Abstract
Background
Chronic red blood cell transfusion is the first-line treatment for severe forms of thalassaemia. This therapy is, however, hampered by a number of adverse effects, including red blood cell alloimmunisation. The aim of this systematic review was to collect the current literature data on erythrocyte alloimmunisation.
Materials and methods
We performed a systematic search of the literature which identified 41 cohort studies involving 9,256 patients.
Results
The prevalence of erythrocyte alloimmunisation was 11.4% (95% CI: 9.3–13.9%) with a higher rate of alloimmunisation against antigens of the Rh (52.4%) and Kell (25.6%) systems. Overall, alloantibodies against antigens belonging to the Rh and Kell systems accounted for 78% of the cases. A higher prevalence of red blood cell alloimmunisation was found in patients with thalassaemia intermedia compared to that among patients with thalassaemia major (15.5 vs 12.8%).
Discussion
Matching transfusion-dependent thalassaemia patients and red blood cell units for Rh and Kell antigens should be able to reduce the risk of red blood cell alloimmunisation by about 80%.
Keywords: thalassaemia, alloimmunisation, red blood cells, transfusion, complications
Introduction
Thalassaemia, a congenital haemolytic disorder caused by a partial or complete defect in the synthesis of α- or β-globin chains, is one of the major health issues worldwide, considering that every year 60,000 babies are born with severe forms of thalassaemia1. The primary therapy for thalassaemia major is lifelong transfusion of red blood cells (RBC), which has the aim of suppressing ineffective erythropoiesis and improving the growth and development of affected children2. However, although this treatment is life-saving, it is encumbered by a number of complications, including haemosiderosis, transfusion reactions, infections (potentially caused also by emerging or re-emerging infectious disease agents3,4, as the residual risk of transfusion-transmitted infections has consistently declined over time5), and alloimmunisation6–11. In particular, alloimmunisation is a challenging occurrence which increases the risk of delayed haemolytic transfusion reactions, complicates cross-matching and contributes to a delay in the identification and provision of compatible RBC transfusions10,11. Transfusion therapy may also be required by patients with thalassaemia intermedia in particular circumstances (growth failure, extramedullary haematopoiesis, pregnancy, infections, operations) so that a consistent proportion of such patients are at risk of developing transfusion-associated complications, including alloimmunisation12.
The purpose of this systematic review is to summarise the current literature on erythrocyte alloimmunisation in patients with transfusion-dependent thalassaemia, with the aim of improving our knowledge on the associated risk factors and optimising the approach to patients’ transfusion therapy.
Materials and methods
Search strategy
A computer-assisted literature search of the MEDLINE (through PUBMED), EMBASE, SCOPUS, OVID and Cochrane Library electronic databases was performed (latest search August 30, 2018) to identify studies on RBC alloimmunisation in patients with transfusion-dependent thalassaemia. A combination of the following text words was used to maximise the specificity and sensitivity of the search: “red blood cells”, “erythrocyte”, “alloimmunization”, “alloantibodies”, “reactions”, “thalassemia major”, “thalassemia intermedia”, “beta thalassemia”, “β thalassemia”, “transfusion”, “transfusion-dependent”, “patients”. In addition, we checked the reference lists of the most relevant items (original studies and reviews) in order to identify potentially eligible studies not captured by the initial literature search.
Study selection and inclusion criteria
Studies were selected independently by two reviewers (MF and MC), with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (GM). Assessment of the eligibility of studies was based on the title or abstract and on the full text, if required. Articles were eligible if they reported erythrocyte alloimmunisation in transfusion-dependent thalassaemia patients (i.e., patients with thalassaemia major or thalassaemia intermedia) in either the title or the abstract. Other required inclusion criteria were that the article be: (i) original, (ii) randomised, cohort, cross-sectional or case-control, (iii) retrospective or prospective, and (iv) published in full in English in the last 20 years (1998–2018).
Data extraction
For each study included in the systematic review13–53, the following data were extracted independently by two reviewers (MF and MC): first author, year of publication, origin of the population studied, study design, sample size and disease type, median age and range, rate and type of RBC alloantibodies detected, type of test used for alloantibody detection, transfusion protocol and evaluated variables (RBC phenotype matching, sex, age at enrolment, age at first transfusion, years of transfusions, RBC units received, splenectomy, RBC leucoreduction) with the main results of the studies. Disagreement was resolved by consensus and by the opinion of a third reviewer (GM), if necessary.
Assessment of risk of bias in the included studies
Since no randomised controlled trials were found for this systematic review, we assessed the methodological quality of the observational studies following the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions on assessing the quality of non-randomised studies54. We used the Newcastle-Ottawa quality assessment scale (NOS) to assess the quality of cohort studies55.
The methodological quality of cohort studies is assessed by examining selection (four questions relating to the representativeness of the cohort, selection of the unexposed cohort, ascertainment of exposure and demonstration that the outcome of interest was not present at the start of the study), comparability (exposed and unexposed individuals must be matched in the design and/or confounders must be adjusted for in the analysis) and outcome (three questions relating to the assessment of outcome, follow up long enough for outcomes to occur and losses to follow up).
These assessments were performed independently in duplicate (MC, MF) and a third reviewer (GM) resolved any disagreements to reach consensus. Using the NOS, a study can be awarded a maximum of four stars for selection, a maximum of two stars for comparability, and a maximum of three stars for outcome (Online Supplementary Content, Table SI).
Statistical analysis
Qualitative data are presented as numbers and percentages, while quantitative data are expressed statistically in frequencies and percentages. A mean prevalence of RBC alloantibodies was estimated by meta-analysis of the proportions method. This method is based on meta-analytical pooling of the logit-transformed prevalences at a study level. These are used to produce summary measures. The meta-analytical methods used were: the inverse variance method; DerSimonian-Laird estimator for tau2; logit transformation; Clopper-Pearson confidence interval for individual studies; and a continuity correction of 0.5 in studies with zero cell frequencies. Both fixed effects and random effects models were used. The heterogeneity between studies was assessed by the heterogeneity χ2 test (Cochran’s Q), the I2 statistics, and the DerSimonian-Laird estimate of between-study variance (τ2). When the size of the population affected by thalassaemia major or thalassaemia intermedia clinical variants was reported along with the alloantibody prevalence, the relevant information was used to perform subgroup analyses. The random effects model is reported as it is preferred in subgroup meta-analyses, or when heterogeneity tests are significant. All calculations were done using R-3.5.1 for windows.
Results
Literature search and characteristics of the studies
In total, 478 articles were identified through the initial electronic and manual searches (Figure 1). Three hundred and fifty-two of them were excluded because they focused on topics outside the scope of this systematic review. Thus, 126 potentially relevant articles were selected and the next screening led to the exclusion of 85 of these (reviews, duplicates, studies not evaluating patients with transfusion-dependent thalassaemia, studies containing no informative data). Finally, 41 studies13–53 were included in the systematic review (see Table I for the main characteristics and results of the studies included). The studies contained data from 9,256 individuals (children and adults) from different countries and originated in India (n=12), Iran (n=6), Egypt (n=6), Pakistan (n=3), Oman (n=3), Taiwan (n=2), Asia (n=2) and the USA, Malaysia, Tunisia, Hong Kong, Turkey, Albania, Kuwait (each with 1 study) (Table I). No studies conducted in European countries were included in this systematic review.
Figure 1.
Flow chart of the selection of the studies.
Table I.
Characteristics and main results of the studies included on alloimmunisation in transfusion-dependent thalassaemia.
| Study (year)ref | Origin | Study design | Cases (N) | Median age, years (range) | Alloantibodies | Transfusion policy | Risk factors analysis | |
|---|---|---|---|---|---|---|---|---|
| N (%) | Type (%) | |||||||
| Singer (2000)13 | Asian | Cohort, retrospective | 64 (30 TM; 34 others) | 15 (2–39) | 14/64 (21.9) | Anti-K (42.9); anti-Kpa (7.1); anti-E (28.6); anti-c (14.3); anti-i (14.3); anti-M (7.1); anti-Jkb (7.1); anti-Lea (7.1) | ABO-D or ABO-Rh and Kell matched RBC. Bedside leucofiltered blood in 90% of patients. |
A positive association was found between splenectomy and alloimmunisation. Transfusion of ABO-Rh-Kell matched RBC was effective in preventing alloimmunisation. |
| Ameen (2003)14 | Kuwait | Cohort, prospective | 190 TM | 12.7 (0.25–33) | 57/190 (30.0) | Anti-K (72); anti-E (45.6); anti-D (21.1); anti-v (21.1); anti-C (15.8); anti-Lea (12.3); anti-Jka (10.5); anti-c (8.8); anti-Cw (8.8); anti-Kpa (5.3); anti-S (5.3); anti-Jsa (3.5); anti-Fya (3.5); anti-Fyb (3.5); anti-Leb (3.5); anti-Jkb (1.8); anti-Lua (1.8); anti-Cob (1.8) | ABO-Rh matched RBC for patients with antibodies. Post-storage leucoreduction. |
ABO-Rh and Kell matching along with pre-storage RBC leucodepletion would reduce the risk of alloimmunisation. |
| Bilwani (2005)15 | Pakistan | Cohort, retrospective | 97 TM | 10.6 (2–24) | 9/97 (9.2) | Anti-K (22); anti-D (11.1); anti-E (11.1); anti-D+C (11.1); anti-E+K (11.1) | ABO-D matched RBC. | - |
| Wang (2006)16 | Taiwan | Cohort, cross-sectional | 30 (28 TM; 2 others) | 20 (4–31) | 11/30 (36.7) | Anti-E (36); anti-E+c (18); anti-Mia (18); anti-Mia+anti-c (9); anti-D (9); anti-S (9) | ABO matched RBC. Bedside leucoreduction. |
- |
| Noor Haslina (2006)17 | Malaysia | Cohort, prospective | 58 (8 TM; 50 others) | NA | 5/58 (8.6) | Anti-E (40); anti-E+anti-c+anti-S+anti-N (20); anti-E+anti-Jka (20); anti-K (20) | - | - |
| Karimi (2007)18 | Iran | Cohort, cross-sectional | 711 TDT | 14.4 (1–41) | 38/711 (5.3) | Anti-K (50); anti-D (15.8); anti-E (10.5); anti-D+C (7.9); anti-C (5.3); anti-c (5.3); anti-e (2.6); anti-Lua (2.6) | ABO-D matched RBC. Bedside leucofiltered blood in 73% of patients. |
A positive correlation was found between alloimmunisation and duration of blood transfusion (> 6 years) |
| Sadeghian (2009)19 | Iran | Cohort, cross-sectional | 313 TDT | 14.4 (0.7–38) | 9/313 (2.9) | Anti-D (88.9); anti-C (33.3); anti-E (11.1) | ABO-D matched RBC. Bedside leucofiltered blood in a subset of patients. |
A higher frequency of alloimmunisation was observed in female and splenectomised patients. |
| Ahmed (2010)20 | Egypt | Cohort, prospective | 448 (389 TM; 59 TI) | TM: 9.3 (2–24) TI: 12.7 (4–26) |
TM: 33/389 (8.4) TI: 4/59 (6.7) |
Anti-K (42); anti-E (24); anti-C (18), anti-Fya (6), anti-Kpa (6), anti-M (3) Anti-K (6), anti-E (6) |
ABO-D matched RBC. None for the majority of patients. |
A positive association was found between splenectomy and alloimmunisation. |
| Pahuja (2010)21 | India | Cohort, cross-sectional | 211 TM | 10.4 (6–28) | 9/211 (3.8) | Anti-E (33); anti-K (22); anti-D (11); anti-Kpa (11); anti-Cw (11); anti-c (11); anti-Jka (11) | ABO-D matched RBC. Bedside leucofiltered blood in a subset of patients. |
- |
| Gupta (2011)22 | India | Cohort, cross-sectional | 116 TM | 14 (2–27) | 11/116 (9.5) | Anti-E (36.4); anti-K (27.2); anti-Kpa (18.2); anti-Cw (18.2); anti-D (9); anti-E+anti-Kpa (9) | Leucoreduced RBC. | Higher incidence of alloantibody development if first transfusion > 2 years of age. |
| Chaudhari (2011)23 | India | Cohort, cross-sectional | 32 TM | NA (1–18) | 6/32 (18.8) | Anti-E (42.9); anti-c (28.6); anti-Leb (14.3); anti-Jkb (14.3) | ABO-D matched RBC. Non-leucoreduced RBC. |
- |
| Guirat-Dhouib (2011)24 | Tunis | Cohort, prospective | 130 TDT | 10 (1–26) | 10/130 (7.7) | Anti-E (30); anti-C (30); anti-D (10); anti-K (10); anti-E+anti-S (10); anti-D+anti-C (10) | ABO-D matched RBC. Non-leucoreduced RBC for the majority of patients. |
- |
| Azarkeivan (2011)25 | Iran | Cohort, prospective | 835 (707 TM; 128 TI) | NA | 101/835 (12.1) | Anti-K (33.7); anti-Rh (14.9); anti-D (10.9); anti-E (9.9); anti-D+anti-C (7.9); anti-K+anti-E (3); anti-K+anti-Kpa (3); anti-K+anti-D (1); anti-D+anti-E (1) | NA | The alloantibody risk was greater in patients with TI, with increasing age and more exposure to different RBC antigens. |
| Thompson (2011)26 | USA | Cohort, prospective | 697 TDT | NA | 115/697 (16.5) | Anti-E (19); anti-K (18.1); anti-C (9.5); anti-Kidd (7.8); anti-c (6.0); anti-e (5.2); anti-Kpa (5.2); anti-Le (3.4); anti-D (3.4); anti-S (2.6); anti-Duffy (1.7); anti-M (1.7) | Extended phenotype-matched RBC in a minority of patients. Leucoreduced RBC. |
Splenectomy and duration of transfusion were independent risk factors for alloimmunisation. |
| Saied (2011)27 | Egypt | Cohort, retrospective | 95 (74 TM; 21 TI) | 17.1 (1–45) | 27/95 (28.4) | Anti-Kell (23.6); anti-E (23.6); anti-C (7.3); anti-D (5.5); anti-c (5.5); anti-S (5.5); anti-Fya (5.5); anti-Jka (3.6); anti-Jkb (3.6); anti-Lea (3.6); anti-M (3.6); anti-s (1.8); anti-Leb (1.8); anti-Fyb (1.8) | ABO-D matched RBC. Leucoreduced RBC in 67% of patients. |
Older age, transfusion frequency and non-leucodepleted RBC were associated with a higher rate of alloimmunisation. |
| Cheng (2012)28 | Hong Kong | Cohort, retrospective | 382 TM | 23 (0.25–52) | 77/382 (20.2) | Anti-E (37); anti-Mia/Mur (29); anti-c (12); anti-Jka (6); anti-S (1.8); anti-Di (1.8); anti-C (0.9); anti-e (0.9); anti-f (0.9); anti-K (0.9); anti-Jkb (0.9) | Leucoreduction within 3 days of production. | - |
| El-Danasoury (2012)29 | Egypt | Cohort, prospective | 235 TM | 12 (6–34) | 46/235 (19.5) | Anti-Kell (23.9); anti-E (23.9); anti-C (15.2); anti-D (13); anti-Cw (11); anti-N (4.3); anti-Jka (4.3); anti-Jkb (2.1); anti-Lea (2.1) | ABO-D matched RBC. | Development of alloantibodies was associated with older age > 12 years, higher transfusion frequency, splenectomy. |
| Chao (2013)30 | Taiwan | Cohort, cross-sectional | 64 TM | 19.2 (+6.7)1 | 6/64 (9.4) | Anti-E (66.6); anti-C (16.7); anti-Mia (16.7) | ABO-D matched RBC. | - |
| Makroo (2013)31 | India | Cohort, prospective | 462 TDT | NA (0.7–38) | 19/462 (4.1) | Anti-K (42.1); anti-E (36.8); anti-D (5.3); anti-Cw (3.5); anti-E+anti-C (3.5) | NA | - |
| Al-Riyami (2014)32 | Oman | Cohort, retrospective | 37 TI | 27 (11–59) | 7/24 (29.2) | Anti-E (57.1); anti-c (28.6); anti-K (28.6); anti-Jkb (28.6); anti-D (14.3) | Extended phenotype-matched RBC. Leucoreduced RBC. |
A high proportion of patients (24/37, 65%) required RBC transfusion and about one third of them developed an alloantibody. |
| Dhawan (2014)33 | India | Cohort, cross-sectional | 319 TM | 15.2 (1.5–27) | 18/319 (5.6) | Anti-E (17); anti-D (13); anti-C (13); anti-Cw (9); anti-K (35); anti-Kidd (9); anti-Xg (4) | ABO-D matched RBC. Antigen matched in patients with alloantibodies. | A lower rate of alloimmunisation was recorded in patients aged > 2 years at first transfusion. |
| Jain (2014)34 | India | Cohort, prospective | 96 TM | NA | 5/96 (5.2) | Anti-K (60); anti-E (20); anti-Kpa (20) | ABO-D matched RBC. | A higher rate of alloimmunisation was observed in female patients. |
| Hussein (2014)35 | Egypt | Cohort, retrospective | 272 (207 TM; 65 TI) | NA | 62/272 (22.8) | Anti-E (14.6); anti-D (8.9); anti-C (8.9); anti-c (4.9); anti-K (26); anti-MNS (9.8); anti-Kidd (8.9); anti-Duffy (8.1); anti-Le (5.7); anti-Lu (2.4); anti-P1 (1.6) | ABO-D matched RBCs. Pre-storage leucoreduction in 40% of patients. |
The risk of alloimmunisation increased with male gender, the number of units transfused, in patients splenectomised and receiving non-leucoreduced RBC. |
| Philip (2014)36 | India | Cohort, retrospective | 82 TDT | NA | 6/82 (7.3) | Anti-E (43); anti-D (14); anti-c (14); anti-Lea (14) | ABO-D matched RBC. Leucoreduced RBC. |
- |
| Koçyiğit (2014)37 | Turkey | Cohort, retrospective | 139 (109 TM; 30 TI) | 18.3 (+8.7)1 | 9/139 (6.4) | Anti-K (27); anti-C (27); anti-D (18); anti-Jka (18); anti-E (9) | Leucoreduced RBC. | The risk of alloimmunisation increased in patients with TI and in those who received a first transfusion > 2 years of age. |
| Elhence (2014)38 | India | Cohort, prospective | 280 TM | 10 (0.7–32) | 24/280 (8.6) | Anti-E (39.3); anti-K (21.4); anti-c (10.8); anti-D (7.1); anti-C (7.1); anti-Jka (7.1); anti-N (3.5); anti-S (3.5) | ABO-D matched RBC. | - |
| Vichinsky (2014)39 | Asian and Caucasian | Cohort, prospective | 365 (278 TM; 28 TI; 59 others) | NA | 68/365 (19) | Anti-E (29); anti-K (17); anti-C (12); anti-Jka (6); anti-c (5); anti-Kpa (4); anti-D (3); anti-S (3); anti-Cw (2); anti-e (2); anti-Jkb (2); anti Lea (2); anti-Fya (1); anti-Fyb (1); anti-Lua (1); anti-M (1) | ABO-D matched RBC (31%); ABO-Rh-K matched RBC (38%); extended phenotype-matched RBC (10%). Leucoreduced RBC (94%). | Years of transfusion was an independent predictor of alloimmunisation. |
| Dogra (2015)40 | India | Cohort, cross-sectional | 70 (59 TM; 8 TI; 3 others) | NA (2–17) | 6/70 (8.6) | Anti E (37.5); anti-K (25); anti-D (12.5) | ABO-D matched RBC. | - |
| Datta (2015)41 | India | Cohort, prospective | 500 (333 TM; 167 TI) | NA | 28/500 (5.6) | Anti-c (28.6); anti-E (21.4); anti-c+anti-E (17.8); anti-Jkb (7.1); anti-Jka (3.6); anti-C (3.6), anti-D (3.6); anti-D+anti-C (3.6); anti-E+anti-Fyb (3.6), anti-s (3.6) | ABO-D matched RBC. | - |
| Jansuwan (2015)42 | India | Cohort, cross-sectional | 143 TM | 16 (11–21) | 25/143 (17.5) | Anti-E (52); Anti-c (16); anti.M (16); anti-Lea (16); anti-Mia (16); anti-Jka (8) | Leucoreduced RBC. | Alloimmunisation correlated with splenectomy. |
| Seferi (2015)43 | Albania | Cohort, prospective | 118 TM | 17 (2–34) | 14/118 (11.8) | Anti-K (23.9); anti-D (19.1); anti-C (14.3); anti-E (14.3); anti-c (14.3); anti-e (4.7); anti-Jkb (4.7); anti-Cw (4.7) | Post-storage leucoreduced RBC. | Rhesus and Kell matching greatly reduced the development of new alloantibodies. |
| Vaziri (2015)44 | Iran | Cohort, cross-sectional | 100 TDT | 15 (+7.9)1 | 4/100 (4) | Anti-K (75); anti-D+anti-C (25) | Leucoreduced RBC. | - |
| Zaidi (2015)45 | Pakistan | Cohort, cross-sectional | 162 TM | 6.7 (0.5–25) | 14/162 (8.6) | Anti-E (28.6); anti-K (21.4); anti-e (14.3); anti-D (7.1); anti-D+anti-C (7.1); anti-E+ anti-K (7.1); anti-D+anti-E+anti-C (7.1); anti-E+anti-K+anti-C+anti-M+anti-Kpa(7.1) | ABO-D matched RBC. | Matching RBC for Rh and Kell antigens reduced the risk of alloimmunisation by 90%. |
| Obaid (2015)46 | Egypt | Cohort, prospective | 40 (26 TM, 14 TI) | TM: 20.3 (+7.3) TI: 36.9 (+11.4) |
17/40 (42.5) | Anti-Lub (19); anti-Kpb (19); anti-Lua (9.5); anti-Kpa (9.5); anti-D (4.8); anti-c (4.8); anti-K (4.8) | Non-leucoreduced RBC. | - |
| Davari (2016)47 | Iran | Cohort, prospective | 49 TM | 18.6 (+8.16)1 | 8/49 (16.3) | Anti-K (75); anti-E (12.5); anti-c (12.5); anti-Leb (12.5) | ABO-D matched RBC. Leucoreduced RBC in the majority of patients. |
Lower rate in patients who received leucoreduced RBC. |
| Abdelrazik (2016)48 | Egypt | Cohort, prospective | 188 (147 TM; 41 TI) | 10 (2–45) | 15/188 (8.0) | Anti-D (53); anti-E (13); anti-C (13); anti-c (13); anti-Fya (13); anti-K (6.6) | ABO-D matched RBC. Non-leucoreduced RBC. |
High rate of alloimmunisation in female patients, with TI, splenectomised, Rh-D neg. and who received a first transfusion at > 3 years of age. |
| Bhuva (2017)49 | India | Cohort, prospective | 250 TM | NA (1–80) | 8/250 (3.2) | Anti-K (50); anti-D (25); anti-E (12.5); anti-Kpa (12.5) | Non-leucoreduced RBC. | - |
| Davoudi-Kiakalayeh (2017)50 | Iran | Cohort, retrospective | 190 TM | 26 (+5.9)1 | 47/190 (24.7) | Anti-K (33); anti-E (25); anti-D (19) | Post-storage leucoreduced RBC. | - |
| Alkindi (2017)51 | Oman | Cohort, prospective | 129 TM | NA (5–32) | 26/129 (20) | Anti-K (26.9); anti-E (23.1); anti-c (7.7); anti-C+anti-D (7.7); anti-D (3.8); anti-e (3.8); anti-Kpa (3.8); anti-E+anti-K (3.8); anti-C+anti-e (3.8); anti-C+anti-D+anti-Jka (3.8); anti-E-anti-Cw (3.8) | NA | - |
| Al-Riyami (2018)52 | Oman | Cohort, retrospective | 268 TDT | 22 (2–43) | 25/268 (9.3) | Anti-E (24); anti-K (24); anti-D (12); anti-c (12); anti-C (12); anti-e (8); anti-Kpa (8) | Extended phenotype-matched RBC. Leucoreduced RBC. |
An association was found between alloimmunisation and age of the patients and the number of units transfused. |
| Moeen (2018)53 | Pakistan | Cohort, cross-sectional | 302 TM | NA | 12/302 (4.0) | Anti-Cw (33); anti-K (17); anti-k (17); anti-S (17); anti-Lua (17) | Non leucoreduced RBC. | - |
Mean (standard deviation).
RBC: red blood cells; NA: not available; TM: thalassaemia major; TI: thalassaemia intermedia; TDT: transfusion-dependent thalassaemia.
All the studies were classified as cohort studies. The design was retrospective in 11 studies13,15,27,28,32,35–37,50–52 and prospective in 1714,17,20,24–26,29,31,34,38,39,41,43,46–49. Thirteen studies16,18,19,21–23,30,33,40,42,44,45,53 in which the investigator sampled a source population cross-sectionally and then retrospectively assessed subjects’ histories of exposures and outcomes over a specified time period were defined as cross-sectional cohort studies56.
The sample size of the included studies ranged from 30 patients in the study by Wang and colleagues16 to 835 patients in the study by Azarkeivan and colleagues25. Transfusion-dependent thalassaemia was β-thalassaemia major in the majority of cases (n=4,829); patients with thalassaemia intermedia (n=491), α-thalassaemia syndromes as well as haemoglobin E/β thalassaemia (n=148) and otherwise undefined cases (n=3,788) were also included.
Bias assessment
We present the results of the NOS in the Online Supplementary Content, Table SI. The quality of cohort studies was assessed by examining selection, comparability and outcome. Of the 41 cohort studies included, all achieved the maximum four stars for selection, and the maximum three stars for outcome; for comparability, 39 studies achieved the maximum two stars, but two studies merited only one star because they did not control for confounders16,23.
Frequency of erythrocyte alloimmunisation
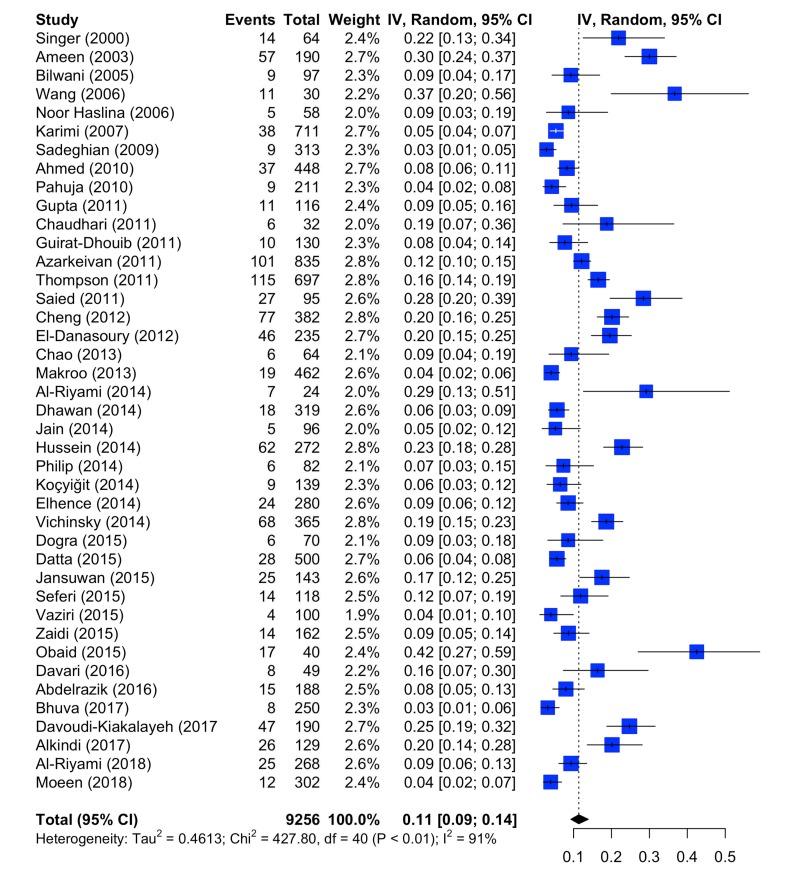
Online Supplementary Table SII reports the detailed analysis of RBC alloantibodies for each study. The great majority of the studies included used high sensitivity agglutination microcolumn assays (Online Supplementary Content, Table SIII). The prevalence of alloantibodies in the thalassaemia patients, without distinguishing between the clinical variants, is depicted in Figure 2. As stated above, 41 studies were included. The random effects model resulted in a mean prevalence of 0.1137 (95% CI: 0.0928; 0.1386). The heterogeneity was substantial: Cochran’s Q was 427.80, with degrees of freedom (d.o.f.)=40 (p<0.0001), τ2=0.4613, I2=90.6% (88.3%; 92.6%). The variation of erythrocyte alloimmunisation ranged from 2.9% (Sadeghian et al.19) to 37.0% (Wang et al.16). Taking into account all selected studies, a higher rate of alloimmunisation against antigens of the Rh system (52.4%), especially against E antigen (22.0%), was observed, showing that these antigens are among the most immunogenic. Alloantibodies against D, C, c and e antigens accounted for 6.9%, 5.5%, 5.5% and 1.3% of the cases, respectively. The second highest rate was found for alloantibody antigens of the Kell system (25.6%), especially against K antigen (22.5%), followed by the Kidd system (3.9%) and the MNS system (2.4%) (Online Supplementary Content, Table SII). Notably, alloimmunisation against antigens of the Rh and Kell systems accounted for 78% of the total cases. It should be noted that 9.4% of alloimmunised patients had antibodies directed against more than one RBC antigen (mostly, alloantibodies against antigens of the Rh and Kell systems). Four studies27,28,35,39 reported only the frequency per single antigen without a description of the presence of more than one antigen in the same subject.
Figure 2.
Forest plot of the prevalence of red blood cell alloantibodies in patients with thalassaemia.
CI: confidence intervals.
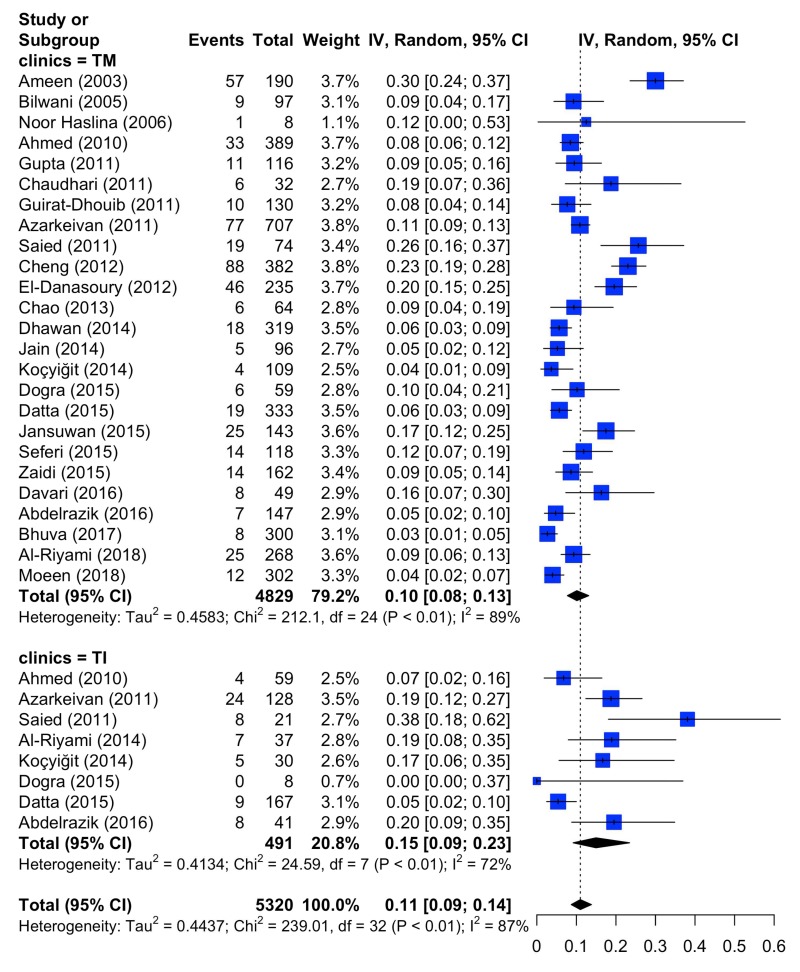
The comparison of the prevalence of erythrocyte alloantibodies in patients with thalassaemia major (total 25 studies evaluated) and thalassaemia intermedia (total 8 studies evaluated) is presented in Figure 3 and Table II. In patients with thalassaemia major, Q was 212.1, τ2 0.4583, I2 88.7%, whereas in those with thalassaemia intermedia, Q was 24.59, τ2 0.4134, and I2 71.5%. In the aggregate set of patients with either thalassaemia major or thalassaemia intermedia, Q was 239.1, τ2 0.4437, and I2 86.6%. The results of the test for subgroup differences (random effects model) were: Q=1.95, d.o.f.=1, p=0.1629 (not significant). Thus, the prevalence of alloantibodies in thalassaemia major and thalassaemia intermedia did not appear to differ significantly. The rate of erythrocyte alloimmunisation in patients with thalassaemia major ranged from 2.7% (Bhuva et al.49) to 30.0% (Ameen et al.14) and from 0% (Dogra et al.40) to 38.1% (Saied et al.27) in patients with thalassaemia intermedia (Online Supplementary Table SIV).
Figure 3.
Forest plots of the prevalence of red blood cell alloantibodies in patients with thalassaemia major (TM) or thalassaemia intermedia (TI).
CI: confidence intervals.
Table II.
Comparison of the prevalence of erythrocyte alloimmunisation between patients with thalassaemia major and thalassaemia intermedia.
| Subgroup | Model | N. of studies | Mean proportion | 95% C.I. | |
|---|---|---|---|---|---|
| TM | Random effects | 25 | 0.1012 | 0.0773 | 0.1313 |
| TI | Random effects | 8 | 0.1497 | 0.0918 | 0.2345 |
| All | Random effects | 33 | 0.1100 | 0.0871 | 0.1380 |
Studies distinguishing the clinical variants, thalassaemia major (TM) and thalassaemia intermedia (TI).The mean proportion (prevalence) of alloantibodies is reported for patients with TM and TI with 95% confidence intervals (C.I.). The same indexes are reported on the aggregate (all) set of studies.
Analysis of risk factors associated with erythrocyte alloimmunisation
The main risk factors for RBC alloimunisation that emerged from the analysis of the studies included (Table II) were: age25,27,29,52, female gender19,34,48, splenectomy13,20,26,29,35,42,48, first transfusion before 2–3 years of age22,33,37,48, and RBC units received/duration of blood transfusion/transfusion frequency18,26,27,29,35,39,52. By contrast, increased antigen matching for Rh and Kell and the use of leucoreduced RBC were found to have a protective effect against alloantibody development13,14,25,27,35,43,45,47.
A meta-analytical approach to weigh the risk of RBC alloimmunisation associated with each factor was not possible because of the clinical heterogeneity and inconsistency of reporting among the different studies.
Discussion
To our knowledge, this is the first published systematic review on the prevalence of RBC alloimmunisation in patients with transfusion-dependent thalassaemia. After the identification of 41 eligible cohort studies involving 9,256 thalassaemia patients, we determined a prevalence of erythrocyte alloimmunisation of 11.4%. The wide variation in the rate of RBC alloimmunisation (2.9 to 37%) observed among the different studies probably reflects the heterogeneity of the studies, which is common in reviews addressing prevalence and incidence data. There are three types of heterogeneity: clinical, methodological, and statistical57. Statistical heterogeneity is the variation of effects sizes between studies. Statistical heterogeneity may arise because of clinical heterogeneity, methodological heterogeneity, or simply by chance. In the current systematic review, the statistical heterogeneity was substantial; this was addressed by using a random effects model. A random effects model allows more flexibility, assuming that there may be other factors influencing the data than error or chance, within and between studies. Beside the statistical heterogeneity, there was clinical and methodological heterogeneity in the studies included in the review. Differences in study designs and methodological quality (risk of bias) represent methodological heterogeneity. All the studies included in this review were classified as cohort studies, although the design of the studies varied. There were prospective, retrospective and cross-sectional cohorts. However, our assessment of bias using the NOS shows that the quality of the included studies was high. There was also clinical heterogeneity in the studies considered in the analysis related to specific covariates (e.g., age, gender, ethnicity), transfusion policy (including the use of non-leucoreduced RBC units and the degree of antigen-matching techniques), different methods of antibody identification, and heterogeneity of the donor and the recipient populations. Unfortunately, due to limitations and inconsistencies in reporting alloantibody prevalences according to these covariates, we could not perform subgroup analyses or meta-regressions for all the relevant covariates. However, our subgroup analysis of clinical variants showed that the prevalences of alloantibodies in thalassaemia major and thalassaemia intermedia did not differ significantly.
It has been consistently reported that the ethnic correlation between donors and recipients influences the frequency of erythrocyte alloimmunisation in both thalassaemia and sickle cell anaemia patients58. Lower rates of RBC alloimmunisation were reported in studies with more homogeneous donor and patient populations, while higher rates were observed in studies with ethnic/racial disparity between donors and recipients. For example, in the study by Abdelrazik and colleagues48, the relatively low rate of alloimmunisation (8%) was attributed to the fact that blood donors and thalassaemic patients were from the same ethnic Egyptian group.
Among the other risk factors associated with RBC alloimmunisation in patients with transfusion-dependent thalassaemia, splenectomy was that most frequently reported. For instance, Singer and colleagues13 reported that patients who underwent splenectomy had a higher rate of alloimmunisation compared to non-splenectomised patients (36 vs 12.8%). In multivariate analysis, Thompson and co-workers26 found that splenectomy was an independent risk factor for alloimmunisation. The mechanism by which removal of the spleen increases alloantibody formation is unclear. One possible explanation is that the absence of the spleen may further enhance the immune response to infused foreign blood antigens that are not effectively filtered. Other studies, however, did not find such an association37,52. In any case, although frequently performed in the past, the currently lower tendency to perform splenectomy in transfusion-dependent thalassaemia patients might mitigate such risk.
Other important risk factors for RBC alloimmunisation that emerged from our systematic analysis of the literature were the duration of transfusions and the age at the start of transfusion therapy, with older patients being at increased risk. While the former finding is obviously related to the amount of exposure to RBC antigens during the years of blood transfusions1, the latter is quite intriguing. Some authors have in fact reported a lower rate of alloimmunisation in patients who began transfusion before 2–3 years of age22,33. The still immature immune system of young patients could be responsible for a lower risk of alloimmunisation by creating a kind of immunotolerance. While the early institution of transfusion therapy after diagnosis could be an interesting approach to minimise the risk of alloimmunisation risk, on the other hand it could expose patients with thalassaemia to an increased risk of other transfusion-related complications. An unresolved issue regards the different risk of RBC alloimmunisation in transfusion-dependent patients with thalassaemia major or thalassaemia intermedia. Various studies found a higher percentage of RBC alloantibodies among patients with thalassaemia intermedia than among those with thalassaemia major20,25,48. The results of our systematic review confirm this trend (Table II): although the difference was not statistically significant, we observed a higher prevalence of alloantibodies in patients with thalassaemia intermedia. While patients with thalassaemia major usually start RBC transfusions earlier than patients with thalassaemia intermedia and thus could benefit from the protective effect of the younger age reported above, this finding could also reflect differences in transfusion policies between the two types of thalassaemia. Indeed, it is conceivable that patients with thalassaemia intermedia, having lower and less regular transfusion requirements, could be transfused more frequently with non-extended phenotype matched RBC units than are patients with thalassaemia major29. On the other hand, the large differences in sample sizes among the two cohorts of patients evaluated (4,829 patients with thalassaemia major vs 491 patients with thalassaemia intermedia) could represent an important bias for the subgroup analysis of the results.
No definitive association between female gender and alloimmunisation risk could be found because of the conflicting results reported from the different studies included in this systematic analysis.
Finally, in this systematic review the most frequent antibodies identified in patients with transfusion-dependent thalassaemia were directed against Rh and Kell systems, accounting for 78% of all cases. This means that matching RBC units for these antigens will reduce the risk of RBC alloimunisation by about 80% in such patients. With the aim of risk minimisation, we therefore recommend, in accordance with the main standard guidelines59–62, the implementation of an early diagnosis of thalassaemia with the determination of baseline extended RBC phenotype and the provision of RBC units matched for Rh and Kell antigens.
Supplementary Information
Acknowledgements
The Authors thank Professor Marilyn Scopes (Italian Foundation for Research on Anaemia and Haemoglobinopathies, Genoa, Italy) for her precious assistance with the English editing that greatly improved this manuscript.
Footnotes
Diclosure of conflicts of interest
Giancarlo M. Liumbruno is the Editor-in-Chief of Blood Transfusion. As a result, this manuscript was subjected to an additional external review. The Authors declare no conflicts of interest.
References
- 1.Vichinsky E, Neumayr L, Trimble S, et al. CDC Thalassemia Investigators. Transfusion complications in thalassemia patients: a report from the Center of Disease Control and Prevention. Transfusion. 2014;54:972–81. doi: 10.1111/trf.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Compernolle V, Chou ST, Tanael S, et al. for the International Collaboration for Transfusion Medicine Guidelines. Red blood cell specifications for patients with hemoglobinopathies: a systematic review and guideline. Transfusion. 2018;58:1555–66. doi: 10.1111/trf.14611. [DOI] [PubMed] [Google Scholar]
- 3.Marano G, Pupella S, Pati I, et al. Ten years since the last Chikungunya virus outbreak in Italy: history repeats itself. Blood Transfus. 2017;15:489–90. doi: 10.2450/2017.0215-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musso D, Aubry M, Broult J, et al. Zika virus: new emergencies, potential for severe complications, and prevention of transfusion-transmitted Zika fever in the context of co-circulation of arboviruses. Blood Transfus. 2017;15:272–3. doi: 10.2450/2016.0003-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velati C, Romanò L, Piccinini V, et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis C virus and human immunodeficiency virus after the implementation of nucleic acid testing in Italy: a 7-year (2009–2015) survey. Blood Transfus. 2018;16:422–32. doi: 10.2450/2018.0069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchero A, Marchetti M, Giaccherini C, et al. Platelet haemostatic properties in β-thalassaemia: the effect of blood transfusion. Blood Transfus. 2017;15:413–21. doi: 10.2450/2016.0033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricchi P, Ammirabile M, Costantini S, et al. Soluble form of transferrin receptor as a biomarker of overall morbidity in patients with non-transfusion-dependent thalassaemia: a cross-sectional study. Blood Transfus. 2016;14:538–40. doi: 10.2450/2016.0279-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrzad V, Khajouei AS, Fahami E. Correlation of N-terminal pro-B-type natriuretic peptide levels and cardiac magnetic resonance imaging T2* in patients with β-thalassaemia major. Blood Transfus. 2016;14:516–20. doi: 10.2450/2016.0120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M, Marano G, Mengoli C, et al. Red blood cell transfusion policy: a critical literature review. Blood Transfus. 2017;15:307–17. doi: 10.2450/2017.0059-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matteocci A, Pierelli L. Red blood cell alloimmunization in sickle cell disease and in thalassemia: current status, future perspectives and potential role of molecular typing. Vox Sang. 2013;106:197–208. doi: 10.1111/vox.12086. [DOI] [PubMed] [Google Scholar]
- 11.Chou ST, Liem RI, Thompson AA. Challenges of alloimmunization in patients with haemoglobinopathies. Br J Haematol. 2012;159:394–404. doi: 10.1111/bjh.12061. [DOI] [PubMed] [Google Scholar]
- 12.Taher A, Ismaeel H, Cappellini MD. Thalassemia intermedia: revisited. Blood Cells Mol Dis. 2006;37:12–20. doi: 10.1016/j.bcmd.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Singer ST, Wu V, Mignacca R, et al. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96:3369–73. [PubMed] [Google Scholar]
- 14.Ameen R, Al-Shemmari S, Al-Humood S, et al. RBC alloimmunization and autoimmunization among transfusion dependent Arab thalassemia patients. Transfusion. 2003;43:1604–10. doi: 10.1046/j.1537-2995.2003.00549.x. [DOI] [PubMed] [Google Scholar]
- 15.Bilwani F, Kakepoto GN, Adil SN, et al. Frequency of irregular red cell alloantibodies in patients with thalassemia major: a bicenter study. J Pak Med Assoc. 2005;55:563–5. [PubMed] [Google Scholar]
- 16.Wang LY, Liang DC, Liu HC, et al. Alloimmunization among patients with transfusion-dependent thalassemia in Taiwan. Transfus Med. 2006;16:200–3. doi: 10.1111/j.1365-3148.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 17.Noor Haslina MN, Ariffin N, Illuni Hayati I, Rosline H. Red cell immunization in multiply transfused Malay thalassemic patients. Southeast Asian J Trop Med Public Health. 2006;37:1015–20. [PubMed] [Google Scholar]
- 18.Karimi M, Nikrooz P, Kashef S, et al. RBC alloimmunization in blood transfusion-dependent beta-thalassemia patients in southern Iran. Int J Lab Hematol. 2007;29:321–6. doi: 10.1111/j.1365-2257.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 19.Sadeghian MH, Keramati MR, Badiei Z, et al. Alloimmunization among transfusion-dependent thalassemia patients. Asian J Transfus Sci. 2009;3:95–8. doi: 10.4103/0973-6247.53884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed AM, Hasan NS, Ragab SH, et al. Red cell alloimmunization and autoantibodies in Egyptian transfusion dependent thalassaemia patients. ArchMed Sci. 2010;6:592–8. doi: 10.5114/aoms.2010.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pahuja S, Pujani M, Gupta SK, et al. Alloimmunization and red cell autoimmunization in multitransfused thalassemics of Indian origin. Hematology. 2010;15:174–7. doi: 10.1179/102453309X12583347114013. [DOI] [PubMed] [Google Scholar]
- 22.Gupta R, Singh DK, Singh B, et al. Alloimmunization to red cells in thalassemics: emerging problem and future strategies. Transfus Apher Sci. 2011;45:167–70. doi: 10.1016/j.transci.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhari CN. Red cell alloantibodies in multiple transfused thalassaemia patients. Med J Armed Forces India. 2011;67:34–7. doi: 10.1016/S0377-1237(11)80008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guirat-Dhouib N, Mezri M, Hmida H, et al. High frequency of autoimmunization among transfusion-dependent Tunisian thalassemia patients. Transfus Apheresis Sci. 2011;45:199–202. doi: 10.1016/j.transci.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Azarkeivan A, Ansari S, Ahmadi MH, et al. Blood transfusion and alloimmunization in patients with thalassemia: multicenter study. Pediatr Hematol Oncol. 2011;28:479–85. doi: 10.3109/08880018.2011.568595. [DOI] [PubMed] [Google Scholar]
- 26.Thompson AA, Cunningham MJ, Singer ST, et al. Thalassemia Clinical Research Network Investigators. Red cell alloimmunization in a diverse population of transfused patients with thalassaemia. Br J Haematol. 2011;153:121–8. doi: 10.1111/j.1365-2141.2011.08576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saied DA, Kaddah AM, Badr Eldin RM, Mohaseb SS. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent Egyptian thalassemic patients. J Pediatr Hematol Oncol. 2011;33:409–14. doi: 10.1097/MPH.0b013e3182208154. [DOI] [PubMed] [Google Scholar]
- 28.Cheng CK, Lee CK, Lin CK. Clinically significant red blood cell antibodies in chronically transfused patients: a survey of Chinese thalassemia major patients and literature review. Transfusion. 2012;52:2220–4. doi: 10.1111/j.1537-2995.2012.03570.x. [DOI] [PubMed] [Google Scholar]
- 29.El-Danasoury AS, Eissa DG, Abdo RM, et al. Red blood cell alloimmunization in transfusion-dependent Egyptian patients with thalassemia in a limited donor exposure program. Transfusion. 2012;52:43–7. doi: 10.1111/j.1537-2995.2011.03234.x. [DOI] [PubMed] [Google Scholar]
- 30.Chao YH, Wu KH, Lu JJ, et al. Red blood cell alloimmunisation among Chinese patients with β-thalassaemia major in Taiwan. Blood Transfus. 2013;11:71–4. doi: 10.2450/2012.0153-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makroo RN, Arora JS, Chowdhry M, et al. Red cell alloimmunization and infectious marker status (human immunodeficiency virus, hepatitis B virus and hepatitis C virus) in multiply transfused thalassemia patients of North India. Indian J Pathol Microbiol. 2013;56:378–83. doi: 10.4103/0377-4929.125295. [DOI] [PubMed] [Google Scholar]
- 32.Al-Riyami AZ, Al-Mahrooqi S, Al-Hinai S, et al. Transfusion therapy and alloimmunization in thalassemia intermedia: a 10 year experience at a tertiary care university hospital. Transfus Apher Sci. 2014;51:42–6. doi: 10.1016/j.transci.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Dhawan HK, Kumawat V, Marwaha N, et al. Alloimmunization and autoimmunization in transfusion dependent thalassemia major patients: study on 319 patients. Asian J Transfus Sci. 2014;8:84–8. doi: 10.4103/0973-6247.137438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain R, Choudhury N, Chudgar U, et al. Detection and identification of red cell alloantibodies in multiply transfused thalassemia major patients: a prospective study. Indian J Hematol Blood Transfus. 2014;30:291–6. doi: 10.1007/s12288-013-0282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussein E, Desooky N, Rihan A, et al. Predictors of red cell alloimmunization in multitransfused Egyptian patients with beta-thalassemia. Arch Pathol Lab Med. 2014;138:684–8. doi: 10.5858/arpa.2013-0016-OA. [DOI] [PubMed] [Google Scholar]
- 36.Philip J, Biswas AK, Hiregoudar S, Kushwaha N. Red blood cell alloimmunization in multitransfused patients in a tertiary care center in Western India. Lab Med. 2014;45:324–30. doi: 10.1309/LMUCV97YUWQKAHU4. [DOI] [PubMed] [Google Scholar]
- 37.Koçyiğit C, Eliaçık K, Kanık A, et al. Frequency of red cell allo- and autoimmunization in patients with transfusion-dependent beta thalassemia and affecting factors. Turk J Pediatr. 2014;56:487–92. [PubMed] [Google Scholar]
- 38.Elhence P, Solanki A, Verma A. Red blood cell antibodies in thalassemia patients in northern India: risk factors and literature review. Indian J Hematol Blood Transfus. 2014;30:301–8. doi: 10.1007/s12288-013-0311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vichinsky E, Neumayr L, Trimble S, et al. CDC Thalassemia Investigators. Transfusion complications in thalassemia patients: a report from the Centers for Disease Control and Prevention (CME) Transfusion. 2014;54:972–81. doi: 10.1111/trf.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dogra A, Sidhu M, Kapoor R, Kumar D. Study of red blood cell alloimmunization in multitransfused thalassemic children of Jammu region. Asian J Transfus Sci. 2015;9:78–81. doi: 10.4103/0973-6247.150958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datta SS, Mukherjee S, Talukder B, et al. Frequency of red cell alloimmunization and autoimmunization in thalassemia patients: a report from Eastern India. Adv Hematol. 2015;2015 doi: 10.1155/2015/610931. 610931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansuwan S, Tangvarasittichai O, Tangvarasittichai S. Alloimmunization to red cells and the association of alloantibodies formation with splenectomy among transfusion-dependent b-thalassemia major/HbE patients. Ind J Clin Biochem. 2015;30:198–203. doi: 10.1007/s12291-014-0424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seferi I, Xhetani M, Face M, et al. Frequency and specificity of red cell antibodies in thalassemia patients in Albania. Int J Lab Hematol. 2015;37:569–74. doi: 10.1111/ijlh.12362. [DOI] [PubMed] [Google Scholar]
- 44.Vaziri M, Javadzadeh Shahshahani H, Moghaddam M, Taghvaee N. Prevalence and specificities of red cell alloantibodies in transfusion-dependent beta thalassemia patients in Yazd. Iran J Ped Hematol Oncol. 2015;5:93–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Zaidi U, Borhany M, Ansari S, et al. Red cell alloimmunisation in regularly transfused beta thalassemia patients in Pakistan. Transfus Med. 2015;25:106–10. doi: 10.1111/tme.12196. [DOI] [PubMed] [Google Scholar]
- 46.Obaid JM, Abo El-Nazar SY, Ghanem AM, et al. Red blood cells alloimmunization and autoimmunization among transfusion-dependent beta-thalassemia patients in Alexandria province, Egypt. Transfus Apher Sci. 2015;53:52–7. doi: 10.1016/j.transci.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Davari K, Soltanpour MS. Study of alloimmunization and autoimmunization in Iranian beta-thalassemia major patients. Asian J Transfus Sci. 2016;10:88–92. doi: 10.4103/0973-6247.172179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelrazik AM, Elshafie SM, El Said MN, et al. Study of red blood cell alloimmunization risk factors in multiply transfused thalassemia patients: role in improving thalassemia transfusion practice in Fayoum, Egypt. Transfusion. 2016;56:2303–7. doi: 10.1111/trf.13695. [DOI] [PubMed] [Google Scholar]
- 49.Bhuva DK, Vachhani JH. Red cell alloimmunization in repeatedly transfused patients. Asian J Transfus Sci. 2017;11:115–20. doi: 10.4103/0973-6247.214347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davoudi-Kiakalayeh A, Mohammadi R, Pourfathollah AA, et al. Alloimmunization in thalassemia patients: new insight for healthcare. Int J Prev Med. 2017;8:101. doi: 10.4103/ijpvm.IJPVM_246_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alkindi S, AlMahrooqi S, AlHinai S, et al. Alloimmunization in patients with sickle cell disease and thalassemia: experience of a single centre in Oman. Mediterr J Hematol Infect Dis. 2017;9:e2017013. doi: 10.4084/MJHID.2017.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Riyami AZ, Al-Muqbali A, Al-Sudiri S, et al. Risks of red blood cell alloimmunization in transfusion-dependent β-thalassemia in Oman: a 25-year experience of a university tertiary care reference center and a literature review. Transfusion. 2018;58:871–8. doi: 10.1111/trf.14508. [DOI] [PubMed] [Google Scholar]
- 53.Moeen S, Farooq N, Irshad R, et al. Red cell alloimmunization in multitransfused thalassaemia major patients. J Ayub Med Coll Abbottabad. 2018;30:81–4. [PubMed] [Google Scholar]
- 54.Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Accessed on: 12/11/2018]. Available at www.handbook.cochrane.org. [Google Scholar]
- 55.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analyses. [Accessed on: 12/11/2018]. Available at wwwohrica/programs/clinical_epidemiology/oxford.asp.
- 56.Hudson JI, Pope HG, Jr, Glynn RJ. The cross-sectional cohort study: an underutilized design. Epidemiology. 2005;16:355–9. doi: 10.1097/01.ede.0000158224.50593.e3. [DOI] [PubMed] [Google Scholar]
- 57.The Joanna Bring Institute Reviewers manual 2014. The systematic review of prevalence and incidence data. [Accessed on: 12/11/2018]. Available at: http://joannabriggs.org/assets/docs/sumari/ReviewersManual_2014-The-Systematic-Review-of-Prevalence-and-Incidence-Data_v2.pdf.
- 58.Da Cunha Gomes EG, Machado LAF, de Oliveira LC, Neto JFN. The erythrocyte alloimunisation in patients with sickle cell anaemia: a systematic review. Transfus Med. 2018 doi: 10.1111/tme.12543. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Cappellini MD, Cohen A, Porter J, et al. Guidelines for the management of transfusion dependent thalassaemia (TDT) 3rd edition. Nicosia (CY): Thalassaemia International Federation; 2014. [PubMed] [Google Scholar]
- 60.Vichinsky E, Levine L, Bhatia S, et al. Standards of care guidelines for thalassemia. Oakland: Children’s Hospital & Research Center; 2008. [Google Scholar]
- 61.Anne Yardumian PT, Shah F, Ryan K, et al. Standards for the clinical care of children and adults with thalassaemia in the UK. [Accessed on: 12/11/2018]. Available at: http://ukts.org/standards/Standards-2016final.pdf.
- 62.Bonomo P, Carta MP, Forni GL, et al. Raccomandazioni per le strategie trasfusionali nelle emoglobinopatie. Società Italiana di Medicina Trasfusionale (SIMTI), Società Italiana Talassemie ed Emoglobinopatie (SITE); Milano: 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.