ABSTRACT
Allergen immunotherapy is a rapidly evolving field. Although subcutaneous immunotherapy has been practiced for over a hundred years, improved understanding of the underlying immunological mechanisms has led to the development of new, efficacious and better tolerated allergen-derivatives, adjuvants and encapsulated allergens. Diverse routes of allergen immunotherapy – oral, sublingual, epicutanoeus and intralymphatic – are enabling immunotherapy for anaphylactic food allergies and pollen-food allergy syndrome, while improving the tolerability and effectiveness of aeroallergen immunotherapy. The addition of Anti-IgE therapy decreases adverse effects of subcutaneous and oral immunotherapy.
Keywords: Allergen Immunotherapy, Oral Immunotherapy, Subcutaneous Immunotherapy, Sublingual Immunotherapy, Epicutaneous Immunotherapy, adjuvants, Anti-IgE
Introduction
In this review we will address non-registered forms of allergen immunotherapy for various kinds of IgE mediated hypersensitivity, including aeroallergies, food allergy and pollen food allergy syndrome. Allergen Immunotherapy has been in use for over a hundred years. At its core, subcutaneous immunotherapy (SCIT) for aeroallergies and stinging insect hypersensitivity has changed very little since inception. However, the field has moved forward with exciting new developments in immunotherapy including novel routes of delivery, modified allergens, allergen derivatives and combination therapy with biologics.
Our understanding of the immunological basis of allergy and the changes associated with allergen immunotherapy has vastly improved over the last three to four decades. In parallel, advances in molecular characterization have led to a better understanding of allergens at the protein and sequence level.
The process of preparing extracts from various naturally occurring source materials and periodic subcutaneous injection has remained the same. The evidence supporting subcutaneous immunotherapy for aeroallergies is plentiful but patchy.1,2 In the United States, standardization of extracts across manufacturers and allergens is limited to a few allergens. Despite these limitations, the overwhelming consensus and the practice parameters back the use of subcutaneous immunotherapy (SCIT) for inhalant allergies and Hymenoptera venom allergies.3–7 However, there is a risk of local and systemic reactions and poor adherence with SCIT.8 Several approaches have been adopted to remedy this including alternate routes and rapid build-up schedules. SCIT has been abandoned for food allergies due to a high rate of adverse reactions.9,10
In this review, we will evaluate newer modalities of immunotherapy. Conventional SCIT for aeroallergens and venom anaphylaxis have been reviewed extensively elsewhere. We will not discuss FDA approved products such as newly approved SLIT products that are outside the purview of this review article. We will present data based on some products that are in development (such as Epicutaneous immunotherapy), as they best illustrate a new technology. Here we will briefly summarize the evidence. We will discuss updates to our understanding of the immunological mechanism underlying immunotherapy. We evaluate the evidence supporting the treatment of food allergies using oral immunotherapy (OIT), sublingual immunotherapy (SLIT), and epicutaneous immunotherapy (EPIT). We will assess the evidence backing the use of anti-IgE in combination with other forms of immunotherapy. Finally, we will examine novel methods of antigen preparation and delivery such as intralymphatic immunotherapy.
Immunological mechanism of allergen immunotherapy
Despite a century of successful immunotherapy, the molecular and cellular mechanisms of immunotherapy remain poorly understood. There is some understanding of the immunological changes associated with SCIT. These include desensitization of mast cells and basophils, changes in immunoglobulin production, and the generation of regulatory T cells (Treg). We refer you to excellent reviews on the topic by Shamji and Durham,11 Soyer OU et al.12 and, Berin and Shreffler.13 Here we briefly summarize some of the theories and critical findings that impact the modalities of immunotherapy discussed below.
Aeroallergen SCIT, SLIT and OIT are associated with changes in mast cell activation. While the phenomenon of the decreased mast cell and basophil responsiveness is well established,14 the mechanism is debatable and not directly observable. Partial degranulation, endocytosis of surface IgE15 and actin remodeling,16 have all been proposed based on in vitro and animal studies
Treg suppressor activity mediated by TGFβ and IL10 are responsible for late effects on mast cell number and function. Allergen-specific activation of peripheral basophil activity is diminished particularly in OIT and SLIT where it has the potential for use as a marker of successful desensitization.17–20 This, however, is not universal. Peanut epicutaneous immunotherapy did not show significant changes in basophil activation.14,21,22
Dendritic cell mediated induction of regulatory T and B cells of various kinds – natural Tregs, inducible Tregs, T follicular regulatory cells, B regs have all been described in various forms of immunotherapy. However, the role of dendritic cells is highly complex and varies tremendously by the site. Allergen (is) first encountered by different populations of mature and immature dendritic and Langerhans cells depending on the route of allergen immunotherapy. While epicutaneous immunotherapy relies on dermal resident dendritic cell populations, intralymphatic immunotherapy entirely bypasses them. With mucosal exposure as in SLIT mucosal Langerhans-like dendritic cells play a critical role by capturing the allergen within the oral mucosa. In response, Th2 cytokine production is decreased with concurrent increase in Th1 and suppressive cytokines (TGFβ and IL10) and various T reg subpopulations – natural Treg, inducible T reg and T follicular regulatory cells. Natural and inducible T regs suppress the development of allergic diseases via (a) induction of suppressive cytokines, such as interleukin-10 (IL-10) and transforming growth factor β (TGF- β); (b) suppression of allergen-specific IgE and induction of IgG4 and IgA; (c) suppression of mast cells, basophils, eosinophils, and inflammatory dendritic cells and (d) by suppression of effector TH1, Th2 and Th17 cells.12,23 Multiple studies have shown that aeroallergen immunotherapy leads to an increase in local FOXP3+ CD25+ T cells in the nasal mucosa in allergic rhinitis and airways of asthmatics.24–27 Similarly, OIT is also thought to increase the migratory potential of Tregs cells towards intestinal epithelial cell and induce epigenetic changes enhancing their function via FoxP3 + locus.28 It increases CD4+ CD25+ FoxP3+ Tregs and, Foxp3 + mRNA and protein expression, in CD4 cells from mesenteric lymph nodes in the jejunum with OIT.29 Besides Tregs, a population of newly described IgG4 producing IL10 secreting, regulatory B-cells (Bregs) have been observed in subjects on venom immunotherapy and may have a role in other forms of immunotherapy.30
Subcutaneous immunotherapy produces humoral changes. Initially, there is an early increase in specific IgE, but blunting of the seasonal rise in specific IgE, and is followed by a gradual decline in serum specific IgE over months to years of immunotherapy.31 Immunotherapy results in a 10–100-fold increase in allergen-specific antibodies in IgG1 and IgG4 subclasses, particularly IgG4.
A vital issue in food allergen immunotherapy is the development of sustained unresponsiveness, a way station for the development of true immunological tolerance. A limited number of participants in OIT, SLIT and EPIT trials develop sustained unresponsiveness.14,17,32 Individuals who haven’t developed this state, have a rapid loss of desensitization with the return of basophil reactivity whereas those who develop sustained unresponsiveness do not react for prolonged periods and continue to have suppressed basophil reactivity.32,33 The real locus of sustained unresponsiveness is not known. Subjects who fail to develop sustained unresponsiveness have higher starting specific IgE levels, suggesting a humoral component.33 The use of anti-IgE therapy can decrease basophil reactivity but does not improve rates of sustained unresponsiveness.34
The diversity of allergen immunotherapy options
Immunotherapy has evolved beyond SCIT to a panoply of routes and allergen preparations including OIT, SLIT, EPIT, and peptide-based immunotherapy. (See Figure 1) While the bulk of immunotherapy is based on native extracts, allergoids35 and recombinant allergens36 have been trialed.. Modified allergens can be associated with reduced risk of reactions.35 Acetone precipitated dog extracts have increased the concentration of major allergens compared to native allergen extracts and consequently achieved better desensitization.37 Peptide Immunotherapy bypasses IgE mediated mast cell activation and induces Tregs and IL-10 mediated immunological tolerance.38,39
Figure 1.
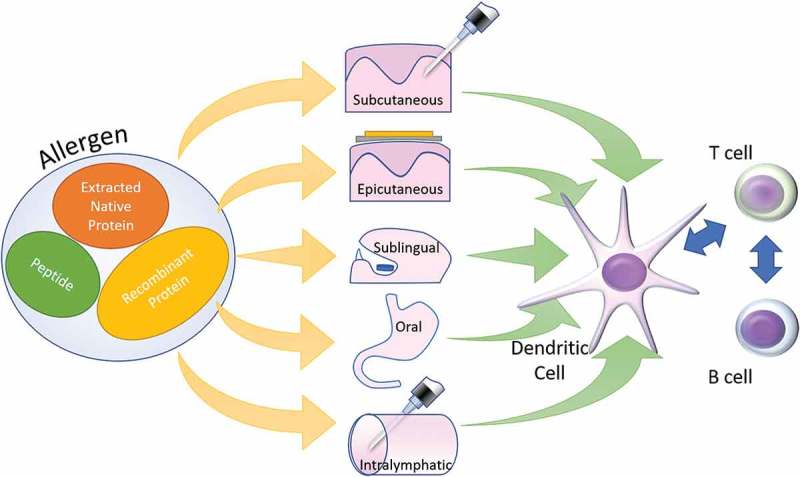
Different allergen forms can be administered through a variety of routes eventually leading to similar immunological changes. Subcutaneous – Subcutaneous Immunotherapy, Sublingual – Sublingual Immunotherapy, Oral – Oral Immunotherapy, Epicutanoeus – Epicutaneous Immunotherapy and Intralymphatic – Intralymphatic immunotherapy.
Summary of evidence supporting subcutaneous immunotherapy for inhalant allergies
Translating research involving Immunotherapy into clinical practice is challenging. Subcutaneous immunotherapy as is practiced in the United States often comprises multiple allergens in allergen mixes reflecting polysensitization commonly seen in clinical practice. Studies, however, are confined to single allergens. Aggregated evidence from meta-analyses40,41 and expert opinion form the basis of guideline documents for subcutaneous immunotherapy.3 However, the quality of the included studies limits meta-analyses. Differences in outcome measures, duration of therapy, allergens and treatment regimens limit comparisons. The Cochrane review, suggests that aero-allergen SCIT lowers symptom and medication scores.42 Unlike other meta-analyses and systematic reviews, it calculates statistics based on studies meeting their inclusion criteria but also cite data from studies that did not meet inclusion criteria but had significant results. Their principal conclusions are supported by the statistical analysis and the majority of rejected trials as well. The AAAAI/ACAAI Practice Parameters now endorse SLIT Immunotherapy for the treatment of aeroallergies.43 Several commercial products are available for co-seasonal and year-round use.
Food allergy – oral and sublingual immunotherapy
Investigations into the treatment of food allergies have lagged the treatment of aeroallergies. However, the explosion in the incidence and awareness of food allergies has led to a push for immunotherapy. Initial SCIT studies had high rates of reactions.9,10 Many things set food allergy immunotherapy apart from respiratory allergies or venom allergies. The route of exposure is different (oral vs, inhaled or parenteral). The dose per exposure, for food, is several orders of magnitude larger. Partial desensitization or tolerance (i.e., Mild food allergic reactions) is not an acceptable outcome. Strict avoidance is possible but has nutritional, social and financial consequences. Though there are few head-to-head comparisons, OIT is the most efficacious therapy for food allergy. OIT has the highest rates of treatment-associated adverse reactions. Most studies address, peanut,28,32,33,44–49 milk50–53 and egg allergies.51,54–58 Limited studies have been carried out with wheat,59,60 multi-food immunotherapy61 and as discussed elsewhere pollen-food allergy syndrome (PFAS). OIT and SLIT comparison studies have demonstrated higher rates of desensitization and adverse effects with OIT.32 In designing oral immunotherapy for food allergies it is important to define the objective – the ability to consume conventional portions of the food or prevention of allergic reactions due to small or accidental exposures. There hasn’t been a systematic effort to qualify the objectives and outcomes in these terms. Most milk and egg trials target the ability of the patient to consume conventional portions of the food as the goal of treatment. All oral immunotherapy trials for all foods published to date cumulatively include approximately 1500 subjects. Several major food allergens are yet to be in clinical trials. This lack of data despite enormous efforts, across a large number of sites and at great cost is the single greatest limitation to our understanding of oral immunotherapy.
OIT proceeds through three phases – initial rapid up-dosing (especially with omalizumab as premedication), a slow escalation phase and a maintenance phase. Studies assess desensitization (a food challenge while on daily dosing) and sustained unresponsiveness (food challenge after a period of abstinence after achieving desensitization. The duration maintenance therapy and the duration of abstinence before a sustained unresponsiveness challenge vary amongst studies.
Oral immunotherapy for peanut (Table 1A) serves as a prototype for the study of OIT for anaphylactic food allergies. However, the generalizability of these findings will not be known until a large cohort of foods has been studied. Desensitization rates have varied from 60 to 80%, and this has been the case for most foods. Early studies used a large goal dose for desensitization (about 4000mg) However, data suggests that lower maintenance doses such as 1000mg or 300mg of peanut protein may achieve higher rates of desensitization, with lower rates of adverse effects and similar ability to tolerate more substantial amounts upon challenge. High rates of desensitization and sustained unresponsiveness were associated with the use of lactobacillus in combination with peanut.45 However, the trial involved younger subjects (mean age about six years), and sustained unresponsiveness challenges were carried an earlier than other studies (2–5 weeks)
Table 1A.
Peanut oral immunotherapy.
| Study | Food allergen | N | Age Range | Study design | Maintenance Dose | Desensitization Outcome | Long-term results | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Hofmann et al. 200962 Jones et al. 200946 |
Peanut | 39 | 1–16 years | Open-label, not controlled. | 300–1800 mg | 71% reached 300 mg daily dose. | 69% (27/39) passed OFC at 1800 mg (total 3.9 g). | Escalation: 92% symptomatic, 15% needed epinephrine. 3.7 % of 14,773 doses during up-dosing or maintenance, mostly minor. |
| Blumchen et al. 201047 | Peanut | 23 | 3–14 years | Open label, not controlled. | ?500 mg | 61% (14/23) reached 500 mg daily for 8 weeks. | After 2 weeks off OIT, 14/23 (100% who reached maintenance) tolerated 500 mg in DBPCFC, 17% tolerated 4 g. | Escalation: subjective symptoms with 25/317 (7.9 %) doses. Reactions with 160/6137 (2.6 %) of buildup/maintenance doses. |
| Varshney et al. 201148 | Peanut | 28 (2:1 OIT: placebo) | 1–16 years | RCT, placebo-controlled, double-blind. | Up to 4000 mg daily | 80% on OIT tolerated 4000 mg daily for 1 year, 80% on OIT passed 5000 mg DBPCFC after 1 year. | N/A | Escalation: 47% (9/19) of OIT had symptoms, 2 required epinephrine. Reactions with 1.2 % of buildup/maintenance doses. |
| Vickery et al. 201433 | Peanut | 39 | 1–16 years | Open label, not controlled (enrolled patients from53 and24). | 300 mg OR 1800 mg (if passed 3900mg OFC), then 4000 mg daily | 66% (26/39) reached 4000 mg daily. | After 4 weeks off OIT, 31% (12/39) tolerated 5000 mg OFC and open dose. | 15% withdrew for allergic side effects. |
| Anagnostou et al. 201463 | Peanut | 99 | 7–16 years | RCT, placebo-controlled, double-blind. (OIT/placebo), control group crossed over to active OIT. | 800 mg | Phase 1: 84% OIT, 0 % placebo passed 1400 mg DBPCFC. Phase 2: placebo patients switched to OIT, 54% passed the same DBPCFC. | Phase 1: 62% OIT, 0 % placebo tolerated 800 mg daily at 26 weeks. Phase 2: placebo patients switched to OIT, 91% tolerated 800 mg daily at 26 weeks. QoL improved in both groups. | Up-dosing: Mild adverse events in 6.3 % of doses. 1 patient received epinephrine for 2 separate reactions. |
| Narisety et al. 201532 | Peanut | 21 | 7–13 years | RCT, parallel intervention, double blind (1:1 OIT/SLIT). | 2000 mg OIT, 3.7 mg SLIT | 50% SLIT, 45% OIT passed OFC 10g at 6–12 months. | Sustained unresponsiveness: 50% (2/4) OIT, 20% (1/5) SLIT passed repeat OFC 10 g after 4 weeks peanut avoidance. | Reactions to 43% of OIT, 9 % of SLIT. 5 OIT reactions required epinephrine. 1 OIT patient developed eosinophilic esophagitis and withdrew. |
| Syed et al. 201428 | Peanut | 43 | 5–45 years | RCT, open label (OIT/peanut avoidance). | 4000 mg | 20/23 OIT, 0 % (0/20) control desensitized (passed DBPCFC 4 g) at 24 months. | Sustained unresponsiveness: after off peanut OIT for 3 months, 30% (7/23) passed DBPCFC at 27 months. After off peanut for 6 months, 13% (3/7 SU, 3/23 ITT) remained tolerant (passed DBPCFC). | N/A |
| Tang et al. 201545 | Peanut and lactobacillus | 62 | 1–10 years | RCT, placebo controlled, double blind (Probiotic?+?OIT vs. placebo?+?placebo OIT). | 2000 mg | 90% probiotic?+?OIT, 7 % placebo desensitized (DBPCFC). | Sustained unresponsiveness: 82% probiotic, 4 % placebo (DBPCFC 2–5 weeks after probiotic discontinued). | 45% probiotic, 32% placebo patients had ?1 severe adverse event. 1 patient had anaphylaxis to probiotic. |
In milk and egg allergies, the introduction of baked milk or egg products in baked milk or baked egg-tolerant subjects respectively has revolutionized care. It is associated with high rates of resolution of allergy to unheated milk and egg.64 Paradoxically immunotherapy with baked milk in baked milk-reactive subjects has had dismal results due to high rates of reactions.65 Milk powder and fluid milk have been used for milk oral immunotherapy. (Table 1B) Fluid milk doses have varied from 100 to 200 ml. The limited number of trials and small populations preclude systematic analysis of the best strategy. Likewise, egg oral immunotherapy trials (Table 1C) have varied in dose used for desensitization and maintenance. Most clinical trials use dried or lyophilized egg white as agent for desensitization and challenge to one egg. However, egg clinical trials show the greatest variability in how maintenance dosing is conducted; using raw, undercooked or cooked egg; 1/3rd to a whole egg in quantity and daily to twice weekly dosing intervals. As with other foods, there is a paucity head to head comparisons. However, high rates of desensitization (80–94%) have been achieved in many trials.54,55,77,78,80 Only two clinical trials of wheat OIT Table 1D), with a total 24 subjects have been published. These serve as little more than proof of concept currently.
Table 1B.
Milk oral immunotherapy.
| Study | Food allergen | N | Age Range | Study design | Maintenance Dose | Desensitization Outcome | Long-term results | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Meglio et al. 200850 Meglio et al. 200466 | Fluid milk | 21 | 5–10 years | Open-label, not controlled. | Up to 200 mL milk or milk-containing foods | 71% (15/21) tolerated 200 mL, 14% (3/21) reached 40–80 mL. Mean duration 201 days (range 183–234 days). | After 48–51 months, 70% in follow-up (14/20) tolerating some milk. 43% (9/14) taking milk ad lib. | Desensitization: 3/21 (14%) had mild symptoms so discontinued; 3/21 (14%) had symptoms so took a lower daily dose. Follow-up: no reactions requiring epinephrine. |
| Pajno et al. 201367 | Fluid milk | 32 | 4–13 years | RCT, open-label, comparison of maintenance regimens. | 150–200 mL daily (group A) or twice weekly (group B) and milk ad lib | 100% in both groups continued maintenance, a similar frequency of allergic symptoms. | N/A | 8/15 in group A and 9/15 in group B had recorded events. |
| Salmivesi et al. 201368 Paassilta et al. 201669 |
Fluid milk | 24 | 6–14 years (from initial study 2013) | RCT, placebo control, double-blind, open-label crossover. | 200 mL (6400 mg) daily | 89% (16/18) OIT desensitized, not assessed in placebo. 100% (10/10) control patients desensitized in the crossover to open-label OIT. At 12 months, 13/18 (72%) of OIT taking 6400 mg CM daily. | At 3 years, 85% OIT (including original OIT and crossover) tolerating milk daily, 58% at 7 years. | Desensitization: 100% of OIT, 80% crossover, 63% of placebo patients reported symptoms. 6–12 months maintenance: 62% had symptoms, none severe; 50% at 3 years, 19% at 7 years. |
| Longo et al. 200852 | Fluid milk, then foods with milk. | 60 | 5–17 years | RCT, open-label, (OIT/milk-free diet). | 150 mL (4800 mg) then milk containing foods | At 1 year, 37% (11/30) OIT fully desensitized to 150 ml daily, 53% (16/30) OIT partially (5–15), 0 % control passed DBPCFC. | N/A | Desensitization: 4 patients required IM epinephrine. Home dosing: 2 needed epinephrine. 20% control had an adverse reaction to accidental milk. |
| Morisset et al. 200751 | Fluid milk. | 57 | 1–6 years | RCT, open-label (OIT/milk-free diet). | After 6 months, 11% OIT and 40% control had positive SBPCFC (< 200 mL). | N/A | Reactions in 3 OIT, 0 control patients. | |
| Martorell et al. 201170 | Fluid milk. | 60 | 2–3.5 years | RCT, open label. (OIT/milk-free diet). | 200 mL (6400 mg) daily | At 1 year, 90% milk OIT tolerant to 200 mL daily, 13% (3/23) of control passed DBPCFC. RR 7.7 for milk tolerance in OIT vs. placebo. | N/A | 80% OIT patients had ?1 reaction, all mild-moderate. Reactions with 15% (114/738) doses. |
| Skpirak et al. 200853 Narisety et al. 200971 Keet et al. 201372 | Milk powder. | 20 | 6–21 years | RCT, placebo controlled, double blind, then open-label crossover. (2:1 OIT/placebo) | 500 mg daily for 13 weeks, then 7000 mg (if pass 8g DBPFC). | 23% OIT, 0 % placebo, 67% crossover OIT passed DBPCFC (8000 mg); 92% active OIT, 0% placebo, 83% of cross-over OIT tolerant to ?2540 mg. | At 5 years: 19% of 13 in follow-up tolerating unlimited milk, 31% 1 serving/day, 34% limited amounts, 16% no milk/avoiding completely. | Desensitization: reactions to 45% of 2437 doses in OIT, 11% of 1193 in placebo patients. 4 patients required epinephrine. Follow-up open-label OIT: reactions in 17% of 2465 home doses. |
| Goldberg et al. 201565 | Baked milk. | 15 | 6–12 years | Open-label, uncontrolled. | Baked milk products daily as tolerated. | 21% tolerated 1.3 g baked milk OFC. Maximum tolerated dose 900 mg unheated milk. | N/A | 8/15 did not complete desensitization due to IgE-mediated reactions. 2 had anaphylaxis requiring epinephrine with home doses. |
Table 1C.
Egg oral immunotherapy.
| Study | Food allergen | N | Age Range | Study design | Maintenance Dose | Desensitization Outcome | Long-term results | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Staden et al. 200773 | Lyophilized egg white. | 45 (11 egg, 10 egg avoidance control, 24 other foods) | 0.6–12 years. | RCT, open-label (OIT/egg-free diet). | 1600 mg egg daily plus deliberate intake | 64% (16/25) fully or partially desensitized (milk and egg OIT assessed jointly), 35% of control later tolerant. | 36% of egg OIT had sustained tolerance (passed DBPCFC after stopping OIT for 2 months) vs. 35% of controls. | All OIT patients had some symptoms; 20% (4/20) of control had symptoms with accidental ingestion. |
| Buchanan et al. 200774 Burks et al. 200875 |
Dried egg white. | 21 (initially 7) | 1–16 years. | Open-label, no control. | 300 mg daily | 90% of 21 reached 300 mg daily, 57% of 7 initial patients passed DBPCFC for 8 g egg. | 26% of 7 had sustained unresponsiveness (pass DBPCFC after stopping OIT 3–4 months). | Up-dosing: 1/7 initial patients reacted. Maintenance: no reactions in 7/7 patients, 3 did not react to accidental egg exposure. |
| Vickery et al. 201076 | Dried egg white. | 8 | 3–13 years. | Open-label, no control (same protocol as,32 different patients). | 300 mg–3.6 g daily | 75% (6/8) OIT reached maintenance. After 4 months, 62.5 % (5/5 tested) passed OFC to 3.9 g egg, 1 not tested. After median 34 months, 75% (6/6 who reached maintenance) passed DBPCFC. | Sustained unresponsiveness: After 1 month off OIT, 75% (6/6) passed 2nd DBPCFC. All incorporated egg into the diet. | Buildup:4/6 who completed had reactions. Maintenance: no reactions. No reactions requiring epinephrine. |
| Burks et al. 201217 | Dried egg white. | 55 | 5–11 years. | RCT, placebo-controlled, double-blind (OIT/placebo). | 2 g daily (approx 1/3 egg) | At 10 months, 55% on OIT, 0% on placebo desensitized (OFC 5 g). At 22 months, 75% on OIT desensitized (OFC 10 g). | Sustained unresponsiveness: at 24 months, after off egg for 4–6 weeks, 27.5% OIT passed 10 g OFC. Not tested in placebo. | Desensitization: adverse events with 25% of 11,680 OIT doses, 4 % 4018 placebo doses. No adverse events in patients with SU consuming egg ad lib. |
| Fuentes-Aparicio et al. 2013 57 | Dried egg white. | 72 | 5–15 years. | RCT, open-label (OIT/egg-free diet). | 10 g daily | After 1 month: 92% (37/40) OIT, 22% (7/32) control desensitized to or tolerated egg. | 54% (20/37) OIT passed OFC to 10 g raw egg and introduced into the diet. | Desensitization: 21/37 had symptoms. 5 reactions required epinephrine. 1 patient withdrew with eosinophilic esophagitis. |
| Caminiti et al. 2015 55 | Dried egg white. | 31 | 4–10 years. | RCT, placebo control, double blind (OIT/placebo). | 1 cooked egg twice weekly | 94% (16/17) OIT, 0% control (0/14) desensitized (passed DBPCFC 4 g). | After 6 months of egg diet then 3 months avoidance, 29% (5/17) OIT, 7% (1/14) placebo had sustained tolerance (DBPCFC). | Desensitization: 3 patients had adverse events, 1 discontinued OIT. Egg containing diet phase: 2 patients had symptoms. |
| Escudero et al. 201554 | Dried egg white. | 61 | 5–17 years. | RCT, open label (OIT/egg avoidance). | 1 undercooked egg every 2 days and egg ad lib | 93% (28/30) on OIT desensitized. At 4 months, 37% OIT passed DBPCFC 2808 mg (11/25 challenged); 1% control passed DBPCFC (1/31 challenged). | After 1 month avoidance, 37% OIT, 3% control had sustained unresponsiveness (DBPCFC 2808 mg). | 145 reactions in OIT, 14% in dose escalation, 54% in buildup, 31% in maintenance. 70% of patients had ?1 reaction. 1 required epinephrine. |
| Ruiz-Garcia et al. 201277 | Dried egg white – OVODES NM kit. | 17 | 6–38 years. | Open-label, not controlled. | 3 eggs per week | 82% (14/17) reached maintenance dose and tolerated for 9 months. | N/A | 70.5% (12/17) symptomatic during OIT. 3 required epinephrine during up-dosing or at home. |
| Tortajada et al. 201278 | Raw e79gg. | 19 | 3–14 years. | Open-label, prospective, no control. | 0.5 egg twice weekly for several months, then 1 egg weekly. | 89% (17/19) desensitized to 30 g, no control. | N/A | 2/19 had anaphylactic reactions requiring epinephrine, discontinued OIT. |
| Ojeda et al. 201280 | Raw egg. | 31 | 6–15 years. | Open-label, controlled (not randomized). | 0.5 raw (pasteurized) egg 3 times weekly. | 81% (25/31) in OIT reached maximum dose, 74% passed OFC for 1 unpasteurized egg. 3% partially desensitized, 10% not desensitized but increased reactivity threshold. Control outcomes N/A. | N/A | 74% of OIT patients reacted. 180 adverse events, 86% were grades 1–2. 4 required epinephrine at home. |
| Letran et al. 201279 | Raw egg. | 17 | 4–14 years. | Open label, not controlled. | 1 egg omelet (or fraction tolerated) | 35% (6–17) fully desensitized. | N/A | Up-dosing: 4 reactions reported. Maintenance: 47% (8/17) had mild symptoms, 1 patient required emergency room visit. |
| Meglio et al. 201381 | Raw egg. | 20 | 4–14 years. | RCT, open label (OIT/egg-free diet) | Raw egg or food with eggs 3 times/week | 80% (8/10) OIT, 0% control tolerated 25 mL (13.6 g) daily; 10% OIT had partial tolerance. | N/A | 7/10 OIT had some symptoms during desensitization, 5 resolved, 1 tolerated limited amounts, 1 discontinued. |
| Dello Iacono et al. 201282 | Raw egg. | 20 | 5–11 years. | RCT, open label (OIT/egg free diet) | 10–40 mL egg daily. | After 6 months, 0% (0/10) in egg OIT desensitized to 1 raw egg (40 mL); 90% partially desensitized (tolerated 10–40 mL). 0% control passed DBPCFC. | N/A | 100% OIT patients had 53 total adverse events during desensitization. 24% were grades 1–2. None required epinephrine. 30% control patients had 5 total adverse reactions from accidental egg ingestion. |
| Vazquez-Ortiz et al. 201456 | Raw egg. | 82 | 5–18 years | Non-randomized, controlled, open label. (Parallel OIT/avoidance). | 1 egg twice weekly | Desensitization: 80% (40/50) OIT reached 2 eggs/week. | At 12 months of maintenance, 56% (28/50) OIT passed OFC for 1 raw egg, 8% (4/50) of OIT partially desensitized (tolerated 0.8–1.7 g). 16% (5/32) of control spontaneously developed tolerance at 12 months (passed DBPCFC). | Dose-related reactions in 45/50 (90%) and 7.6% (1024/13,551) of egg-OIT doses. 61% were mild (grades 1–2), 18 required epinephrine (in 13 patients). |
| Garcia-Rodriguez et al. 201183 | Raw and cooked egg. | 23 | 5–17 years. | Open-label, no control. | 1 cooked egg daily for 3 months, then every 2–3 days for 12 months. | 87% (20/23) OIT desensitized (tolerated 1 cooked egg), 61% (14/23) within 5 days. | 2 patients (9%) who failed initial desensitization switched to slow protocol, desensitized in 60–80 days. | 78% (18/23) had an allergic reaction during desensitization. Total reactions 55, 35 mild and 20 moderate. 2 patients had reactions during daily cooked egg maintenance. |
| Morisset et al. 200751 | Hard-boiled egg. | 84 | 1–8 years. | RCT (OIT/egg-free diet). (Both egg and milk in the same study). | Daily intake including creamy desserts and flan | 69% OIT (32/49), 51% (18/35) control patients desensitized and passed SBPCFC (7 g raw egg white). | N/A | N/A |
| Itoh et al. 201084 | Scrambled egg. | 6 | 7–12 years. | Open-label, no control. | 1 heated whole egg twice weekly | 100% desensitized to 1 scrambled egg (60 g), meantime 12 days, 50% also passed OFC 1 g powdered egg after 9–12 months maintenance. | 16–21 months after rush desensitization, all continuing to eat 1 heated egg twice weekly. | 100% had allergic symptoms during desensitization. None required epinephrine. |
| Leonard et al. 201285 | Baked egg. | 70 | 0.5–25 years. | Prospective, historical control. | 1–3 servings of baked egg product daily | 53% (42/70) tolerated partially cooked egg (OFC 6.5 g), 36% (28/70) did not but continued baked egg. | Retrospective: 28% (13/47) tolerant to partially cooked egg, 13% (6/47) to baked egg, 59% (28/47) avoidant. | No reactions to baked egg consumption, 1 patient reacted to accidental exposure to unbaked egg. |
Table 1D.
Wheat oral immunotherapy.
| Study | Food allergen | N | Age Range | Study design | Maintenance Dose | Desensitization Outcome | Long-term results | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Sato et al. 201560 | Wheat noodles. | 18 | 5–13 years. | Open-label, historical controls. | 5.2 g daily | 89% reached maintenance and tolerating 5.2 g daily. | After 2 years off OIT, 61% passed OFC, 9.1% in historical control. | Rush/dose escalation: 26% (42/143) doses resulted in symptoms; none required epinephrine. Maintenance: 6.8% (486/5778) doses with symptoms, 1 required epinephrine. |
| Rodriguez del Rio et al. 201459 | Wheat porridge or pasta. | 6 | 5–11 years | Open-label, no control. | 100 g daily | 83% (5/6) completed maintenance, 80% also tolerant of oat. | N/A | Up-dosing: 6 adverse reactions in 2 patients (6.25%, 6/96 doses). Maintenance: 1 patient had exercise-induced symptoms. |
Two related limitations in oral immunotherapy have been the need for indefinite daily maintenance dosing and low rates of “sustained unresponsiveness.” It is the maintenance of desensitization despite withholding the food. The rates across foods and studies have been low. Therefore, most subjects need to consume the food daily to maintain desensitization. A small trial with 17 subjects suggests that intermittent twice weekly dosing may be as effective as a daily dosing regimen after desensitization. The incidence of allergic reactions was the same.67 However, this is going to need further validation. Longer duration of therapy is associated with higher rates of sustained unresponsiveness.86 This would be particularly significant because many allergic subjects have an aversion to the food. Even in the absence of food aversion, daily dosing is onerous. Sustained unresponsiveness has been assessed after 3–6 weeks of withdrawal. We do not know what would happen if the subjects did not consume the food for more extended periods of time. The only way to assess sustained unresponsiveness is the withdrawal of the food and challenge which carries the risk of anaphylaxis. There is a desperate need for a surrogate marker. IgG4 levels and BAT responses are potential markers but will need extensive validation for each food.86
Determinants of successful desensitization and sustained unresponsiveness are not known. A long-term study suggests that the starting specific IgE level and peak IgE level during desensitization may determine the long-term sustained unresponsiveness.33 Trials of very young children with peanut and milk allergies has demonstrated that in this age group OIT was associated with higher rates of desensitization and was associated with lower rates of reactions.51,87 When coupled with studies of the primary and secondary prevention of peanut allergy by the early introduction of peanut88 it suggests that there may be a continuum of immunological plasticity, which applies to peanut allergy and possibly other foods. This degree of plasticity might decrease with age resulting in lower desensitization rates with age. Studies are needed to determine the generalizability to other foods and different forms of immunotherapy.
Pollen food allergy syndrome
Pollen-food allergy syndrome (PFAS) is caused by anti-pollen antibodies cross-reacting with antigenically similar protein in several plant-based foods principally, fruits, vegetables, and nuts. It is unique because both the inciting pollen and the secondary food are targets for immunotherapy. The most commonly studied is the birch-apple allergy caused by cross-reactivity between, the PR10 family proteins, Bet v1 in birch and Mal d 1 in apple. Seven studies have investigated three modalities of therapy, subcutaneous immunotherapy using birch pollen, SLIT using birch pollen and OIT using raw apple. Fundamental limitations in these studies include the lack of uniformity in intervention, the lack of randomization and placebo controls and the frequent use of open challenges with apple. These studies have varied in outcomes. (see Table 2) Unlike prior studies, Hansen et al. were the first to perform a double-blind, double-dummy placebo-controlled study.93 However, this study ultimately suffered from a small number of subjects who had challenge confirmed PFAS in each group and threshold-dose insensitive challenge protocol.93 Likewise, the only study using apple DBPFC for diagnosis did not use a graded challenge but instead relied on a VAS score for symptoms with a fixed dose of apple. This method likely impaired their ability to measure changes in the degree of apple sensitivity as determined by threshold dose for eliciting symptoms.94
Table 2.
Therapy for pollen food syndrome.
| Author/Year of publication | Food | Pollen | Route | Intervention | Age (years) | N | Outcome | Limitations |
|---|---|---|---|---|---|---|---|---|
| Moller C 1989 89 | Apple | Birch | SCIT | SCIT (Birch Pollen) vs OIT (Birch pollen) | 21–47 | 15 | neither intervention improved food sensitivity significantly | |
| Herrmann D et al. 199590 | Apple | Birch | SCIT | Observational study | Adults | 20 | improvement in 56% of patients | not a controlled study |
| Asero et al. 199891 | Apple | Birch | SCIT | SCIT (aluminum hydroxide-adsorbed birch pollen extracts) vs. no treatment | Mean age 34.4 years | SCIT: 49 Controls 22 | treatment group 45% complete resolution, 39% partial reduction, 16% unchanged control group 0% unchanged | No information about food challenge methodology, age or gender of subject allocation |
| Bucher et al 200492 | Apple/Hazelnut | Birch | SCIT | SCIT (birch-hazel-alder± ash pollen extract) vs no treatment | SCIT: 15 Controls: 12 | 87% of the treatment group and 8% of control group improved tolerance after treatment | no sham group; no randomization | |
| Hansen et al. 200493 | Apple | Birch | SCIT & SLIT | Double-blind, double-dummy placebo-controlled Birch pollen SCIT and Birch pollen SLIT | mean 32 | M: 42, F: 32. Actual number of challenge confirmed PFAS subjects SCIT 10, SLIT 4, Placebo 10 | No significant change in number of subjects who passed a food challenge | a small number of subjects for SLIT, Two-step challenge 10g of apple and whole apple not sensitive for detecting changes in tolerated dose. |
| Kinaciyan et al. 200794 | Apple | Birch | SLIT | Birch pollen SLIT | 21–47 mean 33.2 | M: 5 F:15 | No significant change in DBPFC (VAS) score | DBPFC used VAS to fixed dose not threshold dose of reactivity |
| Mauro et al 201195 | Apple | Birch | SCIT & SLIT | Birch pollen SCIT and SLIT | 18–60 mean 37.8 | SLIT (M: 11,F: 9) SCIT (M: 10, F: 10) | 25% of SCIT and 14.2% of SLIT complete tolerance, 37.5% of SCIT and 28.6% of SLIT developed increase in the provocative dose | No Placebo group |
| Kopac et al 201296 | Apple | Birch | OIT | Apple OIT | 18–61 | OIT (M: 9, F:18); Control (M: 3, F: 10) | 17/27 subjects in treatment and 0/13 subjects in control achieved desensitization | Open challenges |
| Kinaciyan et al. 2017 97 | Apple | Birch | SLIT | Bet v1 SLIT, Mal d1 SLIT or Placebo | 18–65 | Bet v1 SLIT: 20, Mal d1 SLIT: 20 and Placebo: 20 | Mal d1 SLIT performed significantly better than Bet v1 SLIT or placebo in Mal d 1 sublingual challenge | No Apple challenge |
SCIT – Subcutaneous Immunotherapy, SLIT – Sublingual Immunotherapy, OIT – Oral Immunotherapy, Bet v1 SLIT – SLIT using the recombinant birch pollen protein Bet v1, Mal d1 SLIT – SLIT using the recombinant apple protein Mal d1
The lack of improvement in apple PFAS with Birch pollen SLIT is explained by lack change IgE, IgG4 or T-cell responses with the intervention despite concomitant amelioration in Bet v1 specific responses.94 However, a recent study by the same group using Mal d1 SLIT demonstrated significant improvement in Mal d1-specific sublingual challenge, IgE, and IgG4 responses.93 OIT with apple and Mal d1 SLIT suggest that though the pathogenesis of birch-apple PFAS involves birch pollen sensitization desensitization with apple rather than Birch may be a superior approach to therapy.
Epicutaneous immunotherapy
Epicutaneous immunotherapy involves transdermal administration of allergen under an occlusive dressing that promotes allergen absorption. Three allergens, grass pollen,98 milk99 and peanut14,100 have been studied using this modality. Skin preparation before application of the patch has also varied – no preparation, abrasion with a foot file and tape stripping. In a head-to-head comparison of the two skin prep methods, the more aggressive abrasion method was associated with a higher risk of systemic allergic reactions.101 Mouse studies have demonstrated the uptake of allergen by dendritic cells after prolonged application and resulting immunological changes.102 Trials involving milk and peanut without skin preparation, use a different proprietary patch, precluding direct comparison, however, the rates of systemic reactions were low.100 Comparison of different protein doses in patches shows a dose effect100 Local reactions, most commonly patch site pruritus, was virtually universal.99–101,103 All trials compare efficacy compared to placebo. There are no trials comparing grass pollen EPIT to SCIT or SLIT. Similarly, there no trials comparing milk or peanut EPIT to SLIT or OIT. However, comparison of completed studies shows a clear distinction in the rates of systemic adverse effects favoring EPIT. Peanut EPIT resulted in desensitization in 48% subjects (passing a 1000mg of peanut food challenge or 10-fold increase in maximum tolerated dose). It was more efficacious in 4–11 age group than in older subjects.100 In comparison, the largest peanut OIT trial which used similar entry criteria resulted in desensitization in 62% of subjects (passing a 1400mg challenge).44 Excluding subjects on EPIT who did not pass a 1000mg challenge but did have tenfold increase in threshold further widens the gap between OIT and EPIT, suggesting OIT is likely more efficacious than EPIT.44 Like OIT, EPIT outcomes appear to be better in younger subjects.87
Intralymphatic immunotherapy
Intralymphatic immunotherapy (ILIT) is a new modality of injectable allergen immunotherapy. It involves the repeated injection of allergen directly into the lymph node. The dose of allergen is lower than in subcutaneous therapy.104 It involves fewer injections and has fewer side effects.104 Injections were performed under ultrasound guidance.104 Tolerance achieved was long lasting and equivalent to SCIT.104 It has been used for Grass pollen,104 Birch pollen,105,106 Cat,103,107,108 Dog,107,108 Dust mite.107,108 (Key studies are summarized in Table 3) However, unlike SCIT expertise and studies are confined to a limited number of centers. Though the results are encouraging and carry the promise of improved compliance, studies are limited and without replication. Another limitation is that the studies are randomized but open-label. Ideally, SCIT and ILIT comparison studies should be double-blind double-dummy. However, this may not be ethically feasible. The methodology and optimal injection schedule are open to debate.109 At this time four clinical trials of ILIT are recruiting or active. (ClinicalTrials.gov)
Table 3.
Table intralymphatic immunotherapy.
| Author/Year | Disease | Allergen | Intervention | Age (years) | Number of Patients | Outcome |
|---|---|---|---|---|---|---|
| Hylander et al. 2016106 | Allergic Rhinitis | Birch or grass pollen | Double Blind Randomized Placebo Control | 20–54 | 21 (8 females) (15 controls) | Overall met the primary endpoint of symptom reduction by VAS. |
| Senti et al. 2008104 | Allergic Rhinitis | Grass pollen | ILT vs. SCIT | 32 ± 8.7 years | 58 ILT (20 females)54 SCIT | ILT was safe, effective, induced tolerance faster and tolerance was durable |
| Senti et al. 2012103 | Allergic Rhinitis | Cat (MAT-Fel d 1) | Randomized Placebo controlled | 34.6 ± 11.9 | 12 (8 Females) 8 Placebo | Met the primary end point of increased nasal tolerance |
| Lee et al. 2015104 | Allergic Rhinitis | Dust mite cat, dog | Dust mite, cat and dog ILT | Not reported | 10 | ILT can rapidly improve rhinitis symptoms, however severe systemic reactions (2) and severe local reaction (1) occurred. |
Combination immunotherapy with biologics
Anti-IgE therapy has found application in settings where there are high rates of reactions to allergen immunotherapy – food OIT, aeroallergen rush immunotherapy, and venom immunotherapy. Compared to OIT alone, the addition of Omalizumab significantly reduces the risk of mild and severe reactions.49,110 (See Table 4) Anti-IgE monotherapy with a different biologic (TNX-901) suggests that it increases the threshold of reactivity to the food allergen.116 The combination with OIT appears to confirm this benefit, facilitating faster desensitization using rush protocols with low rates of adverse reactions.49,111,112,114 Carefully designed placebo-controlled studies suggest that it does not improve the overall rate of desensitization over OIT alone. A case series suggested that it enables desensitization in subjects who have previously failed immunotherapy with conventional protocols.115 However, another small case series opposes this. Here patients refractory to OIT were successfully desensitized on Omalizumab but reverted to allergy after discontinuing anti-IgE therapy.113 Mechanistic studies confirm a transient decrease in basophil reactivity but no change in the number of Tregs.34 Such findings are mirrored in aeroallergen SCIT where Omalizumab has been shown to decrease the adverse effect and anaphylaxis related to ragweed rush immunotherapy;117 have a synergistic effect in decreasing seasonal allergic rhinitis symptoms,118 and improve the safety and efficacy of SCIT in allergic asthma.119–121 Newer biologics such as Dupilumab (Anti-IL4 Receptor) that have a more direct effect on atopy might have a greater effect on T-cells improving rates of desensitization and are the subjects of ongoing studies.
Table 4.
Oral immunotherapy combined with Anti-IgE therapy.
| Study | Food | N | Age | Study Design | Duration of Omalizumab | Target Maintenance Dose | Adverse effects | Desensitization |
|---|---|---|---|---|---|---|---|---|
| Nadeau et al. 2011111 | Milk | 11 | 7–17 years | Open-label Single arm, Rush OIT, Total duration of OIT 24 weeks | 16 wk (Pre OIT 8 wks) | 2000 mg of Milk protein | Total 1.8% (41/2301 doses) Severe 0.1% Epi 3/2301 | Desensitization: 82% (9/11) achieved the primary objective of desensitization to a daily dose of 2000 mg milk |
| Wood et al. 2016110** | Milk | 57 (O 28, P 29) | 7–32 years | Randomized double-blind placebo controlled for Omalizumab. Unblinded milk administration, dose escalation 22–40 weeks, Duration of OIT 28 months | 28 months (Pre-OIT 4 months) | 3800 mg of Milk Protein | Total during escalation O: 8.5% (442/5226doses) P: 26.1% (1634/6252 doses) Maintenance O: 0.7% (110/15418 doses) P: 14.4% (1983/13745 doses) Epi O: 2 subjects (2 doses) P: 9 subjects (18 doses) | No significant difference in desensitization Omalizumab (24/27; 88.9%) and Placebo (20/28; 71.4%) (P = .18) |
| Schneider et al. 2013112 | Peanut | 13 | 7–15 years | Open-label Single arm, Rush escalation, Duration of OIT: from 12 weeks to 30–32 week | 20 weeks (Pre-OIT 12 wks) | 4000 mg of peanut protein | Total 2% (72/3502) Epi 3 subjects 5 doses | Desensitization: 92% (12/13) tolerated oral food challenge with 8000 mg peanut flour (about 20 peanuts) |
| MacGinnitie et al. 201749 | Peanut | 37 (O 29, P 8) | 6–19 years | Randomized double-blind placebo-controlled for Omalizumab. Unblinded peanut administration, Open-Label Omalizumab (2 active, 6 control) who failed to reach 250mg of peanut by week 8 | 19 weeks (Pre-OIT 12 wks) | 2000 mg of peanut protein | Epi – 8 subjects (14 reactions) P: 2 subjects (3 reactions), O: 3 (4 reactions), Open-label 7 doses. EoE 8% (3/37) (2 in the active group and 1 in control) | Desensitization to 2000 mg of peanut protein 6 wk after withdrawal of omalizumab. Omalizumab 79% (23/29) Placebo 12.5% (1/8) p < 0.01 |
| Lafuente et al. 2014113 | Egg | 3 | 9–10 years | Case series in OIT failures | 4–7 months (Pre-OIT 8–12 wks) | 1 egg 3 times a week | 3/3 patients had recurrence of symptoms 3–4 mo after omalizumab as discontinued | Desensitization to 50 ml egg white: 100% (3/3) |
| Begin et al. 2014114 | Multiple | 25 | 4–15 years | Open-label single-arm Phase 1. Up to 5 allergens; rush escalation | 16 wk (Pre OIT 8 wks) | 4000 mg of protein for each food | rush phase 52% of subjects; escalation 5.7% (13/227 doses) Maintenance 5.3% (401/7530 doses) Epi 1 dose | 76% (19/25) tolerated all 6 steps of the initial escalation day (up to 1250 mg of combined food proteins), requiring minimal or no rescue therapy. All subjects achieved 4000mg/food by 9 months |
| Martorell-Calatayud et al. 2016115 | Milk, Egg | N = 14 (Egg 9, Milk 5) | 3–13 years | Case series Open-label single arm OIT in patients who failed conventional OIT | Variable till 2 months after maintenance dose is achieved (Pre-OIT 9 wks) | Milk: 200ml (6600 mg Milk protein), Egg white 17 ml (1800mg egg protein) | Rush phase: 28% (4/14) Late maintenance (2.5–4 months after discontinuation of omalizumab): 42% (6/14) | Desensitization: 100% after end of induction phase (egg and milk) |
Key: O – Omalizumab, P – Placebo, OIT – Oral Immunotherapy, Epi – Epinephrine administration, wk – weeks.
**Key study.
Encapsulated allergen
Encapsulation of allergen is a new concept in the allergy world but has been studied and used extensively in pharmacology. Nanoparticles or microparticles can be used to enclose allergens. These can then be injected or ingested like other forms of AIT. The chief advantage of encapsulating allergen is that it can help potentially shield the allergen from mast cells. Other benefits may include reduced allergen dose, co-encapsulation of adjuvants, targeting and improved uptake. Encapsulating agents include Liposomes, Virus-Like particles, natural polymers (Chitosan, Dextran)122 and synthetic polymers (poly (lactic acid), poly (lactic-co-glycolic acid) (PLGA))
A randomized placebo-controlled trial of Liposome encapsulated dust mite extract was noted to be safe and effective in 55 asthmatics.123 Over 12 months of therapy, medication scores were reduced and healthy days increased. The study documented reduced allergen-specific responses in skin tests and bronchial challenges. The study did not include extensive mechanistic investigations. It did not compare responses (or adverse effects) against conventional subcutaneous immunotherapy.123
In an older study, which aggregated four small studies assessing the cutaneous tolerance of the allergens, the safety of empty and allergen filled liposomes and lastly the safety and efficacy of liposomal therapy. The study concluded that systemic safety was poor.124 However, their sample sizes were limited (12 patients in 4 treatment arms). The same trial noted reduced local reaction upon subcutaneous injection. Interestingly, alpha-tocopherol used as an antioxidant in the production of these liposomes caused contact dermatitis in two patients. Despite the promise of lower rates of allergic reactions and extensive development of liposome-based therapies in other fields such as oncology there have been no new trials in this field. It is not clear if this is due to unpublished negative data, lack of funding or lack of interest.
The use of TLR ligands
Besides liposomes, studies have used virus-like particles for allergic rhinitis.1,125,126 VLP with CpG, a TLR9 ligand has been used with125 and without allergen immunotherapy. In a dose-ranging study of 299 subjects, subjects treated with the highest dose a VLP containing CpG, without an allergen, had a significantly reduced combined symptom and medication score.126 In a subsequent study, 20 patients underwent open-label therapy with the CpG containing VLP and conventional house dust mite extract in increasing doses over ten weeks. Conjunctival provocation eliciting dose and protective changes IgE and IgG levels improved.125 In this study House dust mite was adsorbed on alum which is a confounder. A 2006 study that utilized ragweed conjugated to CpG (without Alum) showed similar promise with significant improvement in symptom scores over two seasons following a single treatment. However, a follow-up phase 2 study for Amb a 1 CpG conjugate was prematurely withdrawn due to no meaningful effect in the first ragweed season (ClincalTrials.gov NCT00387738). The same company has since launched a clinical trial using a proprietary TLR9 agonist for eosinophilic asthma without a conjugated allergen (ClincalTrials.gov NCT02898662)
Several animal studies have explored various biodegradable, and non-biodegradable substrates have been studies in animal models. These studies are summarized by Pohlit et al.127
Conclusion
Immunotherapy of allergic disorders is entering a new phase. New therapies are available, particularly for food allergies. There is an improved understanding of the immunological changes that occur during immunotherapy. This has led to the development of new formulations and methods of administering immunotherapy. While local effects on the immune milieu occur with sublingual, oral and epicutaneous immunotherapy, the core immunological changes in mast cells, antibody production, and T-cell changes follow a similar pattern. Our understanding of immunology has led to the investigation of adjuvants such as CpG to improve efficacy, anti-IgE therapy to reduce adverse effects, and encapsulating-agents that do both.
The route of allergen administration influences efficacy and adverse effects. As illustrated in the treatment of food allergies there are tradeoffs between efficacy and adverse effects. Further studies would be needed to determine if therapy could be started with a low adverse effect modality such as EPIT, and then transitioned to OIT.
Looking ahead, one of the significant challenges confronting us is the patchwork of studies, without uniform methodologies, limited comparability and a shortage of well-designed head-to-head comparisons of different treatment modalities. The multitude of allergens further exacerbates this problem. As an example, all the studies on pollen-food allergy syndrome use one of two pollen-food combinations, birch-apple or birch-hazelnut. The question remains – are the results of birch-apple generalizable to all pollen-food combinations, or even to all birch related foods?
The other major challenge is the long duration of therapy. SCIT, SLIT, OIT, and EPIT are all associated with treatments that last months to years, and for food allergies may even be life-long. Intralymphatic therapy promises to shorten the duration of therapy, but the relevant studies are limited to a handful of aeroallergens.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, Zaman H, Agarwal A, Netuveli G, Roberts G, Pfaar O, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72(11):1597–1631. doi: 10.1111/all.13201. [DOI] [PubMed] [Google Scholar]
- 2.Turkalj M, Banic I, Anzic SA.. A review of clinical efficacy, safety, new developments and adherence to allergen-specific immunotherapy in patients with allergic rhinitis caused by allergy to ragweed pollen (Ambrosia artemisiifolia). Patient Prefer Adherence. 2017;11:247–257. doi: 10.2147/PPA.S70411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1, Supplement):S1–S55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Fass PT. American academy of otolaryngic allergy endorses the allergen immunotherapy practice parameter. J Allergy Clin Immunol. 2008;121(1):269–70; author reply 270. doi: 10.1016/j.jaci.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Golden DB, Demain J, Freeman T, Graft D, Tankersley M, Tracy J, Blessing-Moore J, Bernstein D, Dinakar C, Greenhawt M, et al. Stinging insect hypersensitivity: A practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118(1):28–54. doi: 10.1016/j.anai.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Golden DB, Moffitt J, Nicklas RA, Freeman T, Graft DF, Reisman RE, Tracy JM, Bernstein D, Blessing-Moore J, Cox L, et al. Stinging insect hypersensitivity: a practice parameter update 2011. J Allergy Clin Immunol. 2011;127(4):852–4 e1-23. doi: 10.1016/j.jaci.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Joint Task Force on Practice, P., et al. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120(3 Suppl):S25–85. doi: 10.1016/j.jaci.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Cox LS, Hankin C, Lockey R. Allergy immunotherapy adherence and delivery route: location does not matter. J Allergy Clin Immunol Pract. 2014;2(2):156–160. doi: 10.1016/j.jaip.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90(2):256–262. [DOI] [PubMed] [Google Scholar]
- 10.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99(6 Pt 1):744–751. [DOI] [PubMed] [Google Scholar]
- 11.Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140(6):1485–1498. doi: 10.1016/j.jaci.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Soyer OU, Akdis M, Akdis CA. Mechanisms of subcutaneous allergen immunotherapy. Immunol Allergy Clin North Am. 2011;31(2):175–90, vii–viii. doi: 10.1016/j.iac.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Berin MC, Shreffler WG. Mechanisms underlying induction of tolerance to foods. Immunol Allergy Clin North Am. 2016;36(1):87–102. doi: 10.1016/j.iac.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Jones SM, Sicherer SH, Burks AW, Leung DYM, Lindblad RW, Dawson P, Henning AK, Berin MC, Chiang D, Vickery BP, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol. 2017;139(4):1242–1252 e9. doi: 10.1016/j.jaci.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka T, Rios EJ, Tsai M, Kalesnikoff J, Galli SJ. Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells. J Allergy Clin Immunol. 2013;132(4):922–932.e16. doi: 10.1016/j.jaci.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang WXG, Church AM, Kulis M, Choi HW, Burks AW, Abraham SN. Mast cell desensitization inhibits calcium flux and aberrantly remodels actin. J Clin Invest. 2016;126(11):4103–4118. doi: 10.1172/JCI87492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, Stablein D, Henning AK, Vickery BP, Liu AH, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367(3):233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burks AW, Wood RA, Jones SM, Sicherer SH, Fleischer DM, Scurlock AM, Vickery BP, Liu AH, Henning AK, Lindblad R, et al. Sublingual immunotherapy for peanut allergy: long-term follow-up of a randomized multicenter trial. J Allergy Clin Immunol. 2015;135(5):1240–8 e1-3. doi: 10.1016/j.jaci.2014.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swamy RS, Reshamwala N, Hunter T, Vissamsetti S, Santos CB, Baroody FM, Hwang PH, Hoyte EG, Garcia MA, Nadeau KC. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol. 2012;130(1):215–24 e7. doi: 10.1016/j.jaci.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caruso M, Cibella F, Emma R, Campagna D, Tringali G, Amaradio MD, Polosa R. Basophil biomarkers as useful predictors for sublingual immunotherapy in allergic rhinitis. Int Immunopharmacol. 2018;60:50–58. doi: 10.1016/j.intimp.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Devillier P, Montagut A, Mélac M, Galvain S, Jean-Alphonse S, Van Overtvelt L, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009;124(3):471–7, 477 e1. doi: 10.1016/j.jaci.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Patil SU, Shreffler WG. Immunology in the Clinic Review Series; focus on allergies: basophils as biomarkers for assessing immune modulation. Clin Exp Immunol. 2012;167(1):59–66. doi: 10.1111/j.1365-2249.2011.04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123(4):735–46; quiz 747–8. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Aslam A, Chan H, Warrell DA, Misbah S, Ogg GS, Unutmaz D. Tracking antigen-specific T-cells during clinical tolerance induction in humans. PLoS One. 2010;5(6):e11028. doi: 10.1371/journal.pone.0011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121(6):1467–72, 1472 e1. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Tsai YG, Chiou Y-L, Chien J-W, Wu H-P, Lin C-Y. Induction of IL-10+ CD4+ CD25+ regulatory T cells with decreased NF-kappaB expression during immunotherapy. Pediatr Allergy Immunol. 2010;21(1 Pt 2):e166–73. doi: 10.1111/j.1399-3038.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 27.Tsai YG, Yang KD, Niu D-M, Chien J-W, Lin C-Y. TLR2 agonists enhance CD8+Foxp3+ regulatory T cells and suppress Th2 immune responses during allergen immunotherapy. J Immunol. 2010;184(12):7229–7237. doi: 10.4049/jimmunol.1000083. [DOI] [PubMed] [Google Scholar]
- 28.Syed A, Garcia MA, Lyu S-C, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O’Riordan G, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol. 2014;133(2):500–510. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Yang IV, Davidson EJ, Joetham A, Takeda K, O'Connor BP, Gelfand EW. Forkhead box protein 3 demethylation is associated with tolerance induction in peanut-induced intestinal allergy. J Allergy Clin Immunol. 2017;141(2):659–670.e2. doi: 10.1016/j.jaci.2017.04.020. Epub 2017 May 4. PubMed PMID: 28479331" [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van De Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, Rückert B, Akdis CA, Akdis M. IgG4 production is confined to human IL-10–producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131(4):1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Norman PS, Lichtenstein LM, Marsh DG. Studies on allergoids from naturally occurring allergens IV. Efficacy and safety of long-term allergoid treatment of ragweed hay fever. J Allergy Clin Immunol. 1981;68(6):460–470. [DOI] [PubMed] [Google Scholar]
- 32.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, Wood RA. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol. 2015;135(5):1275–82.e1–6. doi: 10.1016/j.jaci.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, Burk C, Hiegel A, Carlisle S, Christie L, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frischmeyer-Guerrerio PA, Masilamani M, Gu W, Brittain E, Wood R, Kim J, Nadeau K, Jarvinen KM, Grishin A, Lindblad R, et al. Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2017;140(4):1043. doi: 10.1016/j.jaci.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales M, Gallego M, Iraola V, Taulés M, De Oliveira E, Moya R, Carnés J. In vitro evidence of efficacy and safety of a polymerized cat dander extract for allergen immunotherapy. BMC Immunol. 2017;18(1):10. doi: 10.1186/s12865-017-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallner M, Pichler U, Ferreira F. Recombinant allergens for pollen immunotherapy. Immunotherapy. 2013;5(12):1323–1338. doi: 10.2217/imt.13.114. [DOI] [PubMed] [Google Scholar]
- 37.Lent AM, Harbeck R, Strand M, Sills M, Schmidt K, Efaw B, Lebo T, Nelson HS. Immunologic response to administration of standardized dog allergen extract at differing doses. J Allergy Clin Immunol. 2006;118(6):1249–1256. doi: 10.1016/j.jaci.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 38.Verhoef A, Alexander C, Kay AB, Larché M, Platts-Mills T. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. Plos Medicine. 2005;2(3):253–261. doi: 10.1371/journal.pmed.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WLG, Stern LJ, Grönlund H, Van Hage M, Reynolds CJ, Boyton RJ, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J Exp Med. 2009;206(7):1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cingi C, Muluk NB, Hanci D, Ulusoy S, Sahin F. Updating the role played by immunotherapy for allergic rhinitis: meta-analysis. Int Forum Allergy Rhinol. 2015;5(2):132–142. doi: 10.1002/alr.2015.5.issue-2. [DOI] [PubMed] [Google Scholar]
- 41.Abramson M, Puy R, Weiner J. Immunotherapy in asthma: an updated systematic review. Allergy. 1999;54(10):1022–1041. [DOI] [PubMed] [Google Scholar]
- 42.Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Evid Based Child Health. 2010;5(3):1279–1379. PubMed PMID: 17253469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenhawt M, Oppenheimer J, Nelson M, Nelson H, Lockey R, Lieberman P, Nowak-Wegrzyn A, Peters A, Collins C, Bernstein DI, et al. Sublingual immunotherapy: A focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118(3):276–282 e2. doi: 10.1016/j.anai.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, Palmer C, Deighton J, Ewan P, Clark A. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297–1304. doi: 10.1016/S0140-6736(13)62301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang ML, Ponsonby A-L, Orsini F, Tey D, Robinson M, Su EL, Licciardi P, Burks W, Donath S. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J Allergy Clin Immunol. 2015;135(3):737–44.e8. doi: 10.1016/j.jaci.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 46.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, Shreffler WG, Steele P, Henry KA, Adair M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300, 300 e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, De Oliveira LCL, Shreffler WG, Sampson HA, Niggemann B, Wahn U, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126(1):83–91 e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 48.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, Hiegel A, Kamilaris J, Carlisle S, Yue X, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, Heimall J, Makhija M, Robison R, Chinthrajah RS, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. 2017;139(3):873–881.e8. doi: 10.1016/j.jaci.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meglio P, Giampietro PG, Gianni S, Galli E. Oral desensitization in children with immunoglobulin E-mediated cow’s milk allergy–follow-up at 4 yr and 8 months. Pediatr Allergy Immunol. 2008;19(5):412–419. doi: 10.1111/j.1399-3038.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 51.Morisset M, Moneret-Vautrin DA, Guenard L, Cuny JM, Frentz P, Hatahet R, Hanss C, Beaudouin E, Petit N, Kanny G. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow’s milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol. 2007;39(1):12–19. [PubMed] [Google Scholar]
- 52.Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, Ventura A. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol. 2008;121(2):343–347. doi: 10.1016/j.jaci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 53.Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, Matsui EC, Burks AW, Wood RA. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122(6):1154–1160. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escudero C, Rodríguez Del Río P, Sánchez-García S, Pérez-Rangel I, Pérez-Farinós N, García-Fernández C, Ibáñez MD. Early sustained unresponsiveness after short-course egg oral immunotherapy: a randomized controlled study in egg-allergic children. Clin Exp Allergy. 2015;45(12):1833–1843. doi: 10.1111/cea.12604. [DOI] [PubMed] [Google Scholar]
- 55.Caminiti L, Pajno GB, Crisafulli G, Chiera F, Collura M, Panasci G, Ruggeri P, Guglielmo F, Passalacqua G. Oral Immunotherapy for Egg Allergy: A Double-Blind Placebo-Controlled Study, with Postdesensitization Follow-Up. J Allergy Clin Immunol Pract. 2015;3(4):532–539. doi: 10.1016/j.jaip.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Vazquez-Ortiz M, Alvaro M, Piquer M, Dominguez O, Machinena A, Martín-Mateos MA, Plaza AM. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg-allergic children. Clin Exp Allergy. 2014;44(1):130–141. doi: 10.1111/cea.12233. [DOI] [PubMed] [Google Scholar]
- 57.Fuentes-Aparicio V, Alvarez-Perea A, Infante S, Zapatero L, D’Oleo A, Alonso-Lebrero E. Specific oral tolerance induction in paediatric patients with persistent egg allergy. Allergol Immunopathol (Madr). 2013;41(3):143–150. doi: 10.1016/j.aller.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Dello Iacono I, Tripodi S, Calvani M, Panetta V, Verga MC, Miceli Sopo S. Specific oral tolerance induction with raw hen’s egg in children with very severe egg allergy: a randomized controlled trial. Pediatr Allergy Immunol. 2013;24(1):66–74. doi: 10.1111/j.1399-3038.2012.01349.x. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez Del Rio P, Díaz-Perales A, Sanchez-García S, Escudero C, Do Santos P, Catarino M, Ibañez MD. Oral immunotherapy in children with IgE-mediated wheat allergy: outcome and molecular changes. J Investig Allergol Clin Immunol. 2014;24(4):240–248. [PubMed] [Google Scholar]
- 60.Sato S, Utsunomiya T, Imai T, Yanagida N, Asaumi T, Ogura K, Koike Y, Hayashi N, Okada Y, Shukuya A, et al. Wheat oral immunotherapy for wheat-induced anaphylaxis. J Allergy Clin Immunol. 2015;136(4):1131–3.e7. doi: 10.1016/j.jaci.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 61.Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, Rudman Spergel A, Desai M, Galli SJ, Nadeau KC, Chinthrajah RS. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2018. Feb;3(2):85–94. doi: 10.1016/S2468-1253(17)30392-8. Epub 2017 Dec 12. PubMed PMID: 29242014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, Kamilaris J, Burks AW. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009;124(2):286–91, 291 e1–6. doi: 10.1016/j.jaci.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anagnostou K, Clark A. Peanut immunotherapy. Clin Transl Allergy. 2014;4:30. doi: 10.1186/2045-7022-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonard SA. Baked egg and milk exposure as immunotherapy in food allergy. Curr Allergy Asthma Rep. 2016;16(4):32. doi: 10.1007/s11882-016-0604-y. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg MR, Nachshon L, Appel MY, Elizur A, Levy MB, Eisenberg E, Sampson HA, Katz Y. Efficacy of baked milk oral immunotherapy in baked milk-reactive allergic patients. J Allergy Clin Immunol. 2015;136(6):1601–1606. doi: 10.1016/j.jaci.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 66.Meglio P, Bartone E, Plantamura M, Arabito E, Giampietro PG. A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy. 2004;59(9):980–987. doi: 10.1111/j.1398-9995.2004.00542.x. [DOI] [PubMed] [Google Scholar]
- 67.Pajno GB, Caminiti L, Salzano G, Crisafulli G, Aversa T, Messina MF, Wasniewska M, Passalacqua G. Comparison between two maintenance feeding regimens after successful cow’s milk oral desensitization. Pediatr Allergy Immunol. 2013;24(4):376–381. doi: 10.1111/pai.12077. [DOI] [PubMed] [Google Scholar]
- 68.Salmivesi S, Korppi M, Mäkelä MJ, Paassilta M. Milk oral immunotherapy is effective in school-aged children. Acta Paediatr. 2013;102(2):172–176. doi: 10.1111/j.1651-2227.2012.02815.x. [DOI] [PubMed] [Google Scholar]
- 69.Paassilta M, Salmivesi S, Mäki T, Helminen M, Korppi M. Children who were treated with oral immunotherapy for cows’ milk allergy showed long-term desensitisation seven years later. Acta Paediatr. 2016;105(2):215–219. doi: 10.1111/apa.13251. [DOI] [PubMed] [Google Scholar]
- 70.Martorell A, De La Hoz B, Ibáñez MD, Bone J, Terrados MS, Michavila A, Plaza AM, Alonso E, Garde J, Nevot S, et al. Oral desensitization as a useful treatment in 2-year-old children with cow’s milk allergy. Clin Exp Allergy. 2011;41(9):1297–1304. doi: 10.1111/j.1365-2222.2011.03749.x. [DOI] [PubMed] [Google Scholar]
- 71.Narisety SD, Skripak JM, Steele P, Hamilton RG, Matsui EC, Burks AW, Wood RA. Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2009;124(3):610–612. doi: 10.1016/j.jaci.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keet CA, Seopaul S, Knorr S, Narisety S, Skripak J, Wood RA. Long-term follow-up of oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2013;132(3):737–739 e6. doi: 10.1016/j.jaci.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy. 2007;62(11):1261–1269. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 74.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, Steele PH, Pons L, Helm RM, Lee LA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119(1):199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 75.Burks AW, Jones SM. Egg oral immunotherapy in non-anaphylactic children with egg allergy: follow-up. J Allergy Clin Immunol. 2008;121(1):270–271. doi: 10.1016/j.jaci.2007.07.066. [DOI] [PubMed] [Google Scholar]
- 76.Vickery BP, Pons L, Kulis M, Steele P, Jones SM, Burks AW. Individualized IgE-based dosing of egg oral immunotherapy and the development of tolerance. Ann Allergy Asthma Immunol. 2010;105(6):444–450. doi: 10.1016/j.anai.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruiz Garcia M, Haroun E, Landivar ME, Torres Hernandez JA, Sastre J. Commercial dehydrated egg white for specific oral tolerance induction (SOTI): an easier treatment for egg allergy. J Investig Allergol Clin Immunol. 2012;22(7):529–531. [PubMed] [Google Scholar]
- 78.Tortajada-Girbes M, Porcar-Almela M, Martorell-Giménez L, Tallón-Guerola M, Gracia-Antequera M, Codoñer-Franch P. Specific oral tolerance induction (SOTI) to egg: our experience with 19 children. J Investig Allergol Clin Immunol. 2012;22(1):75–77. [PubMed] [Google Scholar]
- 79.Letran A, Espinazo M, López MC, Caro FJ, Gómez L, Lobatón P, Dafonte J, Moreno F. Threshold doses in specific oral tolerance induction in children with egg allergy. J Investig Allergol Clin Immunol. 2012;22(2):147–149. [PubMed] [Google Scholar]
- 80.Ojeda P, Ojeda I, Rubio G, Pineda F. Home-based oral immunotherapy protocol with pasteurized egg for children allergic to hen’s egg. Isr Med Assoc J. 2012;14(1):34–39. [PubMed] [Google Scholar]
- 81.Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE-mediated hen’s egg allergy: a new protocol with raw hen’s egg. Pediatr Allergy Immunol. 2013;24(1):75–83. doi: 10.1111/j.1399-3038.2012.01341.x. [DOI] [PubMed] [Google Scholar]
- 82.Dello Iacono I, Verga MC, Tripodi S. Oral immunotherapy for egg allergy in children. N Engl J Med. 2012;367(15):1471; author reply 1472–3. [DOI] [PubMed] [Google Scholar]
- 83.Garcia Rodriguez R, Urra JM, Feo-Brito F, Galindo PA, Borja J, Gómez E, Lara P, Guerra F. Oral rush desensitization to egg: efficacy and safety. Clin Exp Allergy. 2011;41(9):1289–1296. doi: 10.1111/j.1365-2222.2011.03722.x. [DOI] [PubMed] [Google Scholar]
- 84.Itoh N, Itagaki Y, Kurihara K. Rush specific oral tolerance induction in school-age children with severe egg allergy: one year follow up. Allergol Int. 2010;59(1):43–51. doi: 10.2332/allergolint.09-OA-0107. [DOI] [PubMed] [Google Scholar]
- 85.Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, Nowak-Węgrzyn A. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012;130(2):473–80 e1. doi: 10.1016/j.jaci.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA, Liu AH, Sicherer SH, Henning AK, Lindblad RW, et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol. 2016;137(4):1117–1127.e10. doi: 10.1016/j.jaci.2015.12.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, Keet CA, Kulis M, Orgel KG, Guo R, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. 2017;139(1):173–181.e8. doi: 10.1016/j.jaci.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moller C. Effect of pollen immunotherapy on food hypersensitivity in children with birch pollinosis. Ann Allergy. 1989;62(4):343–345. [PubMed] [Google Scholar]
- 90.Herrmann D, Henzgen M, Frank E, Rudeschko O, Jäger L. Effect of hyposensitization for tree pollinosis on associated apple allergy. J Investig Allergol Clin Immunol. 1995;5(5):259–267. [PubMed] [Google Scholar]
- 91.Asero R. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. 1998;28(11):1368–1373. [DOI] [PubMed] [Google Scholar]
- 92.Bucher X, Pichler WJ, Dahinden CA, Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004;59(12):1272–1276. doi: 10.1111/j.1398-9995.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- 93.Hansen KS, Khinchi MS, Skov PS, Bindslev-Jensen C, Poulsen LK, Malling H-J. Food allergy to apple and specific immunotherapy with birch pollen. Mol Nutr Food Res. 2004;48(6):441–448. doi: 10.1002/mnfr.200400037. [DOI] [PubMed] [Google Scholar]
- 94.Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwölfer B, Schreiber C, Francis JN, Ebner C, Bohle B. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007;119(4):937–943. doi: 10.1016/j.jaci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 95.Mauro M, Russello M, Incorvaia C, Gazzola G, Frati F, Moingeon P, Passalacqua G. Birch-apple syndrome treated with birch pollen immunotherapy. Int Arch Allergy Immunol. 2011;156(4):416–422. doi: 10.1159/000323909. [DOI] [PubMed] [Google Scholar]
- 96.Kopac P, Rudin M, Gentinetta T, Gerber R, Pichler C, Hausmann O, Schnyder B, Pichler WJ. Continuous apple consumption induces oral tolerance in birch-pollen-associated apple allergy. Allergy. 2012;67(2):280–285. doi: 10.1111/j.1398-9995.2011.02744.x. [DOI] [PubMed] [Google Scholar]
- 97.Kinaciyan T, Nagl B, Faustmann S, Frommlet F, Kopp S, Wolkersdorfer M, Wöhrl S, Bastl K, Huber H, Berger U, et al. Efficacy and safety of 4 months of sublingual immunotherapy with recombinant Mal d 1 and Bet v 1 in patients with birch pollen-related apple allergy. J Allergy Clin Immunol. 2018. Mar;141(3):1002–1008. doi: 10.1016/j.jaci.2017.07.036. Epub 2017 Sep 1. PubMed PMID: 28870463. [DOI] [PubMed] [Google Scholar]
- 98.Senti G, Von Moos S, Tay F, Graf N, Sonderegger T, Johansen P, Kündig TM. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: A double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129(1):128–135. doi: 10.1016/j.jaci.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 99.Dupont C, Kalach N, Soulaines P, Legoué-Morillon S, Piloquet H, Benhamou P-H. Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol. 2010;125(5):1165–1167. doi: 10.1016/j.jaci.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 100.Sampson HA, Shreffler WG, Yang WH, Sussman GL, Brown-Whitehorn TF, Nadeau KC, Cheema AS, Leonard SA, Pongracic JA, Sauvage-Delebarre C, et al. Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: a randomized clinical trial. JAMA. 2017;318(18):1798–1809. doi: 10.1001/jama.2017.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Von Moos S, Johansen P, Tay F, Graf N, Kündig TM, Senti G. Comparing safety of abrasion and tape-stripping as skin preparation in allergen-specific epicutaneous immunotherapy. J Allergy Clin Immunol. 2014;134(4):965–7.e4. doi: 10.1016/j.jaci.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 102.Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Benhamou P-H, Dupont C. Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol. 2011;186(10):5629–5637. doi: 10.4049/jimmunol.1003134. [DOI] [PubMed] [Google Scholar]
- 103.Senti G, Crameri R, Kuster D, Johansen P, Martinez-Gomez JM, Graf N, Steiner M, Hothorn LA, Grönlund H, Tivig C, et al. Intralymphatic immunotherapy for cat allergy induces tolerance after only 3 injections. J Allergy Clin Immunol. 2012;129(5):1290–1296. doi: 10.1016/j.jaci.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 104.Senti G, Prinz Vavricka BM, Erdmann I, Diaz MI, Markus R, McCormack SJ, Simard JJ, Wüthrich B, Crameri R, Graf N, et al. Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial. Proc Natl Acad Sci U S A. 2008;105(46):17908–17912. doi: 10.1073/pnas.0803725105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hjalmarsson E, Bierrenbach AL, Calderon MA, Sheikh A, Simons FER, Demoly P. Intralymphatic immunotherapy (ilit) with both grass and birch allergen- a randomized double-blind placebo controlled trial. Allergy. 2017;72:120. doi: 10.1111/all.13006. [DOI] [PubMed] [Google Scholar]
- 106.Hylander T, Larsson O, Petersson-Westin U, Eriksson M, Kumlien Georén S, Winqvist O, Cardell L-O. Intralymphatic immunotherapy of pollen-induced rhinoconjunctivitis: a double-blind placebo-controlled trial. Respir Res. 2016;17:10. doi: 10.1186/s12931-016-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee SM, Jung JH, Choi SJ, Joe E, Kang SM, Kim YJ, Kyung SY, Park J-W, Jeong SH, Lee SP. The evaluation of efficacy and adverse effect in intralymphatic allergen-specific immunotherapy against house dust mite, cat, and dog allergens in allergic rhinitis. J Allergy Clin Immunol. 2015;135(2):AB159–AB159. doi: 10.1016/j.jaci.2014.12.1460. [DOI] [Google Scholar]
- 108.Lee SP, Choi SJ, Joe E, Lee SM, Lee MW, Shim JW, Kim YJ, Kyung SY, Park JW, Jeong SH, et al. A pilot study of intralymphatic immunotherapy for house dust mite, cat, and dog allergies. Allergy Asthma Immunol Res. 2017;9(3):272–277. doi: 10.4168/aair.2017.9.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kundig TM, Johansen P, Bachmann MF, Cardell LO, Senti G. Intralymphatic immunotherapy: time interval between injections is essential. J Allergy Clin Immunol. 2014;133(3):930–931. doi: 10.1016/j.jaci.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 110.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, Plaut M, Sampson HA. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol. 2016;137(4):1103–1110.e11. doi: 10.1016/j.jaci.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127(6):1622–1624. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. 2013;132(6):1368–1374. doi: 10.1016/j.jaci.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lafuente I, Mazon A, Nieto M, Uixera S, Pina R, Nieto A. Possible recurrence of symptoms after discontinuation of omalizumab in anti-IgE-assisted desensitization to egg. Pediatr Allergy Immunol. 2014;25(7):717–719. doi: 10.1111/pai.12259. [DOI] [PubMed] [Google Scholar]
- 114.Begin P, Shams-Ghahfarokhi M, Postigo I, Razzaghi-Abyaneh M, Eslamifar A, Gutiérrez A, Suñén E, Martínez J. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin Immunol. 2014;10(1):7. doi: 10.1186/1710-1492-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martorell-Calatayud C, Michavila-Gómez A, Martorell-Aragonés A, Molini-Menchón N, Cerdá-Mir JC, Félix-Toledo R, De Las Marinas-Álvarez MD. Anti-IgE-assisted desensitization to egg and cow’s milk in patients refractory to conventional oral immunotherapy. Pediatr Allergy Immunol. 2016;27(5):544–546. doi: 10.1111/pai.12567. [DOI] [PubMed] [Google Scholar]
- 116.Leung DY, Sampson HA, Yunginger JW, Burks AW, Schneider LC, Wortel CH, Davis FM, Hyun JD, Shanahan WR. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348(11):986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 117.Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, Townley RG, Mokhtarani M, Seyfert-Margolis V, Asare A, Bateman K, et al. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117(1):134–140. doi: 10.1016/j.jaci.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 118.Kuehr J, Brauburger J, Zielen S, Schauer U, Kamin W, Von Berg A, Leupold W, Bergmann K-C, Rolinck-Werninghaus C, Gräve M, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109(2):274–280. [DOI] [PubMed] [Google Scholar]
- 119.Lambert N, Guiddir T, Amat F, Just J. Pre-treatment by omalizumab allows allergen immunotherapy in children and young adults with severe allergic asthma. Pediatr Allergy Immunol. 2014;25(8):829–832. doi: 10.1111/pai.12306. [DOI] [PubMed] [Google Scholar]
- 120.Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, Zeldin RK. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125(2):383–389. doi: 10.1016/j.jaci.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 121.Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann K-C, Sieder C, Stenglein S, Seyfried S, Wahn U. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy. 2009;39(2):271–279. doi: 10.1111/j.1365-2222.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 122.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5(4):387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 123.Basomba A, Tabar AI, De Rojas DHF, García BE, Alamar R, Olaguíbel JM, Del Prado JM, Martín S, Rico P. Allergen vaccination with a liposome-encapsulated extract of Dermatophagoides pteronyssinus: a randomized, double-blind, placebo-controlled trial in asthmatic patients. J Allergy Clin Immunol. 2002;109(6):943–948. [DOI] [PubMed] [Google Scholar]
- 124.Galvain S, André C, Vatrinet C, Villet B. Safety and efficacy studies of liposomes in specific immunotherapy. Curr Ther Res-Clin Exp. 1999;60(5):278–294. doi: 10.1016/S0011-393X(99)80004-6. [DOI] [Google Scholar]
- 125.Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Müller P, Pfister T, Maurer P, Bachmann MF, Graf N, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39(4):562–570. doi: 10.1111/j.1365-2222.2008.03191.x. Epub 2009 Feb 16. PubMed PMID: 19226280. [DOI] [PubMed] [Google Scholar]
- 126.Klimek L, Willers J, Hammann-Haenni A, Pfaar O, Stocker H, Mueller P, Renner WA, Bachmann MF. Assessment of clinical efficacy of CYT003-QbG10 in patients with allergic rhinoconjunctivitis: a phase IIb study. Clin Exp Allergy. 2011;41(9):1305–1312. doi: 10.1111/j.1365-2222.2011.03783.x. [DOI] [PubMed] [Google Scholar]
- 127.Pohlit H, Bellinghausen I, Frey H, Saloga J. Recent advances in the use of nanoparticles for allergen-specific immunotherapy. Allergy. 2017;72(10):1461–1474. doi: 10.1111/all.13199. [DOI] [PubMed] [Google Scholar]


