Abstract
Red blood cells (RBCs) are a specialised organ that enabled the evolution of multicellular organisms by supplying a sufficient quantity of oxygen to cells that cannot obtain oxygen directly from ambient air via diffusion, thereby fueling oxidative phosphorylation for highly efficient energy production. RBCs have evolved to optimally serve this purpose by packing high concentrations of haemoglobin in their cytosol and shedding nuclei and other organelles. During their circulatory lifetimes in humans of approximately 120 days, RBCs are poised to transport oxygen by metabolic/redox enzymes until they accumulate damage and are promptly removed by the reticuloendothelial system. These elaborate evolutionary adaptions, however, are no longer effective when RBCs are removed from the circulation and stored hypothermically in blood banks, where they develop storage-induced damages (“storage lesions”) that accumulate over the shelf life of stored RBCs. This review attempts to provide a comprehensive view of the literature on the subject of RBC storage lesions and their purported clinical consequences by incorporating the recent exponential growth in available data obtained from “omics” technologies in addition to that published in more traditional literature. To summarise this vast amount of information, the subject is organised in figures with four panels: i) root causes; ii) RBC storage lesions; iii) physiological effects; and iv) reported outcomes. The driving forces for the development of the storage lesions can be roughly classified into two root causes: i) metabolite accumulation/depletion, the target of various interventions (additive solutions) developed since the inception of blood banking; and ii) oxidative damages, which have been reported for decades but not addressed systemically until recently. Downstream physiological consequences of these storage lesions, derived mainly by in vitro studies, are described, and further potential links to clinical consequences are discussed. Interventions to postpone the onset and mitigate the extent of the storage lesion development are briefly reviewed. In addition, we briefly discuss the results from recent randomised controlled trials on the age of stored blood and clinical outcomes of transfusion.
Keywords: blood transfusion, blood banking, storage lesion, clinical sequelae
Introduction
Approximately 25 trillion red blood cells (RBCs) circulate in the bloodstream of an adult individual, each one packed with ~260 million haemoglobin molecules. To make room for haemoglobin, erythroblasts and reticulocytes progressively lose nuclei and organelles during maturation, which impairs the erythrocytes’ capacity to synthesise new proteins during their 120 days lifespan in the human bloodstream. Indeed, haemoglobin occupies 95% of the ~110 fL mean RBC volume1, making up ~670 g of the 25 kg dry body weight of an average adult individual2. Each alpha and beta globin subunit in the haemoglobin tetramer contains one ferrous iron, which can bind one molecule of oxygen. Evolution has shaped haemoglobin and the RBC as a highly specialised carrier of oxygen in the body3, enabling large warm-blooded vertebrates to thrive. When RBCs are fully oxygenated, concentrations of both iron and oxygen approximate 16 mM, a very high concentration considering the highly reactive nature of ferrous iron and oxygen. To mitigate oxidative stress and oxygen consumption, mature RBCs lose mitochondria and strengthen their antioxidant systems to specifically maintain haemoglobin iron in a reduced state, even in the presence of high oxygen concentrations. Oxidised (ferric state, +III) haemoglobin (i.e., methaemoglobin) can thus be either reduced back to ferrous state (+II) by enzymes such as methaemoglobin reductase, or denatured/aggregated (Heinz bodies) before removal via vesiculation (e.g., aging RBCs shed one microvesicle per hour)4. Senescent erythrocytes are usually characterised by higher oxidative stress than young erythrocytes and are readily removed from the bloodstream via phagocytosis by reticuloendothelial system macrophages in the liver and spleen.
In blood centers, donated blood is separated into a red cell concentrate (RCC) from which white blood cells (WBCs) are filtered in most cases, as well as platelets (log 2 WBC removal via buffy coat depletion or log 3.5–4 WBC and log 2.5 removal if leucofiltration is performed, respectively). Isolated RBCs are resuspended in an acidic additive solution at approximately 60% haematocrit and stored under refrigerated conditions (1–6 °C hypothermic storage) for up to 3–7 weeks. In cryopreserved RBCs with a shelf life of over 10 years, chemical reactions in RBCs are virtually suspended by storing at −65 °C with a cryo-protectant. However, the latter process is time-consuming and cumbersome, and it promotes the lysis of the older, more fragile RBC population that was originally frozen5. In contrast, during hypothermic storage (1–6 °C), chemical reactions proceed - albeit at reduced rates6 - without the full benefit of the protective mechanisms that operate in the circulation. Thus, gradual degradation of various components of RBCs, collectively referred to as the “storage lesion”, accumulates during hypothermic storage resulting in a limited shelf life of up to 7 weeks. This contribution provides an overview of the RBC storage lesion and its potential clinical implications by examining causative elements, damage to RBCs, consequences in vitro and in animal models, and finally, associated clinical sequelae based on a thorough and extensive review of the existing literature.
Elements of the storage lesion and downstream consequences
Reviews7–9 of recent randomised controlled trials (RCTs)10–15 indicated that transfusion of the freshest available blood does not decrease the risk of mortality in several categories of recipients (including a small number of massively transfused critically ill or sickle cell disease patients) when compared to the standard of care. Despite reassuring evidence from RCTs, there is a burgeoning literature on the potential clinical sequelae other than mortality to transfusion of packed RBCs16,17 and on the potential etiological link between the storage lesion and untoward consequences upon transfusion.
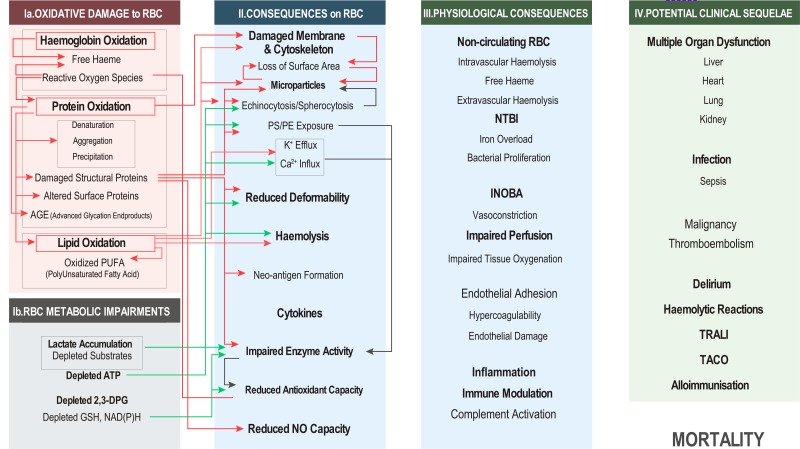
In Figure 1 we summarise elements of the RBC storage lesion - from causes to associated clinical sequelae - in four vertical panels, including root causes (Panel I); effects on RBCs (i.e., storage lesions) (Panel II); physiological consequences deduced from in vitro experiments or animal models (Panel III); and finally, potential clinical sequelae of RBC transfusion as gleaned from retrospective or prospective studies (Panel IV). Representative references for each of the elements in Figure 1 are provided. Our categorisations, though helpful from a systematic perspective, may at times appear arbitrary, owing to the labile boundary between cause and effect for some of the extensively reported lesions. For example, ion homeostasis is controlled by energy-dependent mechanisms, which are in turn affected by redox and energy metabolism. Nonetheless, storage temperature alone negatively affects proton pumps, and dysregulation of ion homeostasis (e.g. calcium18) affects kinase activity and metabolic signalling, making it difficult to conclude whether some of the proposed connections (if any) are only unidirectional. Nonetheless, we firmly believe that such a systematic overview of the storage lesion is unprecedented and will, at least, fuel further debate on the most relevant etiological factors to be targeted by next generation storage strategies/additives designed to improve RBC storage quality, as well as analytical strategies to provide pre-clinical insights regarding RBC safety and efficacy.
Figure 1.
Elements of red blood cell storage lesions from root causes to potential clinical sequelae.
Representative references for each element are shown within the figure. RBC: red blood cell; ATP: adenosine triphosphate (ATP); DPG: diphosphoglycerate; GSH: glutathione; NAD(P)H: nicotinamide adenine dinucleotide phosphate; PS: phosphatidylserine; PE: phosphatidylethanolamine; NTBI: non-transferrin bound iron; INOBA: insufficient nitric oxide bioavailability; TRALI: transfusion-related acute lung injury; TACO: transfusion-associated circulatory overload.
Root causes [Figure 2, Panel I]
Figure 2.
Causes of red blood cell damage and impairments during storage, and effects on red blood cell as storage lesions.
RBC: red blood cell; ATP: adenosine triphosphate (ATP); DPG: diphosphoglycerate; GSH: glutathione; NAD(P)H: nicotinamide adenine dinucleotide phosphate; PS: phosphatidylserine; PE: phosphatidylethanolamine; NTBI: non-transferrin bound iron; INOBA: insufficient nitric oxide bioavailability; TRALI: transfusion-related acute lung injury; TACO: transfusion-associated circulatory overload.
For hypothermic storage, RBCs are isolated from plasma and suspended in an acidic solution containing a high concentration of glucose. During storage, RBCs are exposed to plasticisers in the storage bag as well as oxygen diffusing in from ambient air, while accumulating metabolic waste resulting in further acidification throughout the shelf life. RBCs have evolved to cope with oxidative and mechanical stresses they encounter while performing their vital function as oxygen carriers in vivo. However, during their isolated state under hypothermic storage ex vivo, RBCs face a different set of chemical and mechanical stresses. Since RBCs were never exposed to evolutionary pressure to cope with such conditions, no physiological countermeasures evolved to address the stresses that create the storage lesions. The root causes of the development of the RBC storage lesion can be roughly classified into two categories: (i) arising from isolation of RBCs, dilution of plasma with an additive solution, and extended hypothermic storage in a closed bag; and (ii) arising from storage ex vivo in the presence of oxygen, resulting in oxidative stress and loss of biochemical countermeasures that were functional in vivo. Both causes result in physical damage and biochemical impairment to stored RBCs.
Oxidative damage as a cause for RBC storage lesions [Figure 2, Panel Ia]
Chemical oxidation of iron in haemoglobin is the central reaction that initiates oxidative stress, the major element for the development of the storage lesion, in stored RBCs. RBCs contain high concentrations of reactive ferrous iron in the haeme prosthetic group of haemoglobin together with a high concentration of dissolved oxygen. Four iron moieties (ferrous state) in haemoglobin react chemically with oxygen to form methaemoglobin (ferric state). As a byproduct, superoxide anion is generated, which is converted by superoxide dismutase to form H2O2, a major reactive oxygen species (ROS) and a substrate for hydroxyl radical (OH•) generation. In vivo, methaemoglobin is reduced back to haemoglobin by reductase enzymes, but these enzyme activities are curtailed under hypothermic storage conditions. Coupled with higher dissolved oxygen concentrations stemming from increased solubility at low temperature, this phenomenon results in enhanced production of methaemoglobin and superoxide anion. However, methaemoglobin does not accumulate to high levels in stored RBCs, and instead denatures into globin and haemin or free haeme due to its instability at hypothermic temperature. Ferric iron in haeme is reduced by the superoxide derived from methaemoglobin formation. The resulting ferrous ion then reacts with H2O2 by the Fenton reaction to produce net OH• by the Haber-Weiss reaction19. H2O2 also reacts with oxyhaemoglobin to produce ferryl (+IV) haemoglobin. Both OH• and ferryl haemoglobin are highly reactive, and oxidise nearby enzymes and lipids. The occurance of this cascade of events exponentially increases after two weeks of storage in the SAGM additive, resulting in reversible and irreversible oxidationsa of structural (e.g. ankyrin and spectrin20), functional (band 320 and haemoglobin21), and metabolic enzymesb, exacerbating metabolic impairments22. These events lead to the exposure of phosphatidylserine on RBC surfaces (a phenomenon counteracted through the expenditure of high energy phosphate compounds, such as adenosine triphosphate (ATP), and imbalances in K+ and Ca2+ ions23. Oxidised proteins, including denatured haemoglobin, bind to the cytoskeleton and disrupt its network structure causing morphological changes and reduced in deformability24,25. Oxidised and denatured proteins aggregate and precipitate in/on the RBC membrane. ROS also oxidises haemoglobin at the critical amino acid residue, β-92 histidine, destroying its ability to bind oxygen21. Another critical aspect of haemoglobin oxidation/ROS generation is lipid oxidation. Ferryl-haemoglobin and OH• are powerful ROS that can initiate the lipid peroxidation cycle26, which is sustained by the availability of oxygen, thereby disrupting the membrane bilayer and producing biologically active, oxidised polyunsaturated fatty acids (oxylipins)27. Of note, in a murine model, lipid peroxidation is a predictor of post-transfusion recovery; that is, the percentage of transfused erythrocytes that still circulate at 24 h from transfusion, a minimum but not sufficient condition for transfused RBCs to exert their function28.
Metabolic impairments as a cause for storage lesion development [Figure 2, Panel Ib]
Metabolic impairments of stored RBCs occur as a consequence of removing RBCs from a donor’s circulation, isolating them from plasma, and storing them in acidic solution, with a limited solution volume, at hypothermic temperature. Depletion of critical substrates (i.e., extracellular nutrients, such as glucose and intracellular purine derivatives, such as urate29) and accumulation of metabolic waste products, dominated by lactic acid, occur in component processing and storage. The consequences of glycolysis are reduced pH and impaired activities of critical enzymes that supply energy and antioxidant defense30, as reported since the 1940s31. Several metabolic pathways not expected in RBCs because of their lack of organelles have been discovered during the last two decades, especially with the advancement provided by “omics” sciences32–35. Down- and up-regulated pathways were quantified in MAP and phosphate-adenine-glucose-guanosine-gluconate-mannitol (PAGGGM) additive solutions with differences between the 0–7 day and 8–35 day periods36,37, and a three-phase temporal evolution in metabolic pathways was reported in saline-adenine-glucose-mannitol (SAGM) and AS-36,38–43.
Low pH additive solutions (5.5–6.0) reduce the activities of the rate-limiting enzymes of glycolysis, the pentose phosphate pathway (PPP), and the Rapoport-Luebering shuntc, and contribute to the rapid depletion of 2,3-diphosphoglycerate (2,3-DPG) and a gradual reduction of ATP during storage. Glycolytic enzymes are reversibly and irreversibly oxidised progressively during storage44–46, and 2,3-DPG breakdown fuels ATP generation at the expense of haemoglobin capacity to off-load oxygen, due to the ensuing low 2,3-DPG levels. As 2,3-DPG is consumed and haemoglobin oxygen saturation is increased by storage weeks 2–3, ROS accumulation reaches its plateau in classic additives, such as SAGM20.
It is worth noting that glucose availability is not a limiting factor, since all of the currently available additives are loaded with glucose to such an extent (>50 mM and up to 154 mM in the case of AS-1) that glucose autoxidation and non-enzymatic glycation of haemoglobin (HbA1c47) and membrane proteins48 are observed in end-of-storage RBCs.
Depletion of ATP and nicotinamide adenine dinucleotide phosphate (NADP)+ becomes a limiting factor in glutathione synthesis (an ATP-dependent process), resulting in reduced glutathione pools during storage. As the γ-glutamyl cycle is incomplete in mature RBCs, the lack of oxoprolinase activity results in 5-oxoproline accumulation in stored RBCs and supernatants, representing a key marker of RBC metabolic age during storage42. These considerations explain why attempts to replenish glutathione reservoirs by feeding RBCs in additives supplemented with glutathione precursors (e.g. glutamine) have failed to date49,50.
Of note, redox and energy metabolism in stored RBCs are more intertwined than was generally assumed for decades. Recent evidence suggests that hypoxanthine accumulates in stored RBCs as a result of ATP breakdown into adenosine monophosphate (AMP), whose deamination to the hypoxanthine-precursor inosine monophosphate (IMP) is enzymatically catalysed by RBC-specific AMP deaminase 3 in response to oxidative stress51. This is relevant in that hypoxanthine is a predictor of post-transfusion recovery in mice and, preliminary data suggest, in humans51. At the end of storage, hypoxanthine in RBCs and supernatants are at mM levels; hypoxanthine at ~100 μM is a substrate for generating hydrogen peroxide and urate in the presence of xanthine oxidase in the circulation of transfusion recipients. This prompts the consideration that circulating levels of post-transfusion hypoxanthine above the 100 μM threshold (i.e., the equivalent of a single end of storage ~450 mL unit diluted in 5 L of blood in the recipient) may be sufficient to catalyse oxidative stress, negatively impacting the recipient including the recipient’s RBCs52.
Some of the metabolic lesions that RBCs undergo during refrigerated storage are somewhat reversible following transfusion. For example, ATP and 2,3-DPG levels recover by 7–72 h after circulating in the recipient53, though at a rate that may be insufficient to meet the sudden and supra-physiological metabolic demand of trauma or critically ill recipients. Furthermore, RBCs that were near senescent when collected for storage, with reduced ATP and enzyme activities, may not be able to recover from the metabolic impairment and be removed by the recipient’s reticuloendothelial system after transfusion, as studies on the effect of the storage lesion on RBC populations sorted through density gradients seem to suggest54.
Consequences for RBCs - storage lesions [Figure 2, Panel II]
Extensive metabolomic investigations revealed that levels of high-energy compounds, such as ATP and 2,3-DPG, as well as reducing equivalents, glutathione (GSH) and (NAD(P)H), are reduced with a shift in the overall metabolic state after approximately two weeks of hypothermic storage55. Depleted ATP impacts several enzymatic functions and ion pumps, such as Ca2+ pumps. Decreased ATP deregulates cation homeostasis and disrupts membrane asymmetry, triggering the exposure of phosphatidylserine (PS) and phosphatidylethanolamine (PE), normally confined to the inner bilayer, and leading to microparticle formation. ATP depletion also alters the ability of kinases to phosphorylate proteins, as revealed by the restored membrane protein phosphorylation capacity after rejuvenation of long-stored RBCs56. Depleted ATP also causes reorganisation of the cytoskeleton, leading to echinocytosis57. Depletion of reducing equivalents results in reduced anti-oxidant capacity, further exacerbating damage from oxidative stress during storage and in RBC recipients after transfusion. Rapid loss of S-nitrosylation (SNO) of haemoglobin in stored RBCs is hypothesised to interfere with vasodilation in transfused patients58 by causing insufficient nitric oxide bioavailability (INOBA), although SNO-haemoglobin’s relevance remains controversial59,60.
A unit of RBC contains a continuum of cell ages from those just released into the circulation to those that are senescent and at the end of their circulatory life. Most in vitro parameters described in this panel are averaged values from this inhomogeneous RBC population in which the rate of damage accumulation may not be linear with storage time nor consistent from donor to donor. Thus, a unit of stored RBCs at any storage time contains some senescent cells that have lost excess membrane area by vesiculation and have decreased antioxidant capacity (e.g. glucose 6-phosphate dehydrogenase activity decreases in older circulating RBCs61). Morphological analyses report 6–9% of RBCs with irreversible changes62,63. Additionally, those cells’ metabolic status may have exhausted their capacity to handle oxidative stress during ex vivo storage for an extended time54, explaining the average ~17.6% loss of potency of end of storage packed RBCs when transfused back to a healthy autologous donor64 as gleaned from extensive post-transfusion recovery studies65. Reorganisation and damage to the RBC membrane and cytoskeleton, binding of haemoglobin and oxidised proteins, degradation of band 3, and variations in raft proteins66,67 are consequences of hypothermic storage. Accumulation of denatured methaemoglobin and damage caused by ROS result in changing RBC morphology from discocytes to echinocytes, then irreversibly to spherocytes, by releasing microparticles (MPs), resulting in reduced deformability that is not reversible after transfusion. Oxidation of membrane lipids and proteins exposes inner membrane phospholipids (PS and PE)68–70, contributing to membrane re-organisation and promoting MP formation. Proportionally higher loss of membrane area compared to volume occurs with MP formation, leading to loss of the excess surface area needed to allow passage of RBCs through narrow splenic capillaries71. Minimisation of surface to volume ratios also increases RBC osmotic fragility62. Cross-linking cytoskeleton and membrane proteins72, dysregulating cytoskeletal protein phosphorylation73, and dehydration caused by Ca2+ influx and K+ efflux, also contribute to reduced RBC deformability74. Oxidation of the membrane cytoskeleton disturbs anchoring between membrane proteins and the cytoskeleton, leading to eryptosis signal formation by band 3 clustering25. Oxidation of CD47 leads to eryptosis signal formation75, while storage-dependent depletion of membrane CD4776,77 - a “do not eat me” signal - makes transfused RBCs more prone to removal mediated by the recipients’ reticuloendothelial system. RBCs that are nearly senescent at the time of blood collection are therefore likely to comprise the majority of cells that haemolyse over the course of the storage period, though the occurrence of haemolysis is very low, typically less than 0.8%. Biologic response modifiers (BRMs), such as cytokines, chemokines, bioactive lipids, and metabolites, accumulate during storage52,79,80; most of these BRMs function as pro-inflammatory agents for transfusion recipients.
Finally, processing methods and additive solutions used to prepare RCC impact the storage lesion. The buffy-coat process leads to lower haemolysis at the end of the storage period than whole blood filtration81, and apheresis techniques generally generate more platelet-derived MPs as compared to whole blood donation82.
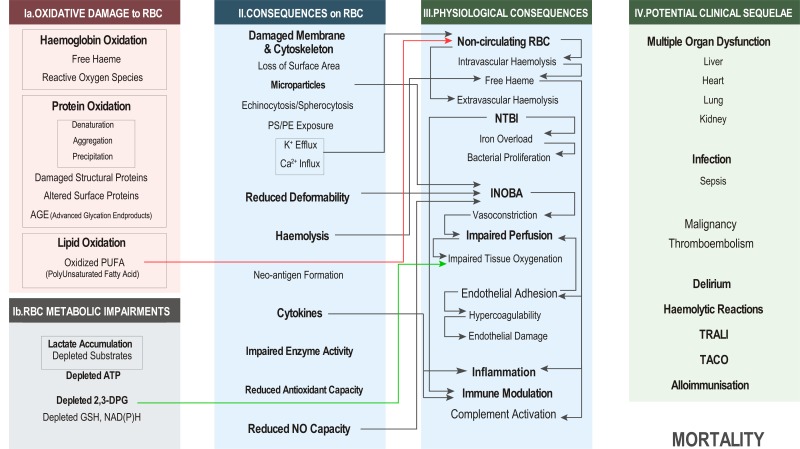
Physiological consequences of transfusing RBC with storage lesions [Figure 3]
Figure 3.
Physiological consequences of specific storage lesions elucidated by in vitro and animal model experiments.
RBC: red blood cell; ATP: adenosine triphosphate (ATP); DPG: diphosphoglycerate; GSH: glutathione; NAD(P)H: nicotinamide adenine dinucleotide phosphate; PS: phosphatidylserine; PE: phosphatidylethanolamine; NTBI: non-transferrin bound iron; INOBA: insufficient nitric oxide bioavailability; TRALI: transfusion-related acute lung injury; TACO: transfusion-associated circulatory overload.
When stored RBCs are transfused to autologous healthy volunteers, a significant fraction (median 17.6%) of RBCs is cleared from circulation within 24-hr65. Although a small fraction of mechanically damaged cells may haemolyse intravascularly after transfusion, the majority of non-viable RBCs display eryptosis markers and are phagocytosed extravascularly by macrophages in the recipient’s reticuloendothelial system83,84. Mechanisms of programmed cell death may activate during storage in parallel, resulting in eryptosis, which is induced by calcium influx and K+ efflux23,85, cell shrinkage, exposure of PS86 and PE68 from inner membrane bilayer, vesiculation of MPs with loss of excess surface area87, activation of calpains and caspases88, and reduced deformability89–92. In general, the portion of removed cells increases linearly with storage duration starting from over 6% after 1 week to 11% after 6 weeks93 in healthy volunteers, though non-linear exponential increases are observed after storage day 35, especially with respect to circulating iron metabolites, such as non-transferrin bound iron (NTBI) originating from cleared RBCs93.
Free iron in the circulation is tightly regulated in the body not only due to its catalytic activity in ROS production, but also as the major nutrient constraining growth of siderophilic bacteria94, a consideration relevant in patients with sepsis or bacteremia. In vivo, RBCs haemolysed in the circulation release iron from haeme but sequestered quickly by transferrin95. However, with transfusion of one unit of RBCs in healthy volunteers, up to 60 mL of damaged RBCs (25% of a unit) are removed extravascularly within 24 hours and iron is recycled. Since only 1 mL of senescent RBCs are removed hourly normally, transfusion of one unit can overwhelm both the reticuloendothelial system and the capacity of transferrin, thereby releasing NTBI into the circulation, which can result in bacterial proliferation96. The harmful consequences of uncontrolled NTBI in the circulation are magnified with multiple-unit transfusions and long-stored RBC units with a higher portion of non-viable cells. Additionally, for chronically transfused patients, the recipient’s capacity to handle the excess iron from non-viable RBCs included in every transfused unit is overwhelmed, resulting in iron overload of tissues and subsequent organ dysfunction97,98. Ferryl-haemoglobin, an oxidation product of methaemoglobin by ROS, is also a proinflammatory agonist that can also cause endothelial damage99.
NO is a signal for vasodilation that is generated by endothelial nitric oxide synthase (eNOS) near pre-capillary arterioles. Dysregulation of blood flow by disrupting NO-mediated vasoregulation is another major physiological effect of transfusing stored RBCs. Free haeme and MPs scavenge NO100, causing INOBA and disrupting signals for increasing flow for higher oxygen delivery to hypoxic tissues. Less deformable RBCs are also implicated in causing INOBA by scanvenging NO, resulting from their tendency to flow closer to the endothelial wall, as compared to normal RBC101. Additionally, RBCs play a direct role in regulating their flow in capillaries via NO: RBC haemoglobin reduces nitrite in plasma to produce NO102,103 and endothelial eNOS can be stimulated by RBC-released ATP104–107. Storage under conventional conditions alters the latter mechanism and the reduced glucose concentration in additive solution enables better NO production from endothelial cells stimulated by ATP release107.
Transfused stored RBCs can provoke a pro-inflammatory response108,109 by the cytokines, eicosanoids, and free haeme within a unit, as well as the NTBI produced by extravascular haemolysis in the recipient110. Storage lesions also promote adhesion to endothelial cells111,112, complement system activation113,114, and changes in coagulability115–117 in studies in vitro and in animal models. These effects also damage the endothelial lining to cause capillary leakage. In addition to the pro-inflammatory nature of stored RBCs, immune modulatory effects are also reported, attributed to NTBI118, RBC phagocytosis, and interaction with T-cells119.
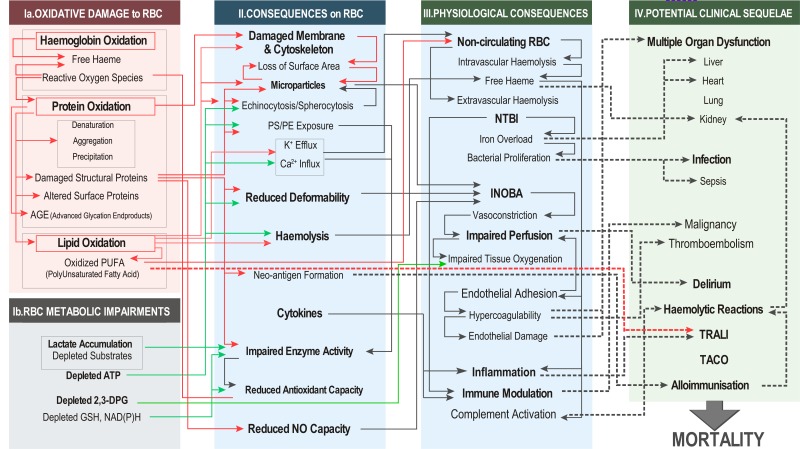
Potential clinical sequelae and upstream linkages [Figure 4]
Figure 4.
Potential linkages between elements of RBC storage lesion and reported clinical sequelae of RBC transfusion.
RBC: red blood cell; ATP: adenosine triphosphate (ATP); DPG: diphosphoglycerate; GSH: glutathione; NAD(P)H: nicotinamide adenine dinucleotide phosphate; PS: phosphatidylserine; PE: phosphatidylethanolamine; NTBI: non-transferrin bound iron; INOBA: insufficient nitric oxide bioavailability; TRALI: transfusion-related acute lung injury; TACO: transfusion-associated circulatory overload.
Panel IV of Figure 1 summarises numerous clinical outcomes suggested to be linked to transfusion of stored RBCs. Figure 4 illustrates possible linkages between elements of the three left panels to these reported clinical sequelae. It should be noted that RBC transfusion cannot be directly implicated, outside of a few exceptional cases for the entries in panel IV. Exceptions where a causal relationship can be established between a transfused RBC unit and clinical outcomes include: pathogen transmission, host vs graft disease, intravascular haemolytic reactions arising from mismatched RBCs, febrile responses, and some transfusion-related acute lung injury (TRALI) cases involving blood components containing incompatible plasma. For these cases, causal links to transfusion outcomes can be proven by pathogens in the donor RBCs, a high leucocyte burden, and antibodies or other BRMs in the transfused RBC unit. On the other hand, links can be only inferred between potential sequelae and transfusion of RBCs with accumulated storage lesions. The literature on this topic can be classified into four types of studies depending on how they are linked to the transfusion of stored RBCs:
retrospective or prospective studies examining transfusion triggers in different settings; incidences of specific morbidity are recorded as a function of a high or low transfusion trigger-where the quantity or absence of RBC transfusion is compared with specific morbidity or severity of negative outcome. A classical example is the TRICC study120.
Retrospective studies in which the age of stored RBCs, as a surrogate for the extent of the storage lesion, is compared to specific morbidity or mortality outcomes. A classical example is Koch’s study in cardiac patients121 or the meta-analysis of retrospective studies conducted by a group at the NIH122.
Mechanistic in vitro studies, where consequences of specific storage lesions are examined on suspected target cell types107,123.
Animal model studies where RBCs with specific storage lesions or RBCs stored for extended time are infused into an animal prepared to simulate specific recipient conditions and overall mortality or organ-specific parameters are examined. Murine110,119,124, canine125, and ovine126 models are classical examples.
Multiple organ dysfunction or individual organ failure are potential sequelae of RBC transfusion in complex surgery or in critical illness and are often used as the primary outcome measures for numerous RCTs studying the effects of transfusion trigger or storage age, a surrogate for the level of the accumulated storage lesion14,127. Impaired tissue oxygenation, hypercoagulability, and endothelial damage are attributed to physiological consequences of transfusing storage-damaged RBCs. Although transfusion of RBCs with storage lesions could exacerbate critical illness, it is virtually impossible to pinpoint one unit of transfused RBCs with a specific storage lesion as the major culprit. However, there is abundant literature drawing specific inferences between transfusion of stored RBCs and clinical sequelae based on clinical observations combined with results from in vitro experiments or animal model studies (link from Panel III to Panel IV).
- Specific organ damage, such as to the liver and heart, are attributed to iron overload in chronically transfused patients97,128,129. Massive intravascular haemolysis from transfusion of mismatched RBC to alloimunised patients can cause acute kidney failure121,130–137.
- Free iron concentration in circulation is limited and tightly controlled as iron is a major limiting substrate for bacterial proliferation. Transfusing a large quantity of non-viable RBCs can easily overwhelm a recipient’s ability to process excess iron, resulting in the release of NTBI into the circulation. Thus, increased infection125,135,138–155 and sepsis94,121,125,149,156,157 in transfused patients are attributed to bacterial proliferation arising from the availability of NTBI. Of note, extravascular haemolysis, not intravascular haemolysis, was recently associated with the transfusion of RBCs (an increased level of transferrin saturation as a hallmark) in cardiac surgery (the age of RCCs was not recorded in this observational study158).
- RBC transfusion reduces rate of graft rejection by recipients of organ transplants159,160, and exhibits immunosuppressive effects, termed transfusion-telated immune modulation (TRIM)161. In vitro studies demonstrated suppression of monocyte function when incubated with stored RBCs162,163. Suppression of innate immunity was observed when critically ill children were transfused with RCC stored more than 21 days164. Those observations demonstrate immunomodulatory effects of stored RBCs, and link RBC transfusion to increased rate of recurring malignancy as well as infections165.
- Transfusion of stored RBCs may exacerbate the occurrences of post-operative delirium and confusion that may be caused by impaired brain tissue perfusion and oxygen delivery148,166–171.
- Hypercoagulability109,115,172,173, endothelial adhesion112,173–181, and endothelial damage attributed182 to stored RBCs may contribute to thromboembolism in RBC transfusion recipients142,183–185.
- TRALI involves pulmonary inflammation and can be caused by BRMs in stored RBCs, such as oxidised lipids186–193. The blood processing methods might have an impact since various levels of residual plasma were reported.
- Haemolytic reactions may be caused by complement activation and alloimmunisation in which neo-antigens formed in stored RBCs may contribute194–197; this may lead to acute kidney injury121,130,134.
- TACO (transfusion-associated circulatory overload) is attributed to fluid overload in patients with underlying morbidity198,199. No links between stored RBCs quality and the incidence of TACO is apparent.
- Alloimmunisation119,195,200–215 is often caused by transfusion of RBCs with a mismatch of minor antigens in chronically transfused patients200,202,206–208,216, which may result in delayed haemolytic reactions194,209. Enhanced inflammation, stemming from transfusion of RCCs, can enhance alloimmunisation responses203,205,217. Additionally, modification of the RBC surface during hypothermic storage could result in erythrophagocytosis218, which may promote alloimmunisation.
Countermeasures to reduce storage lesion
Efforts to retard haemolysis during hypothermic storage and to extend the shelf life were started at the same time as the blood banking infrastructure was established. Anticoagulant/storage solutions for whole blood were explored, culminating in approval of CPDA-1 for storage of whole blood up to 35 days. When blood component separation became the mainstay, additive solutions to replace plasma in RBC units were developed. A review by Hogman and Meryman219 summarises efforts in the period leading up to late 1980s in which the volume, osmolality, inorganic phosphate and non-permeable ion content of additive solutions were examined to yield the additive solutions commonly used today, such as SAGM, PAGGSM, MAP, AS-1, AS-5, and AS-3. Since then, in addition to haemolysis, ATP, and 2,3-DPG levels, new parameters were increasingly measured to evaluate the quality of stored RBCs, such as microparticle release, deformability, membrane fluctuations, PS exposure, and osmotic fragility63,220,221. Since 2000, “omics” technologies were introduced into the field, starting with proteomics222,223, followed by metabolomics55,224,225, genomics226,227 and lipidomics28, and accompanied by system biology or in-silico modelling36,228–232, greatly expanding the scope of understanding of the mechanisms underlying storage lesion progression and its genetic and epigenetic effects228–231, as reviewed recently55,233. Research into better additive solutions continues to the present day in order to maintain high 2,3-DPG and ATP levels while minimising haemolysis during storage by manipulating RBC intracellular pH, either with high pH additive solution containing bicarbonate or with Donnan equilibrium employing hypotonic solution with non-permeant ions234. These new additive solutions remain experimental except for AS-7235–237, which gained US FDA approval but is not available commercially. The additive solutions described above were formulated to reduce storage lesions arising from metabolic impairments; the subsequent consequences that are suggested in Figure 4. In parallel to developing RBC additive solutions, rejuvenation solutions based on pyruvate, inosine, phosphate and adenine, yielding levels of 2,3-DPG and ATP comparable to freshly collected RBCs when processed at the end of storage238,239, are available. More recently, adding such solutions was shown to be effective during hypothermic storage240,241 by reactivating critical metabolic pathways of stored RBCs241.
Addressing metabolic impairments by adjusting additive composition and pH positively affected maintenance of RBC energetics as well as levels of some antioxidant metabolites. However, since biochemical reactions are limited during hypothermic storage, reducing metabolic impairments alone may not fully reduce storage lesions caused by oxidative stress. Diffusion of oxygen from ambient air through the storage bag coupled with its higher solubility at storage temperature leads to an increase in oxygen concentration, the main reactant in oxidative reactions. Thus, in addition to addressing metabolic impairments, provisions to reduce the direct sources of oxidative damage should be included in comprehensive solutions to reduce the development of the RBC storage as illustrated in Figure 4, where oxidative damage due to O2 (Panel Ia) affects every item downstream (Panel II–IV).
Two general approaches were proposed to reduce oxidative damage during hypothermic storage: i) inclusion of anti-oxidants in the additive solution; and ii) reduction of pro-oxidants in stored RBCs by hypoxic storage. Chemicals such as nicotinic acid242, melatonin243, L-carnosine244, ascorbic acid245,246, quercetin247, iron chelators248, and N-acetylcysteine249,250 have been suggested as antioxidants to be included in additive solutions, but the improvements appeared insufficient and none has been tested extensively for commercial production. Supplementation with antioxidants, such as ascorbic acid (which can only be up taken by RBCs in its oxidised form, dehydroascorbate) and/or N-acetylcysteine, while boosting RBC antioxidant capacity246,250,251, ended up depleting glutathione pools by favoring reduced glutathione conversion to disulfide during storage250 and limiting energy metabolism. The latter is attributed to the competitive uptake of dehydroascorbate by RBCs via the transporter GLUT1 at the expense of glucose51. Alternative additives, including non-glucose sugars such as fructose and mannose225, in the formulation may obviate this problem. Lipophilic antioxidants, such as vitamin E, may be valuable alternatives to specifically mitigate lipid peroxidation252,253, but they also induce morphological changes.
Hypoxic storage, where the oxygen content of RBC units is reduced to low levels (e.g., less than 4% oxy-haemoglobin [%SO2]254) prior to refrigeration and maintained throughout storage, was proposed as an alternative to antioxidant-based additive solutions to reduce oxidative stress during hypothermic RBC storage254–259. The rationale for implementing hypoxic storage is to reduce oxygen, the essential substrate for haemoglobin oxidation that generates a multitude of ROS as byproducts. Additionally, oxygen is required for sustaining the lipid peroxidation cycle catalysed by ROS.
Hypoxic storage was shown to counteract some metabolic impairments without requiring novel additive ingredients256 independent of the reduction in oxidative stress. During pre-storage processing to reduce oxygen content in the RCC, carbon dioxide is also reduced. Carbon dioxide depletion increases cytosolic pH that was lowered from its physiological level by exposure to acidic anticoagulant and additive solutions. The resulting more neutral pH in the early phase of storage results in sustained flux through the glycolytic pathway and elevated 2,3-DPG levels, which are highly sensitive to pH and normally depleted early during hypothermic storage256,260. Additionally, deoxyhaemoglobin causes metabolic modulation to release glycolytic enzymes, such as phosphofluctokinase and glyceraldehyde dehydrogenase, sequestered at the band 3 binding domain, thereby enhancing overall glycolytic flux during hypoxic storage44,261,262. Under reduced oxygen concentration, hexokinase output to the PPP is partially blocked due to metabolic modulation as well as limiting the concentration of NADP+ (limiting substrate for PPP), which may have resulted in curtailed glutathione levels260.
Factors affecting storage lesion development: donor characteristics and RCC preparation methods
RBC responses to hypothermic storage, such as maintenance of glutathione levels and the extent of haemolysis, are heritable traits and depend on genetic makeup of the donors227,263–265. Evolutionary selection pressure in different geographical environments resulted in multiple strategies to optimise human survival, which included subtle differences in RBC physiology, resulting in a wide variation of RBCs in the current-day blood donors. For example, selection against malaria resulted in various mutations providing a survival advantage in heterozygous carriers, but their RBCs may be less suitable for hypothermic storage266–268 or less efficacious for transfusion in specific patient categories269. An additional large variability is the oxygen saturation of the collected whole blood, resulting in a wide distribution of oxygen content in the prepared RCCs prior to hypothermic storage. Although RCC preparation procedures, donor gender, and the elevation of blood donation sites affect median percent haemoglobin oxygen saturation (%SO2), a very wide %SO2 distribution ranging from below 20% to above 80% was observed270,271. This variation in the initial oxygen content of RCCs could contribute to overall variability of stored RCC quality, since oxygen is the major substrate for oxidative reactions resulting in oxidative storage lesions, and oxygen concentration profoundly affects RBC metabolism during hypothermic storage44,51,261.
The specific method used to prepare RCCs from donated whole blood for hypothermic storage, independent of donor-dependent factors (gender, donor age, iron status etc.), affects the characteristics of stored RBCs81,272,273,500 and may contribute to non-uniform clinical outcomes274. There are various procedures in blood donation (whole blood or apheresis), leucocyte filtration (whole blood, RBC, no filtration), component preparation (buffy coat, hard or soft spin), additive solutions (AS-1, AS-3, AS-5, SAGM, MAP, PAGGSM), and process time before refrigeration (8 hours or 24 hours after overnight room temperature hold). Leucocyte filtration could positively affect the quality of stored RBC by reducing ROS produced in leucocytes275. Gamma irradiation276–281 and pathogen inactivation processes282–286 generate ROS, causing oxidative damage and exacerbating the rate of storage lesion development during subsequent hypothermic storage. Washing RCCs removes damaged RBCs and other potentially harmful byproducts, such as potassium, MPs, and cytokinesd,178,287–289. Aliquoting a single RCC unit throughout its shelf life for repeated transfusion in neonates is acceptable in order to limit multiple donor exposure290. Cryopreservation overcomes the 6–7 week storage limit of refrigerated RCCs by deep-freezing RBC in cryoprotectant solutions containing glycerol291. This technology was developed in 1950s–1980s, as summarised in early292 and more recent293 reviews. Subsequent development of a closed deglycerolisation system allows for extended storage duration of post-thaw RCC from 24 hours to 14 days294. Numerous publications provide a detailed characterisation of thawed RBCs including rheologic properties295, microvesiculation296, and their potential superiority over conventionally stored RBCs in trauma settings297. Cryopreserved RCC is routinely used for rare blood types and in military settings in the United States of America and Europe.
Quality of stored RBCs: results from randomised controlled trials on age of blood and their implications
In nearly all cases, the damage RBCs sustain while stored hypothermically ex vivo accumulates throughout the storage period, albeit at different rates depending on the genetic makeup, and possibly, the dietary and environmental exposure of each donor. Therefore, to evaluate the clinical consequences of transfusing RBCs with storage lesions, numerous RCTs have taken place, including five large scale ones in the past five years10–14 and two smaller trials earlier15,298, all using the age of stored RBCs as a surrogate for the extent of the accumulated storage lesion. These large studies compared fresh (6–12 days on average) to standard-issue or moderately aged RBC units (~3 weeks; except for one study at 5 weeks12) under various patient conditions and found no differences in mortality or the development of selected morbidities, indicating that there is no inferiority in transfusing RBCs using standard practice (oldest units available) when compared to transfusing fresher RBCs (freshest available). For details, readers are referred to the original reports, several meta-analyses8,9,299–301, and recent commentaries7,302. These results dispelled blood establishments’ potentially critical safety concerns for recipients of their products. However, the debate is still open on transfusion of end-of-storage RCCs (older than 28 or 35 days)16,303–305, since the outcomes of patients receiving a large quantity of old RCCs on one occasion or those receiving transfusions chronically over an extended period of time have not been examined by RCTse. Additionally, different RBC manufacturing methods or donor factors could affect the quality of stored RBCs81,274,285,306,307, potentially confounding the results of multicentered RCTs. Therefore, questions of safety remain for vulnerable patient populations receiving transfusions of RCCs with a high storage lesion burden.
Conclusions and future directions
Placed outside of the donor’s circulation and stored in a blood bank refrigerator, RBCs incur storage lesions. The recent application of “omics” technologies has made available vast quantities of new information, providing new paths to formulate, reduce, and test hypotheses on the mechanisms of storage lesion development. However, even with the current relative abundance of data, such an undertaking would not be easy as there is wide genetic variability of performance in donors’ RBC under hypothermic storage conditions29,81,306,308,309. “Omics” studies scopes need to expand from a small number of subjects currently examined in detail to a much larger and diverse population. Different blood processing strategies need to be considered to optimise the function of donors’ characteristics and patients’ needs, keeping in mind the economic, technical, and logistical issues, and that our donors are of primary importance to sustain the transfusion chain310,311. As reviewed in previous sections, a body of evidence exists in animal models and humans suggesting that physiological responses to transfusion of damaged RBCs are adversely affecting the recipients. However, a direct link between transfusion of RBCs with specific storage lesions and observed negative outcomes in diverse recipients with different reasons for requiring transfusion therapy is difficult to establish unequivocally. This challenge is further complicated by pre-existing comorbidity as well as genetic variability in recipient responses to transfusion.
A large number of individuals will still receive stored RBCs (e.g. 4–5 million patients annually in the USA alone), even though RCC consumption is steadily decliningf,312, and the potential harmful effects of transfusions with high storage lesion burden remains a concern. Additionally, selected categories of patients are potentially more vulnerable: patients with less common blood groups (e.g. AB) often receive older RCCs as compared to other groups, and patients who are massively or chronically transfused receive a disproportionately large fraction of RBC units that may include older RCC. Especially for the latter patients, the potential sequelae of transfusion could be amplified because of high levels of exposure to RBCs stored over an extended period. The published large-scale RCTs on “age of RBC” do not provide objective evidence to assure safety of exposure to RBCs with high storage lesion burden in massive or chronic transfusion recipients. Because of these considerations, continued efforts to improve RBC processing/storage methods in order to reduce the storage lesion should benefit recipients and improve the overall cost-effectiveness of the patient-care system.
Acknowledgement
Martin Cannon is acknowledged for encouraging the initial development and continued support for organizing the existing literature into causes and potential consequences of RBC storage lesions. We would also like to thank Andrew Dunham, Ph.D., and Helen Hultin for their critical review of the manuscript.
Footnotes
For example, beta-elimination of cysteine thiols to generate dehydroalanine or carbonylation on side chains of lysine, arginine, proline and threonine residues20,44,45,314–316.
For example, phosphofructokinase, glucose 6-phosphate dehydrogenase and biphosphoglycerate mutase.
Negative outcome was reported with washing older RCCs, presumably from inflicting mechanical damage on older fragile RBCs during the process155.
A secondary analysis of data from an RCT (PROPPR trial on trauma) suggested an association between transfusion of 10 or more units of RCCs older than 22 days, and an increased liklihood of mortality within 24 hours501.
Shifing demographics might change this trend in a decade313.
Funding and resources
Funded in part by an SBIR grant from NHLBI: 2R44HL132172.
Diclosure of conflicts of interest
TY is an employee and equity holder of Hemanext Inc., and the company is commercialising a hypoxic RCC storage technology. MP declares that there are no conflicts of interest associated with this publication, but that he receives financial support (analytical measurements) from Hemanext for a research project on Hemanext bags. ADA is a founder of Omix Technologies Inc. and a consultant for Hemanext Inc.
References
- 1.Kanias T, Acker JP. Biopreservation of red blood cells--the struggle with hemoglobin oxidation. FEBS J. 2010;277:343–56. doi: 10.1111/j.1742-4658.2009.07472.x. [DOI] [PubMed] [Google Scholar]
- 2.D’Alessandro A, Zolla L. Proteomic analysis of red blood cells and the potential for the clinic: what have we learned so far? Expert Rev Proteomics. 2017;14:243–52. doi: 10.1080/14789450.2017.1291347. [DOI] [PubMed] [Google Scholar]
- 3.Winslow RM. Oxygen: the poison is in the dose. Transfusion. 2013;53:424–37. doi: 10.1111/j.1537-2995.2012.03774.x. [DOI] [PubMed] [Google Scholar]
- 4.Willekens FL, Werre JM, Groenen-Dopp YA, et al. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–56. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 5.D’Alessandro A, Gray AD, Szczepiorkowski ZM, et al. Red blood cell metabolic responses to refrigerated storage, rejuvenation, and frozen storage. Transfusion. 2017;57:1019–30. doi: 10.1111/trf.14034. [DOI] [PubMed] [Google Scholar]
- 6.Yurkovich JT, Zielinski DC, Yang L, et al. Quantitative time-course metabolomics in human red blood cells reveal the temperature dependence of human metabolic networks. J Biol Chem. 2017;292:19556–64. doi: 10.1074/jbc.M117.804914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belpulsi D, Spitalnik SL, Hod EA. The controversy over the age of blood: what do the clinical trials really teach us? Blood Transfus. 2017;15:112–5. doi: 10.2450/2017.0328-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cushing MM, Kelley J, Klapper E, et al. Critical developments of 2017: a review of the literature from selected topics in transfusion. A committee report from the AABB Clinical Transfusion Medicine Committee. Transfusion. 2018;58:1065–75. doi: 10.1111/trf.14520. [DOI] [PubMed] [Google Scholar]
- 9.McQuilten ZK, French CJ, Nichol A, et al. Effect of age of red cells for transfusion on patient outcomes: a systematic review and meta-analysis. Transfus Med Rev. 2018;32:77–88. doi: 10.1016/j.tmrv.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Cooper DJ, McQuilten ZK, Nichol A, et al. Age of red cells for transfusion and outcomes in critically Ill adults. N Engl J Med. 2017;377:1858–67. doi: 10.1056/NEJMoa1707572. [DOI] [PubMed] [Google Scholar]
- 11.Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375:1937–45. doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 12.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL randomized clinical trial. JAMA. 2015;314:2514–23. doi: 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- 13.Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–8. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 14.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–29. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fergusson DA, Hebert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–51. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 16.Hod EA, Francis RO, Spitalnik SL. Red blood cell storage lesion-induced adverse effects: more smoke; is there fire? Anesth Analg. 2017;124:1752–4. doi: 10.1213/ANE.0000000000001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehrie EA, Tobian AAR. Finally, what we have been waiting for: evidence that transfusion of RBCs at the extreme of the storage spectrum is safe. Lancet Haematol. 2017;4:e504–5. doi: 10.1016/S2352-3026(17)30179-5. [DOI] [PubMed] [Google Scholar]
- 18.Flatt JF, Bawazir WM, Bruce LJ. The involvement of cation leaks in the storage lesion of red blood cells. Front Physiol. 2014;5:214. doi: 10.3389/fphys.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kehrer JP. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50. doi: 10.1016/s0300-483x(00)00231-6. [DOI] [PubMed] [Google Scholar]
- 20.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion. 2016;56:421–6. doi: 10.1111/trf.13363. [DOI] [PubMed] [Google Scholar]
- 22.Tavazzi B, Amorini AM, Fazzina G, et al. Oxidative stress induces impairment of human erythrocyte energy metabolism through the oxygen radical-mediated direct activation of amp-deaminase. J Biol Chem. 2001;276:48083–92. doi: 10.1074/jbc.M101715200. [DOI] [PubMed] [Google Scholar]
- 23.Lang F, Abed M, Lang E, Foller M. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal. 2014;21:138–53. doi: 10.1089/ars.2013.5747. [DOI] [PubMed] [Google Scholar]
- 24.Fortier N, Snyder LM, Garver F, et al. The relationship between in vivo generated hemoglobin skeletal protein complex and increased red cell membrane rigidity. Blood. 1988;71:1427–31. [PubMed] [Google Scholar]
- 25.Wolfe LC. Oxidative injuries to the red cell membrane during conventional blood preservation. Semin Hematol. 1989;26:307–12. [PubMed] [Google Scholar]
- 26.Alayash AI, Patel RP, Cashon RE. Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid Redox Signal. 2001;3:313–27. doi: 10.1089/152308601300185250. [DOI] [PubMed] [Google Scholar]
- 27.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion. 2016;56:2560–70. doi: 10.1111/trf.13748. [DOI] [PubMed] [Google Scholar]
- 28.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica. 2016;101:578–86. doi: 10.3324/haematol.2015.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardyn M, Maye S, Lesch A, et al. The antioxidant capacity of erythrocyte concentrates is increased during the first week of storage and correlated with the uric acid level. Vox Sang. 2017;112:638–47. doi: 10.1111/vox.12563. [DOI] [PubMed] [Google Scholar]
- 30.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 31.Rapoport S. Dimensional, osmotic, and chemical changes of erythrocytes in stored blood cells separated from plasma. J Clin Invest. 1947;26:629–35. [PubMed] [Google Scholar]
- 32.Wilson MC, Trakarnsanga K, Heesom KJ, et al. Comparison of the proteome of adult and cord erythroid cells, and changes in the proteome following reticulocyte maturation. Mol Cell Proteomics. 2016;15:1938–46. doi: 10.1074/mcp.M115.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Alessandro A, Dzieciatkowska M, Nemkov T, Hansen KC. Red blood cell proteomics update: is there more to discover? Blood Transfusion. 2017;15:182–7. doi: 10.2450/2017.0293-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Alessandro A, Righetti PG, Zolla L. The red blood cell proteome and interactome: an update. J Proteome Res. 2010;9:144–63. doi: 10.1021/pr900831f. [DOI] [PubMed] [Google Scholar]
- 35.Bryk AH, Wisniewski JR. Quantitative analysis of human red blood cell proteome. J Proteome Res. 2017;16:2752–61. doi: 10.1021/acs.jproteome.7b00025. [DOI] [PubMed] [Google Scholar]
- 36.Nishino T, Yachie-Kinoshita A, Hirayama A, et al. Dynamic simulation and metabolome analysis of long-term erythrocyte storage in adenine-guanosine solution. PLoS One. 2013;8:e71060. doi: 10.1371/journal.pone.0071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishino T, Yachie-Kinoshita A, Hirayama A, et al. In silico modeling and metabolome analysis of long-stored erythrocytes to improve blood storage methods. J Biotechnol. 2009;144:212–23. doi: 10.1016/j.jbiotec.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Nemkov T, Sun K, Reisz JA, et al. Metabolism of citrate and other carboxylic acids in erythrocytes as a function of oxygen saturation and refrigerated storage. Front Med (Lausanne) 2017;4:175. doi: 10.3389/fmed.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rolfsson O, Johannsson F, Magnusdottir M, et al. Mannose and fructose metabolism in red blood cells during cold storage in SAGM. Transfusion. 2017;57:2665–76. doi: 10.1111/trf.14266. [DOI] [PubMed] [Google Scholar]
- 40.Paglia G, Sigurjonsson OE, Bordbar A, et al. Metabolic fate of adenine in red blood cells during storage in SAGM solution. Transfusion. 2016;56:2538–47. doi: 10.1111/trf.13740. [DOI] [PubMed] [Google Scholar]
- 41.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion. 2016;56:852–62. doi: 10.1111/trf.13460. [DOI] [PubMed] [Google Scholar]
- 42.Paglia G, D’Alessandro A, Rolfsson O, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood. 2016;128:e43–50. doi: 10.1182/blood-2016-06-721688. [DOI] [PubMed] [Google Scholar]
- 43.Prudent M, Rochat B, Marvin L, et al. Targeted metabolomics of SAGM red blood cell storage. Clinical Laboratory. 2014;60:S3. [Google Scholar]
- 44.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128:e32–42. doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 45.Delobel J, Prudent M, Crettaz D, et al. Cysteine redox proteomics of the hemoglobin-depleted cytosolic fraction of stored red blood cells. Proteomics Clin Appl. 2016;10:883–93. doi: 10.1002/prca.201500132. [DOI] [PubMed] [Google Scholar]
- 46.Delobel J, Prudent M, Tissot JD, Lion N. Proteomics of the red blood cell carbonylome during blood banking of erythrocyte concentrates. Proteomics Clin Appl. 2016;10:257–66. doi: 10.1002/prca.201500074. [DOI] [PubMed] [Google Scholar]
- 47.D’Alessandro A, Mirasole C, Zolla L. Haemoglobin glycation (Hb1Ac) increases during red blood cell storage: a MALDI-TOF mass-spectrometry-based investigation. Vox Sang. 2013;105:177–80. doi: 10.1111/vox.12029. [DOI] [PubMed] [Google Scholar]
- 48.Sparrow RL, Veale MF, Healey G, Payne KA. Red blood cell (RBC) age at collection and storage influences RBC membrane-associated carbohydrates and lectin binding. Transfusion. 2007;47:966–8. doi: 10.1111/j.1537-2995.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 49.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57:325–36. doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 50.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion. 2011;51:1450–9. doi: 10.1111/j.1537-2995.2010.03026.x. [DOI] [PubMed] [Google Scholar]
- 51.Nemkov T, Sun K, Reisz JA, Song A, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103:361–72. doi: 10.3324/haematol.2017.178608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casali E, Berni P, Spisni A, et al. Hypoxanthine: a new paradigm to interpret the origin of transfusion toxicity. Blood Transfus. 2015;14:555–6. doi: 10.2450/2015.0177-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heaton A, Keegan T, Holme S. In vivo regeneration of red cell 2,3-diphosphoglycerate following transfusion of DPG-depleted AS-1, AS-3 and CPDA-1 red cells. Br J Haematol. 1989;71:131–6. doi: 10.1111/j.1365-2141.1989.tb06286.x. [DOI] [PubMed] [Google Scholar]
- 54.Tuo WW, Wang D, Liang WJ, Huang YX. How cell number and cellular properties of blood-banked red blood cells of different cell ages decline during storage. PLoS One. 2014;9:e105692. doi: 10.1371/journal.pone.0105692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion. 2016;56:980–93. doi: 10.1111/trf.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prudent M, Rappaz B, Hamelin R, et al. Indirect activity of kinases on protein phosphorylation: loss of protein Tyr-phosphorylation during in vitro storage of human erythrocytes: impact on RBC morphology. Transfusion. 2014;54:49A–50A. [Google Scholar]
- 57.Park Y, Best CA, Auth T, et al. Metabolic remodeling of the human red blood cell membrane. Proc Natl Acad Sci U S A. 2010;107:1289–94. doi: 10.1073/pnas.0910785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds JD, Ahearn GS, Angelo M, et al. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winslow RM, Intaglietta M. Red cell age and loss of function: advance or SNO-job? Transfusion. 2008;48:411–4. doi: 10.1111/j.1537-2995.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 60.Isbell TS, Sun CW, Wu LC, et al. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med. 2008;14:773–7. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodgers GP, Lichtman HC, Sheff MF. Red blood cell glucose-6-phosphate dehydrogenase activity in aged humans. J Am Geriatr Soc. 1983;31:8–11. doi: 10.1111/j.1532-5415.1983.tb06281.x. [DOI] [PubMed] [Google Scholar]
- 62.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 63.Bardyn M, Rappaz B, Jaferzadeh K, et al. Red blood cells ageing markers: a multi-parametric analysis. Blood Transfus. 2017;15:239–48. doi: 10.2450/2017.0318-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mays JA, Hess JR. Modelling the effects of blood component storage lesions on the quality of haemostatic resuscitation in massive transfusion for trauma. Blood Transfus. 2017;15:153–7. doi: 10.2450/2017.0310-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 66.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 67.Prudent M, Delobel J, Hubner A, et al. Proteomics of stored red blood cell membrane and storage-induced microvesicles reveals the association of Flotillin-2 with band 3 complexes. Front Physiol. 2018;9:421. doi: 10.3389/fphys.2018.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larson MC, Karafin MS, Hillery CA, Hogg N. Phosphatidylethanolamine is progressively exposed in RBCs during storage. Transfus Med. 2017;27:136–41. doi: 10.1111/tme.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geldwerth D, Kuypers FA, Butikofer P, et al. Transbilayer mobility and distribution of red cell phospholipids during storage. J Clin Invest. 1993;92:308–14. doi: 10.1172/JCI116568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verhoeven AJ, Hilarius PM, Dekkers DW, et al. Prolonged storage of red blood cells affects aminophospholipid translocase activity. Vox Sang. 2006;91:244–51. doi: 10.1111/j.1423-0410.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 71.Safeukui I, Buffet PA, Deplaine G, et al. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood. 2012;120:424–30. doi: 10.1182/blood-2012-01-404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hebbel RP, Leung A, Mohandas N. Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood. 1990;76:1015–20. [PubMed] [Google Scholar]
- 73.Rinalducci S, Longo V, Ceci LR, Zolla L. Targeted quantitative phosphoproteomic analysis of erythrocyte membranes during blood bank storage. J Mass Spectrom. 2015;50:326–35. doi: 10.1002/jms.3531. [DOI] [PubMed] [Google Scholar]
- 74.Liu TZ, Lin TF, Hung IJ, et al. Enhanced susceptibility of erythrocytes deficient in glucose-6-phosphate dehydrogenase to alloxan/glutathione-induced decrease in red cell deformability. Life Sci. 1994;55:PL55–60. doi: 10.1016/0024-3205(94)00888-4. [DOI] [PubMed] [Google Scholar]
- 75.Burger P, Hilarius-Stokman P, de Korte D, et al. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood. 2012;119:5512–21. doi: 10.1182/blood-2011-10-386805. [DOI] [PubMed] [Google Scholar]
- 76.Anniss AM, Sparrow RL. Expression of CD47 (integrin-associated protein) decreases on red blood cells during storage. Transfus Apher Sci. 2002;27:233–8. doi: 10.1016/s1473-0502(02)00070-8. [DOI] [PubMed] [Google Scholar]
- 77.Stewart A, Urbaniak S, Turner M, Bessos H. The application of a new quantitative assay for the monitoring of integrin-associated protein CD47 on red blood cells during storage and comparison with the expression of CD47 and phosphatidylserine with flow cytometry. Transfusion. 2005;45:1496–503. doi: 10.1111/j.1537-2995.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 78.Burger P, de Korte D, van den Berg TK, van Bruggen R. CD47 in erythrocyte ageing and clearance - the Dutch point of view. Transfus Med Hemother. 2012;39:348–52. doi: 10.1159/000342231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anniss AM, Glenister KM, Killian JJ, Sparrow RL. Proteomic analysis of supernatants of stored red blood cell products. Transfusion. 2005;45:1426–33. doi: 10.1111/j.1537-2995.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 80.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jordan A, Chen D, Yi QL, et al. Assessing the influence of component processing and donor characteristics on quality of red cell concentrates using quality control data. Vox Sang. 2016;111:8–15. doi: 10.1111/vox.12378. [DOI] [PubMed] [Google Scholar]
- 82.Bakkour S, Acker JP, Chafets DM, et al. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang. 2016;111:22–32. doi: 10.1111/vox.12390. [DOI] [PubMed] [Google Scholar]
- 83.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–82. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wojczyk BS, Kim N, Bandyopadhyay S, et al. Macrophages clear refrigerator storage-damaged red blood cells and subsequently secrete cytokines in vivo, but not in vitro, in a murine model. Transfusion. 2014;54:3186–97. doi: 10.1111/trf.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lion N, Crettaz D, Rubin O, Tissot JD. Stored red blood cells: a changing universe waiting for its map(s) J Proteomics. 2010;73:374–85. doi: 10.1016/j.jprot.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 86.Burger P, Kostova E, Bloem E, et al. Potassium leakage primes stored erythrocytes for phosphatidylserine exposure and shedding of pro-coagulant vesicles. Br J Haematol. 2013;160:377–86. doi: 10.1111/bjh.12133. [DOI] [PubMed] [Google Scholar]
- 87.Roussel C, Dussiot M, Marin M, et al. Spherocytic shift of red blood cells during storage provides a quantitative whole cell-based marker of the storage lesion. Transfusion. 2017;57:1007–18. doi: 10.1111/trf.14015. [DOI] [PubMed] [Google Scholar]
- 88.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–20. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 89.Daly A, Raval JS, Waters JH, et al. Effect of blood bank storage on the rheological properties of male and female donor red blood cells. Clin Hemorheol Microcirc. 2014;56:337–45. doi: 10.3233/CH-131754. [DOI] [PubMed] [Google Scholar]
- 90.Salaria ON, Barodka VM, Hogue CW, et al. Impaired red blood cell deformability after transfusion of stored allogeneic blood but not autologous salvaged blood in cardiac surgery patients. Anesth Analg. 2014;118:1179–87. doi: 10.1213/ANE.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 92.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J Clin Invest. 2017;127:375–82. doi: 10.1172/JCI90837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prestia K, Bandyopadhyay S, Slate A, et al. Transfusion of stored blood impairs host defenses against Gram-negative pathogens in mice. Transfusion. 2014;54:2842–51. doi: 10.1111/trf.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rapido F. The potential adverse effects of haemolysis. Blood Transfus. 2017;15:218–21. doi: 10.2450/2017.0311-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hod EA, Spitalnik SL. Stored red blood cell transfusions: iron, inflammation, immunity, and infection. Transfus Clin Biol. 2012;19:84–9. doi: 10.1016/j.tracli.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wood JC, Cohen AR, Pressel SL, et al. Organ iron accumulation in chronically transfused children with sickle cell anaemia: baseline results from the TWiTCH trial. Br J Haematol. 2016;172:122–30. doi: 10.1111/bjh.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bennett JM MDS Foundation’s Working Group on Transfusional Iron Overload. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol. 2008;83:858–61. doi: 10.1002/ajh.21269. [DOI] [PubMed] [Google Scholar]
- 99.Silva G, Jeney V, Chora A, et al. Oxidized hemoglobin is an endogenous proinflammatory agonist that targets vascular endothelial cells. J Biol Chem. 2009;284:29582–95. doi: 10.1074/jbc.M109.045344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yalcin O, Ortiz D, Tsai AG, et al. Microhemodynamic aberrations created by transfusion of stored blood. Transfusion. 2014;54:1015–27. doi: 10.1111/trf.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen K, Piknova B, Pittman RN, et al. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide. 2008;18:47–60. doi: 10.1016/j.niox.2007.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piknova B, Keszler A, Hogg N, Schechter AN. The reaction of cell-free oxyhemoglobin with nitrite under physiologically relevant conditions: implications for nitrite-based therapies. Nitric Oxide. 2009;20:88–94. doi: 10.1016/j.niox.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bogle RG, Coade SB, Moncada S, et al. Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells. Biochem Biophys Res Commun. 1991;180:926–32. doi: 10.1016/s0006-291x(05)81154-4. [DOI] [PubMed] [Google Scholar]
- 105.Sprague RSEM, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275:H1726–H32. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 106.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–61. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Giebink A, Spence DM. Microfluidic evaluation of red cells collected and stored in modified processing solutions used in blood banking. Integr Biol (Camb) 2014;6:65–75. doi: 10.1039/c3ib40187a. [DOI] [PubMed] [Google Scholar]
- 108.Radwanski K, Garraud O, Cognasse F, et al. The effects of red blood cell preparation method on in vitro markers of red blood cell aging and inflammatory response. Transfusion. 2013;53:3128–38. doi: 10.1111/trf.12143. [DOI] [PubMed] [Google Scholar]
- 109.Zecher D, Cumpelik A, Schifferli JA. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler Thromb Vasc Biol. 2014;34:313–20. doi: 10.1161/ATVBAHA.113.302378. [DOI] [PubMed] [Google Scholar]
- 110.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wagener FA, Eggert A, Boerman OC, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–11. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 112.Sparrow RL, Sran A, Healey G, et al. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: a paired study. Transfusion. 2014;54:560–8. doi: 10.1111/trf.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu X, Patel RP, Weinberg JA, et al. Membrane attack complex generation increases as a function of time in stored blood. Transfus Med. 2014;24:114–6. doi: 10.1111/tme.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neal MD, Raval JS, Triulzi DJ, Simmons RL. Innate immune activation after transfusion of stored red blood cells. Transfus Med Rev. 2013;27:113–8. doi: 10.1016/j.tmrv.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 115.Aung HH, Tung JP, Dean MM, et al. Procoagulant role of microparticles in routine storage of packed red blood cells: potential risk for prothrombotic post-transfusion complications. Pathology. 2017;49:62–9. doi: 10.1016/j.pathol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Subramaniam K, Spilsbury K, Ayonrinde OT, et al. Red blood cell transfusion is associated with further bleeding and fresh-frozen plasma with mortality in nonvariceal upper gastrointestinal bleeding. Transfusion. 2016;56:816–26. doi: 10.1111/trf.13446. [DOI] [PubMed] [Google Scholar]
- 117.Larsen AM, Leinoe EB, Johansson PI, et al. Haemostatic function and biomarkers of endothelial damage before and after RBC transfusion in patients with haematologic disease. Vox Sang. 2015;109:52–61. doi: 10.1111/vox.12249. [DOI] [PubMed] [Google Scholar]
- 118.Yazdanbakhsh K, Bao W, Zhong H. Immunoregulatory effects of stored red blood cells. Hematology Am Soc Hematol Educ Program. 2011;2011:466–9. doi: 10.1182/asheducation-2011.1.466. [DOI] [PubMed] [Google Scholar]
- 119.Vallion R, Bonnefoy F, Daoui A, et al. Transforming growth factor-beta released by apoptotic white blood cells during red blood cell storage promotes transfusion-induced alloimmunomodulation. Transfusion. 2015;55:1721–35. doi: 10.1111/trf.13031. [DOI] [PubMed] [Google Scholar]
- 120.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 121.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 122.Wang D, Sun J, Solomon SB, et al. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–95. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152–65. doi: 10.1016/j.freeradbiomed.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 124.Amireault P, Bayard E, Launay JM, et al. Serotonin is a key factor for mouse red blood cell survival. PLoS One. 2013;8:e83010. doi: 10.1371/journal.pone.0083010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–72. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simonova G, Tung JP, Fraser JF, et al. A comprehensive ovine model of blood transfusion. Vox Sang. 2014;106:153–60. doi: 10.1111/vox.12076. [DOI] [PubMed] [Google Scholar]
- 127.Hebert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care. 2001;29:227–9. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 128.Shander A, Sazama K. Clinical consequences of iron overload from chronic red blood cell transfusions, its diagnosis, and its management by chelation therapy. Transfusion. 2010;50:1144–55. doi: 10.1111/j.1537-2995.2009.02551.x. [DOI] [PubMed] [Google Scholar]
- 129.Berdoukas V, Coates TD, Cabantchik ZI. Iron and oxidative stress in cardiomyopathy in thalassemia. Free Radic Biol Med. 2015;88:3–9. doi: 10.1016/j.freeradbiomed.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 130.Brown CD, Ghali HS, Zhao Z, et al. Association of reduced red blood cell deformability and diabetic nephropathy. Kidney International. 2005;67:295–300. doi: 10.1111/j.1523-1755.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 131.Weinberg JA, McGwin G, Jr, Marques MB, et al. Transfusions in the less severely injured: does age of transfused blood affect outcomes? J Trauma. 2008;65:794–8. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 132.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–58. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gerber DR. Risks of packed red blood cell transfusion in patients undergoing cardiac surgery. J Crit Care. 2012;27:737 e1–9. doi: 10.1016/j.jcrc.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 134.Kaukonen KM, Vaara ST, Pettila V, et al. Age of red blood cells and outcome in acute kidney injury. Crit Care. 2013;17:R222. doi: 10.1186/cc13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spadaro S, Taccone FS, Fogagnolo A, et al. The effects of storage of red blood cells on the development of postoperative infections after noncardiac surgery. Transfusion. 2017;57:2727–37. doi: 10.1111/trf.14249. [DOI] [PubMed] [Google Scholar]
- 136.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 137.Weinberg JA, McGwin G, Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–82. doi: 10.1097/TA.0b013e31817c9687. discussion 82–4. [DOI] [PubMed] [Google Scholar]
- 138.Agarwal N, Murphy JG, Cayten CG, Stahl WM. Blood transfusion increases the risk of infection after trauma. Arch Surg. 1993;128:171–6. doi: 10.1001/archsurg.1993.01420140048008. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 139.Andreasen JJ, Dethlefsen C, Modrau IS, et al. Storage time of allogeneic red blood cells is associated with risk of severe postoperative infection after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2011;39:329–34. doi: 10.1016/j.ejcts.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 140.Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39:694–700. doi: 10.1046/j.1537-2995.1999.39070694.x. [DOI] [PubMed] [Google Scholar]
- 141.Claridge JA, Sawyer RG, Schulman AM, et al. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68:566–72. [PubMed] [Google Scholar]
- 142.Grimshaw K, Sahler J, Spinelli SL, et al. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion. 2011;51:874–80. doi: 10.1111/j.1537-2995.2011.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hill GE, Frawley WH, Griffith KE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908–14. doi: 10.1097/01.TA.0000022460.21283.53. [DOI] [PubMed] [Google Scholar]
- 144.Horvath KA, Acker MA, Chang H, et al. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg. 2013;95:2194–201. doi: 10.1016/j.athoracsur.2012.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Juffermans NP, Prins DJ, Vlaar AP, et al. Transfusion-related risk of secondary bacterial infections in sepsis patients: a retrospective cohort study. Shock. 2011;35:355–9. doi: 10.1097/SHK.0b013e3182086094. [DOI] [PubMed] [Google Scholar]
- 146.Juffermans NP, Vlaar AP, Prins DJ, et al. The age of red blood cells is associated with bacterial infections in critically ill trauma patients. Blood Transfus. 2012;10:290–5. doi: 10.2450/2012.0068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Karafin MS, Carpenter E, Pan A, et al. Older red cell units are associated with an increased incidence of infection in chronically transfused adults with sickle cell disease. Transfus Apher Sci. 2017;56:345–51. doi: 10.1016/j.transci.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 148.Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 149.Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 150.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–6. doi: 10.1001/archsurg.137.6.711. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 151.Ozment CP, Mamo LB, Campbell ML, et al. Transfusion-related biologic effects and free hemoglobin, heme, and iron. Transfusion. 2013;53:732–40. doi: 10.1111/j.1537-2995.2012.03837.x. [DOI] [PubMed] [Google Scholar]
- 152.Rachoin JS, Daher R, Schorr C, et al. Microbiology, time course and clinical characteristics of infection in critically ill patients receiving packed red blood cell transfusion. Vox Sang. 2009;97:294–302. doi: 10.1111/j.1423-0410.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 153.Rogers MA, Micic D, Blumberg N, et al. Storage duration of red blood cell transfusion and Clostridium difficile infection: a within person comparison. PLoS One. 2014;9:e89332. doi: 10.1371/journal.pone.0089332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang D, Cortes-Puch I, Sun J, et al. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion. 2014;54:1712–24. doi: 10.1111/trf.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cholette JM, Pietropaoli AP, Henrichs KF, et al. Longer RBC storage duration is associated with increased postoperative infections in pediatric cardiac surgery. Pediatr Crit Care Med. 2015;16:227–35. doi: 10.1097/PCC.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stubbs JR, Reddy RL, Elg SA, et al. Fatal Yersinia enterocolitica (serotype 0:5,27) sepsis after blood transfusion. Vox Sang. 1991;61:18–23. doi: 10.1111/j.1423-0410.1991.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 157.Kopko PM, Holland PV. Mechanisms of severe transfusion reactions. Transfus Clin Biol. 2001;8:278–81. doi: 10.1016/s1246-7820(01)00113-6. [DOI] [PubMed] [Google Scholar]
- 158.Karkouti K, Callum JL, Acker JP, et al. Red cell transfusion-associated hemolysis in cardiac surgery: an observational cohort study. Anesth Analg. 2017;124:1986–91. doi: 10.1213/ANE.0000000000001807. [DOI] [PubMed] [Google Scholar]
- 159.Fernandez FG, Jaramillo A, Ewald G, et al. Blood transfusions decrease the incidence of acute rejection in cardiac allograft recipients. J Heart Lung Transplant. 2005;24:S255–61. doi: 10.1016/j.healun.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 160.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978;299:799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 161.Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101:299–308. doi: 10.1016/S0002-9343(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 162.Muszynski J, Nateri J, Nicol K, et al. Immunosuppressive effects of red blood cells on monocytes are related to both storage time and storage solution. Transfusion. 2012;52:794–802. doi: 10.1111/j.1537-2995.2011.03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Muszynski JA, Bale J, Nateri J, et al. Supernatants from stored red blood cell (RBC) units, but not RBC-derived microvesicles, suppress monocyte function in vitro. Transfusion. 2015;55:1937–45. doi: 10.1111/trf.13084. [DOI] [PubMed] [Google Scholar]
- 164.Muszynski JA, Frazier E, Nofziger R, et al. Red blood cell transfusion and immune function in critically ill children: a prospective observational study. Transfusion. 2015;55:766–74. doi: 10.1111/trf.12896. [DOI] [PubMed] [Google Scholar]
- 165.Cata JP, Wang H, Gottumukkala V, et al. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown CH, 4th, Grega M, Selnes OA, et al. Length of red cell unit storage and risk for delirium after cardiac surgery. Anesth Analg. 2014;119:242–50. doi: 10.1213/ANE.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kawatani Y, Nakamura Y, Hayashi Y, et al. Development of delirium in the intensive care unit in patients after endovascular aortic repair: a retrospective evaluation of the prevalence and risk factors. Crit Care Res Pract. 2015;2015 doi: 10.1155/2015/405817. 405817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Risk factors of delirium after cardiac surgery: a systematic review. Eur J Cardiovasc Nurs. 2011;10:197–204. doi: 10.1016/j.ejcnurse.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 169.Xue FS, Liu GP, Yang GZ, Sun C. Is longer storage time of red blood cells really not associated with risks of delirium and complications after hip fracture surgery? Injury. 2016;47:1359–60. doi: 10.1016/j.injury.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 170.Zhang ZY, Gao DP, Yang JJ, et al. Impact of length of red blood cells transfusion on postoperative delirium in elderly patients undergoing hip fracture surgery: a cohort study. Injury. 2016;47:408–12. doi: 10.1016/j.injury.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 171.Tan H, Bi J, Wang Y, et al. Transfusion of old RBCs induces neuroinflammation and cognitive impairment. Crit Care Med. 2015;43:e276–86. doi: 10.1097/CCM.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rubin O, Delobel J, Prudent M, et al. Red blood cell-derived microparticles isolated from blood units initiate and propagate thrombin generation. Transfusion. 2013;53:1744–54. doi: 10.1111/trf.12008. [DOI] [PubMed] [Google Scholar]
- 173.Said AS, Doctor A. Influence of red blood cell-derived microparticles upon vasoregulation. Blood Transfus. 2017;15:522–34. doi: 10.2450/2017.0353-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barshtein G, Manny N, Yedgar S. Circulatory risk in the transfusion of red blood cells with impaired flow properties induced by storage. Transfus Med Rev. 2011;25:24–35. doi: 10.1016/j.tmrv.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 175.Wagener FADTG, Abraham NG, van Kooyk Y, et al. Heme-induced cell adhesion in the pathogenesis of sickle-cell disease and inflammation. Trends Pharmacol Sci. 2001;22:52–4. doi: 10.1016/s0165-6147(00)01609-6. [DOI] [PubMed] [Google Scholar]
- 176.Zhu H, Zennadi R, Xu BX, et al. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med. 2011;39:2478–86. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Straat M, van Hezel ME, Boing A, et al. Monocyte-mediated activation of endothelial cells occurs only after binding to extracellular vesicles from red blood cell products, a process mediated by beta-integrin. Transfusion. 2016;56:3012–20. doi: 10.1111/trf.13851. [DOI] [PubMed] [Google Scholar]
- 178.Loh YS, Tan S, Kwok M, et al. Reduction of biological response modifiers in the supernatant of washed paediatric red blood cells. Vox Sang. 2016;111:365–73. doi: 10.1111/vox.12442. [DOI] [PubMed] [Google Scholar]
- 179.Kirby BS, Hanna G, Hendargo HC, McMahon TJ. Restoration of intracellular ATP production in banked red blood cells improves inducible ATP export and suppresses RBC-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2014;307:H1737–44. doi: 10.1152/ajpheart.00542.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Anniss AM, Sparrow RL. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion. 2006;46:1561–7. doi: 10.1111/j.1537-2995.2006.00944.x. [DOI] [PubMed] [Google Scholar]
- 181.Luk CS, Gray-Statchuk LA, Cepinkas G, Chin-Yee IH. WBC reduction reduces storage-associated RBC adhesion to human vascular endothelial cells under conditions of continuous flow in vitro. Transfusion. 2003;43:151–6. doi: 10.1046/j.1537-2995.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 182.Rifkind JM, Mohanty JG, Nagababu E. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front Physiol. 2014;5:500. doi: 10.3389/fphys.2014.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg. 2007;142:126–32. doi: 10.1001/archsurg.142.2.126. discussion 33. [DOI] [PubMed] [Google Scholar]
- 184.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Goel R, Patel EU, Cushing MM, et al. Association of perioperative red blood cell transfusions with venous thromboembolism in a North American Registry. JAMA Surg. 2018;1543:826–33. doi: 10.1001/jamasurg.2018.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Babaev A, Pozzi F, Hare G, Zhang H. Storage of red blood cells and transfusion-related acute lung injury. J Anesth Crit Care. 2014;1:1–14. doi: 10.15406/jaccoa.2014.01.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fung YL, Silliman CC. The role of neutrophils in the pathogenesis of transfusion-related acute lung injury. Transfus Med Rev. 2009;23:266–83. doi: 10.1016/j.tmrv.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Menis M, Anderson SA, Forshee RA, et al. Transfusion-related acute lung injury and potential risk factors among the inpatient US elderly as recorded in Medicare claims data, during 2007 through 2011. Transfusion. 2014;54:2182–93. doi: 10.1111/trf.12626. [DOI] [PubMed] [Google Scholar]
- 190.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105:2266–73. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 191.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–62. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 192.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vlaar AP, Hofstra JJ, Levi M, et al. Supernatant of aged erythrocytes causes lung inflammation and coagulopathy in a “two-hit” in vivo syngeneic transfusion model. Anesthesiology. 2010;113:92–103. doi: 10.1097/ALN.0b013e3181de6f25. [DOI] [PubMed] [Google Scholar]
- 194.Chadebech P, Habibi A, Nzouakou R, et al. Delayed hemolytic transfusion reaction in sickle cell disease patients: evidence of an emerging syndrome with suicidal red blood cell death. Transfusion. 2009;49:1785–92. doi: 10.1111/j.1537-2995.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 195.Desai PC, Deal AM, Pfaff ER, et al. Alloimmunization is associated with older age of transfused red blood cells in sickle cell disease. Am J Hematol. 2015;90:691–5. doi: 10.1002/ajh.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sanz C, Nomdedeu M, Belkaid M, et al. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013;53:710–5. doi: 10.1111/j.1537-2995.2012.03819.x. [DOI] [PubMed] [Google Scholar]
- 197.Vamvakas EC, Blajchman MA. Blood still kills: six strategies to further reduce allogeneic blood transfusion-related mortality. Transfus Med Rev. 2010;24:77–124. doi: 10.1016/j.tmrv.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Alam A, Lin Y, Lima A, et al. The prevention of transfusion-associated circulatory overload. Transfus Med Rev. 2013;27:105–12. doi: 10.1016/j.tmrv.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 199.Lieberman L, Maskens C, Cserti-Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27:206–12. doi: 10.1016/j.tmrv.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 200.Abdelrazik AM, Elshafie SM, El Said MN, et al. Study of red blood cell alloimmunization risk factors in multiply transfused thalassemia patients: role in improving thalassemia transfusion practice in Fayoum, Egypt. Transfusion. 2016;56:2303–7. doi: 10.1111/trf.13695. [DOI] [PubMed] [Google Scholar]
- 201.Belsito A, Magnussen K, Napoli C. Emerging strategies of blood group genotyping for patients with hemoglobinopathies. Transfus Apher Sci. 2017;56:206–13. doi: 10.1016/j.transci.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 202.Gehrie EA, Ness PM, Bloch EM, et al. Medical and economic implications of strategies to prevent alloimmunization in sickle cell disease. Transfusion. 2017;57:2267–76. doi: 10.1111/trf.14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gibb DR, Calabro S, Liu D, et al. The NLRP3 inflammasome does not regulate alloimmunization to transfused red blood cells in mice. EBioMedicine. 2016;9:77–86. doi: 10.1016/j.ebiom.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hendrickson JE, Hod EA, Hudson KE, et al. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion. 2011;51:2695–702. doi: 10.1111/j.1537-2995.2011.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hendrickson JE, Hod EA, Spitalnik SL, et al. Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion. 2010;50:642–8. doi: 10.1111/j.1537-2995.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Matteocci A, Pierelli L. Red blood cell alloimmunization in sickle cell disease and in thalassaemia: current status, future perspectives and potential role of molecular typing. Vox Sang. 2014;106:197–208. doi: 10.1111/vox.12086. [DOI] [PubMed] [Google Scholar]
- 207.Miller ST, Kim HY, Weiner DL, et al. Red blood cell alloimmunization in sickle cell disease: prevalence in 2010. Transfusion. 2013;53:704–9. doi: 10.1111/j.1537-2995.2012.03796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nickel RS, Hendrickson JE, Fasano RM, et al. Impact of red blood cell alloimmunization on sickle cell disease mortality: a case series. Transfusion. 2016;56:107–14. doi: 10.1111/trf.13379. [DOI] [PubMed] [Google Scholar]
- 209.Noizat-Pirenne F. Relevance of alloimmunization in haemolytic transfusion reaction in sickle cell disease. Transfus Clin Biol. 2012;19:132–8. doi: 10.1016/j.tracli.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 210.Ryder AB, Zimring JC, Hendrickson JE. Factors Influencing RBC alloimmunization: lessons learned from murine models. Transfus Med Hemother. 2014;41:406–19. doi: 10.1159/000368995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vichinsky E, Neumayr L, Trimble S, et al. Transfusion complications in thalassemia patients: a report from the Centers for Disease Control and Prevention (CME) Transfusion. 2014;54:972–81. doi: 10.1111/trf.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zimring JC, Stowell SR, Johnsen JM, Hendrickson JE. Effects of genetic, epigenetic, and environmental factors on alloimmunization to transfused antigens: Current paradigms and future considerations. Transfus Clin Biol. 2012;19:125–31. doi: 10.1016/j.tracli.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 213.Fasano RM, Leong T, Kaushal M, et al. Effectiveness of red blood cell exchange, partial manual exchange, and simple transfusion concurrently with iron chelation therapy in reducing iron overload in chronically transfused sickle cell anemia patients. Transfusion. 2016;56:1707–15. doi: 10.1111/trf.13558. [DOI] [PubMed] [Google Scholar]
- 214.Sins JW, Biemond BJ, van den Bersselaar SM, et al. Early occurrence of red blood cell alloimmunization in patients with sickle cell disease. Am J Hematol. 2016;91:763–9. doi: 10.1002/ajh.24397. [DOI] [PubMed] [Google Scholar]
- 215.Fasano RM, Booth GS, Miles M, et al. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol. 2015;168:291–300. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- 216.da Cunha Gomes EG, Machado LAF, de Oliveira LC, Neto JFN. The erythrocyte alloimmunisation in patients with sickle cell anaemia: a systematic review. Transfus Med. 2018 doi: 10.1111/tme.12543. [DOI] [PubMed] [Google Scholar]
- 217.Hendrickson JE, Desmarets M, Deshpande SS, et al. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–36. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 218.Veale MF, Healey G, Sparrow RL. Longer storage of red blood cells is associated with increased in vitro erythrophagocytosis. Vox Sang. 2014;106:219–26. doi: 10.1111/vox.12095. [DOI] [PubMed] [Google Scholar]
- 219.Hogman CF, Meryman HT. Storage parameters affecting red blood cell survival and function after transfusion. Transfus Med Rev. 1999;13:275–96. doi: 10.1016/s0887-7963(99)80058-3. [DOI] [PubMed] [Google Scholar]
- 220.Gov NS, Safran SA. Red blood cell membrane fluctuations and shape controlled by ATP-induced cytoskeletal defects. Biophys J. 2005;88:1859–74. doi: 10.1529/biophysj.104.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kozlova E, Chernysh A, Moroz V, et al. Morphology, membrane nanostructure and stiffness for quality assessment of packed red blood cells. Sci Rep. 2017;7:7846. doi: 10.1038/s41598-017-08255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Low TY, Seow TK, Chung MC. Separation of human erythrocyte membrane associated proteins with one-dimensional and two-dimensional gel electrophoresis followed by identification with matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Proteomics. 2002;2:1229–39. doi: 10.1002/1615-9861(200209)2:9<1229::AID-PROT1229>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 223.Kakhniashvili DG, Bulla LA, Goodman SR. The human erythrocyte proteome. Mol Cell Proteomics. 2004;3:501–9. doi: 10.1074/mcp.M300132-MCP200. [DOI] [PubMed] [Google Scholar]
- 224.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76(Spec No):168–80. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 225.Rolfsson O, Sigurjonsson OE, Magnusdottir M, et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang. 2017;112:326–35. doi: 10.1111/vox.12506. [DOI] [PubMed] [Google Scholar]
- 226.Zimring JC, Smith N, Stowell SR, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–48. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Weisenhorn EM, van TETJ, Riley NM, et al. Multi-omics evidence for inheritance of energy pathways in red blood cells. Mol Cell Proteomics. 2016;15:3614–23. doi: 10.1074/mcp.M116.062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rapoport TA, Heinrich R, Jacobasch G, Rapoport S. A linear steady-state treatment of enzymatic chains. A mathematical model of glycolysis of human erythrocytes. Eur J Biochem. 1974;42:107–20. doi: 10.1111/j.1432-1033.1974.tb03320.x. [DOI] [PubMed] [Google Scholar]
- 229.Rapoport TA, Heinrich R. Mathematical analysis of multienzyme systems. I. Modelling of the glycolysis of human erythrocytes. Biosystems. 1975;7:120–9. doi: 10.1016/0303-2647(75)90049-0. [DOI] [PubMed] [Google Scholar]
- 230.Kuchel PW. Current status and challenges in connecting models of erythrocyte metabolism to experimental reality. Prog Biophys Mol Biol. 2004;85:325–42. doi: 10.1016/j.pbiomolbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 231.Bordbar A, Jamshidi N, Palsson BO. iAB-RBC-283: a proteomically derived knowledge-base of erythrocyte metabolism that can be used to simulate its physiological and patho-physiological states. BMC Syst Biol. 2011;5:110. doi: 10.1186/1752-0509-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yurkovich JT, Bordbar A, Sigurjonsson OE, Palsson BO. Systems biology as an emerging paradigm in transfusion medicine. BMC Syst Biol. 2018;12:31. doi: 10.1186/s12918-018-0558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zolla L, D’Alessandro A, Rinalducci S, et al. Classic and alternative red blood cell storage strategies: seven years of “-omics” investigations. Blood Transfus. 2015;13:21–31. doi: 10.2450/2014.0053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.D’Alessandro A, Reisz JA, Culp-Hill R, et al. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion. 2018;58:1992–2002. doi: 10.1111/trf.14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cancelas JA, Dumont LJ, Maes LA, et al. Additive solution-7 reduces the red blood cell cold storage lesion. Transfusion. 2015;55:491–8. doi: 10.1111/trf.12867. [DOI] [PubMed] [Google Scholar]
- 236.D’Alessandro A, Nemkov T, Hansen KC, et al. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015;55:2955–66. doi: 10.1111/trf.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dumont LJ, Cancelas JA, Maes LA, et al. Overnight, room temperature hold of whole blood followed by 42-day storage of red blood cells in additive solution-7. Transfusion. 2015;55:485–90. doi: 10.1111/trf.12868. [DOI] [PubMed] [Google Scholar]
- 238.Oski FA, Travis SF, Miller LD, et al. The in vitro restoration of red cell 2,3-diphosphoglycerate levels in banked blood. Blood. 1971;37:52–8. [PubMed] [Google Scholar]
- 239.Valeri CR, Zaroulis CG. Rejuvenation and freezing of outdated stored human red cells. N Engl J Med. 1972;287:1307–13. doi: 10.1056/NEJM197212282872601. [DOI] [PubMed] [Google Scholar]
- 240.Yoshida T, AuBuchon JP, Dumont LJ, et al. The effects of additive solution pH and metabolic rejuvenation on anaerobic storage of red cells. Transfusion. 2008;48:2096–105. doi: 10.1111/j.1537-2995.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- 241.Gehrke S, Srinivasan AJ, Culp-Hill R, et al. Metabolomics evaluation of early-storage red blood cell rejuvenation at 4 degrees C and 37 degrees C. Transfusion. 2018;58:1980–91. doi: 10.1111/trf.14623. [DOI] [PubMed] [Google Scholar]
- 242.Arun P, Padmakumaran Nair KG, Manojkumar V, et al. Decreased hemolysis and lipid peroxidation in blood during storage in the presence of nicotinic acid. Vox Sang. 1999;76:220–5. doi: 10.1159/000031055. [DOI] [PubMed] [Google Scholar]
- 243.Aydogan S, Yerer MB, Yapislar H. In vitro effects of melatonin on the filtrability of erythrocytes in SNP-induced oxidative stress. Clin Hemorheol Microcirc. 2004;30:317–22. [PubMed] [Google Scholar]
- 244.Aydogan S, Yapislar H, Artis S, Aydogan B. Impaired erythrocytes deformability in H2O2-induced oxidative stress: protective effect of L-carnosine. Clin Hemorheol Microcirc. 2008;39:93–8. [PubMed] [Google Scholar]
- 245.Stowell SR, Smith NH, Zimring JC, et al. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion. 2013;53:2248–57. doi: 10.1111/trf.12106. [DOI] [PubMed] [Google Scholar]
- 246.Sanford K, Fisher BJ, Fowler E, et al. Attenuation of red blood cell storage lesions with vitamin C. Antioxidants (Basel) 2017;6 doi: 10.3390/antiox6030055. pii: E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zbikowska HM, Antosik A, Szejk M, et al. A moderate protective effect of quercetin against gamma-irradiation- and storage-induced oxidative damage in red blood cells for transfusion. Int J Radiat Biol. 2014;90:1201–10. doi: 10.3109/09553002.2013.877173. [DOI] [PubMed] [Google Scholar]
- 248.Knight JA, Voorhees RP, Martin L, Anstall H. Lipid peroxidation in stored red cells. Transfusion. 1992;32:354–7. doi: 10.1046/j.1537-2995.1992.32492263451.x. [DOI] [PubMed] [Google Scholar]
- 249.Amen F, Machin A, Tourino C, et al. N-acetylcysteine improves the quality of red blood cells stored for transfusion. Arch Biochem Biophys. 2017;621:31–7. doi: 10.1016/j.abb.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 250.Pallotta V, Gevi F, D’Alessandro A, Zolla L. Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: a metabolomics overview. Blood Transfus. 2014;12:376–87. doi: 10.2450/2014.0266-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Soumya R, Vani R. Vitamin C as a modulator of oxidative stress in erythrocytes of stored blood. Acta Haematol Pol. 2017;48:350–6. [Google Scholar]
- 252.Leonart MS, Weffort-Santos AM, Munoz EM, et al. Effect of vitamin E on red blood cell preservation. Braz J Med Biol Res. 1989;22:85–6. [PubMed] [Google Scholar]
- 253.Silva CAL, Azevedo Filho CA, Pereira G, Silva DCN, et al. Vitamin E nanoemulsion activity on stored red blood cells. Transfus Med. 2017;27:213–7. doi: 10.1111/tme.12394. [DOI] [PubMed] [Google Scholar]
- 254.Yoshida T, AuBuchon JP, Tryzelaar L, et al. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007;92:22–31. doi: 10.1111/j.1423-0410.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 255.Dumont LJ, Yoshida T, AuBuchon JP. Anaerobic storage of red blood cells in a novel additive solution improves in vivo recovery. Transfusion. 2009;49:458–64. doi: 10.1111/j.1537-2995.2008.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8:220–36. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zolla L, D’Alessandro A. An efficient apparatus for rapid deoxygenation of erythrocyte concentrates for alternative banking strategies. J Blood Transfus. 2013;2013 doi: 10.1155/2013/896537. 896537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.D’Amici GM, Rinalducci S, Zolla L. Proteomic analysis of RBC membrane protein degradation during blood storage. J Proteome Res. 2007;6:3242–55. doi: 10.1021/pr070179d. [DOI] [PubMed] [Google Scholar]
- 259.Prudent M, Stauber F, Rapin A, et al. Small-scale perfusion bioreactor of red blood cells for dynamic studies of cellular pathways: proof-of-concept. Front Mol Biosci. 2016;3:11. doi: 10.3389/fmolb.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dumont LJ, D’Alessandro A, Szczepiorkowski ZM, Yoshida T. CO2 -dependent metabolic modulation in red blood cells stored under anaerobic conditions. Transfusion. 2016;56:392–403. doi: 10.1111/trf.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Messana I, Orlando M, Cassiano L, et al. Human erythrocyte metabolism is modulated by the O2-linked transition of hemoglobin. FEBS Lett. 1996;390:25–8. doi: 10.1016/0014-5793(96)00624-2. [DOI] [PubMed] [Google Scholar]
- 262.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci USA. 2009;106:18515–20. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.van’t Erve TJ, Doskey CM, Wagner BA, et al. Heritability of glutathione and related metabolites in stored red blood cells. Free Radic Biol Med. 2014;76:107–13. doi: 10.1016/j.freeradbiomed.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.van’t Erve TJ, Wagner BA, Martin SM, et al. The heritability of metabolite concentrations in stored human red blood cells. Transfusion. 2014;54:2055–63. doi: 10.1111/trf.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.van’t Erve TJ, Wagner BA, Martin SM, et al. The heritability of hemolysis in stored human red blood cells. Transfusion. 2015;55:1178–85. doi: 10.1111/trf.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Osei-Hwedieh DO, Kanias T, Croix CS, et al. Sickle cell trait increases red blood cell storage hemolysis and post-transfusion clearance in mice. EBioMedicine. 2016;11:239–48. doi: 10.1016/j.ebiom.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Francis RO, Jhang JS, Pham HP, et al. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox Sang. 2013;105:271–82. doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Reisz JA, Tzounakas VL, Nemkov T, et al. Metabolic linkage and correlations to storage capacity in erythrocytes from glucose 6-phosphate dehydrogenase-deficient donors. Front Med (Lausanne) 2017;4:248. doi: 10.3389/fmed.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sagiv E, Fasano RM, Luban NLC, et al. Glucose-6-phosphate-dehydrogenase deficient red blood cell units are associated with decreased posttransfusion red blood cell survival in children with sickle cell disease. Am J Hematol. 2018;93:630–4. doi: 10.1002/ajh.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yoshida T, Blair A, D’Alessandro A, et al. Enhancing uniformity and overall quality of red cell concentrate with anaerobic storage. Blood Transfus. 2017;15:172–81. doi: 10.2450/2017.0325-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Prudent M, Martin A, Abonnenc M, et al. Oxygen in red blood cell concentrates: influence of donor’s characteristics, location and blood processing. Vox Sang. 2018;113:166. [Google Scholar]
- 272.Hansen AL, Kurach JD, Turner TR, et al. The effect of processing method on the in vitro characteristics of red blood cell products. Vox Sang. 2015;108:350–8. doi: 10.1111/vox.12233. [DOI] [PubMed] [Google Scholar]
- 273.Alshalani A, Howell A, Acker JP. Impact of blood manufacturing and donor characteristics on membrane water permeability and in vitro quality parameters during hypothermic storage of red blood cells. Cryobiology. 2018;80:30–7. doi: 10.1016/j.cryobiol.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 274.Heddle NM, Arnold DM, Acker JP, et al. Red blood cell processing methods and in-hospital mortality: a transfusion registry cohort study. Lancet Haematol. 2016;3:e246–54. doi: 10.1016/S2352-3026(16)00020-X. [DOI] [PubMed] [Google Scholar]
- 275.Nogueira D, Rocha S, Abreu E, et al. Biochemical and cellular changes in leukocyte-depleted red blood cells stored for transfusion. Transfus Med Hemother. 2015;42:46–51. doi: 10.1159/000370140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kim YK, Kwon EH, Kim DH, et al. Susceptibility of oxidative stress on red blood cells exposed to gamma rays: hemorheological evaluation. Clin Hemorheol Microcirc. 2008;40:315–24. [PubMed] [Google Scholar]
- 277.Katharia R, Chaudhary R, Agarwal P. Prestorage gamma irradiation induces oxidative injury to red cells. Transfus Apher Sci. 2013;48:39–43. doi: 10.1016/j.transci.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 278.de Oliveira GC, Maia GA, Cortes VF, et al. The effect of gamma-radiation on the hemoglobin of stored red blood cells: the involvement of oxidative stress in hemoglobin conformation. Ann Hematol. 2013;92:899–906. doi: 10.1007/s00277-013-1719-z. [DOI] [PubMed] [Google Scholar]
- 279.Antosik A, Czubak K, Gajek A, et al. Influence of pre-storage irradiation on the oxidative stress markers, membrane integrity, size and shape of the cold stored red blood cells. Transfus Med Hemother. 2015;42:140–8. doi: 10.1159/000371596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zbikowska HM, Antosik A. Irradiation dose-dependent oxidative changes in red blood cells for transfusion. Int J Radiat Biol. 2012;88:654–60. doi: 10.3109/09553002.2012.705223. [DOI] [PubMed] [Google Scholar]
- 281.Moreira OC, Oliveira VH, Benedicto LB, et al. Effects of gamma-irradiation on the membrane ATPases of human erythrocytes from transfusional blood concentrates. Ann Hematol. 2008;87:113–9. doi: 10.1007/s00277-007-0378-3. [DOI] [PubMed] [Google Scholar]
- 282.Cancelas JA, Gottschall JL, Rugg N, et al. Red blood cell concentrates treated with the amustaline (S-303) pathogen reduction system and stored for 35 days retain post-transfusion viability: results of a two-centre study. Vox Sang. 2017;112:210–8. doi: 10.1111/vox.12500. [DOI] [PubMed] [Google Scholar]
- 283.Brixner V, Kiessling AH, Madlener K, et al. Red blood cells treated with the amustaline (S-303) pathogen reduction system: a transfusion study in cardiac surgery. Transfusion. 2018;58:905–16. doi: 10.1111/trf.14528. [DOI] [PubMed] [Google Scholar]
- 284.Qadri SM, Chen D, Schubert P, et al. Pathogen inactivation by riboflavin and ultraviolet light illumination accelerates the red blood cell storage lesion and promotes eryptosis. Transfusion. 2017;57:661–73. doi: 10.1111/trf.13959. [DOI] [PubMed] [Google Scholar]
- 285.Ning S, Heddle NM, Acker JP. Exploring donor and product factors and their impact on red cell post-transfusion outcomes. Transfus Med Rev. 2018;32:28–35. doi: 10.1016/j.tmrv.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 286.Chen D, Schubert P, Devine DV. Identification of potential protein quality markers in pathogen inactivated and gamma-irradiated red cell concentrates. Proteomics Clin Appl. 2017;11:1600121. doi: 10.1002/prca.201600121. [DOI] [PubMed] [Google Scholar]
- 287.Lannan KL, Sahler J, Spinelli SL, et al. Transfusion immunomodulation--the case for leukoreduced and (perhaps) washed transfusions. Blood Cells Mol Dis. 2013;50:61–8. doi: 10.1016/j.bcmd.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cortes-Puch I, Wang D, Sun J, et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2014;123:1403–11. doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Cholette JM, Henrichs KF, Alfieris GM, et al. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med. 2012;13:290–9. doi: 10.1097/PCC.0b013e31822f173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Nalbant D, Cancelas JA, Mock DM, et al. In premature infants there is no decrease in 24-hour posttransfusion allogeneic red blood cell recovery after 42 days of storage. Transfusion. 2018;58:352–8. doi: 10.1111/trf.14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Valeri CR, Vecchione JJ, Pivacek LE, et al. Viability and function of outdated human red blood cells after biochemical modification to improve oxygen transport function, freezing, thawing, washing, postthaw storage at 4 C, perfusion in vitro through a bubble oxygenator, and autotransfusion. Transfusion. 1980;20:39–46. doi: 10.1046/j.1537-2995.1980.20180125039.x. [DOI] [PubMed] [Google Scholar]
- 292.Rowe AW. Cryopreservation of red blood cells. Vox Sang. 1994;67:201–6. doi: 10.1111/j.1423-0410.1994.tb04576.x. [DOI] [PubMed] [Google Scholar]
- 293.Henkelman S, Noorman F, Badloe JF, Lagerberg JW. Utilization and quality of cryopreserved red blood cells in transfusion medicine. Vox Sang. 2015;108:103–12. doi: 10.1111/vox.12218. [DOI] [PubMed] [Google Scholar]
- 294.Valeri CR, Pivacek LE, Cassidy GP, Ragno G. Posttransfusion survival (24-hour) and hemolysis of previously frozen, deglycerolized RBCs after storage at 4 degrees C for up to 14 days in sodium chloride alone or sodium chloride supplemented with additive solutions. Transfusion. 2000;40:1337–40. doi: 10.1046/j.1537-2995.2000.40111337.x. [DOI] [PubMed] [Google Scholar]
- 295.Henkelman S, Lagerberg JW, Graaff R, et al. The effects of cryopreservation on red blood cell rheologic properties. Transfusion. 2010;50:2393–401. doi: 10.1111/j.1537-2995.2010.02730.x. [DOI] [PubMed] [Google Scholar]
- 296.Holovati JL, Wong KA, Webster JM, Acker JP. The effects of cryopreservation on red blood cell microvesiculation, phosphatidylserine externalization, and CD47 expression. Transfusion. 2008;48:1658–68. doi: 10.1111/j.1537-2995.2008.01735.x. [DOI] [PubMed] [Google Scholar]
- 297.Hampton DA, Wiles C, Fabricant LJ, et al. Cryopreserved red blood cells are superior to standard liquid red blood cells. J Trauma Acute Care Surg. 2014;77:20–7. doi: 10.1097/TA.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 298.Kor DJ, Kashyap R, Weiskopf RB, et al. Fresh red blood cell transfusion and short-term pulmonary, immunologic, and coagulation status: a randomized clinical trial. Am J Respir Crit Care Med. 2012;185:842–50. doi: 10.1164/rccm.201107-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Remy KE, Sun J, Wang D, et al. Transfusion of recently donated (fresh) red blood cells (RBCs) does not improve survival in comparison with current practice, while safety of the oldest stored units is yet to be established: a meta-analysis. Vox Sang. 2016;111:43–54. doi: 10.1111/vox.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Chai-Adisaksopha C, Alexander PE, Guyatt G, et al. Mortality outcomes in patients transfused with fresher versus older red blood cells: a meta-analysis. Vox Sang. 2017;112:268–78. doi: 10.1111/vox.12495. [DOI] [PubMed] [Google Scholar]
- 301.Martí-Carvajal AJ, Simancas-Racines D, Peña-Gonźalez BS. Prolonged storage of packed red blood cells for blood transfusion. Cochrane Database Syst Rev. 2015;7:CD009330. doi: 10.1002/14651858.CD009330.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Garraud O. Clinical trials in transfusion medicine and hemotherapy: worth moving forward in evaluating ‘fresh’ versus ‘old’ blood cell components? Transfus Apher Sci. 2017;56:98–9. doi: 10.1016/j.transci.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 303.Ng MSY, David M, Middelburg RA, et al. Transfusion of packed red blood cells at the end of shelf life is associated with increased risk of mortality - a pooled patient data analysis of 16 observational trials. Haematologica. 2018;103:1542–8. doi: 10.3324/haematol.2018.191932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Prudent M, Tissot JD, Lion N. In vitro assays and clinical trials in red blood cell aging: lost in translation. Transfus Apher Sci. 2015;52:270–6. doi: 10.1016/j.transci.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 305.Goel R, Johnson DJ, Scott AV, et al. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion. 2016;56:1690–8. doi: 10.1111/trf.13559. [DOI] [PubMed] [Google Scholar]
- 306.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Donor variation effect on red blood cell storage lesion: a multivariable, yet consistent, story. Transfusion. 2016;56:1274–86. doi: 10.1111/trf.13582. [DOI] [PubMed] [Google Scholar]
- 307.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 2017;1:1132–41. doi: 10.1182/bloodadvances.2017004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kanias T, Wang L, Lippert A, et al. Red blood cell endothelial nitric oxide synthase does not modulate red blood cell storage hemolysis. Transfusion. 2013;53:981–9. doi: 10.1111/j.1537-2995.2012.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Dern RJ, Gwinn RP, Wiorkowski JJ. Studies on the preservation of human blood. I. Variability in erythrocyte storage characteristics among healthy donors. J Lab Clin Med. 1966;67:955–65. [PubMed] [Google Scholar]
- 310.Prudent M, Tissot JD, Lion N. The 3-phase evolution of stored red blood cells and the clinical trials: an obvious relationship. Blood Transfus. 2017;15:188. doi: 10.2450/2017.0317-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Bardyn M, Tissot JD, Prudent M. Oxidative stress and antioxidant defenses during blood processing and storage of erythrocyte concentrates. Transfus Clin Biol. 2018;25:96–100. doi: 10.1016/j.tracli.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 312.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion. 2017;57(Suppl 2):1588–98. doi: 10.1111/trf.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Volken T, Buser A, Castelli D, et al. Red blood cell use in Switzerland: trends and demographic challenges. Blood Transfus. 2018;16:73–82. doi: 10.2450/2016.0079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Harper VM, Oh JY, Stapley R, et al. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxid Redox Signal. 2015;22:294–307. doi: 10.1089/ars.2014.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Delobel J, Prudent M, Rubin O, et al. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J Proteomics. 2012;76(Spec No):181–93. doi: 10.1016/j.jprot.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 316.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Membrane protein carbonylation in non-leukodepleted CPDA-preserved red blood cells. Blood Cells Mol Dis. 2006;36:279–82. doi: 10.1016/j.bcmd.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 317.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 318.Advani R, Rubin E, Mohandas N, Schrier SL. Oxidative red blood cell membrane injury in the pathophysiology of severe mouse beta-thalassemia. Blood. 1992;79:1064–7. [PubMed] [Google Scholar]
- 319.Berlett BS. Protein oxidation in aging, disease, and oxidative stress. JBC. 1997;272:20313–6. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 320.Buehler PW, Karnaukhova E, Gelderman MP, Alayash AI. Blood aging, safety, and transfusion: capturing the “radical” menace. Antioxid Redox Signal. 2011;14:1713–28. doi: 10.1089/ars.2010.3447. [DOI] [PubMed] [Google Scholar]
- 321.Chaudhary R, Katharia R. Oxidative injury as contributory factor for red cells storage lesion during twenty eight days of storage. Blood Transfus. 2012;10:59–62. doi: 10.2450/2011.0107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Jarolim P, Lahav M, Liu SC, Palek J. Effect of hemoglobin oxidation products on the stability of red cell membrane skeletons and the associations of skeletal proteins: correlation with a release of hemin. Blood. 1990;76:2125–31. [PubMed] [Google Scholar]
- 323.Karon BS, van Buskirk CM, Jaben EA, et al. Temporal sequence of major biochemical events during blood bank storage of packed red blood cells. Blood Transfus. 2012;10:453–61. doi: 10.2450/2012.0099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J Cell Mol Med. 2007;11:148–55. doi: 10.1111/j.1582-4934.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol. 2014;5:84. doi: 10.3389/fphys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Browne P, Shalev O, Hebbel RP. The molecular pathobiology of cell membrane iron: the sickle red cell as a model. Free Radic Biol Med. 1998;24:1040–8. doi: 10.1016/s0891-5849(97)00391-2. [DOI] [PubMed] [Google Scholar]
- 327.Kono M, Saigo K, Takagi Y, et al. Heme-related molecules induce rapid production of neutrophil extracellular traps. Transfusion. 2014;54:2811–9. doi: 10.1111/trf.12700. [DOI] [PubMed] [Google Scholar]
- 328.Minneci PC, Deans KJ, Zhi H, Yuen PS, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–17. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shaklai N, Shviro Y, Rabizadeh E, et al. Accumulation and drainage of hemin in the red cell membrane. Biochim Biophys Acta. 1985;821:355–66. doi: 10.1016/0005-2736(85)90106-3. [DOI] [PubMed] [Google Scholar]
- 330.Stapley R, Owusu BY, Brandon A, et al. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. Biochem J. 2012;446:499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wang D, Piknova B, Solomon SB, et al. In vivo reduction of cell-free methemoglobin to oxyhemoglobin results in vasoconstriction in canines. Transfusion. 2013;53:3149–63. doi: 10.1111/trf.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Antonelou MH, Kriebardis AG, Papassideri IS. Aging and death signalling in mature red cells: from basic science to transfusion practice. Blood Transfus. 2010;8(Suppl 3):s39–47. doi: 10.2450/2010.007S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Carroll J, Raththagala M, Subasinghe W, et al. An altered oxidant defense system in red blood cells affects their ability to release nitric oxide-stimulating ATP. Mol Biosyst. 2006;2:305–11. doi: 10.1039/b604362n. [DOI] [PubMed] [Google Scholar]
- 334.Cluitmans JC, Hardeman MR, Dinkla S, et al. Red blood cell deformability during storage: towards functional proteomics and metabolomics in the Blood Bank. Blood Transfus. 2012;10(Suppl 2):s12–8. doi: 10.2450/2012.004S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–19. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 336.Dumaswala UJ, Zhuo L, Mahajan S, et al. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol Cell Physiol. 2001;280:C867–73. doi: 10.1152/ajpcell.2001.280.4.C867. [DOI] [PubMed] [Google Scholar]
- 337.Pallotta V, Rinalducci S, Zolla L. Red blood cell storage affects the stability of cytosolic native protein complexes. Transfusion. 2015;55:1927–36. doi: 10.1111/trf.13079. [DOI] [PubMed] [Google Scholar]
- 338.Rael LT, Bar-Or R, Ambruso DR, et al. The effect of storage on the accumulation of oxidative biomarkers in donated packed red blood cells. J Trauma. 2009;66:76–81. doi: 10.1097/TA.0b013e318191bfe0. [DOI] [PubMed] [Google Scholar]
- 339.Rinalducci S, Zolla L. Biochemistry of storage lesions of red cell and platelet concentrates: a continuous fight implying oxidative/nitrosative/phosphorylative stress and signaling. Transfus Apher Sci. 2015;52:262–9. doi: 10.1016/j.transci.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 340.Wagner GM, Chiu DT, Qju JH, et al. Spectrin oxidation correlates with membrane vesiculation in stored RBCs. Blood. 1987;69:1777–81. [PubMed] [Google Scholar]
- 341.Low PS, Waugh SM, Zinke K, Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985;227:531–3. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- 342.Privalov PL. Cold denaturation of proteins. Crit Rev Biochem Mol Biol. 1990;25:281–305. doi: 10.3109/10409239009090612. [DOI] [PubMed] [Google Scholar]
- 343.Chiu DT, Liu TZ. Free radical and oxidative damage in human blood cells. J Biomed Sci. 1997;4:256–9. doi: 10.1007/BF02253426. [DOI] [PubMed] [Google Scholar]
- 344.Chen D, Schubert P, Devine DV. Proteomic analysis of red blood cells from donors exhibiting high hemolysis demonstrates a reduction in membrane-associated proteins involved in the oxidative response. Transfusion. 2017;57:2248–56. doi: 10.1111/trf.14188. [DOI] [PubMed] [Google Scholar]
- 345.Dzieciatkowska M, Silliman CC, Moore EE, et al. Proteomic analysis of the supernatant of red blood cell units: the effects of storage and leucoreduction. Vox Sang. 2013;105:210–8. doi: 10.1111/vox.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Rinalducci S, Ferru E, Blasi B, et al. Oxidative stress and caspase-mediated fragmentation of cytoplasmic domain of erythrocyte band 3 during blood storage. Blood Transfus. 2012;10(Suppl 2):s55–62. doi: 10.2450/2012.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Snyder LM, Fred Garver F, Liu SC, et al. Demonstration of haemoglobinassociated with isolated, purified spectrinfrom senescent human red cells. Brit J Haematol. 1985;61:415–9. doi: 10.1111/j.1365-2141.1985.tb02845.x. [DOI] [PubMed] [Google Scholar]
- 348.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion. 2010;50:376–89. doi: 10.1111/j.1537-2995.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 349.Bosman GJ. Survival of red blood cells after transfusion: processes and consequences. Front Physiol. 2013;4:376. doi: 10.3389/fphys.2013.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Lutz HU. Naturally occurring anti-band 3 antibodies in clearance of senescent and oxidatively stressed human red blood cells. Transfus Med Hemother. 2012;39:321–7. doi: 10.1159/000342171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Lutz HU, Bogdanova A. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol. 2013;4:387. doi: 10.3389/fphys.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Lysenko L, Mierzchala M, Gamian A, et al. The effect of packed red blood cell storage on arachidonic acid and advanced glycation end-product formation. Arch Immunol Ther Exp (Warsz) 2006;54:357–62. doi: 10.1007/s00005-006-0042-y. [DOI] [PubMed] [Google Scholar]
- 353.Mangalmurti NS, Chatterjee S, Cheng G, et al. Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products. Transfusion. 2010;50:2353–61. doi: 10.1111/j.1537-2995.2010.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Straat M, van Bruggen R, de Korte D, Juffermans NP. Red blood cell clearance in inflammation. Transfus Med Hemother. 2012;39:353–61. doi: 10.1159/000342229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Voziyan PA, Khalifah RG, Thibaudeau C, et al. Modification of proteins in vitro by physiological levels of glucose: pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. J Biol Chem. 2003;278:46616–24. doi: 10.1074/jbc.M307155200. [DOI] [PubMed] [Google Scholar]
- 356.Collard K, White D, Copplestone A. The influence of storage age on iron status, oxidative stress and antioxidant protection in paediatric packed cell units. Blood Transfus. 2014;12:210–9. doi: 10.2450/2013.0142-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Dumaswala UJ, Zhuo L, Jacobsen DW, et al. Protein and lipid oxidation of banked human erythrocytes: role of glutathione. Free Radic Biol Med. 1999;27:1041–9. doi: 10.1016/s0891-5849(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 358.Hirsch RE, Sibmooh N, Fucharoen S, Friedman JM. HbE/beta-thalassemia and oxidative stress: the key to pathophysiological mechanisms and novel therapeutics. Antioxid Redox Signal. 2017;26:794–813. doi: 10.1089/ars.2016.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Longo V, D’Alessandro A, Zolla L. Deoxygenation of leucofiltered erythrocyte concentrates preserves proteome stability during storage in the blood bank. Blood Transfus. 2014;12:599–604. doi: 10.2450/2014.0335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Tavazzi B, Di Pierro D, Amorini AM, et al. Energy metabolism and lipid peroxidation of human erythrocytes as a function of increased oxidative stress. Eur J Biochem. 2000;267:684–9. doi: 10.1046/j.1432-1327.2000.01042.x. [DOI] [PubMed] [Google Scholar]
- 361.Montuschi P, Barnes PJ, Roberts LJ., 2nd Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 362.Silliman CC, Elzi DJ, Ambruso DR, et al. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol. 2003;73:511–24. doi: 10.1189/jlb.0402179. [DOI] [PubMed] [Google Scholar]
- 363.Spinelli SL, Lannan KL, Casey AE, et al. Isoprostane and isofuran lipid mediators accumulate in stored red blood cells and influence platelet function in vitro. Transfusion. 2014;54:1569–79. doi: 10.1111/trf.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Qi Z, Roback JD, Voit EO. Effects of storage time on glycolysis in donated human blood units. Metabolites. 2017;7:12. doi: 10.3390/metabo7020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Roback JD, Josephson CD, Waller EK, et al. Metabolomics of ADSOL (AS-1) red blood cell storage. Transfus Med Rev. 2014;28:41–55. doi: 10.1016/j.tmrv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55:1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.D’Alessandro A, Nemkov T, Hansen KC. Rapid detection of DEHP in packed red blood cells stored under European and US standard conditions. Blood Transfusion. 2016;14:140–4. doi: 10.2450/2015.0210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Knutson F, Loof H, Hogman CF. Pre-separation storage of whole blood: the effect of temperature on red cell 2,3-diphosphoglycerate and myeloperoxidase in plasma. Transfus Apher Sci. 1999;21:111–5. doi: 10.1016/s0955-3886(99)00081-8. [DOI] [PubMed] [Google Scholar]
- 369.Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion. 2011;51:1574–9. doi: 10.1111/j.1537-2995.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 370.Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–47. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 371.de Korte D, Kleine M, Korsten HG, Verhoeven AJ. Prolonged maintenance of 2,3-diphosphoglycerate acid and adenosine triphosphate in red blood cells during storage. Transfusion. 2008;48:1081–9. doi: 10.1111/j.1537-2995.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- 372.Hogman CF, de Verdier CH, Ericson A, et al. Studies on the mechanism of human red cell loss of viability during storage at +4 degrees C in vitro. I. Cell shape and total adenylate concentration as determinant factors for posttransfusion survival. Vox Sang. 1985;48:257–68. doi: 10.1111/j.1423-0410.1985.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 373.Kulandavelu S, Balkan W, Hare JM. Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc Nat Acadf Sci. 2015;112:6254–5. doi: 10.1073/pnas.1506523112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Leonart MS, Nascimento AJ, Nonoyama K, et al. Correlation of discocyte frequency and ATP concentration in preserved blood. A morphological indicator of red blood cell viability. Braz J Med Biol Res. 1997;30:745–7. doi: 10.1590/s0100-879x1997000600007. [DOI] [PubMed] [Google Scholar]
- 375.Nakao K, Wada T, Kamiya T, et al. A direct relationship between adenosine triphosphate-level and in vivo viability of erythrocytes. Nature. 1962;194:877–8. doi: 10.1038/194877a0. [DOI] [PubMed] [Google Scholar]
- 376.Sun K, D’Alessandro A, Xia Y. Purinergic control of red blood cell metabolism: novel strategies to improve red cell storage quality. Blood Transfus. 2017;15:535–42. doi: 10.2450/2017.0366-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.van de Watering L. Red cell storage and prognosis. Vox Sang. 2011;100:36–45. doi: 10.1111/j.1423-0410.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 378.Zimmermann R, Heidenreich D, Weisbach V, et al. In vitro quality control of red blood cell concentrates outdated in clinical practice. Transfus Clin Biol. 2003;10:275–83. doi: 10.1016/s1246-7820(03)00032-6. [DOI] [PubMed] [Google Scholar]
- 379.Apstein CS, Dennis RC, Briggs L, et al. Effect of erythrocyte storage and oxyhemoglobin affinity changes on cardiac function. Am J Physiol. 1985;248:H508–15. doi: 10.1152/ajpheart.1985.248.4.H508. [DOI] [PubMed] [Google Scholar]
- 380.Hamasaki N, Yamamoto M. Red blood cell function and blood storage. Vox Sang. 2000;79:191–7. doi: 10.1159/000056729. [DOI] [PubMed] [Google Scholar]
- 381.Hogman CF. Preparation and preservation of red cells. Vox Sang. 1998;74(Suppl 2):177–87. doi: 10.1111/j.1423-0410.1998.tb05419.x. [DOI] [PubMed] [Google Scholar]
- 382.Hogman CF, Knutson F, Loof H. Storage of whole blood before separation: the effect of temperature on red cell 2,3 DPG and the accumulation of lactate. Transfusion. 1999;39:492–7. doi: 10.1046/j.1537-2995.1999.39050492.x. [DOI] [PubMed] [Google Scholar]
- 383.Kimura H, Hamasaki N, Yamamoto M, Tomonaga M. Circulation of red blood cells having high levels of 2,3-bisphosphoglycerate protects rat brain from ischemic metabolic changes during hemodilution. Stroke. 1995;26:1431–7. doi: 10.1161/01.str.26.8.1431. [DOI] [PubMed] [Google Scholar]
- 384.Li Y, Xiong Y, Wang R, et al. Blood banking-induced alteration of red blood cell oxygen release ability. Blood Transfus. 2016;14:238–44. doi: 10.2450/2015.0055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Shander A, Javidroozi M, Ozawa S, Hare GM. What is really dangerous: anaemia or transfusion? Br J Anaesth. 2011;107(Suppl 1):i41–59. doi: 10.1093/bja/aer350. [DOI] [PubMed] [Google Scholar]
- 386.Srinivasan AJ, Morkane C, Martin DS, Welsby IJ. Should modulation of p50 be a therapeutic target in the critically ill? Expert Rev Hematol. 2017;10:449–58. doi: 10.1080/17474086.2017.1313699. [DOI] [PubMed] [Google Scholar]
- 387.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfus Apher Sci. 2010;43:69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Weiskopf RB. The efficacy and safety of liquid stored blood and storage duration: a confused subject; are patients confused? Anesth Analg. 2014;119:224–9. doi: 10.1213/ANE.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 389.Weiskopf RB, Feiner J, Hopf H, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104:911–20. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 390.D’Alessandro A, Nemkov T, Blair A, et al. Anaerobic storage condition enhances GSH levels while maintaining pentose phosphate pathway activity. Transfusion. 2016;56:51A. [Google Scholar]
- 391.Snyder LM, Fortier NL, Trainor J, et al. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin-hemoglobin cross-linking. J Clin Invest. 1985;76:1971–7. doi: 10.1172/JCI112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Wolfe LC, Byrne AM, Lux SE. Molecular defect in the membrane skeleton of blood bank-stored red cells. Abnormal spectrin-protein 4.1-actin complex formation. J Clin Invest. 1986;78:1681–6. doi: 10.1172/JCI112762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Safeukui I, Buffet PA, Perrot S, et al. Surface area loss and increased sphericity account for the splenic entrapment of subpopulations of Plasmodium falciparum ring-infected erythrocytes. PLoS One. 2013;8:e60150. doi: 10.1371/journal.pone.0060150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Moon I, Yi F, Lee YH, et al. Automated quantitative analysis of 3D morphology and mean corpuscular hemoglobin in human red blood cells stored in different periods. Opt Express. 2013;21:30947–57. doi: 10.1364/OE.21.030947. [DOI] [PubMed] [Google Scholar]
- 395.Almizraq R, Tchir JD, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion. 2013;53:2258–67. doi: 10.1111/trf.12080. [DOI] [PubMed] [Google Scholar]
- 396.Almizraq RJ, Seghatchian J, Acker JP. Extracellular vesicles in transfusion-related immunomodulation and the role of blood component manufacturing. Transfus Apher Sci. 2016;55:281–91. doi: 10.1016/j.transci.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 397.Burnouf T, Chou ML, Goubran H, et al. An overview of the role of microparticles/microvesicles in blood components: Are they clinically beneficial or harmful? Transfus Apher Sci. 2015;53:137–45. doi: 10.1016/j.transci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 398.Cognasse F, Hamzeh-Cognasse H, Laradi S, et al. The role of microparticles in inflammation and transfusion: a concise review. Transfus Apher Sci. 2015;53:159–67. doi: 10.1016/j.transci.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 399.Danesh A, Inglis HC, Jackman RP, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123:687–96. doi: 10.1182/blood-2013-10-530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Greenwalt TJ, Zehner Sostok C, Dumaswala UJ. Studies in red blood cell preservation. 2. Comparison of vesicle formation, morphology, and membrane lipids during storage in AS-1 and CPDA-1. Vox Sang. 1990;58:90–3. doi: 10.1111/j.1423-0410.1990.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 401.Jank H, Salzer U. Vesicles generated during storage of red blood cells enhance the generation of radical oxygen species in activated neutrophils. ScientificWorldJournal. 2011;11:173–85. doi: 10.1100/tsw.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Kent MW, Kelher MR, West FB, Silliman CC. The pro-inflammatory potential of microparticles in red blood cell units. Transfus Med. 2014;24:176–81. doi: 10.1111/tme.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kriebardis A, Antonelou M, Stamoulis K, Papassideri I. Cell-derived microparticles in stored blood products: innocent-bystanders or effective mediators of post-transfusion reactions? Blood Transfus. 2012;10(Suppl 2):s25–38. doi: 10.2450/2012.006S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–53. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 406.Liu C, Zhao W, Christ GJ, et al. Nitric oxide scavenging by red cell microparticles. Free Radic Biol Med. 2013;65C:1164–73. doi: 10.1016/j.freeradbiomed.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Prudent M, Crettaz D, Delobel J, et al. Differences between calcium-stimulated and storage-induced erythrocyte-derived microvesicles. Transfus Apher Sci. 2015;53:153–8. doi: 10.1016/j.transci.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 408.Rubin O, Crettaz D, Tissot JD, Lion N. Microparticles in stored red blood cells: submicron clotting bombs? Blood Transfus. 2010;8(Suppl 3):s31–8. doi: 10.2450/2010.006S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Saas P, Angelot F, Bardiaux L, et al. Phosphatidylserine-expressing cell by-products in transfusion: a pro-inflammatory or an anti-inflammatory effect? Transfus Clin Biol. 2012;19:90–7. doi: 10.1016/j.tracli.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 410.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol. 2008;84:1316–25. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 411.Xiong Z, Cavaretta J, Qu L, et al. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011;51:610–21. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Mittag D, Sran A, Chan KS, et al. Stored red blood cell susceptibility to in vitro transfusion-associated stress conditions is higher after longer storage and increased by storage in saline-adenine-glucose-mannitol compared to AS-1. Transfusion. 2015;55:2197–206. doi: 10.1111/trf.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Piety NZ, Reinhart WH, Pourreau PH, et al. Shape matters: the effect of red blood cell shape on perfusion of an artificial microvascular network. Transfusion. 2016;56:844–51. doi: 10.1111/trf.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Usry RT, Moore GL, Manalo FW. Morphology of stored, rejuvenated human erythrocytes. Vox Sang. 1975;28:176–83. doi: 10.1111/j.1423-0410.1975.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 415.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci U S A. 1998;95:3077–81. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Gottlieb Y, Topaz O, Cohen LA, et al. Physiologically aged red blood cells undergo erythrophagocytosis in vivo but not in vitro. Haematologica. 2012;97:994–1002. doi: 10.3324/haematol.2011.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Mandal D, Moitra PK, Saha S, Basu J. Caspase 3 regulates phosphatidylserine externalization and phagocytosis of oxidatively stressed erythrocytes. FEBS Lett. 2002;513:184–8. doi: 10.1016/s0014-5793(02)02294-9. [DOI] [PubMed] [Google Scholar]
- 418.Bhaduri B, Kandel M, Brugnara C, et al. Optical assay of erythrocyte function in banked blood. Sci Rep. 2014;4:6211. doi: 10.1038/srep06211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Burns JM, Yoshida T, Dumont LJ, et al. Deterioration of red blood cell mechanical properties is reduced in anaerobic storage. Blood Transfus. 2016;14:80–8. doi: 10.2450/2015.0241-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Card RT, Mohandas N, Mollison PL. Relationship of post-transfusion viability to deformability of stored red cells. Br J Haematol. 1983;53:237–40. doi: 10.1111/j.1365-2141.1983.tb02016.x. [DOI] [PubMed] [Google Scholar]
- 421.Collard K, White D, Copplestone A. The effect of maximum storage on iron status, oxidative stress and antioxidant protection in paediatric packed cell units. Blood Transfus. 2013;11:419–25. doi: 10.2450/2012.0046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Gkoumassi E, Dijkstra-Tiekstra MJ, Hoentjen D, de Wildt-Eggen J. Hemolysis of red blood cells during processing and storage. Transfusion. 2012;52:489–92. doi: 10.1111/j.1537-2995.2011.03298.x. [DOI] [PubMed] [Google Scholar]
- 423.Hess JR, Sparrow RL, van der Meer PF, et al. Red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion. 2009;49:2599–603. doi: 10.1111/j.1537-2995.2009.02275.x. [DOI] [PubMed] [Google Scholar]
- 424.McAteer MJ, Dumont LJ, Cancelas J, et al. Multi-institutional randomized control study of haemolysis in stored red cell units prepared manually or by an automated system. Vox Sang. 2010;99:34–43. doi: 10.1111/j.1423-0410.2010.01313.x. [DOI] [PubMed] [Google Scholar]
- 425.Sowemimo-Coker SO. Red blood cell hemolysis during processing. Transfus Med Rev. 2002;16:46–60. doi: 10.1053/tmrv.2002.29404. [DOI] [PubMed] [Google Scholar]
- 426.Beppu M, Mizukami A, Nagoya M, Kikugawa K. Binding of anti-band 3 autoantibody to oxidatively damaged erythrocytes. Formation of senescent antigen on erythrocyte surface by an oxidative mechanism. J Biol Chem. 1990;265:3226–33. [PubMed] [Google Scholar]
- 427.Bracci R, Perrone S, Buonocore G. Oxidant injury in neonatal erythrocytes during the perinatal period. Acta Paediatr Suppl. 2002;91:130–4. doi: 10.1111/j.1651-2227.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 428.Benson DD, Beck AW, Burdine MS, et al. Accumulation of pro-cancer cytokines in the plasma fraction of stored packed red cells. J Gastrointest Surg. 2012;16:460–8. doi: 10.1007/s11605-011-1798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Fujihara M, Wakamoto S, Ikebuchi K, et al. [Changes in cytokine levels in blood components during storage]. Japanese Journal of Transfusion Medicine. 2002;47:829–36. [in Japanese.] [Google Scholar]
- 430.McFaul SJ, Corley JB, Mester CW, Nath J. Packed blood cells stored in AS-5 become proinflammatory during storage. Transfusion. 2009;49:1451–60. doi: 10.1111/j.1537-2995.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 431.Muylle L. The role of cytokines in blood transfusion reactions. Blood Rev. 1995;9:77–83. doi: 10.1016/s0268-960x(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 432.Nagura Y, Tsuno NH, Ohkawa R, et al. Inhibition of lysophosphatidic acid increase by prestorage whole blood leukoreduction in autologous CPDA-1 whole blood. Transfusion. 2013;53:3139–48. doi: 10.1111/trf.12152. [DOI] [PubMed] [Google Scholar]
- 433.Sowemimo-Coker SO. Evaluation of an experimental filter designed for improving the quality of red blood cells (RBCs) during storage by simultaneously removing white blood cells and immunomodulators and improving RBC viscoelasticity and Band 3 proteins. Transfusion. 2014;54:592–601. doi: 10.1111/trf.12330. [DOI] [PubMed] [Google Scholar]
- 434.Tasaki T, Gotoh K, Fujii K, et al. Accumulated cytokines in stored autologous blood do not cause febrile nonhemolytic transfusion reactions. Transfus Apher Sci. 2008;39:15–9. doi: 10.1016/j.transci.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 435.Wadhwa M, Seghatchian MJ, Dilger P, et al. Cytokine accumulation in stored red cell concentrates: effect of buffy-coat removal and leucoreduction. Transfus Sci. 2000;23:7–16. doi: 10.1016/s0955-3886(00)00049-7. [DOI] [PubMed] [Google Scholar]
- 436.Wei J, Zhao J, Schrott V, et al. Red blood cells store and release interleukin-33. J Investig Med. 2015;63:806–10. doi: 10.1097/JIM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Guppy M, Attwood PV, Hansen IA, et al. pH, temperature and lactate production in human red blood cells: implications for blood storage and glycolytic control. Vox Sang. 1992;62:70–5. doi: 10.1111/j.1423-0410.1992.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 438.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion. 2015;55:2659–71. doi: 10.1111/trf.13211. [DOI] [PubMed] [Google Scholar]
- 439.Preston K, Harm S, Dreyfus N, et al. Packed red blood cells accumulate oxidative stress with increased storage duration. Shock. 2017;48:270–1. doi: 10.1097/SHK.0000000000000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Dumaswala UJ, Wilson MJ, Wu YL, et al. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic Res. 2000;33:517–29. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- 441.Reynolds JD, Hess DT, Stamler JS. The transfusion problem: role of aberrant S-nitrosylation. Transfusion. 2011;51:852–8. doi: 10.1111/j.1537-2995.2011.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Reynolds JD, Bennett KM, Cina AJ, et al. S-nitrosylation therapy to improve oxygen delivery of banked blood. Proc Natl Acad Sci USA. 2013;110:11529–34. doi: 10.1073/pnas.1306489110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proc Natl Acad Sci USA. 2005;102:2531–6. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Pieracci FM, Moore EE, Chin T, et al. The age of transfused blood predicts hematocrit response among critically ill surgical patients. Am J Surg. 2012;204:269–73. doi: 10.1016/j.amjsurg.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.van Bruggen R. CD47 functions as a removal marker on aged erythrocytes. ISBT Sci Ser. 2013;8:153–6. [Google Scholar]
- 446.Arashiki N, Kimata N, Manno S, et al. Membrane peroxidation and methemoglobin formation are both necessary for band 3 clustering: mechanistic insights into human erythrocyte senescence. Biochemistry. 2013;52:5760–9. doi: 10.1021/bi400405p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, et al. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–85. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 448.Camus SM, De Moraes JA, Bonnin P, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125:3805–14. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Risbano MG, Kanias T, Triulzi D, et al. Effects of aged stored autologous red blood cells on human endothelial function. Am J Respir Crit Care Med. 2015;192:1223–33. doi: 10.1164/rccm.201501-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest. 2012;122:1205–8. doi: 10.1172/JCI62972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Stapley R, Rodriguez C, Oh JY, et al. Red blood cell washing, nitrite therapy, and antiheme therapies prevent stored red blood cell toxicity after trauma-hemorrhage. Free Radic Biol Med. 2015;85:207–18. doi: 10.1016/j.freeradbiomed.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Hod EA, Spitalnik SL. Harmful effects of transfusion of older stored red blood cells: iron and inflammation. Transfusion. 2011;51:881–5. doi: 10.1111/j.1537-2995.2011.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kalhan TG, Bateman DA, Bowker RM, et al. Effect of red blood cell storage time on markers of hemolysis and inflammation in transfused very low birth weight infants. Pediatr Res. 2017;82:964–9. doi: 10.1038/pr.2017.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Brissot P, Ropert M, Le Lan C, Loreal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2012;1820:403–10. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 455.Owusu BY, Stapley R, Honavar J, Patel RP. Effects of erythrocyte aging on nitric oxide and nitrite metabolism. Antioxid Redox Signal. 2013;19:1198–208. doi: 10.1089/ars.2012.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Neuman R, Hayek S, Rahman A, et al. Effects of storage-aged red blood cell transfusions on endothelial function in hospitalized patients. Transfusion. 2015;55:782–90. doi: 10.1111/trf.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Liu C, Liu X, Janes J, et al. Mechanism of faster NO scavenging by older stored red blood cells. Redox Biol. 2014;2:211–9. doi: 10.1016/j.redox.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Alexander JT, El-Ali AM, Newman JL, et al. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013;53:2619–28. doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Berra L, Coppadoro A, Yu B, et al. Transfusion of stored autologous blood does not alter reactive hyperemia index in healthy volunteers. Anesthesiology. 2012;117:56–63. doi: 10.1097/ALN.0b013e31825575e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfus Apher Sci. 2008;38:117–25. doi: 10.1016/j.transci.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 461.Escobar GA, Cheng AM, Moore EE, et al. Stored packed red blood cell transfusion up-regulates inflammatory gene expression in circulating leukocytes. Ann Surg. 2007;246:129–34. doi: 10.1097/01.sla.0000264507.79859.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Weinberg JA, Maclennan PA, Vandromme-Cusick MJ, et al. The deleterious effect of red blood cell storage on microvascular response to transfusion. J Trauma Acute Care Surg. 2013;75:807–12. doi: 10.1097/TA.0b013e3182a74a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Cabrales P. Effects of erythrocyte flexibility on microvascular perfusion and oxygenation during acute anemia. Am J Physiol Heart Circ Physiol. 2007;293:H1206–15. doi: 10.1152/ajpheart.00109.2007. [DOI] [PubMed] [Google Scholar]
- 464.Ayhan B, Yuruk K, Koene S, et al. The effects of non-leukoreduced red blood cell transfusions on microcirculation in mixed surgical patients. Transfus Apher Sci. 2013;49:212–22. doi: 10.1016/j.transci.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 465.Arslan E, Sierko E, Waters JH, Siemionow M. Microcirculatory hemodynamics after acute blood loss followed by fresh and banked blood transfusion. Am J Surg. 2005;190:456–62. doi: 10.1016/j.amjsurg.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 466.Stowell CP, Whitman G, Granger S, et al. The impact of red blood cell storage duration on tissue oxygenation in cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:610–9.e2. doi: 10.1016/j.jtcvs.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Bennett-Guerrero E, Lockhart EL, Bandarenko N, et al. A randomized controlled pilot study of VO2 max testing: a potential model for measuring relative in vivo efficacy of different red blood cell products. Transfusion. 2017;57:630–6. doi: 10.1111/trf.13918. [DOI] [PubMed] [Google Scholar]
- 468.Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. J Trauma. 2009;67:29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 469.Wagener BM, Hu PJ, Oh JY, et al. Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: a preclinical experimental study. PLoS Med. 2018;15:e1002522. doi: 10.1371/journal.pmed.1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Silliman CC, Kelher MR, Khan SY, et al. Supernatants and lipids from stored red blood cells activate pulmonary microvascular endothelium through the BLT2 receptor and protein kinase C activation. Transfusion. 2017;57:2690–700. doi: 10.1111/trf.14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Kent MW, Kelher MR, West FB, CCS The pro-inflammatory potential of microparticles in red blood cell units. Transfus Med. 2014;24:176–81. doi: 10.1111/tme.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Callan MB, Patel RT, Rux AH, et al. Transfusion of 28-day-old leucoreduced or non-leucoreduced stored red blood cells induces an inflammatory response in healthy dogs. Vox Sang. 2013;105:319–27. doi: 10.1111/vox.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Mangalmurti NS, Xiong Z, Hulver M, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–66. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Torrance HD, Vivian ME, Brohi K, et al. Changes in gene expression following trauma are related to the age of transfused packed red blood cells. J Trauma Acute Care Surg. 2015;78:535–42. doi: 10.1097/TA.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 475.Sadallah S, Eken C, Schifferli JA. Ectosomes as modulators of inflammation and immunity. Clin Exp Immunol. 2011;163:26–32. doi: 10.1111/j.1365-2249.2010.04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Theodoraki K, Markatou M, Rizos D, Fassoulaki A. The impact of two different transfusion strategies on patient immune response during major abdominal surgery: a preliminary report. J Immunol Res. 2014;2014 doi: 10.1155/2014/945829. 945829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Long K, Meier C, Bernard A, et al. T-cell suppression by red blood cells is dependent on intact cells and is a consequence of blood bank processing. Transfusion. 2014;54:1340–7. doi: 10.1111/trf.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 479.Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002;16:144–60. doi: 10.1053/tmrv.2002.31463. [DOI] [PubMed] [Google Scholar]
- 480.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 481.Karam O, Tucci M, Bateman ST, et al. Association between length of storage of red blood cell units and outcome of critically ill children: a prospective observational study. Crit Care. 2010;14:R57. doi: 10.1186/cc8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Moore FA, Moore EE, Sauaia A. Blood transfusion an independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–5. [PubMed] [Google Scholar]
- 483.Surgenor SD, DeFoe GR, Fillinger MP, et al. Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;114:I43–8. doi: 10.1161/CIRCULATIONAHA.105.001271. [DOI] [PubMed] [Google Scholar]
- 484.Oduor H, Minniti CP, Brofferio A, et al. Severe cardiac iron toxicity in two adults with sickle cell disease. Transfusion. 2017;57:700–4. doi: 10.1111/trf.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–26. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Janz DR, Zhao Z, Koyama T, et al. Longer storage duration of red blood cells is associated with an increased risk of acute lung injury in patients with sepsis. Ann Intensive Care. 2013;3:33. doi: 10.1186/2110-5820-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Toy P, Bacchetti P, Grimes B, et al. Recipient clinical risk factors predominate in possible transfusion-related acute lung injury. Transfusion. 2015;55:947–52. doi: 10.1111/trf.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Atzil S, Arad M, Glasner A, et al. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology. 2008;109:989–97. doi: 10.1097/ALN.0b013e31818ddb72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Bennett S, Baker LK, Martel G, et al. The impact of perioperative red blood cell transfusions in patients undergoing liver resection: a systematic review. HPB (Oxford) 2017;19:321–30. doi: 10.1016/j.hpb.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 490.Busch OR, Hop WC, Hoynck van Papendrecht MA, et al. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–6. doi: 10.1056/NEJM199305133281902. [DOI] [PubMed] [Google Scholar]
- 491.Luan H, Ye F, Wu L, et al. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surgery. 2014;14:34. doi: 10.1186/1471-2482-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Qiu L, Wang DR, Zhang XY, et al. Impact of perioperative blood transfusion on immune function and prognosis in colorectal cancer patients. Transfus Apher Sci. 2016;54:235–41. doi: 10.1016/j.transci.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 493.Riedl R, Engels EA, Warren JL, et al. Blood transfusions and the subsequent risk of cancers in the United States elderly. Transfusion. 2013;53:2198–206. doi: 10.1111/trf.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Tzounakas VL, Seghatchian J, Grouzi E, et al. Red blood cell transfusion in surgical cancer patients: targets, risks, mechanistic understanding and further therapeutic opportunities. Transfus Apher Sci. 2017;56:291–304. doi: 10.1016/j.transci.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 495.Dubovoy T, Engoren M. Thrombotic risks in red blood cell transfusions. Semin Thromb Hemost. 2016;42:102–11. doi: 10.1055/s-0035-1569069. [DOI] [PubMed] [Google Scholar]
- 496.Tung JP, Fraser JF, Nataatmadja M, et al. Age of blood and recipient factors determine the severity of transfusion-related acute lung injury (TRALI) Crit Care. 2012;16:R19. doi: 10.1186/cc11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Hopewell S, Omar O, Hyde C, et al. A systematic review of the effect of red blood cell transfusion on mortality: evidence from large-scale observational studies published between 2006 and 2010. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002154. pii: e002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Parsons EC, Hough CL, Seymour CW, et al. Red blood cell transfusion and outcomes in patients with acute lung injury, sepsis and shock. Crit Care. 2011;15:R221. doi: 10.1186/cc10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Robinson SD, Janssen C, Fretz EB, et al. Red blood cell storage duration and mortality in patients undergoing percutaneous coronary intervention. Am Heart J. 2010;159:876–81. doi: 10.1016/j.ahj.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 500.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS-III-Omics. Transfusion. 2018 doi: 10.1111/trf.14979. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Jones AR, Patel RP, Marques MB, et al. Older blood Is associated with increased mortality and adverse events in massively transfused trauma patients: secondary analysis of the PROPPR trial. Ann Emerg Med. 2018 doi: 10.1016/j.annemergmed.2018.09.033. pii: S0196-0644(18)31326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]