ABSTRACT
CYP27A1, an enzyme involved in regulating cellular cholesterol homeostasis, converts cholesterol into 27-hydroxycholesterol (27-HC). The relationship between CYP27A1 and cell proliferation was studied to determine the role of CYP27A1 in bladder cancer. The expression of CYP27A1 in three bladder cancer cell lines (T24, UM-UC-3 and 5637) were assessed by qRT-PCR and Western blotting, and cells with stable CYP27A1 expression were generated by lentiviral infection. Cell proliferation was detected by MTT assays, colony formation assays and a tumor xenograft model in vitro and in vivo, and the intracellular 27-HC and cholesterol secretion levels were detected by enzyme-linked immunosorbent assays (ELISA). The results revealed that CYP27A1 expression was downregulated in androgen receptor (AR)-positive T24/UM-UC-3 cells compared with AR-negative 5637 cell. After CYP27A1 expression was restored, cell proliferation was inhibited in vitro and in vivo because much more intracellular 27-HC was produced in the CYP27A1-overexpressing cells than in the control cells. Both T24 and UM-UC-3 cells treated with 27-HC showed similar results. In addition, CYP27A1/27HC could reduce the cellular cholesterol level in both T24 and UM-UC-3 cells by upregulating ATP-binding cassette transporters G1 and A1 (ABCG1 and ABCA1) through Liver X receptors (LXRs) pathway and downregulating low-density lipoprotein receptor (LDLR) expression. These findings all suggest that CYP27A1 is a critical cholesterol sensor in bladder cancer cells that may contribute significantly to bladder cancer proliferation.
KEYWORDS: CYP27A1, 27-hydroxycholesterol, bladder cancer, ATP-binding cassette transporters, low-density lipoprotein receptor
Introduction
Bladder cancer is one of the most common malignant tumors in the urogenital system, and associated with a significant recurrence rate [1]. Researchers have shown that the incidence of bladder cancer in males is three to four times greater than that in females [2]. Although the bladder is not an accessory sex organ, it originates from the urogenital sinus, similar to other accessory sex organs, such as the prostate [3]. Androgen receptor (AR) which is androgen ligand-regulated transcription factor, androgen-AR signaling plays an important role in the development and progression of bladder cancer and may explain the gender differences in bladder cancer incidence [4,5].
In addition, cholesterol is central for proper cellular functions and increased to meet the needs of tumor cell proliferation. High dietary cholesterol intake increased the risk of several cancer types, including bladder cancer [6,7]. Multiple studies have also suggested that hypercholesterolemia is associated with an increased risk of high-grade metastatic disease, while the potential mechanisms regulating this association remain unclear [8–10]. Due to increased cholesterol levels have been reported to play important roles in the progression of cancer. Therefore, research on cholesterol homeostasis in bladder cancer has become very important and can provide new strategies and methods for treating bladder cancer.
CYP27A1 encodes 27-hydroxylase, a cytochrome P450 oxidase family member that converts cholesterol into 27-hydroxycholesterol (27-HC). CYP27A1 primarily catalyzes the hydroxylation step required for bile acid synthesis in the classical and acid pathways, maintains the acid balance in the body and catalyzes the biological activation of vitamin D3 [11–13]. Notably, the loss of CYP27A1 expression results in a number of problems associated with cholesterol and bile acid metabolism. In addition, CYP27A1 expression is closely related to the proliferation of multiple tumor cells, such as prostate, breast and colon cancer [14–16]. Furthermore, 27-HC, serves as a cholesterol metabolite that acts as a selective estrogen receptor (ER) modulator and an agonist of Liver X receptors to regulate cellular cholesterol homeostasis and further affect cell proliferation [17]. In breast cancer, 27-HC was the first identified ER modulator, and many studies have shown that 27-HC can activate ERs and increase ER (+) breast cancer cell proliferation [10,18]. This molecule increases the growth of human MCF7 cell xenografts propagated in an ER-dependent manner [10]. However, in prostate cancer, the effect of 27-HC on cell proliferation is controversial. A recent paper showed that 27-HC promotes the proliferation of non-transformed RWPE-1 prostate epithelial cells in an ER- and AR-dependent manner [19]. Additionally, 27-HC has an important role in the intratumoral expression and activity of CYP27A1 in prostate cancer pathogenesis. Overexpression of CYP27A1 increased intracellular 27-HC levels, which directly attenuated the proliferation of LNCaP and 22RV1 prostate cancer cells as well as CYP27A1-overexpressing 22RV1 cell-derived xenografts [14]. However, the CYP27A1 expression level in bladder cancer and the relationship between CYP27A1/27-HC and bladder cancer proliferation have not been studied. Hence, the role of CYP27A1 expression in bladder cancer development has become a popular research topic.
In this study, the effect of CYP27A1 on cholesterol homeostasis was confirmed. Furthermore, bladder cancer cells proliferation was detected following CYP27A1 overexpression or exogenous 27-HC treatments. This study aimed to clarify that CYP27A1 is a critical cellular cholesterol sensor for regulating bladder cancer proliferation.
Materials and methods
Cell lines and culture conditions
The T24, UM-UC-3, 5637 and 293T cell lines were obtained from the Cell Bank of the Chinese Academy of Science (Shanghai, China) and were passaged no more than 25 times in the lab. All cells were cultured in DMEM with 10% FBS (fetal bovine serum) and 1% P/S (penicillin/streptomycin) at 37°C with 5% CO2. T24/UM-UC-3 cells overexpressing CYP27A1 or the pLVX control were cultured in 10% DMEM with 1 μg/ml puromycin.
Construction of stable cell lines
CYP27A1-pcDNA3.1 was synthesized by GENEWIZ (Suzhou, China), and the insert fragment was subcloned into the plasmid pLVX-IRES-Puro (Clontech, USA) using standard molecular biology technologies to obtain the plasmid CYP27A1-pLVX -IRES-Puro. The pLenti pLVX-IRES-Puro vector (1 µg) or pLenti CYP27A1-pLVX -IRES -Puro (1 µg) was cotransfected with psPAX2 (1 µg) and pMD2.G vectors (0.5 µg) into 293T cells in a 6 cm dish and further cultured. Lipofectamine 3000 (Invitrogen, USA) was used as a transfection reagent according to the manufacturer’s instructions. After the cells were cultured for 48h, the viral supernatants were collected and centrifuged for 5 min at 1500 rpm. Next, T24 or UM-UC-3 cells were seeded in a 12-well plate, and 8 µg/ml polybrene was added to the viral supernatants before infection. After infection for 24 h, cells were then selected with 2 µg/ml puromycin, yielding CYP27A1- or pLVX-overexpressing cell lines. Cells were engineered to stably overexpress CYP27A1 by CYP27A1-pLVX-IRES-Puro lentivirus infection, and pLVX-IRES-Puro virus infected cells served as controls.
qRT-PCR
Total RNA from the cells was extracted by RNAiso Plus (TaKaRa, Japan) according to the manufacturer’s recommendations. For detection of mRNA, the total RNA (1 μg) was transcribed into cDNA using PrimeScript™ RT reagent kit (Perfect Real Time) (TaKaRa, Japan). The primers for CYP27A1, AR, LXRα, LXRβ, ABCG1, ABCA1, LDLR and GAPDH were as follows: CYP27A1-forward (F): AGC TGC GCT TCT TCT TTC AG and CYP27A1-reverse (R): GCT CCA TGT CGT TCC GTA CT; AR-F: AAG GCT ATG AAT GTC AGC CCA and AR-R: CAT TGA GGC TAG AGA GCA AGG C; LXRα-F: CGG GCT TCC ACT ACA ATG TTC TG and LXRα-R: TCA GGC GGA TCT GTT CTT CT; LXRβ-F: TAA GCA AGT GCC TGG TTT CC and LXRβ-R: GAA CTC GAA GAT GGG GTT GA; ABCG1-F: ACG CAG TTC TGC ATC CTC TTC A and ABCG1-R: CGG AGT TGC TCA AGA CCT TCT T; ABCA1-F: GCT TTC AAT CAT CCC CTG AA and ABCA1-R: TGA CAG GCT TCA CTC CAC TG; LDLR-F: GCT TGT CTG TCA CCT GCA AA and LDLR-R: AAC TGC CGA GAG ATG CAC TT; GAPDH-F: TCA TGG GTG TGA ACC ATG AGA A and GAPDH-R: GGC ATG GAC TGT GGT CAT GAG. All primers were synthesized by Huada Gene (Beijing, China). qRT-PCR was performed using a Roche LightCycler 480II real-time PCR detection system (Roche, Switzerland). The fold changes in expression of each gene were calculated by the comparative threshold cycle (Ct) method using the 2-(ΔΔCt) formula.
Western blotting
Total protein from the cells was extracted by SDS buffer, and the protein concentration was measured by a bicinchoninic acid (BCA) kit (Thermo, USA). Afterwards, the proteins (20 μg) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore, Billerica, USA). After blocking with 5% nonfat milk, the membranes were incubated with primary antibody at 4°C overnight, using antibodies for CYP27A1 (Abcam, 1:1000 dilution), AR (Cell Signaling Technology, 1:1000 dilution) and GAPDH (Cell Signaling Technology, 1:1000 dilution). Then, the membranes were incubated with horseradish peroxidase conjugated secondary antibodies (Jackson Immuno Research, 1:10,000 dilution) for an additional 1 h at room temperature. The immune complexes were detected by an enhanced chemiluminescence kit (Millipore, Billerica, USA). The results were normalized using GAPDH to correct for differences in protein loading.
MTT assay
Different treatments were applied to detect the effects of LDL (Yiyuan Biotechnologies, Guangzhou, China)/CYP27A1/27-HC (Santa Cruz, USA) on the proliferation of bladder cancer cell lines. T24/5637 cells were seeded in 96-well plates and incubated in 200 μl of DMEM with 10% FBS. The next day, the wells were treated with different LDL concentrations (0, 6.25, 12.5, 25, 50, 100 μg/ml), and the cells continued to be cultured. Subsequently, on the indicated day, 20 μl of MTT solution (5 mg/ml) was added to each well, and the plates were incubated for an additional 4 h at 37°C. The MTT formazan precipitate was dissolved in DMSO. Absorbance at 490 nm was measured using a microplate reader (Thermo, USA). Likewise, T24/UM-UC-3 cells overexpressing CYP27A1 or the pLVX control were seeded into 96-well plates, and cell proliferation was detected on the indicated days according to the methods described above. Finally, to study the effects of 27-HC on T24/UM-UC-3 cell proliferation, cells were seeded into 96-well plates and treated with 10 μM 27-HC and DMSO (control) the next day, and proliferation was evaluated on the indicated days according to the methods described above. Experiments were performed three times. The error bars represent the standard error of the mean (*p < 0.05, **p < 0.01, ***p < 0.001 (two-way ANOVA)).
Colony formation
Different treatments were applied to determine the effects of CYP27A1/27-HC on colony formation ability in T24/UM-UC-3 cell lines. Cells overexpressing CYP27A1 or the pLVX control (1,000, 500 cells/well) were seeded into 6-well plates, 10% DMEM containing 1 μg/ml puromycin was added to each well, and the treated medium was replaced every three days. Two weeks later, the cells were washed with PBS three times, fixed with 4% polyformaldehyde for 15 min and washed by distilled water 3 times. Crystal violet was incubated for 15 min at room temperature and washed by distilled water. The colonies were stained with crystal violet and counted. The results were statistically analyzed by GraphPad. Similarly, the effect of 27-HC on the colony formation ability of T24/UM-UC-3 cell lines was detected. Cells (1,000, 500 cells/well) were seeded into 6-well plates, 10% DMEM containing DMSO or 27-HC (1 μM) was added to each well, and the treated medium was replaced every three days, in accordance with the above methods. Experiments were performed three times. The error bars represent the standard error of the mean (*p < 0.05, **p < 0.01, ***p < 0.001 (unpaired t-test)).
Enzyme-linked immunosorbent assay (ELISA)
To analyze the intracellular 27-HC/cholesterol secretion level, human 27-HC and cholesterol ELISA kits were used. T24/UM-UC-3 cells overexpressing CYP27A1 or the pLVX control (2.5 × 104 cells/well) were seeded into 6-well plates and cultured for 6 d. Briefly, the samples were lysed with RIPA; then, the lysates (10 μl) or standard samples (10 μl) were premixed with dilution buffer (40 μl). The mixtures were loaded and incubated at 37°C for 30 min, and the plates were washed three times with washing buffer. Next, streptavidin-HRP was added. The plates were incubated at 37°C for 30 min and washed three times with washing buffer. The reaction was visualized by adding 100 μl of tetramethylbenzidine (TMB) substrate solution, and the plates were incubated at 37°C for 10 min in a dark environment. The reaction was stopped with the addition of 50 μl Stop Solution into each well. The colorimetric reaction was determined by measuring the absorbance at 450 nm with a microplate reader (Thermo, USA). The intracellular 27-HC and cholesterol levels were calculated according to standard curve and normalized to the total protein amount.
Tumor xenograft model in vivo
Sixteen BALB/c nude mice (4–5 wk, female) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. All mice were allowed to acclimatize to the surroundings for 1 wk before the experiments, and divided into two groups equally. Each mouse was subcutaneously injected into its left back with T24-pLVX or T24-CYP27A1 cells (8 × 106 cells per site). After injecting for 1 wk, tumor size was individually measured 3 times/wk using a Vernier’s caliper to measure 2 perpendicular diameters,and tumor volume was calculated according to the following formula: (Length×Width2)/2. After 30 d of measurement, all animals were sacrificed. Tumors were stripped and kept intact, and then weighed.
RNA interference
Small interfering RNAs (SiRNAs) directed against both LXRα and LXRβ (5ʹ-CAU CAA CCC CAU CUU CGA GTT-3ʹ) [20]or negative control SiRNA (5ʹ-UUC UCC GAA CGU GUC ACG UTT-3ʹ) were synthesized by GenePharma (Shanghai, China). T24 or UM-UC-3 cells were seeded into 24-well plates at a density of 1 × 104 cells/well. The next day, cells were transfected with either LXR SiRNA (SiLXRα/β, 20 pM) or NC SiRNA (SiNC, 20 pM) using HiPerFect transfection reagent (QIAGEN, Germany) according to the manufacturer’s instructions. Two days later, the cells were treated with DMSO/27-HC (10 μM)/T1317 (10 μM) for 24 h. RNA was extracted by RNAiso Plus, and the expression of ABCG1, ABCA1 was detected by qRT-PCR.
Statistical analysis
All experiments were independently conducted 3 times, and the error bars represent the standard error of the mean. Statistical analyzes were performed using Graph Prism 6.0 software. The results were analyzed using unpaired t-test or two-way ANOVA to assess statistical significance, and values of p < 0.05 were considered statistically significant.
Results
CYP27A1 expression in bladder cancer cell lines
According to the reported literature [21,22], three types of bladder cancer cell lines were selected to determine CYP27A1 expression. The results revealed that AR was lowly expressed in 5637 cell but highly expressed in T24 and UM-UC-3 cells at both the mRNA and protein levels (Figure 1(a–c)). However, only AR-negative 5637 cell expressed detectable levels of CYP27A1 at both the mRNA and protein levels, but almost no CYP27A1 was expressed in the AR-positive T24 and UM-UC-3 cells (Figure 1(b,c)). Because AR plays an important role in the development and progression of bladder cancer, T24 and UM-UC-3 cells were selected for further analyzes of the relationship between CYP27A1 expression and bladder cancer.
Figure 1.
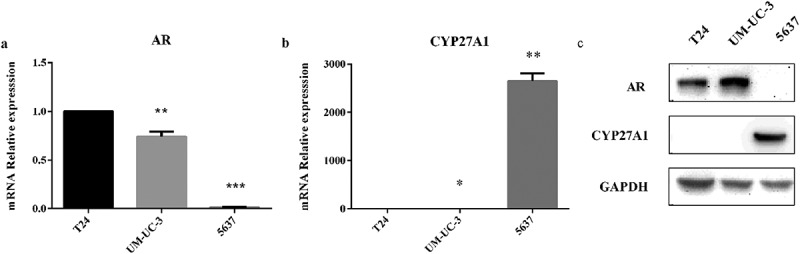
The mRNA and protein levels of CYP27A1 in bladder cancer cells.
(a, b) T24, UM-UC-3 and 5637 cell lines (2.5 × 104/well) were seeded in 24-well plates and cultured for 48 h. RNA was extracted by RNAiso Plus. Expression of androgen receptor (AR) and CYP27A1 was assessed using qRT-PCR. Experiments were repeated three times, and error bars represent the standard error of the mean and *p < 0.05, **p < 0.01, ***p < 0.001 (unpaired t-test). (c)T24, UM-UC-3 and 5637 cell lines (2.5 × 104/well) were seeded in 24-well plates and normally cultured for 72 h. Cell lysates were harvested for Western blotting to analyze the protein levels of AR and CYP27A1.
Cholesterol promotes bladder cancer cells proliferation in vitro
As an important step in this study, the impact of altered cholesterol levels on bladder cancer cells proliferation was assessed. Among lipoproteins, LDL has highest the cholesterol content. Therefore, the effect of different LDL concentrations (0, 6.25, 12.5, 25, 50, 100 μg/ml) on T24 and 5637 cells proliferation was evaluated. There were no obvious differences for all groups after 24h. However, when the concentration of LDL was 100 μg/ml, proliferation of T24 cell was increased 1.31-fold and 1.43-fold at 48 and 72 h, respectively, in the LDL-treated cells compared with the control cells (Figure 2(a)). Similarly, proliferation of 5637 cell was increased 1.33-fold and 1.41-fold at 48 and 72 h, respectively (Figure 2(b)). All these results revealed that a high LDL concentration can promote bladder cancer cells proliferation. These results established a relationship between cholesterol availability and bladder cancer cells proliferation.
Figure 2.
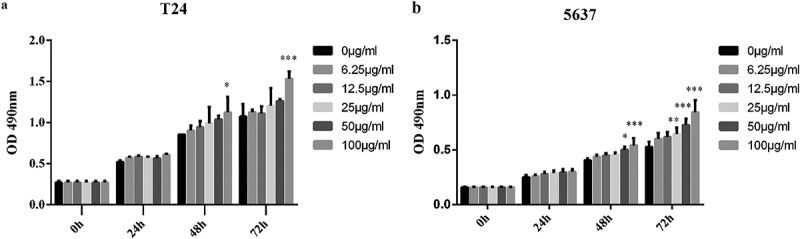
Cholesterol can promote the proliferation of bladder cancer cells in vitro.
T24 or 5637 cells were seeded in four 96-well plates and normally cultured for 24 h. The next day, one plate was assessed by MTT assays to confirm the cell density and cell difference between wells. The others were treated with different concentrations of LDL (0, 6.25, 12.5, 25, 50, 100 μg/ml), and cell proliferation was assessed at 24 h, 48 h and 72 h by MTT assays. Experiments were repeated three times, and error bars represent the standard error of the mean and *p < 0.05, **p < 0.01, ***p < 0.001 (2-way ANOVA).
CYP27A1 can affect intracellular 27-HC and cholesterol secretion
The CYP27A1 mRNA and protein levels were both substantially increased in the T24 or UM-UC-3 cells with CYP27A1 overexpression, and compared with the mRNA level in the normal control cells, the mRNA levels were increased 153,784-fold and 42,964-fold, respectively (Figure 3(a,b)). The CYP27A1 gene encodes sterol 27-hydroxylase, which converts cholesterol into 27-HC. 27-HC is involved in regulating cellular cholesterol homeostasis, so the intracellular secretion of 27-HC was assessed. As expected, comparing with the pLVX vector control cells, the CYP27A1-overexpressing cells presented with increased intracellular 27-HC production and decreased cholesterol levels at 6 d in both T24 and UM-UC-3 cells (Figure 3(c,d)). These results revealed that CYP27A1 expression is closely related to cholesterol homeostasis in bladder cancer cells.
Figure 3.
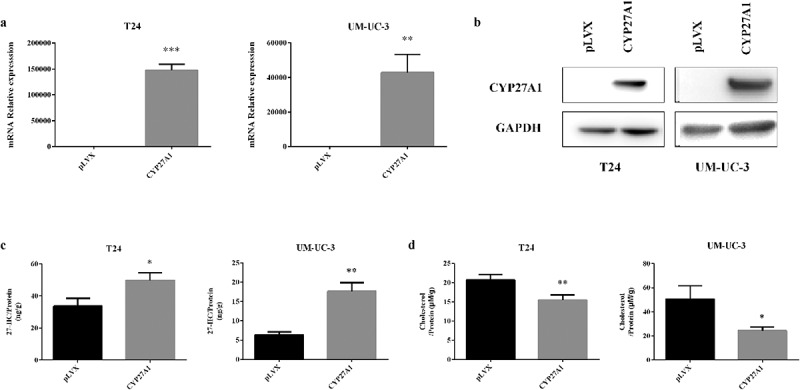
CYP27A1 affects intracellular 27-HC and cholesterol secretion.
(a, b) T24 or UM-UC-3 cells stably expressing CYP27A1 or the pLVX control (2.5 × 104/well) were seeded in 24-well plates and cultured for 48 h and 72 h, RNA and protein were extracted by RNAiso Plus and SDS buffer respectively. mRNA levels of CYP27A1 were assessed using qRT-PCR, cell lysates were harvested and Western blotting was performed to analyze the protein levels of CYP27A1. Experiments were repeated three times, and error bars represent the standard error of the mean and **p < 0.01, ***p < 0.001 (unpaired t-test). (c, d) T24 or UM-UC-3 cells stably expressing CYP27A1 or the pLVX control (2.5 × 104/well) were seeded in 6-well plates and cultured for 6 d and cell lysates were harvested. 27-HC/Cholesterol levels were measured by ELISAs and normalized to total protein. Experiments were repeated three times, error bars represent the standard error of the mean and *p < 0.05, **p < 0.01 (unpaired t-test).
CYP27A1 inhibits bladder cancer cells proliferation in vitro and vivo
Using T24 and UM-UC-3 cells with or without stable CYP27A1 expression, the effect of CYP27A1 overexpression on bladder cancer cells proliferation in vitro was detected on the indicated days. After 6 d, proliferation was significantly impeded in both T24 and UM-UC-3 cells stably expressing CYP27A1 compared with the pLVX control cells (Figure 4(a)). Furthermore, these CYP27A1-overexpressing cells demonstrated a significantly reduced colony formation ability (Figure 4b). To further confirm the inhibitory effect of CYP27A1 on bladder cancer cells, CYP27A1 overexpression T24 cell-derived tumors were detected in vivo with pLVX T24-derived tumors as the control. Tumor growth was assessed by caliper measurements 3 times per week. Starting from the 25th day, CYP27A1-tumors were significantly smaller than the control tumors (Figure 4(c–e)). When all animals were sacrificed to collect tumors, the weight of the control tumors was 1.38-fold that of CYP27A1-tumors (Figure 4(d–e)). All of these results showed that restoring the expression of CYP27A1 could obviously inhibit bladder cancer progression in vitro and vivo.
Figure 4.
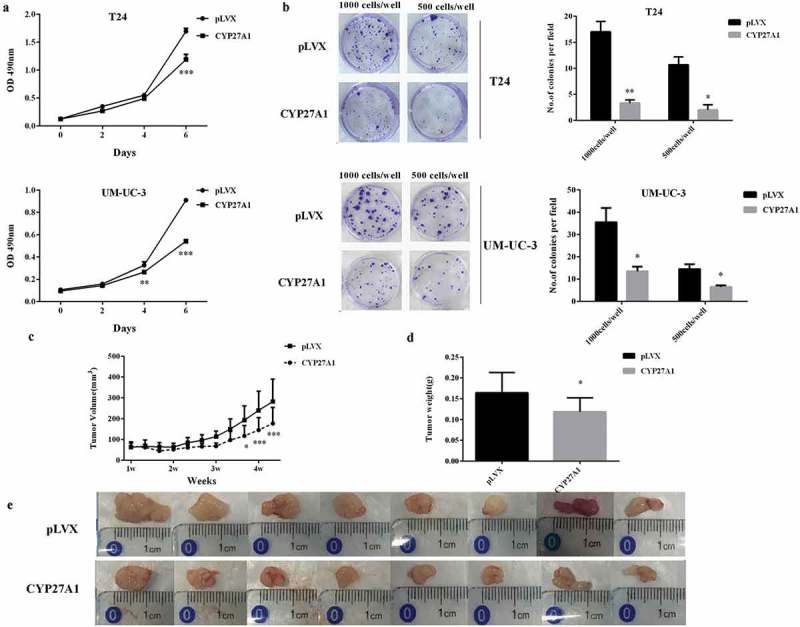
CYP27A1 inhibits bladder cancer cells proliferation in vitro and vivo.
T24 or UM-UC-3 cells overexpressing CYP27A1 or the pLVX control (1 × 103/well) were seeded in 96-well plates and cell proliferation was detected by MTT assays at the indicated days. At day 0, one plate was assessed by MTT assays to confirm the cell density and cell difference between wells. Experiments were performed three times, and error bars represent the standard error of the mean and **p < 0.01, ***p < 0.001(2-way ANOVA). (b) T24 or UM-UC-3 cells overexpressing CYP27A1 or the pLVX control cells (1,000, 500 cells/well) were seeded in 6-well plates and cultured in 10% DMEM with 1 μg/ml puromycin for 2 wk. Colonies were stained with crystal violet. Experiments were performed three times. A representative well showing colony growth is also shown in the graph. Error bars represent the standard error of the mean and *p < 0.05, **p < 0.01 (unpaired t-test). Tumor volume (c) and weights (d) of CYP27A1-T24- and pLVX-T24-derived xenografts in vivo were compared. After the 25th day, CYP27A1-tumor size was significantly smaller than the control (pLVX) and *p < 0.05, ***p < 0.001(2-way ANOVA). After 30 d, all animals were sacrificed and tumors were collected (e).
CYP27A1 inhibits bladder cancer cells proliferation via producing 27-HC
Since CYP27A1 overexpression inhibited bladder cancer proliferation and increased intracellular 27-HC production, the ability of the 27-HC treatment to induce similar results was important to investigate. 27-HC, produced by CYP27A1, is thought to influence cell proliferation via cholesterol synthesis. The results revealed that proliferation was significantly impeded in both T24 and UM-UC-3 cells treated with 10 µM 27-HC compared with control cells treated with DMSO. Clear differences were observed in T24 cell cultured for 2 d and UM-UC-3 cultured for 4 d (Figure 5(a)). Furthermore, compared with the control cells, both T24 and UM-UC-3 cells treated with 1 µM 27-HC demonstrated significantly reduced colony formation ability (Figure 5(b)).
Figure 5.
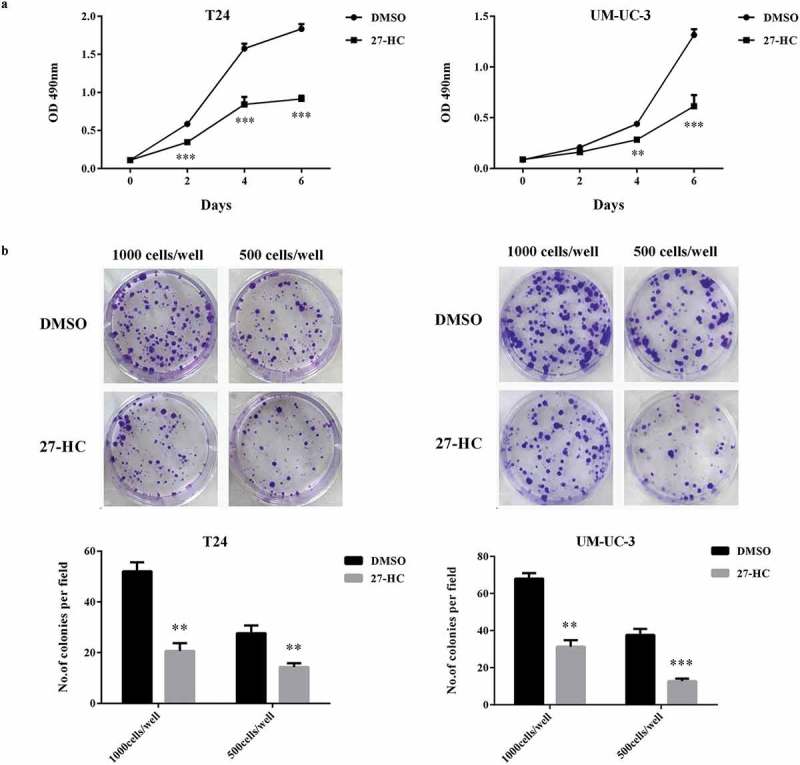
CYP27A1 inhibits bladder cancer cells proliferation via producing 27- HC.
T24 or UM-UC-3 cells (1 × 103/well) were seeded in 96-well plates for 24 h and then treated with 10 µM of 27-HC or DMSO (control). Cell proliferation was detected by MTT assays at the indicated days. At day 0, one plate was assessed by MTT assays to confirm the cell density and cell difference between wells. The data shown are representative of three independent experiments. Error bars represent the standard error of the mean and **p < 0.01, ***p < 0.001 (2-way ANOVA). (b) T24 or UM-UC-3 cells (1,000, 500 cells/well) were seeded in 6-well plates for 24 h and then treated with 1 µM of 27-HC or DMSO (control) and cultured for 2 wk. Colonies were stained with crystal violet. Experiments were repeated three times. A representative well showing colony growth is also shown in the graph. Error bars represent the standard error of the mean and **p < 0.01, ***p < 0.001 (unpaired t-test).
27-HC inhibits bladder cancer cells proliferation by upregulating ABCG1/ABCA1 through LXRs pathway
Since 27-HC inhibited bladder cancer cells proliferation, exploring the mechanisms by which 27-HC affects cells proliferation via cholesterol homestasis was an important pursuit. Intracellular cholesterol homeostasis is regulated by two kinds of transcription factors: LXRs and sterol regulatory element-binding proteins (SREBPs). LXRs promote intracellular cholesterol efflux by inducing the expression of related genes, such as ABCG1 and ABCA1, while SREBPs promote intracellular cholesterol synthesis and extracellular cholesterol uptake by inducing the expression of related gene, such as LDLR [23–26]. Our results revealed that compared with the control cells, both T24 and UM-UC-3 cells treated with 10 µM 27-HC displayed upregulated ABCG1 and ABCA1 and downregulated LDLR at mRNA levels (Figure 6(a,b)). Furthermore, 27-HC, as LXRs ligand, can increase LXRs-dependent metastasis, which has an anti-proliferative effect. To determine the role of LXRs as a mediator on bladder cancer cells proliferation, we assessed the effect of LXRs agonist T0901317 (T1317) on the proliferation of T24 and UM-UC-3 cells. Similar to 27-HC, T1317 obviously inhibited the proliferation of T24 and UM-UC-3 at 6 d (Figure 6(c)). Next, the mechanisms of LXRs on the cholesterol homeostasis in bladder cancer cells were explored. First, knockdown of LXRs by RNA interference was performed (Figure 6(d)). Like 27-HC, T1317 could upregulate ABCG1 and ABCA1 in both T24 and UM-UC-3 cells. On the other hand, RNAi induced downregulation of LXRs attenuated the above effluence upon ABCG1 and ABCA1 (Figure 6(a)). These results revealed that upregulation of ABCG1 and ABCA1 by 27-HC occur through LXRs pathway in bladder cancer cells.
Figure 6.
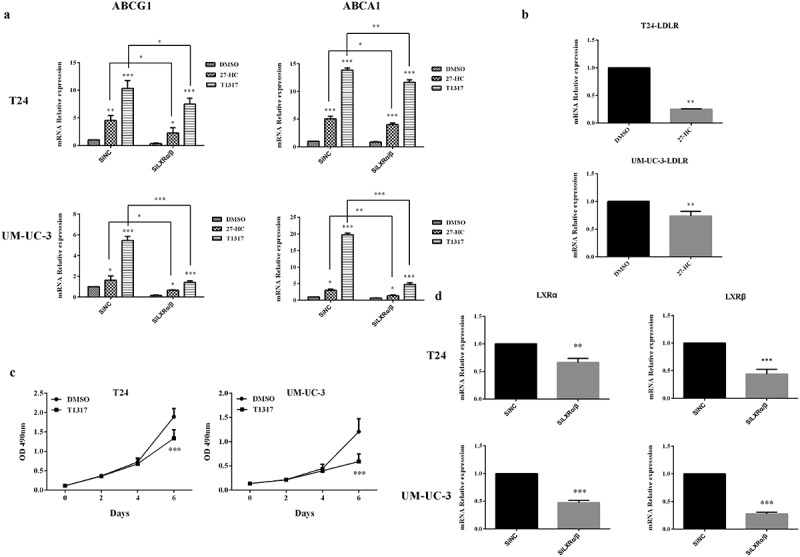
27-HC inhibits bladder cancer cells proliferation by upregulating ABCG1 and ABCA1 through LXRs pathway.
(a) T24 or UM-UC-3 cells were seeded into 24-well plates at a density of 1 × 104 cells/well. The next day, cells were transiently transfected with either SiNC (20 pM) or SiLXRα/β (20 pM). Two days later, the cells were treated with DMSO/27-HC (10 μM)/T1317 (10 μM) for 24 h. The expression of ABCG1, ABCA1 was assessed by qRT-PCR, *p < 0.05, **p < 0.01, ***p < 0.001 (2-way ANOVA and unpaired t-test).(b) T24/UM-UC-3 cells were seeded in 24-well plates and then treated with 10 μM of 27-HC and DMSO (control) at next day and continue cultured for 4 d, mRNA level of LDLR was assessed using qRT-PCR. Experiment were repeated three times, error bars represent the standard error of the mean and **p < 0.01 (unpaired t-test). (c) T24 or UM-UC-3 cells (1 × 103/well) were seeded in 96-well plates for 24 h and then treated with 10 µM of T1317 or DMSO (control). Cell proliferation was detected by MTT assays at the indicated days. At day 0, one plate was assessed by MTT assays to confirm the cell density and cell difference between wells. The data shown are representative of three independent experiments. Error bars represent the standard error of the mean and ***p < 0.001 (2-way ANOVA). (d) T24 or UM-UC-3 cells were transiently transfected with SiNC (negative control) or SiLXRα/β, knockdown effects of LXRα and LXRβ were detected, **p < 0.01, ***p < 0.001 (unpaired t-test).
Discussion
CYP27A1, a cytochrome P450 oxidase, is an enzyme expressed in liver and other tissues. The main roles of CYP27A1 are regulation of cellular cholesterol homeostasis and vitamin D3 metabolism [12,13]. In addition, CYP27A1 can convert cholesterol into 27-HC which acts as a selective ER modulator and an agonist of Liver X receptors and further affects cell proliferation [17]. AR, a ligand-regulated transcription factor, plays an important role in the development and progression of bladder cancer [4,5]. Although there was no difference of AR expression between low-grade and high-grade bladder cancer clinical specimens [27], many independent studies demonstrated that androgens-AR signals can increase the proliferation of bladder cancer cells [4] . Beside, knockdown of AR in bladder cancer cells results in cell apoptosis [28]. Therefore, AR positive bladder cancer cell lines which were elected for studying the relationship between CYP27A1 and bladder cancer proliferation were appropriate, and this relationship was explored in this study to confirm that the CYP27A1/27-HC axis is involved in cholesterol homeostasis and affects cell proliferation. CYP27A1 and AR levels were first assessed in three bladder cancer cell lines. We found that CYP27A1 was barely expressed in the AR-positive T24/UM-UC-3 cell lines, as evidenced by the CYP27A1 expression levels in the bladder cancer cells.
Cholesterol is essential for cell proliferation, intake of a high cholesterol diet correlates with an increased risk of many cancer types, including bladder cancer [6,7] . Elevated cholesterol has been implicated in disease development in prostate cancer [29]. Furthermore, the link between cholesterol and tumor progression has been reported in melanoma and breast cancer [30,31]. To further confirm the role of cholesterol in bladder cancer cells proliferation, we used LDL, a type of lipoprotein with the highest cholesterol content, for the study. We found that a high LDL concentration can promote bladder cancer cells proliferation. This conclusion established a relationship between cholesterol availability and bladder cancer cells proliferation. As such, it is important to understand the molecular mechanisms used by bladder cancer cells to regulate intracellular cholesterol content.
Previous reports showed that 27-HC, which serves as a primary metabolite of cholesterol and is synthesized by the action of 27-hydroxylase (CYP27A1), regulates cholesterol biosynthesis to affect cell proliferation. Similar studies have focused on prostate cancer and breast cancer, but the effect on cell proliferation was different [17]. In breast cancer, 27-HC could increase ER (+) breast cell proliferation [10,18]. However, in prostate cancer, the effect of 27-HC on cell proliferation is controversial [14,19]. Because the effect of 27-HC on the proliferation of bladder cancer cells has not been reported, it was studied in this paper. To validate the role of oxysterols in the regulation of cholesterol homeostasis, bladder cancer cells accomplish this activity by synthesizing 27-HC as a consequence of CYP27A1 gene overexpression. In support of this hypothesis, we showed that overexpression of CYP27A1 in bladder cancer cells increases intracellular 27-HC production and decreases the cellular cholesterol level. In addition, restoring CYP27A1 expression inhibited the progression of T24 and UM-UC-3 bladder cancer cells as well as CYP27A1-overexpressing T24 cell-derived xenografts. Similarly, we found that exogenous 27-HC, as a negative feedback regulatory factor, also inhibited cell growth.
In cancer, cholesterol homeostasis regulation is aberrant, and growth is abnormally rapid. For these reasons, we speculated that cancer cells may develop new mechanisms to bypass the pathways regulating cholesterol metabolism homeostasis [32]. As mentioned above, the level of cholesterol in cells was regulated by uptake, biosynthesis and efflux primarily through the coordinated activity of SREBPs and LXRs. At present, LXRs have been proposed as a new anticancer target, and the agonists T0901317 and GW3965 were reported to inhibit cell proliferation of prostate and breast cancer cells in vitro [33,34]. To the best of our knowledge, the effect of LXRs in bladder cancer cells has not been reported. To clarify the effect of LXRs in bladder cancer cells, the ability of 27-HC/T1317 to affect the expression of LXRs target genes (ABCG1 and ABCA1) was explored [24,26]. The results from this experiment confirmed that the inhibitory effect of 27-HC on bladder cancer cells growth was probably mediated by ABCG1/ABCA1 expression upregulation and LDLR expression downregulation. Furthermore, the LXRs activation and knockdown results revealed that upregulation of ABCG1 and ABCA1 by 27-HC may occur through the LXRs pathway.
In summary, bladder cancer cells must bypass the regulation of cholesterol balance to achieve rapid proliferation. In this study, we found that this can be implemented by downregulating the expression of CYP27A1 and further inhibiting the expression of 27-HC. In addition, 27-HC acts as an endogenous agonist for the LXRs and induces the expression of genes involved in cholesterol efflux, and in turn, inhibits the growth of bladder cancer cells. Therefore, our findings suggest that CYP27A1 is a critical cellular cholesterol sensor in bladder cancer cells and that CYP27A1/27-HC dysregulation contributes significantly to bladder cancer cells proliferation.
Funding Statement
This work was supported by the China Postdoctoral Science Foundation [2017M622144];National Natural Science Foundation of China [81472411];National Natural Science Foundation of China [81772713];National Natural Science Foundation of China [81372752];National Natural Science Foundation of China [81401899];Natural Science Foundation of Shandong Province [ZR2014HM088];Natural Science Foundation of Shandong Province [ZR2016HQ18];Key Research and Development Program of Shandong Province [2018GSF118197];Qingdao Postdoctoral Application Research Project;Qingdao Young Scientist Applied Basic Research Fund [15-9-1-51-jch];Qingdao Young Scientist Applied Basic Research Fund [15-9-1-105-jch];Taishan Scholar Foundation of Shandong Province [tsqn20161077].
Acknowledgments
This work was supported by the National Natural Science Foundations of China under Grant 81772713, 81472411, 81372752, 81401899; Taishan Scholar Program of Shandong Province under Grant tsqn20161077; Natural Science Foundation of Shandong Province under Grant ZR2014HM088, ZR2016HQ18; Key Research and Development Program of Shandong Province under Grant 2018GSF118197; China Postdoctoral Science Foundation under Grant 2017M622144; Qingdao Postdoctoral Application Research Project; and Qingdao Young Scientist Applied Basic Research Fund under Grant 15-9-1-51-jch, 15-9-1-105-jch.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Nicholas Munro F, Peter Whelan F.. Bladder cancer. Surgery. 2000;24:1349–1358. [Google Scholar]
- [2].Hemelt M, Yamamoto H, Cheng KK, et al. The effect of smoking on the male excess of bladder cancer: a meta-analysis and geographical analyses. Int J Cancer. 2009;124:412–419. PMID:18792102 [DOI] [PubMed] [Google Scholar]
- [3].Chung LW, Zhau HE, Ro JY.. Morphologic and biochemical alterations in rat prostatic tumors induced by fetal urogenital sinus mesenchyme. Prostate. 1990;17:165–174. PMID:2399192 [DOI] [PubMed] [Google Scholar]
- [4].Miyamoto H, Yang Z, Chen YT, et al. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. PMID:17406000 [DOI] [PubMed] [Google Scholar]
- [5].Lombard AP, Mudryj M. The emerging role of the androgen receptor in bladder cancer. Endocr Relat Cancer. 2015;22:R265–R77. PMID:26229034 [DOI] [PubMed] [Google Scholar]
- [6].Hamm R, Chen YR, Seo EJ, et al. Induction of cholesterol biosynthesis by archazolid B in T24 bladder cancer cells. Biochem Pharmacol. 2014;91:18–30. PMID:24976507 [DOI] [PubMed] [Google Scholar]
- [7].Hu J, La Vecchia C, de Groh M, et al. Dietary cholesterol intake and cancer. Ann Oncol. 2012;23:491–500. PMID:21543628 [DOI] [PubMed] [Google Scholar]
- [8].Shah RR, Bono P, Oudard S, et al. Outcomes in patients with metastatic renal cell carcinoma who develop everolimus-related hyperglycemia and hypercholesterolemia: combined subgroup analyses of the RECORD-1 and REACT trials. Clin Genitourin Cancer. 2016;15:e53–e54. 2017 PMID:27524511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Magura L, Blanchard R, Hope B, et al. Hypercholesterolemia and prostate cancer: a hospital-based case-control study. Cancer Causes Control. 2008;19:1259–1266. PMID:18704722 [DOI] [PubMed] [Google Scholar]
- [10].Nelson ER, Wardell SE, Jasper JS, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. PMID:24288332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goodwin B, Gauthier KC, Umetani M, et al. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc Natl Acad Sci U S A. 2003;100:223–228. PMID:12509506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gottfried E, Rehli M, Hahn J, et al. Monocyte-derived cells express CYP27A1 and convert vitamin D3 into its active metabolite. Biochem Biophys Res Commun. 2006;349:209–213. PMID:16930540 [DOI] [PubMed] [Google Scholar]
- [13].Sawada N, Sakaki T, Ohta M, et al. Metabolism of vitamin D(3) by human CYP27A1. Biochem Biophys Res Commun. 2000;273:977–984. PMID:10891358 [DOI] [PubMed] [Google Scholar]
- [14].Alfaqih MA, Nelson ER, Liu W, et al. CYP27A1 loss dysregulates cholesterol homeostasis in prostate’ cancer. Cancer Res. 2017;77:1662–1673. PMID:28130224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kimbung S, Chang CY, Bendahl PO, et al. Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr Relat Cancer. 2017;24:339–349. PMID:28442559 [DOI] [PubMed] [Google Scholar]
- [16].Ji YC, Liu C, Zhang X, et al. Intestinal bacterium-derived cyp27a1 prevents colon cancer cell apoptosis. Am J Transl Res. 2016;8:4434–4439. PMID:27830027 [PMC free article] [PubMed] [Google Scholar]
- [17].Marwarha G, Raza S, Hammer K, et al. 27-hydroxycholesterol: A novel player in molecular carcinogenesis of breast and prostate cancer. Chem Phys Lipids. 2017;207:108–126. PMID:28583434 [DOI] [PubMed] [Google Scholar]
- [18].Cruz P, Torres C, Ramirez ME, et al. Proliferation of human mammary cancer cells exposed to 27-hydroxycholesterol. Exp Ther Med. 2010;1:531–536. PMID:22993572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raza S, Meyer M, Schommer J, et al. 27-Hydroxycholesterol stimulates cell proliferation and resistance to docetaxel-induced apoptosis in prostate epithelial cells. Med Oncol. 2016;33:12 PMID:26732475 [DOI] [PubMed] [Google Scholar]
- [20].Endo-Umeda K, Uno S, Fujimori K, et al. Differential expression and function of alternative splicing variants of human liver X receptor alpha. Mol Pharmacol. 2012;81:800–810. PMID:22399489 [DOI] [PubMed] [Google Scholar]
- [21].Jitao W, Jinchen H, Qingzuo L, et al. Androgen receptor inducing bladder cancer progression by promoting an epithelial-mesenchymal transition. Andrologia. 2014;46:1128–1133. PMID:24329492 [DOI] [PubMed] [Google Scholar]
- [22].Zheng Y, Izumi K, Yao JL, et al. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr Relat Cancer. 2011;18:451–464. PMID:21613411 [DOI] [PubMed] [Google Scholar]
- [23].Huber MD, Vesely PW, Datta K, et al. Erlins restrict SREBP activation in the ER and regulate cellular cholesterol homeostasis. J Cell Biol. 2013;203:427–436. PMID:24217618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ouvrier A, Cadet R, Vernet P, et al. LXR and ABCA1 control cholesterol homeostasis in the proximal mouse epididymis in a cell-specific manner. J Lipid Res. 2009;50:1766–1775. PMID:19395734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tamehiro N, Shigemoto-Mogami Y, Kakeya T, et al. Sterol regulatory element-binding protein-2- and liver X receptor-driven dual promoter regulation of hepatic ABC transporter A1 gene expression: mechanism underlying the unique response to cellular cholesterol status. J Biol Chem. 2007;282:21090–21099. PMID:17526932 [DOI] [PubMed] [Google Scholar]
- [26].Tarling EJ, Edwards PA. Dancing with the sterols: critical roles for ABCG1, ABCA1, miRNAs, and nuclear and cell surface receptors in controlling cellular sterol homeostasis. Biochim Biophys Acta. 2012;1821:386–395. PMID:21824529 [DOI] [PubMed] [Google Scholar]
- [27].Mir C, Shariat SF, van der Kwast TH, et al. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi-institutional study. BJU Int. 2011;108:24–30. PMID:21070579 [DOI] [PubMed] [Google Scholar]
- [28].Wu JT, Han BM, Yu SQ, et al. Androgen receptor is a potential therapeutic target for bladder cancer. Urology. 2010;75:820–827. PMID:WOS:000276258300011 [DOI] [PubMed] [Google Scholar]
- [29].Di Vizio D, Solomon KR, Freeman MR. Cholesterol and cholesterol-rich membranes in prostate cancer: an update. Tumori. 2008;94:633–639. PMID:WOS:000261045000001 [DOI] [PubMed] [Google Scholar]
- [30].Baruthio F, Quadroni M, Ruegg C, et al. Proteomic analysis of membrane rafts of melanoma cells identifies protein patterns characteristic of the tumor progression stage. Proteomics. 2008;(8):4733–4747. DOI: 10.1002/pmic.200800169 PMID:18942674 [DOI] [PubMed] [Google Scholar]
- [31].Llaverias G, Danilo C, Mercier I, et al. Role of cholesterol in the development and progression of breast cancer. Am J Pathol. 2011;178:402–412. PMID:21224077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Adlakha YK, Khanna S, Singh R, et al. Pro-apoptotic miRNA-128-2 modulates ABCA1, ABCG1 and RXRalpha expression and cholesterol homeostasis. Cell Death Dis. 2013;4:e780 PMID:23990020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fukuchi J, Kokontis JM, Hiipakka RA, et al. Antiproliferative effect of liver X receptor agonists on LNCaP human prostate cancer cells. Cancer Res. 2004;64:7686–7689. PMID:15520170 [DOI] [PubMed] [Google Scholar]
- [34].Vedin LL, Lewandowski SA, Parini P, et al. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis. 2009;30:575–579. PMID:19168586 [DOI] [PubMed] [Google Scholar]


