ABSTRACT
Lung cancer is one of the most common cancers and the leading cause of cancer-related death worldwide. Despite encouraging results achieved with targeted therapy in recent years, the early diagnosis and treatment of lung cancer remains a major problem. Circular RNA (circRNA), a type of RNA with covalently closed continuous loop structures, has structural stability and certain tissue specificity. Recent studies have found that circRNAs have an important role in tumor development and are expected to be revealed as new targets for tumor prediction and treatment. Research on the biological functions and regulation mechanisms of circRNAs in lung cancer is in its infancy but is gathering momentum. In this review, we discuss the properties, biogenesis, biological function, and research progress of circRNAs in lung cancer to provide a theoretical foundation and new directions for studies on circRNAs in lung cancer.
KEYWORDS: Lung cancer, circular RNA, microRNA sponge, back-splicing, therapeutic target, biogenesis, biomarkers
Lung cancer is one of the most common cancers and is the leading cause of cancer-related deaths globally.1 The 5-year survival rate of lung cancer is approximately 15%, mainly as a result of late-stage diagnosis and the limitations of surgery, radiotherapy and chemotherapy2 In recent years, targeted therapy has hugely improved. Although drugs targeting proteins encoded by driver genes such as EGFR, KRAS, and ALK, along with immune checkpoints such as PD-1, PD-L1, and CTLA-4, have reignited hopes for patients with advanced lung cancer, the ensuing drug-resistant problems mean that new therapeutic approaches are required. In-depth study of the biological mechanisms involved, along with the discovery of new therapeutic targets, are the subject of current lung cancer research. Circular RNAs (circRNAs) are a novel class of non-coding RNAs (ncRNAs), representing a new paradigm of gene regulation in many biological processes. Unlike linear RNAs, circRNAs form covalently closed continuous loop structures without terminal 5ʹ caps and 3ʹ polyadenylated tails. This structure makes it a more stable and conserved molecule that is often expressed in a tissue- and cancer-specific manner. Therefore, the potential of circular RNA as a novel tumor marker and therapeutic target is limitless.
1. History of circrnas
As early as the 1970s, Sanger et al. initially identified circular RNAs in RNA viruses such as plant viruses.3 Based on their structural specificity, unknown function, and low abundance, circRNAs were originally considered as experimental interference products in the formation of erroneous splicing of exon transcripts.4 However, with advances in RNA high-throughput sequencing and other biotechnology, increasing numbers of studies have confirmed that circRNAs are not splicing byproducts but biological molecules that can be stably expressed in a wide range of biological cells in humans, mice, fruit flies, and nematodes.5,6 CircRNAs are highly conserved and specific molecules that are widely expressed in human tissue including the nervous system, circulatory system, digestive system, urinary system, lung, breast, and skin.7 Several studies revealed that circRNAs exert a regulatory function in different diseases via different mechanisms, triggering a research boom in circRNAs.
2. Characteristics of circrnas
Reflecting their unique covalently closed continuous loop structures, circRNAs have several noteworthy properties. First, circRNAs are widely expressed. A total of 5.8% to 23% of actively transcribed human genes reportedly produce circRNAs.8,9.Second, the lack of exposed 3ʹ and 5ʹ terminals makes circRNAs more stable and less susceptible to degradation by ribonuclease R (RNase R) or other exonucleases. Third, the majority of circRNAs are evolutionarily conserved. The expression of many circRNAs is not only conserved across mammals, but is even conserved in evolutionarily distant Drosophila.10,11 Fourth, most circRNAs are located in the cytoplasm, while circRNAs from introns are abundant in the nucleus.12 Fifth, circRNAs are often expressed in a tissue- and cancer-specific manner. As potential tumor markers, circRNAs have high sensitivity and specificity.13 Chen et al. reported that the sensitivity and specificity of hsa_circ_0000190 is much better than that of CEA and CA19-9 for the diagnosis of gastric cancer.14 Last, while circRNAs were originally regarded as a novel class of ncRNAs, recent studies revealed that several circRNAs can produce functional proteins.7,12
3. Biogenesis of circrnas
Early studies found that most circRNAs are derived from the exons of protein-coding genes. With further research, it has been found that introns, non-coding regions, and antisense regions can also participate in their formation.5,15 CircRNAs are generated by spliceosome-mediated precursor mRNA (pre-mRNA) back-splicing, which connects an upstream 3ʹ splice site to a downstream 5ʹ splice site.7,12 Similar to canonical splicing, back-splicing also appears to be regulated by canonical cis-acting splicing regulatory elements and trans-acting splicing factors. However, the same combinations of splicing regulatory elements and factors may have distinct or even opposing activity during the regulatory process of back-splicing.16 Moreover, through alternative back-splice site selection, a single locus can produce a wide variety of circRNAs.17 According to their origin from different genomic regions and difference in RNA sequences, circRNAs can be divided into four categories: exonic circRNAs (ecircRNAs), circular intronic RNAs (ciRNAs), retained-intron or exon-intron circRNAs (EIciRNAs), and intergenic circRNAs.5,7,12 Although the exact mechanism of circRNA production remains unclear, researchers proposed three models of exon circRNA formation (Figure 1) including lariat-driven circularization, intron-pair-driven circularization, and resplicing-driven circularization.7,12,19 Their formation is regulated by many factors. Flanking long introns containing reverse complementary sequences such as the Alu sequence may promote intron pairing and exon circularization.10,20 RNA binding proteins (RBPs) also play an important role in the regulation of circRNA formation.8 QKI and MBL/MBNL1 proteins can promote circularization by combining with the specific sites of preRNAs,8,21 while the RNA splicing enzyme ADAR binds to RNA and inhibits the formation of circRNAs by the action of adenosine-to-inosine (A-to-I) editing.22 CiRNA biogenesis, which occurs via a lariat-derived mechanism, depends mainly on a 7-nt GU-rich element near the 5ʹ splice site and an 11-nt C-rich element near the branch point site.15,20 In addition, John et al. found that precursor tRNAs can be cleaved into loops to form tricRNAs.23 Furthermore, sequence analyses have shown a weak but significant enrichment of conserved nucleotides between few ciRNAs and intergenic circRNAs.5 However, there is currently very little information on the overall characteristics and biogenesis processes of intergenic circRNAs.
Figure 1.
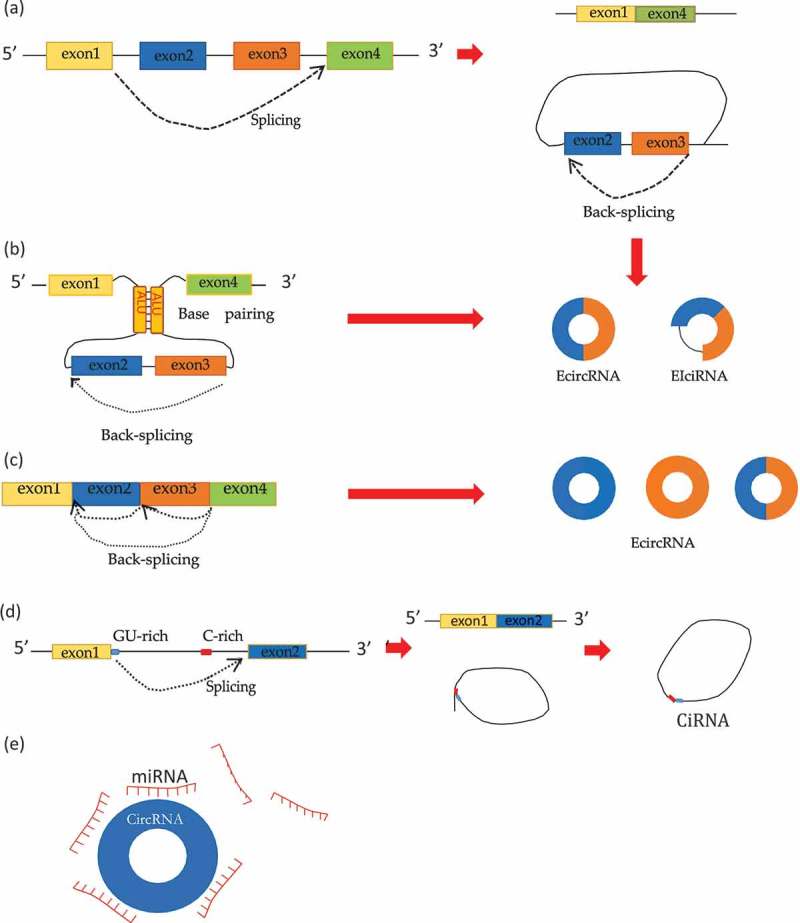
(a)Lariat- driven circularization (exon skipping):Following canonical splicing, exons in exon-containing lariats undergo back-splicing and circularization, which results in the formation of ecircRNA or EIciRNA molecules. (b) Intron pairing-driven circularization: Flanking long introns containing reverse complementary sequences such as the Alu sequence may promote intron pairing and exon circularization. (c) Resplicing-driven circularization:mature mRNA exons may form EcircRNAs by back-splicing and circularization (d) Following canonical splicing, introns may form lariats to escape the usual intron debranching and degradation. Then ciRNAs are formed. (e) CircRNAs function as miRNA sponges. This figure is adapted from Wang et al.18.
4. Circrna function
4.1. Mirna sponge
miRNA is a type of lncRNA that has an important role in the post-transcriptional regulation of gene expression, binding to specific sites of mRNA to prevent its translation or promote its degradation.24 A number of circRNAs contain miRNA response elements (MREs), which acts as miRNA sponges through competitive binding to miRNAs, thereby weakening the role of miRNAs in the regulation of mRNAs. CDR1as/ciRS-7 was the first circRNA to be demonstrated to have an miRNA sponge function, which can significantly inhibit the activity of miR-7. Further studies revealed that ciRS-7 contains more than 70 selective binding sites for miR-7, and its ability to bind to miR-7 is 10 times higher than other known transcripts.5,25 In addition, ciRS-7 can also combine with miR-671 and induce its self-degradation to release miR-7.26 Zheng et al. found that circHIPK3 has multiple binding sites for nine miRNAs, suggesting that circRNAs can act as sponges for various miRNAs.27 The miRNA sponge effect of circRNAs is not limited to humans. Researchers found that Sry circRNA has 16 miR-138 binding sites in mouse testes, acting as a sponge to regulate its expression.25 Meanwhile, circRNA can act as a competitive endogenous RNA (ceRNA) to regulate miRNA levels, thereby affecting upstream and downstream gene networks, providing a new direction for the treatment of human diseases but especially tumors.28
4.2. Interactions with rbps
Albrecht et al. first reported that circRNAs can bind to RBPs such as IMP3.29 Several circRNAs can bind, store, and even insulate RBPs from specific subcellular sites.30 CircRNAs can also act as competitive elements to influence the function of RBPs. Recent studies have also found that circRNAs may be assembled as scaffolds for larger protein complexes.25,31
4.3. Regulation of gene expression
Unlike ecircRNAs, ciRNAs and EIciRNAs are mostly located in the nucleus and tend to function at the transcriptional level. EIciRNAs, such as circEIF3J, upregulate their parental gene expression in cis by interacting with U1 nuclear ribonucleoprotein (U1 snRNP) and RNA polymerase II upstream of transcription start sites.32 Furthermore, Ashwal-Fluss et al. found that circRNAs such as circMbl can act on gene expression in trans by competing with linear splicing.21 During circMbl formation, back-splicing can compete with the classical linear splicing of MBL pre-mRNA, thus affecting the formation of linear RNA. Additionally, during the formation of ecircRNAs, some ecircRNAs may sequester translation start sites, leading to the production of non-coding linear transcripts and thereby reducing protein expression.31
4.4. Translation
Although circRNAs have long been considered as a type of ncRNA, researchers have not stopped exploring their translational capacity. Initial studies found that synthetic ecircRNAs containing internal ribosome entry sites or prokaryotic binding sites have protein-coding abilities in vitro and in vivo, leading to the question of whether these translational products exist endogenously.16,33 Recent studies have found that a series of circRNAs in Drosophila have a function in translation. N6-methyladenosine (m6A) modifications are enriched in many circRNAs, while m6A recognition protein YTHDF3 can bind to the modification sites of certain circRNAs and recruit the translation initiation factors eIF4G2 and eIF3A to start translation of circRNAs in a cap-independent manner.34 Legnini et al. indicated that circZNF609 can be translated into proteins in a splicing- and cap-independent manner.35 In addition, the association of many circRNAs with polysomes has been determined.34 These emerging evidences indicate that circRNAs may also function as protein-coding RNAs, thus further studies may ultimately reveal a series of uncharacterized proteins and clarify the processes in which they are involved.
5. Circrnas in lung cancer
CircRNAs play an important role in the development of human diseases, and their potential in diagnosis and treatment is encouraging. Despite the discovery of thousands of circRNAs in lung cancer tissues and cell lines through second-generation sequencing technology, many circRNAs are found to be abnormally expressed in lung cancer, but the study of their specific functional mechanisms in the development of lung cancer has just begun (Table 1). Wan et al. found that the expression of cir-ITCH was decreased in lung cancer cell lines.36 Further mechanistic studies showed that cir-ITCH can act as an miR-7 and miR-214 sponge in lung cancer, thus enhancing the expression of its parental tumor suppressor gene ITCH and blocking activation of the Wnt/β-catenin signaling pathway, eventually inhibiting the proliferation of lung cancer cells. Interestingly, miR-7 and miR-214, in turn, can induce cir-ITCH degradation. Hansen et al. found that CDR1as can have a tumor suppressing role by sponging a large amount of miR-7, though miR-617 can bind with CDR1as to induce its cleavage and release miR-7.26 MA et al. proposed that circMAN2B2 promotes the expression of FOXK1 through sponging miR-1275, which in turn promotes cell proliferation and invasion in a cancer-promoting role.37 Liu et al. found that circZEB1.5, circ-ZEB1.19, circ-ZEB1.17, and circ-ZEB1.33 were downregulated in lung cancer tissues.38 These circRNAs may act as miR-200 sponges, which has been reported to target ZEB1 and to promote cancer initiation, and then regulate ZEB1 gene expression in lung adenocarcinoma. Tian et al. illustrated that circHIPK3 could promote cell proliferation through miR-379-regulated insulin-like growth factor (IGF1) expression in NSCLC cell lines NCI-H1299 and NCI-H2170.39 In addition, another of their studies found that cinnamaldehyde interfered with NSCLC through the hsa_circ_0043256/miR-1252/ITCH axis.40 Wang et al. found that hsa_circ_0012673 acts as a miR-22 sponge and regulates the expression of erb-b2 receptor tyrosine kinase 3 (ErbB3), thereby promoting the proliferation of adenocarcinoma cells.41 Jiang et al. highlighted that hsa_circ_0007385 is related to cell proliferation, invasion, and metastasis, and may be related to miR-181.42 Yin et al. found that circUBAP2 plays an important role in the proliferation and invasion of lung cancer in vitro, and its specific mechanism may be related to miR-339-5p, miR-96-3p, and miR-135b-3p.43
Table 1.
CircRNAs and lung cancer.
| CircRNAs | Expression | Function | Refs |
|---|---|---|---|
| cir-ITCH | Down | miR-7, miR-214 sponge, inhibiting Wnt/β- catenin signaling |
35 |
| CDR1as | Up | miR-7 sponge | 25 |
| circMAN2B2 | Up | miR-1275 sponge | 36 |
| circZEB1.5 circ-ZEB1.19 circ-ZEB1.17 circ-ZEB1.33 |
Down | miR-200 sponge | 37 |
| circHIPK3 | Highest expression in NCI-H2170 cells and lowest in NCI-H1299 cells |
miR-379 sponge | 38 |
| hsa_circ_0012673 | Up | miR-22 sponge | 40 |
| hsa_circ_0007385 | Up | - | 41 |
| circUBAP2 | Up | - | 42 |
| circRNA-100876 | Up | - | 44 |
| hsa_circ_0013958 hsa_circ_0000064 hsa_circ_0014130 circPRKCI |
Up Up Up Up |
MiR-134 sponge - - miR-545,miR-589 sponge |
45 46 47 48 |
Researchers have also explored the diagnostic significance of circRNAs. CircRNAs may be stably expressed and present in relatively high quantities in human blood, saliva and exosomes.44 Combined with its tissue and tumor specificity, circRNAs are expected to become novel tumor markers for lung cancer. Yao et al. revealed that circRNA-100876 is highly expressed in NSCLC tissues, and is closely related to tumor stage and lymph node metastasis.45 Patients with high circRNA-100876 expression have significantly shorter overall survival. This study suggested that circRNA-100876 may regulate MMP-13 expression through sponge function, thus affecting tumor proliferation and metastasis. Zhu et al. indicated that hsa_circ_0013958 is significantly upregulated in lung adenocarcinoma and acts as a miR-134 sponge to upregulate the oncogene CCND1 (cyclin D1), thereby promoting lung adenocarcinoma cell proliferation, invasion, and inhibition of cell apoptosis.46 Meanwhile, the level of hsa_circ_0013958 is closely related to TNM stage and lymphatic metastasis. Luo et al. demonstrated that hsa_circ_0000064 is upregulated in lung cancer and can promote cell proliferation and metastasis.47 Hence, its abnormal expression is significantly associated with lymph node metastasis and TNM staging of lung cancer. Zhang et al. found that the expression of hsa_circ_0014130 is also significantly associated with lung cancer staging and lymph node metastasis.48
In addition, studies also found that mutations of genes that produce circRNAs may be associated with the occurrence of lung cancer, suggesting that circRNAs could have a role in the genetics of lung cancer. Qiu et al. found that amplicon 3q26.2, which is susceptible to gene mutation, can produce circPRKCI, and thus promote cell proliferation and migration through the circPRKCI-miR-545/589-gene E2F7 axis in a tumor-promoting function.49 At the same time, circPRKCI is associated with TNM staging and prognosis of lung cancer.
6. Circrna databases
The enthusiasm for circRNA research has contributed to the development of circRNA databases to help researchers conduct more extensive and in-depth studies (Table 2). CircBase collects and integrates circRNA information from six species including humans, mice, and fruit flies, and has gene annotations for researchers to browse and download for free.50 CircNet provides information on tissue-specific circRNA expression profiles and circRNA–miRNA–gene regulatory networks.38 CircIntercome can help researchers search for miRNA and RBP binding sites on circRNAs, and design circRNAs detection primers and siRNAs for circRNAs silencing.51 Circ2Traits classifies circRNAs by different diseases and provides putative miRNA-circRNA-mRNA interaction networks. In addition, it can also locate disease-associated SNPs on circRNAs locus.52 StarBase v2.0 integrates published circRNA data to construct miRNA–circRNA and cicrRNA–RBP interaction networks.53 DeepBase v2.0 builds a comprehensive transcript profile of circRNAs.54 Moreover, the emergence of databases such as circRNADb55 and CIRCexplorer56 provides researchers with more options. Although these databases have their own advantages, there are still several problems including little overlap in prediction and no clear gold standard. Therefore, an important step for using these bioinformatics approaches is to adjust the search strategy to elevate the confidence with an appropriate threshold.
Table 2.
CircRNAs Databases.
| Databases | Characteristics | URL | Refs |
|---|---|---|---|
| circBase circNet circIntercome circ2Traits starBase v2.0 deepBase v2.0 circRNADb CIRCexplorer |
The merged and unified data sets of circRNAs Tissue-specific circRNAs expression profiles and circRNA-miRNA-gene regulatory networks Predicting and mapping RBP and miRNA binding sites on reported circRNAs A disease-circRNA association database providing putative miRNA-circRNA-mRNA interaction networks Providing miRNA-circRNA, cicrRNA-RBP interaction networks Providing a comprehensive transcript profile of circRNAs Human circRNAs with protein-coding annotations circRNAs annotations |
http://www.circbase.org/ http://circnet.mbc.nctu.edu.tw/ https://circinteractome.nia.nih.gov/ http://gyanxet-beta.com/circdb/ http://starbase.sysu.edu.cn/ http://deepbase.sysu.edu.cn/ http://reprod.njmu.edu.cn/circrnadb http://yanglab.github.io/CIRCexplorer/ |
49 37 50 51 52 53 54 55 |
7. Conclusions and perspectives
In recent years, although the progress of targeted therapy and immunotherapy has given new hope for lung cancer patients, the ensuing problems of drug resistance require new approaches for early diagnosis and treatment of lung cancer. The recent revelation of the widespread existence of circRNAs casts an attractive light on the research and therapy of lung cancer. Mechanistic and functional studies have transformed circRNAs from useless splice byproducts into potential targets for tumor diagnosis and treatment. Although current knowledge of the biological function and mechanism of circRNAs remains limited, continuous exploration may bring oncologists closer to tumor cures. Current studies have found that circRNAs may have diagnostic significance in TNM stages and metastasis of lung cancer. Their role as miRNA sponges in the development of lung cancer provides a new direction for the treatment of lung cancer. However, there are still many gaps and problems in the study of circRNAs in lung cancer. First, the high cost of second-generation sequencing technology limits the sample size of studies, making the results inconclusive or unconvincing. Meanwhile, there is a lack of comparative studies on circRNAs in the different types of lung cancer and in precancerous lesions of lung cancer. Second, the research is mostly limited to the miRNA sponge function of circRNAs, and there are few studies on other functions of circRNA such as protein coding. Third, the interaction of circRNAs with miRNAs and RBPs in lung cancer is very important for the establishment of a complete regulatory network and requires further study. Fourth, although circRNAs can be detected in human blood, saliva, and exosomes, it remains unclear as to whether they can be detected in the sputum of lung cancer patients. Also, current studies often use lung cancer tissues or cell lines as research specimens, and there is lack of research on blood or exosomes. Diagnostic experiments also need to contrast or combine with common tumor markers such as CEA and NSE, etc. Fifth, the occurrence and development of lung cancer are closely related to immune regulation, and few circRNAs studies have been performed in the field of lung cancer immunity. Sixth, lung cancer is a disease with a genetic predisposition, but there are limited studies concerning the occurrence of circRNAs producing gene mutations associated with lung cancer. Finally, little is known about the clearance of circRNAs. Lasda et al.57 found that cells may be able to clear circRNAs by releasing vesicles, but other pathways are not yet clear. Although the features of circRNAs and their functional mechanisms make them potentially useful for targeted therapy, the study of circRNAs in lung cancer has just begun. The number of circRNAs studied to date is minimal, and the functional mechanisms are unclear. Their use in lung cancer treatment through translational medicine requires far more study.
In future, with the maturation of the second-generation sequencing technology and the reduction in cost, along with the emergence of new detection technologies and the perfection of the circRNA database, researchers are expected to develop circRNAs as a clinically available lung cancer marker through large-scale multiple control studies. The regulatory network of circRNAs–miRNAs–mRNAs will also be improved by incorporating more factors. CircRNA drugs such as artificial sponges will be applied to lung cancer treatment through translational medicine. In conclusion, circRNA has great potential in the diagnosis, prediction, and treatment of lung cancer. It is believed that through the continuous efforts of researchers, circRNA will become another powerful tool to overcome lung cancer.
Funding Statement
This work was supported by the Liaoning Fundamental Research Funds for Universities (No. LZDK201702).
Acknowledgments
We thank H. Nikki March, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D.. Global cancer statistics. CA: a cancer. J Clinician. 2011;61:.69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther. 2016;9:.1023–1028. doi: 10.2147/OTT.S100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–3856. http://www.ncbi.nlm.nih.gov/pubmed/1069269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J: Official Publication of the Federation of American Soc for Exp Biol. 1993;7:155–160. http://www.ncbi.nlm.nih.gov/pubmed/7678559. [DOI] [PubMed] [Google Scholar]
- 5.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, SD Mackowiak, LH Gregersen, Munschauer M, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 6.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PloS one. 2012;7:.e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han B, Chao J, Circular YH. RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018; doi: 10.1016/j.pharmthera.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:.1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Kelly S, Greenman C, Cook PR, Papantonis A. Exon Skipping Is Correlated with Exon Circularization. J Mol Biol. 2015;427:.2414–2417. doi: 10.1016/j.jmb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. Rna. 2013;19:.141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rybak-Wolf A, Stottmeister C, Glazar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function. Biochim Biophys Acta. 2016;1859:.163–168. doi: 10.1016/j.bbagrm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Xu Z, Yan Y, Zeng S, Dai S, Chen X, Wei J, Gong Z. Circular RNAs: clinical relevance in cancer. Oncotarget. 2018;9:.1444–1460. doi: 10.18632/oncotarget.22846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:.167–171. doi: 10.1016/j.cca.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang X-O, Chen T, Xiang J-F, Yin Q-F, Xing Y-H, Zhu S, Yang L, Chen -L-L. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:.792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. Rna. 2015;21:.172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X-O, Dong R, Zhang Y, Zhang J-L, Luo Z, Zhang J, Chen -L-L, Yang L. Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26:.1277–1287. doi: 10.1101/gr.202895.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Nazarali AJ, Ji S. Circular RNAs as potential biomarkers for cancer diagnosis and therapy. Am J Cancer Res. 2016;6:1167–1176. http://www.ncbi.nlm.nih.gov/pubmed/27429839. [PMC free article] [PubMed] [Google Scholar]
- 19.Kameyama T, Suzuki H, Mayeda A. Re-splicing of mature mRNA in cancer cells promotes activation of distant weak alternative splice sites. Nucleic Acids Res. 2012;40:.7896–7906. doi: 10.1093/nar/gks520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:.134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:.55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:.170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Noto JJ, Schmidt CA, Matera AG. Engineering and expressing circular RNAs via tRNA splicing. RNA Biology. 2017;14:.978–984. doi: 10.1080/15476286.2017.1317911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beermann J, Piccoli MT, Viereck J, Non-Coding TT. RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96:.1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 25.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:.384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 26.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. Embo J. 2011;30:.4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:.11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:.344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider T, Hung L-H, Schreiner S, Starke S, Eckhof H, Rossbach O, Reich S, Medenbach J, Bindereif A. CircRNA-protein complexes: IMP3 protein component defines subfamily of circRNPs. Sci Rep. 2016;6:.31313. doi: 10.1038/srep31313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du WW, Zhang C, Yang W, Yong T, Awan FM, Yang BB. Identifying and Characterizing circRNA-Protein Interaction. Theranostics. 2017;7:.4183–4191. doi: 10.7150/thno.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:.453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 33.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. http://www.ncbi.nlm.nih.gov/pubmed/7536344. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, LL Chen, Wang Y, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A., Santini T., Andronache A., Wade M, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37 e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan L, Zhang L, Fan K, Cheng ZX, Sun QC, Wang JJ. Circular RNA-ITCH Suppresses Lung Cancer Proliferation via Inhibiting the Wnt/beta-Catenin Pathway. Biomed Res Int. 2016;2016:.1579490. doi: 10.1155/2016/1579490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma X, Yang X, Bao W, Li S, Liang S, Sun Y, Zhao Y, Wang J, Zhao C. Circular RNA circMAN2B2 facilitates lung cancer cell proliferation and invasion via miR-1275/FOXK1 axis. Biochem Biophys Res Commun. 2018;498:.1009–1015. doi: 10.1016/j.bbrc.2018.03.105. [DOI] [PubMed] [Google Scholar]
- 38.Liu YC, Li JR, Sun CH, Andrews E, Chao RF, Lin FM, SL Weng, SD Hsu, CC Huang, Cheng C, et al. CircNet: a database of circular RNAs derived from transcriptome sequencing data. Nucleic Acids Res. 2016;44:D209–15. doi: 10.1093/nar/gkv940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian F, Wang Y, Xiao Z, Zhu X. Circular RNA CircHIPK3 Promotes NCI-H1299 and NCI-H2170 Cell Proliferation through miR-379 and its Target IGF1]. Zhongguo Fei Ai Za Zhi. 2017;20:.459–467. doi: 10.3779/j.issn.1009-3419.2017.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian F, Yu CT, Ye WD, Wang Q. Cinnamaldehyde induces cell apoptosis mediated by a novel circular RNA hsa_circ_0043256 in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;493:.1260–1266. doi: 10.1016/j.bbrc.2017.09.136. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Zhu X, Zhang H, Wei S, Chen Y, Chen Y, Wang F, Fan X, Han S, Wu G. Increased circular RNA hsa_circ_0012673 acts as a sponge of miR-22 to promote lung adenocarcinoma proliferation. Biochem Biophys Res Commun. 2018;496:.1069–1075. doi: 10.1016/j.bbrc.2018.01.126. [DOI] [PubMed] [Google Scholar]
- 42.Jiang MM, Mai ZT, Wan SZ, Chi YM, Zhang X, Sun BH, BH Sun, QG Di. Microarray profiles reveal that circular RNA hsa_circ_0007385 functions as an oncogene in non-small cell lung cancer tumorigenesis. J Cancer Res Clin Oncol. 2018;144:667–674. doi: 10.1007/s00432-017-2576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin Y, Gao H, Guo J, Gao Y. [Effect of Circular RNA UBAP2 silencing on proliferation and invasion of human lung cancer A549 cells and its mechanism]. Zhongguo Fei Ai Za Zhi. 2017;20:.800–807. doi: 10.3779/j.issn.1009-3419.2017.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolha L, Ravnik-Glavac M, Circular GD. RNAs: biogenesis, function, and a role as possible cancer biomarkers. Int J Genomics. 2017;2017:.6218353. doi: 10.1155/2017/6218353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao JT, Zhao SH, Liu QP, Lv MQ, Zhou DX, Liao ZJ, KJ Nan. Over-expression of CircRNA_100876 in non-small cell lung cancer and its prognostic value. Pathol Res Pract. 2017;213:453–456. doi: 10.1016/j.prp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Zhu X, Wang X, Wei S, Chen Y, Chen Y, Fan X, Han S, Wu G. hsa_circ_0013958: a circular RNA and potential novel biomarker for lung adenocarcinoma. FEBS J. 2017;284:.2170–2182. doi: 10.1111/febs.14132. [DOI] [PubMed] [Google Scholar]
- 47.Luo Y-H, Zhu X-Z, Huang K-W, Zhang Q, Fan Y-X, Yan P-W, Wen J. Emerging roles of circular RNA hsa_circ_0000064 in the proliferation and metastasis of lung cancer. Biomed Pharmacother. 2017;96:.892–898. doi: 10.1016/j.biopha.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Zeng X, Ding T, Guo L, Li Y, Ou S, Yuan H. Microarray profile of circular RNAs identifies hsa_circ_0014130 as a new circular RNA biomarker in non-small cell lung cancer. Sci Rep. 2018;8:.2878. doi: 10.1038/s41598-018-21300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu M, Xia W, Chen R, Wang S, Xu Y, Ma Z, Xu W, Wang J, Fang T, Hu J, et al. The circular RNA circPRKCI promotes tumor growth in lung adenocarcinoma. Cancer Res. 2018; doi: 10.1158/0008-5472.CAN-17-2808. [DOI] [PubMed] [Google Scholar]
- 50.Glazar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. Rna. 2014;20:.1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, CircInteractome: GM. A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biology. 2016;13:.34–42. doi: 10.1080/15476286.2015.1128065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosal S, Das S, Sen R, Basak P, Chakrabarti J. Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits. Front Genet. 2013;4:.283. doi: 10.3389/fgene.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:.D92–7. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng -L-L, Li J-H, Wu J, Sun W-J, Liu S, Wang Z-L, Zhou H, Yang J-H, Qu L-H. deepBase v2.0: identification, expression, evolution and function of small RNAs, LncRNAs and circular RNAs from deep-sequencing data. Nucleic Acids Res. 2016;44:.D196–202. doi: 10.1093/nar/gkv1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Han P, Zhou T, Guo X, Song X, Li Y. circRNADb: A comprehensive database for human circular RNAs with protein-coding annotations. Sci Rep. 2016;6:.34985. doi: 10.1038/srep34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galasso M, Costantino G, Pasquali L, Minotti L, Baldassari F, Corra F, Agnoletto C, Volinia S. Profiling of the predicted circular RNAs in ductal in situ and invasive breast cancer: a pilot study. Int J Genomics. 2016;2016:4503840. doi: 10.1155/2016/4503840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lasda E, Circular PR. RNAs co-precipitate with extracellular vesicles: a possible mechanism for circRNA clearance. PloS one. 2016;11:.e0148407. doi: 10.1371/journal.pone.0148407. [DOI] [PMC free article] [PubMed] [Google Scholar]


