ABSTRACT
To investigate the molecular mechanism of TGF-β1 in regulating chondrocyte proliferation through MSC-exosomes, an osteoarthritis (OA) rat model was established. Cartilage degradation was quantified by using OARSI score. TGF-β1 was used to stimulate MSCs. The expressions of miR-135b and Sp1 in MSCs, MSC-exosomes and C5.18 cells were detected. The cell viability of C5.18 cells was measured using MTT assay. We found that TGF-β1 stimulation enhanced miR-135b expression in MSC-exosomes, and MSC-exosomes derived miR-135b increased the cell viability of C5.18 cells. Moreover, miR-135b negatively regulated Sp1 expression. The cell viability of C5.18 cells in TGF-β1+ miR-135b inhibitor+si-control group was reduced, while the cell viability in TGF-β1+ miR-135b inhibitor+si-Sp1 group was enhanced. In rat experiments, OARSI score was decreased and the number of chondrocytes was increased in OA+TGF-β1+ MSC-exosome group, while the score and the number had an opposite trend in OA+TGF-β1+ MSC-miR135b inhibitor-exosome group. These results demonstrated that TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b, then promoted cartilage repair.
KEYWORDS: Osteoarthritis, chondrocyte, MSC-exosomes, miR-135b, Sp1
1. Introduction
Osteoarthritis (OA) is the most common form of chronic joint disease, and its basic pathological feature is the degeneration of articular cartilage [1]. The knee, as the body weight-bearing joint, is the predilection site of osteoarthritis. At present, the clinical treatment is only to alleviate the pain symptoms of patients with OA, and it cannot repair and regenerate damaged articular cartilage [2]. Therefore, exploring the molecular mechanism in promoting the repair of cartilage tissue has great significance for the treatment of OA.
Mesenchymal stem cells (MSCs), a type of multipotent cells, can exert the function of anti-inflammatory reaction, the regeneration and reparation of tissues by deriving exosomes. Studies have found that MSC-exosomes can promote cartilage repair via promoting the proliferation of chondrocytes and inducing apoptosis of chondrocytes in OA [2,3]. Transforming growth factor β1 (TGF-β1) has been showed an important role in cartilage repair of OA [4]. Wu et al. [5] reported that MSC-exosomes induced by TGF-β1 could can repair cartilage defects more effectively that MSC-exosomes alone. However, the mechanisms of TGF-β1 on cartilage repair in OA through MSC-exosomes have not been reported.
Specificity Protein 1 (Sp1) is one member of transcription factor family and can regulate cell apoptosis and proliferation [6,7]. Researches have shown a regulatory relationship between Sp1 and chondrocytes [8]. Watanabe et al [9] found that Sp1 inhibited the proliferation of chondrocytes via up-regulating the mouse collagen α1(XI) gene. Yang et al [10] showed that miR‐140 promoted chondrocyte proliferation by suppressing Sp1 expression. These studies indicated that inhibition of Sp1 could promote the proliferation of chondrocytes. Bioinformatics analysis showed that miR-135b could target the 3ʹUTR of Sp1. MiR-135b, an oncogene, can regulate the development of various tumors [11–13]. Xu et al [14] found that miR-135b was expressed in MSCs and it was involved in the osteogenic differentiation of MSCs. However, whether miR-135b is expressed in MSC-exosomes and participates in chondrogenesis is not yet known.
Therefore, in the current study, we tested miR-135b expression in MSC-exosomes and investigated the molecular mechanisms of TGF-β1 in regulating chondrocyte proliferation through MSC-exosomes, aiming to find new therapeutic targets for cartilage repair in OA.
2. Materials and methods
2.1. Ethics
All experimental protocols were approved by the Animal Research Committee of The 1st affiliated hospital of Anhui Medical University.
2.2. Cell culture
MSCs from male SD rats were purchased from Cyagen Biosciences (Guangzhou, China). Rat chondrocyte line (C5.18 cell) was purchased from Cellcook (Guangzhou, China). The 2 cell lines were cultured in DMEM, including 10% fetal bovine serum (FBS, Gibco-BRL, Grand Island, New York), 100 U/ml of penicillin and 100 μg/ml of streptomycin (Sigma-Aldrich, St. Louis, MO) at 37°C in a humidified atmosphere containing 5% CO2.
2.3. Isolation of MSC-exosomes
MSC-exosomes were purified from the culture medium as previously described [3]. The culture medium of MSCs was collected. According to the manufacturer’s instructions, exosome extraction kit (Geneseed, Guangzhou, China) was used to isolate MSC-exosomes. Western blot was used to assay the exosome-associated markers including CD63, CD9, CD81 and ALIX.
2.4. Cell treatment
Following overnight incubation, MSCs were stimulated with TGF-β1 (10 ng/ml) for 24 h [15]. Then, MSCs transfection was performed using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instruction. MiR-135b inhibitor was synthesized by Invitrogen (Shanghai, China). After 24 h transfection, MSC-exosomes were isolated by using the method described above. C5.18 cells were co-cultured with 10 μg/ml MSC-exosomes for 3 d.
2.5. Quantitative real time PCR (qRT-PCR)
Total RNA was isolated from cells or tissues using TRIzol reagent (Invitrogen) according to manufacturer’s instructions. RNA was reverse transcribed using the SuperScript First Strand Synthesis System for RT-PCR (Invitrogen). qRT-PCR reactions were performed by using an ABI Prism 5700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). ABI Prism 5700 SDS software (Applied Biosystems) was used for analyzing the data. The comparative method 2−ΔΔCt was used to calculate the relative expression of miR-6125, miR-4281, miR-8069, miR-6800 and miR-135b.
2.6. Western blot
Total proteins from tissues or cells were extracted using RIPA Lysis Buffer (Beyotime, Beijing, China) on the ice for 15 min and centrifuged at 12,000 rpm for 15 min. The concentrations of proteins were detected by BCA Protein Assay kit (Pierce Biotechnology). Western blot was performed according to standard procedures. Anti-CD63 (Santa Cruz, USA), Anti-CD9 (Santa Cruz), Anti-CD81 (Santa Cruz), anti-ALIX (Santa Cruz), anti-Sp1 (Cell Signaling Technology, USA) and anti-β-actin were used as the first primary antibody at 1:1000 dilutions. The corresponding horseradish peroxidase-conjugated secondary antibody (Santa Cruz) was incubated at room temperature for 1 h. Protein-antibody complexes were detected by the enhanced chemiluminescence system (ECL, Roche Molecular Biochemicals). The protein expression of Sp1 was normalized to β-actin levels.
2.7. MTT assay
The cell viability of C5.18 cells was measured using MTT assay. Briefly, cells were seeded into 96-well culture plate (1 × 105 cells/well). Next, 10 μl MTT solution was added into each well. After 4 h incubation at 37°C, 150 μl DMSO was added into each well and the culture plate was placed in a rocking bed for 10 min at a low speed oscillation. Finally, the optical density (OD) values were measured by a microplate reader at 490 nm.
2.8. Dual-luciferase reporter (DLR) assay
Sp1 3ʹ-UTR fragments with wild type (WT) or mutant (Mut) miR-135b binding sites were cloned by PCR. PCR products were inserted into the pmir-REPORT-Sp1-3ʹUTR plasmid (Invitrogen). Reporter plasmids and miR-135b mimic or inhibitor were co-transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, Firefly and Renilla luciferase activities were sequentially measured using the Dual-Glo™ Luciferase Assay system (Promega)
2.9. The OA model
Twenty-four male Sprague-Dawley (SD) rats (12 weeks old, 300–330 g) were purchased from Shanghai experimental animal research center. The OA model was established as descripted before [16]. Briefly, the medial collateral ligament and the medial meniscus were transected completely. Then, the meniscus at the narrowest point was cut without damaging the tibial surface. After that, the anterior cruciate ligament was transected. After the operation, all rats were received 0.05 mg/kg buprenorphine and 5 mg/kg gentamicin for pain relief. All rats were randomly divided into 4 groups: (1) OA+MSC-exosome group, n = 6, rats received articular cavity injection of 100 μl MSC-exosome, 1 × 1011 MSC-exosome particles/ml; (2) OA+TGF-β1+ MSC-exosome group, n = 6, rats received articular cavity injection of 100 μl TGF-β1-exosome, 1 × 1011 TGF-β1-exosome particles/ml; (3) OA+TGF-β1+ MSC-NC-exosome group, n = 6, rats received articular cavity injection of 100 μl TGF-β1-NC-exosome, 1 × 1011 TGF-β1-NC-exosome particles/ml; (4) OA+TGF-β1+ MSC-miR135b inhibitor-exosome group, n = 6, rats received articular cavity injection of 100 μl TGF-β1-miR135b inhibitor-exosome, 1 × 1011 TGF-β1-miR135b inhibitor-exosome particles/ml. Twelve weeks after surgery, rats were sacrificed and the knee samples were harvested to evaluate disease progression.
2.10. Osteoarthritis research society international (OARSI) score
The knee samples were harvested, and the tibiofemoral joints were removed. Then, the femoral condyles were fixed in 10% neutral-buffered formalin (containing 4% formaldehyde) for 24 h. After washed with water for 2 h, the fixed femoral condyles were decalcified in 10% EDTA for 21 d. Then, graded ethanol dehydration, dimethylbenzene vitrification, paraffin embedding and tissue sectioning (5 μm) were performed. Cartilage degradation was quantified by using OARSI score. The total score was 24. The higher the score, the more serious the destruction of articular cartilage.
2.11. Chondrocytes count
The process of slice preparation was same as above. The number of chondrocytes was detected under high magnification.
2.12. Statistical analysis
Data are presented as mean ± standard deviation (SD). Student’s t test or one-way analysis of variance (one-way ANOVA) were performed for normally distributed data. All statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, USA). Statistical significance was set as P < 0.05.
3. Results
3.1. MiRNAs expression in MSC-exosomes treated with TGF-β1
In this experiment, we aimed to investigate which miRNA expression could be changed in MSC-exosomes after the treatment of TGF-β1. We firstly isolated MSC-exosomes from MSCs. Transmission electron microscopy (TEM) and western blot were used to characterize the particles secreted from MSCs. These hollow spherical microvesicles are MSC-exosomes (Figure 1(a)). The expression of the exosome markers CD63, CD81 and CD9 (Figure 1(b)) were significantly enriched in exosomes. All these data indicate that exosomes were successfully isolated. Then, we isolated MSC-exosomes from MSCs that stimulated by 10 ng/ml TGF-β1 (TGF-β1-exosome), and tested the exosome-associated markers using western blot. Results showed that TGF-β1-exosomes expressed high levels of CD81 and ALIX (Figure 1(c)). Then, we detected the miRNAs expression in MSC-exosomes. Compared with control-exosome, there was no significant change in the expressions of miR-6125, miR-4281, miR-8069 and miR-6800, while miR-135b expression increased significantly in TGF-β1-exosome (Figure 1(d)).
Figure 1.
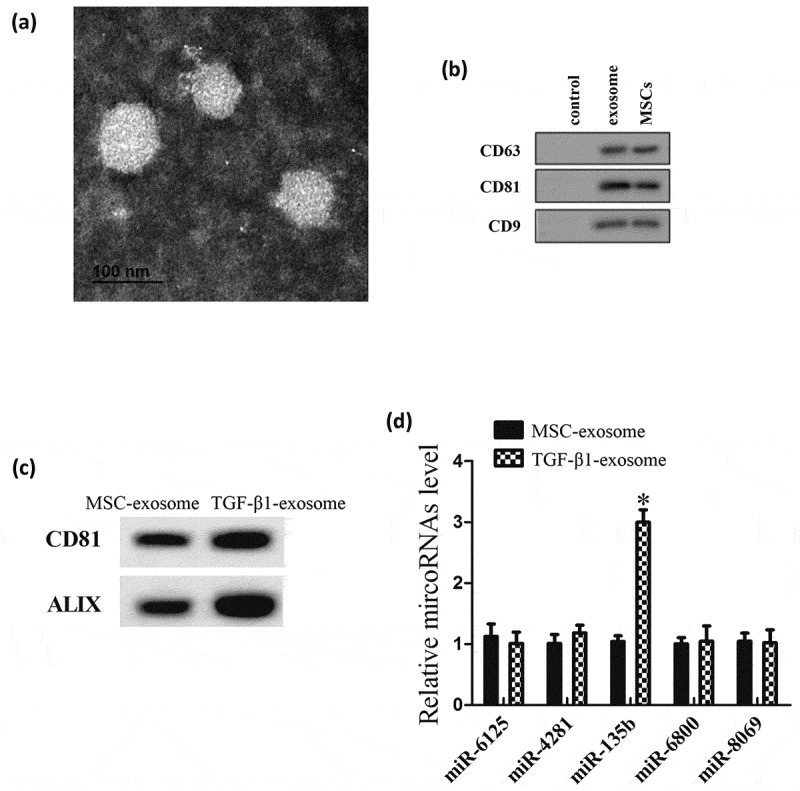
MiRNAs expression in MSC-exosomes treated with TGF-β1. MSC-exosomes were isolated from MSCs or MSCs that stimulated by 10 ng/ml TGF-β1 (TGF-β1-exosome). (a) Morphology of exosomes observed by transmission electron microscopy (TEM). Scale bar: 100 nm. (b) Exosome surface markers (CD63, CD81 and CD9) measured using western blot. Control group is culture medium. (c) The expression of the surface markers of MSC-exosomes. (d) MiRNAs expression in MSC-exosomes. *P < 0.05, vs control-exosomes.
3.2. MSC-exosomes derived miR-135b promoted chondrocyte proliferation
In this experiment, we explored the effect of MSC-exosomes derived miR-135b on chondrocyte proliferation. MSC-exosomes were isolated from MSCs that transfected with miR-135b mimic or pre-NC (a control of miR-135b mimic). C5.18 cells were co-cultured with pre-NC-exosome or miR-135b mimic-exosome for 3 d. Then, miR-135b expressions in MSC-exosomes and C5.18 cells were measured using qRT-PCR, and results showed that miR-135b expressions in miR-135b mimic-exosome and C5.18 cells that co-cultured with miR-135b mimic-exosome were increased (Figure 2(a)). Moreover, MTT assay showed that the cell viability of C5.18 cells that co-cultured with miR-135b mimic-exosome was higher than C5.18 cells that co-cultured with pre-NC-exosome (Figure 2(b)).
Figure 2.
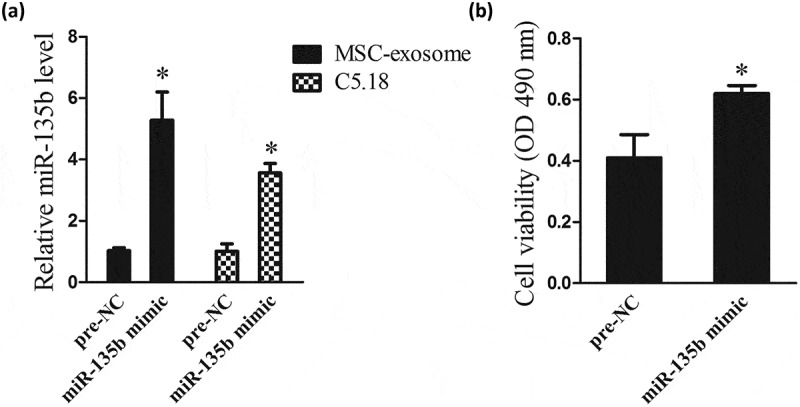
MSC-exosomes derived miR-135b promoted chondrocyte proliferation. MSC-exosomes were isolated from MSCs that transfected with miR-135b mimic or pre-NC. C5.18 cells were co-cultured with pre-NC-exosome or miR-135b mimic-exosome for 3 d. (a) MiR-135b expressions in MSC-exosomes and C5.18 cells. (b) The cell viability of C5.18 cells. Pre-NC was a control of miR-135b mimics. *P < 0.05, vs Pre-NC.
3.3. TGF-β1 promoted chondrocyte proliferation via stimulating MSCs to secrete exosomes containing miR-135b
In the part, we investigated how TGF-β1 regulated chondrocyte proliferation through MSC-exosomes. MSCs were divided into 4 groups, namely control group (MSCs), TGF-β1 group (MSCs were stimulated by 10 ng/ml TGF-β1), TGF-β1+ NC group (after stimulated by 10 ng/ml TGF-β1, MSCs were transfected with NC, a control of miR-135b inhibitor,), and TGF-β1+ miR-135b inhibitor group (after stimulated by 10 ng/ml TGF-β1, MSCs were transfected with miR-135b inhibitor). MSC-exosomes were isolated from the MSCs of the 4 groups. C5.18 cells were respectively co-cultured with the four groups of MSC-exosomes. As shown in Figure 3(a) left, TGF-β1 upregulated the level of miR-135b in MSC-exosomes, whereas miR-135b inhibitor countered the promotion effect. Meanwhile, miR-135b expression in C5.18 cells that co-cultured with TGF-β1-exosome was increased, but its expression was decreased in C5.18 cells that co-cultured with TGF-β1+ miR-135b inhibitor-exosome (Figure 3(a) right). Results of MTT assay showed that TGF-β1-exosome enhanced the cell viability of C5.18 cells, while the beneficial effects of TGF-β1 were abolished by miR-135b inhibitor (Figure 3(b)).
Figure 3.
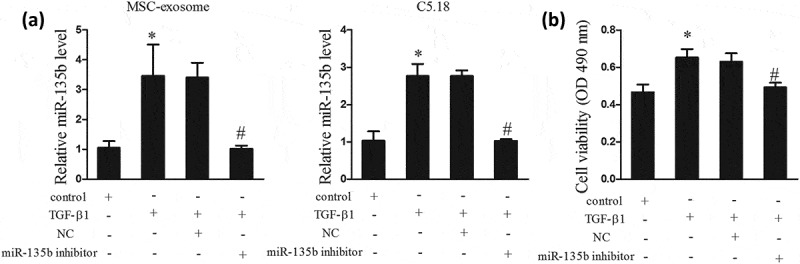
TGF-β1 promoted chondrocyte proliferation via stimulating MSCs to secrete exosomes containing miR-135b. MSCs were divided into 4 groups, namely control group (MSCs), TGF-β1 group (MSCs were stimulated by 10 ng/ml TGF-β1), TGF-β1+ NC group (after stimulated by 10 ng/ml TGF-β1, MSCs were transfected with NC, a control of miR-135b inhibitor), and TGF-β1+ miR-135b inhibitor group (after stimulated by 10 ng/ml TGF-β1, MSCs were transfected with miR-135b inhibitor). MSC-exosomes were isolated from the MSCs of the 4 groups. C5.18 cells were respectively co-cultured with the four groups of MSC-exosomes. (a) MiR-135b expressions in MSC-exosomes and C5.18 cells. (b) The cell viability of C5.18 cells. *P < 0.05, vs control; #P < 0.05, vs TGF-β1+ NC.
3.4. MiR-135b negatively regulated Sp1
Bioinformatics software prediction (microRNA.org and TargetScan) found that Sp1 3ʹUTR had a binding site with miR-135b (Figure 4(a)). Then, we tested the regulatory relationship between miR-135b and Sp1 using DLR assay. Results showed that the luciferase activity of Sp1 3ʹUTR-WT in HEK293 cells co-transfected with miR-135b mimics was significantly decreased, and its luciferase activity was significantly increased in HEK293 cells co-transfected with miR-135b inhibitor (Figure 4(b)). Moreover, in C5.18 cells, miR-135b mimics up-regulated Sp1 expression, while miR-135b inhibitor down-regulated Sp1 expression (Figure 4(c)).
Figure 4.
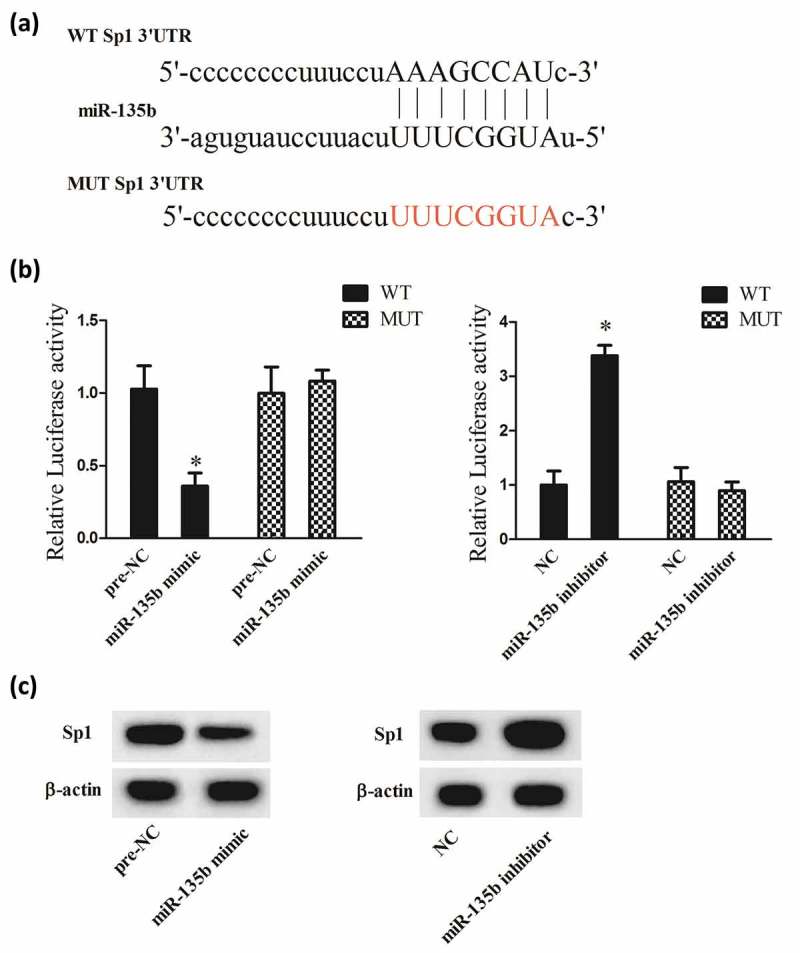
MiR-135b negatively regulated Sp1. (a) The binding site between miR-135b and Sp1 3ʹUTR. (b) The luciferase activity of Sp1 3ʹUTR in HEK293 cells co-transfected with miR-135b mimics or miR-135b inhibitor. (c) The protein expression of Sp1 in C5.18 cells. Pre-NC was a control of miR-135b mimics; NC was a control of miR-135b inhibitor. *P < 0.05, vs Pre-NC or NC.
3.5. TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b
To investigate whether TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b, MSCs were divided into TGF-β1+ NC group (treatment method was same as section 3.3), and TGF-β1+ miR-135b inhibitor group (treatment method was same as section 3.3). MSC-exosomes were isolated from the 2 groups. C5.18 cells were divided into TGF-β1+ NC+si-control group (after transfected with si-control, C5.18 cells were co-cultured with TGF-β1-exosome), TGF-β1+ miR-135b inhibitor+si-control group (after transfected with si-control, C5.18 cells were co-cultured with TGF-β1+ miR-135b inhibitor-exosome), TGF-β1+ NC+si-Sp1 group (after transfected with si-Sp1, C5.18 cells were co-cultured with TGF-β1-exosome), and TGF-β1+ miR-135b inhibitor+si-Sp1 group (after transfected with si-Sp1, C5.18 cells were co-cultured with TGF-β1+ miR-135b inhibitor-exosome). Western blot assay showed that Sp1 expression in C5.18 cells of TGF-β1+ miR-135b inhibitor+si-control group was increased, and its expression was decreased in C5.18 cells of TGF-β1+ miR-135b inhibitor+si-Sp1 group (Figure 5(a)). Moreover, the cell viability of C5.18 cells in TGF-β1+ miR-135b inhibitor+si-control group was reduced, while the cell viability in TGF-β1+ miR-135b inhibitor+si-Sp1 group was enhanced (Figure 5(b)).
Figure 5.
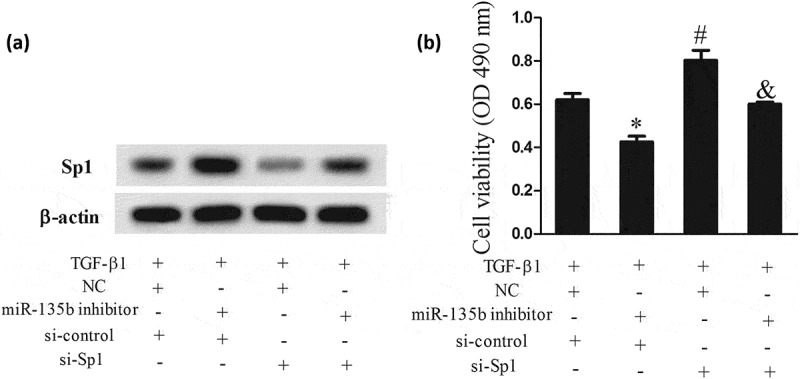
TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. MSCs were divided into TGF-β1+ NC group (treatment method was same as Figure 3), and TGF-β1+ miR-135b inhibitor group (treatment method was same as Figure 3). MSC-exosomes were isolated from the 2 groups. C5.18 cells were divided into TGF-β1+ NC+si-control group (after transfected with si-control, C5.18 cells were co-cultured with TGF-β1-exosome), TGF-β1+ miR-135b inhibitor+si-control group (after transfected with si-control, C5.18 cells were co-cultured with TGF-β1+ miR-135b inhibitor-exosome), TGF-β1+ NC+si-Sp1 group (after transfected with si-Sp1, C5.18 cells were co-cultured with TGF-β1-exosome), and TGF-β1+ miR-135b inhibitor+si-Sp1 group (after transfected with si-Sp1, C5.18 cells were co-cultured with TGF-β1+ miR-135b inhibitor-exosome). (a) Sp1 expression in C5.18 cells. (b) The cell viability of C5.18 cells. *P < 0.05 or #P < 0.05, vs TGF-β1+ NC+si-control; &P < 0.05, vs TGF-β1+ miR-135b inhibitor+si-control group.
3.6. TGF-β1 promoted cartilage repair in vivo
To verify the molecular mechanism of TGF-β1 in promoting cartilage repair in vivo, rats were divided into OA+MSC-exosome group, OA+TGF-β1+ MSC-exosome group, OA+TGF-β1+ MSC-NC-exosome group, and OA+TGF-β1+ MSC-miR135b inhibitor-exosome group. Results showed that the OARSI score in OA+TGF-β1+ MSC-exosome group was decreased, and the OARSI score in OA+TGF-β1+ MSC-miR135b inhibitor-exosome group was increased (Figure 6(a)). As expected, the number of chondrocytes in OA+TGF-β1+ MSC-exosome group was increased, and the number was decreased in OA+TGF-β1+ MSC-miR135b inhibitor-exosome group was increased (Figure 6(b)). In addition, as shown in Figure 6(c and d), miR-135b expression was elevated and Sp1 expression was reduced in cartilage of OA+TGF-β1+ MSC-exosome group, while their expressions had an opposite trend in cartilage of OA+TGF-β1+ MSC-miR135b inhibitor-exosome group.
Figure 6.
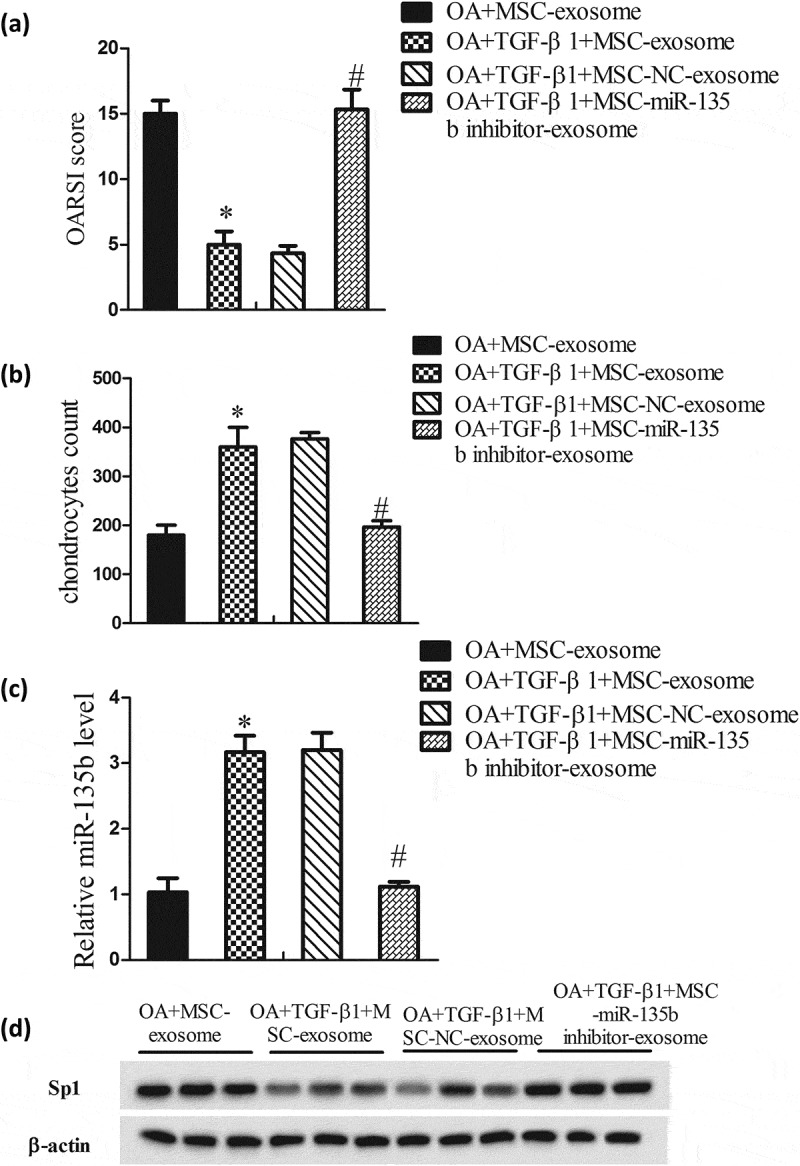
TGF-β1 promoted the reparation of cartilage tissues in vivo. Rats were divided into OA+MSC-exosome group (rats received articular cavity injection of MSC-exosome, 100 μl; 1 × 1011 MSC-exosome particles/ml; n = 6), OA+TGF-β1+ MSC-exosome group (rats received articular cavity injection of TGF-β1-exosome, 100 μl; 1 × 1011 TGF-β1-exosome particles/ml; n = 6), OA+TGF-β1+ MSC-NC-exosome group (rats received articular cavity injection of TGF-β1-NC-exosome, 100 μl; 1 × 1011 TGF-β1-NC-exosome particles/ml; n = 6), and OA+TGF-β1+ MSC-miR135b inhibitor-exosome group (rats received articular cavity injection of TGF-β1-miR135b inhibitor-exosome, 100 μl; 1 × 1011 TGF-β1-miR135b inhibitor-exosome particles/ml; n = 6). Twelve weeks after surgery, rats were sacrificed and the knee samples were harvested to evaluate disease progression. (A) OARSI score. (B) Chondrocytes count. (C) MiR-135b expressions in cartilage. (D) Sp1 expression in cartilage. *P < 0.05, vs OA+MSC-exosome; #P < 0.05, vs OA+TGF-β1+ MSC-exosome.
4. Discussions
OA is a degenerative disease that can be caused by multiple reasons, and it seriously affects the lives of patients. Cartilage degradation is the basic clinicopathology of OA [17]. However, there is no specific treatment for inhibiting cartilage degradation at present in clinic. Therefore, it is meaningful to reveal the underlying mechanisms of cartilage repair.
Recently, MSC-based therapy has been considered as a novel therapy for the treatment of tissue repair. MSC transplantation could protect cartilage from degeneration in OA [18–20]. Besides that, growing evidence suggests that MSCs mediate tissue repair through its secretion [2,21]. Among the factors exist in the MSC secretion, MSC-exosome was first identified and reported as the main active component. Several studies demonstrated that MSC-exosomes participated in the regulation of cartilage repair. For example, Zhang et al. [3] revealed that MSC-exosomes could promote cartilage repair and regeneration by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Moreover, studies reported that MSC-exosomes stimulated by TGF-β1 had could promote cartilage repair better [5], while the regulation mechanism have not been understood.
In the current study, we found that TGF-β1 stimulation could up-regulate miR-135b expression in MSC-exosomes, and the cell viability of C5.18 cells that co-cultured with miR-135b overexpression-MSC-exosomes was increased, which indicated that TGF-β1 might promoted chondrocyte proliferation by up-regulating miR-135b expression in MSC-exosomes. To verify this hypothesis, we co-cultured C5.18 cells with TGF-β1-exosomes and found that TGF-β1 promoted chondrocyte proliferation via stimulating MSCs to secrete exosomes containing miR-135b. However, this promotion is performed by which molecules in the chondrocytes?
In general, miRNAs regulate cell proliferation, apoptosis and differentiation by binding the miRNAs regulatory elements located in 3ʹ-UTR of target mRNAs [22]. Bioinformatics software analysis revealed that Sp1 might be a target gene of miR-135b. Sp1 was regarded as an inhibitor of chondrocyte proliferation [9,10], but its upstream mechanism has not been investigated deeply. In the present study, we found that miR-135b could down-regulated Sp1 expression in chondrocyte. Moreover, further study revealed that Sp1 expression in C5.18 cells that co-cultured with TGF-β1-exosomes was decreased and the cell viability of the cells was enhanced, while si-Sp1 changed the effects, which indicated that TGF-β1 could promote chondrocyte proliferation by down-regulating Sp1 through MSC-exosomes derived miR-135b. In vivo, we used TGF-β1-exosomes in an OA rat model. The cartilage degradation caused by OA was reduced by TGF-β1-exosomes, while TGF-β1-miR135b inhibitor-exosomes had an opposite effect.
In conclusion, TGF-β1 could promote chondrocyte proliferation by down-regulating Sp1 through MSC-exosomes derived miR-135b, and then promoted cartilage repair in OA. This might provide a new direction for the treatment of OA.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Loeser RF, Goldring SR, Scanzello CR, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Toh WS, Lai RC, Hui JHP, et al. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64. [DOI] [PubMed] [Google Scholar]
- [3].Zhang S, Chuah SJ, Lai RC, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. [DOI] [PubMed] [Google Scholar]
- [4].Ying B, Chen K, Hu J, et al. Effect of different doses of transforming growth factor-beta(1) on cartilage and subchondral bone in osteoarthritic temporomandibular joints. Br J Oral Maxillofac Surg. 2013;51(3):241–246. [DOI] [PubMed] [Google Scholar]
- [5].Wu G, Cui Y, Ma L, et al. Repairing cartilage defects with bone marrow mesenchymal stem cells induced by CDMP and TGF-beta1. Cell Tissue Bank. 2014;15(1):51–57. [DOI] [PubMed] [Google Scholar]
- [6].Wang J, Kang M, Qin YT, et al. Sp1 is over-expressed in nasopharyngeal cancer and is a poor prognostic indicator for patients receiving radiotherapy. Int J Clin Exp Pathol. 2015;8(6):6936–6943. [PMC free article] [PubMed] [Google Scholar]
- [7].Luo J, Wang X, Xia Z, et al. Transcriptional factor specificity protein 1 (SP1) promotes the proliferation of glioma cells by up-regulating midkine (MDK). Mol Biol Cell. 2015;26(3):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dharmavaram RM, Liu G, Mowers SD, et al. Detection and characterization of Sp1 binding activity in human chondrocytes and its alterations during chondrocyte dedifferentiation. J Biol Chem. 1997;272(43):26918–26925. [DOI] [PubMed] [Google Scholar]
- [9].Watanabe K, Hida M, Sasaki T, et al. Sp1 upregulates the proximal promoter activity of the mouse collagen alpha1(XI) gene (Col11a1) in chondrocytes. In Vitro Cell Dev Biol Anim. 2016;52(2):235–242. [DOI] [PubMed] [Google Scholar]
- [10].Yang J, Qin S, Yi C, et al. MiR-140 is co-expressed with Wwp2-C transcript and activated by Sox9 to target Sp1 in maintaining the chondrocyte proliferation. FEBS Lett. 2011;585(19):2992–2997. [DOI] [PubMed] [Google Scholar]
- [11].Wu W, Wang Z, Yang P, et al. MicroRNA-135b regulates metastasis suppressor 1 expression and promotes migration and invasion in colorectal cancer. Mol Cell Biochem. 2014;388(1–2):249–259. [DOI] [PubMed] [Google Scholar]
- [12].Hua K, Jin J, Zhao J, et al. miR-135b, upregulated in breast cancer, promotes cell growth and disrupts the cell cycle by regulating LATS2. Int J Oncol. 2016;48(5):1997–2006. [DOI] [PubMed] [Google Scholar]
- [13].Xu Y, Zhao S, Cui M, et al. Down-regulation of microRNA-135b inhibited growth of cervical cancer cells by targeting FOXO1. Int J Clin Exp Pathol. 2015;8(9):10294–10304. [PMC free article] [PubMed] [Google Scholar]
- [14].Xu S, Cecilia Santini G, De Veirman K, et al. Upregulation of miR-135b is involved in the impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients. PLoS One. 2013;8(11):e79752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lerrer S, Liubomirski Y, Bott A, et al. Co-inflammatory roles of TGFbeta1 in the presence of TNFalpha drive a pro-inflammatory fate in mesenchymal stem cells. Front Immunol. 2017;8:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tao SC, Yuan T, Zhang YL, et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Francin PJ, Abot A, Guillaume C, et al. Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):519–526. [DOI] [PubMed] [Google Scholar]
- [18].Lim CT, Ren X, Afizah MH, et al. Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model. Tissue Eng Part A. 2013;19(15–16):1852–1861. [DOI] [PubMed] [Google Scholar]
- [19].Ham O, Lee CY, Kim R, et al. Therapeutic potential of differentiated mesenchymal stem cells for treatment of osteoarthritis. Int J Mol Sci. 2015;16(7):14961–14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Canadas RF, Pirraco RP, Oliveira JM, et al. Stem cells for osteochondral regeneration. Adv Exp Med Biol. 2018;1059:219–240. [DOI] [PubMed] [Google Scholar]
- [21].Cosenza S, Ruiz M, Toupet K, et al. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]


