ABSTRACT
Background: Human papillomavirus (HPV) vaccination for young women up to age 26 is highly cost-effective and has been implemented in 65 countries globally. We investigate the cost-effectiveness for HPV vaccination program in older women (age > 26 years), heterosexual men and men who have sex with men (MSM).
Method: A targeted literature review was conducted on PubMed for publications between January 2000 and January 2017 according to the PRISMA guidelines. We included English-language articles that reported the incremental cost-effectiveness ratio (ICER) of HPV vaccination programs for women over age 26, heterosexual men, and MSM and identified the underlying factors for its cost-effectiveness.
Results: We included 36 relevant articles (six, 26 and four in older women, heterosexual men and MSM, respectively) from 17 countries (12 high-income (HICs) and five low- and middle-income (LMICs) countries). Most (4/6) studies in women over age 26 did not show cost-effectiveness ($65,000–192,000/QALY gained). Two showed cost-effectiveness, but only when the vaccine cost was largely subsidised and protection to non-naïve women was also considered. Sixteen of 26 studies in heterosexual men were cost-effective (ICER = $19,600–52,800/QALY gained in HICs; $49–5,860/QALY gained in LMICs). Nonavalent vaccines, a low vaccine price, fewer required doses, and a long vaccine protection period were key drivers for cost-effectiveness. In contrast, all four studies on MSM consistently reported cost-effectiveness (ICER = $15,000-$43,000/QALY gained), particularly in MSM age < 40 years and those who were HIV-positive. Countries’ vaccination coverage did not significantly correlate with its per-capita Gross National Income.
Conclusion: Targeted HPV vaccination for MSM should be next priority in HPV prevention after having established a solid girls vaccination programme. Vaccination for heterosexual men should be considered when 2-dose 4vHPV/9vHPV vaccines become available with a reduced price, whereas targeted vaccination for women over age 26 is unlikely to be cost-effective.
Keywords: Human papillomavirus, vaccine, cost-effectiveness, men who have sex with men
Introduction
Human Papillomavirus (HPV) infection is a common sexually transmitted infection (STI) and a necessary cause for cervical cancer in women.1 It is also responsible for anal, vaginal, vulvar, oropharyngeal and penile cancers.2 Cervical cancer was the fourth most common cancer among women globally, and second (only after breast cancer) in women in low- and middle- income countries (LMICs).3 According to the World Health Organization (WHO), an estimated 530,000 cervical cancers were diagnosed in 2012, and approximately 270,000 women per year died from cervical cancer worldwide. More than 90% of deaths occur in low- and middle- income countries (LMICs) due to poor access to screening and treatment services.4 However, HPV infection is vaccine-preventable, and currently approved vaccines have achieved an excellent safety and efficacy profile.5
National HPV vaccination programs have been initiated over a decade ago, but there are large disparities in coverage and targeted populations of vaccination strategies between countries where the program has been introduced. By mid-2016, national HPV vaccination programs have been established in 65 countries globally, most of which are high-income countries (HICs). Strong momentum has been observed to expand HPV vaccination programs to LMICs, where the majority of HPV-related cancers occur.6
The type of HPV vaccination program that countries choose to implement depends on the countries’ economic status, disease priorities, and the cost-effectiveness of the programs. Most HPV vaccination programs target 9–14 year old schoolgirls before sexual debut and it is cost-effective if more than 70% of young women are vaccinated.7 There remain lots of debate around whether it is cost-effective to expand the existing vaccination programs to also include women older than 26 years, heterosexual men, and men who have sex with men (MSM). Unlike HPV vaccination for adolescent girls and women up to 26 years which has been shown to be highly cost-effective in many studies,8–13 relatively fewer cost-effectiveness analyses (CEA) on HPV vaccination have been conducted in other population groups. This study aims to investigate the cost-effectiveness of HPV vaccination program for women older than 26 years, heterosexual men and MSM and the factors that drive its cost-effectiveness through a literature review.
Results
Study selection and characteristics
A total of 407 published articles were identified through PubMed (Figure 1). Initial screening eliminated 14 duplicated articles and a further 253 articles were excluded because they were not cost-effectiveness analyses of HPV vaccination. The remaining 140 articles were reviewed in full-text for eligibility according to our inclusion and exclusion criteria. Another 104 articles were excluded and 36 papers were eventually selected for our literature review. Among these 36 studies, six reported on women over age 26, 26 on heterosexual men, four on MSM and one reported on both women over age 26 and heterosexual men. These studies were conducted in 17 countries (12 high-income countries (HICs) and five low- and middle- income countries (LMICs), Table 1). Most (64%, n = 23) selected studies were published in 2011 or later.
Figure 1.
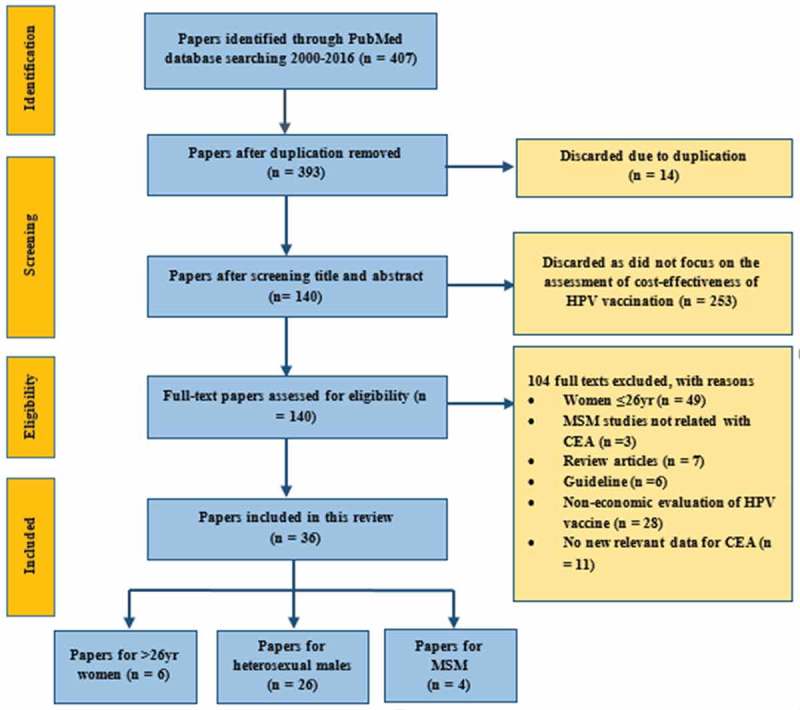
Selection of papers with PRISMA Statement.
Table 1.
Gross National Income, HPV-related Disease Burden and Current HPV Prevention Programs in 17 countries.
| Country (2015) | GNI per capita (PPP int $) 2013–2014 | *Incidence rate of CC (2012) | *Mortality rate of CC (2012) | Existence of national HPV vaccination program | Year of introduction for national HPV vaccination program | targeted Age group (year), mostly GF |
Vaccination coverage, mostly GF | Existence of national CC screening program | Coverage of national CC screening program (%) | Most widely used CC screening method |
|---|---|---|---|---|---|---|---|---|---|---|
| High-income countries | ||||||||||
| Norway | 65,970 | 11.9 | 2.3 | Yes | GF: 2009 | 12 | 63% (2011) | Yes | > 70% | PAP smear |
| USA | 55,860 | 6.6 | 2.7 | Yes (GF & GM) | GF: 2006; GM: 2011 | 11–12 | GM: 50%; GF: 63% (2016) | Yes | > 70% | PAP smear |
| Netherlands | 47,660 | 6.8 | 1.6 | Yes | GF: 2010; | 12 | ~ 60% | Yes | > 70% | PAP smear |
| Germany | 46,840 | 8.2 | 1.7 | Yes | GF: 2007 | 9–14 | ~ 60% | Yes | > 70% | PAP smear |
| Denmark | 46,160 | 10.6 | 1.9 | Yes | GF: 2009 | 12 | 79% (2011) | Yes | 50–70% | HPV test |
| Austria | 45,040 | 5.8 | 2 | Yes (GF & GM) | GF & GM: 2013 | 9 | N/A | Yes | > 70% | PAP smear |
| Canada | 43,400 | 6.3 | 1.7 | Yes (GF & GM) | GF: 2007; GM 2013 | 9–14 | 75% (2013) | Yes | > 70% | PAP smear |
| Belgium | 43,030 | 8.6 | 1.9 | Yes | GF: 2007 | 12–13 | 29–80% (2012) | Yes | 50–70% | PAP smear |
| Australia | 42,880 | 5.5 | 1.6 | Yes (GF & GM) | GF: 2007; GM: 2013 | 12–26 | 73.1% (2014) | Yes | 50–70% | PAP smear |
| United Kingdom | 38,370 | 7.1 | 1.8 | Yes (GF & GM) | GF: 2008; GM: 2014 | GF:9–26; GM: 9–15 | 80% (2009) | Yes | > 70% | PAP smear |
| Italy | 34,710 | 9.4 | 1.5 | Yes | GF: 2007 | 12 | 61% (2011) | Yes | > 70% | PAP smear |
| New Zealand | 33,760 | 5.3 | 1.4 | Yes | GF: 2008 | 12 | 56% (2014) | Yes | > 70% | PAP smear |
| Low- and middle-income countries | ||||||||||
| Mexico | 16,710 | 23.3 | 8 | Yes | GF: 2009 | 10 | N/A | Yes | 36% | PAP smear |
| Brazil | 15,900 | 16.3 | 7.3 | Yes | GF:2014 | 9 | N/A | Yes | > 70% | PAP smear |
| China | 13,130 | 7.5 | 3.4 | No | N/A | N/A | N/A | Yes | N/A | PAP smear |
| Viet Nam | 5350 | 10.6 | 5.2 | No^ | N/A | N/A | N/A | Yes | > 10% | PAP smear |
| Lao PDR | 4910 | 12.5 | 7.4 | Yes | GF: 2013 | N/A | N/A | No | N/A | N/A |
GNI = Gross National Income; PPP int $ = Purchasing Power Parity International Dollars; HPV = Human Papillomavirus; CC = Cervical Cancer; yr = years; GF = general women; GM = general men; N/A = Data not available; * Rate per 100,000 women per year; ^with Pilot Vaccination Program
Cost-effectiveness of HPV vaccination for ≥ 26-year-old women
Six studies15-20 evaluated the cost-effectiveness of 2vHPV vaccine in women > 26 years. Four studies15,16,18,20 found the costs for targeted vaccination for women > 26 years (ICER = US$65,000–192,000/QALY gained, Table S1) were beyond their respective cost-effectiveness thresholds (~$50,000/QALY gained) (Figure 2a). Four studies assumed vaccination cost US$283–400/3-dose vaccination schedule and concluded the program as not cost-effective. However, one study from the UK15 showed marginal cost-effectiveness when vaccine price was below £20/dose and life-time vaccine protection for women when no loss of immunity over time was considered. Another study from Lao PDR19 showed the program to be cost-effective with a catch-up vaccination for women up to age 75 years and the existing schoolgirls vaccination program was strongly subsidised by GAVI, the Vaccine Alliance (US$8.5/dose). Only one Belgium study17 demonstrated their program to be very cost-effective with the 2vHPV for women age up to 33 years (Table S1). Both the Lao PDR and Belgium studies assumed high vaccination coverage (≥ 70%). All studies assumed 3-dose vaccination strategies and none compare it with a 2-dose vaccination strategy.
Figure 2.
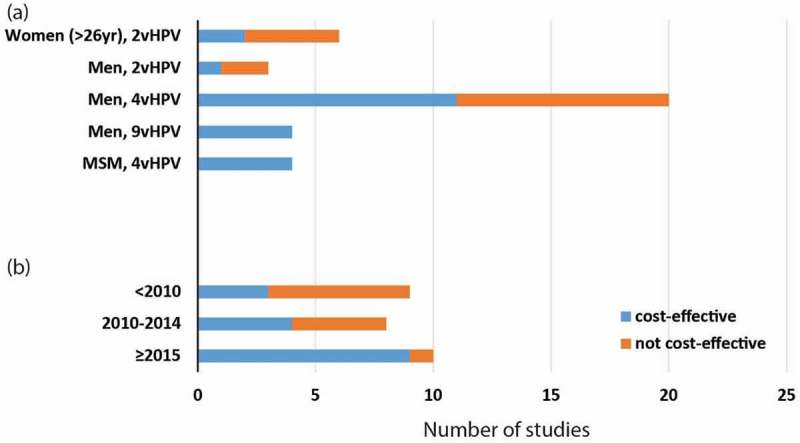
Cost-effectiveness of included studies for women (> 26yr), heterosexual men and MSM.
Cost-effectiveness of HPV vaccination for heterosexual men
Of 26 selected studies12,21–44 on gender-neutral vaccination (three in LMICs and 23 in HICs), two studies examined 2vHPV vaccine, 20 on 4vHPV, and four on 9vHPV vaccines. Sixteen studies22-25,27,36–46 demonstrated that HPV vaccination for heterosexual men with an existing female program was cost-effective (ICER = $19,600–52,800/QALY gained in HICs and $49–5,860/QALY gained in LMICs, Table S1) with respect to their respective cost-effectiveness thresholds (Figure 2a).
All four studies that assessed 9vHPV36,38,40,42 vaccine concluded that the vaccine for both girls and boys was cost-effective (ICER = $8600–49,800/QALY gained, Table S1) in comparison with 2vHPV or 4vHPV vaccination for both women and/or men. The majority (2/3) of studies with 2vHPV vaccination24,30 was not cost-effective, while 11/20 studies with 4vHPV vaccination were cost-effective. Interestingly, when stratified by five-year time periods (< 2010, 2010–2014 and ≥ 2015, Figure 2b), increasing proportion of studies demonstrated cost-effectiveness of HPV vaccination for heterosexual men in recent years (p-value = 0.035).
The assumed price of HPV vaccines varied substantially across studies (US $10–130/dose), and our analysis did not show any correlation between vaccine price and program cost-effectiveness in heterosexual men. While 3-dose vaccination strategy showed mixed results (14 cost-effective and 11 not), both studies with a 2-dose vaccination strategy showed cost-effectiveness.45,46 Longer duration of vaccine protection (life time protection) and program evaluation (100 years horizon) led to lower ICERs in these studies.
Age was an important factor for vaccine cost-effectiveness. Eight studies showed it was cost-effective to expand existing schoolgirl program to cover schoolboys at the same age (< 15 years). However, a UK study28 and a Danish study31 demonstrated that in the presence of a schoolgirl program, catch-up vaccination for young women up to 26 was a more cost-effective option than expanding schoolgirl program to cover the same age schoolboys. Eight studies showed that vaccination program for schoolboys and heterosexual men was no longer cost-effective if the vaccination coverage in women was beyond 70–75%. There was no evidence that the countries’ economic development status and vaccine efficacy had any impact on the cost-effectiveness of vaccination program for heterosexual men.
Cost-effectiveness of HPV vaccination for MSM
Four studies47-50 evaluated the cost-effectiveness of 4vHPV vaccine for MSM. All four studies demonstrated that the 4vHPV vaccine for MSM compared with no vaccination was cost-effective ($15,000–43,000/QALY gained) (Figure 2 a, Table S1), and it showed lower ICERs, hence better cost-effectiveness, for vaccination against MSM at a young age (< 40 years) or against those who were HIV-positive. A good cost-effectiveness of HPV vaccination for MSM was also associated with a high vaccination coverage (at least 55–80%), a potent vaccine efficacy (50–90%), a low vaccine price of 4vHPV (US$180–360/3-doses), a long duration of evaluation (life-time/100 years’ time horizon) (Table 2). In all MSM studies, there was no evidence that the socio-economic development status of the countries and vaccine dosage influenced the cost-effectiveness of MSM vaccination.
Table 2.
Summary of cost-effectiveness of targeted HPV vaccination program in MSM,women over age 26 and heterosexual men (all age) in the presence of a background vaccination program for young women.
| Targeted vaccination for women over age 26(n = 6) | Additional vaccination for heterosexual men (n = 26) | MSM (n = 4) | |
|---|---|---|---|
| 1. Cost-effectiveness | • Mostly not cost-effective. | • 16 studies indicated cost-effectiveness, while 11 papers concluded not. | • All cost-effective. |
| • Marginally cost-effective only when vaccine price was low and protection to non-naïve women was considered. | • Cost-effectiveness was associated with low background vaccination coverage, low price, good efficacy, high valency and long protection. | • Very cost-effective/cost-saving to vaccinate MSM at a young age or those who are HIV+ . | |
| Contributing factors | |||
| 2. Developed/developing countries status | • Of six studies, only one HIC (Belgium) was cost-effective. One LMIC (Lao) was cost-effective due to GAVI subsides for a low vaccine price | • Two out of four studies in LMICs (Brazil and Vietnam) demonstrated cost-effectiveness. | • All four studies were in HICs, there was no evidence that socioeconomic status affects the cost-effectiveness of vaccination program for MSM. |
| • Developmental status did not influence the cost-effectiveness of vaccination program for women over age 26. | • The 22 studies in HICs demonstrated mixed results (14 cost-effective and 9 not cost-effective) | ||
| 3. Vaccination age | • The younger age of vaccination is associated with lower ICERs. | • Vaccinating both preadolescent boys and girls was cost-effective compared with cervical cancer screening alone in 5/5 studies. | • The younger the age of vaccination, the lower ICER was the targeted MSM vaccination. |
| • Most (4/6) studies showed vaccinating women up to 26 years was cost-effective. | • Compared with preadolescent girl vaccination alone, 8/14 studies were cost-effective in expanding the program to preadolescent boys. | • Three studies appeared to justify cost-effectiveness for vaccination up to the age of 26, 55, 75 years respectively. One study among MSM who attended genitourinary medicine (GUM) clinics suggested the cost-effective age was up to 40 years. | |
| • Only 2 studies demonstrated vaccinating women up to 40 years would be cost-effective 17,19. | • Eight studies on male catch-up programs demonstrated that adding female catch-up up to 26 years would be more cost-effective than male catch-up program. | ||
| 4. Vaccination coverage | • Four out of six studies showed it was not cost-effective despite high vaccination coverage (70–100%). | • Eleven out of 26 studies demonstrated not cost-effective even with high vaccination coverage (70–80%) among women. | • Four studies demonstrated that vaccination coverage needed to be at least 55–80% to be cost-effective. |
| • In the Lao study, vaccination coverage for heterosexual women was 70% and Belgium study was 100%. | • Among 16 studies showing cost-effectiveness, eight related cost-effectiveness with vaccination coverage and showed a reducing cost-effectiveness with increasing vaccination coverage, these studies consistently showed that vaccination program was no longer cost-effective if coverage in female with/without male was beyond 70–75%. | ||
| 5. Duration of evaluation | • Lifetime or 100-year time horizon was used in all six studies, yet four were not cost-effective. | • Four of 8 studies with 50–60 years of evaluation period showed male vaccination was not cost-effective. | • Lifetime or 100 years’ time horizon was used for evaluation in four studies, which all showed cost-effectiveness. |
| • The majority (10/15) of studies with lifetime or 100-year as evaluation period demonstrated cost-effectiveness. | |||
| 6. Vaccine efficacy | • Despite 93–100% vaccine efficacy was used in these studies, 4/6 studies were not cost-effective. | • Most studies assumed high vaccine efficacy (80–100%) but showed mixed results (16 cost-effective, 11 not). | • All four studies assumed vaccine efficacies between 50–90% and all demonstrated cost-effectiveness. |
| • One study demonstrated that vaccination for heterosexual men was still cost-effective even with 50% vaccine efficacy. | • One of them demonstrated that 50% vaccine efficacy is sufficient to achieve cost-effectiveness in MSM 48. | ||
| 7. Vaccine price | • Four studies assumed vaccination cost US$283–400/3-dose for individual and concluded as not cost-effective. | • Among the 16 studies that demonstrated cost-effectiveness, the vaccine price was $50–500/3-dose. | • Two studies demonstrated vaccination at low price ($180–360/3-dose) to be more cost-effective 48, 64. |
| • One study from UK was not cost-effective even with a low vaccine price ($150/3-dose). | • In contrast, among the 11 studies that demonstrated otherwise, the vaccine price was $30–500/3-dose. | • Vaccine prices ($700/3-dose) higher than base scenarios ($364–500/3-dose) were still cost-effective at | |
| • Only Lao study with a very low GAVI price ($30/3-dose) cost-effectiveness in women above 26. | • The assumed vaccine price did not differ between the two, indicating it alone may not play a major role in its cost-effectiveness. | ||
| 8. Vaccine dosage (2 versus 3 doses) | • 3-dose vaccination strategies were used in all studies and no comparison with 2-dose vaccination was found. | • Two studies analyzed the cost-effectiveness of the 2-dose vaccination and both showed cost-effectiveness, suggesting lowering vaccine price may improve cost-effectiveness 45,46. | • 3-dose vaccination strategies were used in all studies and no comparison with 2-dose vaccination was found. |
| • Other 25 studies used 3-dose vaccination and showed mixed results (14 cost-effective and 11 not). | |||
| 9. Vaccine type (2v-, 4v- and 9vHPV) | • 2vHPV vaccines were used for female vaccination and no comparison was found. | • 9vHPV vaccine was found to be more cost-effective than 4vHPV and 2vHPV vaccines in 4 studies 36–38,40. | • 4vHPV vaccines were used for all MSM studies and no comparison was found. |
| • 10 out of 19 studies showed 4vHPV vaccination for males was cost-effective. | |||
| • 2 out of 4 studies demonstrated that 2vHPV vaccination to males was not cost-effective. | |||
| • No comparison between 2vHPV and 4vHPV vaccines were found. | |||
| 10. Duration of vaccine protection | • Generally not cost-effective even with lifetime vaccine protection. | • 12/18 studies showed cost-effectiveness with lifetime vaccine protection. | • Less than 30 year of vaccine protection was not cost-effective in 3 studies, however, the remaining one showed > 6–8 years of protection was cost-effective among MSM who attended GUM. |
| • 3/3 male vaccination with less than 20 years of vaccine protection was found to be not cost-effective. | |||
Vaccination and cervical cancer screening in included countries
The HPV vaccination and cervical cancer screening programs from the selected studies were described in Table 1. The annual cervical cancer incidence was generally higher (9.4–23.7 versus 5.5–12.9 per 100,000) in women from LMIC than HIC, as was the age standardized mortality rate for cervical cancer (3.4–8.0 versus 1.4–2.1 per 100,000). Cervical cancer mortality rates were significantly and negatively correlated with Gross National Income (GNI) (Spearman, r = -0.75, p < 0.001). Cervical cancer screening coverage among targeted women in HIC was more than 50–70%. In contrast, among LMIC, only Brazil reached a similar screening coverage as in HIC, while other countries were consistently below 40%. All National HPV vaccination programs for schoolgirls (up to age 14) were introduced before 2011 in HIC, and some programs included a catch-up program for young women up to age 26. To date, Austria, Australia, Canada, and the US, have expanded the vaccination program to schoolboys (age 9–14 years). In contrast, HPV vaccination began much later in LMICs, typically between 2013 and 2015 and China and Vietnam did not implement any vaccination programs until 2017. Vaccination coverage for women ranged from 40–80% in developed countries, where Germany had the lowest (40%) and the United Kingdom the highest (80%) coverage. We found no significant correlation between GNI per capita and vaccination coverage (R = -0.0049, p = 0.9877).
Discussion
Our targeted literature review indicated that HPV vaccine for women > 26 years would not be cost-effective, and this is consistent with current policy and practice. In contrast, HPV vaccination for heterosexual men demonstrated mixed results: programs proposing 9vHPV (compared with 4vHPV and 2vHPV), those assuming a long duration of vaccine effectiveness and those vaccinating young heterosexual men (< 26) demonstrated cost-effectiveness. Further, it suggested that targeted HPV vaccination for MSM is cost-effective in all four included studies. A previous systematic review on the cost-effectiveness of HPV vaccination among adolescent girls in LMICs has shown that vaccine price is one of the key determinant of vaccination cost-effectiveness.51 Our review further confirms this is also true in heterosexual men and MSM. In addition, we also identified a broad genotype coverage (9vHPV), less required doses and longer vaccine protection are important determinants for cost-effectiveness.
Our findings suggests that targeted HPV vaccination for MSM should be a priority worldwide. Unlike heterosexual men, MSM may benefit to a lesser extent from the herd immunity that heterosexual men may receive from the female vaccination programs.52 On the other hand, MSM are much more at-risk than heterosexual men to HPV infection in particular anogenital warts and anal cancer. In contrast to vaccination program in women where the vaccination coverage required (~ 70%) is well established, the vaccination coverage required in MSM to achieve the same level of herd immunity that heterosexual men may experience is not known. Since the reproductive rate of HPV infection in MSM is much greater than heterosexual men, it is likely that a higher level of vaccination coverage will be required.53
Despite only 16 of 26 studies in heterosexual men demonstrating cost effectiveness, our data suggest that a gender neutral vaccination strategy may become increasingly cost-effective for a number of reasons. First, recent literatures reported that 1- or 2-doses vaccination is as effective as 3-doses vaccination for people age 9–14 years, which means a potential 30% cost reduction per head if this is implemented in any school age vaccination programs.54–56 Second, it is anticipated that the mean price of HPV vaccine for LMICs will continue to decline over time, especially with significant subsidies and influence from major international health organizations such as GAVI, UNICEF and Pan American Health Organization (PAHO).57,58
Our analysis shows no correlation between individual country’s socio-economic status and vaccination coverage. However, we argue that the rollout of a universal HPV vaccination program in LMICs may face more challenges. Given limited resources, LMICs generally have a lower willingness-to-pay threshold for a vaccination program. Therefore, vaccine cost needs to be substantially lowered in LMICs, not only for the consideration of cost-effectiveness, but also the upfront investment cost must not become an excessive financial burden to the country budget. The initial rollout of the program often require a one-time investment for health facilities, establishment of an efficient implementation system and training for healthcare staff. Further, in resource-poor settings, an efficient healthcare provision system is often absent to provide the scheduled vaccination program, which is an essential infrastructure for additional HPV vaccination programs. For these settings, resources from the international community should be directed to provide point-of-care vaccination where primary healthcare is absent, and 2-dose HPV vaccine should be promoted to improve vaccination coverage in the population.
A number of limitations need to be considered when interpreting our results. As a targeted literature review, we excluded studies not published in English and therefore, our study may be subject to publication bias. Second, we could not conduct a meta-analysis due to limited data available from targeted reviews. Similarly, we could not prove the robustness of outcomes because of the variations in models applied in the included studies where different assumptions and parameters were used. For instance, population impact was not reported in a consistent form across the studies, however, we emphasized that all cost-effectiveness studies included a baseline scenario and the analysis was conducted by comparing the scenarios in the presence and absence scenario. Therefore, we summarized the absolute number of studies and the factors influencing the cost-effectiveness instead. Despite these limitations, we believe our findings would be a springboard for further studies of the cost-effectiveness of HPV vaccination for these currently untargeted populations.
Conclusion
Targeted HPV vaccination for MSM should be next priority in HPV prevention after having established a solid girls vaccination programme. Vaccination for heterosexual men should be considered when 2-dose 4vHPV/9vHPV vaccines become available with a reduced price. Vaccination for women over age 26 may not be cost-effective until the vaccine price is further reduced.
Method
Search
The full electronic search was conducted in PubMed for related articles and reviews on February 15th 2017, which were published in the English language from January 1, 2000 to December 31, 2016. The search strategy was conducted using the following key words: “Human Papillomavirus” AND “Cost-effectiveness” AND “Vacc*” in MeSH terms AND “HPV” OR “Human Papillomavirus” AND “Cost-effective*” AND “Vacc*” in titles and abstracts AND “English” in language.
Eligibility criteria
This review included English-language articles (published between 2000–2016) that assessed the incremental cost-effectiveness ratio (ICER) of HPV vaccination to the female population older than 26 years, heterosexual men and MSM, in comparison with the cost-effectiveness of existing cervical cancer screening or vaccination in young adolescent girls with a catch-up program for women age up to 26 years. In this review, the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta- Analyses) statement59 was followed (Figure 1). Articles were excluded if they (1) were in a language other than English; (2) did not report ICER of the HPV vaccination program; and (3) only focused on young female vaccination program.
Data collection
We collected demographic data, HPV epidemiological data, impact and cost-effectiveness data from aforementioned literature review. In addition, based on the countries identified from the selected studies, we further collected data on country-specific HPV-related programs and country incomes that were not available in the literature research.
First, demographic data included age and sex of the targeted population, period of analysis (retrospective or prospective study) and country of the study population. Second, epidemiological data included status quo HPV disease burden, subtypes and vaccination coverage. Third, population impact data included the type of model used, reduction in HPV infections, number of genital warts, pre-cancerous lesions CIN-1, −2, and −3 cases, cervical cancer cases and mortality. Fourth, cost-effectiveness data included incremental cost associated with HPV vaccination programs; incremental cost-effectiveness ratios (ICERs); incremental life-years gained (LYGs) or Quality-adjusted life-years (QALYs) gained from a vaccination program. Fifth, we identified 17 countries from the selected 36 publications. For these 17 countries, we collected other HPV-related program and income data from these well-known online HPV databases: HPV Information Centre;60 National Cancer Institute;61 and International Agency for Research on Cancer.62 Specific country data included: gross National Income per capita (GNI); age-standardized incidence rate of cervical cancer; age-standardized mortality rate of cervical cancer; existence of national cervical cancer screening and HPV vaccination programs; years of introduction of the national HPV vaccination program; targeted age and gender of current HPV vaccination program; vaccination coverage; and cervical cancer screening coverage. Double-entry was performed to extract these data by two independent investigators (NNS, FC). Microsoft excel 2013 was used to store and analyse these data.
Quality assessment
The quality assessment of each included study was conducted by two independent investigators (NNS, FC). Any conflicting opinions were resolved by a third reviewer (LZ). The quality check for each included study was assessed by three domains: study design, data collection, and analysis and interpretation of the results (Cost-effectiveness study quality checklist,63 Table S2).
Data analysis
Descriptive statistics were conducted for each study population group (older women, heterosexual men and MSM) to inform HPV program, impact and cost-effectiveness indicators. First, for each population, we categorized the selected studies that showed proposed strategy was cost-effective according to their stated willingness-to-pay threshold, and those showed it was not cost-effective. Second, the major contributing factors influencing the cost-effectiveness, including vaccination age and coverage, vaccine efficacy, price and dosage, duration of vaccine protection, and the time horizon of evaluation, were identified in both cost-effective and non-cost-effective studies. A Spearman’s correlation test was used to analyse the correlation between the GNI and HPV-burden of the included countries. In addition, chi-square tests were conducted to investigate the time trend of cost-effectiveness of HPV vaccination for heterosexual males.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
References
- 1.Walboomers JMM, Jacobs MV, Mnos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Munoz N.. Human Papillomavirus is a necessary cause of invasive cervical cancer worldwide. 1999; Sep;189(1):12-9. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention HPV and men- CDC fact sheet. 2015. February https://www.cdc.gov/std/hpv/hpv-and-men-factsheet-feb-2015.pdf.
- 3.World Health Organization Cervical cancer. 2018. http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/.
- 4.World Health Organization Human papillomavirus (HPV) and cervical cancer. 2016. June http://www.who.int/mediacentre/factsheets/fs380/en/.
- 5.World Health Organization Safety update of HPV vaccines. 2017. accessed http://www.who.int/vaccine_safety/committee/topics/hpv/June_2017/en/.
- 6.Stanley M. HPV vaccination in boys and men. Hum Vaccin Immunother. 2014;10(7):2109–2111. doi: 10.4161/hv.29137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canfell K, Harrell Chesson SL, Kulasingam JB, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine. 2012;30(Suppl 5):F157–67. doi: 10.1016/j.vaccine.2012.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldie SJ, Carol Levin N, Mosqueira-Lovón R, Ortendahl J, Kim J, O’Shea M, Sanchez MD, Araujo MAM. Health and economic impact of human papillomavirus 16 and 18 vaccination of preadolescent girls and cervical cancer screening of adult women in Peru. Rev Panam Salud Publica. 2012;32(6):426–434. [PubMed] [Google Scholar]
- 9.Luttjeboer J, Westra TA, Wilschut JC, Nijman HW, Daemen T, Postma MJ. Cost-effectiveness of the prophylactic HPV vaccine: an application to the Netherlands taking non-cervical cancers and cross-protection into account. Vaccine. 2013;31(37):3922–3927. doi: 10.1016/j.vaccine.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Uusküla A, Müürsepp A, Kawai K, Raag M, Jürisson M, Pillsbury M. 2013. The epidemiological and economic impact of a quadrivalent human papillomavirus (hpv) vaccine in Estonia. BMC Infect Dis. 13:304. doi: 10.1186/1471-2334-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walwyn L, Janusz CB, Clark AD, Prieto E, Waight E, Largaespada N. Cost-effectiveness of HPV vaccination in Belize. Vaccine. 2015;33(Suppl 1):A174–81. [DOI] [PubMed] [Google Scholar]
- 12.Insingaa RP, Dasbach EJ, Elbasha EH, Puig A, Reynales-Shigematsu LM. Cost-effectiveness of quadrivalent human papillomavirus (HPV) vaccination in Mexico: a transmission dynamic model-based evaluation. Vaccine. 2007;26(1):128–139. doi: 10.1016/j.vaccine.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 13.Kawai K, de Araujo GTB, Fonseca M, Pillsbury M, Singhal PK. 2012. Estimated health and economic impact of quadrivalent HPV (types 6/11/16/18) vaccination in Brazil using a transmission dynamic model. BMC Infect Dis. 12:250. doi: 10.1186/1471-2334-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control Introduction of HPV vaccines in EU countries – an update. Stockholm: ECD; 2012. doi: 10.2900/60814 [DOI] [Google Scholar]
- 15.Turner HC, Baussano L, Garnett GP. Vaccinating women previously exposed to human papillomavirus: a cost-effectiveness analysis of the bivalent vaccine. PLoS One. 2013;8(9):e75552. doi: 10.1371/journal.pone.0075552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JJ, Ortendahl J, Goldie SJ. Cost-effectiveness of human papillomavirus vaccination and cervical cancer screening in women older than 30 years in the United States. Ann Intern Med. 2009;151(8):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demarteaua N, Van Kriekinge G, Simon P. Incremental cost-effectiveness evaluation of vaccinating girls against cervical cancer pre- and post-sexual debut in Belgium. Vaccine. 2013;31(37):3962–3971. doi: 10.1016/j.vaccine.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Westra TA, Rozenbaum MH, Rogoza RM, Nijman HW, Daemen T, Postma MJ, Wilschut JC. Until which age should women be vaccinated against HPV infection? Recommendation based on cost-effectiveness analyses. J Infect Dis. 2011;204(3):377–384. doi: 10.1093/infdis/jir281. [DOI] [PubMed] [Google Scholar]
- 19.Chanthavilay P, Reinharz D, Mayxay M, Phongsavan K, Marsden DE, Moore L, White LJ. The economic evaluation of human papillomavirus vaccination strategies against cervical cancer in women in Lao PDR: a mathematical modelling approach. BMC Health Serv Res. 2016;16(1):418. doi: 10.1186/s12913-016-1662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y-J, Zhang Q, Shang-Ying H, Zhao F-H. 2016. Effect of vaccination age on cost-effectiveness of human papillomavirus vaccination against cervical cancer in China. BMC Cancer. 16:164. doi: 10.1186/s12885-016-2207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis. 2004. Nov;10(11):1915-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuliano AR. Human papillomavirus vaccination in males. Gynecol Oncol. 2007;107(2 Suppl 1):S24–6. doi: 10.1016/j.ygyno.2007.07.075. [DOI] [PubMed] [Google Scholar]
- 23.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: a cost-effectiveness analysis in a low-resource setting. Br J Cancer. 2007;97(9):1322–1328. doi: 10.1038/sj.bjc.6604023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulasingam S, Connelly L, Conway E, Hocking JS, Myers E, Regan DG, Roder D, Ross J, Wain G. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian National Cervical Cancer Screening Program. Sex Health. 2007;4(3):165. doi: 10.1071/SH07043. [DOI] [PubMed] [Google Scholar]
- 26.Pearson AL, Kvizhinadze G, Wilson N, Smith M, Canfell K, Blakely T. 2014. Is expanding HPV vaccination programs to include school-aged boys likely to be value-for-money: a cost-utility analysis in a country with an existing school-girl program. BMC Infect Dis. 14:351. doi: 10.1186/1471-2334-14-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Chessona HW, Ekwueme DU, Saraiyab M, Dunnea EF, Markowitza LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29(46):8443–8450. doi: 10.1016/j.vaccine.2011.07.096. [DOI] [PubMed] [Google Scholar]
- 29.Zechmeister I, de Blasio BF, Garnett G, RaeNeilson A, Siebert U. Cost-effectiveness analysis of human papillomavirus-vaccination programs to prevent cervical cancer in Austria. Vaccine. 2009;27(37):5133–5141. doi: 10.1016/j.vaccine.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 30.Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. Bmj. 339;2009:b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen J, Jepsen MR. Human papillomavirus transmission and cost-effectiveness of introducing quadrivalent HPV vaccination in Denmark. Int J Technol Assess Health Care. 2010;26(2):183–191. doi: 10.1017/S0266462310000085. [DOI] [PubMed] [Google Scholar]
- 32.Jit M, Choi YH, John Edmunds W. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ (Clinical research ed) 2008; 337: a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laprise J-FO, Drolet M, Boily M-C, Jit M, Sauvageau C, Franco EL, Lemieux-Mellouki P, Malagón T, Brisson M. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modelling study. Vaccine. 2014;32(44):5845–5853. doi: 10.1016/j.vaccine.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 34.Isaranuwatchai W, Graham DM, Siu LL, Hoch JS. Could the human papillomavirus vaccination be cost-effective in males for the prevention of oropharyngeal cancer? Expert Rev Pharmacoecon Outcomes Res. 2014;14(6):763–765. doi: 10.1586/14737167.2014.946012. [DOI] [PubMed] [Google Scholar]
- 35.Sinisgalli E, Bellini I, Indiani L, Sala A, Bechini A, Bonanni P, Boccalini S. HPV vaccination for boys? A systematic review of economic studies. Epidemiol Prev. 2015;39(4 Suppl 1):51–58. [PubMed] [Google Scholar]
- 36.Durhama DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci U S A. 2016;113(18):5107–5112. doi: 10.1073/pnas.1515528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham DM, Isaranuwatchai W, Habbous S, de Oliveira C, Liu G, Siu LL, Hoch JS. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer. Cancer. 2015;121(11):1785–1792. doi: 10.1002/cncr.29111. [DOI] [PubMed] [Google Scholar]
- 38.Chesson HW, Markowitz LE, Hariri S, Ekwueme DU, Saraiya M. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: estimates from a simplified transmission model. Hum Vaccin Immunother. 2016;12(6):1363–1372. doi: 10.1080/21645515.2016.1140288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haeussler K, Marcellusi A, Mennini FS, Favato G, Picardo M, Garganese G, Bononi M, Costa S, Scambia G, Zweifel P, et al. Cost-effectiveness analysis of universal human papillomavirus vaccination using a dynamic bayesian methodology: the BEST II study. Value Health. 2015;18(8):956–968. doi: 10.1016/j.jval.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Boiron L, Joura E, Largeron N, Prager B, Uhart M. 2016. Estimating the cost-effectiveness profile of a universal vaccination programme with a nine-valent HPV vaccine in Austria. BMC Infect Dis. 16:153. doi: 10.1186/s12879-016-1987-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma M, Sy S, Kim JJ. The value of male human papillomavirus vaccination in preventing cervical cancer and genital warts in a low-resource setting. Bjog. 2016;123(6):917–926. doi: 10.1111/1471-0528.13503. [DOI] [PubMed] [Google Scholar]
- 42.Largeron N, Petry KU, Jacob J, Bianic F, Anger D, Uhart M. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. JMIR Res Protoc. 2017; 17(1): 85–98. [DOI] [PubMed] [Google Scholar]
- 43.Kotsopoulos N, Connolly MP, Remy V. Quantifying the broader economic consequences of quadrivalent human papillomavirus (HPV) vaccination in Germany applying a government perspective framework. Health Econ Rev. 2015;5(1):54. doi: 10.1186/s13561-015-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bresse X, Goergen C, Prager B, Joura E. Universal vaccination with the quadrivalent HPV vaccine in Austria: impact on virus circulation, public health and cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):269–281. doi: 10.1586/14737167.2014.881253. [DOI] [PubMed] [Google Scholar]
- 45.Burger EA, Stephen S, Nygard M, Kristiansen IS, Lim JJ. Prevention of HPV-related cancers in Norway: cost-effectiveness of expanding the HPV vaccination program to include pre-adolescent boys. PLoS One. 2014;9(3):e89974. doi: 10.1371/journal.pone.0089974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen J, Jørgensen TR. 2015. Revisiting the cost-effectiveness of universal HPV-vaccination in Denmark accounting for all potentially vaccine preventable HPV-related diseases in males and females. Cost Eff Resour Alloc. 13:4. doi: 10.1186/s12962-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deshmukh AA, Jagpreet Chhatwal EY, Chiao AG, Nyitray PD, Cantor SB. Long-term outcomes of adding HPV vaccine to the anal intraepithelial neoplasia treatment regimen in HIV-positive men who have sex with men. Clin Infect Dis. 2015;61(10):1527–1535. doi: 10.1093/cid/civ628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deshmukh AA, Chiao EY, Dasc P, Cantord SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32(51):6941–6947. doi: 10.1016/j.vaccine.2014.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin A, Ong KJ, Hobbelen P, King E, Mesher D, Edmunds WJ, Sonnenberg P, Gilson R, Bains I, Choi YH, et al. Impact and cost-effectiveness of selective human papillomavirus vaccination of men who have sex with men. Clin Infect Dis. 2016. doi: 10.1093/cid/ciw845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10(12):845–852. doi: 10.1016/S1473-3099(10)70219-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fesenfelda M, Hutubessya R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: a systematic review. Vaccine. 2013;31(37):3786–3804. doi: 10.1016/j.vaccine.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 52.Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, McNamee K, Garefalakis M, Phillip S, Cummins E, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14(10):958–966. doi: 10.1016/S1473-3099(14)70841-2. [DOI] [PubMed] [Google Scholar]
- 53.Fairley CK, Zou H, Zhang L, Chow EPF. Human papillomavirus vaccination in men who have sex with men - what will be required by 2020 for the same dramatic changes seen in heterosexuals. Sex Health. 2017;14(1):123–125. doi: 10.1071/SH16067. [DOI] [PubMed] [Google Scholar]
- 54.Markowitz LE. 2017. Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination-updated recommendations of the advisory committee on immunization practices. American Journal Of Transplantation : Official Journal Of The American Society Of Transplantation and The American Society Of Transplant Surgeons. 17(3): 834–837. [Google Scholar]
- 55.World Health Organization Weekly epidemiological record. 2014. http://www.who.int/wer/2014/wer8921.pdf.
- 56.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, Garland SM, Herrero R, David M-P, Wheeler CM, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015;16(7):775–786. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dasbach EJ, Insinga RP, Elbasha EH. The epidemiological and economic impact of a quadrivalent human papillomavirus vaccine (6/11/16/18) in the UK. Bjog. 2008;115(8):947–956. doi: 10.1111/j.1471-0528.2008.01743.x. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization WHO price report: vaccine product, price and procurement. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 59.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 60.HPV Information Center HPV and cervical cancer prevention statistics. 2016. http://www.hpvcentre.net/references.php.
- 61.National Cancer Institute Cancer statistics. 2016. https://seer.cancer.gov/.
- 62.International Agency for Research on Cancer Data visualization tools that present current national estimates of cancer incience, mortality, and prevalence. 2016. http://gco.iarc.fr/today/home.
- 63.Deshpande SN, van Asselt AD, Tomini F, Armstrong N, Allen A, Noake C, Khan K, Severens JL, Kleijnen J, Westwood ME, Rapid fetal fibronectin testing to predict preterm birth in women with symptoms of premature labour: a systematic review and cost analysis. Health Technology Assessment 2013. accessed May 5 https://www.ncbi.nlm.nih.gov/books/NBK261009/. doi: 10.3310/hta17400. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


