Abstract
Antineutrophil cytoplasmic antibodies (ANCAs) are autoantibodies specific for antigens located in the cytoplasmic granules of neutrophils and lysosomes of monocytes. ANCAs are associated with a spectrum of necrotizing vasculitis that includes granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis. Pulmonary vasculitis and related extravascular inflammation and fibrosis are frequent components of ANCA vasculitis. In this review, we detail the factors that have been associated with the origin of the ANCA autoimmune response and summarize the most relevant clinical observations, in vitro evidence, and animal studies strongly indicating the pathogenic potential of ANCA. In addition, we describe the putative sequence of pathogenic mechanisms driven by ANCA-induced activation of neutrophils that result in small vessel necrotizing vasculitis and extravascular granulomatous necrotizing inflammation.
Keywords: ANCA, granulomatosis with polyangiitis, microscopic polyangiitis, pulmonary vasculitis
Antineutrophil cytoplasmic antibodies (ANCAs) are autoantibodies specific for antigens located in the cytoplasmic granules of neutrophils and lysosomes of monocytes.1 The two major autoantigen targets of ANCA are myeloperoxidase (MPO) and proteinase 3 (PR3).1 The term ANCA vasculitis refers to a particular group of autoimmune disorders characterized by necrotizing vasculitis, absence or paucity of immune deposits, and predominant involvement of small vessels, that is, capillaries, venules, arterioles, and small arteries.2 Granulomatosis with polyangiitis (GPA, formerly called Wegener’s granulomatosis), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA, formerly Churg–Strauss’ syndrome) are the major clinicopathologic forms of small vessel vasculitis associated with ANCA. Some patients with ANCAvasculitis have no evidence for lungdisease, for example, patient with renal limited vasculitis (RLV). Optimum clinical classification of ANCA vasculitis includes both the serotype (PR3-ANCA, MPO-ANCA, or ANCA negative) and the clinico-pathologic phenotype (MPA, GPA, EGPA, RLV).2
ANCA vasculitides are the major cause of vasculitis affecting the lung. Pulmonary involvement has been reported to occur in almost half of GPA patients at diagnosis. Characteristic acute pulmonary pathologic lesions include hemorrhagic capillaritis, necrotizing arteritis, and necrotizing granulomatous inflammation (►Fig. 1). Approximately 70 to 90% of GPA patients will eventually develop evidence of lung disease during the course of their disease, for example, pulmonary hemorrhage, nodules, masses, cavities, or airway inflammatory lesions.3,4 In MPA, the overall prevalence of pulmonary vasculitis, typically presented as diffuse alveolar hemorrhage caused by pulmonary capillaritis, is around 35 to 50%.5,6 Pulmonary vasculitis is less frequently associated with other primary vasculitides, systemic autoimmune disorders, infections, and drugs (►Table 1). This article reviews the current understanding of the origin and pathogenesis of ANCA vasculitis, with a special focus on GPA and MPA. EGPA is reviewed by Guillevin et al on page 471–481.
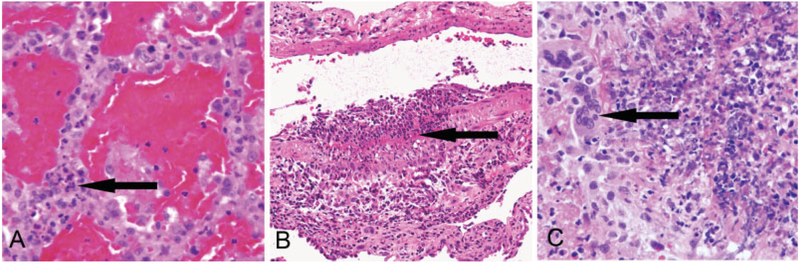
Fig. 1.
ANCA vasculitis lung lesions (H&E stain). (A) Pulmonary hemorrhagic capillaritis showing numerous neutrophils within alveolar septal capillaries (arrow) and red blood cells in air spaces (H&E stain). (B) Intense segmental acute arteritis in lung with transmural inflammation (arrow). (C) Granulomatous inflammation with multinucleated giant cells (arrow) (H&E stain). ANCA, antineutrophil cytoplasmic antibody.
Table 1.
Etiology of pulmonary vasculitis
| Most frequent cause |
| ANCA vasculitis and granulomatosis |
| Granulomatosis with polyangiitis |
| Microscopic polyangiitis |
| Eosinophilic granulomatosis with polyangiitis |
| Less frequent causes |
| Other systemic vasculitis |
| Antiglomerular basement membrane disease |
| IgA vasculitis |
| Behcet’s disease |
| Cryoglobulinemic vasculitis |
| Takayasu arteritis |
| Autoimmune diseases |
| Systemic lupus erythematosus |
| Antiphospholipid syndrome |
| Sarcoidosis |
| Rheumatoid arthritis |
| Infections |
| Bacteria, e.g., mycobacteria, syphilis, rickettsias |
| Fungus, e.g., aspergillosis, histoplasmosis |
| Virus, e.g., herpes zoster |
| Malignancy |
| Drugs-induced ANCA vasculitis |
| Propylthiouracil, diphenylhydantoin, levamisole-adulterated cocaine |
Genesis of the ANCA Response
The precise cause of ANCA immune genesis remains unclear, but probably involves a complex process where multiple elements converge, that is, infections, genetic influences, environmental exposures, and abnormalities of the innate and acquired immune system.7 The role that each of these factors plays in the induction of the autoantibodies and the persistence of the ANCA pathogenic response varies among individuals.
Infections
As the lungs are affected in most patients with ANCA vasculitis, respiratory pathogens have been largely implicated in the pathogenesis of these diseases, particularly in GPA. One proposed mechanism for the development of ANCA suggests the initiation of the autoimmune response by peptides that are complementary to peptides in the target antigens, such as PR3. Putatively, the adaptive immune response against a complimentary peptide of PR3 (cPR3) elicits its cognate antibody, which in turn evokes an antiidiotype of this antibody with specificity for PR3.8 Findings that support this theory included the detection of antibodies directed against antisense peptides that are complementary to autoantigen epitopes on PR3 in patients with PR3-ANCA vasculitis, the presence of circulating anti-cPR3 specific memory T cells, and the production of anti-PR3 antibodies after immunization of mice with cPR3.8,9
Of particular interest for lung vasculitis is the fact that Staphylococcus aureus has peptides that mimic cPR3, and thus, in theory, infection by this microorganism might initiate or augment an immune response to the peptide mimic of antisense PR3, which in turn would result in anti-idiotypic antibodies that react with PR3.8 It is important to realize that S. aureus has long been known to be associated with GPA. Previous studies have shown that 60 to 70% of PR3-ANCA GPA patients are chronic nasal carriers of S. aureus, a state that has been associated with an increased risk of relapses, including pulmonary flares.10,11 Furthermore, the risk for reactivation in these patients is highest in the presence of S. aureus strains producing the toxic shock syndrome toxin-1, superantigen with a strong immunosti-mulatory capacity.12 Of relevance, the addition of trimethoprim/sulfamethoxazole to standard remission maintenance reduced the risk of nonsevere relapses in GPA patients, probably by preventing respiratory tract infections.13,14
Entamoeba histolytica and Ross River virus are other pathogens with known peptides that mimic cPR3.8 In addition, ANCA has also been detected in patients with bacterial endocarditis. In these rare cases, ANCA are usually directed to both MPO and PR3 and are associated with other autoantibodies, such as antiphospholipid and antinuclear antibodies.15,16
Drugs
Certain drugs such as propylthiouracil, minocycline, allopurinol, levamisole-adulterated cocaine, D-penicillamine, and diphenylhydantoin have been associated with the induction of ANCA and the development of pulmonary capillaritis, diffuse alveolar hemorrhage, and glomerulonephritis.17,18 In contrast with primary ANCA disease, drugs usually induce a heterogeneous ANCA response with antibodies that are not only directed to MPO and PR3 but also to other neutrophil granule proteins, for example, azurocidin, cathepsin G, elastase, or lactoferrin.19,20
Environmental Factors
As for pulmonary vasculitis, the inhalation of an external agent seems to be an interesting, although still unproven possible trigger event.21,22 In this sense, previous epidemiological studies have shown a significant association between ANCA vasculitis and high exposure to dust and heavy metals, that is, silica, mercury, and lead.21,23–25 Extensive exposure to silica might result in its accumulation in antigen-presenting cells and macrophages of the reticuloendothelial system, where it can act as an effective adjuvant enhancing the autoimmune ANCA response. Strong occupational exposures to inhaled fumes, pesticides, and hydrocarbons have also been reported in GPA patients.26,27 Further, as the incidence of GPA correlates inversely with ambient ultraviolet (UV) radiation, some authors have suggested a causative role for low UV radiation exposure (possibly acting via vitamin D status) in the development of ANCA vasculitis.28,29
Genetic Factors
Genetic predisposition influences the onset and development of ANCA vasculitis. Results of two large-scale genomewide association studies (GWASs) have implicated a genetic role on the pathogenesis of ANCA disease, that is, PR3-ANCA vasculitis was associated with single nucleotide polymorphisms (SNP) in the HLA-DP1 area and genes encoding PR3 (PRTN3) and α1-antitrypsin (SERPINA1), the predominant inhibitor of PR3; for patients with MPO-ANCA serotype, a significant association was identified with SNP in HLA-DQ.30,31 The strong association of ANCA disease with distinct HLA molecules suggests a central role of autoimmunity in ANCA implicating that HLA-determined immune response against PR3, MPO, and cPR3 influences the initiation of the ANCA autoimmune response.31,32
In these GWASs, the genetic associations were stronger for ANCA specificity than for clinical phenotype, consistent with two distinct immune responses initiated by recognition by HLA receptors of two different types of initiating antigens. Some of the marked clinical heterogeneity observed in ANCA patients may be influenced at least in part by genetic factors. In this sense, it is interesting to note that certain pulmonary manifestations, that is, lung nodules and cavities, predominate in PR3-ANCA cases, whereas alveolar capillaritis with pulmonary hemorrhage, bronchiectasis, and lung fibrosis are much more associated to MPO-ANCA.33–37
Other relevant associations that support the importance of genetics in ANCA vasculitis include an increased prevalence of MHC class II allele HLA-DRB1–15 in PR3-ANCA disease among African American patients as well as polymorphism variants of PTPN22 and CTLA4 genes.32,38 A genetic influence has also been suggested by a greater frequency in first-degree relatives and sporadic reports of familial occurrence.39
Finally, epigenetic factors also seem to influence the pathogenesis of ANCA vasculitis. ANCA patients have an abnormal epigenetic regulation of PR3 and MPO genes that results in the continued overexpression of both autoantigens in circulating neutrophils.40 Theoretically, the increased availability of PR3 and MPO could facilitate the loss of tolerance to these proteins or facilitate pathogenic events.41
Abnormalities in the Regulation of the ANCA Autoimmune Response
The ability of the immune system to distinguish between self- and non-self-antigens is known as self-tolerance, an important process finely regulated by regulatory B cells and regulatory T cells (Treg). In the context of ANCA vasculitis, the autoimmune response leading to the development of ANCA appears to be facilitated on one hand by impaired T cell and B cell suppression, and on the other hand by enhanced B cell stimulation by ANCA-activated neutrophils.
Clinical and experimental studies have identified several abnormalities in the number and function of specific T cell subsets in ANCA vasculitis patients.42–52 Former studies have reported a persistent increase of CD4+ effector memory T cells (TEM) in the peripheral blood of GPA patients.51 CD4+ TEM cells are probably involved in tissue damage and kidney injury, as suggested by the detection of an elevated number of these cells in urine sediments of active patients and their disappearance during remission.49–52 Analysis of the CD4+ T cell compartment has also disclosed an increased frequency of a distinct proinflammatory CD4+ effector T cell (CD25int) subset that is resistant to Treg suppression.44 An expanded population of CD4+T cells lacking the costimulatory molecule CD28 has been reported in GPA granulomatous lesions.46,53 In addition to TEM, the suppressive capacity of regulatory T cells is markedly decreased in these patients, thus probably contributing to loss of tolerance and emergence of a pathogenic ANCA response.42,44 Aberrant T-helper cell polarization has also been described in ANCA disease, that is, patients have a significant elevation of serum levels of interleukin (IL)-17, IL-21, and IL-23, in addition to increased autoantigen-specific Th17 cells.47,48,54 Overall, these data indicate that T cells participate in the genesis of autoantibody production and development of ANCA disease by providing active B cell help, through ineffective suppression of the ANCA autoimmune response, by directly participating in the damage of tissue organs, and by sustaining the autoimmune reaction through interactions with dendritic cells and B cells within the granulomatous lesions of the respiratory tract.55
B cells are the precursors for plasma cells that produce ANCA, and undoubtedly are involved in the pathogenesis of ANCA vasculitis. However, several abnormalities in the phenotype of circulating B cells have also been reported, for example, quantitative and qualitative defects of B-regulatory lymphocytes,56,57 or the overexpression of CD19+, possibly leading to B cell hyperactivation.58 In addition, activated neutrophils release mediators that enhance the proliferation and inhibit apoptosis of B lymphocytes.59
It is important to realize that a certain proportion of mature B cells in healthy individuals are actually autoreactive and able to secrete “natural autoantibodies” against MPO and PR3.60 In this regard, some authors have suggested that endogenous and exogenous influences (as those previously described in this section) might modify intrinsic characteristics, for example, epitope specificity, of nonpathogenic natural autoantibodies to render them pathogenic.61,62
Clinical and In Vitro Evidence for the Pathogenicity of ANCA
Several clinical observations support the participation of ANCA in the pathogenesis of ANCA-associated systemic vasculitis.62 A frequently cited example is the report of a prematurely born infant who developed pulmonary hemorrhage and renal disease within days after birth following transplacental passage of MPO-ANCA from his mother affected with MPA. Although this report provides direct evidence for the pathogenic role of MPO-ANCA in humans, no other similar cases have been published.63
Also consistent with an important role for ANCA in the pathogenesis of human disease is the finding of high levels of these autoantibodies in the vast majority of patients with active generalized GPA and MPA, the fall of ANCA titers during therapy, and the correlation of disease activity with higher levels of PR3 or MPO on the surface of circulating neutrophils.64–66 Although the correlation is not absolute, several studies have reported that ANCA titers correlate with disease activity and recurrences in specific subsets of patients, for example, an increase of PR3-ANCA level has been associated with the development of severe relapses in patients with previous alveolar hemorrhage or renal involvement and in those treated with rituximab.67
The efficacy of rituximab and plasma exchange, targeted therapies that reduce B-lymphocytes and deplete circulating autoantibodies, also argues in favor of the participation of ANCA in the pathogenesis of systemic vasculitis.68–70 In fact, severe reactivations of ANCA vasculitis following complete remission with rituximab are rare exceptions in the absence of detectable ANCA.71 In this line, a recent clinical trial showed that the pre-emptive use of rituximab based on rising ANCA levels (or repopulation of CD19+ B lymphocytes) was an effective therapeutic strategy for remission maintenance.72
The close association of the development of ANCA vasculitis following specific drug exposures and more importantly, the resolution of clinical manifestations and clearance of the autoantibodies after discontinuation of the offending medication is another observation that adds circumstantial evidence for the pathogenicity of ANCA.73–76
Extensive in vitro evidence demonstrates mechanisms by which ANCA could cause vascular inflammation.77–79 For example, incubation of ANCA immunoglobulin G (IgG) with primed neutrophils and monocytes induces the release of mediators of inflammation such as toxic reactive oxygen species and lytic granule enzymes.78,80,81 Also, several studies have demonstrated that surface expression of ANCA autoantigens is low or absent in resting neutrophils, but it is rapidly up-regulated after inflammatory stimuli such as tumor necrosis factor (TNF)-α, bacterial lipopolysaccharide (LPS), or the complement anaphylatoxin C5a.80,82,83 In this regard, a reasonable hypothesis is that in ANCA patients, an inflammatory process, for example, a respiratory tract infection, may cause increased levels of circulating cytokines, which in turn prime neutrophils to interact with circulating ANCA to induce vasculitis.79 The identification of a “flu-like syndrome” (clinical evidence for elevated circulating cytokines) occurring in most ANCA patients shortly before the onset ofovert clinical manifestations is in line with this hypothetical scenario.79,84 Experimental evidence also supports this hypothesis, as injection of bacterial LPS into mice prior to induction of glomerulonephritis with anti-MPO IgG causes more severe injury.85
In vitro experiments have also been used to demonstrate that ANCA-activated neutrophils cause the death of endothelial cells; that ANCA IgG stimulates the adherence and rolling of neutrophils to endothelial monolayers through integrin-mediated mechanisms; that ANCA antigen targets and ANCA-containing immune complexes may become planted in vessel walls, where they might become toxic or contribute to further targeting of endothelial cells by activated neutrophils and monocytes; and that ANCA stimulates the release of neutrophil extracellular traps (NETs), which can cause damage to endothelial cells.86–91 The effect of ANCA on stimulation of leukocyte adhesion and migration across the endothelium has been confirmed in vivo by intravital microscopy of mice cremasteric vessels.92
Although the major pathogenic effects of ANCA are induced by neutrophil activation after the engagement of Fcγ receptors (FcγR), or cross-linking of F(ab′)2 fragments,93,94 recent studies have suggested that MPO-ANCA antibodies may also cause damage of endothelial cells acting directly through the activation of MPO.95,96
Animal Models of ANCA Vasculitis
Although clinical and in vitro observations support the participation of ANCA in the pathogenesis of systemic vasculitis, the most definite evidence that ANCA are pathogenic comes from animal models. The first convincing animal model for ANCA vasculitis was produced in mice through the intravenous injection of high-affinity anti-MPO IgG.97 In this model, mice with a knockout (KO) of the MPO gene (MPO−/−) are immunized with purified murine MPO and in consequence develop a robust immune response with high titers of circulating antibodies directed against MPO. Once isolated, the intravenous injection of anti-MPO IgG into wild-type (WT) B6 mice induces over the course of 6 days, necrotizing and crescentic glomerulonephritis (NCGN) that is pathologically identical to that observed in ANCA patients. In addition, the passive transfer of anti-MPO antibodies into immunodeficient recombinase-activating gene-2-deficient (Rag2−/−) mice (lacking both functional T and B cells) produces NCGN with similar characteristics to that found in WT B6 animals, indicating that functioning T lymphocytes are not required for the pathogenesis of vasculitis in this model.97 Similar lesions are induced in Rag2−/− mice by transplantation of splenocytes from MPO knockout mice that had been immunized with murine MPO. In the initial experiments with these models, NCGN was the most common vasculitic lesion; however, some animals developed pulmonary lesions including hemorrhagic capillaritis, necrotizing arteritis, and granulomatous inflammation (►Fig. 2).
Fig. 2.
Systemic vasculitis and granulomatous inflammation in Rag2−/− mice 13 days after receiving anti-MPO splenocytes. (Reproduced from Xiao et al.97) (A) Pulmonary hemorrhagic capillaritis showing numerous neutrophils within alveolar septal capillaries (arrow) and red blood cells in air spaces (H&E stain). (B) Intense acute arteritis in lung with transmural (arrow) and perivascular infiltration of predominantly neutrophils (Masson’s trichrome stain). (C) Granulomatous inflammation with multinucleated giant cells (arrow) (H&E stain). MPO, myeloperoxidase.
Studies using the model described earlier, or its variants,85,97–101 have proven to be a valuable tool to demonstrate that (1) MPO-ANCA antibodies alone, in the absence of functional T cells, are sufficient to cause acute disease; (2) depletion of circulating neutrophils protects against the development of NCGN, indicating that these cells are the mainstay effectors of anti-MPO-induced vasculitis; (3) bone marrow–derived cells, that is, MPO-expressing hematopoietic cells, are not only sufficient but also necessary to induce ANCA glomerulonephritis; (4) the synergistic effect of neutrophil priming with proinflammatory stimuli results in an exacerbation of ANCA disease severity; (5) Fc receptors are involved in pathogenesis and disease modulation; (6) genetic background plays a profound effect in the susceptibility and severity of disease; and (7) activation of the alternative complement pathway is a critical mediator of injury in ANCA-associated NCGN, with activation of C5 and engagement of C5a to its receptor playing an essential role in this process.85,97–101 Several observations made in patients have also confirmed the relevance of the complement system in the pathogenesis of ANCA disease, for example, elevated plasma and urinary levels of C5a in active vasculitis; the detection of complement factors in inflamed glomeruli and small blood vessels; and recently, the successful use of avacopan (selective inhibitor of C5a receptor) for remission induction of patients with ANCA glomerulonephritis.102–105
A rat model based on the immunization of animals with human recombinant MPO (hMPO) has confirmed the observations made in mice.106,107 In this model, injection of purified hMPO into Wistar–Kyoto rats lead to the induction of antihuman MPO antibodies that cross-react with rat MPO, resulting in the development of pauci-immune NCGN.106 The role of T cells in ANCA pathogenesis has been studied in a mouse model of autoimmune glomerulonephritis that results from the synergy between anti-MPO and antiglomerular basement antibodies.108
The discussion so far focused on the pathogenic properties of MPO-ANCA as unfortunately, a clear-cut animal model for PR3-ANCA disease is still lacking. Although several putative models have been proposed,109 the most promising method for modeling ANCA-PR3 disease involves the development of mice with a humanized immune system.110 In a recently described model, NOD-SCID IL-2 receptor KO mice (lacking native T, B, and NK cells and the IL-2 receptor), which received human hematopoietic stem cells thus developing a chimeric human–mouse immune system developed mild glomerulonephritis following the administration of human PR3-ANCA IgG.110 Although the observed lesions identified in the latter model do not closely resemble those in human disease, the use of chimeric mice engineered with human immune machinery is an exciting future approach to investigate the pathogenesis of ANCA vasculitis.
The aforementioned animal models are centered on the induction of glomerular inflammatory lesions; however, the development of pulmonary vasculitis has been reported only sporadically in these experimental rodents. In the passive transfer model, less than 30% of mice developed mild pulmonary alveolar capillaritis usually affecting less than 5% of the lung parenchyma.97 In addition, hemorrhagic pulmonary capillaritis was reported only in small proportion of the chimeric NOD-SCID mice and in rats after immunization with human MPO.106,110
Until recently, previous attempts to reproduce the pulmonary manifestations of ANCA vasculitis in animal models have been unsuccessful.111,112 For example, infusion of monoclonal anti-PR3 antibodies and TNF-α-primed human neutrophils into isolated rat lungs resulted in marked edema, but not overt vasculitis.112 In a different approach, the immunization of BALB/c mice with human IgG C-ANCA generated an anti-idiotypic reaction that resulted in the development of nonspecific foci of inflammation in the lungs of some animals.111 These pulmonary lesions, however, did not present histologic evidence of vasculitis or granulomatous inflammation and importantly, were mediated by immune complex deposits, clearly contrasting with the pauci-immune nature of ANCA inflammatory injury.
In unpublished studies, our group has observed that MPO-ANCA can cause severe pulmonary vasculitis in mice if they have adequate predisposing characteristics of the innate immune system and synergistic lung inflammation, for example, concurrent influenza infection.113,114 Furthermore, on the basis of these observations, we recently developed a reproducible method to induce extravascular lung granulomatous inflammatory lesions and necrotizing vasculitis in WT B6 mice.115 This new GPA model is detailed in the Pathogenesis of Granulomatosis section.
Pathogenesis of Vascular Inflammation
The hypothetical sequence of pathogenic events leading to the development of ANCA-associated vasculitis is illustrated in ►Fig. 3. Once pathogenic ANCA are in the circulation, they activate neutrophils by reacting with ANCA antigens. As previously mentioned, priming of neutrophils by an inflammatory stimuli facilitates the interaction of MPO-ANCA and PR3-ANCA with their target antigens.62 Binding of ANCA to ANCA antigens on the surface of neutrophils results in neutrophil activation through Fcγ receptor engagement and through cross-linking of F(ab′)2 fragments.93,94 Then, activated neutrophils adhere and penetrate vessel walls, releasing toxic oxygen radicals; destructive enzymes that cause apoptosis and necrosis of adjacent cells; and granule proteins, which might become planted in vessel walls by a charge-dependent mechanism, allowing their further interaction with additional ANCA.62,65,80,89,116 Neutrophils also secrete factors that activate the alternative complement pathway leading to the generation of C5a, which recruits and activates more neutrophils to the site of inflammation, establishing an inflammatory amplification loop that increases destructive necrotizing inflammation.98 The activation of the complement system by neutrophils is probably mediated by the inhibitory effect of MPO on factor H, a key regulator of the alternative pathway.117 ANCA-induced NETs probably also play a role in complement activation.118
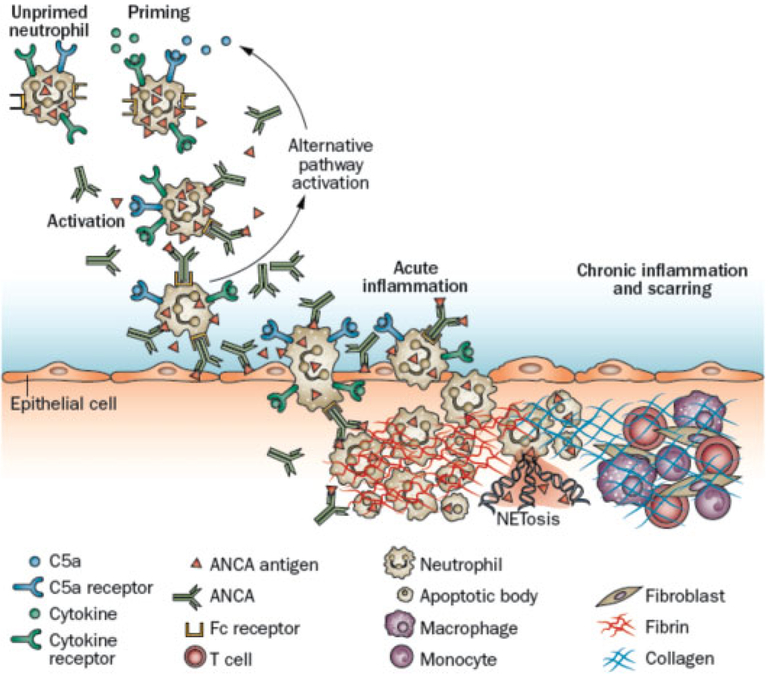
Fig. 3.
Putative sequence of pathogenic events in ANCA-mediated vasculitis. (Reproduced from Jennette and Falk.62) Circulating neutrophils are primed for activation by ANCA by inflammatory cytokines or C5a derived from complement activation (note that monocytes can be similarly primed and activated but are not illustrated). Primed neutrophils release ANCA antigens at the cell surface and into the microenvironment, where they interact with ANCA. Fc receptor engagement by ANCA bound to ANCA antigens as well as F(ab′)2 binding to ANCA antigens on neutrophil surfaces cause neutrophil activation. ANCA-activated neutrophils release factors that activate the alternative complement pathway, generating C5a. C5a and ANCA create an inflammatory amplification loop, with C5a attracting and priming more neutrophils for activation by ANCA, which causes, in turn, further activation of the alternative complement pathway and production of more C5a. ANCA-activated neutrophils marginate and penetrate vessel walls and undergo respiratory burst, degranulation, NETosis, apoptosis, and necrosis. Disruption of endothelium allows plasma to spill into vascular and perivascular tissue where activation of the coagulation cascade produces the fibrin strands of fibroid necrosis. The innate inflammatory response orchestrates the conversion of the initial acute neutrophil-rich inflammation into mononuclear leukocyte-rich inflammation and subsequent collagen deposition and fibrosis. ANCA, antineutrophil cytoplasmic antibody.
As neutrophils, monocytes are also activated by ANCA, resulting in the production of proinflammatory cytokines, for example, IL-8, and chemokines, for example, monocyte chemoattractant protein 1 (MCP-1).119–121 On one hand, IL-8 can attract and activate neutrophils thus amplifying neutrophil-mediated injury, and on the other hand, MCP-1 attracts monocytes and macrophages, probably participating in the transition from neutrophil-rich inflammation to predominant monocyte-rich inflammation and in the development of granulomatous lesions.119 Macrophages are also able to produce inflammatory chemokines in response to phagocytosis of PR3-expressing apoptotic cells.122
As a result of the intense necrotizing injury induced by activated neutrophils, blood vessel walls are disrupted producing local hemorrhage and releasing plasma proteins into vascular and perivascular tissues, where activation of coagulation cascade results in the formation of typical fibrinoid necrosis.62,87 Within days, the severe acute injury elicits an innate inflammatory response and the initial acute neutrophil-rich inflammation and necrosis is replaced by inflammation with a predominance of monocytes, macrophages, and later Tcells.97 When tissue injury is mild, it resolves with remodeling of the vessel to normal structure. In contrast, severe damage turns into fibroblast activation with collagen deposit, resulting in fibrosis and sclerosis of injured vessels and adjacent tissue.
The pathogenic features of necrotizing vasculitis described here seem to be the same in the skin, kidney, nerve, and every other organ. In the particular case of the lungs, the intense neutrophilic infiltration of the alveolar septa (alveolar capillaritis) produces necrosis, leukocytoclasia, and loss of the capillary integrity with spilling of red blood cells into the alveolar space and interstitium. The clinical expression of this pathogenic injury, that is, diffuse alveolar hemorrhage, is a prominent and life-threatening manifestation of ANCA vasculitis.
Pathogenesis of Granulomatosis
In addition to vasculitis, GPA is characterized by multiple foci of extravascular necrotizing granulomatous inflammation most often affecting the upper and lower respiratory tracts. In the lung, these granulomatous lesions are clinically presented as multiple bilateral parenchymal nodules and masses that may become cavitated.
The pathogenic mechanisms involved in the development of ANCA necrotizing granulomatous inflammation are not completely understood. Based on detailed pathology descriptions123,124 and a recently described animal model,115 the leading hypothesis is that in a process analogous to vascular inflammation, granulomatous lesions start with the activation of extravascular primed neutrophils—that have been positioned in the airway mucosa by a respiratory infection or other inflammatory condition—by ANCA located in the interstitial compartment (►Fig. 4). Once activated, neutrophils liberate reactive oxygen species and destructive enzymes that result in an intense localized tissue necrosis with abundant fibrin formation, initially resembling microabscesses. In these early granulomatous pulmonary lesions, acute parenchymal damage would elicit a high influx of mononuclear cells. Over time, the neutrophil-rich acute necrotizing lesions would accrue large numbers of mononuclear leukocytes evolving into more typical granulomatous appearance, that is, the center of the lesion contains necrotic debris that is walled off by a well-defined band of numerous epithelioid macrophages. In addition to macrophages, granulomatous inflammation is typically composed by a mixed inflammatory infiltrate of dendritic cells, B and T lymphocytes, eosinophils, and plasma cells.62,116,125 Weeks after the initial insult, necrotic debris and macrophage-rich lesions are replaced by deposition of collagen, fibrous tissue, and organized lymphoid follicles.62
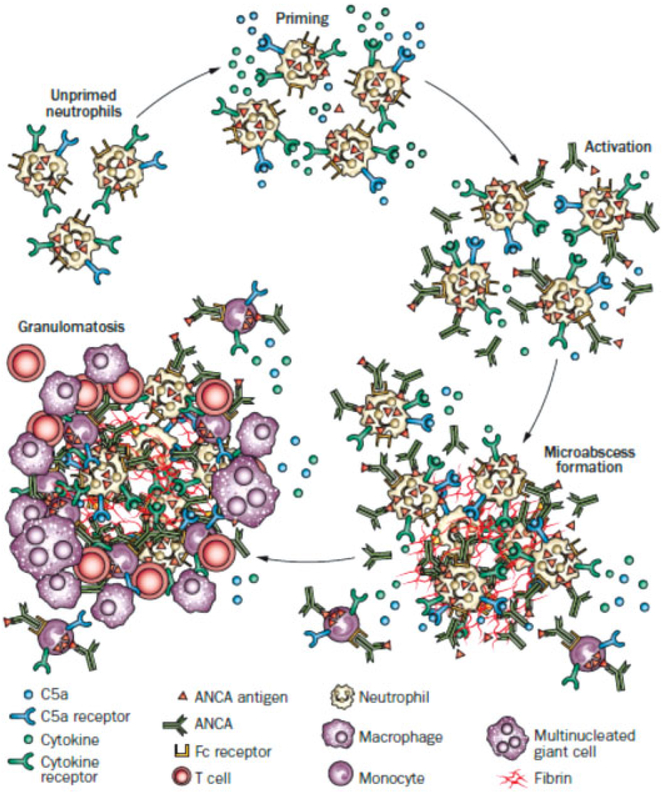
Fig. 4.
Putative sequence of pathogenic events in ANCA-mediated necrotizing granulomatosis. (Reproduced from Jennette and Falk.62) An inflammatory prodrome, such as an infectious or allergic inflammatory respiratory tract disease, positions increased numbers of primed neutrophils in extravascular interstitial tissue. ANCA immunoglobulin in the interstitial fluid would activate primed neutrophils and initiate an inflammatory amplification loop that wouldattract and activate more neutrophils, resulting in the formation of a necrotizing microabscess. This acute inflammation and necrosis would initiate an innate inflammatory response that would wall off the necrotic zone with granulomatous inflammation containing a predominance of monocytes and macrophages with admixed lymphocytes. ANCA, antineutrophil cytoplasmic antibody.
In a recent mouse model of anti-MPO-ANCA-induced pulmonary granulomatosis, early granulomatous lesions initiate as neutrophil-rich microabscesses containing neutrophils in varying stages of necrosis and apoptosis;115 these lesions were identical to those described as an early form of parenchymal necrosis in lung specimens obtained from GPA patients.123 The time course of this model showed that later, by the end of the first week, the extensive tissue necrosis and fibrinoid material were totally replaced by monocytes, macrophages, and scattered giant cells.115 Although the participation of functional T cells were not required for induction of granulomatous lesions in this animal model, it is probably that lymphocytes may indeed play a role in human disease as organized lymphoid follicles with clusters of PR3 cells surrounded by mature B lymphocytes and effector memory T cells have been described in lung specimens from GPA patients.46,126
An alternative proposal for the pathogenesis of ANCA granulomatous inflammation suggests that in contrast to the exaggerated antigen-independent innate response described earlier, GPA granulomas are indeed a consequence of an antigen-specific T cell immune response.127 In this theory, PR3 and MPO released from activated neutrophils are processed and presented by antigen-presenting cells to autoreactive T-lymphocytes. As Treg fails to inhibit this autoimmune response, a T cell effector memory response eventually lead to the development of granulomatous lesions, some of which displaying features of ectopic lymphoid-like tissue.46,127,128 According to some authors, the lymphocyte clusters in these granulomatous lesions may provide a place to sustain the autoimmunity response to ANCA autoantigens.127
Pathogenesis of Interstitial Lung Disease
Pulmonary fibrosis is an uncommon although severe complication of ANCA vasculitis. The disease is strongly associated with ANCA specific to MPO and therefore has been reported more frequently in MPA patients. The etiology of ANCA-associated lung fibrosis remains obscure, although recurrent episodes of subclinical alveolar hemorrhage have been incriminated as the leading factor in the pathogenesis of this condition.129–131 In addition, MPO-ANCA seems to play a direct role in the development of interstitial lung disease (ILD), as suggested by the presence of positive MPO-ANCA autoantibodies in the vast majority of patients who develop this pulmonary manifestation. Further, recent evidence has demonstrated that anti-MPO-ANCA, acting through the activation of MPO, are able to trigger fibroblast proliferation.95 Local release proteolytic enzymes by ANCA-activated neutrophils and chronic pulmonary parenchymal ischemia are other putative contributors of ANCA-associated ILD.34
Conclusion
Clinical, in vitro and experimental animal model evidence supports the pathogenic role for ANCA in the pathogenesis of ANCA-associated systemic necrotizing vasculitis. In the future, a better understanding of the pathogenic mechanisms leading to the development and progression of pulmonary vasculitis and lung necrotizing granulomatous lesions will enable more targeted and effective therapies, and hopefully, to the cure of these diseases.
Acknowledgments
M.A. Alba was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico.
References
- 1.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 1988;318(25):1651–1657 [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65(01):1–11 [DOI] [PubMed] [Google Scholar]
- 3.Hoffman GS, Kerr GS, Leavitt RY,et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116(06):488–498 [DOI] [PubMed] [Google Scholar]
- 4.Anderson G, Coles ET, Crane M, et al. Wegener’s granuloma. A series of 265 British cases seen between 1975 and 1985. A report by a sub-committee of the British Thoracic Society Research Committee. QJ Med 1992;83(302):427–438 [PubMed] [Google Scholar]
- 5.Guillevin L, Durand-Gasselin B, Cevallos R, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum 1999;42(03):421–430 [DOI] [PubMed] [Google Scholar]
- 6.Collins CE, Quismorio FP Jr. Pulmonary involvement in microscopic polyangiitis. Curr Opin Pulm Med 2005;11(05):447–451 [DOI] [PubMed] [Google Scholar]
- 7.Flores-Suárez LF, Alba MA, Mateos-Toledo H, Ruiz N. Pulmonary involvement in systemic vasculitis. Curr Rheumatol Rep 2017;19 (09):56. [DOI] [PubMed] [Google Scholar]
- 8.Pendergraft WFIII, Preston GA, Shah RR, et al. Autoimmunity is triggered by cPR-3(105–201), a protein complementary to human autoantigen proteinase-3. Nat Med 2004;10(01):72–79 [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Bautz DJ, Lionaki S, et al. ANCA patients have T cells responsive to complementary PR-3 antigen. Kidney Int 2008;74 (09):1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tadema H, Heeringa P, Kallenberg CG. Bacterial infections in Wegener’s granulomatosis: mechanisms potentially involved in autoimmune pathogenesis. Curr Opin Rheumatol 2011;23(04):366–371 [DOI] [PubMed] [Google Scholar]
- 11.Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med 1994;120(01):12–17 [DOI] [PubMed] [Google Scholar]
- 12.Popa ER, Stegeman CA, Abdulahad WH, et al. Staphylococcal toxic-shock-syndrome-toxin-1 as a risk factor for disease relapse in Wegener’s granulomatosis. Rheumatology (Oxford) 2007;46 (06):1029–1033 [DOI] [PubMed] [Google Scholar]
- 13.Zycinska K, Wardyn KA, Zielonka TM, Krupa R, Lukas W. Co-trimoxazole and prevention of relapses of PR3-ANCA positive vasculitis with pulmonary involvement. Eur J Med Res 2009;14 (Suppl 4):265–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG; Dutch Co-Trimoxazole Wegener Study Group. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. N Engl J Med 1996;335(01):16–20 [DOI] [PubMed] [Google Scholar]
- 15.Bell EK, Chugh SS, Cook WJ. A case of infection-associated anti-proteinase-3-negative cytoplasmic antineutrophil cytoplasmic antibody pauci-immune focal necrotizing glomerulonephritis. Nephrol Dial Transplant 2010;25(09):3119–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaci-Nikolic B, Andrejevic S, Pavlovic M, Dimcic Z, Ivanovic B, Nikolic M. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenge. Clin Rheumatol 2010;29(08):893–904 [DOI] [PubMed] [Google Scholar]
- 17.Dhillon SS, Singh D, Doe N, Qadri AM, Ricciardi S, Schwarz MI. Diffuse alveolar hemorrhage and pulmonary capillaritis due to propylthiouracil. Chest 1999;116(05):1485–1488 [DOI] [PubMed] [Google Scholar]
- 18.Yermakov VM, Hitti IF, Sutton AL. Necrotizing vasculitis associated with diphenylhydantoin: two fatal cases. Hum Pathol 1983;14(02):182–184 [DOI] [PubMed] [Google Scholar]
- 19.Yu F, Chen M, Gao Y, et al. Clinical and pathological features of renal involvement in propylthiouracil-associated ANCA-positive vasculitis. Am J Kidney Dis 2007;49(05):607–614 [DOI] [PubMed] [Google Scholar]
- 20.Merkel PA. Drug-induced vasculitis. Rheum Dis Clin North Am 2001;27(04):849–862 [DOI] [PubMed] [Google Scholar]
- 21.Hogan SL, Satterly KK, Dooley MA, Nachman PH, Jennette JC, Falk RJ; Glomerular Disease Collaborative Network. Silica exposure in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and lupus nephritis. J Am Soc Nephrol 2001;12(01): 134–142 [DOI] [PubMed] [Google Scholar]
- 22.Nuyts GD, Van Vlem E, De Vos A, et al. Wegener granulomatosis is associated to exposure to silicon compounds: a case-control study. Nephrol Dial Transplant 1995;10(07):1162–1165 [PubMed] [Google Scholar]
- 23.Beaudreuil S, Lasfargues G, Lauériere L, et al. Occupational exposure in ANCA-positive patients: a case-control study. Kidney Int 2005;67(05):1961–1966 [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Puerta JA, Gedmintas L, Costenbader KH. The association between silica exposure and development of ANCA-associated vasculitis: systematic review and meta-analysis. Autoimmun Rev 2013;12(12):1129–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert D, Clarkin C, Komoroski J, Brensinger CM, Berlin JA. Wegener’s granulomatosis: possible role of environmental agents in its pathogenesis. Arthritis Rheum 2004;51(04):656–664 [DOI] [PubMed] [Google Scholar]
- 26.Pai P, Bone JM, Bell GM. Hydrocarbon exposure and glomerulonephritis due to systemic vasculitis. Nephrol Dial Transplant 1998;13(05):1321–1323 [DOI] [PubMed] [Google Scholar]
- 27.Duna GF, Cotch MF, Galperin C, Hoffman DB, Hoffman GS. Wegener’s granulomatosis: role of environmental exposures. Clin Exp Rheumatol 1998;16(06):669–674 [PubMed] [Google Scholar]
- 28.Gatenby PA, Lucas RM, Engelsen O, Ponsonby AL, Clements M. Antineutrophil cytoplasmic antibody-associated vasculitides: could geographic patterns be explained by ambient ultraviolet radiation? Arthritis Rheum 2009;61(10):1417–1424 [DOI] [PubMed] [Google Scholar]
- 29.Kemna MJ, Cohen Tervaert JW, Broen K, Timmermans SAMEG, van Paassen P, Damoiseaux JGMC. Seasonal influence on the risk of relapse at a rise of antineutrophil cytoplasmic antibodies in vasculitis patients with renal involvement. J Rheumatol 2017;44 (04):473–481 [DOI] [PubMed] [Google Scholar]
- 30.Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med 2012;367(03): 214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkel PA, Xie G, Monach PA, et al. ; Vasculitis Clinical Research Consortium. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol 2017; 69(05):1054–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y, Schmitz JL, Yang J, et al. DRB1 *15 allele is a risk factor for PR3-ANCA disease in African Americans. J Am Soc Nephrol 2011; 22(06):1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lionaki S, Blyth ER, Hogan SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum 2012;64(10):3452–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alba MA, Flores-Suárez LF, Henderson AG, et al. Interstital lung disease in ANCAvasculitis. Autoimmun Rev 2017;16(07):722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geffriaud-Ricouard C, Noël LH, Chauveau D, Houhou S, Grünfeld JP, Lesavre P. Clinical spectrum associated with ANCA of defined antigen specificities in 98 selected patients. Clin Nephrol 1993; 39(03):125–136 [PubMed] [Google Scholar]
- 36.Shin MS, Young KR, Ho KJ. Wegener’s granulomatosis upper respiratory tract and pulmonary radiographic manifestations in 30 cases with pathogenetic consideration. Clin Imaging 1998;22 (02):99–104 [DOI] [PubMed] [Google Scholar]
- 37.Néel A, Espitia-Thibault A, Arrigoni PP, et al. Bronchiectasis is highly prevalent in anti-MPO ANCA-associated vasculitis and is associated with a distinct disease presentation. Semin Arthritis Rheum 2018;48(01):70–76 [DOI] [PubMed] [Google Scholar]
- 38.Alberici F, Martorana D, Vaglio A. Genetic aspects of antineutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2015;30(Suppl 1):i37–i45 [DOI] [PubMed] [Google Scholar]
- 39.Knight A, Sandin S, Askling J. Risks and relative risks of Wegener’s granulomatosis among close relatives of patients with the disease. Arthritis Rheum 2008;58(01):302–307 [DOI] [PubMed] [Google Scholar]
- 40.Ciavatta DJ, Yang J, Preston GA, et al. Epigenetic basis for aberrant upregulation of autoantigen genes in humans with ANCA vasculitis. J Clin Invest 2010;120(09):3209–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdgawad M, Gunnarsson L, Bengtsson AA, et al. Elevated neutrophil membrane expression of proteinase 3 is dependent upon CD177 expression. Clin Exp Immunol 2010;161(01):89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morgan MD, Day CJ, Piper KP, et al. Patients with Wegener’s granulomatosis demonstrate a relative deficiency and functional impairment of T-regulatory cells. Immunology 2010;130(01): 64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rimbert M, Hamidou M, Braudeau C, et al. Decreased numbers of blood dendritic cells and defective function of regulatory T cells in antineutrophil cytoplasmic antibody-associated vasculitis. PLoS One 2011;6(04):e18734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Free ME, Bunch DO, McGregor JA, et al. Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum 2013;65(07):1922–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamprecht P, Erdmann A, Mueller A, et al. Heterogeneity of CD4 and CD8+ memory T cells in localized and generalized Wegener’s granulomatosis. Arthritis Res Ther 2003;5(01):R25–R31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komocsi A, Lamprecht P, Csernok E, et al. Peripheral blood and granuloma CD4(+)CD28(−) T cells are a major source of interferon-gamma and tumor necrosis factor-alpha in Wegener’s granulomatosis. Am J Pathol 2002;160(05):1717–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nogueira E, Hamour S, Sawant D, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 2010;25(07):2209–2217 [DOI] [PubMed] [Google Scholar]
- 48.Abdulahad WH, Lepse N, Stegeman CA, et al. Increased frequency of circulating IL-21 producing Th-cells in patients with granulomatosis with polyangiitis (GPA). Arthritis Res Ther 2013;15(03): R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutfleisch J, Baumert E, Wolff-Vorbeck G, Schlesier M, Strutz HJ, Peter HH. Increased expression of CD25 and adhesion molecules on peripheral blood lymphocytes of patients with Wegener’s granulomatosis (WG) and ANCA positive vasculitides. Adv Exp Med Biol 1993;336:397–404 [DOI] [PubMed] [Google Scholar]
- 50.Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis.J Allergy Clin Immunol 1999;103(5 Pt 1):885–894 [DOI] [PubMed] [Google Scholar]
- 51.Abdulahad WH, van der Geld YM, Stegeman CA, Kallenberg CG. Persistent expansion of CD4+ effector memory T cells in Wegener’s granulomatosis. Kidney Int 2006;70(05):938–947 [DOI] [PubMed] [Google Scholar]
- 52.Abdulahad WH, Kallenberg CG, Limburg PC, Stegeman CA. Urinary CD4+ effector memory T cells reflect renal disease activity in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2009;60(09):2830–2838 [DOI] [PubMed] [Google Scholar]
- 53.Moosig F, Csernok E, Wang G, Gross WL. Costimulatory molecules inWegener’s granulomatosis (WG): lack of expression of CD28 and preferential up-regulation of its ligands B7-1 (CD80) and B7-2 (CD86) on T cells. Clin Exp Immunol 1998;114(01):113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum 2008;58(07):2196–2205 [DOI] [PubMed] [Google Scholar]
- 55.Lamprecht P, Csernok E, Gross WL. Effector memory T cells as driving force of granuloma formation and autoimmunity in Wegener’s granulomatosis. J Intern Med 2006;260(03):187–191 [DOI] [PubMed] [Google Scholar]
- 56.Bunch DO, McGregor JG, Khandoobhai NB, et al. Decreased CD5+ B cells in active ANCA vasculitis and relapse after rituximab. Clin J Am Soc Nephrol 2013;8(03):382–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lepse N, Abdulahad WH, Rutgers A, Kallenberg CG, Stegeman CA, Heeringa P. Altered B cell balance, but unaffected B cell capacity to limit monocyte activation in anti-neutrophil cytoplasmic antibody-associated vasculitis in remission. Rheumatology (Oxford) 2014;53(09):1683–1692 [DOI] [PubMed] [Google Scholar]
- 58.Culton DA, Nicholas MW, Bunch DO, et al. Similar CD19 dysregulation in two autoantibody-associated autoimmune diseases suggests a shared mechanism of B-cell tolerance loss. J Clin Immunol 2007;27(01):53–68 [DOI] [PubMed] [Google Scholar]
- 59.Schneeweis C, Rafalowicz M, Feist E, et al. Increased levels of BLyS and sVCAM-1 in anti-neutrophil cytoplasmatic antibody (ANCA)-associated vasculitides (AAV). Clin Exp Rheumatol 2010;28(01, Suppl 57):62–66 [PubMed] [Google Scholar]
- 60.Cornec D, Berti A, Hummel A, Peikert T, Pers JO, Specks U. Identification and phenotyping of circulating autoreactive proteinase 3-specific B cells in patients with PR3-ANCA associated vasculitis and healthy controls. J Autoimmun 2017;84:122–131 [DOI] [PubMed] [Google Scholar]
- 61.Roth AJ, Ooi JD, Hess JJ, et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 2013;123(04):1773–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 2014;10 (08):463–473 [DOI] [PubMed] [Google Scholar]
- 63.Silva F, Specks U, Sethi S, Irazabal MV, Fervenza FC. Successful pregnancy and delivery of a healthy newborn despite transplacental transfer of antimyeloperoxidase antibodies from a mother with microscopic polyangiitis. Am J Kidney Dis 2009;54(03):542–545 [DOI] [PubMed] [Google Scholar]
- 64.Eisenberger U, Fakhouri F, Vanhille P, et al. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant 2005;20(07):1392–1399 [DOI] [PubMed] [Google Scholar]
- 65.Xiao H, Hu P, Falk RJ, Jennette JC. Overview of the pathogenesis of ANCA-associatedvasculitis.Kidney Dis (Basel) 2016;1(04):205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schreiber A, Busjahn A, Luft FC, Kettritz R. Membrane expression of proteinase 3 is genetically determined. J Am Soc Nephrol 2003;14(01):68–75 [DOI] [PubMed] [Google Scholar]
- 67.Fussner LA, Hummel AM, Schroeder DR, et al. ; Rituximab in ANCA-Associated Vasculitis-Immune Tolerance Network Research Group. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol 2016;68(07):1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jayne DR, Gaskin G, Rasmussen N, et al. ; European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007;18(07):2180–2188 [DOI] [PubMed] [Google Scholar]
- 69.Jones RB, Tervaert JW, Hauser T, et al. ; European Vasculitis Study Group. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010;363(03):211–220 [DOI] [PubMed] [Google Scholar]
- 70.Stone JH, Merkel PA, Spiera R, et al. ; RAVE-ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363(03):221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miloslavsky EM, Specks U, Merkel PA, et al. ; Rituximab in ANCA-Associated Vasculitis-Immune Tolerance Network Research Group. Rituximab for the treatment of relapses in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2014;66(11):3151–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Charles P, Terrier B, Perrodeau É, et al. ; French Vasculitis Study Group. Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2). Ann Rheum Dis 2018;77(08): 1143–1149 [DOI] [PubMed] [Google Scholar]
- 73.Han WK, Choi HK, Roth RM, McCluskey RT, Niles JL. Serial ANCA titers: useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int 2003;63(03):1079–1085 [DOI] [PubMed] [Google Scholar]
- 74.Kallenberg CG, Brouwer E, Weening JJ, Tervaert JW. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int 1994;46(01):1–15 [DOI] [PubMed] [Google Scholar]
- 75.Falk RJ, Jennette JC. ANCA disease: where is this field heading? J Am Soc Nephrol 2010;21(05):745–752 [DOI] [PubMed] [Google Scholar]
- 76.Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol 2004;93(04): 398–401 [DOI] [PubMed] [Google Scholar]
- 77.Williams JM, Kamesh L, Savage CO. Translating basic science into patient therapy for ANCA-associated small vessel vasculitis. Clin Sci (Lond) 2005;108(02):101–112 [DOI] [PubMed] [Google Scholar]
- 78.Rarok AA, Limburg PC, Kallenberg CG. Neutrophil-activating potential of antineutrophil cytoplasm autoantibodies. J Leukoc Biol 2003;74(01):3–15 [DOI] [PubMed] [Google Scholar]
- 79.Jennette JC, Falk RJ. Pathogenesis of the vascular and glomerular damage in ANCA-positive vasculitis. Nephrol Dial Transplant 1998;13(Suppl 1):16–20 [DOI] [PubMed] [Google Scholar]
- 80.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A 1990;87(11):4115–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leukoc Biol 1991;50(06):539–546 [DOI] [PubMed] [Google Scholar]
- 82.Savage CO, Gaskin G, Pusey CD, Pearson JD. Myeloperoxidase binds to vascular endothelial cells, is recognized by ANCA and can enhance complement dependent cytotoxicity. Adv Exp Med Biol 1993;336:121–123 [DOI] [PubMed] [Google Scholar]
- 83.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 2009;20(02):289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Falk RJ, Hogan S, Carey TS, Jennette JC; The Glomerular Disease Collaborative Network. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. Ann Intern Med 1990;113(09):656–663 [DOI] [PubMed] [Google Scholar]
- 85.Huugen D, Xiao H, van Esch A, et al. Aggravation of antimyeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am J Pathol 2005;167(01):47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Radford DJ, Savage CO, Nash GB. Treatment of rolling neutrophils with antineutrophil cytoplasmic antibodies causes conversion to firm integrin-mediated adhesion. Arthritis Rheum 2000;43(06): 1337–1345 [DOI] [PubMed] [Google Scholar]
- 87.Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int 1992;41(02):375–383 [DOI] [PubMed] [Google Scholar]
- 88.Savage CO, Pottinger BE, Gaskin G, Pusey CD, Pearson JD. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am J Pathol 1992;141(02):335–342 [PMC free article] [PubMed] [Google Scholar]
- 89.Vargunam M, Adu D, Taylor CM, et al. Endothelium myeloperoxidase-antimyeloperoxidase interaction in vasculitis. Nephrol Dial Transplant 1992;7(11):1077–1081 [PubMed] [Google Scholar]
- 90.Gupta AK, Joshi MB, Philippova M, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETo-sis-mediated cell death. FEBS Lett 2010;584(14):3193–3197 [DOI] [PubMed] [Google Scholar]
- 91.Kessenbrock K, Krumbholz M, Schönermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009;15(06):623–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nolan SL, Kalia N, Nash GB, Kamel D, Heeringa P, Savage CO. Mechanisms of ANCA-mediated leukocyte-endothelial cell interactions in vivo. J Am Soc Nephrol 2008;19(05):973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porges AJ, Redecha PB, Kimberly WT, Csernok E, Gross WL, Kimberly RP. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol 1994;153(03):1271–1280 [PubMed] [Google Scholar]
- 94.Kettritz R, Jennette JC, Falk RJ. Crosslinking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol 1997;8(03):386–394 [DOI] [PubMed] [Google Scholar]
- 95.Guilpain P, Chéreau C, Goulvestre C, et al. The oxidation induced by antimyeloperoxidase antibodies triggers fibrosis in microscopic polyangiitis. Eur Respir J 2011;37(06):1503–1513 [DOI] [PubMed] [Google Scholar]
- 96.Guilpain P, Servettaz A, Goulvestre C, et al. Pathogenic effects of antimyeloperoxidase antibodies in patients with microscopic polyangiitis. Arthritis Rheum 2007;56(07):2455–2463 [DOI] [PubMed] [Google Scholar]
- 97.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002;110(07):955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 2007;170(01):52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao H, Heeringa P, Liu Z, et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 2005;167(01):39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schreiber A, Xiao H, Falk RJ, Jennette JC. Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J Am Soc Nephrol 2006;17(12):3355–3364 [DOI] [PubMed] [Google Scholar]
- 101.Xiao H, Ciavatta D, Aylor DL, et al. Genetically determined severity of anti-myeloperoxidase glomerulonephritis. Am J Pathol 2013; 182(04):1219–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xing GQ, Chen M, Liu G, et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol 2009; 29(03):282–291 [DOI] [PubMed] [Google Scholar]
- 103.Gou SJ, Yuan J, Wang C, Zhao MH, Chen M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol 2013;8(11):1884–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yuan J, Gou SJ, Huang J, Hao J, Chen M, Zhao MH. C5a and its receptors in human anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Arthritis Res Ther 2012;14(03): R140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jayne DRW, Bruchfeld AN, Harper L, et al. ; CLEAR Study Group. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 2017;28(09):2756–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Little MA, Smyth L, Salama AD, et al. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol 2009; 174(04):1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Little MA, Bhangal G, Smyth CL, et al. Therapeutic effect of anti-TNF-alpha antibodies in an experimental model of anti-neutrophil cytoplasm antibody-associated systemic vasculitis. J Am Soc Nephrol 2006;17(01):160–169 [DOI] [PubMed] [Google Scholar]
- 108.Ruth AJ, Kitching AR, Kwan RY, et al. Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 2006;17(07):1940–1949 [DOI] [PubMed] [Google Scholar]
- 109.Salama AD, Little MA. Animal models of antineutrophil cytoplasm antibody-associated vasculitis. Curr Opin Rheumatol 2012;24(01):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Little MA, Al-Ani B, Ren S, et al. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS One 2012;7 (01):e28626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tomer Y, Gilburd B, Blank M, et al. Characterization of biologically active antineutrophil cytoplasmic antibodies induced in mice. Pathogenetic role in experimental vasculitis. Arthritis Rheum 1995;38(10):1375–1381 [DOI] [PubMed] [Google Scholar]
- 112.Hattar K, Oppermann S, Ankele C, et al. c-ANCA-induced neutrophil-mediated lung injury: a model of acute Wegener’s granulomatosis. Eur Respir J 2010;36(01):187–195 [DOI] [PubMed] [Google Scholar]
- 113.Charles Jennette J, Falk RJ. L1. Pathogenesis of ANCA-associated vasculitis: observations, theories and speculations. Presse Med 2013;42(4 Pt 2):493–498 [DOI] [PubMed] [Google Scholar]
- 114.Xiao H, Morrison T, Heise M, Falk R, Jennette J. MPO-ANCA IgG and influenza: a viral infection acts in concert to induce severe hemorrhagic pulmonary capillaritis. Clin Exp Rheumatol 2007; 25:S-91 [Google Scholar]
- 115.Hu P, Xiao X, Alba MA, Falk R, Jennette C. Anti-MPO antibodies cause granulomatosis in mice: an animal model of GPA. Rheumatology 2017;56:iii29–iii31 [Google Scholar]
- 116.Schönermarck U, Csernok E, Gross WL. Pathogenesis of antineutrophil cytoplasmic antibody-associated vasculitis: challenges and solutions 2014. Nephrol Dial Transplant 2015;30(Suppl 1): i46–i52 [DOI] [PubMed] [Google Scholar]
- 117.Chen SF, Wang FM, Li ZY, Yu F, Chen M, Zhao MH. Myeloperoxidase influences the complement regulatory activity of complement factor H. Rheumatology (Oxford) 2018 [DOI] [PubMed] [Google Scholar]
- 118.Wang H, Wang C, Zhao MH, Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin Exp Immunol 2015;181(03):518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J Clin Invest 1997;100(06):1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jennette JC, Falk RJ. ANCAs are also antimonocyte cytoplasmic autoantibodies. Clin J Am Soc Nephrol 2015;10(01):4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Casselman BL, Kilgore KS, Miller BF, Warren JS. Antibodies to neutrophil cytoplasmic antigens induce monocyte chemoattractant protein-1 secretion from human monocytes. J Lab Clin Med 1995;126(05):495–502 [PubMed] [Google Scholar]
- 122.Millet A, Martin KR, Bonnefoy F, et al. Proteinase 3 on apoptotic cells disrupts immune silencing in autoimmune vasculitis. J Clin Invest 2015;125(11):4107–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener’s granulomatosis. Review of 87 open lung biopsies from 67 patients. Am J Surg Pathol 1991;15 (04):315–333 [DOI] [PubMed] [Google Scholar]
- 124.Gaudin PB, Askin FB, Falk RJ, Jennette JC. The pathologic spectrum of pulmonary lesions in patients with anti-neutrophil cytoplasmic autoantibodies specific for anti-proteinase 3 and anti-myeloperoxidase. Am J Clin Pathol 1995;104(01):7–16 [DOI] [PubMed] [Google Scholar]
- 125.Jennette JC, Falk RJ, Hu P, Xiao H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol 2013;8:139–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mueller A, Holl-Ulrich K, Lamprecht P, Gross WL. Germinal centre-like structures in Wegener’s granuloma: the morphological basis for autoimmunity? Rheumatology (Oxford) 2008;47 (08):1111–1113 [DOI] [PubMed] [Google Scholar]
- 127.Lamprecht P, Gross WL. Current knowledge on cellular interactions in the WG-granuloma. Clin Exp Rheumatol 2007;25(01, Suppl 44):S49–S51 [PubMed] [Google Scholar]
- 128.Voswinkel J, Müller A, Lamprecht P. Is PR3-ANCA formation initiated in Wegener’s granulomatosis lesions? Granulomas as potential lymphoid tissue maintaining autoantibody production. Ann N YAcad Sci 2005;1051:12–19 [DOI] [PubMed] [Google Scholar]
- 129.Birnbaum J, Danoff S, Askin FB, Stone JH. Microscopic polyangiitis presenting as a “pulmonary-muscle” syndrome: is subclinical alveolar hemorrhage the mechanism of pulmonary fibrosis? Arthritis Rheum 2007;56(06):2065–2071 [DOI] [PubMed] [Google Scholar]
- 130.Schnabel A, Reuter M, Csernok E, Richter C, Gross WL. Subclinical alveolar bleeding in pulmonary vasculitides: correlation with indices of disease activity. Eur Respir J 1999;14(01): 118–124 [DOI] [PubMed] [Google Scholar]
- 131.Pineton de Chambrun M, Nunes H, Brochériou I, Hertig A. Idiopathic lung fibrosis and anti myeloperoxidase glomerulonephritis: the tree that hides the forest. BMC Pulm Med 2015; 15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Franks TJ, Koss MN. Pulmonary capillaritis. Curr Opin Pulm Med 2000;6(05):430–435 [DOI] [PubMed] [Google Scholar]
- 133.Heeringa P, Schreiber A, Falk RJ, Jennette JC. Pathogenesis of pulmonary vasculitis. Semin Respir Crit Care Med 2004;25(05): 465–474 [DOI] [PubMed] [Google Scholar]