Abstract
Soybean (Glycine max) is the most important dicot crop worldwide, and is increasingly used as a model legume due to the wide availability of genomic soybean resources; however, the slow generation times of soybean plants are currently a major hindrance to research. Here, we demonstrate a method for accelerating soybean breeding in compact growth chambers, which greatly shortens the generation time of the plants and accelerates breeding and research projects. Our breeding method utilizes commonly used fluorescent lamps (220 �mol m–2 s–1 at the canopy level), a 14 h light (30�C)/10 h dark (25�C) cycle and carbon dioxide (CO2) supplementation at >400 p.p.m. Using this approach, the generation time of the best-characterized elite Japanese soybean cultivar, Enrei, was shortened from 102–132 d reported in the field to just 70 d, thereby allowing up to 5 generations per year instead of the 1–2 generations currently possible in the field and/or greenhouse. The method also facilitates the highly efficient and controlled crossing of soybean plants. Our method uses CO2 supplementation to promote the growth and yield of plants, appropriate light and temperature conditions to reduce the days to flowering, and the reaping and sowing of immature seeds to shorten the reproductive period greatly. Thus, the appropriate parameters enable acceleration of soybean breeding in the compact growth chambers commonly used for laboratory research. The parameters used in our method could therefore be optimized for other species, cultivars, accessions and experimental designs to facilitate rapid breeding in a wide range of crops.
Keywords: Accelerated generation, CO2 supplementation, Crossing, Glycine max, Growth chamber
Introduction
Soybean (Glycine max L. Merr.) originated in East Asia, including Japan, and is the most important dicot crop worldwide. The global production of soybean was the fourth highest of all crops in 2016, after maize (Zea mays), wheat (Triticum aestivum) and rice (Oryza sativa) (FAOSTAT database). Over the past two decades, worldwide soybean production and the area harvested have increased more rapidly than those of the other staple crops (FAOSTAT database), with soybean being increasingly used as a food oil, especially in emerging economies [United States Department of Agriculture (USDA) 2018]. Although the current major producers of soybean are the USA, Brazil and Argentina, soybean is an ancient food crop in China, Korea and Japan (Ohyama et�al. 2013), cultivated for thousands of years, and numerous wild and cultivated soybean accessions originated in East Asia (Xu et�al. 2002, Kaga et�al. 2012). Soybean seeds are not only a major source of nutritious food for humans and livestock, but are also used for industrial materials, due to their high protein and oil contents (Yamada et�al. 2012a).
The multifaceted importance of soybean has led to the development of a wide range of tools and genomic resources, which are easily accessible from public databases such as SoyBase (Grant et�al. 2010), SoyKB (Joshi et�al. 2012) and Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html#) (Schmutz et�al. 2010). Plant researchers can utilize a variety of robust molecular tools, including transformation (Hinchee et�al. 1988, McCabe et�al. 1988, Finer and McMullen 1991), virus-induced gene silencing (Zhang and Ghabrial 2006, Yamagishi and Yoshikawa 2009, Ogata et�al. 2017) and CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9) genome editing (Cai et�al. 2015, Jacobs et�al. 2015, Li et�al. 2015). Artificial and natural mutant resources have been developed, including ethyl methanesulfonate (EMS) mutant libraries (Cooper et�al. 2008, Hoshino et�al. 2014, Tsuda et�al. 2015, Lakhssassi et�al. 2017, Espina et�al. 2018) and the collation of selected sets of germplasm into core collections of soybean accessions (Kaga et�al. 2012). These tools and resources have established soybean as a model legume crop. However, many plant researchers believe that soybean needs more light than model plants, and that crossing genotypes is more laborious (Talukdar and Shivakumar 2012). Moreover, the slow generation times of soybean remain a major barrier to soybean studies.
Recently, Watson et�al. (2018) developed a method for speed breeding long-day crops such as wheat and barley (Hordeum vulgare) using a prolonged photoperiod, which reduces the generation time and facilitates efficient breeding; however, the speed breeding methods have not been reported in short-day soybean plants. Here, we describe a new method for accelerating soybean breeding in widely used compact growth chambers, which greatly reduces its generation time and facilitates soybean breeding and research programs.
Results
CO2 supplementation enhances the growth and yield of soybean plants in growth chambers
Here, we aimed to develop a method for accelerating soybean breeding using compact, environmentally controlled growth chambers (internal volumes of approximately 0.4 m3), which are widely used in laboratory-based plant research (Fig.�1A). The chambers were fitted with commonly used fluorescent lamps, which provided a light intensity of around 220 �mol m–2 s–1 at the canopy level. As the appropriate cultivation area of each soybean cultivar is limited to relatively narrow latitudes (Watanabe et�al. 2012), a single cultivar, the well-characterized elite Japanese soybean cultivar Enrei, was mainly used in this study (Supplementary Fig. S1). The genome sequence of Enrei has been published (Shimomura et�al. 2015), and its genomic information is available in a public database, DaizuBase (Katayose et�al. 2012). Moreover, a high-density mutant library, a high-density linkage map and chromosomal segment substitution lines have been developed for Enrei (Tsuda et�al. 2015, Watanabe et�al. 2018).
Fig. 1.
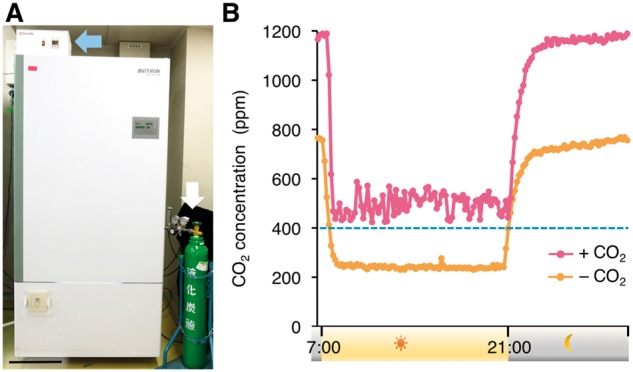
Growth chamber CO2 conditions over time. (A) A growth chamber used in this study. Blue and white arrows indicate the CO2 regulator and the CO2 cylinder, respectively. Scale bar = 30 cm. (B) The internal CO2 concentrations within CO2-supplemented (pink) and control (orange) growth chambers containing mature soybean plants. The data were collected every 10 min over a single day, 25 d after flower initiation in the soybean plants.
To evaluate soybean growth, 12 plants were grown in a single growth chamber, which was programmed for a photoperiod of 14 h of light at a temperature of 30�C, followed by 10 h of darkness at 25�C. These conditions are comparable with those of mid-summer in the plain regions of Japan (Japan Meteorological Agency, https://www.data.jma.go.jp/obd/stats/data/en/smp/index.html). Previous studies have shown that CO2 concentrations inside growth chambers, which comprise a closed and limited space, were much lower than those of the ambient atmosphere (at approximately 400 p.p.m.) during the active growth phase of rice, and that CO2 supplementation improved the growth and yields of rice grown in plant growth chambers (Ohnishi et�al. 2011, Tanaka et�al. 2016). To examine how exogenous CO2 supplementation affects the CO2 concentration inside growth chambers in the presence of soybean plants, the internal CO2 concentrations were recorded. During the light period, the internal CO2 concentration ranged from 400 to 600 p.p.m. in the growth chambers supplemented with CO2, whereas the CO2 concentration in the unsupplemented growth chamber was consistently around 200 p.p.m. (Fig.�1B) at 25 d after flowering. During the dark period, the CO2 concentration markedly increased to around 1,200 and 750 p.p.m. in the growth chambers with and without supplemental CO2, respectively (Fig.�1B). The CO2 concentrations in the growth chamber were changing depending on the soybean growth (Supplementary Fig. S2). These data suggest that the CO2 concentration inside growth chambers is strongly affected by the photosynthesis and respiration of the soybean plants. In accordance with previous observations in rice (Ohnishi et�al. 2011, Tanaka et�al. 2016), our findings show that CO2 concentrations within growth chambers are lower than the ambient CO2 concentration during the day, which may retard plant growth.
We therefore investigated the effects of CO2 supplementation on soybean growth in the growth chamber. Consistent with previous reports in rice (Ohnishi et�al. 2011, Tanaka et�al. 2016), we found that CO2 supplementation enhanced the growth and development of soybean in the growth chamber in terms of total leaf area, plant height, total length of branches, dry leaf weight, dry stem weight and dry root weight (Fig.�2;Supplementary Fig. S3). We also evaluated the seed yields of the soybean plants, and found that the average seed numbers per plant were 53.4 � 9.1 when supplemented with CO2 and 26.6 � 2.5 in the unsupplemented chamber (Supplementary Fig. S4A–F). In contrast, no significant difference was observed in the number of seeds per pod produced by plants grown in chambers with or without supplemental CO2 (Supplementary Fig. S4G). We further evaluated the quality of the harvested seeds and found that the average seed weight was significantly increased by CO2 supplementation (Supplementary Fig. S4E, H). These results therefore indicate that CO2 supplementation enhances the growth and yield of soybean plants in the growth chamber.
Fig. 2.
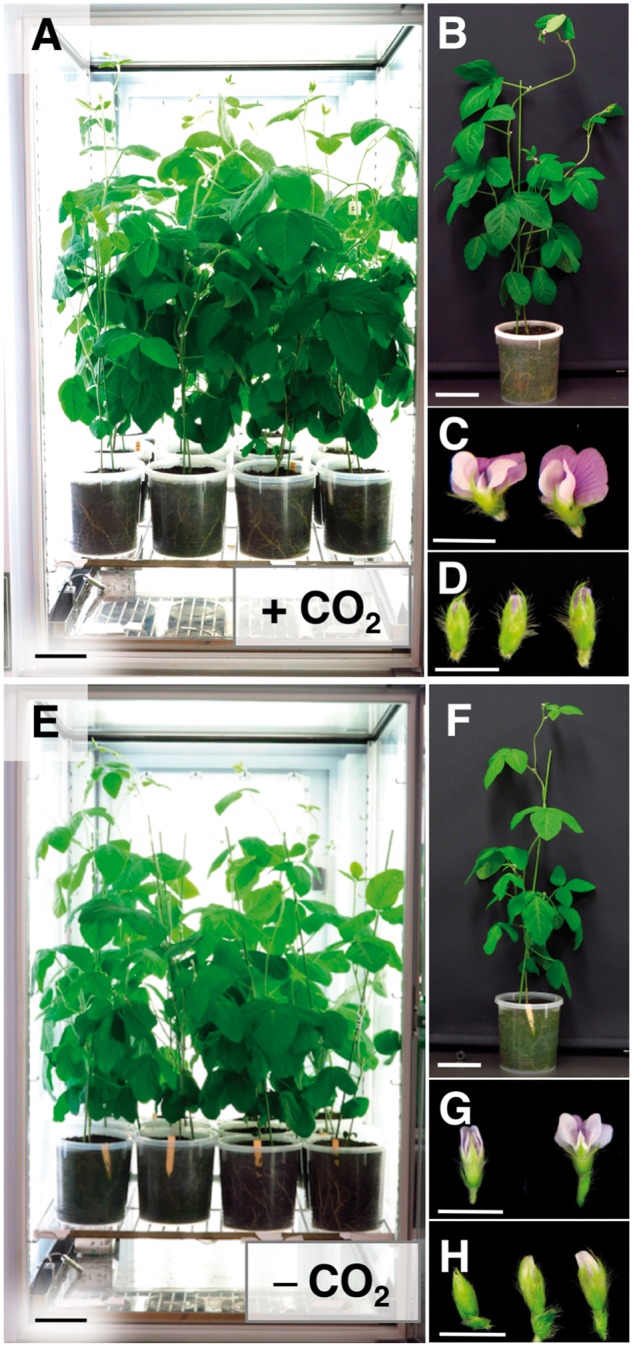
CO2 supplementation enhances soybean growth in growth chambers. Images show 27-day-old soybean plants and flowers grown in the compact growth chambers with (+; A–D) or without (−; E–H) supplemental CO2. The growth chamber (0.4 m3) was programmed to run a 14 h light (30�C)/10 h dark (25�C) cycle. Twelve soybean plants in 2.0 liter pots were cultured in each growth chamber. Scale bars = 10 cm (A, B, E and F), 5 mm (C, D, G and H).
Light and temperature regulate the days to flowering in soybean in growth chambers
Reducing the number of days to flowering is one of the most important factors for shortening generation times and accelerating breeding. Previous studies showed that CO2 supplementation in growth chambers reduced the flowering time in rice (Ohnishi et�al. 2011, Tanaka et�al. 2016) and that low CO2 concentrations delayed flowering in Arabidopsis (Li et�al. 2014); therefore, we examined whether CO2 supplementation affects the days to flowering in soybean plants grown in growth chambers. The number of days between sowing and flower initiation for plants grown in chambers with or without supplemental CO2 was 25.5 � 0.5 and 25.3 � 0.5 d, respectively (Fig.�3A); thus, the differences in vegetative growth rates caused by CO2 supplementation (Fig.�2) did not decrease the number of days to flowering. These results are consistent with the previous observations that flowering time is strictly controlled by temperature and photoperiod in soybean (Hadley et�al. 1984, Cober et�al. 2014). The data also indicate that the number of days to flowering of Enrei markedly decreased from 33–59 d reported in the field (Yamada et�al. 2012b) to around 25 d in the temperature and light conditions used in this study. Likewise, the similar reductions in the days to flowering can be observed in the sequenced model US cultivar Williams 82 (Schmutz et�al. 2010) (Supplementary Fig. S1) and the Brazilian cultivar BR16 (Supplementary Fig. S1), which are widely used in basic studies in Brazil (Rodrigues et�al. 2015). The number of days between sowing and flower initiation decreased from >40–60 to 28.0 � 0 d in Williams82 (Schon and Blevins 1990, Kong et�al. 2018) (Supplementary Fig. S5), and also decreased from 33–44 to 30.4 � 0.9 d in BR16 (Carpentieri-Pipolo et�al. 2014). Thus, our results indicate that light and temperature conditions greatly contribute to reduce the days to flowering in soybean in growth chambers.
Fig. 3.
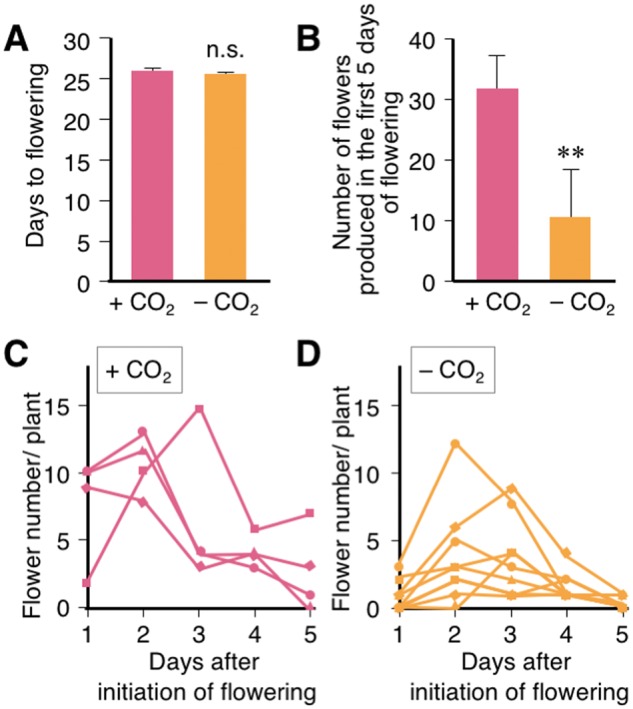
CO2 supplementation improves the quantity and quality of soybean flowers in the growth chamber. (A) Number of days to flower initiation after sowing soybean seeds in growth chambers with (+) or without (−) supplemental CO2. Error bar = SD. n = 8. (B) Number of healthy flowers produced during the first 5 d of flowering in growth chambers with (+; n = 4) or without (−; n = 8) supplemental CO2. Error bar = SD. (C, D) The number of healthy flowers produced each day for the first 5 d of flowering for plants grown with (C, n = 4) or without (D, n = 8) supplemental CO2. **P < 0.01, n.s. no significant difference.
CO2 supplementation improves the quantity and quality of soybean flowers, enabling higher crossing efficiency
The quantity and quality of flower buds in the female parent are important factors for efficient crossing. We analyzed the numbers of healthy flowers (Fig.�2C) produced by soybean plants grown in growth chambers with or without CO2 supplementation. The average number of healthy flowers produced in the first 5 d of flowering was 32.0 � 5.6 and 10.9 � 7.9 for plants grown in growth chambers with and without supplemental CO2, respectively (Fig.�3B). The peak numbers of healthy flowers were observed in the first 3 d of flowering (Fig.�3C, D). Abnormal flowers, which had immature or deformed corollas with insipid coloration, were often observed in the unsupplemented growth chamber (Fig.�2G, H), whereas abnormal flowers were almost never seen during the first 5 d of flowering in plants grown in the CO2-supplemented growth chamber (Fig.�2C, D).
Next, we examined whether healthy flowers could be obtained from plants grown in smaller pots (0.4 liter volume, 10 cm diameter) than those used throughout the rest of this study (2.0 liter volume, 15 cm diameter) in the CO2-supplemented growth chamber. Many healthy flowers (25.3 � 5.9 in the first 5 d of flowering) were observed in the vigorous soybean plants grown in these smaller pots (Supplementary Fig. S6). These observations suggest that the soybean plants grown in smaller pots produced almost as many healthy flowers as those plants grown in the larger pots, indicating that at least 30 plants could be grown for accelerating breeding in each growth chamber. These results show that CO2 supplementation increases the amount of high-quality flowers produced by soybean plants in growth chambers, indicating that the flowers of soybean grown in the CO2-supplemented growth chamber might be useful for performing effective crossings. Until now, conducting effective crossings out of season in artificial conditions was considered to be more challenging than doing so in the field in summer.
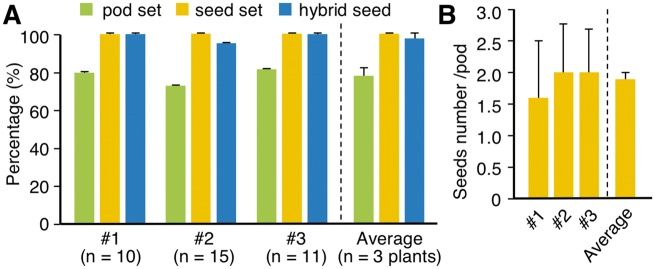
Therefore, to test the quality of the flowers produced by soybean plants grown in the CO2-supplemented growth chambers, we investigated their crossing efficiency. Ten days after emasculating and pollinating the female flowers, 78.4 � 4.5% of the crossed flowers had formed pods (Fig.�4A). The frequency of seed set within the pods was 100%, and the average number of seeds per pod was 1.9 � 0.1 (Fig.�4B). The seeds were genotyped using a mutation as a marker of hybridization (Supplementary Fig. S7), revealing that 98.5 � 2.6% of the harvested seeds were hybrids (Fig.�4A). The crossing efficiency was therefore 77.3 � 6.4% for soybean plants grown in CO2-supplemented growth chambers, which was much higher than that of field-grown soybean (Walker et�al. 1979).
Fig. 4.
The high crossing efficiencies in soybean plants grown in growth chambers supplemented with CO2. (A) The percentage of pod set determined as the number of pods arising from the total number of crossings, and the percentages of seed set and hybrid seeds in the three individual female soybean parent plants. The total number of crossings in each plant was 10 in #1, 15 in #2 and 11 in #3. The average percentages from all three plants are shown on the right. Error bar = SD. (B) Seed numbers per pod in three individual female parent plants. The total numbers of pods in the three plants were 8 in #1, 10 in #2 and 9 in #3. The average seed numbers per pod from all three plants are shown on the right. Error bar = SD.
Air-dried immature soybean seeds display a high germination rate and normal seedling growth
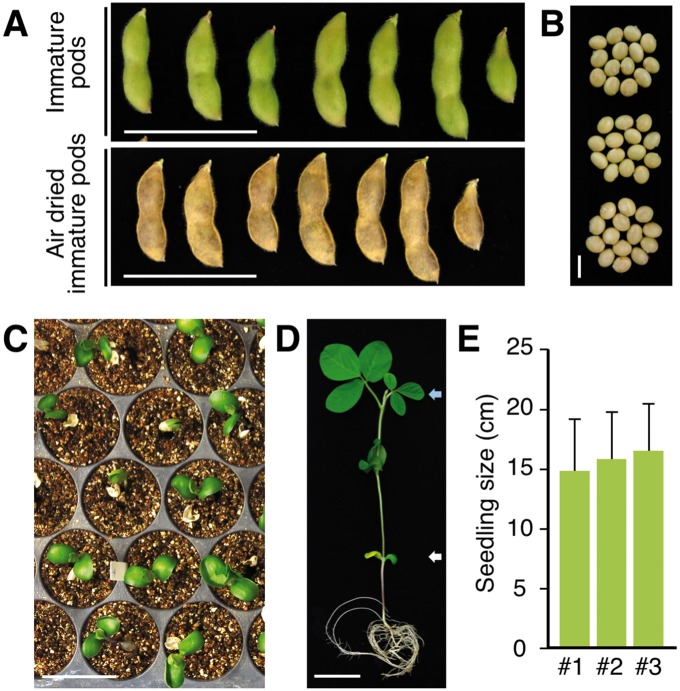
The reproductive period of soybean comprises more than half its total life span (Roumet and Morin 1997). In the field in Japan, Enrei was reported to take 65–92 d to complete seed maturation after flowering (Yamada et�al. 2012b). To shorten this reproductive period, we examined whether the previously reported methods for germinating immature soybean seeds (Obendorf et�al. 1980, Carandang et�al. 2006) could be used to shorten the reproductive period of plants grown in CO2-supplemented growth chambers. We harvested fully swollen immature pods at 37 d after flowering, as they started to change color from green to yellow (Fig.�5A). After air-drying the pods for 8 d, their color changed to light brown, and the seeds in the pods matured (Fig.�5B). Their germination rate at 7 d after sowing was 100% (Fig.�5C). By 15 d after germination, the seedlings had produced one or two trifoliate leaves (Fig.�5D, E). We have usually used the progeny grown from immature seeds as well as mature seeds for crossing. These data showed that the germination ratio and subsequent growth rate of these immature seeds were comparable with those of mature seeds, indicating that the harvesting and germination of immature seeds could be used to shorten further the generation time of soybean plants grown in CO2-supplemented growth chambers.
Fig. 5.
Air-dried immature soybean seeds display high germination rates and normal seedling growth. (A) Immature soybean pods harvested 37 d after flower initiation, imaged both at the time of harvest and after 8 d of air-drying. Scale bars = 5 cm. (B) Soybean seeds removed from immature soybean pods air-dried for 8 d, taken from three independent plants. Scale bar = 1 cm. (C) Seven-day-old seedlings germinated from the air-dried immature seeds. The germination ratio of the air-dried immature seeds was 100% for seeds derived from three individual plants. n = 14 per plant. Scale bar = 5 cm. (D) A 15-day-old seedling derived from an air-dried immature seed grown on vermiculite. Scale bar = 5 cm. (E) The seedling size, determined as the stem length from the cotyledon [white arrowhead in (D)] to the top of the plant [blue arrowhead in (D)], of 15-day-old plants grown from immature seeds harvested from three individual plants. n = 14 per plant. Error bar = SD.
Discussion
Here, we present a method for accelerating breeding of soybean in a compact growth chamber, which greatly reduces the generation time and facilitates rapid breeding and research projects. The recently reported speed breeding methods (Watson et�al. 2018) have not been applied to short-day soybean plants; however, our method can reduce the generation time of the soybean cultivar Enrei, the best-characterized Japanese cultivar, to just 70 d (Fig.�6). This breeding approach therefore allows up to 5 generations per year, instead of the 1–2 generations currently possible under field and greenhouse conditions. Moreover, our method allows the highly efficient controlled crossing of soybean lines under laboratory conditions, enabling researchers and breeders to work consistently year-round, whatever the weather or season.
Fig. 6.
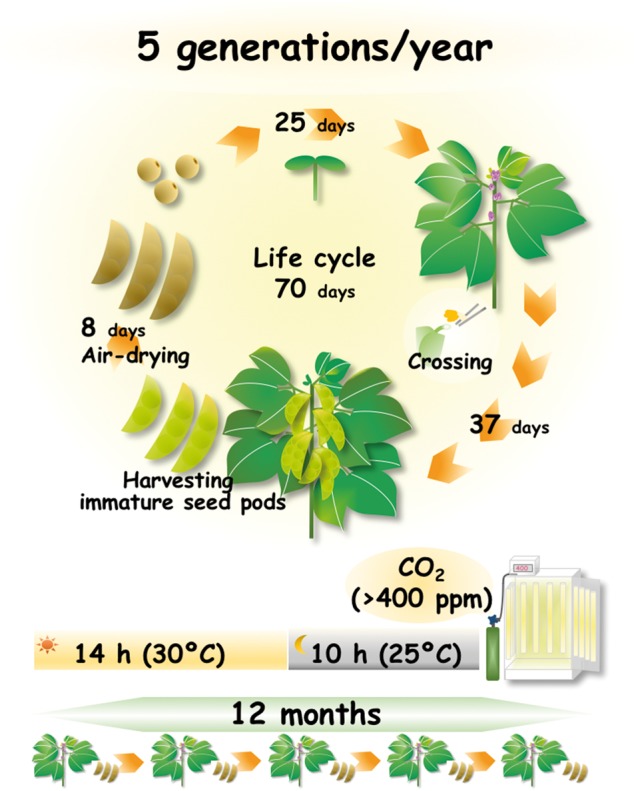
Schematic representation of our method for accelerating breeding in soybean (cv. Enrei) in a growth chamber supplemented with CO2. The appropriate combination of a 14 h light (30�C)/10 h dark (25�C) photoperiod and >400 p.p.m. CO2 concentration, with the use of immature seeds, reduces the generation time to 70 d, thereby achieving up to 5 generations per year in soybean. See main text for details.
Our breeding method uses supplemental CO2 in combination with appropriate light and temperature cycles to accelerate the growth and development of plants, and the reaping and sowing of immature seeds to greatly shorten the generation time further in the growth chamber. The compact chambers (0.4 m3) used in this study are widely used for Arabidopsis research, thereby enabling plant researchers to shift their focus from this weedy model plant to soybean, a staple crop. Since the light intensity derived from general purpose fluorescent lamps was just 220 �mol m–2 s–1 at the canopy level in the growth chamber used in this study, the lighting costs (for both the light source and the electricity) would be lower than those described in recent speed breeding protocols, which use high-power light-emitting diodes (LEDs) providing light intensities of 340–650 �mol m–2 s–1 (Watson et�al. 2018). Furthermore, our method uses a 14 h light period, which is much shorter than the 22 h period required by other speed breeding methods (Watson et�al. 2018), contributing further cost savings.
So far, it has been difficult to obtain enough high-quality soybean flowers to perform effective crossings in artificial conditions, due to the less vigorous growth of plants compared with those in the field. We demonstrated that CO2 supplementation enhances the quantity and quality of both vegetative and reproductive growth in soybean plants in the growth chamber (Figs.�2, 3; Supplementary Figs. S3, S4), and allows for the highly efficient controlled crossing of soybean. However, CO2 supplementation is not directly involved in shortening the soybean life cycle. Instead, appropriate light and temperature cycles can be used to decrease the number of days to soybean flower initiation from 33–59 d (Yamada et�al. 2012b) to 25 d, while the reaping and sowing of immature seeds greatly shortens the reproductive period from 65–92 d (Yamada et�al. 2012b) to 45 d. Thus, the appropriate combination of supplemental CO2, light and temperature conditions with the use of immature seeds would allow reduction of the generation time of Enrei from 102–132 d in the field (Yamada et�al. 2012b) to just 70 d.
Considering the previous findings that Enrei is a weakly photosensitive cultivar (Ozawa et�al. 2017) and that comparatively higher temperatures accelerate the flowering times in soybean plants (Hadley et�al. 1984), our data suggest that the temperature setting used in this study plays a more important role in the reduction in the days to flowering of Enrei. On the other hand, soybean plants are generally photosensitive, and thus there are variations in the days to flowering in soybean cultivars. However, although the cultivars used in this study, Enrei, Williams82 and BR16, greatly vary in cultivation areas (Alliprandini et al. 2009, Yamada et al. 2012b, Wolfgang and An 2017) (Supplementary Fig. S1), we showed that in these distinct cultivars, the days to flowering can be reduced with our method (Supplementary Fig. S5). Their growth habits are also different; Enrei and BR16 are determinate cultivars, whereas Williams 82 is an indeterminate cultivar (Oya et�al. 2004, Tian et�al. 2010, Yamada et�al. 2012b) (Supplementary Fig. S1). As the latitude of cultivation areas and growth habits mainly depend on specific genotypes (Bernard 1972, Tian et�al. 2010, Maldonado dos Santos et�al. 2016, Langewisch et�al. 2017), these findings suggest the possibility that our technique is of general application in a wide range of soybean cultivars. Given that each soybean cultivar can be cultivated only in limited latitudes (Watanabe et�al. 2012), the photoperiod conditions among the parameters in our method needs to be adapted for each cultivar. Alongside other protocols for speed breeding and effective phenotyping (Kuroda and Ikenaga 2015, Watson et�al. 2018), the parameters used in our method could be optimized for a variety of species, cultivars, accessions and experimental designs to facilitate cutting-edge breeding in a wide range of crops.
Materials and Methods
Plant materials and growth conditions
Soybean (Glycine max L. Merr.) cv. Enrei, Williams 82 and BR16 plants were grown in a 14 h light (30�C)/10 h dark (25�C) photoperiod for Enrei or in a 10 h light (30�C)/14 h dark (25�C) photoperiod for Williams 82 and BR16 in LH-410S growth chambers (Nippon Medical & Chemical Instruments) or LH-350S growth chambers equipped with the CO2 regulator AMC-CO2-1S (Nippon Medical & Chemical Instruments) and general purpose white light fluorescent tubes. Dedicated CO2 regulators controlled the addition of CO2 from external CO2 cylinders to the growth chambers to maintain their internal CO2 concentration at >400 p.p.m. The internal CO2 concentrations were recorded every 10 min using a data logger (GL220-UM-801; GRAPHTEC). The light intensity (photosynthetic photon flux) in the growth chamber was around 220 �mol m–2 s–1 at the canopy level, while the relative humidity was not regulated and ranged from 50% to 80%.
The seeds were germinated on moistened vermiculite, and 10-day-old seedlings were transferred to individual plastic 2.0 liter pots filled with a mixture of Nippi soil (Nihon Hiryo) and Tsuchitaro soil (Sumitomo Forestry). Twelve 2.0 liter plant pots were placed in each growth chamber, and the positions of the plants were rearranged during watering. Additional fertilizer was not supplied after the seeds were sown.
Measurements of soybean growth
For the analyses of soybean growth in growth chambers with or without supplemental CO2, 31-day-old soybean cv. Enrei plants were used. Total leaf area was measured using an automatic area meter (AAM-9; Hayashi Denko Co. Ltd.). For dry weight measurements, the parts of the plants were measured after being dried for 48 h at 60�C in an oven. The soybean plants were harvested at about 2.5 months after sowing, and then air-dried. Dried seeds were collected from the plants, which were used for the measurements of the seed weight.
Quantification of the days to flowering and the number of healthy flowers
The number of the days from sowing to the opening of the first flower was defined as the days to flower initiation. Newly opened healthy flowers (Fig.�2C) were counted each morning for the first 5 d after flower initiation. Aberrant flowers, with immature or deformed corollas and insipid coloration, were not counted.
Crossing soybean lines
Healthy flower buds of plants grown in the growth chamber, which were expected to open the next day and were swollen with a visible purple corolla through the calyx (Fig.�2D), were selected for crossing, and all the other flowers and buds at that node were removed using forceps. The calyx and corolla were detached, after which the immature anthers were carefully removed (emasculation). The pollen-containing anthers of male parents from newly opened flowers, which have a single nucleotide substitution in the Enrei genome background, were then gently attached to the stigma of the female emasculated flowers. Crossing was conducted for 3 d after the initiation of flowering using three independent female soybean parent plants in the growth chamber.
Evaluation of crossing efficiency
Almost 10 d after crossing, pods >1 cm in length were considered to be set, and the percentage of pod set was determined as the number of pods arising from the total number of crossings. The total number of seeds as a percentage of the total number of ovules in a pod at 30 d after crossing was also calculated to determine the seed set. To determine the number of hybrid seeds, a C-to-T mutation site derived from male parents was used as a marker. Total DNAs were extracted from 52 seeds harvested from the soybean plants 30 d after crossing, using a simple and rapid DNA extraction method (Edwards et�al. 1991). A 207 bp sequence including the mutation site was amplified from the total DNAs using PCR and the primer pair 5′-CAAAACAAGCTTGACTCTTAAACTC-3′ and 5′-CGAAATAATACAAAACGGAGAATG-3′. The resulting PCR products were sequenced using the primer 5′-CAAAACAAGCTTGACTCTTAAACTC-3′. The sequences were aligned, and a heterozygous sequence at the mutation site indicated the hybrid seed derived from a successful cross.
Air-drying immature seed
Immature pods were harvested from 62-day-old soybean plants grown in the CO2-supplemented growth chambers, 37 d after flower initiation. Fully swollen pods were collected as they began to change color from green to yellow (Fig.�5A). The harvested immature pods were air-dried for 8 d at room temperature at around 27�C. The immature pods were opened by hand after drying, and the seeds were sown on moistened vermiculite in the greenhouse at 27 � 5�C and with a relative humidity of 50 � 15%. The germination ratio of the seeds was determined 7 d after sowing. The seedling growth, which was measured as the length of the stem from the cotyledon to the top of the plant, was measured in 15-day-old seedlings.
Funding
This work was supported by the Japan Society for the Promotion of Science [KAKENHI Nos. 24510312 and 16K07412 to Y.F.; No. 18K05379 to YN] and the Ministry of Agriculture, Forestry and Fisheries (MAFF) of Japan.
Supplementary Material
Acknowledgments
We thank I. Saito, M. Ikegami, K. Ozawa, K. Shimizu, N. Hisatomi, M. Kishimoto, E. Ohgawara, N. Takano, R. Motohashi and K. Mogami for their excellent technical assistance, M. Toyoshima for skillful editorial support, D. Xu and N. Yamanaka for their kind advice and support in soybean cultivation, S. Hata (Ryukoku University) for helpful advice about cultivating soybean in a growth chamber, A. Kaga, M. Ishimoto and T. Sayama (Institute of Crop Science, NARO) for their kind advice regarding the cultivation of soybean, J. Tanaka (Institute of Crop Science, NARO) for the helpful instructions regarding the Biotron Breeding System in rice, Y. Nakaizumi and T. Ozawa (Nippon Medical & Chemical Instruments Co., Ltd.) for setting and maintaining the growth chambers, M. Fujita (RIKEN), T. Ogata, C. Yamamizo, Y. Kobayashi and T. Kashiwa for critical reading of the manuscript, and Plant Editors for skillful language editing support of the manuscript.
Y.N. and Y.F. designed the research; Y.N. performed the experiments and analyzed the data; and Y.N. and Y.F. wrote the manuscript.
Disclosures
The authors have no conflicts of interest to declare.
References
- Alliprandini L.F., Abatti C., Bertagnolli P.F., Cavassim J.E., Gabe H.L., Kurek A. (2009) Understanding soybean maturity groups in Brazil: environment, cultivar classification, and stability. Crop Sci. 49: 801. [Google Scholar]
- Bernard R.L. (1972) Two genes affecting stem termination in soybeans. Crop Sci. 12: 235–239. [Google Scholar]
- Cai Y., Chen L., Liu X., Sun S., Wu C., Jiang B., et al. (2015) CRISPR/Cas9-mediated genome editing in soybean hairy roots. PLoS One 10: e0136064.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandang F.M., Shanmugasundaram S., Carpena A.L. (2006) Rapid generation advancement in soybeans using immature seeds. Philipp. J. Crop Sci. 31: 53–59. [Google Scholar]
- Carpentieri-Pipolo V., de Almeida A.L., de Souza Kiihl R.A., Stefani Pagliosa E. (2014) Inheritance of late flowering in natural variants of soybean cultivars under short-day conditions. Pesq. Agropec. Bras. 49: 796–803. [Google Scholar]
- Cober E.R., Curtis D.F., Stewart D.W., Morrison M.J. (2014) Quantifying the effects of photoperiod, temperature and daily irradiance on flowering time of soybean isolines. Plants 3: 476–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J.L., Till B.J., Laport R.G., Darlow M.C., Kleffner J.M., Jamai A., et al. (2008) TILLING to detect induced mutations in soybean. BMC Plant Biol. 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K., Johnstone C., Thompson C. (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 19: 1349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espina M.J., Ahmed C.M.S., Bernardini A., Adeleke E., Yadegari Z., Arelli P., et al. (2018) Development and phenotypic screening of an ethyl methane sulfonate mutant population in soybean. Front. Plant Sci. 9: doi:10.3389/fpls.2018.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer J.J., McMullen M.D. (1991) Transformation of soybean via particle bombardment of embryogenic suspension culture tissue. In Vitro Cell. Dev. Biol. Plant 27: 175–182. [Google Scholar]
- Grant D., Nelson R.T., Cannon S.B., Shoemaker R.C. (2010) SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res. 38: D843–D846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley P., Roberts E.H., Summerfield R.J., Minchin F.R. (1984) Effects of temperature and photoperiod on flowering in soya bean [Glycine max (L.) Merrill]: a quantitative model. Ann. Bot. 53: 669–681. [Google Scholar]
- Hinchee M.A.W., Connor-Ward D.V., Newell C.A., McDonnell R.E., Sato S.J., Gasser C.S., et al. (1988) Production of transgenic soybean plants using Agrobacterium-mediated DNA transfer. Nat. Biotechnol. 6: 915–922. [Google Scholar]
- Hoshino T., Watanabe S., Takagi Y., Anai T. (2014) A novel GmFAD3-2a mutant allele developed through TILLING reduces alpha-linolenic acid content in soybean seed oil. Breed. Sci. 64: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs T.B., LaFayette P.R., Schmitz R.J., Parrott W.A. (2015) Targeted genome modifications in soybean with CRISPR/Cas9. BMC Biotechnol. 15: 16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi T., Patil K., Fitzpatrick M.R., Franklin L.D., Yao Q., Cook J.R., et al. (2012) Soybean Knowledge Base (SoyKB): a web resource for soybean translational genomics. BMC Genomics 13: S15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga A., Shimizu T., Watanabe S., Tsubokura Y., Katayose Y., Harada K., et al. (2012) Evaluation of soybean germplasm conserved in NIAS genebank and development of mini core collections. Breed. Sci. 61: 566–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayose Y., Kanamori H., Shimomura M., Ohyanagi H., Ikawa H., Minami H., et al. (2012) DaizuBase, an integrated soybean genome database including BAC-based physical maps. Breed. Sci. 61: 661–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Lu S., Wang Y., Fang C., Wang F., Nan H., et al. (2018) Quantitative trait locus mapping of flowering time and maturity in soybean using next-generation sequencing-based analysis. Front. Plant Sci. 9: 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M., Ikenaga S. (2015) Single-tube hydroponics as a novel idea for small-scale production of crop seed in a plant incubator. Biosci. Biotechnol. Biochem. 79: 63–67. [DOI] [PubMed] [Google Scholar]
- Lakhssassi N., Zhou Z., Liu S., Colantonio V., AbuGhazaleh A., Meksem K. (2017) Characterization of the FAD2 gene family in soybean reveals the limitations of gel-based TILLING in genes with high copy number. Front. Plant Sci. 8: 324.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langewisch T., Lenis J., Jiang G.L., Wang D., Pantalone V., Bilyeu K. (2017) The development and use of a molecular model for soybean maturity groups. BMC Plant Biol. 17: 91.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu J., Haq N.U., Zhang H., Zhu X.G. (2014) Was low CO2 a driving force of C4 evolution: Arabidopsis responses to long-term low CO2 stress. J. Exp. Bot. 65: 3657–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu Z.B., Xing A., Moon B.P., Koellhoffer J.P., Huang L., et al. (2015) Cas9-guide RNA directed genome editing in soybean. Plant Physiol. 169: 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado dos Santos J.V., Valliyodan B., Joshi T., Khan S.M., Liu Y., Wang J., et al. (2016) Evaluation of genetic variation among Brazilian soybean cultivars through genome resequencing. BMC Genomics 17: 110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe D.E., Swain W.F., Martinell B.J., Christou P. (1988) Stable transformation of soybean (Glycine max) by particle acceleration. Nat. Biotechnol. 6: 923–926. [Google Scholar]
- Obendorf R.L., Ashworth E.N., Rytko G.T. (1980) Influence of seed maturation on germinability in soybean. Crop Sci. 20: 483. [Google Scholar]
- Ogata T., Nagatoshi Y., Yamagishi N., Yoshikawa N., Fujita Y. (2017) Virus-induced down-regulation of GmERA1A and GmERA1B genes enhances the stomatal response to abscisic acid and drought resistance in soybean. PLoS One 12: e0175650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Yoshino M., Yamakawa H., Kinoshita T. (2011) The biotron breeding system: a rapid and reliable procedure for genetic studies and breeding in rice. Plant Cell Physiol. 52: 1249–1257. [DOI] [PubMed] [Google Scholar]
- Ohyama T., Takahashi Y., Joh T., Whitaker A.C., Nishiwaki T., Morohashi K., et al. (2013) Traditional and Modern Japanese Soy Foods: Manufacturing, Nutrition and Cuisine of a Variety of Soy Foods for Health and Joy of Taste. Nova Science Publishers, New York. [Google Scholar]
- Oya T., Nepomuceno A.L., Neumaier N., Bou�as Farias J.R., Tobita S., Ito O. (2004) Drought tolerance characteristics of Brazilian soybean cultivars— valuation and characterization of drought tolerance of various Brazilian soybean cultivars in the field. Plant Prod. Sci. 7: 129–137. [Google Scholar]
- Ozawa S., Hatakeyama K., Takahata Y., Yokoi S. (2017) The transition time from the juvenile phase to the adult phase differs in soybean cultivars ‘Enrei’ and ‘Peking’. Plant Breed. 136: 386–392. [Google Scholar]
- Rodrigues F.A., Fuganti-Pagliarini R., Marcolino-Gomes J., Nakayama T.J., Molinari H.B.C., Lobo F.P., et al. (2015) Daytime soybean transcriptome fluctuations during water deficit stress. BMC Genomics 16: 505.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumet P., Morin F. (1997) Germination of immature soybean seeds to shorten reproductive cycle duration. Crop Sci. 37: 521. [Google Scholar]
- Schmutz J., Cannon S.B., Schlueter J., Ma J., Mitros T., Nelson W., et al. (2010) Genome sequence of the palaeopolyploid soybean. Nature 463: 178–183. [DOI] [PubMed] [Google Scholar]
- Schon M.K., Blevins D.G. (1990) Foliar boron applications increase the final number of branches and pods on branches of field-grown soybeans. Plant Physiol. 92: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura M., Kanamori H., Komatsu S., Namiki N., Mukai Y., Kurita K., et al. (2015) The Glycine max cv. Enrei genome for improvement of Japanese soybean cultivars. Int. J. Genomics 2015: 358127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar A., Shivakumar M. (2012) Pollination without emasculation: an efficient method of hybridization in soybean (Glycine max (L.) Merrill). Curr. Sci. 103: 629–630. [Google Scholar]
- Tanaka J., Hayashi T., Iwata H. (2016) A practical, rapid generation-advancement system for rice breeding using simplified biotron breeding system. Breed. Sci. 66: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z., Wang X., Lee R., Li Y., Specht J.E., Nelson R.L., et al. (2010) Artificial selection for determinate growth habit in soybean. Proc. Natl. Acad. Sci. USA 107: 8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Kaga A., Anai T., Shimizu T., Sayama T., Takagi K., et al. (2015) Construction of a high-density mutant library in soybean and development of a mutant retrieval method using amplicon sequencing. BMC Genomics 16: 1014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture (USDA). (2018) Oilseeds: World Markets and Trade. Foreign Agricultural Service; Washington, DC. [Google Scholar]
- Walker A.K., Cianzio S.R., Bravo J.A., Fehr W.R. (1979) Comparison of emasculation and nonemasculation for hybridization of soybeans. Crop Sci. 19: 285. [Google Scholar]
- Watanabe S., Harada K., Abe J. (2012) Genetic and molecular bases of photoperiod responses of flowering in soybean. Breed. Sci. 61: 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Shimizu T., Machita K., Tsubokura Y., Xia Z., Yamada T., et al. (2018) Development of a high-density linkage map and chromosome segment substitution lines for Japanese soybean cultivar Enrei. DNA Res. 25: 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A., Ghosh S., Williams M.J., Cuddy W.S., Simmonds J., Rey M.D., et al. (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 4: 23–29. [DOI] [PubMed] [Google Scholar]
- Wolfgang G., An Y.C. (2017) Genetic separation of southern and northern soybean breeding programs in North America and their associated allelic variation at four maturity loci. Mol. Breed. 37: 8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Abe J., Gai Y., Shimamoto Y. (2002) Diversity of chloroplast DNA SSRs in wild and cultivated soybeans: evidence for multiple origins of cultivated soybean. Theor. Appl. Genet. 105: 645–653. [DOI] [PubMed] [Google Scholar]
- Yamada T., Takagi K., Ishimoto M. (2012a) Recent advances in soybean transformation and their application to molecular breeding and genomic analysis. Breed. Sci. 61: 480–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Hajika M., Yamada N., Hirata K., Okabe A., Oki N., et al. (2012b) Effects on flowering and seed yield of dominant alleles at maturity loci E2 and E3 in a Japanese cultivar, Enrei. Breed. Sci. 61: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N., Yoshikawa N. (2009) Virus-induced gene silencing in soybean seeds and the emergence stage of soybean plants with Apple latent spherical virus vectors. Plant Mol. Biol. 71: 15–24. [DOI] [PubMed] [Google Scholar]
- Zhang C., Ghabrial S.A. (2006) Development of Bean pod mottle virus-based vectors for stable protein expression and sequence-specific virus-induced gene silencing in soybean. Virology 344: 401–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.