Abstract
The target molecules of antibodies against falciparum malaria remain largely unknown. Recently we have identified multiple proteins as targets of immunity against Plasmodium falciparum using African serum samples. To investigate whether potential targets of clinical immunity differ with transmission intensity, we assessed immune responses in residents of low malaria transmission region in Thailand. Malaria asymptomatic volunteers (Asy: n = 19) and symptomatic patients (Sym: n = 21) were enrolled into the study. Serum immunoreactivity to 186 wheat germ cell-free system (WGCFS)-synthesized recombinant P. falciparum asexual-blood stage proteins were determined by AlphaScreen, and subsequently compared between the study groups. Forty proteins were determined as immunoreactive with antibody responses to 35 proteins being higher in Asy group than in Sym group. Among the 35 proteins, antibodies to MSP3, MSPDBL1, RH2b, and MSP7 were significantly higher in Asy than Sym (unadjusted p < 0.005) suggesting these antigens may have a protective role in clinical malaria. MSP3 reactivity remained significantly different between Asy and Sym groups even after multiple comparison adjustments (adjusted p = 0.033). Interestingly, while our two preceding studies using African sera were conducted differently (e.g., cross-sectional vs. longitudinal design, observed clinical manifestation vs. functional activity), those studies similarly identified MSP3 and MSPDBL1 as potential targets of protective immunity. This study further provides a strong rationale for the application of WGCFS-based immunoprofiling to malaria vaccine candidate and biomarker discovery even in low or reduced malaria transmission settings.
Keywords: Blood-stage vaccine, High-throughput immunoscreening, Malaria, Plasmodium falciparum, Thailand, Wheat germ cell-free system (WGCFS)
1. Introduction
Malaria remains a global health problem in the tropical and subtropical region of the world. An estimated 212 million cases of malaria occur annually with 429,000 deaths reported in 2015 [1]. Malaria control efforts, such as long-lasting insecticidal nets, indoor residual spraying and expanding access to the artemisinin-based combination therapies, are undoubtedly effective [1]. However, the recent spread of parasite resistance to artemisinins and mosquito resistance to insecticides may instigate a reversion of the positive trend [1,2]. One promising complementary measure is vaccination. However, a sole phase 3 clinical trial of a leading malaria vaccine, RTS,S/AS01, showed moderate efficacy [3]. Moreover, malaria vaccine development efforts to date have yielded only a handful of vaccine candidates, with few that progressed to clinical trials having no or marginal efficacies [4] [as reviewed in [5]]. The major challenge for the identification of novel vaccine candidates remains to be poor understanding of the Plasmodium falciparum antigens that are targets of naturally acquired protective immunity.
Although studies in malaria endemic regions clearly show that protective immunity, at least for clinical malaria, can be developed in individuals exposed to repeated P. falciparum infections [6], the mechanism of this protective immunity remains largely unknown. However, antibodies are key in protection against high parasitemia and clinical malaria, as demonstrated by passive-transfer studies in humans [7,8]. Therefore, there is an urgent need to objectively profile antibody responses against P. falciparum antigens for deeper understanding of the mechanism of acquired immunity as well as identification of novel vaccine candidates.
To identify the key targets of protective immunity, population-based immuno-epidemiological studies have of recent been employed to establish temporal associations between antibody profiles and subsequent clinical outcome [9–14]. In these studies, recombinant plasmodia proteins expressed using in vitro bacterial translation systems and printed on microarray chip were probed with sera obtained from malaria-exposed individuals [10,11]. Using these approaches, several antigens were identified as potential vaccine candidates. However, the major limitation of this strategy is the possibility of proteins expressed by the bacterial expression systems not attaining natural conformations [15,16]. On the other hand, only few proteins expressed by eukaryotic expression system have been profiled against natural immunity [9,12]. Importantly, we have recently demonstrated that wheat germ cell-free system (WGCFS), a robust eukaryotic alternative to express plasmodial proteins, overcomes these limitations [17,18]. It has been shown that animal antibodies raised against multiple WGCFS-synthesized recombinant proteins recognize their respective native proteins and have parasite growth-inhibition activity in vitro [19]. The recombinant proteins also strongly react with sera obtained from individuals exposed to P. falciparum [reviewed in [19]]. These data clearly shows that the proteins are to a great extent correctly folded and can be considered as representative of native proteins [19]. We thus, in our previous study, created a WGCFS-synthesized protein library consisting of 1827 recombinant proteins, representing ~ 35% of the P. falciparum proteome. A 12-month prospective study conducted in a malaria high-endemic area in Uganda, where entomological inoculation rate (EIR) of > 100 infective bites/year, identified antibody responses against 53 proteins, which are natively expressed mainly in asexual-blood and sporozoite stages, significantly associated with protection from clinical malaria [20]. With decreasing malaria transmission and as we gear towards malaria elimination, it is of great importance to understand whether targets of protective immunity against falciparum malaria are similar with those in low transmission areas such as in South East Asia (www.aplma.org).
In the current study, we attempted to identify P. falciparum asexual-blood stage proteins that are potential targets of antibodies associated with clinical protection in western Thailand; a region of low malaria endemicity where EIR of 1–3 infective bites/year [21]. Antibody levels to 186 WGCFS-synthesized recombinant proteins were determined by a high-throughput AlphaScreen method [22]. Antibody responses to 35 proteins in asymptomatic (Asy) group were significantly higher than those in symptomatic (Sym) group. This study further provides a strong rationale for the application of WGCFS-based immunoprofiling approach to malaria vaccine candidate discovery even in regions of low malaria endemicity.
2. Materials and methods
2.1. Parasite culture, total RNA isolation, and cDNA preparation
P. falciparum asexual stage parasite (3D7) was maintained in vitro according to Trager-Jensen method [23] using healthy human erythrocytes (blood group O +) obtained from the Japanese Red Cross Society, supplemented with 5% heat-inactivated human plasma and 0.5% AlbuMAX™ I (Thermo Scientific, Waltham, MA) in an atmosphere of 5% O2, 5% CO2, and 90% N2. Total RNA was extracted from frozen infected RBC (iRBC) pellet rich in late-trophozoite and schizont by TRIzol™ reagent (Invitrogen, Carlsbad, CA) and then treated with DNase I (Invitrogen) at 37 °C for 15 min. cDNA was generated with random hexamers by using Superscript III™ (Invitrogen) at 25 °C for 10 min followed by at 50 °C for 50 min and at 85 °C for 5 min.
2.2. In silico gene selection and cDNA cloning
We selected a set of P. falciparum genes from the malaria genome database (PlasmoDB; http://plasmodb.org/plasmo/) which are expressed either (1) at least twice as much in schizont than in trophozoite at the level of transcription [24–26], or (2) exclusively at the merozoite stage [27]. The resulting list of ~ 200 genes was narrowed down to 192 based on ascending molecular size (< 5.9 kbp, Table S1). To create a cDNA library, 5′ primers were designed as 46-mers: 16-mer Sl-tag sequence followed by a 30-mer of unique sequence covering each 5′ open reading frame containing the start codon [28]. For the 3′ primers, 30-mer nucleotide sequences covering each unique sequence upstream of the termination codon were prepared. The cDNA PCR amplification was performed with Phusion™ High-Fidelity DNA Polymerase (Thermo Scientific). The PCR products were then cloned into the pCR™2.1 plasmid using a TOPO TA cloning kit (Invitrogen), and their nucleotide sequences were confirmed by sequencing both 5′ and 3′ ends. Finally, we obtained 186 cDNA clones with cloning of the remaining 6 cDNA being unsuccessful.
2.3. Construction of P. falciparum biotinylated protein library using WGCFS
Transcription templates were prepared by amplification of the P. falciparum cDNA clones by a two-step split-primer method as previously described [22,28]. Briefly, the first PCR was conducted with 10 nM of the following primers: sense primer corresponding to the S1-tag followed by a start codon: S1-ATG; 5′-CCACCCACCACCACCAATG-3′, and antisense primers corresponding to the vector (pCR™2.1, Invitrogen) sequence: 1931A; 5′-CGGCCACAGTCGATGAATCC-3′ or 221 IS; S’-GCTGGTGAAAGTAAAAGATG-S’ depending on the orientation of each cDNA insert in the plasmid. The second PCR for transcription templates were constituted with 100 nM SPu: 5′-GCGTAGCATTTAGG TGACACT-3′, 1 nM deSP6E02bls-Sl: 5′-GGTGACACTATAGAACTCACC TATCTCTCTACACAAAACATTTCCCTACATACAACTTTCAACTTCCTAT TATGGGCCTGAACGACATCTTCGAGGCCCAGAAGATCGAGTGGCACGA ACTCCACCCACCACCACCAATG-3′ and 100 nM 1923A: 5′-GTCGATGA ATCCAGAAAAGC-3′, or 2214S: 5′-GGTGAAAGAAAAAGATGCTG-3′, depending on the orientation of each cDNA insert in the plasmid. With the “split-primer PCR” strategy, mono-biotin ligation site (bis) was inserted upstream of each cDNA for subsequent protein biotinylation. The WGCFS protocol was modified with addition of WGCFS-expressed crude BirA and 500 nM D-biotin (Nacalai Tesque, Kyoto, Japan) to the translation mixture for simultaneous biotinylation as described [22]. Consecutive in vitro transcription and translation by WGCFS was carried out using the GenDecoder1000 robotic protein synthesizer (Cell-Free Sciences, Matsuyama, Japan) as described [29]. Expression of each recombinant protein in the protein library was confirmed by separation on 12.5% SDS-PAGE, stained with fluorescein avidin D (Vector Laboratories, Peterborough, U.K.) and scanned with Typhoon9400 imager at 488 nm laser excitation (GE Healthcare, Piscataway, NJ) [22]. The assembled P. falciparum protein library consisting of 186 proteins was used for detecting humoral immune responses without any purification.
2.4. Serum samples
The study was designed to compare antibody responses in serum samples of adults with asymptomatic P. falciparum malaria (Asy) on one hand and uncomplicated symptomatic P. falciparum malaria patients (Sym) on the other. Briefly, 19 serum samples were collected from Asy residents of Kong Mong Tha, Kanchanaburi Province, western Thailand in an active follow-up study from June 2001 to May 2005 [30]. Asymptomatic P. falciparum malaria was defined as having no reported fever (≤ 37.5 °C) at the time of serum collection but with any parasitemia either by microscopy or nested PCR [30]. The study was approved by the Ethics Committee of the Thai Ministry of Public Health and the Institutional Review Board of the Walter Reed Army Institute of Research (WRAIR 802) [30]. Additional serum samples were obtained between May and June 2005 from 21 adults with uncomplicated symptomatic P. falciparum malaria (Sym) who were residents of Tak Province neighboring to Kanchanaburi Province, western Thailand. Of note, all the 21 Sym cases had had at least one confirmed and recorded falciparum malaria episode before the one corresponding to sample collection. Sym was defined as having fever (> 37.5 °C) at the time of sample collection with any parasitemia either by microscopy or nested PCR but without any manifestation of signs of complicated malaria based on the WHO guideline. Control subjects were 11 healthy malaria naive adult residents (Nor) in malaria-free Bangkok who had no history of travelling outside the region (and no medical history of malaria infection). This study with Sym and Nor groups, received ethical approval from the ethical review committees of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University (ID: 09-46-10). All the Asy and Sym samples (Table 1) were diagnosed as P. falciparum single species (mono) infection by PCR. All individuals enrolled in this study provided written informed consent. Protocol to use the serum samples in this study was also approved by the Institutional Review Boards of Ehime University Hospital, Japan.
Table 1.
Characteristics of individuals enrolled in this study in Thailand.
| Characteristics | Nora | Asyb | Symc |
|---|---|---|---|
| (n = 11) | (n = 19) | (n = 21) | |
| Age in years at sampling, median (range) | NA | 30 (11–50) | 23 (17–50) |
| Gender (male %) | NA | 63 | 90 |
| Median parasitemia % (range) | 0 | 0.003d (0.0006–0.2020) | 0.600 (0.002–2.900) |
Healthy malaria naive adult residents in malaria-free Bangkok who had no history of travelling outside the region. NA: not available.
Asymptomatic malaria defined as body temperature ≤ 37.5 °C with any parasitemia.
Symptomatic malaria defined as body temperature > 37.5 °C with any parasitemia, but not signs of complicated malaria.
Statistically significant by Mann Whitney U test.
2.5. Detecting humoral immune responses to P. falciparum proteins by AlphaScreen
AlphaScreen (amplified luminescent proximity homogeneous assay) was performed according to the manufacturer's protocol (PerkinElmer Life and Analytical Sciences, Boston, MA). Specifically, the reactions were carried out in a final volume of 19 μl in 384-well Optiwell microtiter plates (PerkinElmer). For the antigen-antibody reaction, 3 μl of each P. falciparum biotinylated protein was mixed with 6 μl of the human serum sample (described below) diluted 1:400 in the reaction buffer [100 mM Tris-HCl (pH 8.0), 0.01% (v/v) polyoxyethylene (20) sorbitan monolaurate (Wako Pure Chemical, Osaka, Japan, an equivalent of Tween-20) and 0.1% (w/v) bovine serum albumin (Wako)] and incubated at 30 °C for 30 min. Subsequently, 10 μl of the detection mixture [12 μg/ml streptavidin-coated donor beads (PerkinElmer), 12 μg/ml protein G-conjugated acceptor beads (PerkinElmer) in the reaction buffer] was added and incubated at 26 °C for 1 h in a dark box. Fluorescence emission in the range of 580–620 nm was measured using Envision plate reader (PerkinElmer) with the 680-nm laser excitation. The data was captured by the AlphaScreen detection program and presented as AlphaScreen counts (ASC) (PerkinElmer). All antibody measurements were performed in replicates.
2.6. Enzyme-linked immunosorbent assay (ELISA)
Ninety-six-well ELISA plates were coated with 50 μl of the WGCFS-expressed crude MSP3 protein diluted 1:50 in the coating buffer (20 mM boric acid, pH 8.9) in duplicate and incubated at 4 °C overnight. The plates were washed with phosphate-buffered saline with 0.1% (v/v) Tween-20 (PBS-T) and then blocked with 2 mg/ml of gelatin in coating buffer for 1 h at room temperature. Selected human serum samples (n = 20) were diluted at 1:600 in PBS-T, added to antigen-coated wells in duplicate, and incubated for 1 h at room temperature. After washing, the plates were incubated with 1:3000-diluted HRP-conjugated rabbit anti-human IgG (DakoCytomation, Glostrup, Denmark) in PBS-T for 1 h at room temperature. The plates were again washed with PBS-T followed by incubation with 0.5 mg/ml azino-bis-3-ethylbenthiazoline-6-sulfonic acid (Wako) diluted in citrate buffer (0.1 M citric acid, pH 4.1) for 20 min at room temperature. The reaction was stopped with 0.1 M citric acid, and optical densities (ODs) were determined at 415 nm using precision microplate reader (Molecular Devices, Sunnyvale, CA).
2.7. Statistical analyses
All original ASC were log-transformed and the data from assay replicates normalized using the median of log-transformed ASC of all proteins. The arithmetic mean of normalized log-transformed ASC (mASC) from two assays was calculated for each antibody-protein response. To determine background signal level, three negative control proteins (Non-M pro) were included in each plate: biotinylated non-malarial proteins - flowering locus T (FT) and dihydrofolate reductase (DHFR), and cell-free translation mixture without template mRNA (WGE). Background signal was then set as the average of log-transformed ASC of the three proteins. Malaria-specific response for each malaria protein, final value (fASC), was determined by subtracting the average background signal from the mASC. A protein with significantly higher fASC in Asy and Sym groups than in Nor group was defined as immunoreactive protein, and only immunoreactive proteins were subjected to further analysis. Welch's t-test was performed to compare fASC between Asy and Sym groups and Holm's corrections were applied for multiple comparison adjustments.
All statistical tests were performed by R (version 3.2.3) or Prism 6 (GraphPad Software Inc., La Jolla, CA) with probability value < 0.05 being considered significant.
3. Results
3.1. Characteristics of the malaria cases
Serum samples were obtained from adult volunteers in Thailand. As shown in Table 1, median age of the Asy and Sym groups were similar. However, gender was biased towards male for Sym (90% male) than in Asy group (63% male). Median parasitemia in Asy group was significantly lower than in Sym group; 0.003% (0.0006–0.202% range) and 0.6% (0.002–2.9% range) respectively (Mann Whitney U test, p < 0.0001).
3.2. Determination of immunoreactivity profiles to P. falciparum proteins
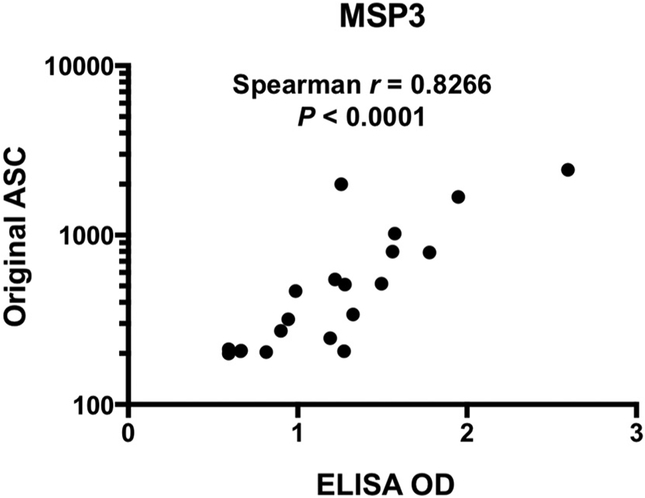
To compare antibody responses measured by conventional ELISA and high-throughput AlphaScreen system, randomly selected human serum samples (Asy; n = 13 and Sym; n = 7) were tested against P. falciparum MSP3; a representative of well-known blood-stage vaccine candidates. Fig. 1 clearly shows that original ASC values and ELISA OD values against MSP3 were significantly and positively correlated (Spearman r = 0.8266, p < 0.0001). The results demonstrated that AlphaScreen system could offer a better high-throughput approach to assessing antibody responses against multiple proteins in malaria-exposed individuals.
Fig. 1.
Correlation between original ASC and ELISA OD values.
Original ASC values and ELISA OD values against MSP3 were analyzed by Spearman's rank correlation. Number of serum samples is 20.
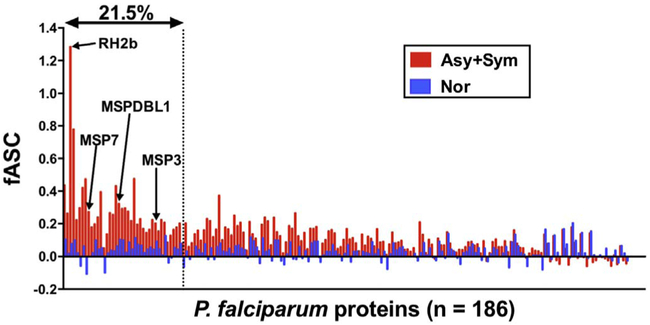
To identify which parasite proteins that are immunogenic in natural infection, we determined immunoreactivity of P. falciparum proteins to sera obtained from Asy, Sym, and Nor individuals. Of the 186 recombinant proteins synthesized by WGCFS and analyzed by AlphaScreen system, antibody responses to 158 proteins were higher in malaria positive (Asy + Sym) group than in Nor group (Table S1; Y indicates Asy + Sym > Nor). Out of those 158, 40 proteins showed significantly higher fASC in Asy + Sym group than in Nor group (Table S1; Asy + Sym vs. Nor (Adjusted p): adjusted p-values for 158 comparisons), by this virtue, the 40 (21.5%) proteins were considered as immunoreactive (Fig. 2).
Fig. 2.
Immunoreactivity of WGCFS-synthesized protein library of Plasmodium falciparum. Each bar represents mean fASC (malaria-specific response) of either Asy + Sym or Nor groups. Further explanation of fASC is provided in Materials and methods Section 2.7. Number of proteins in horizontal axis is 186. Forty (21.5%) proteins were defined as immunoreactive proteins. Four selected antigens, MSP3, MSPDBL1, RH2b, and MSP7, are indicated.
3.3. Identification of P. falciparum proteins associated with higher immunoreactivity in Asy than Sym
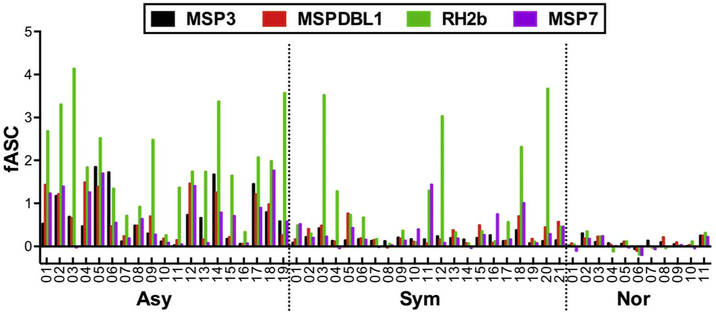
For the 40 immunoreactive proteins, fASC between Asy and Sym groups were compared to identify potential targets of clinical immunity. Out of the 40, 35 proteins had relatively higher median fASC in Asy than Sym. Four of the 35 proteins, namely; MSP3, MSPDBL1, RH2b, and MSP7, had significantly higher fASC in Asy than Sym group (unadjusted p < 0.05 by Welch's t-tests, Table 2). However, only MSP3 remains statistically significant after adjusting for the multiple comparisons (n = 35, adjusted p = 0.0327). The distributions of fASC to the 4 selected antigens by individual serum sample in Asy, Sym, and Nor groups are shown in Fig. 3. All 4 (out of 40) selected antigens had predicted signal peptide (SP) and/or transmembrane domain(s) (TM) based on PlasmoDB (http://plasmodb.org/).
Table 2.
Comparative antibody responses to the selected 4 antigens between Asy and Sym group.
| Prot.no. | Gene ID | Protein name (PlasmoDB, release 27) | SPa | TMb | fASC difference (p-value: Asy > Sym)c |
|
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| 26 | PF3D7_1035400 | Merozoite surface protein 3 (MSP3) | Y | N | 0.0009 | 0.0327 |
| 56 | PF3D7_1035700 | Duffy binding-like merozoite surface protein (MSPDBL1) | Y | N | 0.0018 | 0.0613 |
| 165 | PF3D7_1335300 | Reticulocyte binding protein 2 homologue b (RH2b) | Y | Y | 0.0052 | 0.1724 |
| 23 | PF3D7_1335100 | Merozoite surface protein 7 (MSP7) | Y | N | 0.0168 | 0.5369 |
SP: signal peptide. Y: predicted.
TM: transmembrane domain. Y: predicted. N: unpredicted.
Welch's t-test was performed to compare fASC between Asy and Sym for each malaria protein, and Holm's correction was used for multiple comparisons. Statistically significant values are shown in bold.
Fig. 3.
Distribution of fASC of the 4 selected antigens. Individual fASC values to the 4 selected antigens, MSP3, MSPDBL1, RH2b, and MSP7 among Asy (n = 19), Sym (n = 21), or Nor (n = 11) group are shown.
4. Discussion
We hereby attempted to identify P. falciparum asexual blood-stage proteins that are targets of humoral immunity against clinical malaria by analyzing serum samples obtained from Thailand; a region of low malaria endemicity. Immunoreactivities to 186 WGCFS-synthesized recombinant proteins from a P. falciparum asexual blood-stage were determined by high-throughput AlphaScreen method [22]. Forty proteins were immunoreactive, and antibody responses against 35 proteins in Asy group were relatively higher than responses in Sym group. Among the 35, antibody responses to 4 proteins (MSP3, MSPDBL1, RH2b, and MSP7) were significantly higher in Asy than Sym individuals. With the study site, Kong Mong Tha in western Thailand, experiencing low but stable malaria transmission intensity, decreasing parasite densities with increasing age and preponderance of asymptomatic infections in the area was previously observed [30]. We therefore hypothesized that adults in Asy group had acquired some level of protective immunity against P. falciparum malaria. Thus, the results obtained here suggest that the 4 antigens are potential targets of protective humoral immunity and could be prospective malaria vaccine candidates. MSP3 was selected with significantly higher fASC in Asy than Sym group even after adjustment for multiple comparisons (adjusted p-value = 0.0327), with MSPDBL1 leaning towards significance threshold (adjusted p-value = 0.0613). This study therefore, further strengthens the usefulness of WGCFS-based immunoprofiling approach to malaria vaccine candidate discovery even in the low endemic settings.
This being a proof-of-concept study, the number of serum samples in each group was small relative to the number of proteins tested. Therefore, unadjusted p-values were utilized for testing significance when comparing fASC levels between groups (between Asy + Sym vs. Nor, or between Asy vs. Sym) (Table 2, and Table S1). The small sample size used in this study may have under- or over-estimated immune-associations, hence a future study with larger and well-matched samples and/or a targeted study with fewer study proteins are required to confirm the current observations. In addition, such study may identify more subtle associations between antibody responses and protection from symptomatic malaria.
By using similar platform consisting of WGCFS-synthesized protein library and AlphaScreen, we recently reported high immunoreactivity of upto 49% or 51% of 1827 P. falciparum proteins against Malian semiimmune adult sera [31] and Ugandan young adult sera (6 to 20 years' old) [20], respectively. In contrast, immunoreactivity of 186 P. falciparum proteins in this study was at 21.5% (Fig. 2). The lower immunoreactivity observed here could be explained, in part, by the low malaria transmission intensity in the Thai study site, with an EIR of 1–3 infective bites/year [21], compared to high malaria transmission intensity of EIR > 100 infective bites/year in the African (Ugandan) field site [20].
Although all three studies used similar screening platform, sample selection and definition of clinical malaria used in the current study was completely different from those of our two recent studies with African serum samples. However, to our surprise, all three studies identified same proteins, MSP3 and MSPDBL1. The Ugandan study was a longitudinal cohort study (n = 66) where the associations between baseline antibody reactivity and time to the first subsequent malaria episode were assessed. Reactivity to MSP3 and MSPDBL1 significantly associated with reduced risk of symptomatic malaria before adjusting for age and bednet use [20]. In another study, immunoreactivity of Malian adult total IgGs (n = 51) with various levels of in vitro growth-inhibition activity was determined. The study similarly revealed that the reactivity to MSP3 and MSPDBL1 significantly correlated with functional activity before adjusting for multiple comparisons. Furthermore, the Mali study also identified a significant (unadjusted) association for RH2b protein [31]. The fact that same proteins were identified in totally different settings by using similar platform consisting of WGCFS-synthesized protein library and AlphaScreen, suggests that this technology advancement is universally useful for biomarker discovery in malaria.
The data presented here is squarely consistent with previous study in naturally acquired immunity that led to the identification of the MSP3 [32]. Antibodies to MSP3 have consistently associated with a reduction in clinical malaria episodes [20,33–36]. More specifically, increase of cytophilic subclasses (IgGl and/or IgG3) was significantly associated with reduced risk of clinical malaria after adjusting for age in several studies [33,34,36]. However, a recent phase 2b clinical trial of GMZ2 malaria vaccine, a blood-stage vaccine containing Glutamate-Rich Protein (GLURP) and MSP3, resulted in the low vaccine efficacy (14%) [37]. Therefore, MSP3-based vaccine still needs improvement. MSPDBL1 is a member of the MSP3 family and has both a Duffy binding-like (DBL) and a secreted polymorphic antigen associated with merozoites (SPAM) domains. Previously, we observed that anti-MSPDBL1 rabbit antibodies raised against recombinant full-length MSPDBL1 inhibited erythrocyte invasion in vitro [38], suggesting that MSPDBL1 is also a potential blood-stage malaria vaccine candidate. These studies further highlight the vaccine candidacy of both MSP3 and MSPDBL1 and clearly demonstrate that WGCFS-synthesized proteins could greatly contribute towards the successful identification and prioritization of multiple blood-stage antigens for vaccine candidate discovery.
In conclusion, this study demonstrated that, generation of protein library using the WGCFS coupled with high-throughput AlphaScreen system provides an ideal platform for rational discovery and prioritization of potential blood-stage malaria vaccine candidates using serum samples collected from both low endemic areas (such as Thailand) and high endemic areas (such as Sub-Saharan Africa).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parint.2017.12.002.
Supplementary Material
Acknowledgments
We thank volunteers in Thailand who participated in the epidemiology study. We also thank the staff of the Department of Entomology, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand, for their technical assistance. We acknowledge the Japanese Red Cross Society for providing human erythrocytes and human plasma.
The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Funding
This work was supported in part by MEXT KAKENHI (JP23117008) and JSPS KAKENHI (JP25460517, JP26253026, JP26670202, JP26860279, JP15H05276, JP16K15266) in Japan, and Thailand Research Fund (5TMU45H1) to RU. KM was also supported in part by the Intramural Research Program of NIAID, NIH. The funding source was not involved in any part of the study design, analysis, or interpretation of the data.
Abbreviations:
- WGCFS
Wheat germ cell-free system
- ASC
AlphaScreen Counts
- Asy
asymptomatic malaria Thai volunteers
- Sym
symptomatic malaria Thai volunteers
- Nor
normal (malaria naïve) Thai volunteers
- Asy + Sym
either asymptomatic or symptomatic malaria volunteers
- Adjusted p-value
adjusted p-value post multiple comparisons by a Holm method
- Non-M pro
malaria unrelated proteins, i.e., flowering locus T (FT), dihydrofolate reductase (DHFR), and wheat germ cell-free translation mixture without mRNA (WGE) serving as negative controls used in this study
Footnotes
Conflict of interest disclosure
The authors declare no commercial or financial conflict of interest.
References
- [1].WHO, World Malaria Report 2016, (2016). [Google Scholar]
- [2].Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Taming J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ, Artemisinin resistance in Plasmodium falciparum malaria, N. Engl. J. Med. 361 (5) (2009) 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].S.C.T.P. RTS, Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial, Lancet 386 (9988) (2015) 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thera MA, Doumbo OK, Coulibaly D, Laurens MB, Ouattara A, Kone AK, Guindo AB, Traore K, Traore I, Kouriba B, Diallo DA, Diarra I, Daou M, Dolo A, Tolo Y, Sissoko MS, Niangaly A, Sissoko M, Takala-Harrison S, Lyke KE, Wu Y, Blackwelder WC, Godeaux O, Vekemans J, Dubois MC, Ballou WR, Cohen J, Thompson D, Dube T, Soisson L, Diggs CL, House B, Lanar DE, Dutta S, Heppner DG Jr., Plowe CV, A field trial to assess a blood-stage malaria vaccine, N. Engl. J. Med. 365(11) (2011) 1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miura K, Progress and prospects for blood-stage malaria vaccines, Exp. Rev. Vaccine 15 (6) (2016) 765–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C, Immunity to non-cerebral severe malaria is acquired after one or two infections, Nat. Med. 5 (3) (1999) 340–343. [DOI] [PubMed] [Google Scholar]
- [7].Cohen S, Me GI, Carrington S, Gamma-globulin and acquired immunity to human malaria, Nature 192 (1961) 733–737. [DOI] [PubMed] [Google Scholar]
- [8].McGregor IA, The passive transfer of human malarial immunity, Am. J. Trop. Med. Hyg. 13 (Suppl. 237–9) (1964). [DOI] [PubMed] [Google Scholar]
- [9].Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, Ogada E, McDade B, Rayner JC, Wright GJ, Marsh K, New antigens for a multicomponent blood-stage malaria vaccine, Sci. Transl. Med 6 (247) (2014) 247ral02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Feigner PL, Pierce SK, A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray, Proc. Natl. Acad. Sci. U. S. A. 107 (15) (2010) 6958–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Doolan DL, Mu Y, Unal B, Sundaresh S, Hirst S, Valdez C, Randall A, Molina D, Liang X, Freilich DA, Oloo JA, Blair PL, Aguiar JC, Baldi P, Davies DH, Feigner PL, Profiling humoral immune responses to P. falciparum infection with protein microarrays, Proteomics 8 (22) (2008) 4680–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, Cross N, Langer C, Takeo S, Uboldi AD, Thompson JK, Gilson PR, Coppel RL, Siba PM, King CL, Torii M, Chitnis CE, Narum DL, Mueller I, Crabb BS, Cowman AF, Tsuboi T, Beeson JG, Identification and prioritization of merozoite antigens as targets of protective human immunity to plasmodium falciparum malaria for vaccine and biomarker development, J. Immunol. 191 (2) (2013) 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dent AE, Nakajima R, Liang L, Baum E, Moormann AM, Sumba PO, Vulule J, Babineau D, Randall A, Davies DH, Feigner PL, Kazura JW, Plasmodium falciparum protein microarray antibody profiles correlate with protection from symptomatic malaria in Kenya, J. Infect. Dis. 212 (9) (2015) 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chia WN, Goh YS, Renia L, Novel approaches to identify protective malaria vaccine candidates, Front. Microbiol. 5 (2014) 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aguiar JC, LaBaer J, Blair PL, Shamailova VY, Koundinya M, Russell JA, Huang F, Mar W, Anthony RM, Witney A, Caruana SR, Brizuela L, Sacci JB Jr., Hoffman SL, Carucci DJ, High-throughput generation of P. falciparum functional molecules by recombinational cloning, Genome Res. 14 (10B) (2004) 2076–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vedadi M, Lew J, Artz J, Amani M, Zhao Y, Dong A, Wasney GA, Gao M, Hills T, Brokx S, Qiu W, Sharma S, Diassiti A, Alam Z, Melone M, Mulichak A, Wernimont A, Bray J, Loppnau P, Plotnikova O, Newberry K, Sundararajan E, Houston S, Walker J, Tempel W, Bochkarev A, Kozieradzki I, Edwards A, Arrowsmith C, Roos D, Kain K, Hui R, Genome-scale protein expression and structural biology of Plasmodium falciparum and related Apicomplexan organisms, Mol. Biochem. Parasitol. 151 (1) (2007) 100–110. [DOI] [PubMed] [Google Scholar]
- [17].Tsuboi T, Takeo S, Iriko H, Jin L, Tsuchimochi M, Matsuda S, Han ET, Otsuki H, Kaneko O, Sattabongkot J, Udomsangpetch R, Sawasaki T, Torii M, Endo Y, Wheat germ cell-free system-based production of malaria proteins for discovery of novel vaccine candidates, Infect. Immun. 76 (4) (2008) 1702–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rui E, Fernandez-Becerra C, Takeo S, Sanz S, Lacerda MV, Tsuboi T, del Portillo HA, Plasmodium vivax: comparison of immunogenicity among proteins expressed in the cell-free systems of Escherichia coli and wheat germ by suspension array assays, Malar. J. 10 (2011) 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arumugam TU, Ito D, Takashima E, Tachibana M, Ishino T, Torii M, Tsuboi T, Application of wheat germ cell-free protein expression system for novel malaria vaccine candidate discovery, Exp. Rev. Vaccine 13 (1) (2014) 75–85. [DOI] [PubMed] [Google Scholar]
- [20].Kanoi BN, Takashima E, Morita M, White MT, Palacpac NM, Ntege EH, Balikagala B, Yeka A, Egwang TG, Horii T, Tsuboi T, Antibody profiles to wheat germ cell-free system synthesized Plasmodium falciparum proteins correlate with protection from symptomatic malaria in Uganda, Vaccine 35 (6) (2017) 873–881. [DOI] [PubMed] [Google Scholar]
- [21].Coleman RE, Sithiprasasna R, Kankaew P, Kiaattiut C, Ratanawong S, Khuntirat B, Sattabongkot J, Naturally occurring mixed infection of Plasmodium vivax VK210 and P. vivax VK247 in anopheles mosquitoes (Diptera: Culicidae) in western Thailand, J. Med. Entomol. 39 (3) (2002) 556–559. [DOI] [PubMed] [Google Scholar]
- [22].Matsuoka K, Komori H, Nose M, Endo Y, Sawasaki T, Simple screening method for autoantigen proteins using the N-terminal biotinylated protein library produced by wheat cell-free synthesis, J. Proteome Res. 9 (8) (2010) 4264–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Trager W, Jensen JB, Human malaria parasites in continuous culture, Science 193 (4254) (1976) 673–675. [DOI] [PubMed] [Google Scholar]
- [24].Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA, Discovery of gene function by expression profiling of the malaria parasite life cycle, Science 301 (5639) (2003) 1503–1508. [DOI] [PubMed] [Google Scholar]
- [25].Bozdech Z, Llinas M, Pulliam BL, Wong ED, Zhu J, DeRisi JL, The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum, PLoS Biol. 1 (1) (2003) E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hayward RE, Derisi JL, Alfadhli S, Kaslow DC, Brown PO, Rathod PK, Shotgun DNA microarrays and stage-specific gene expression in Plasmodium falciparum malaria, Mol. Microbiol. 35 (1) (2000) 6–14. [DOI] [PubMed] [Google Scholar]
- [27].Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, Witney AA, Wolters D, Wu Y, Gardner MJ, Holder AA, Sinden RE, Yates JR, Carucci DJ, A proteomic view of the Plasmodium falciparum life cycle, Nature 419 (6906) (2002) 520–526. [DOI] [PubMed] [Google Scholar]
- [28].Sawasaki T, Ogasawara T, Morishita R, Endo Y, A cell-free protein synthesis system for high-throughput proteomics, Proc. Natl. Acad. Sci. U. S. A. 99 (23) (2002) 14652–14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sawasaki T, Morishita R, Gouda MD, Endo Y, Methods for high-throughput materialization of genetic information based on wheat germ cell-free expression system, Methods Mol. Biol. 375 (2007) 95–106. [DOI] [PubMed] [Google Scholar]
- [30].Coleman RE, Kumpitak C, Ponlawat A, Maneechai N, Phunkitchar V, Rachapaew N, Zollner G, Sattabongkot J, Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand, J. Med. Entomol. 41 (2) (2004) 201–208. [DOI] [PubMed] [Google Scholar]
- [31].Morita M, Takashima E, Ito D, Miura K, Thongkukiatkul A, Diouf A, Fairhurst RM, Diakite M, Long CA, Torii M, Tsuboi T, Immunoscreening of Plasmodium falciparum proteins expressed in a wheat germ cell-free system reveals a novel malaria vaccine candidate, Sci. Rep 7 (2017) 46086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P, Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes, J. Exp. Med. 172 (6) (1990) 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Roussilhon C, Oeuvray C, Muller-Graf C, Tall A, Rogier C, Trape JF, Theisen M, Balde A, Perignon JL, Druilhe P, Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3, PLoS Med. 4 (11) (2007) e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Soe S, Theisen M, Roussilhon C, Aye KS, Druilhe P, Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein, Infect. Immun. 72 (1) (2004) 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Greenhouse B, Ho B, Hubbard A, Njama-Meya D, Narum DL, Lanar DE, Dutta S, Rosenthal PJ, Dorsey G, John CC, Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic, J. Infect. Dis. 204 (1) (2011) 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cherif MK, Ouedraogo O, Sanou GS, Diarra A, Ouedraogo A, Tiono A, Cavanagh DR, Michael T, Konate AT, Watson NL, Sanza M, Dube TJT, Sirima SB, Nebie I, Antibody responses to P. falciparum blood stage antigens and incidence of clinical malaria in children living in endemic area in Burkina Faso, BMC Res. Notes 10 (1) (2017) 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sirima SB, Mordmuller B, Milligan P, Ngoa UA, Kironde F, Atuguba F, Tiono AB, Issifou S, Kaddumukasa M, Bangre O, Flach C, Christiansen M, Bang P, Chilengi R, Jepsen S, Kremsner PG, Theisen M, G.M.Z.T.S. Group, A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children, Vaccine 34 (38) (2016) 4536–4542. [DOI] [PubMed] [Google Scholar]
- [38].Sakamoto H, Takeo S, Maier AG, Sattabongkot J, Cowman AF, Tsuboi T, Antibodies against a Plasmodium falciparum antigen PfMSPDBLl inhibit merozoite invasion into human erythrocytes, Vaccine 30 (11) (2012) 1972–1980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.