of the cell that was still connected to the major part of the cell through a thin cytoplasmic bridge that subsequently disrupted. Scale bar, 10 μm. The same cell is also seen in Movie 3.
For a direct comparison, we have scanned the fluorescence intensities of a protrusion in parallel to a neighboring wave, both progressing toward a micropipette (Fig. 5). The initial velocities were 0.14 µm × s−1 for the protrusion outside the wave, and 0.16 µm × s−1 for the wave front (determined as the average of the first five intervals in Fig. 5B). While the protrusion subsequently stopped moving and lost its PIP3 label, the cell border in front of the wave continued to propagate. In general, the PIP3 label within a wave was higher than at a protrusion outside a wave. (For the protrusion in the 268-s frame of Figure 4 see the scan in Figure S2). It should be noted, however, that the front of a cell is usually elevated one or a few µm beyond the substrate surface, such that in the images focused on this surface the front label is under-represented.
Figure 5.
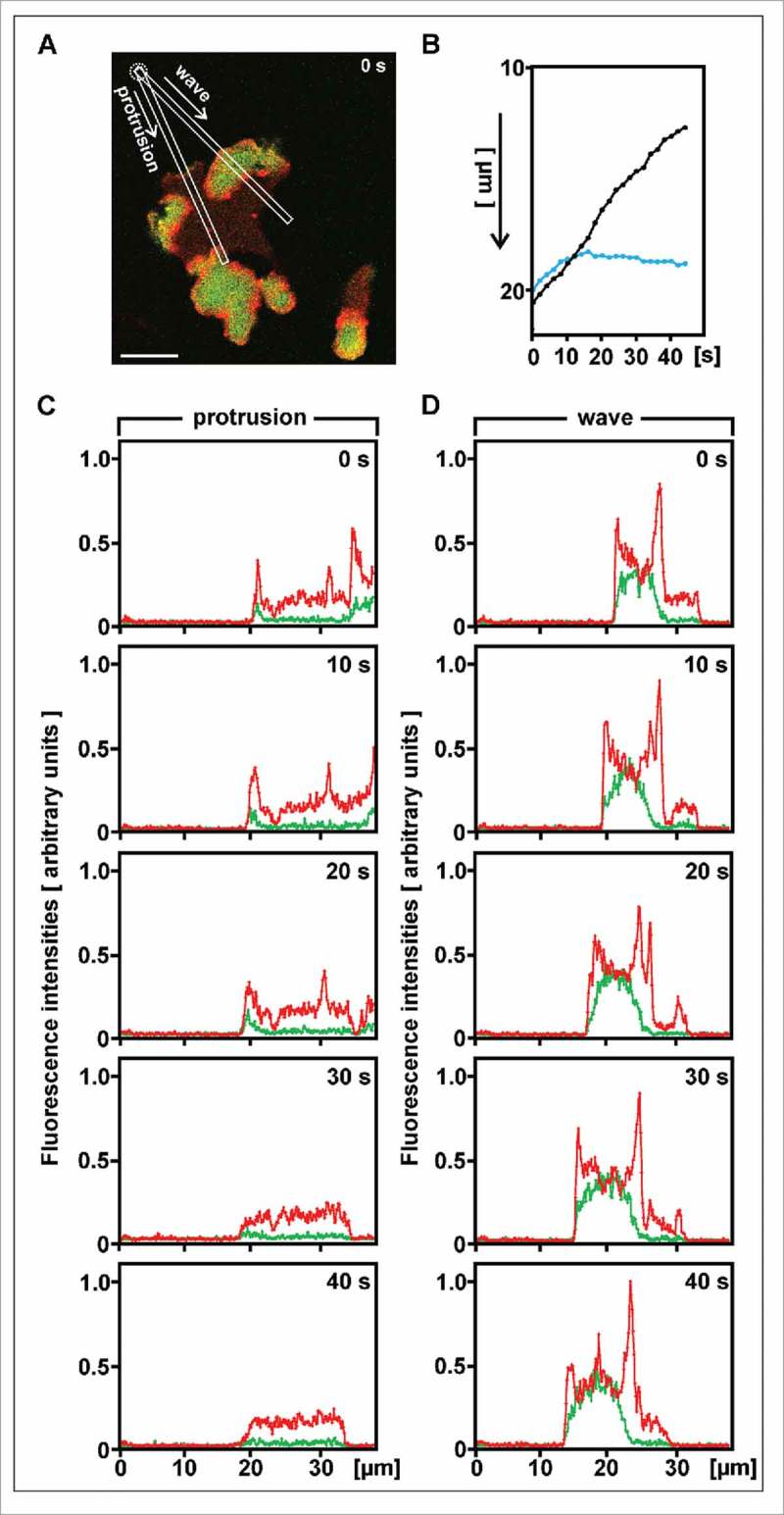
Comparison of velocities and fluorescence intensities between a protrusion outside a wave and the cell border engaged by a wave. The measurements are made in the cell shown in Figure 4; the 0-time here corresponds to 122 s in Figure 4. (A) Direction of scans. Their origin at the position of the micropipette (dotted circle) is set to zero in the graphs of (B) to (D). Scale bar, 10 µm. (B) Progression of the cell border along the two scans shown in (A), either at the protrusion outside the wave (blue) or at the wave (black). On the y-axis the distance from the micropipette is plotted from top to bottom. (C) and (D) Scans of the labels for PIP3 (green) and filamentous actin (red) along the protrusion (C) or the wave (D). Units of fluorescence are the same in (C) and (D). The x-axis indicates distance from the micropipette.
In a report on wave dynamics in the absence of chemoattractant, it has been pointed out that circular waves become unstable when they increase in size.7 The shape transition that breaks the symmetry begins with local opening of the actin wave, turning the circular wave into a horse-shoe shape. For activated Ras this is seen in the 197-s frame of Figure 1. Thus the wave is turned into one or several propagating bands, in which the territory enriched in Ras and PIP3 is confined by a leading and a trailing segment of an actin wave. The circular waves formed in the sequences of Figures 3 and 4 are opening on the opposite side to the source of the gradient, most evidently in the sequence of the 432-s to 450-s frames in Figure 4, which might suggest an influence of the gradient on symmetry breaking.
Discussion
The chemotactic stimulation of large wave-forming cells revealed that gradient sensing is compatible with an autonomous excitable system underlying a wave pattern in the cell membrane and actin cortex. Neither does a chemotactic response suppress the propagation of waves nor do the waves prevent a chemotactic response. A remarkable case of coexistence is illustrated in Figure 2, where a wave is “encaged,” this means propagates within the boundary of a cell that moves in the direction of a gradient and reorients when that direction is altered.
Initiation sites of a wave are not coupled to a protrusion of the cell that is induced by chemoattractant (Figs. 3 and 4). Conversely, at a cell front induced by chemoattractant, Ras can be activated and PIP3 synthesized without eliciting a propagated wave (1228-s and 1336-s frames of Fig. 2 and Movie 1). These data are consistent with the finding that signal transmission in the chemotactic response system is spatially confined, which prevents the response from spreading throughout the cell.11
The independence of wave initiation from the front of a cell distinguishes the wave-propagating system studied here from the signal transduction excitable network (STEN) implicated in the migration of Dictyostelium cells.21 As part of STEN, Ras acts at the front of a cell in promoting sustained protrusions by coupling to the cytoskeletal network, which by itself generates only local oscillations of actin polymerization.
Our data do not provide solid evidence that the direction of wave propagation is biased by the direction of the gradient, although a putative influence of a gradient on the symmetry breaking in circular waves would be consistent with this possibility.
A basic difference between Ras activation in either response to chemoattractant or autonomous wave generation is the involvement of cell-surface cAMP receptors in the response to the external cue but not in the autonomous excitation. Various models of chemotactic signal transduction imply that activated receptors transmit a short-range positive and a long-range negative signal to the interior of the cell.22-25 If the inhibitory signal would act upstream of Ras, the activation of Ras in wave patterns should be suppressed by chemoattractant, and this inhibition should occur at a distance behind the front of a cell. Such a gradient-dependent inhibition of wave formation is not apparent in our recordings: a statistical approach based on more data would be needed to clarify that point.
The waves can be considered as frustrated phagocytic or macropinocytic cups.26 Their co-existence with a chemotactic response is surprising since macropinocytosis has been reported to compete with chemotaxis.27 It may be the membrane invagination associated with vesicle formation that interferes with chemotaxis. On the other hand, single protrusions can turn into the direction of an attractant gradient while they internalize a macropinosome.1
The inner territory surrounded by an actin wave is distinguished from the external area not only by the activation of Ras (Fig. 1) and by its high PIP3 content (Figs. 3 and 4), but also by its dense network of actin filaments together with enrichment in the Arp2/3 complex.28 In contrast, myosin II and the actin-bundling protein cortexillin are more abundant in the external area, similar as in the tail of a migrating cell.28 It is therefore remarkable that regions of the external area can protrude toward a source of attractant (44-s frame of Fig. 3 and 268-s frame of Fig. 4), even though waves that propagate in the right direction tend to over-ride the protrusions formed in the external area (60-s to 174-s frames of Fig. 3, 348-s frame of Fig. 4, and Fig. 5B). The formation of protrusions in the external area may be comparable to the chemotactic response elicited in the tail region of a Dictyostelium cell or a neutrophil by a strong gradient of chemoattractant.29,30
In conclusion, our data indicate that gradient sensing is compatible with the autonomous activation of Ras and the synthesis of PIP3 in dynamic wave patterns on the substrate-attached cell surface.
Material and methods
Cell culture and strains
For dual-color fluorescence imaging, cells of the AX2–214 strain of D. discoideum were transformed to express pair-wise mRFPM-LimEΔ31 and superfolding (sf) GFP-PHcrac,32 mRFPM-LimEΔ and GFP- Raf1-RBD,1 the minimal Ras-binding domain (RBD) of human Raf proto-oncogene serine/threonine-protein kinase (Raf-1) as an activation-specific probe for Ras,33,34 or mRFPM-Raf1-RBD and PTEN-sfGFP.1 The double-transformants were cultivated in nutrient medium containing 10 µg/ml of blasticidin (Invitrogen, Life Technologies, Grand Island, NY, USA) and 10 µg/ml of G418 (Sigma-Aldrich, St. Louis, Missouri, USA) at 21 ± 2°C.
Cell fusion
Cells from four sub-confluent Petri dishes were harvested in 40 ml of 17 mM K/Na-phosphate buffer, pH 6.0 (PB), centrifuged at 4°C for 5 min at 300 × g and washed with 20 ml cold PB. After adjustment to 1.5 × 107 cells / ml, the suspension was gently shaken in a roller tube to allow the cells to agglutinate during a starvation period of 4 h. Subsequently, aliquots of the suspension were transferred to electroporation cuvettes with an electrode distance of 4 mm (VWR International, Radnor, Pennsylvania, USA) using a pipette with the tip cut-off to prevent dissociation. Cells were fused in a BioRad Gene Pulser Model 1652077 (Bio-Rad Laboratories, Hercules, CA, USA) by applying 3 pulses of 1 kV at 1- s intervals with 1 or 3 µF capacity. A 20-µl aliquot of the fused cell suspension was transferred into a tissue-culture dish with a HCl-cleaned cover-glass bottom (FluoroDish, WPI, INC., Sarasota, FL, USA). After 5 minutes, 3 ml of PB supplemented with 2 mM CaCl2 and 2 mM MgCl2 were added. After settling, the cells were subjected to imaging.
Chemotactic stimulation and confocal microscopy
For chemotactic stimulation, a Femtotip microcapillary (Eppendorf, Köln, Germany) was filled with 10−4 M cAMP solution. Using a micromanipulator (Micro Control Instruments Ltd., East Sussex, UK), the pipette tip was lowered until brought into the field of view and moved to the vicinity of a cell.
Confocal images of the substrate-attached membrane region were acquired at a Zeiss LSM 780 equipped with a Plan-Apo 63x / NA 1.46 oil immersion objective (Carl Zeiss Microscopy, Jena, Germany) at 21 ± 2°C. The images were analyzed and processed using the image processing package Fiji (http://Fiji.sc/Fiji) developed by Schindelin et al.35 on the basis of ImageJ http://imagej.nih.gov/ij).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Martin Spitaler and his staff for providing imaging facilities, and Petra Fey and dictyBase for informations, Jana Prassler and Alexandra James for assistance.
Funding
The work was supported by funds of the Max Planck Society.
References
- [1].Lange M, Prassler J, Ecke M, Müller-Taubenberger A, Gerisch G. Local Ras activation, PTEN pattern, and global actin flow in the chemotactic responses of oversized cells. J Cell Sci 2016; 129:3462-72; PMID:27505897; http://dx.doi.org/ 10.1242/jcs.191148 [DOI] [PubMed] [Google Scholar]
- [2].Wang Y, Chen C-L, Iijima M. Signaling mechanisms for chemotaxis. Dev, Growth Differ 2011; 53:495-502; http://dx.doi.org/ 10.1111/j.1440-169X.2011.01265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Insall R. The interaction between pseudopods and extracellular signalling during chemotaxis and directed migration. Curr Opin Cell Biol 2013; 25:526-31; PMID:23747069; http://dx.doi.org/ 10.1016/j.ceb.2013.04.009 [DOI] [PubMed] [Google Scholar]
- [4].Srinivasan K, Wright GA, Hames N, Housman M, Roberts A, Aufderheide KJ, Janetopoulos C. Delineating the core regulatory elements crucial for directed cell migration by examining folic-acid-mediated responses. J Cell Sci 2013; 126:221-33; PMID:23132928; http://dx.doi.org/ 10.1242/jcs.113415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nichols JME, Veltman D, Kay RR. Chemotaxis of a model organism: progress with Dictyostelium. Curr Opin Cell Biol 2015; 36:7-12; PMID:26183444; http://dx.doi.org/ 10.1016/j.ceb.2015.06.005 [DOI] [PubMed] [Google Scholar]
- [6].Gerisch G, Bretschneider T, Müller-Taubenberger A, Simmeth E, Ecke M, Diez S, Anderson K. Mobile actin clusters and traveling waves in cells recovering from actin depolymerization. Biophys J 2004; 87:3493-503; PMID:15347592; http://dx.doi.org/ 10.1529/biophysj.104.047589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gerhardt M, Ecke M, Walz M, Stengl A, Beta C, Gerisch G. Actin and PIP3 waves in giant cells reveal the inherent length scale of an excited state. J Cell Sci 2014; 127:4507-17; PMID:25107368; http://dx.doi.org/ 10.1242/jcs.156000 [DOI] [PubMed] [Google Scholar]
- [8].Shi CJ, Huang CH, Devreotes PN, Iglesias PA. Interaction of motility, directional sensing, and polarity modules recreates the behaviors of chemotaxing cells. Plos Comput Biol 2013; 9:17; http://dx.doi.org/ 10.1371/journal.pcbi.1003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bloomfield G, Traynor D, Sander SP, Veltman DM, Pachebat JA, Kay RR. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. eLife 2015; 4:e04940; http://dx.doi.org/ 10.7554/eLife.04940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gerisch G, Schroth-Diez B, Müller-Taubenberger A, Ecke M. PIP3 waves and PTEN dynamics in the emergence of cell polarity. Biophys J 2012; 103:1170-8; PMID:22995489; http://dx.doi.org/ 10.1016/j.bpj.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gerhardt M, Walz M, Beta C. Signaling in chemotactic amoebae remains spatially confined to stimulated membrane regions. J Cell Sci 2014; 127:5115-25; PMID:25300796; http://dx.doi.org/ 10.1242/jcs.161133 [DOI] [PubMed] [Google Scholar]
- [12].Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol 2004; 167:505-18; PMID:15534002; http://dx.doi.org/ 10.1083/jcb.200406177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kae H, Lim CJ, Spiegelman GB, Weeks G. Chemoattractant-induced Ras activation during Dictyostelium aggregation. EMBO Rep 2004; 5:602-6; PMID:15143344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bolourani P, Spiegelman GB, Weeks G. Delineation of the roles played by RasG and RasC in cAMP-dependent signal transduction during the early development of Dictyostelium discoideum. Mol Biol Cell 2006; 17:4543-50; PMID:16885420; http://dx.doi.org/ 10.1091/mbc.E05-11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gerisch G, Ecke M, Neujahr R, Prassler J, Stengl A, Hoffmann M, Schwarz US, Neumann E. Membrane and actin reorganization in electropulse-induced cell fusion. J Cell Sci 2013; 126:2069-78; PMID:23447671; http://dx.doi.org/ 10.1242/jcs.124073 [DOI] [PubMed] [Google Scholar]
- [16].Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 2002; 109:611-23; PMID:12062104; http://dx.doi.org/ 10.1016/S0092-8674(02)00755-9 [DOI] [PubMed] [Google Scholar]
- [17].Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 2002; 109:599-610; PMID:12062103; http://dx.doi.org/ 10.1016/S0092-8674(02)00745-6 [DOI] [PubMed] [Google Scholar]
- [18].Bretschneider T, Diez S, Anderson K, Heuser J, Clarke M, Müller-Taubenberger A, Köhler J, Gerisch G. Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr Biol 2004; 14:1-10; PMID:14711408; http://dx.doi.org/ 10.1016/j.cub.2003.12.005 [DOI] [PubMed] [Google Scholar]
- [19].Dormann D, Weijer G, Dowler S, Weijer CJ. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J Cell Sci 2004; 117:6497-509; PMID:15572406; http://dx.doi.org/ 10.1242/jcs.01579 [DOI] [PubMed] [Google Scholar]
- [20].Parent CA. Making all the right moves: chemotaxis in neutrophils and Dictyostelium. Curr Opin Cell Biol 2004; 16:4-13; PMID:15037299; http://dx.doi.org/ 10.1016/j.ceb.2003.11.008 [DOI] [PubMed] [Google Scholar]
- [21].Huang C-H, Tang M, Shi C, Iglesias PA, Devreotes PN. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat Cell Biol 2013; 15:1307-16; PMID:24142103; http://dx.doi.org/ 10.1038/ncb2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci 1999; 112:2867-74; PMID:10444381 [DOI] [PubMed] [Google Scholar]
- [23].Meinhardt H, Gierer A. Pattern formation by local self-activation and lateral inhibition. BioEssays 2000; 22:753-60; PMID:10918306; http://dx.doi.org/ 10.1002/1521-1878(200008)22:8%3c753::AID-BIES9%3e3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- [24].Levchenko A, Iglesias PA. Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys J 2002; 82:50-63; PMID:11751295; http://dx.doi.org/ 10.1016/S0006-3495(02)75373-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Devreotes P, Janetopoulos C. Eukaryotic Chemotaxis: Distinctions between directional sensing and polarization. J Biol Chem 2003; 278:20445-8; PMID:12672811; http://dx.doi.org/ 10.1074/jbc.R300010200 [DOI] [PubMed] [Google Scholar]
- [26].Gerisch G, Ecke M, Schroth-Diez B, Gerwig S, Engel U, Maddera L, Clarke M. Self-organizing actin waves as planar phagocytic cup structures. Cell Adhesion Migration 2009; 3:373-82; PMID:19855162; http://dx.doi.org/ 10.4161/cam.3.4.9708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Veltman DM, Lemieux MG, Knecht DA, Insall RH. PIP3-dependent macropinocytosis is incompatible with chemotaxis. J Cell Biol 2014; 204:497-505; PMID:24535823; http://dx.doi.org/ 10.1083/jcb.201309081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schroth-Diez B, Gerwig S, Ecke M, Hegerl R, Diez S, Gerisch G. Propagating waves separate two states of actin organization in living cells. HFSP J 2009; 3:412-27; PMID:20514132; http://dx.doi.org/ 10.2976/1.3239407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gerisch G, Keller HU. Chemotactic reorientation of granulocytes stimulated with micropipettes containing fMet-Leu-Phe. J Cell Sci 1981; 52:1-10; PMID:7037797 [DOI] [PubMed] [Google Scholar]
- [30].Segall JE, Gerisch G. Genetic approaches to cytoskeleton function and the control of cell motility. Curr Opin Cell Biol 1989; 1:44-50; PMID:2560931; http://dx.doi.org/ 10.1016/S0955-0674(89)80035-3 [DOI] [PubMed] [Google Scholar]
- [31].Fischer M, Haase I, Simmeth E, Gerisch G, Müller-Taubenberger A. A brilliant monomeric red fluorescent protein to visualize cytoskeleton dynamics in Dictyostelium. FEBS Lett 2004; 577:227-32; PMID:15527790; http://dx.doi.org/ 10.1016/j.febslet.2004.09.084 [DOI] [PubMed] [Google Scholar]
- [32].Müller-Taubenberger A, Ishikawa-Ankerhold HC. Fluorescent reporters and methods to analyze fluorescent signals In: Eichinger L, Rivero F, eds. Dictyostelium discoideum Protocols. Totowa, NJ: Humana Press, 2013:93-112. [DOI] [PubMed] [Google Scholar]
- [33].Nassar N, Horn G, Herrmann CA, Scherer A, McCormick F, Wittinghofer A. The 2.2 A crystal structure of the Ras-binding domain of the serine/threonine kinase c-Raf1 in complex with RaplA and a GTP analogue. Nature 1995; 375:554-60; PMID:7791872; http://dx.doi.org/ 10.1038/375554a0 [DOI] [PubMed] [Google Scholar]
- [34].de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene 1997; 14:623-5; PMID:9053862; http://dx.doi.org/ 10.1038/sj.onc.1201005 [DOI] [PubMed] [Google Scholar]
- [35].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al.. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9:676-82; PMID:22743772; http://dx.doi.org/ 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


