ABSTRACT
Background: Adeno-associated virus 2 mediated gene transfer of vascular endothelial growth factor Trap (AAV2-VEGF-Trap) has been reported to inhibit the growth of primary tumor as well as distant metastasis in 4T1 metastatic breast cancer models. The aim of this study was to investigate the inhibiting efficacy of AAV2-VEGF-Trap for glioma.
Methods: The intracranial transplanted model of glioma in rats was established. They were treated with AAV2-VEGF-Trap, bevacizumab (BEV), temozolomide (TMZ), TMZ combined with AAV2-VEGF-Trap, TMZ combined with BEV and the control group, respectively. A 7.0 Tesla magnetic resonance (MR) was used to assess the tumor volumes and obtain the apparent diffusion coefficient (ADC) values. Immunohistochemical and terminal dexynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining were used to evaluate the effects on tumor angiogenesis, proliferation and apoptosis.
Results: The combination of TMZ with AAV2-VEGF-Trap or BEV showed greater tumor growth inhibition than the other groups, and the ADC values in these two groups were larger than that of the control group. The decreased microvessel density in treatment groups which contain AAV2-VEGF-Trap or BEV was observed. The reduced proliferation activity in groups containing TMZ and increased apoptotic tumor cells in TMZ combined with AAV2-VEGF-Trap group and TMZ combined with BEV group were detected. In addition, there were no differences in antitumor effect, ADC values, Ki-67 and CD31 staining and apoptosis analysis between the two combined therapy groups.
Conclusion: AAV2-VEGF-Trap has an obvious anti-angiogenic effect and inhibits the growth of glioma just by a single intravenous injection, which is similar to BEV. Moreover, there is a synergistic antitumor effect between AAV2-VEGF-Trap and TMZ.
KEYWORDS: AAV2-VEGF-Trap, glioma, angiogenesis, MR
Introduction
Glioma is the most frequent and lethal primary tumor of the brain in adults. Safe and maximal resection followed by concurrent radiotherapy of daily temozolomide (TMZ) plus 6 cycles of adjuvant chemotherapy with TMZ has become the standard first-line treatment for high-grade gliomas. Despite recent improvements in surgery, radiotherapy and chemotherapy, the overall survival (OS) of patients with this disease has remained strikingly poor1,2.
Angiogenesis is a key process that contributes to solid-tumor growth, survival and metastasis, include glioma, which makes anti-angiogenic therapy a promising strategy for antitumor treatment3-6. At present, it is clear that vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) plays a predominant role in angiogenesis, which makes the inhibition of VEGF or VEGFR a cornerstone of anti-angiogenic therapies7,8.
Bevacizumab (BEV), a monoclonal antibody against VEGF, showed increased progression-free survival (PFS) and durable objective tumor response rate as a single agent in recurrent glioblastoma9-11, which has been approved by the FDA for the treatment of recurrent glioblastoma. Rencently, a phase III study demonstrated that the addition of BEV to standard radiotherapy-TMZ as first-line treatment for glioblastoma lead to a 4.4-month improvement in median PFS, with quality of life and functional status maintained12.
Adeno-associated virus 2 mediated gene transfer of VEGF-Trap (AAV2-VEGF-Trap) is a new drug independently developed by the State Key Laboratory of Biotherapy, West China Hospital of Sichuan University and has been reported long-term expression of VEGF-Trap in vivo via a single intravenous injection, which can simultaneously suppress the growth of primary tumor and lung metastasis in 4T1 metastatic breast cancer models13. This suggested that AAV2-VEGF-Trap may be a new approach to the control of malignant growth. We therefore hypothesized that AAV2-VEGF-Trap can inhibit the growth of glioma. Additionally, a large number of studies have shown that the efficacy of anti-angiogenic therapy alone was limited and the better therapeutic effects could be achieved in association with chemotherapy. This may be due to that the anti-angiogenic drugs can reestablish the tumor vasculature, normalize the tumor vessels and improve the delivery of drugs within the tumor14,15. Thus, we also combined AAV2-VEGF-Trap with TMZ to explore the antitumor effects and determine whether there is a synergistic effect.
In the present study, the antitumor effect of AAV2-VEGF-Trap alone or combined with TMZ on rat C6 glioma models was assessed by a 7.0 Tesla magnetic resonance (MR) scanner. The effect of BEV on C6 glioma models was also evaluated to compare with AAV2-VEGF-Trap.
Materials and methods
Cells and reagents
C6 cell line was purchased from the cell bank of Chinese Academy of Science (Shanghai, China), stored according to supplier’s requirement. AAV2-VEGF-Trap was constructed in the State Key Laboratory of Biotherapy, West China Hospital of Sichuan University. BEV was obtained from Roche. TMZ capsules were purchased from MSD Inc. (USA), contrast agent (Gadopentetic Acid Dimeglumine Salt Injection) from Bayer (Germany). Terminal dexynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) apoptosis detection kit (In Situ Cell Death Detection Kit, Fluorescein) from Roche Diagnostics GmbH (Germany). Anti-Ki-67 antibody and anti-CD31 antibody are rabbit polyclonal and purchased from Abcam (Shanghai, China).
The C6 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% antibiotics (100IU/ml penicillin and 100μg/ml streptomycin) at 37℃ in a 5% CO2 atmosphere.
Animals and xenograft model
All the animal studies were approved by the ethics committee of West China Hospital of Sichuan University and compliance with the regulation on the administration of experimental animals. Male SD rats (body weight 220-280g) were purchased from Chengdu Dashuo Experimental Animal CO.Ltd (Chengdu, China) and housed in a specific pathogen-free grade environment with access to chow and water ad libitum at the Animal Research Center of West China Hospital. For each rat, a total amount of 10μL C6 glioma cell suspension (1 × 106 cells) was injected into the right caudate nucleus at the rate of 1μL/min under stereotaxic apparatus. The day of C6 cells administration was designated as day 0, and observation continued until day 21. The rats were scanned by MR on day 7 after implantation to screen out 36 rats with basically identical tumor size and then divided into 6 groups randomly to receive different treatments. TMZ group (group 1), received intragastrical (IG) administration of TMZ solution (50mg/kg, once daily for 5 days). AAV2-VEGF-Trap group (group 2), received intravenous (IV) injection of AAV2-VEGF-Trap through vena caudalis (1 × 1012vg, only once). BEV group (group 3), received intraperitoneal (IP) injection of BEV (5mg/kg, three times a week). TMZ combined with AAV2-VEGF-Trap group (TMZ+ AAV2-VEGF-Trap group, group 4), received TMZ and AAV2-VEGF-Trap (dose and dosing schedule the same as in group 1 plus group 2). TMZ combined with BEV(TMZ+BEV group, group 5), received TMZ and BEV (dose and dosing schedule the same as in group 1 plus group 3). Control group (group 6), received IG administration of physiological saline (50mg/kg, once daily for 5 days) and IV injection of physiological saline (1 × 1012vg, only once) through vena caudalis.
MR imaging
The MR scan was performed in 6 groups at day 7, day 14 and day 21 respectively after the implantation. All MR images were obtained with a 7.0 Tesla MR scanner (Bruker BioSpec 70/30, Ettlingen, Germany). Multislice multiecho (MSME) T1-weighted images were obtained with the use of following parameters: field of view (FOV) = 30 × 30mm, repetition time (TR) = 561 msec, echo time (TE) = 14 msec, number of excitations (NEX) = 4, matrix size = 256 × 256, Flip angle (FA) = 180°, slice thickness = 1mm, slice gap = 1mm, and acquisition time = 9 minutes, 34 seconds and 502 msec. Turbo Rapid Acquisition Relaxation Enhancement (RARE) T2-weighted images were obtained with the use of following parameters: FOV = 30 × 30mm, TR = 3000 msec, TE = 45 msec, NEX = 4, matrix size = 256 × 256, FA = 180°, slice thickness = 1mm, slice gap = 1mm, acquisition time = 6 minutes 24 seconds. Diffusion-weighted images (DWIs) were collected with following sequence parameters: FOV = 30 × 30mm, TR = 3000 msec, TE = 45 msec, matrix size = 256 × 256, slice thickness = 1mm, slice gap = 1mm, b values = 0, 200, 400, 600, 800, 1000s/mm2. After the completion of scan, rats were injected with contrast agents through vena caudalis and T1-weighted sequences were repeated after 5 minutes.
Apparent diffusion coefficient (ADC) maps were generated automatically by software, and then regions of interest (ROIs) were drawn in the solid and central part of the tumor by two experienced radiologists who did not know the grouping information. The ADC values of 5 ROIs in each rat were measured and the mean value was recorded.
Tumor size was measured for three-dimensional length, width and height, and tumor volume calculated as π/6× length× width× height.
Immunohistochemical and TUNEL staining
After the completion of MR scan at day 21, rats were sacrificed and brain tumor tissues were fixed in 4% paraformaldehyde, embedded in paraffin and then cut into sections. The tumor tissue sections were stained for blood vessels using anti-CD31 antibody, for proliferative cells using anti-Ki-67 antibody. Apoptosis was determined by TUNEL staining.
Scanned the slides at 100× to find out the areas with the highest microvessel density and then randomly selected 5 fields at 200× to count the number of CD31 stained positive vessels. Similarly, 5 different fields were selected randomly at 200×, counting 100 tumor cells and calculating the percentage of Ki-67 stained positive cells. And the percentage of TUNEL stained positive cells was calculated the same as Ki-67 staining.
Statistical analysis
SPSS 16.0 was used for statistical analysis. All quantitative data were expressed as mean values ± SD. One-way analysis of variance and Bonferroni correction were used for comparisons among multiple groups and between two groups, respectively. P values less than 0.05 was considered statistically significant.
Results
Antitumor effect of AAV2-VEGF-Trap
We successfully formed tumors in the right caudate nucleus regions on day 7 after implantation. On day 7, no significant difference was found in tumor volume among six groups (P>0.05). On day 14 and 21, the tumor growth rate of TMZ+ AAV2-VEGF-Trap group and TMZ+ BEV group was significantly slower than that of the other four groups (P<0.01). AAV2-VEGF-Trap monotherapy could slow down the tumor growth compared with control group (P<0.05), which was similar to that of BEV group and TMZ group (Table 1 and Figure 1).
Table 1.
Tumor volume changes in each group at 7d, 14d and 21d.
| Groups | Tumor size (mm3) |
||
|---|---|---|---|
| 7d | 14d | 21d | |
| TMZ+AAV2-VEGF-Trap | 15.31 ± 2.45 | 34.11 ± 3.43** | 79.31 ± 4.05** |
| TMZ+BEV | 16.48 ± 3.28 | 34.09 ± 3.42** | 76.37 ± 4.03** |
| TMZ | 16.40 ± 1.73 | 46.82 ± 3.53* | 93.86 ± 1.85* |
| AAV2-VEGF-Trap | 15.53 ± 2.42 | 46.51 ± 2.75* | 94.17 ± 3.61* |
| BEV | 14.99 ± 2.88 | 46.82 ± 3.08* | 94.02 ± 1.07* |
| Control | 15.78 ± 3.58 | 57.55 ± 2.52 | 104.73 ± 0.72 |
Abbreviations: TMZ, temozolomide; AAV2-VEGF-Trap, adeno-associated virus 2 mediated gene transfer of vascular endothelial growth factor Trap; BEV, bevacizumab.
P values:
**: TMZ+AAV2-VEGF-Trap group and TMZ+BEV group vs. TMZ group, AAV2-VEGF-Trap group, BEV group and control group, <0.01;
*: TMZ group, AAV2-VEGF-Trap group and BEV group vs. control group, <0.05.
Figure 1.
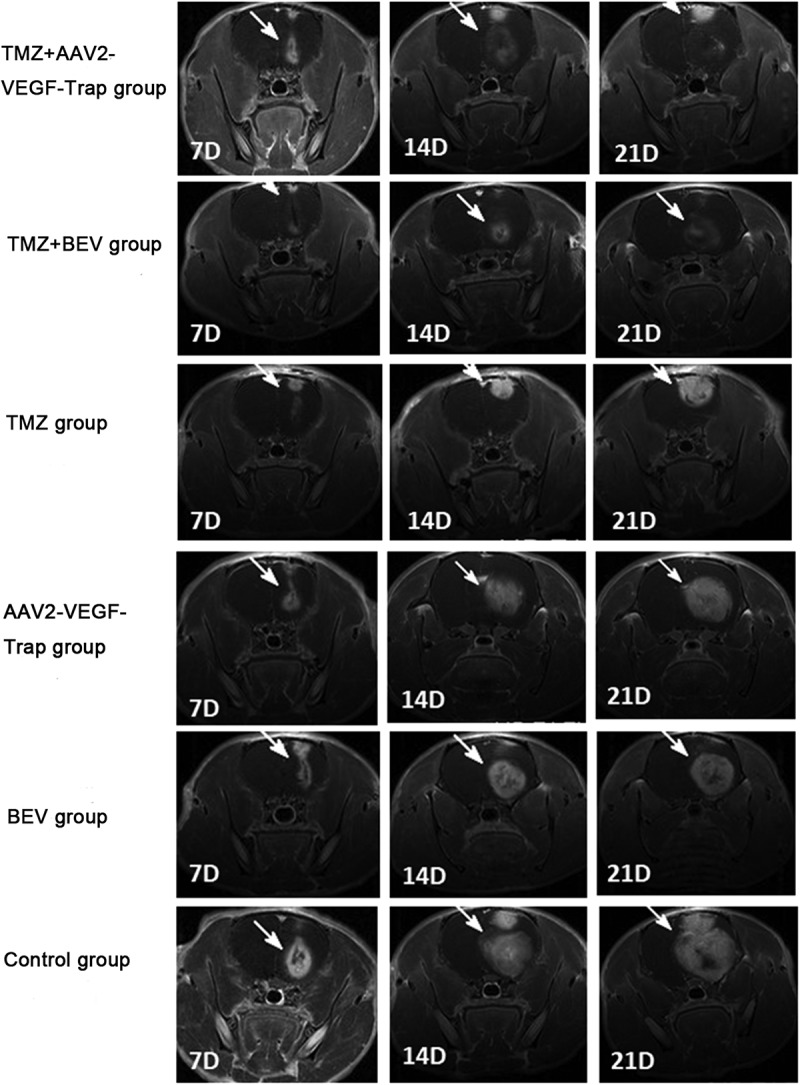
Contrast enhanced T1-weighted images: the tumor areas were obviously enhanced (white arrow) with significant space-occupying effect.
Changes in ADC values of tumor
The mean ADC values of ROIs in each group were recorded at 7d, 14d and 21d to analyze the changing tendency and scopes (Table 2 and Figure 2). No significant difference was found in baseline tumor ADC values among six groups (P > 0.05). Over time, the ADC values of treatment groups increased in different degrees, while that of the control group decreased gradually. The combination of TMZ with AAV2-VEGF-Trap or BEV showed an apparent increase of ADC values compared with control group (P < 0.05). However, there was no significant difference in ADC values among monotherapy groups and the control group (P > 0.05).
Table 2.
Changes in ADC values of tumor in each group at 7d, 14d and 21d.
| Groups | ADC values of tumor (×10−3mm2/s) |
||
|---|---|---|---|
| 7d | 14d | 21d | |
| TMZ+AAV2-VEGF-Trap | 1.02 ± 0.11 | 1.35 ± 0.18** | 1.46 ± 0.19** |
| TMZ+BEV | 0.93 ± 0.19 | 1.31 ± 0.13** | 1.38 ± 0.10** |
| TMZ | 0.93 ± 0.14 | 1.02 ± 0.14* | 1.16 ± 0.21* |
| AAV2-VEGF-Trap | 1.06 ± 0.14 | 1.16 ± 0.12* | 1.17 ± 0.13* |
| BEV | 1.04 ± 0.18 | 1.15 ± 0.20* | 1.19 ± 0.18* |
| Control | 1.07 ± 0.14 | 0.85 ± 0.13 | 0.74 ± 0.13 |
Abbreviations: TMZ, temozolomide; AAV2-VEGF-Trap, adeno-associated virus 2 mediated gene transfer of vascular endothelial growth factor Trap; BEV, bevacizumab.
P values:
**: TMZ+AAV2-VEGF-Trap group and TMZ+BEV group vs. control group, <0.05;
*: TMZ group, AAV2-VEGF-Trap group and BEV group vs. control group, >0.05.
Figure 2.
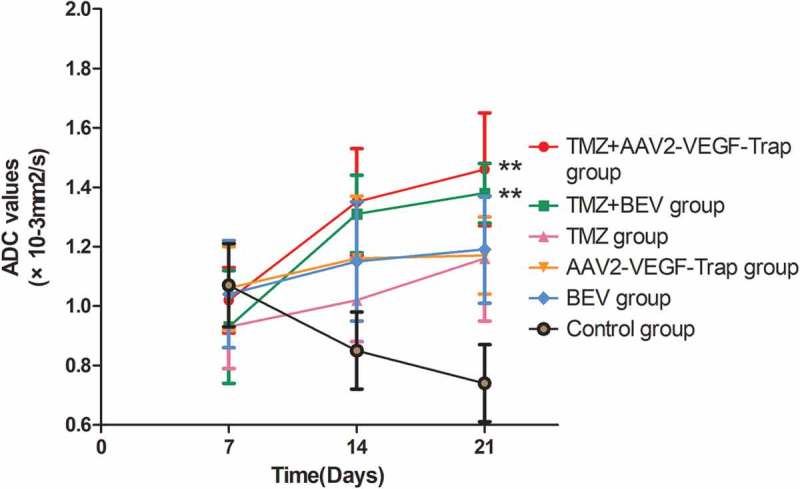
Changes in ADC values of tumor in each group at 7d, 14d and 21d.
Abbreviations: TMZ, temozolomide; AAV2-VEGF-Trap, adeno-associated virus 2 mediated gene transfer of vascular endothelial growth factor Trap; BEV, bevacizumab.On day 14 and 21, the ADC values of TMZ+AAV2-VEGF-Trap group and TMZ+BEV group were larger than that of the control group (**P < 0.05). There was no significant difference among TMZ group, AAV2-VEGF-Trap group, BEV group and the control group (P > 0.05).
Immunohistochemistry and apoptosis analysis
In order to investigate whether the decreased tumor growth was related to a reduction of tumor angiogenesis or cell proliferation, tumor sections were stained with anti-CD31 antibody and anti-Ki-67 antibody (Figure 3A). The treatment groups which contain AAV2-VEGF-Trap or BEV exhibited obvious inhibition of CD31 expression compared with TMZ group (P<0.05) and control group (P<0.01) (Figure 3B). And in the groups containing TMZ, the Ki-67 expression was significantly lower than that of the control group (P<0.05) (Figure 3C).
Figure 3.
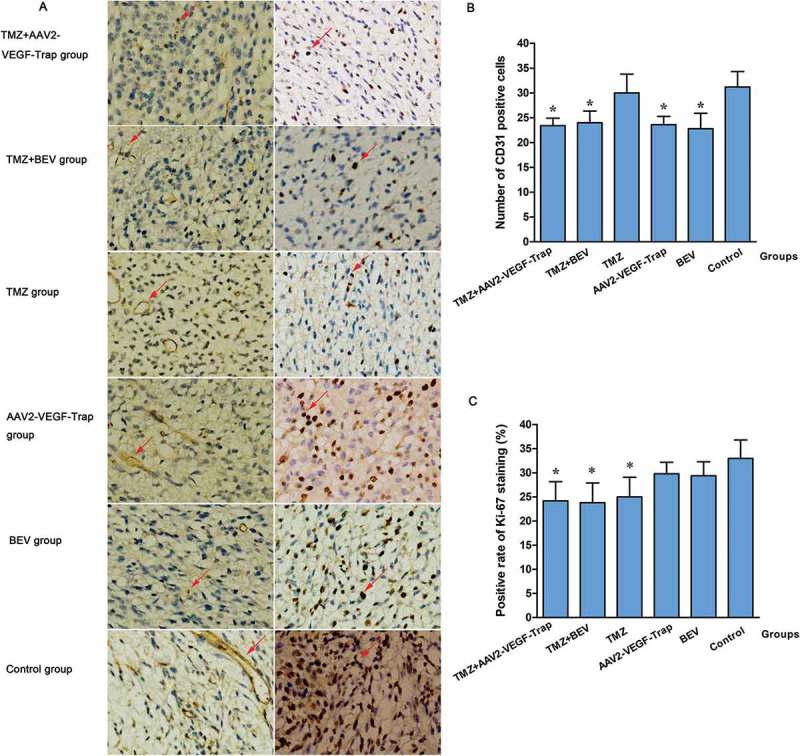
Inhibition of tumor angiogenesis and cell proliferation in tumor sections.
Abbreviations: TMZ, temozolomide; AAV2-VEGF-Trap, adeno-associated virus 2 mediated gene transfer of vascular endothelial growth factor Trap; BEV, bevacizumab.(A) CD31 and Ki-67 staining in each group (magnification, ×200).(B) The number of CD31 stained positive cells in each group.
P values: *: TMZ+AAV2-VEGF-Trap group, TMZ+BEV group, AAV2-VEGF-Trap group and BEV group vs. TMZ group (<0.05) and control group (<0.01); TMZ group vs. control group, >0.05.(C) The positive rate of Ki-67 staining in each group.
P values: *: TMZ+AAV2-VEGF-Trap group, TMZ+BEV group and TMZ group vs. control group, <0.05; No significant difference was found among AAV2-VEGF-Trap group, BEV group and control group (P > 0.05).
To assess the apoptosis of tumor cells, TUNEL staining was conducted (Figure 4A). Obviously, there were more apoptotic cells in the groups treated with TMZ + AAV2-VEGF-Trap or TMZ+ BEV compared with TMZ group (P<0.05), AAV2-VEGF-Trap group (P<0.01), BEV group (P<0.01) and control group (P<0.01). However, monotherapy of AAV2-VEGF-Trap or BEV did not promote tumor cell apoptosis compared with control group (P>0.05) (Figure 4B).
Figure 4.
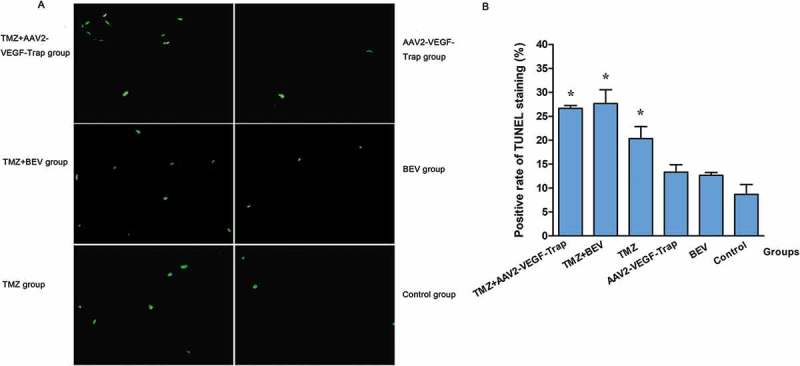
Detection of tumor cell apoptosis by TUNEL staining.
Abbreviations: TMZ, temozolomide; AAV2-VEGF-Trap, adeno-associated virus 2 mediated gene transfer of vascular endothelial growth factor Trap; BEV, bevacizumab.
(A) TUNEL staining in each group (magnification, ×200).
(B) The positive rate of TUNEL staining in each group.
P values: *: TMZ+AAV2-VEGF-Trap group and TMZ+BEV group vs. TMZ group, <0.05; TMZ+AAV2-VEGF-Trap group and TMZ+BEV group vs. AAV2-VEGF-Trap group, BEV group and control group, <0.01. And there was no difference between TMZ+AAV2-VEGF-Trap group and TMZ+BEV group (P > 0.05), among AAV2-VEGF-Trap group, BEV group and the control group (P > 0.05).
Discussion
Angiogenesis plays an important role in the process of growth, survival and metastasis of glioma. And VEGF is the critical factor in tumor angiogenesis, which makes it a potent target for anti-angiogenic therapy. Therefore, scientists have developed a variety of anti-VEGF or VEGFR drugs, of which two classes of drugs were most frequently used in clinical practice: monoclonal antibodies and tyrosine kinase inhibitors (TKIs).
BEV, a humanized monoclonal antibody targeting VEGF with a long elimination half-life (approximately 20 days), received an accelerated approval from FDA in 2009 for the treatment of recurrent glioblastoma based on two phase II studies. One is a single-arm study to evaluate single-agent activity of BEV in 48 patients with recurrent glioblastoma9. Results showed that the median PFS was 16 weeks, the 6-month PFS rate was 29% and the 6-month OS rate was 57%. The other is a multicenter, open-label, noncomparative trial to evaluate the efficacy of BEV alone and in combination with irinotecan in 167 patients with recurrent glioblastoma10. The 6-month PFS and objective response rate (ORR) in BEV-alone and BEV-plus-irinotecan groups were 42.6% vs. 50.3%, and 28.2% vs. 37.8%, respectively. These two studies showed increased PFS and durable ORR in recurrent gliobalstoma, facilitates BEV as an important part of the treatment of glioma.
TKIs are small molecule drugs that inhibit the activity of tyrosine kinases. They can bind tyrosine kinases of VEGFR and compete with ATP to block the downstream signals. It is clear that TKIs have shown potent antitumor effects on many solid tumors. However, it’s efficacy on glioma seems to be unsatisfactory. A variety of clinical trials of TKIs have demonstrated a limited antitumor effect on glioma16-19. Recently, several studies have shown good results, which was encouraging. A randomized phase II study comparing axitinib with the combination of axitinib and lomustine was conducted in 79 patients with recurrent glioblastoma20. The results showed that overall response rate was 28% vs. 38%, 6-month PFS rate was 26% vs. 17% and median OS was 29 weeks vs. 27.4 weeks for patients treated with axitinib versus axitinib combined with lomstine, respectively. This suggested that axitinib improves response rate and PFS in patients with recurrent glioblastoma compared to historical controls. Results of a pilot clinical study evaluating apatinib plus irinotecan in patients with recurrent high-grade glioma were reported21. Among 9 patients available for the efficacy evaluation, 5 with partial response, 2 with stable disease and 2 with progressive disease. The ORR was 55% and median PFS was 8.3 months. This indicated that apatinib combined with irinotecan seems to be an effective therapeutic option for recurrent malignant glioma. A phase II study of cabozantinib was conducted in 152 patients with refractory or recurrent glioblastoma22. Although the study did not meet its primary end point of ORR improvement, cabozantinib showed evidence of clinical activity in patients with refractory or recurrent glioblastoma naive to anti-angiogenic therapy. The results of the above studies need to be further validated in more large-scale clinical trials.
VEGF-Trap, a high-affinity soluble decoy receptor for VEGF, which has been demonstrated one of the most potent and efficacious VEGF blocker23. Compared with BEV, VEGF-Trap binds the VEGF ligand with about 800-fold higher affinity and maintains similar pharmacokinetic properties24. Moreover, VEGF-Trap can bind not only multiple isoforms of VEGF-A like BEV, but also the related VEGFR1 ligands, VEGF-B and placental growth factor (PIGF), which are important factors in promoting pathological angiogenesis25-28. Due to its significant anti-angiogenic effect, it has been approved by the FDA for the treatment of wet macular degeneration and metastatic colorectal cancer that is resistant to or has progressed following an oxaliplatin-containing regimen. A study has shown that VEGF-Trap is effective for both early and advanced gliomas, and can significantly prolong the OS of patients29. However, it has a short half-life, which may increase costs and lead to patients discomfort because of repeated administration.
AAV2-VEGF-Trap, constructed by our lab, State Key Laboratory of Biotherapy, West China Hospital of Sichuan University, has overcome the deficiency of short half-life of VEGF-Trap, which can sustain long-term expression of VEGF Trap via a single intravenous injection and inhibit the growth of primary tumor and pulmonary metastases in 4T1 metastatic breast cancer models13. Furthermore, the study found that AAV2-VEGF-Trap can not only decrease the concentration of VEGF, but also inhibit other angiogenic factors’ function, such as acid fibroblast growth factor (aFGF), basic fibroblast growth factor (bFGF) and angiopoietin-1. In the present study, our results demonstrated that AAV2-VEGF-Trap could inhibit the growth of glioma via a single intravenous administration, and the inhibiting efficacy was similar to that of BEV.
DWI is one of the important imaging methods of magnetic resonance imaging (MRI), which can explore the abnormalities of tissue structure earlier than other traditional imaging techniques by detecting the microscopic changes in the motion of water molecules30,31. ADC is a key parameter derived from DWI, negatively correlated with cell density of tumor and tissue. Because of the superiority of early detection, DWI has been used in many types of tumors to predict and detect the early therapeutic response, including glioma, bone and soft-tissue sarcoma, breast cancer, cervical cancer, head and neck squamous cell carcinoma and rectal cancer30,32–37. Successful treatment in most malignancies is usually associated with an increase of ADC values, so is the glioma32,38–42. In this study, the ADC values of TMZ+AAV2-VEGF-Trap group and TMZ+BEV group increased significantly. We speculated that the treatment of these two groups may induce ischemia, necrosis and apoptosis of tumor cells, reduce cell density, and then result in an increase of ADC values. To confirm our hypothesis, we further performed immunohistochemical and TUNEL staining.
CD31 staining is mainly used to determine microvessel density and assess tumor angiogenesis. The greater the expression of CD31 in tumor tissues, the greater the microvessel density and the smaller the effect of anti-angiogenesis43. In the present study, the expression of CD31 was obviously inhibited in groups containing AAV2-VEGF-Trap or BEV. This indicated that AAV2-VEGF-Trap and BEV have similar and definite anti-angiogenic effects. Moreover, AAV2-VEGF-Trap can achieve the same effect as mutiple injections of BEV with a single administration, which is more convenient. Ki-67 staining was used to evaluate the proliferation activity of tumor cell. Our finds showed that monotherapy of AAV2-VEGF-Trap or BEV did not inhibit the proliferative activity of tumor. It was TMZ that exerts an anti-proliferative effect in this study.
Otherwise, TUNEL staining was conducted to assess the apoptosis of tumor cells. Monotherapy of AAV2-VEGF-Trap or BEV did not increase apoptosis. And monotherapy of TMZ promoted apoptosis compared with AAV2-VEGF-Trap group, BEV group and control group. Interestingly, when combined TMZ with AAV2-VEGF-Trap or BEV, a stronger pro-apoptotic effect than TMZ monotherapy was observed. This suggested that there was a synergistic antitumor effect between AAV2-VEGF-Trap, BEV and TMZ. A number of studies on tumor transplantation models have found that, compared with monotherapy of VEGF Trap, the combination of VEGF Trap and cytotoxic drugs, or radiotherapy can produce a stronger inhibiting efficacy on tumor growth and angiogenesis44,45, which is consistent with our research. There may be a lot of possible mechanisms accountable for this effect. One of the most important explanation is “Normalization of tumor vasculature”, the VEGF Trap and BEV can improve the tumor microenvironment of interstitial hypertension, hypoxia and acidosis, leading to more efficient delivery of TMZ and oxygen to tumor cells14,15. A second possible mechanism is that by reducing vascular permeability, the VEGF Trap and BEV can prevent many serum-derived growth factors that promote tumor cell survival from reaching the tumor parenchyma, thus slowing down tumor cell growth. Thirdly, in addition to its cytotoxic effect, TMZ has a direct anti-angiogenic effect as well as other cytotoxic drugs, and genetically stable endothelial cells of newly forming tumor vessels are considered to be sensitive to chemotherapeutic drugs46-48, thereby the combination of anti-angiogenic agents and TMZ can enhance the antitumor effect. Further experiments will be needed to confirm whether any or all of these mechanisms contribute to this synergistic antitumor effect.
As to the Normalization of tumor vasculature, some scholars believe that there is a “normalization window” in anti-angiogenic therapy, a period during which the addition of chemotherapy and radiotherapy achieves the best therapeutic outcome49. It means that the antitumor efficacy of anti-angiogenic therapy combined with cytotoxic drugs depends on the dose and delivery schedule of each drug. Consequently, the optimal dose and schedule of AAV2-VEGF-Trap needs to be further studied to develop optimal combination therapies and yield the best therapeutic effects.
There is a limitation in this study. The overall survival curves of each groups were not drawn to confirm whether there was a survival benefit. Further studies are necessary to validate our findings and determine whether AAV2-VEGF-Trap monotherapy or combined with TMZ may eventually provide a survival benefit.
Conclusion
The results of this study showed that AAV2-VEGF-Trap can achieve the same anti-angiogenenic effect as BEV just by a single intravenous injection, which is more convenient. MRI is a good method for early and dynamically evaluation of antitumor effect and is worthy of application. Furthermore, combined application of AAV2-VEGF-Trap and TMZ can produce a better antitumor effect.
Funding Statement
This work was supported by the State's Key Project of Research and Development Plan of China (2016YFA0201402), and the National Natural Science Foundation of China (grant number 81520108014, 81771800).
Conflict of interest
There are no potential conflicts of interest to disclose by any of the authors.
References
- 1.Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teo M, Martin S, Owusu-Agyemang K, Nowicki S, Clark B, Mackinnon M, Stewart W, Paul J, St George J.. A survival analysis of GBM patients in the West of Scotland pre- and post-introduction of the Stupp regime. Br J Neurosurg. 2014;28(3):351–355. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175(3):409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–7. [DOI] [PubMed] [Google Scholar]
- 5.Gimbrone MA, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136(2):261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. [DOI] [PubMed] [Google Scholar]
- 7.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–848. [DOI] [PubMed] [Google Scholar]
- 8.Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible In vivo regulatory mechanisms. Int J Cancer. 1994;59(4):520–529. [DOI] [PubMed] [Google Scholar]
- 9.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WKA, Paleologos N, Nicholas MK, Jensen R, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 11.Reardon DA, Turner S, Peters KB, Desjardins A, Gururangan S, Sampson JH, McLendon RE, Herndon JE, Jones LW, Kirkpatrick JP, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Cancer Net. 2011;9(4):414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 13.Lu L, Luo S-T, Shi HS, Li M, Zhang HL, He SS, Liu Y, Pan Y, Yang L. AAV2-mediated gene transfer of VEGF-Trap with potent suppression of primary breast tumor growth and spontaneous pulmonary metastases by long-term expression. Oncol Rep. 2012;28(4):1332–1338. [DOI] [PubMed] [Google Scholar]
- 14.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science (New York, N.Y.). 2005;307(5706):58–62. [DOI] [PubMed] [Google Scholar]
- 15.Siemann DW, Horsman MR. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther. 2015;153:107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalpathy-Cramer J, Chandra V, Da X, Ou Y, Emblem KE, Muzikansky A, Cai X, Douw L, Evans JG, Dietrich J, et al. Phase II study of tivozanib, an oral VEGFR inhibitor, in patients with recurrent glioblastoma. J Neurooncol. 2017;131(3):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S, Ashby LS, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwamoto FM, Lamborn KR, Robins HI, Mehta MP, Chang SM, Butowski NA, DeAngelis LM, Abrey LE, Zhang W-T, Prados MD, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol. 2010;12(8):855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, Duerinck J, Tynninen O, Nupponen N, Michotte A, et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):491–501. [DOI] [PubMed] [Google Scholar]
- 20.Duerinck J, Du Four S, Bouttens F, Andre C, Verschaeve V, Van Fraeyenhove F, Chaskis C, D’Haene N, Le Mercier M, Rogiers A, et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J Neurooncol. 2018;136(1):115–125. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Liang L, Yang T, Qiao Y, Xia Y, Liu L, Li C, Lu P, Jiang X. A pilot clinical study of apatinib plus irinotecan in patients with recurrent high-grade glioma: clinical trial/experimental study. Medicine. 2017;96(49):e9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen PY, Drappatz J, De Groot J, Prados MD, Reardon DA, Schiff D, Chamberlain M, Mikkelsen T, Desjardins A, Holland J, et al. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients naive to antiangiogenic therapy. Neuro Oncol. 2018;20(2):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci. 2002;99(17):11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konner J, Dupont J. Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF Trap) to inhibit vascular endothelial growth factor activity. Clin Colorectal Cancer. 2004;4(Suppl 2):S81–85. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249. [DOI] [PubMed] [Google Scholar]
- 26.Crawford Y, Ferrara N. VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res. 2009;335(1):261–269. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2(59):re1. [DOI] [PubMed] [Google Scholar]
- 28.Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Manzano C, Holash J, Fueyo J, Xu J, Conrad CA, Aldape KD, De Groot JF, Bekele BN, Yung WKA. VEGF trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10(6):940–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmainda KM. Diffusion-weighted MRI as a biomarker for treatment response in glioma. CNS Oncol. 2012;1(2):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI[mdash]a potential new biomarker of response to cancer therapy. Nat Clin Prac Oncol. 2008;5(4):220–233. [DOI] [PubMed] [Google Scholar]
- 32.Hayashida Y, Yakushiji T, Awai K, Katahira K, Nakayama Y, Shimomura O, Kitajima M, Hirai T, Yamashita Y, Mizuta H. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol. 2006;16(12):2637–2643. [DOI] [PubMed] [Google Scholar]
- 33.Sharma U, Danishad KKA, Seenu V, Jagannathan NR. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR Biomed. 2009;22(1):104–113. [DOI] [PubMed] [Google Scholar]
- 34.Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, Doran S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360(9329):307–308. [DOI] [PubMed] [Google Scholar]
- 35.Dudeck O, Zeile M, Pink D, Pech M, Tunn P-U, Reichardt P, Ludwig W-D, Hamm B. Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J Magn Reson Imaging. 2008;27(5):1109–1113. [DOI] [PubMed] [Google Scholar]
- 36.Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111(2):213–220. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, Loevner L, Quon H, Sherman E, Weinstein G, Kilger A, Poptani H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15(3):986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24(7):843–847. [DOI] [PubMed] [Google Scholar]
- 39.Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, Meszoely I, Mayer IA, Herman CR, McManus K, et al. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging. 2007;25(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamel IR, Reyes DK, Liapi E, Bluemke DA, Geschwind J-FH. Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J Vascular Interv Rad. 2007;18(1 Pt 1):49–56. [DOI] [PubMed] [Google Scholar]
- 41.Mardor Y, Pfeffer R, Spiegelmann R, Roth Y, Maier SE, Nissim O, Berger R, Glicksman A, Baram J, Orenstein A, et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol. 2003;21(6):1094–1100. [DOI] [PubMed] [Google Scholar]
- 42.Jain R, Scarpace LM, Ellika S, Torcuator R, Schultz LR, Hearshen D, Mikkelsen T. Imaging response criteria for recurrent gliomas treated with bevacizumab: role of diffusion weighted imaging as an imaging biomarker. J Neurooncol. 2010;96(3):423–431. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Q, Gallo JM. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro Oncol. 2009;11(3):301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wachsberger PR, Burd R, Cardi C, Thakur M, Daskalakis C, Holash J, Yancopoulos GD, Dicker AP. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67(5):1526–1537. [DOI] [PubMed] [Google Scholar]
- 45.Hu L, Hofmann J, Holash J, Yancopoulos GD, Sood AK, Jaffe RB. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res. 2005;11(19 Pt 1):6966–6971. [DOI] [PubMed] [Google Scholar]
- 46.Kerbel RS. Improving conventional or low dose metronomic chemotherapy with targeted antiangiogenic drugs. Cancer Res Treat. 2007;39(4):150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60(7):1878–1886. [PubMed] [Google Scholar]
- 48.Kim JT, Kim J-S, Ko KW, Kong D-S, Kang C-M, Kim MH, Son MJ, Song HS, Shin H-J, Lee D-S, et al. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol Rep. 2006;16(1):33–39. [PubMed] [Google Scholar]
- 49.Winkler F, Kozin SV, Tong RT, Chae -S-S, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, Di Tomaso E, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. [DOI] [PubMed] [Google Scholar]


