ABSTRACT
Caprine Herpesvirus type 1 (CpHV-1) is a species-specific herpes virus able to induce apoptosis in several biological systems. In the present study we aimed to investigate the ability of CpHV-1 to reduce cells viability, to replicate and to cause cell death also in human cancer cell lines. We tested the CpHV-1 effects on HEL-299, Vero, MDA-MB-468, HeLa, U2OS, PC3, A549 and K562 neoplastic cell lines and on MDBK cells. Firstly, we evaluated the effect of CpHV-1 infection on cell viability by MTT assay and our data showed that CpHV-1 can induce a marked cytopathic effect (CPE) in most of cell lines tested, except for HEL-299, Vero and K562 cells. The reduction of cell viability was associated with a significant increase of viral production. We next investigated if CpHV-1 was able to induce cell death and so through western blotting analysis we evaluated cleaved caspase 3, LC3II and p62 protein levels after infection. Caspase 3 activation was detected in MDBK cells and, even if at different times p.i., also in MDA-MB-468, U2OS, and PC3 cell lines, while LC3II increase and concomitant p62 protein reduction were observed only in U2OS, and A549 cells, no significant alteration of these proteins was observed in the other cell lines tested. Finally, to confirm virus ability to trigger apoptosis we performed an Annexin-V apoptosis test after 24 h p.i.
Although we need to further explore mechanisms underlying CpHV-1 treatment, this study could serve as the basis for the development of new treatment options aiming to fight several cancer types.
Keywords: Oncolytic virotherapy, apoptosis, autophagy, human cancer cells
Introduction
Oncolytic virotherapy is a new therapeutic approach based on the use of genetically modified or wild-type viruses able to selectively destroy neoplastic cells.1,2 Recently, virus therapy has become a promising treatment for neoplastic disease, particularly in the cases where methods in use are ineffective or inapplicable.3 During the past decade, several clinical trials have been carried out to document the therapeutic efficacy of these viruses, even though their oncolytic mechanisms are still not-completely understood.4-6
Presently, several DNA and RNA viruses, like herpesviruses, adenovirus, reovirus and poliovirus, have demonstrated an oncolytic potential.3,7–9 Among them, HSV-1 is the first genetically modified virus which has been used in cancer therapy. Several clinical trials have been performed with different HSV-1 mutants demonstrating their safety and efficacy as oncolytic virus in human.10,11 Since HSV-1 is a human pathogen, it is necessary to genetically modify it to attenuate its pathogenetic potential and allow the virus to preferentially replicate in tumour cells. However, viral attenuation decreases viral replication performances and reduces the possibility of therapy success. Moreover, several authors have estimated that the largest part of human population has antibodies against HSV-1, which hampers efficient systemic transport of HSV-1 oncolytic vectors.2,12,13 Therefore, the use of non-human pathogen naïve viruses could be a better and alternative method for cancer therapy2. In this context, Caprine Herpesvirus type 1 (CpHV-1) has been used, in the present study, to kill some neoplastic cell lines of different origin, assuming that CpHV-1 owns oncolytic capacities like other Alphaherpesvirus such as Bovine Herpesvirus Type 1 (BoHV-1) and Equine Herpesvirus Type 1 (EHV-1).13–15
Our group has previously shown that CpHV-1 induces apoptosis in a permissive cell line (Madin Darby Bovine Kidney, MDBK16 and in goat Peripheral Blood Mononuclear Cells (PBMCs),17 and recently, we also have investigated about the apoptotic pathway activation during CpHV-1 infection by gene expression response analysis in a murine neuroblastoma cell line (Neuro 2A).18 Thus, the aim of this study was to investigate the capacity of CpHV-1 to grow in and impair cellular viability of human normal and neoplastic cell lines (HEL-299, Vero, HeLa, U2OS, MDAMB-468, A549, PC3, K562). We also evaluated the CpHV-1 ability to induce cell death (apoptosis or autophagy) and our data suggest that CpHV-1 could be considered a novel useful candidate for oncolytic virotherapy.
Material and methods
Cell line and virus
All cell types, kindly provided by prof. Michele Caraglia (University Luigi Vanvitelli – Campania, Italy) were maintained at 37°C with 5% CO2 in medium supplemented with 10% of foetal bovine serum, 2 mM L-glutamine, 100 U penicillin and 100 U streptomycin. Human embryonic lung fibroblast (HEL-299, ATCC Cat# CCL-137, RRID:CVCL_2480), Cercopithecus aethiops kidney normal(Vero, ATCC Cat# CCL-81, RRID:CVCL_0059), Human breast adenocarcinoma (MDA-MB-468, CLS Cat# 300279/NA, RRID:CVCL_0419), Human cervical adenocarcinoma (HeLa, CLS Cat# 300194/p772_HeLa, RRID:CVCL_0030), Human osteosarcoma (U2OS, CLS Cat# 300364/p489_U-2_OS, RRID:CVCL_0042), Human prostatic adenocarcinoma (PC3, CLS Cat# 300312/p1699_PC-3, RRID:CVCL_0035),Human lung carcinoma (A549, CLS Cat# 300114/p686_A-549, RRID:CVCL_0023), Madin Darby bovine kidney (MDBK, CLS Cat# 600396/p848_MDBK_(NBL-1), RRID:CVCL_0421) were maintained in high glucose Dulbecco’s modified Eagle’s medium (DMEM, Gibco Cat#11965092). Chronic Myelogenous Leukemia (K562, CLS Cat# 300224/p473_K-562, RRID:CVCL_0004) cell lines were maintained in Iscove medium (IMDM, Gibco cat#21056023).
The reference Swiss strain E/CH19 of CpHV-1 was used. It was grown on MDBK cells, viral stocks were obtained by three cycles of freezing and stored in aliquots at −80°C.
Cytopathic effect assay
A panel of two normal, six neoplastic cell lines (HEL-299, Vero, HeLa, U2OS, MDAMB-468, A549, PC3 and K562) and MDBK cell line, used as control, were infected with increasing multiplicity of infection (MOI) of CpHV-1, and cytopathic effect (CPE) was monitored.
Briefly, for adherent cells, 90 – 95% confluent cell monolayers were infected with different MOI between 0.5 and 10 in serum-free medium. After 1 hour of viral adsorption at 37°C, cell monolayers were maintained in medium with 5% of FBS. At 24 and 48 hours post infection cells were fixed in methanol (≥ 99,8% – Sigma-Aldrich Cat#32213–2.5L) and stained with Giemsa to observe and score CPE.
Otherwise, for K562 suspension cells Giemsa staining was not applicable, thus, viability was determined by trypan blue dye exclusion method (Sigma-Aldrich, Cat# T8154). Mock infected and infectedK562cells were collected, and an aliquot of the cell suspension was mixed with an equal volume of 0.4% Trypan-blue solution in 1X phosphate-buffered saline (PBS), and after five minutes of incubation, cells were counted byTC20 automated cell counter (Bio-Rad).
Cytotoxicity
Virus cytotoxicity was tested by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (SERVA Electrophoresis GmbH Cat# 20395.02) assay as previously described.20 The method relies on the reduction of MTT, a soluble tetrazolium salt, that is converted to purple insoluble formazan by mitochondrial dehydrogenases of living cells. The conversion from tetrazolium to formazan is analysed spectrophotometrically after dissolution in dimethylsulfoxide (DMSO) (SERVA Electrophoresis GmbH Cat# 39757.01). The spectrophotometer absorbance at 570 nm was determined. Results are expressed as percentages of cell viability (calculated respect to control cells). The mean and standard deviation of three experiments performed in duplicate are reported in histograms.
Virus production and viral replication
Infectious titres were expressed as median tissue culture infectious doses (TCID50)/ml.21
Nucleic acids were extracted from 200 µl of cellular suspension using QIAsymphony SP automatic extraction system (Qiagen) with the kit DSP Virus/Pathogen Midi (Qiagen). Real time PCR analysis for the detection of CpHV-1 was performed with the protocol described by Elia et al.,22 using reference Swiss strain as positive control.23
Protein extraction and western blot analysis
All cell lines analysed were infected with CpHV-1 at MOI 2.5 and after 12, 24, and 48 h post infection, adherent cells were washed twice with PBS and scraped. Cells were then mixed with cells earlier collected by centrifugation from the supernatant of the same flask and resuspended in PBS. All pellets obtained were then lysed on ice for 30 min in lysis buffer (1 mM EDTA, 150 mM NaCl, 1%NP-40, 50 mM TRIS-HCL pH7.5, supplemented with protease/phosphatase inhibitors) and cell lysates were subjected to SDS-PAGE as previouslydescribed18. Protein concentrations were evaluated using a protein assay kit (Bio-Rad Protein Assay Dye Reagent Concentrate Cat#5000006). 20 µg of proteins were heated at 100°C for 5 min, loaded on bis/acrylamide gels, separated by electrophoresis and proteins were blotted from the gel on Polyvinylidene Difluoride (PVDF) membranes (Trans-Blot® Turbo™ Mini PVDF Transfer Packs – cat#1704156) with a semidry transfer cell (Trans Bot Turbo, Bio-Rad). PVDF membranes were blocked at room temperature (RT) for 2 h in Tris-buffered saline (TBS: 12.5 mM Tris–HCl pH 7.4; 125 mM NaCl) added with 5% of Bovine Serum Albumin (BSA, Cat#AB022), incubated with primary antibodies against Caspase 3, LC3I/II and QSTM1/p62 at + 4°C overnight and then raised with Anti-Rabbit-HRP-linked antibody at RT for 1 h. The blots were stripped and incubated with anti-β-Actin antibodies at 1:2000 dilution to confirm equal loading of proteins in each lane. Blots were visualized and protein expression levels were assessed by densitometry using the ChemiDoc Gel scanner (Bio-Rad).
Rabbit anti-cleaved caspase 3(Cell Signaling Technologies, Cat#9662, RRID:AB_331439; 1:1000 dilution), anti-LC3A/B(Cell Signaling Technologies, Cat#4108, RRID:AB_2137703; 1:1000 dilution), anti-SQSTM1/p62 (Cell Signaling Technologies, Cat#5114, RRID:AB_10624872; 1:1000 dilution), and anti-β-Actin (Cell Signaling Technologies,Cat#4967, RRID:AB_330288; 1:1000 dilution) were used in the present study as primary antibodies. A secondary peroxidise-conjugated anti-rabbit IgG (Cell Signaling Technologies, Cat# 7074S, RRID:AB_2099233) was applied for 1 h at 1:2000 dilution.
Annexin V apoptosis analysis
Cell lines analysed were infected with CpHV-1 at MOI 2.5 and after 24 h post infection the percentage of apoptotic cells was identified by FACS through the Annexin V-FITC kit (Miltenyi Biotec GmbH; https://www.miltenyibiotec.com/~/media/Images/Products/Import/0001300/IM0001386.ashx) according to the manufacturer instructions.
Statistical analysis
Statistical analyses were performed using the GraphPad InStat Version 3.00 for Window 95 (GraphPad Software). Statistically significant differences between the means of multiple matched groups were evaluated by one-way analysis of Variance (ANOVA), followed by Turkey’s post-test. P < 0.05 was considered statistically significant.
Results
CpHV-1 lytic capacity in human neoplastic cell lines
We first assessed the capacity of CpHV-1 to induce cytopathic effect (CPE) in a panel of selected human cancer cells. Our results shown that, although at different MOI and at different time p.i., CpHV-1 was able to induce CPE in almost all adherent human cancer cell line tested. In particular, as shown in Figure 1, at 24 h p.i., in PC3, MDA-MB-468, U2OS, HeLa and MDBK more than 50% CPE was observed between MOI 2.5 and 10, in A549 cell line we observed less than 50% CPE at MOI 10 otherwise in HEL-299 and Vero cells, no CPE was observed at 24 h p.i (Figure 1A). Similarly, at 48 h p.i, as shown in Table 1, we found more than 50% CPE in PC3, MDA-MB-468, U2OS and MDBK at MOI 1, 2.5, 5 and 10; more than 50% CPE in HeLa cells between MOI 0.5 and 10 and 50% CPE in A549 cell line at MOI 5 and 10. In K562 suspension cells, Trypan blue assay did not detect a reduction of the number of living cells at 24 h and 48 h p.i. (Figure 1 B).
Figure 1.
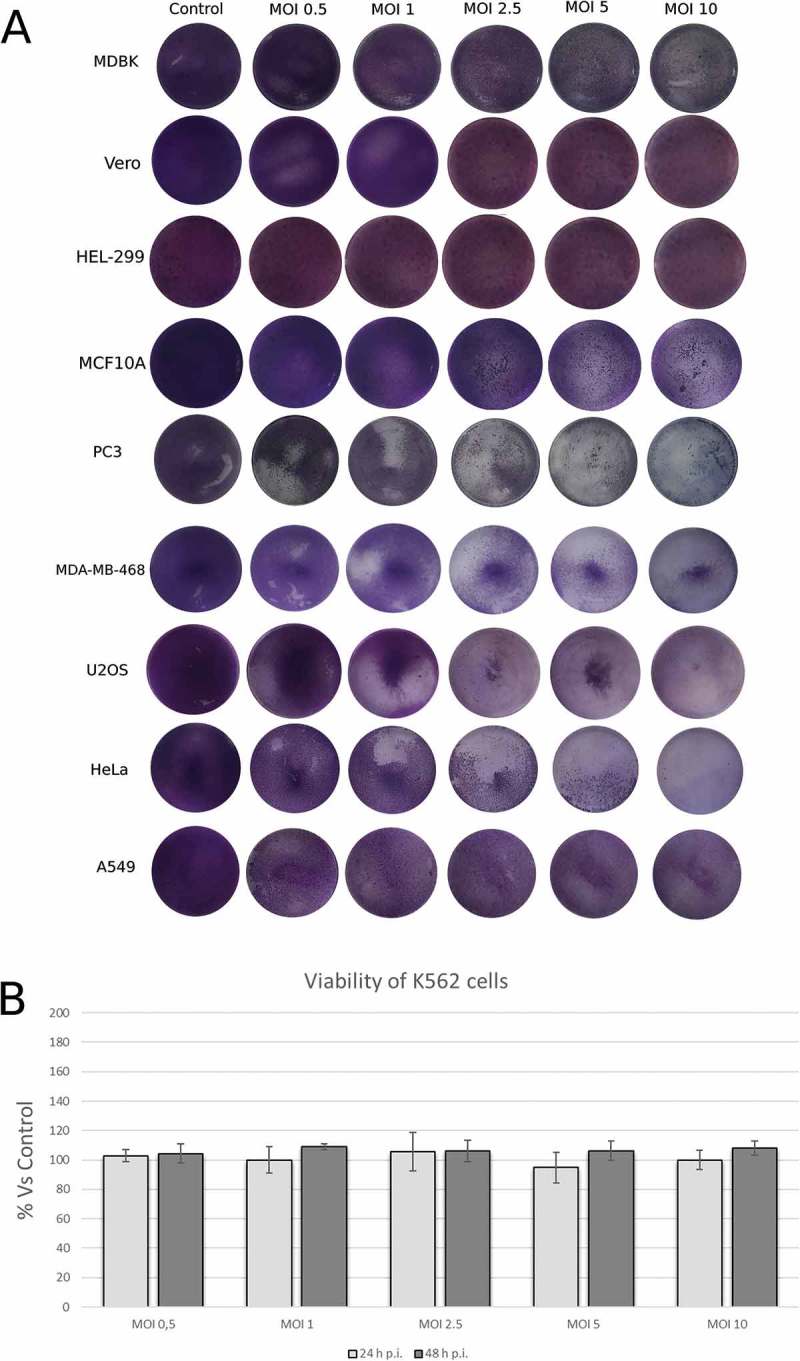
Permissiveness of human neoplastic cell lines to Caprine Herpesvirus 1 (CpHV-1). Cells were infected with the indicated MOI of CpHV-1 at 24 h post infection and stained with Giemsa to observe Cytopathic effect (CPE). Panel B show viability results in cell line determined by trypan blue dye exclusion method at 24 h and 48 h p.i. Results are expressed as the mean ± SD of three independent experiments performed in duplicate. The SD was calculated on the raw data and expressed as a percentage.
Table 1.
In vitro cytotoxicity of CpHV-1 in normal, immortalized and neoplastic cell lines from different origins: +++ indicates very permissive cells, in which more than 50% CPE was seen; ++ indicates a moderately permissive cell type, in which 50% CPE was seen; + indicates a cell type that is not very permissive, in which less than 50% CPE was seen; -indicates a non-permissive cell type in which no CPE was observed at MOI between 0.5 – 10.
| Cell Line | Cell State | Cell Type | CPE 24 h | CPE 48 h |
|---|---|---|---|---|
| HEL-299 | Normal | Embryonic Lung fibroblast | - | - |
| Vero | Normal | Green Monkey Kidney | - | - |
| MDBK | Normal | Bovine Kidney | +++ | +++ |
| PC3 | Adenocarcinoma | Bone metastatis of prostate origin | ++ | ++ |
| MDA-MB-468 | Adenocarcinoma | Adult Breast | ++ | ++ |
| U2OS | Osteosarcoma | Bone sarcoma | ++ | ++ |
| HeLa | Immortalized | Cervix Adenocarcinoma | +++ | +++ |
| A549 | Carcinoma | Adult lung | + | + |
CpHV-1 affects cell viability of several human cancer cell lines
To determine CpHV-1 effect on cell viability in a panel of human cancer cell lines we performed MTT assay by using increasing MOI of virus for 24 h, 48 h and 72 h p.i. CpHV-1 infection resulted in a reduction of cell viability in five of the cell lines tested (PC3, MDA-MB-468, U2OS, HeLa and MDBK) as shown in Figure 2. Specifically in MDA-MB-468 and MDBK cell viability decreases at 24 h p.i. and this effect remains up to 72 h p.i.; U2OS cell viability decreases at 72 h p.i. while in PC3 was observed an irregular trend, characterized by a reduction of cell viability at 48 h p.i. follow by an increase after 72 h infection; finally in HeLa cells the virus induces a decrease of cell viability only at 24 h and 48 h. Whereas in A549, HEL-299 and Vero cells we didn’t observe a statistically significant decrease in cell viability and conversely in K562 the cell viability increases. These results, consistently with the marked CPE observed, demonstrated that CpHV-1 has an oncolytic potential in all cancer cell lines analyzed, except in A549 and K652 cells. This experiment, in addition, allowed us to identify the MOI 2.5 as able to induce a 50% reduction of vitality in most of the cell lines, so we used this MOI 2.5 for the subsequent experiments.
Figure 2.
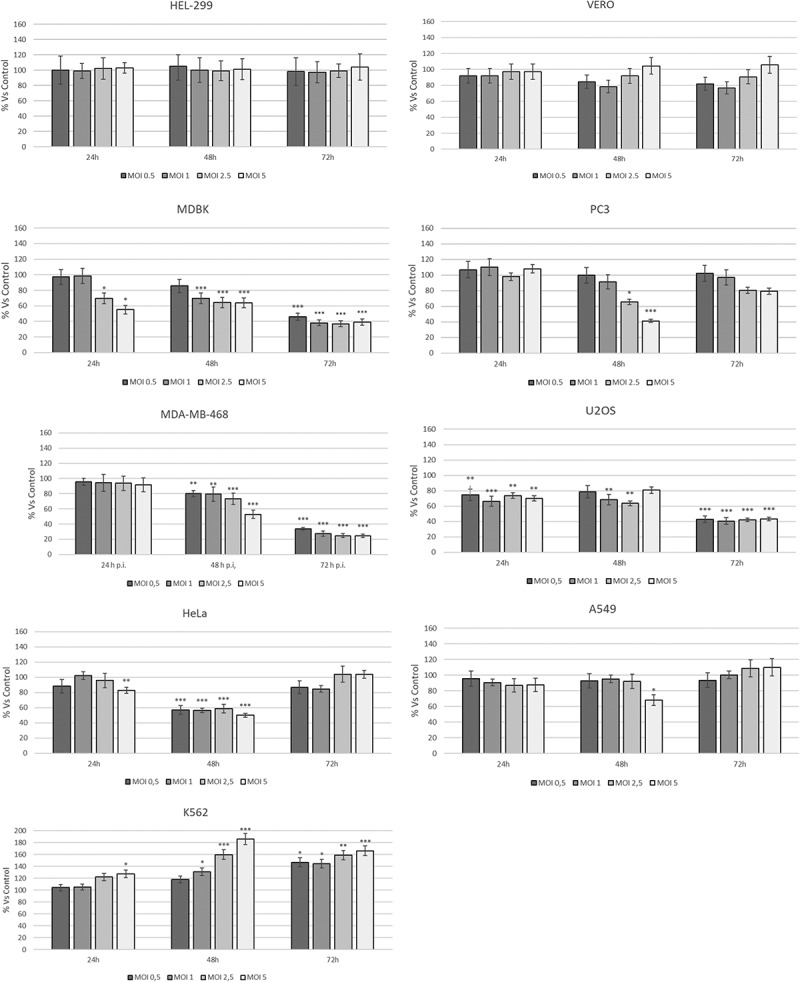
Dose–response curve of human cancer cell lines infected with different MOI of CpHV-1 at different times p.i.. MTT test was performed at different hours post infection and the absorbance assayed as described in the Materials and Methods Section. Data are presented as a percentage of the cell viability calculated respect to control and results are expressed as the mean ± SD of three independent experiments performed in duplicate. Statistical differences between control and CpHV-1 infected groups were evaluated by one-way analysis of Variance (ANOVA), followed by Turkey’s post-test and indicated by probability P. *P < 0.05, **P < 0.01, and ***P < 0.001. The SD was calculated on the raw data and expressed as a percentage.
Virus production and viral replication in neoplastic cell lines
We next determined whether the cytotoxic effects were correlated to a significant increase of viral production. So, we infected human cancer cell lines with CpHV-1 and the virus replication was evaluated by TCID50 assay. Moreover, molecular characterisation of viral replication was performed in human cancer cells by Real-time qRT-PCR. Figure 3 shows a time course of virus yield, in supernatants and cell-associated virus, following exposure to CpHV-1 for 24 h, 48 h and 72 h at a MOI 2.5. MDBK cell line was used as control.
Figure 3.
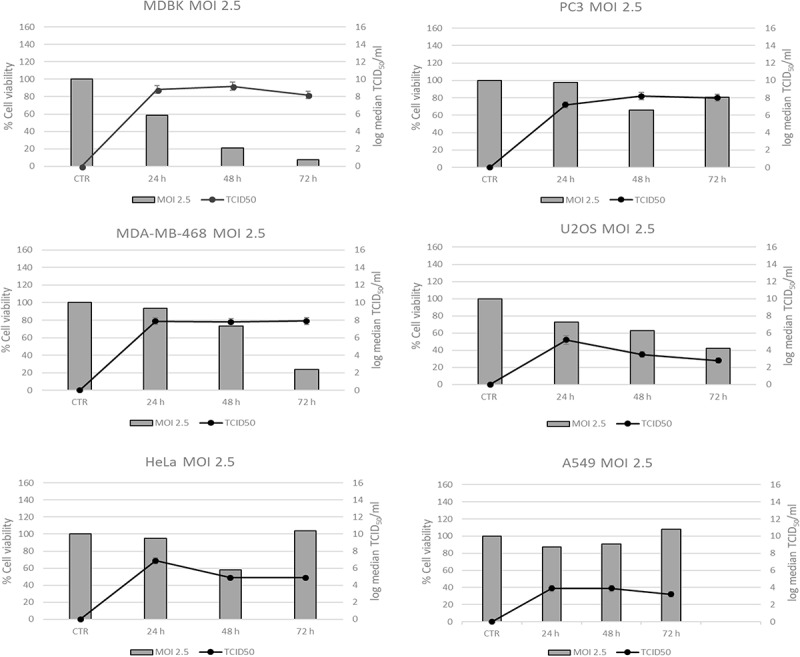
Time course of virus production in Human cancer cell lines infected with CpHV-1. Virus production was titrated at 24 h, 48 h and 72 h after infection with CpHV-1 at a MOI. of 2.5. Results are expressed as Mean ± SD of three independent experiments performed in duplicate.
The acme of viral production was observed at 24 h post infection in PC3, MDA-MB-468, U2OS, HeLa and MDBK cell lines. No efficient viral replication in HEL-299, Vero and A549 cell line was observed. Real-time qRT-PCR data confirmed virus titration assays, showing an analogous trend in human cancer cells (data not shown).
CpHV-1 infection induces cell death in neoplastic cell lines
In order to elucidate the cell death mechanisms responsible for the cytopathic effect during CpHV-1 infection, we evaluated through western blotting, the effect of the treatment on molecular markers of apoptosis and autophagy, such as Caspase 3, LC3II and p62. Caspase-3, a critical executioner of apoptosis, as it is either partially or totally responsible for the proteolytic cleavage of many key proteins.24,25 Activation of caspase-3 requires proteolytic processing of its inactive zymogen into activated p17 and p12 fragments.26 These assays were performed only in those cell lines in which a significant cytotoxic effect was evidenced by the MTT test.
The cleavage of caspase 3 (12–17 kDa) was detected starting from 12 h post infection in MDBK cells, and this effect persisted for up to 48 hours. This scenario agrees with published data demonstrating that, during viral infection, apoptosis can represent a mechanism for maximizing viral progeny, facilitating the virus release from infected cells,27 Figure 4,A/C). In U2OS cells, Caspase 3 cleavage was detected after 12 h while in PC3 after 24 h (Figure 4,A/B). In MDA-MD−468 cleaved Caspase 3 was weakly detected at 24 h p.i and this fragment increased at 48 h p.i. (Figure 4 B/C). Conversely, Caspase 3 activation was not detected in Hela and A549 cells.
Figure 4.
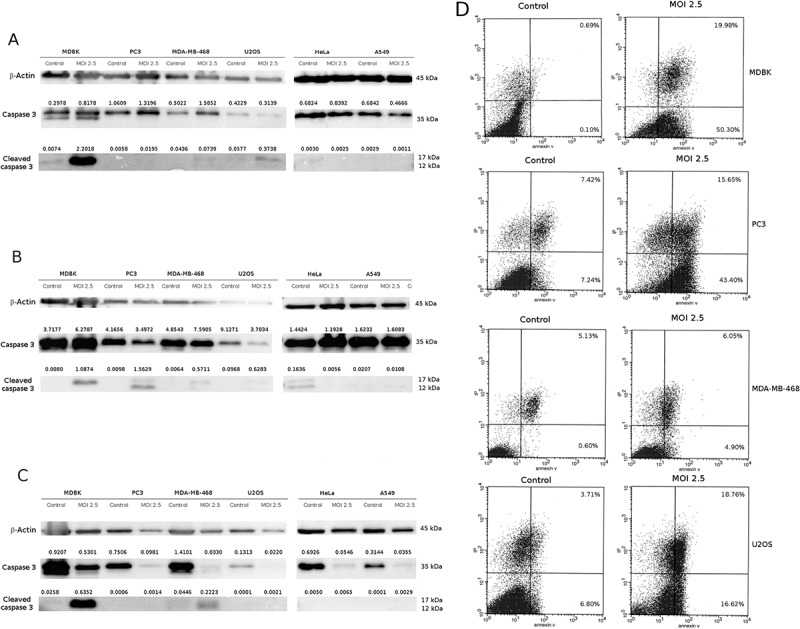
CpHV-1 infection induces apoptosis in neoplastic cell lines. Panel A-C: Western blotting analysis of Caspase and Cleaved caspase 3 levels in MDBK, PC3, MDA-MB-468, U2OS, Hela and A549 cells were performed after mock infection or infection with CpHV-1 at MOI 2.5, at 12 h (panel A), 24 h (panel B) and 48 h (panel C) p.i.. β-Actin were used as a loading control. Band densitometry values indicate caspase 3 and cleaved caspase 3 levels normalized to β-Actin levels. Panel D: Annexin V apoptosis analysis. MDBK, PC3, MDA-MB-468 and U2Os cells were infected with CpHV-1 virus and after 24 h analyzed by annexin-V assay. The graphs (panel D) show the percentages of early apoptosis (population of cells positive for annexin-V staining) and late apoptosis/necrosis (population of cells positive for both annexin-V and propidium iodide staining).
To better characterized the CpHV-1 ability to induce apoptosis in these cell lines we performed also an Annexin V cytofluorimetric assay upon 24 h post infection at the MOI 2.5. As expected, CpHV-1 induced apoptosis in all cell lines in which we previously detected caspase 3 cleavage and a decrease of cell viability (Figure 4D).
Finally, we investigated the activation of autophagic flow by analysis of conversion of LC3I to LC3II and the contemporary reduction of protein p62. The increase of LC3II, with a reduction of p62 were observed in U2OS cell line at 12 h p.i., and surprisingly in A549 cell lines at 48 h p.i. The other cell lines didn’t show typical markers of autophagy (Figure 5).
Figure 5.
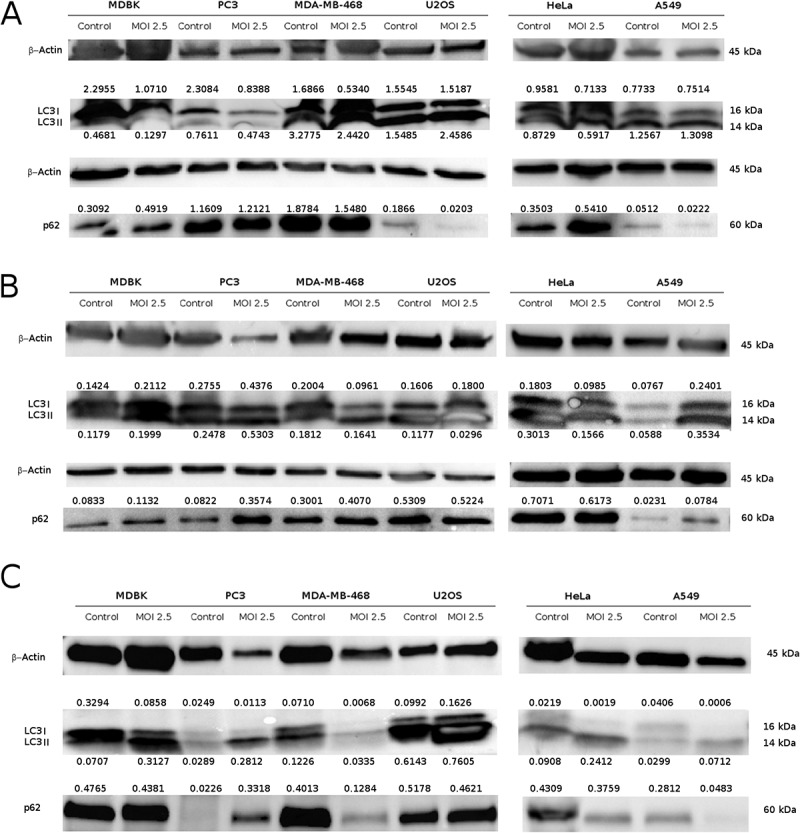
Evaluation of autophagy markers in neoplastic cell lines after CpHV-1 infection. Western blotting analysis of LC3I, LC3II and p62 levels in MDBK, PC3, MDA-MB-468, U2OS, Hela and A549 cells were performed after mock infection or infection with CpHV-1 at MOI 2.5, at 12 h (panel A), 24 h (panel B) and 48 h (panel C) p.i.. β-Actin were used as a loading control. Band densitometry values indicate LC3I, LC3II and p62 levels normalized to β-Actin levels.
Discussion
Neoplastic cells, to multiply indefinitely, have developed mechanisms of apoptosis inhibition and immune system evasion,28,29 moreover, they often present defects in host antiviral response pathways, mainly the interferon pathway, which makes these cells unable to efficiently fight and suppress viral replication. Several oncolytic viruses have been shown to replicate preferentially in cancer cells.30,31 Oncolytic viruses display natural attitude to infect and destroy neoplastic cells, leaving surrounding cells unharmed. Oncolytic viruses show several benefits over classical cancer therapies, such as the safety, the ability of oncolytic viruses to act as tumour vaccines and to work in synergy with conventional cancer therapy.2,29,32,33 Animal oncolytic viruses show many advantages respect to human vectors, like the incapacity to induce disease in humans and the lack of pre-existing immunity.28,34 Thus, in the present study, we investigated the CpHV-1 oncolytic potential in a panel of human cancer cells. First of all, we demonstrated that CpHV-1 is able to induce CPE in PC3, MDA-MB-468, U2OS and HeLa cell lines, otherwise, a higher MOI is needed to induce CPE in the A549 cells. No CPE effects were observed in HEL-299, Vero and K562 cells, suggesting that these cell lines were characterized by low permissiveness to CpHV-1 infection, probably due to the inability of the virus to enter these cell lines, similarly to what has been shown with other oncolytic viruses which preferentially replicate in cancer cells.10,11,29,30 The same trend was observed in cytotoxicity assay experiments.
Virus growth assays demonstrated that CpHV-1 grows in cancer cell lines with a higher titer in PC3, MDA-MB-468, HeLa, U2OS but not in A549 cell lines. These data confirmed that a reduction in viability corresponded to an increase in viral replication, suggesting the permissiveness of infected cell lines.
These results support the data reported in previous studies, which demonstrated the permissiveness of PC3, U2OS and MDA-MB-468 cell lines to oncolytic BoHV-1,2 a genetically related alphaherpesvirus. Several other Herpesviruses have been evaluated as oncolytic viruses, enclosing HSV-1,35 BoHV-12 and Bovine Herpesvirus 4 (a Gammaherpesvirus of the genus Rhadinovirus).36,37 Given the genetic similarity between BoHV-1 and CpHV-1, we believe that CpHV-1 could offer advantageous properties that make it a potential candidate as an anticancer agent. CpHV-1 is a non-pathogenic virus for humans but seems to be able to replicate, with the production of viral progeny, and kill different human cancer cell lines.
CpHV-1 has a brief replicative cycle which results in the death of infected cells, so it easily grows at high titers. Another interesting aspect of CpHV-1 for oncolytic virotherapy is the absence of pre-existing immunity against the virus in most of the human population. This would consent the entry of CpHV-1 into cancer cells and subsequent replication and spread of the virus that would not be delayed by pre-existing antibodies or memory cytotoxic T cells. Furthermore, CpHV-1 is a large virus and therefore has a wide packaging capacity, this feature would allow the relatively easy insertion of therapeutic genes that could be useful for increasing the oncolytic or selective properties of this viral vector.
In our experiments, in order to elucidate the mechanism of cell death involved in the CpHV-1 oncolytic activity, we have investigated the ability to induce apoptosis. Our experiments have demonstrated the activation of apoptotic process in MDA-MB-468, in U2OS and in PC3 dell lines. These results are in accordance with the decrease of viability and the high viral titers in these cell lines, confirming the ability of the virus to induce apoptosis. This is consistent with our previous studies, which demonstrated that CpHV-1 during lytic cycle exert its replicative potential inducing apoptosis in ruminant epithelial cells, in peripheral blood mononuclear cells and in a murine neuroblastoma cell line16,17 and agree with the results obtained by Cardoso et al.,15 who has demonstrated that oncolytic potential of BoHV-1 is mediated by apoptosis induction in glial-derived tumour cell cultures.
As expected, we did not observe the activation of apoptotic process in A549 and this aspect is consistent with the increase of cell viability and the weak viral yield observed in these CpHV-1 infected cells. In HeLa cells, we didn’t observe significant caspase 3 activations, despite the relatively high viral titer detected.
As far as concern the modulation of the autophagy markers, we observed in U2OS and surprisingly also in A549 cells an accumulation of the autophagosome-associated form of LC3, and depletion of p62/SQSTM1 polyubiquitin binding protein asa marker of autophagy flow activation after CpHV-1 infection.38 Our result agrees with other studies about modulation of autophagy by herpesviruses. In fact, Takahashi et al.,39 have demonstrated that VZV infections induce autophagy in a permissive cancer cells line at late stage of infection with depletion of p62. It was also demonstrated that HSV-1 infection induces autophagy in macrophage40 and our previous study demonstrated that BoHV-4 is able to induce autophagy in a permissive cell line.41
Autophagy defends host cells against viral injury by destroying the viruses in autolysosomes, or by activating the immune system and loading viral components onto endosomal sensors such as TLRs.42 In fact, Liang et al.43 suggested that, in some instances, autophagy may produce harmful effects on viral pathogenesis during Sindbis virus infection, and autophagy limits replication of the tobacco mosaic virus.44
On the other hand, viral modulation of autophagy in some circumstances is useful for viral replication, in fact it could facilitates viral spread and protect the viral progeny by cellular defence mechanisms.45,46 Thus, while acting as antiviral defence tool, autophagy could be also subverted by several viruses to increase viral replication.47 Currently, is not possible to clarify if autophagic modulation during CpHV-1 infection in U2OS and A549 cell lines may be considered as a defence mechanism against viral injury or a strategy to increase viral survival, this issue is currently under investigation in our laboratory.
It has been demonstrated that autophagy play a protective role against neoplastic disease48 and deregulation of autophagy is often implicated in several human diseases including cancers. Several studies have shown that tumor cells are defective in autophagy and/or apoptosis pathway.49 Autophagy-defective tumor cells also showed severe genome damage with stress, suggesting that the attenuation of damage by autophagy is a cell mechanism of tumorsuppression.50 It has been shown by Li et al.,51 that agenetically modified adenovirus expressing Beclin-1 gene increase the efficacy of anticancer drugs in multidrug-resistant leukaemia cell lines. Such evidences taken together with our results suggest that modulation of autophagic cell death pathway by CpHV-1 may represent a promising approach for gene virotherapy, used alone or associated with conventional cancer chemotherapy to revert drugs resistance.
Several aspects of the CpHV-1-mediated cell death in neoplastic cells reported herein need to be addressed with further studies. In particular, the activation of intrinsic or extrinsic pathway of apoptosis, the role of interferon pathway in CpHV-1 infection and the mechanism responsible for induction of autophagy in CpHV-1 infection remains to be identified. These issues are currently under investigation in our laboratory.
Overall, our findings showed that CpHV-1 replicates and decreases the viability of several human cancer cells; furthermore, it induces apoptosis and the activation of autophagic pathway and it could represent a new candidate for the oncolytic virus immunotherapy.
Acknowledgments
This work was supported by AIL (Italian Association against Leukemia-Lymphoma and Myeloma Division “Valentina Picazio”). The authors are grateful to Prof. Michele Caraglia for providing cell lines and Dott. Giandomenico Ciaprini for his technical assistance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fountzilas C, Patel S, Mahalingam D.. Oncolytic virotherapy, updates and future directions. Oncotarget. 2017;8(60):102617–102639. doi: 10.18632/oncotarget.18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues R, Cuddington B, Mossman K. Bovine herpesvirus type 1 as a novel oncolytic virus. Cancer Gene Ther. 2010;17:344–355. doi: 10.1038/cgt.2009.77. [DOI] [PubMed] [Google Scholar]
- 3.Courchesne MJ, White MC, Stanfield BA, Frampton AR Jr.. Equine herpesvirus type 1-mediated oncolysis of human glioblastoma multiforme cells. J Virol. 2012;86(5):2882–2886. doi: 10.1128/JVI.06296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2016;14(9):642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 6.Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, McGrath Y, Thomas SK, Thornton M, Bullock P, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- 7.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer A, Feigenbaum F, Tornatore C, Tufaro F, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 8.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 9.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, Petty R, MacLean A, Harland J, McKie E, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. [DOI] [PubMed] [Google Scholar]
- 10.Shen Y, Nemunaitis J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006;13(11):975–992. doi: 10.1038/sj.cgt.7700946. [DOI] [PubMed] [Google Scholar]
- 11.Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: A new era of cancertreatment at dawn. Cancer Sci. 2016;107(10):1373–1379. doi: 10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baringer JR, Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973;288:648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- 13.Corey L, Spear PG. Infections with herpes simplex viruses (1). N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198605153142021. [DOI] [PubMed] [Google Scholar]
- 14.Cuddington BP, Mossman K. Oncolytic bovine herpesvirus type 1 as a broad spectrum cancer therapeutic. Curr Opin Virol. 2015;13:11–16. doi: 10.1016/j.coviro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso TC, Rosa AC, Ferreira HL, Okamura LH, Oliveira BR, Vieira FV, Silva-Frade C, Gameiro R, Flores EF. Bovine herpesviruses induce different cell death forms in neuronal and glial-derived tumor cell cultures. J Neurovirol. 2016;22(6):725–735. doi: 10.1007/s13365-016-0444-5. [DOI] [PubMed] [Google Scholar]
- 16.Longo M, Fiorito F, Marfè G, Montagnaro S, Pisanelli G, De Martino L, Iovane, Pagnini U. Analysis of apoptosis induced by caprine herpesvirus 1 in vitro. Virus Res. 2009;145:227–235. doi: 10.1016/j.virusres.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Pagnini U, Montagnaro S, Sanfelice di Monteforte E, Pacelli F, De Martino L, Roperto S, Florio S, Iovane G. Caprine herpesvirus-1 (CapHV-1) induces apoptosis in goat peripheral blood mononuclear cells. Vet Immunol Immunopathol. 2005;103:283–293. doi: 10.1016/j.vetimm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Montagnaro S, Ciarcia R, De Martinis C, Pacilio C, Sasso S, Puzio MV, De Angelis M, Pagnini U, Boffo S, Kenez I, et al. Modulation of apoptosis by caprine herpesvirus 1 infection in a neuronal cell line. J Cell Biochem. 2013a;114:2809–2822. doi: 10.1002/jcb.v114.12. [DOI] [PubMed] [Google Scholar]
- 19.Mettler F, Engels M, Wild P, Bivetti A. Herpesvirus infection in kids in Switzerland. Schweiz Arch Tierheilkd. 1979;121(12):655–662. [PubMed] [Google Scholar]
- 20.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 21.Pagnini U, Montagnaro S, Pacelli F, De Martino L, Florio S, Rocco D, Iovane G, Pacilio M, Gabellini C, Marsili S, et al. The involvement of oxidative stress in bovine herpesvirus type 4-mediated apoptosis. Front Biosci. 2004;1(9):2106–2114. doi: 10.2741/1320. [DOI] [PubMed] [Google Scholar]
- 22.Elia G, Tarsitano E, Camero M, Bellacicco AL, Buonavoglia D, Campolo M, Decaro N, Thiry J, Tempesta M. Development of a real-time PCR for the detection and quantitation of caprine herpesvirus 1 in goats. J Virol Methods. 2008;148:155–160. doi: 10.1016/j.jviromet.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Amoroso MG, Corrado F, De Carlo E, Lucibelli MG, Martucciello A, Guarino A, Galiero G. Bubaline herpesvirus 1 associated with abortion in a Mediterranean water buffalo. Res Vet Sci. 2013;94:813–816. doi: 10.1016/j.rvsc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptoticprotein with homology to Caenorhabditis elegans cell death protein Ced-3 andmammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269(49):30761–30764. [PubMed] [Google Scholar]
- 25.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391(6662):96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA. Identification andinhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 27.Young LS, Dawson CW, Eliopoulos AG. Viruses and apoptosis. Br Med Bull. 1997;53(3):509–521. [DOI] [PubMed] [Google Scholar]
- 28.Filley AC, Dey M. Immune system, friend or foe of oncolytic virotherapy? Front Oncol. 2017;7:106. doi: 10.3389/fonc.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vähä-Koskela MJ, Heikkilä JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh PK, Doley J, Kumar GR, Sahoo AP, Tiwari AK. Oncolytic viruses & their specific targeting totumour cells. Indian J Med Res. 2012;136:571–584. [PMC free article] [PubMed] [Google Scholar]
- 31.Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, Guo ZS. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervantes-García D, Ortiz-López R, Mayek-Pérez N, Rojas-Martínez A. Oncolytic virotherapy. Ann Hepatol. 2008;7(1):34–45. [PubMed] [Google Scholar]
- 33.Li YM, Song ST, Jiang ZF, Zhang Q, Su CQ, Liao GQ, Qu YM, Xie GQ, Li MY, Ge FJ, et al. Telomerase-specific oncolytic virotherapy for human hepatocellular carcinoma. World J Gastroenterol. 2008;14:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Munck J, Binks A, McNeish IA, Aerts JL. Oncolytic virus-induced cell death and immunity: a match made in heaven? J Leukoc Biol. 2017;102(3):631–643. doi: 10.1189/jlb.5RU0117-040R. [DOI] [PubMed] [Google Scholar]
- 35.Dharmadhikari N, Mehnert JM, Kaufman HL. Oncolytic virus immunotherapy for melanoma. Curr Treat Options Oncol. 2015;16(3):326. doi: 10.1007/s11864-014-0326-0. [DOI] [PubMed] [Google Scholar]
- 36.Gillet L, Dewals B, Farnir F, de Leval L, Vanderplasschen A. Bovine herpesvirus 4 induces apoptosis of human carcinoma cell lines in vitro and in vivo. Cancer Res. 2005;65(20):9463–9472. doi: 10.1158/0008-5472.CAN-05-1076. [DOI] [PubMed] [Google Scholar]
- 37.Redaelli M, Mucignat-Caretta C, Cavaggioni A, Caretta A, D’Avella D, Denaro L, Cavirani S, Donofrio G. Bovine herpesvirus 4 based vector as a potential oncolytic-virus for treatment of glioma. Virol J. 2010;3(7):298. doi: 10.1186/1743-422X-7-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. 3rd ed. Autophagy. 2016;12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi MN, Jackson W, Laird DT, Culp TD, Grose C, Haynes JI 2nd, Benetti L. Varicella-zoster virus infection induces autophagy in both cultured cells and human skin vesicles. J Virol. 2009;83(11):5466–5476. doi: 10.1128/JVI.02670-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.English L, Chemali M, Desjardins M. Nuclear membrane-derived autophagy, a novel process that participates in the presentation of endogenous viral antigens during HSV-1 infection. Autophagy. 2009;5(7):1026–1029. [DOI] [PubMed] [Google Scholar]
- 41.Montagnaro S, Ciarcia R, Pagnini F, De Martino L, Puzio MV, Granato GE, Avino F, Pagnini U, Iovane G, Giordano A. Bovine herpesvirus type 4 infection modulates autophagy in a permissive cell line. J Cell Biochem. 2013b;114(7):1529–1535. doi: 10.1002/jcb.24494. [DOI] [PubMed] [Google Scholar]
- 42.Tallóczy Z, Virgin HW 4th, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy. 2006;2(1):24–29. [DOI] [PubMed] [Google Scholar]
- 43.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Schiff M, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121(4):567–577. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5(6):527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavignac Y, Esclatine A. Herpesviruses and autophagy: catch me if you can! Viruses. 2010;2(1):314–333. doi: 10.3390/v2010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudchodkar SB, Levine B. Viruses and autophagy. Rev Med Virol. 2009;19(6):359–378. doi: 10.1002/rmv.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang MS, Niu FW, Li K. Proflavin suppresses the growth of human osteosarcoma MG63 cells through apoptosis and autophagy. Oncol Lett. 2015;10(1):463–468. doi: 10.3892/ol.2015.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen N, Debnath J. Autophagy and tumorigenesis. FEBS Lett. 2010;584(7):1427–1435. doi: 10.1016/j.febslet.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7(12):961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, You LS, Mao LP, Jin SH, Chen XH, Qian WB. Combing oncolytic adenovirus expressing Beclin-1 with chemotherapy agent doxorubicin synergistically enhances cytotoxicity in human CML cells in vitro. Acta Pharmacol Sin. 2017;39(2):251–260. doi: 10.1038/aps.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]


