ABSTRACT
Objective: The aim of this study was to investigate the mechanism of miR-221 in depression.
Methods: The molecules expressions were measured by qRT-PCR and western blot. The sucrose preference test (SPT), forced swimming test (FST) and tail suspension test (TST) were used to detect depressive-like symptoms. MTT assay and flow cytometric was used to measure the proliferation and apoptosis of hippocampal neuronal.
Results: MiR-221 expression in the cerebrospinal fluid and serum of major depressive disorder patients and the hippocampus of chronic unpredictable mild stress (CUMS) mice were increased, while the expression of Wnt2, p-CREB and BDNF were decreased. Additionally, silence of miR-221 increased sucrose preference of CUMS mice and shortened the immobility time of CUMS mice in SPT and FST. MiR-221 could targeted regulate Wnt2, and knockdown of Wnt2 reversed the effect of miR-221 inhibitor on the proliferation and apoptosis of hippocampal neurons and countered the promoting effect of miR-221 inhibitor on the expression of Wnt2, p-CREB and BDNF.
Conclusion: MiR-221 could promote the development of depression by regulating Wnt2/CREB/BDNF axis.
KEYWORDS: Major depressive disorder, miR-221, Wnt2/CREB/BDNF
1. Introduction
Major depressive disorder (MDD) is a chronic emotional disorder, affecting nearly 300 million people worldwide [1]. Studies have demonstrated that the risk factors for depression development include the change of neurotransmitter expression, susceptibility gene polymorphisms, increased susceptibility to stress, damage to neural function, and synaptic plasticity disorders [2]. Although the research of MDD has not been interrupted, the etiology and pathogenesis of MDD remains unclear.
MicroRNA (miRNA) is a non-coding small molecule single-stranded RNA with a length of 19–23 nucleotides, affecting almost all biological processes [3]. At present, many studies have reported the relationship between miRNAs and depression. For example, Song et al. [4] found that knockdown of miR-16 could inhibit the occurrence of MDD in rats. Zhao et al. [5] revealed that miR-137 could suppress the post-stroke depression by reducing the protein expression of Grin2A. Li et al. [2] suggested that miR-335 exerted antidepressant effects by regulating GRM4. However, the study of miR-221 only found that it was involved in neuronal differentiation [6]. The relationship between miR-221 and depression has not been reported.
Wnt is a class of secretory glycoproteins, playing an essential role in cell proliferation and apoptosis, nervous system development and embryogenesis [7]. Previous studies have shown that the downstream pathway of Wnt/β-catenin signaling is associated with depression [8]. In addition, Wnt2 and Wnt3 have been shown to have antidepressant effects [9]. However, the mechanism of Wnt in depression is not clear. Based on the software’s prediction (MicroRNA.org), there are binding sites between miR-221 and Wnt2. Therefore, we speculated that the role of Wnt2 in depression might be related to miR-221.
In this study, we measured the relationship between miR-221 and depression, and investigated the mechanism of miR-221/Wnt2 in depression, aiming to provide a new target for the treatment of MDD.
2. Materials and methods
2.1. Ethical standards
All protocols used in this study were approved by the Ethics Committee of Zhengzhou University. The written informed consent from was obtained from all patients.
2.2. Patients
The inclusion criteria of MDD patients: major depression diagnosis according to CCMD-3 (Chinese Classification and diagnostic criteria of Mental Disorders, 3rd edition), with Hamilton Depression Scale (HAMD) score of 24 or higher.
The exclusion criteria of MDD patients: primary organic disease, other mental illness, drugs or alcohol dependence, family history of bipolar disorder.
The inclusion criteria of control subjects: no previous or current mental or neurological illness, no mental illness according to CCMD-3. Meanwhile, these control subjects also met the above exclusion criteria.
2.3. Samples preparation
Lumbar puncture was used to collect cerebrospinal fluid (CSF, 4 ml) from 6 MDD patients (3 men and 4 women, average age was 32.05 ± 7.92, age and sex matched) and 6 control subjects (2 men and 4 women, average age was 33.26 ± 8.69, age and sex matched). Fasting venous blood samples (4 ml) from another 22 MDD patients (10 men and 12 women, average age was 35.43 ± 10.27, age and sex matched) and 20 healthy subjects (9 men and 11 women, average age was 34.37 ± 9.36, age and sex matched) were collected between 8:00 a.m and 9:00 a.m. To remove cells and other debris, CSF and blood samples were then immediately centrifuged at 4°C for 10 min, and then stored at −80°C until further analysis.
2.4. Animals
Adult male Kunming mice were provided from Animal Center of Zhengzhou University. The mice were housed in a 12 h light/dark cycle and given food and water ad libitum. All animal experiments were carried out in accordance with the guidelines from the Institutional Animal Care Committee of Zhengzhou University. To confirm the relationship between miR-221 and depression, 12 mice were equally divided into chronic unpredictable mild stress (CUMS) group and control group. To verify the effect of miR-221 on depressive-like symptoms, 24 mice were equally divided into 4 groups (control group, CUMS group, CUMS+antagomir-control group, and CUMS+miR-221 antagomir group). To investigate the effect of miR-221 on the treatment of fluoxetine, 18 mice were equally divided into 3 groups (CUMS group, CUMS+fluoxetine group, and CUMS+fluoxetine+miR-221 agomir group).
MiR-221 antagomir, miR-221 agomir, and their control (Genepharma, Shanghai, China) were dissolved in artificial cerebrospinal fluid (ACSF) and intracerebroventricular (icv) injection into mice (1.0 mm lateral, 2.5 mm ventral and 0.3 mm caudal to bregma) with a dose of 0.5 nmol/mouse [10]. The dose was calculated according to our pre-experiment, so the data were not shown. The treatment was performed once a week for a total of 3 weeks. The fluoxetine group was treated with fluoxetine at the dose of 10 mg/kg by intraperitoneal injection once daily. The drug treatment was performed for a total of 2 weeks.
2.5. Chronic unpredictable mild stress (CUMS)
The CUMS procedure was performed as previously described with minor modifications [11]. Briefly, the mice were subjected to a variety of chronic unpredictable mild stressors as follows: (1) clip the distal 1/3 mice tails for 1 min; (2) foot shock for 10 s; (3) swimming in cold water (4°C) for 5 min; (4) overnight illumination; (5) fast for 24 h; (6) water deprivation for 24 h; (7) wet cage for 24 h; (8) 45°cage tilt for 24 h. Mice were received one to two stressors daily with a completely random order, and lasted for 6 weeks. The control mice subjected no stressor. Subsequently, the mice were subjected to sugar preference test (SPT), forced swimming test (FST) and tail suspension test (TST).
2.6. SPT
SPT was conducted as previously described [9]. Briefly, after CUMS procedure, mice were deprived of water starting from 8:00 p.m. From the second day, mice were accustomed 1% sucrose solution for 3 days (1 hour per day, between 8:00 a.m. and 9:00 a.m.). On the test day, the SPT was performed after 8 h water deprivation. Then, mice were given the free choice to drink from two identical bottles for 1 h. One was filled with 1% sucrose water and the other was filled with water. The test lasted two days, and the positions of the bottles were switched. After the test, the weight loss of the two bottles was liquid consumption. Sucrose preference (SP) was calculated as the formula: SP (%) = sucrose intake/total liquid intake×100. Total liquid intake = sucrose intake + water intake.
2.7. FST
FST was performed in a cylindrical glass beaker with a height of 25 cm and a diameter of 10 cm. In the test, the glass beaker contained 18 cm of water (22°C), and mice were forced to swim in it for 6 min. The sessions were videotaped, and the immobility time of mice was calculated by double blind observers. The immobility time was defined as the time that mice had no movement or made small movements to keep head above water. After FST, the mice were dried and put back into the cage.
2.8. TST
Mice were stuck with a rubberized fabric at 1 cm from the tail tip and suspended from the top of the table 50 cm by the tail for 6 min. The whole course of the test was videotaped, and the immobility time was measured by double blind analysis. The immobility time was considered as the time that mice were completely motionless.
2.9. Hippocampus
To detect miR-221 or the relative protein expression, mice were sacrificed after CUMS procedure. Then, the hippocampus was carefully removed and frozen in liquid nitrogen, and stored at −80°C. The content of corticosterone (CORT) in the serum of mice was detected by ELISA Kit.
2.10. Quantitative real-time PCR (qRT-PCR)
qRT-PCR analysis was used to measure miR-221 expression in hippocampus and hippocampal neuronal. According to the manufacturer’s protocols, Trizol reagent (Invitrogen, CA, USA) was used to extract total RNA. cDNA was synthesized via a reverse transcription reaction. Then, cDNA was used for qRT-PCR analysis. U6 was used as internal control. MiR-221 expression was quantified by 2−ΔΔCt method.
The primers sequences were as follows:
miR-221-F: 5’-GGGAAGCTACATTGTCTGC-3’
miR-221-R: 5’-CAGTGCGTGTCGTGGAGT-3’
U6-F: 5’- GCTTCGGCAGCACATATACTAAAAT
U6-R: 5’- CGCTTCACGAATTTGCGTGTCAT
2.11. Western blot
Hippocampus homogenate was prepared with a lysis buffer, and centrifuged at 4°C for 20 min. Then, the proteins were separated by SDS-PAGE and transferred to PVDF membranes (Amersham, Little Chalfont, UK). Membranes were blocked in 5% skim milk at room temperature for 1 h, and incubated with primary antibodies against Wnt2, cyclic adenosine monophosphate response element binding protein (CREB), phosphorylated CREB (p-CREB), brain-derived neurotrophic factor (BDNF), and β-actin (Santa Cruz Biotechnology Inc., CA, USA) at 4°C overnight. Next, the membranes incubated with horseradish peroxidase-labelled secondary antibody (Invitrogen). The protein bands were detected using an enhanced chemiluminescence kit (Biobyt, UK).
2.12. Hippocampal neuronal cultures and transfection
C57BL/6J newborn (P0) mice were provided from Animal Center of Zhengzhou University. Hippocampal neurons were collected from the newborn mice and cultured in neurobasal media containing L-glutamine and B27 (both from Life Technologies, CA, USA). According to the manufacturer’s protocols, miR-221 mimic, inhibitor and their negative controls (NC), and small interfering RNA (siRNA) targeting Wnt2 (si-Wnt2) and si-NC were transfected into hippocampal neurons using Lipofectamine 2000 (Invitrogen). After 24 h, hippocampal neurons were collected for further experiments.
2.13. MTT assay
Hippocampal neurons were seeded in 96-well plates with fresh medium containing 20 μl MTT (5 mg/ml), and cultured for 4 h at 37°C, 5% CO2. Then, the supernatant was removed and 150 μl dimethyl sulfoxide (DMSO) was added to each well. An ELISA reader (DeTie, Nanjing, China) was used to measure the absorbance at 490 nm.
2.14. Flow cytometric analysis
Flow cytometric analysis was used to detect neurons apoptosis. Hippocampal neuro was centrifuged for 2 min (500–800 r/min). Then, supernatant was removed, and 10 μl Annexin V-FITC and 5 μl Propidium iodide (PI) was added. Hippocampal neuron was stained at 4°C for 30 min (avoided light) and examined by a flow cytometry (Coulter, USA). The apoptosis rate was calculated by Expo 32 analysis software.
2.15. Cell culture and transfection
Human embryonic kidney 293 (HEK293) cells were purchased from Gefan Biotechnology Co., Ltd (Shanghai, China) and cultured in DMEM medium containing 10% fetal bovine serum, 100 mg/L penicillin and 100 mg/L streptomycin. Then, HEK293 cells were incubated at 37°C, 5% CO2.
2.16. Dual-luciferase reporter (DLR) assay
MicroRNA.org was used to analyze the potential binding site of miR-221 and Wnt2. The Wnt2 3’UTR fragment containing miR-221 sequence was amplified and cloned into the PGL3 DLR vector (Promega, WI, USA). Then, Wnt2 3’UTR reporter vector or mutated forms were co-transfected with miR-221 mimic or inhibitor into HEK293 cells. After 48 h, HEK293 cells were lysed. DLR assay kit (Promega) was used to measure luciferase activity following the manufacturer’s instructions.
2.17. Statistical analysis
All data were analyzed by SPSS 19.0 software, and results were represented as means ± SD. Student t test or one-way analysis of variance (ANOVA) was used to compare quantitative variables. P < 0.01 was considered as statistically significant.
3. Results
3.1. miR-221 expression in CSF and serum of MDD patients
To determine which miRNA was associated with depression, we detected the levels of miR-221, miR-126, miR-223, miR-652, miR-744 and miR-151-3p in CSF and serum in MDD patients according to the references [12–16]. Compared with the control patients, the expressions of miR-221, miR-223 and miR-151-3p were all increased in CSF, and the increase of miR-221 expression was more obvious (Figure 1(a)). The same findings were found in the serum (Figure 1(b)).
Figure 1.
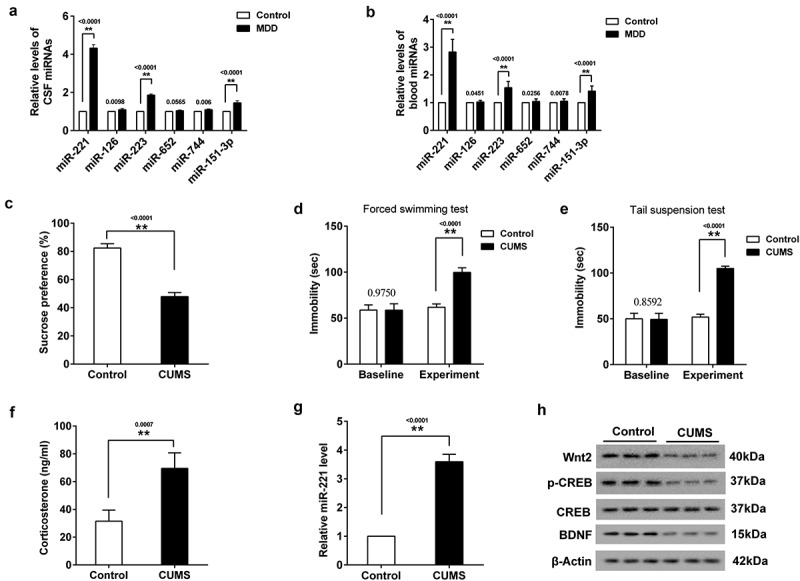
The expression of miR-221 in MDD patients and hippocampus of CUMS mice. (a) The expression of 6 miRNAs in CSF in MDD patients. (b) The expression of 6 miRNAs in serum in MDD patients. (c) SPT. (d) FST. (e) TST. (f) The content of CORT in the serum of CUMS mice. (g) miR-221 expression in hippocampus of CUMS mice. (h) The protein expression of Wnt2, p-CREB and BDNF in hippocampus of CUMS mice. **P < 0.01 vs control.
3.2. Mir-221 expression in hippocampus of CUMS mice
To confirm that miR-221 was indeed associated with depression, the relative level of miR-221 in hippocampus of CUMS mice was detected. Firstly, mice were divided CUMS group and control group. Then, mice were subjected to SPT, FST and TST. As shown in Figure 1(c), a significant decrease of sucrose preference was found in CUMS group. Compared with control group, the immobility time in FST (Figure 1(d)) and TST (Figure 1(e)) were markedly prolonged. Next, serum and hippocampus of mice was collected for the detection of CORT content and miR-221 expression. As shown in Figure 1(f), the content of CORT in the serum of CUMS mice was increased. qRT-PCR revealed that miR-221 expression in the hippocampus of CUMS mice was higher than that of control mice (Figure 1(g)). Western blot assay showed that the protein levels of Wnt2, p-CREB and BDNF in hippocampus of CUMS mice were lower than that of control mice (Figure 1(h)).
3.3. Effects of miR-221 on the proliferation and apoptosis of hippocampal neurons
To further investigate the role of miR-221 in hippocampal neurons, hippocampal neurons of neonatal mice were transfected with miR-221 mimic to promote miR-221 expression (Figure 2(a)) or miR-221 inhibitor to inhibit miR-221 expression (Figure 2(d)). Results showed that overexpression miR-221 inhibited hippocampal neuronal proliferation (Figure 2(b)) and promoted neuronal apoptosis (Figure 2(c)). As expected, silence of miR-221 had the opposite trend (Figure 2(e&f)).
Figure 2.
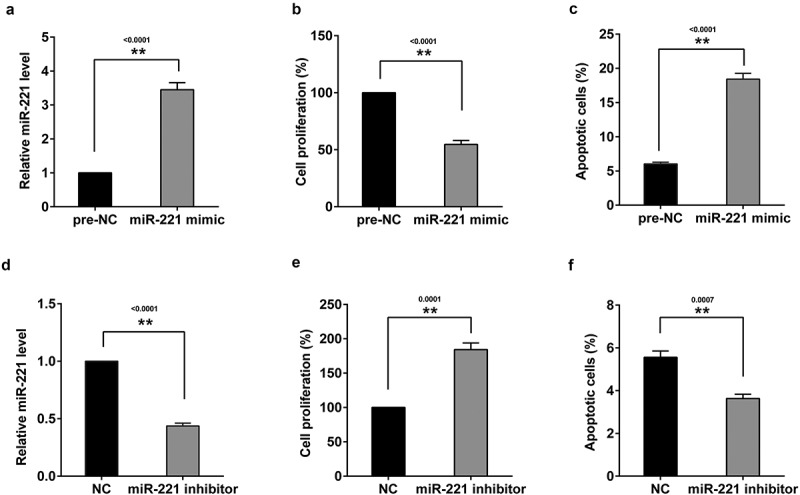
Effects of miR-221 on the proliferation and apoptosis of hippocampal neurons in mice. Hippocampal neurons of neonatal mice were transfected with miR-221 mimic. (a) miR-221 expression. (b) The proliferation of hippocampal neuronal. (c) The apoptosis of hippocampal neuronal. Hippocampal neurons of neonatal mice were transfected with miR-221 mimic. (d) miR-221 expression. (e) The proliferation of hippocampal neuronal. (f) The apoptosis of hippocampal neuronal. **P < 0.01 vs pre-NC or NC.
3.4. miR-221 negatively regulated Wnt2
Online software (MicroRNA.org) predicted potential binding sites between miR-221 and Wnt2 (Figure 3(a)). Therefore, to verify the regulation of miR-221 on the expression of Wnt2, Wnt2 3’-UTR activity was measured in HEK293 cells co-transfection with miR-221 mimic (or miR-221 inhibitor) and Wnt2 3’-UTR-WT or (Wnt2 3’-UTR-Mut) luciferase reporter vectors respectively. DLR assay showed Wnt2 3’-UTR-WT activity was markedly decreased in miR-221 overexpressing cells (Figure 3(b)), while Wnt2 3’-UTR-WT activity was significantly increased in miR-221 silencing cells (Figure 3(c)). Moreover, the protein expressions of Wnt2 (Figure 3(d)), p-CREB (Figure 3(e)) and BDNF (Figure 3(f)) in hippocampal neurons transfected with miR-221 mimic were decreased, and their protein expressions were increased in hippocampal neurons transfected with miR-221 inhibitor.
Figure 3.
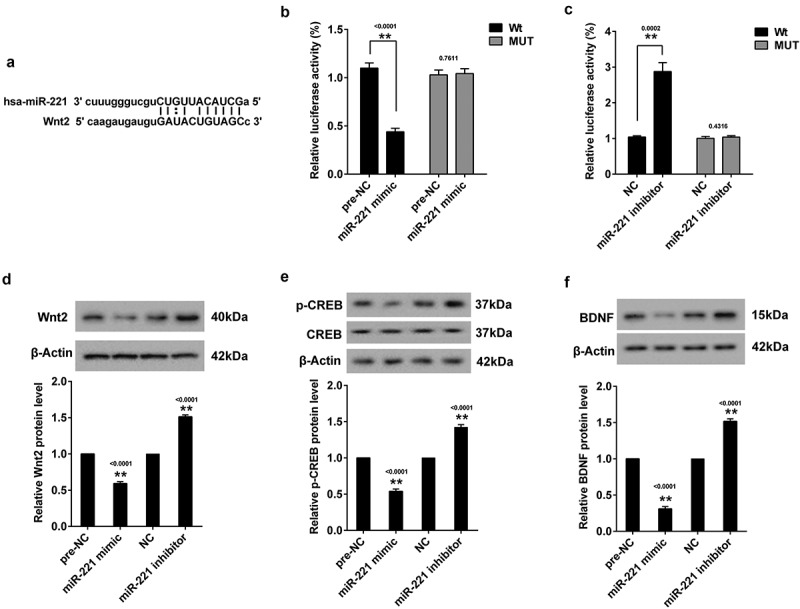
MiR-221 negatively regulated Wnt2. (a) The binding site between miR-221 and Wnt2. (b) Relative luciferase activity of Wnt2 3’-UTR in HEK293 cells transfected with miR-221 mimic. (c) Relative luciferase activity of Wnt2 3’-UTR in HEK293 cells transfected with miR-221 inhibitor. Hippocampal neurons were transfected with miR-221 mimic or miR-221 inhibitor. (d) Wnt2 protein expression. (e) p-CREB protein expression. (f) BDNF protein expression. **P < 0.01vs pre-NC or NC.
3.5. Knockdown of Wnt2 reversed the effect of miR-221 inhibitor on the proliferation and apoptosis of hippocampal neurons
In the experiment, hippocampal neurons were divided into 4 groups, including NC group, miR-221 inhibitor group, miR-221 inhibitor+si-NC group, and miR-221 inhibitor+si-Wnt2 group. As shown in Figure 4(a), silence of miR-221 could promote hippocampal neuronal proliferation, while si-Wnt2 reversed the promoting effect. Moreover, knockdown of Wnt2 reversed the inhibitory effect of miR-221 inhibitor on neuronal apoptosis (Figure 4(b)). Western blot assay showed that silence of miR-221 could increase the protein expression of Wnt2, p-CREB and BDNF, and the promoting effect was then reversed by si-Wnt2 (Figure 4(c)). Next, hippocampal neurons were divided into NC group, miR-221 mimic group, miR-221 mimic+Lenti-NC group, and miR-221 mimic+Lenti-Wnt2 group. Results showed that miR-221 mimic could inhibit hippocampal neuronal proliferation (Figure 4(d)), promote neuronal apoptosis (Figure 4(e)), and down-regulate the protein expression of Wnt2, p-CREB and BDNF (Figure 4(f)), while Lenti-Wnt2 abolished these effects.
Figure 4.
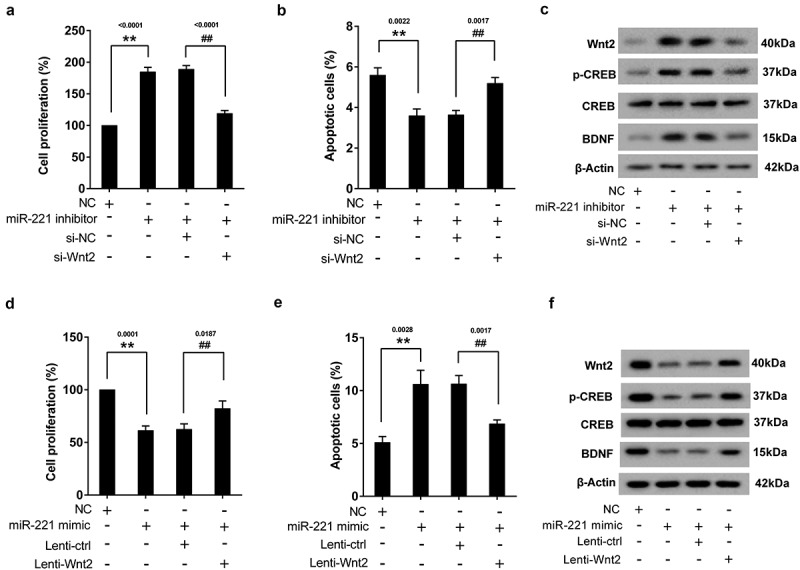
Knockdown of Wnt2 reversed the effect of miR-221 inhibitor on the proliferation and apoptosis of hippocampal neurons. Hippocampal neurons were divided into NC group, miR-221 inhibitor group, miR-221 inhibitor+si-NC group, and miR-221 inhibitor+si-Wnt2 group. (a) The proliferation of hippocampal neuronal. (b) The apoptosis of hippocampal neuronal. (c) The protein expression of Wnt2, p-CREB and BDNF. **P < 0.01 vs NC, ##P < 0.01 vs miR-221 inhibitor+ si-NC. Hippocampal neurons were divided into NC group, miR-221 mimic group, miR-221 mimic+Lenti-NC group, and miR-221 mimic+Lenti-Wnt2 group. (d) The proliferation of hippocampal neuronal. (e) The apoptosis of hippocampal neuronal. (f) The protein expression of Wnt2, p-CREB and BDNF. **P < 0.01 vs NC, ##P < 0.01 vs miR-221 mimic+Lenti-NC.
3.6. Silence of miR-221 alleviated the depressive-like symptoms in CUMS mice
To verify the effect of miR-221 on depressive-like symptoms, mice were divided into 4 groups (control group, CUMS group, CUMS+antagomir-control group, and CUMS+miR-221 antagomir group). MiR-221 antagomir was used to inhibit miR-221 expression. Results showed that miR-221 antagomir altered the effect of CUMS on SP (Figure 5(a)) and the immobility time in FST (Figure 5(b)) and TST (Figure 5(c)). Meanwhile, miR-221 antagomir reversed the increased of CORT (Figure 5(d)) and miR-221 (Figure 5(e)) as well as the decreased of Wnt2, p-CREB and BDNF (Figure 5(f)) induced by CUMS.
Figure 5.
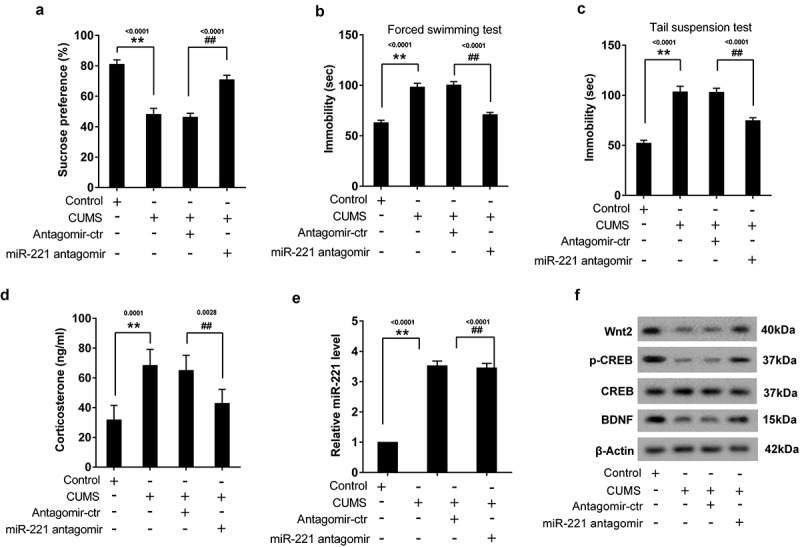
Silence of miR-221 alleviated depressive-like symptoms in CUMS mice. Mice were divided into 4 groups (control group, CUMS group, CUMS+antagomir-control group, and CUMS+miR-221 antagomir group). MiR-221 antagomir was used to inhibit miR-221 expression. (a) SPT. (b) FST. (c) TST. (d) The content of CORT. (e) miR-221 expression. (f) The protein expression of Wnt2, p-CREB and BDNF. **P < 0.01 vs control, ##P < 0.01 vs CUMS+antagomir-ctr.
3.7. Overexpression of miR-221 reversed the treatment of fluoxetine on CUMS mice
To investigate the effect of miR-221 on antidepressant, mice were divided into 3 groups, including CUMS group, CUMS+fluoxetine group, and CUMS+fluoxetine+miR-221 agomir group. MiR-221 agomir was used to up-regulate miR-221 expression. Results showed that fluoxetine could increase the SP of CUMS mice (Figure 6(a)), and reduce the immobility time in FST (Figure 6(b)) and TST (Figure 6(c)). However, overexpression of miR-221 reversed the phenomenon. Additionally, the decreased of CORT (Figure 6(d)) and miR-221 (Figure 6(e)) as well as the increased of Wnt2, p-CREB and BDNF (Figure 6(f)) induced by fluoxetine were all reversed by miR-221 agomir.
Figure 6.
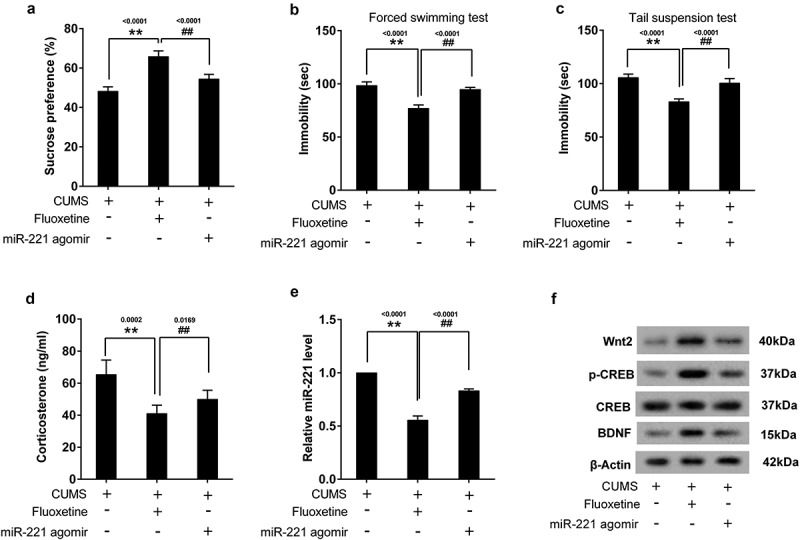
Overexpression miR-221 reversed the treatment of fluoxetine on CUMS mice. Mice were divided into 3 groups, including CUMS group, CUMS+fluoxetine group, and CUMS+fluoxetine+miR-221 agomir group. MiR-221 agomir was used to up-regulate miR-221 expression. (a) SPT. (b) FST. (c) TST. (d) The content of CORT. (e) miR-221 expression. (f) The protein expression of Wnt2, p-CREB and BDNF. **P < 0.01 vs CUMS, ##P < 0.01 vs CUMS+ fluoxetine.
4. Discussion
MDD is the most common and difficult problem in mental disorders, and also a hot topic in neuroscience and psychology. According to experts predict, except for cardiovascular diseases, depression will become the main killer of human health by 2030 [17]. Thus, it is necessary to find an effective way to treat depression.
MiRNAs have been found to be associated with depression [18,19]. It has been reported that miRNAs may participate in the pathophysiology of depression from the aspects of synaptic plasticity [20], neurogenesis [21] and gene expression regulation of key signaling transduction pathways elements [22]. Studies found that miR-124-3p [23] and miR-1202 [24] were related to the pathophysiology of MDD. Additionally, it was also reported that overexpression of miR-132 expression could promote the development of depression [25].
MiR-221 has been considered to act as a tumor regulator [26,27]. In recent years, studies have found that miR-221 is associated with cardiovascular disease. For instance, Su et al. [28] revealed that miR-221 could regulate autophagy and cardiac remodeling via p27/CDK2/mTOR axis. The present study found that miR-221 expression in the CSF and serum of MDD patients and the hippocampus of CUMS mice were abnormally increased, which suggested that miR-221 might be associated with the development of depression. Moreover, the further study found that overexpression of miR-221 could promote hippocampal neurons apoptosis. It has been reported that the loss of hippocampal neurons or the decrease of hippocampal volume is closely related to depression [29,30]. Together, the results confirmed that miR-221 was indeed associated with depression. However, the mechanism of miR-221 in depression is not clear.
Wnt2 is a member of Wnt family and has a certain connection with depression. Okamoto et al. [31] found that Wnt2 had an antidepressant effect. In the present study, we found that miR-221 could targeted regulate Wnt2, and knockdown of Wnt2 could reverse the effect of miR-221 inhibitor on the proliferation and apoptosis of hippocampal neurons. These results indicated that the role of miR-221 in depression might be related to Wnt2.
Moreover, we also found that knockdown of miR-221 could promote the expression of p-CREB and BDNF. BDNF is a vital neurotrophic factor in the treatment of depression, and it has the effects of differentiation, proliferation, nutrition and maturation on various types of neurons, which can affect the plasticity of neurons and the synthesis of neurotransmitters [32]. BDNF is the downstream target gene of CREB, and its promoter region contains CREB sequence [33,34]. p-CREB can promote the expression of BDNF [33,34]. It was reported that CREB played a key role in the prevention of neuronal apoptosis [35]. Motaghinejad et al. [36] found that activation of p-CREB/BDNF signaling could reverse nicotine-induced neuronal apoptosis. Taken together, the role of miR-221 in depression might be related to Wnt2/CREB/BDNF axis.
In mouse experiments, we found that inhibition of miR-221 relieved the depressive-like symptoms in mice and increased the expression of Wnt2, p-CREB and BDNF, which was consistent with the results of in vitro experiments. Fluoxetine is a selective serotonin reuptake inhibitor, which is used in the treatment of MDD [37]. Thus, fluoxetine was used to treat depression mice in the study. We found that overexpression miR-221 could reverse the antidepressant effect of fluoxetine.
In conclusion, we first discovered that miR-221 was associated with MDD, and it could encourage the development of depression by regulating Wnt2/CREB/BDNF signaling pathway. This might provide a novel research target for the treatment of depression.
Funding Statement
This research was supported by the National Natural Science Foundation of China (No.81571318 to XS; No.81401110 to XL), Health and Family Planning Commission of Henan Province (No.201501015 to XS), Department of Science and Technology of Henan Province (No. 162102410061 to XS; No.2017JQ023 to XS), School and Hospital Co-Incubation Funds (No.2017-BSTDJJ-04 to XS).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li J, Meng H, Cao W, et al. MiR-335 is involved in major depression disorder and antidepressant treatment through targeting GRM4. Neurosci Lett. 2015;606:167–172. [DOI] [PubMed] [Google Scholar]
- [3].Kramann R, Moeller MJ.. The next level of complexity: post-transcriptional regulation by microRNAs. Kidney Int. 2011;80(7):692–693. [DOI] [PubMed] [Google Scholar]
- [4].Song MF, Dong J-Z, Wang Y-W, et al. CSF miR-16 is decreased in major depression patients and its neutralization in rats induces depression-like behaviors via a serotonin transmitter system. J Affect Disord. 2015;178:25–31. [DOI] [PubMed] [Google Scholar]
- [5].Zhao L, Li H, Guo R, et al. miR-137, a new target for post-stroke depression? Neural Regen Res. 2013;8(26):2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hamada N, Fujita Y, Kojima T, et al. MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem Int. 2012;60(8):743. [DOI] [PubMed] [Google Scholar]
- [7].Freese JL, Pino D, Pleasure SJ.. Wnt signaling in development and disease. Neurobiol Dis. 2010;38(2):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Voleti B, Duman RS. The roles of neurotrophic factor and Wnt signaling in depression. Clin Pharmacol Ther. 2012;91(2):333–338. [DOI] [PubMed] [Google Scholar]
- [9].Zhou WJ, Xu N, Kong L, et al. The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors. Transl Psychiatry. 2016;6(9):e892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yi LT, Li J, Liu -B-B, et al. BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J Psychiatry Neurosci. 2014;39(5):348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li J, Zhou Y, Liu BB, et al. Nobiletin Ameliorates the Deficits in Hippocampal BDNF, TrkB, and Synapsin I Induced by Chronic Unpredictable Mild Stress. Evid Based Complement Alternat Med. 2013;2013(1):359682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Enatescu VR, Papava I, Enatescu I, et al. Circulating plasma micro RNAs in patients with major depressive disorder treated with antidepressants: a pilot study. Psychiatry Investig. 2016;13(5):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Camkurt MA, Acar Ş, Coşkun S, et al. Comparison of plasma MicroRNA levels in drug naive, first episode depressed patients and healthy controls. J Psychiatr Res. 2015;69:67–71. [DOI] [PubMed] [Google Scholar]
- [14].Rinaldi A, Vincenti S, De Vito F, et al. Stress induces region specific alterations in microRNAs expression in mice. Behav Brain Res. 2010;208(1):265–269. [DOI] [PubMed] [Google Scholar]
- [15].Belzeaux R, Bergon A, Jeanjean V, et al. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry. 2012;2:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kolshus E, Ryan KM, Blackshields G, et al. Peripheral blood microRNA and VEGFA mRNA changes following electroconvulsive therapy: implications for psychotic depression. Acta Psychiatr Scand. 2017;136(6):594–606. [DOI] [PubMed] [Google Scholar]
- [17].Kendler KS, Aggen SH, Li Y, et al. The similarity of the structure of DSM-IV criteria for major depression in depressed women from China, the United States and Europe. Psychol Med. 2015;45(9):1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luikart BW, Perederiy JV, Westbrook GL. Dentate Gyrus Neurogenesis, Integration, and microRNAs. Behav Brain Res. 2012;227(2):348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Saus E, Soria V, Escaramís G, et al. Genetic variants and abnormal processing of pre-miR-182, a circadian clock modulator, in major depression patients with late insomnia. Hum Mol Genet. 2010;19(20):4017–4025. [DOI] [PubMed] [Google Scholar]
- [20].Gao J, Wang W-Y, Mao Y-W, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Choi PS, Zakhary L, Choi W-Y, et al. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron. 2008;57(1):41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu Y, Liu H, Li F, et al. A polymorphism in the microRNA-30e precursor associated with major depressive disorder risk and P300 waveform. J Affect Disord. 2010;127(1–3):332–336. [DOI] [PubMed] [Google Scholar]
- [23].Roy B, Dunbar M, Shelton RC, et al. Identification of MicroRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016;42(4):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lopez JP, Lim R, Cruceanu C, et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med. 2014;20(7):764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ye L, Yang X, Zhao L, et al. Increased miR-132 level is associated with visual memory dysfunction in patients with depression. Neuropsychiatr Dis Treat. 2016;12:2905–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pradhan AK, Talukdar S, Bhoopathi P, et al. mda-7/IL-24 mediates cancer cell-specific death via regulation of miR-221 and the Beclin-1 axis. Cancer Res. 2017;77(4):949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Garofalo M, Quintavalle C, Romano G, et al. miR221/222 in cancer: their role in tumor progression and response to therapy. Curr Mol Med. 2012;12(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Su M, Wang J, Wang C, et al. MicroRNA-221 inhibits autophagy and promotes heart failure by modulating the p27/CDK2/mTOR axis. Cell Death Differ. 2015;22(6):986–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kuhn M, Höger N, Feige B, et al. Fear extinction as a model for synaptic plasticity in major depressive disorder. PLoS One. 2014;9(12):e115280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Boldrini M, Santiago AN, Hen R, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38(6):1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Okamoto H, Voleti B, Banasr M, et al. Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry. 2010;68(6):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10(4):345. [DOI] [PubMed] [Google Scholar]
- [33].Ge L, Liu L, Liu H, et al. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol. 2015;768:49–57. [DOI] [PubMed] [Google Scholar]
- [34].Młyniec K, Budziszewska B, Holst B, et al. GPR39 (Zinc Receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int J Neuropsychopharmacol. 2015;18(3):337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang W, Cao J, Liu X, et al. AMPK plays a dual role in regulation of CREB/BDNF pathway in mouse primary hippocampal cells. J Mol Neurosci. 2015;56(4):782–788. [DOI] [PubMed] [Google Scholar]
- [36].Motaghinejad M, Motevalian M, Fatima S, et al. The neuroprotective effect of curcumin against nicotine-induced neurotoxicity is mediated by CREB–BDNF signaling pathway. Neurochem Res. 2017;42(10):2921–2932. [DOI] [PubMed] [Google Scholar]
- [37].Groot JAN, Ten Bokum L, van den Oever HLA. Late presentation of Torsades de Pointes related to fluoxetine following a multiple drug overdose. J Intensive Care. 2018;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]


