ABSTRACT
In this study, we aimed to reveal the role of miR-191 in apoptosis of renal tubular epithelial cells and in the involvement of renal ischemia-reperfusion injury. Renal transplantation rat model was established. miR-191 and Cystathionine-β-synthase (CBS) were measured by qRT-PCR and Western blot. The regulation of miR-191 on CBS was detected by luciferase reporter assay. We found miR-191 expression in platelets and platelet microvesicles (P-MVs) of patients and model rats was significantly upregulated than that of health and normal rats. Also, mRNA and protein levels of CBS in renal tissues of patients were significantly downregulated than that of health and normal rats. We also found that P-MVs could transfer miR-191 to HK-2 cells. Luciferase reporter assay showed that CBS was a direct target of miR-191. In addition, we proved that P-MVs-secreted miR-191 inhibited CBS expression in HK-2 cells, and P-MVs-secreted miR-191 promoted HK-2 cell apoptosis via CBS. Finally, we verified the trends of CBS expressions, HK-2 cell apoptosis and apoptosis-related proteins in vivo were similar as the trends in vitro. Therefore, CBS was a direct target of miR-191, and miR-191 could transfer to HK-2 cells via P-MVs to decrease the expression of CBS, thus to promote cell apoptosis and renal IR injury.
KEYWORDS: miR-191, Cystathionine-β-synthase, renal transplantation, renal ischemia-reperfusion injury
Introduction
Renal ischemia-reperfusion (IR) injury is a common cause for delayed function of renal transplantation, which is responsible for 20–30% primary graft dysfunction during renal transplantation [1]. There are two main features of IR injury at the renal level, namely apoptosis of tubular cells and interstitial inflammation [2]. H2S plays a protective role as a signaling molecule and antioxidant in kidney tubular epithelial cells that could resist IR-induced lipid peroxidation, reduce cell damage in the kidney tissue, and improve renal function [3]. Recently, researchers found that H2S accelerated kidney recovery that injured by IR through accelerating the regeneration of tubular epithelial cells [4]. In addition, it has been reported that IR decreased the activity of Cystathionine-β-synthase (CBS), which reduced the production of hydrogen sulfide (H2S) [4]. Therefore, CBS may play an important role in renal IR injury.
microRNAs are small non-coding RNA that regulate cell apoptosis, proliferation, and differentiation, which can play roles in many diseases through various pathways [5]. Studies have described that miRNAs are aberrantly expressed in renal IR injury. For example, miR-21 could play a protective effect in renal delayed ischemic preconditioning on vascular endothelial cells [6]. miR-34a can protect myocardial cells against IR injury through regulating the expression of TNFα [7]. In 2017, Ichii [8] et al found that in dogs with renal dysfunction, miR-191 expresssion was remarkably increased with renal dysfunction. Pan et al [9] reported that miR-191 was highly expressed in platelets. Moreover, bioinformatics software predicted that CBS might be a direct target of miR-191. Therefore, we speculated that miR-191 may priticipate in tubular epithelial cell apoptosis via CBS.
How could platelet-secreted miR-191 affect tubular epithelial cell apoptosis? Platelets, as major sources of microvesicles (MVs), can release active MVs, which are small vesicles that can interact with other cells and influence the function of the target cells [10]. It has been reported that platelet MVs (P-MVs) can optionally interact with target cells [9]. Also, MVs can efficiently deliver miRNAs into target cells, which affect the function of recipient cell [11]. Thus, we assumed platelet-secreted miR-191 transferred into tubular epithelial cell, and combined with CBS which caused apoptosis of tubular epithelial cell.
Materials and methods
Blood and renal tissue collection
Venous blood from healthy volunteers (n = 30) and patients with renal allograft rejection (n = 30) were collected according to protocols approved by the ethics committee of Zhengzhou University. Blood samples were centrifuged at 150 g for 15 min at room temperature to obtain platelet-rich plasma. A biopsy was used to obtain renal tissues.
Establishment of allograft renal transplantation rat model
For allograft renal transplantation rat model, male Sprague Dawley (SD) rats (7-week-old, 250‑300 g) were purchased from the Animal Experimental Center of Zhengzhou University, and kept in homeothermal room under a cycle of 12 h of light and 12 h of darkness with free access to food and water. SD rats were divided into two groups, namely sham group (n = 6) and recipient group (n = 6). There were donor rats, and each recipient rat received a left kidney from a donor rat. Donor rats were intraperitoneally injected with 0.01 mg/kg of atropine (Sinopharm Chemical Regent Co., China), 0.04 mg/kg of buprenorphine (Rongbai biological technology co., China) and 10 mg/kg of diazepam (Sinopharm Chemical Regent Co., China). Fifteen min later, 45 mg/kg pentobarbital was used for anesthetization. In donor rats, the blood vessels and ureter were seperated, and the left kidneys were flushed for homogeneously pale using cold Ringer’s lactate solution (3 ml, Henan Provincial People’s hospital) supplemented with heparin (50 U/ml). The harvested kidneys were kept in cold Ringer’s lactate solution for 3 h at 4°C. Before accepting the donated kidneys, left nephrectomy was performed. Then, end-to-end anastomosis of renal artery, vein and ureter was used for orthotopically implantation of donor kidney into the recipient rat. In the sham group, the abdomens of rats were opened without transplantation, the rest procedures were the same as recipient group. Body temperature of the rats was kept between 35°C and 37°C during surgery.
For renal autotransplantation rat model, the harvested kidney was from itself. The rest procedures were just the similar to allograft renal transplantation.
Preservation of blood and kidneys
Blood of sham and recipient rats was collected 24 h after kidney transplantation. Renal tissues were washed with cold PBS with heparin to remove blood cells and clot, and placed in polypropylene tubes and stored at −80°C for molecular analysis.
Platelet isolation
Washing buffer (10 mM HEPES, 136 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 25 mM glucose, 4.2 mM EDTA, 4.2 mM trisodium citrate and 1 mM prostaglandin E1, pH 6.6) was used to dilute the platelet-rich plasma, then the platelet suspension was centrifuged at 750 g for 15 min at 20°C and the pellet was re-suspended in washing buffer without prostaglandin E1 for an additional centrifugation at 750 g for 15 min at 20°C. Suspension buffer (10 mM HEPES, 136 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 25 mM glucose) was used to obtain the platelets.
Cell culture and transfection
Renal tubular epithelial cell line (HK-2 cells) was purchased from Shanghai Cell Bank of Chinese Academy of Sciences, and cultured in DMEM supplemented with 10% fetal bovine serum (Gibco, Invitrogen), 1.2% penicillin-G/streptomycin and 2 mM L-glutamine at 37°C in 5% CO2 incubator. HK-2 cells were seeded in a 6-well plate overnight, and were transfected with miR-191 inhibitor, negative control, si-control and si-CBS using Lipofectamine 2000 (Invitrogen, USA).
Microvesicle isolation and incubation with renal tubular epithelial cells
Platelet microvesicle-riched plasma was collected after centrifuged at 2000 g for 15 min at 4°C, then centrifuged at 100,000 g for 1 h at 4°C. P-MVs were collected from the pellet and resuspended in fetal bovine serum-free RPMI 1640 medium. For incubation of P-MVs with HK-2 cells, HK-2 cells were seeded on 12-well dishes last night, and each well was added with 200 μg P-MVs. Twenty-four hour later, HK-2 cells were collected for the following experiments.
Quantitative real-time PCR (qRT-PCR)
Total RNA from platelet, P-MVs, HK-2 cells and renal tissues was extracted using Trizol reagent (Invitrogen, USA) and reverse transcribed to cDNA by TaqMan miRNA Reverse Transcription Kit (Applied Biosystems, USA) according to manufacturer’s instructions. The expressions of miR-191 and CBS were determined by mirVANA qRT-PCR miRNA Detection Kit (Ambion, USA), and all reactions were performed on ABI Prism 5700 SDS software (Applied Biosystems, USA). The relative expression of miR-191 and CBS gene were normalized to that of the internal control β-Actin by comparative method 2−ΔΔCt.
Western blot analysis
The proteins from HK-2 cells and renal tissues were extracted using RIPA buffer (Beyotime, China), and the concentrations of each sample were determined by BCA Protein Assay kit (Pierce Biotechnology, USA). Protein samples were isolated on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Invitrogen, USA) which were blocked in 5% skim milk for 2 h. Anti-CBS (Invitrogen), anti-cleaved caspase 3 (Merck Millipore), anti-BAX (Invitrogen), anti-Bcl2 (Invitrogen) and anti-β-Actin were used as the first primary antibody at 1:1000 dilutions. Then, cultivalted with secondary antibody (corresponding horseradish peroxidase-conjugated) at room temperature for 1 h. Protein-antibody complexes were detected by ECL System (GE Healthcare, UK).
TUNEL assay
Renal tissues of rats were collected to perform TUNEL staining. After rehydration, slides were treated with proteinase K (20 mg/ml) for 10 min at room temperature, washed with PBS, and re-fixed in 4% paraformaldehyde. Nucleo-tide Mix and rTdT enzyme (Dead End fluorometric Tunnel system, Promega) was used to label the fragmented DNA of apoptotic cells for 60 min at 37°C. Deionized water was used to wash the slides, which then covered with glass coverslips and analyzed under a fluorescence microscope. The percent of apoptotic cells was calculated as the ratio of TUNNEL-positive cells/all cells.
Luciferase reporter assay
Luciferase reporter assay was performed to test the direct binding of miR-191 to the target gene CBS. HEK-293 cells were seeded in 24-well plates at a density of 3.5 × 104 cells per well. CBS 3’UTR sequence was sub-cloned into pGL3 vector and resulted in CBS 3’-UTR construct. Then, the reporter plasmid (CBS 3’-UTR construct) and microRNAs (miR-191 inhibitor, negative control, pre-miR-191, or pre-miR-control) were cotransfected into HEK-293 cells using Lipofectamine 2000 (Invitrogen) for 48 h. Luciferase activity of CBS 3’UTR was detected by dual Luciferase detection system (Promega).
Statistical analysis
All experiments were performed in triplicate. SPSS 17.0 software was used for data analysis. Significance between groups was tested using t test, with P < 0.05 considered statistically significant.
Results
miR-191 and CBS expressions in platelets, P-MVs and renal tissues of patients with renal allograft rejection
To figure out whether miR-191 and CBS were abnormally expressed in patients with renal allograft rejection, blood samples and renal tissues from healthy volunteers and patients with renal allograft rejection were collected. As shown in Figure 1(a), miR-191 expression in platelets and P-MVs of patients was significantly upregulated than that of healthy volunteers. Also, mRNA and protein levels of CBS in renal tissues of patients were significantly downregulated than that of healthy volunteers (Figure 1(b)).
Figure 1.
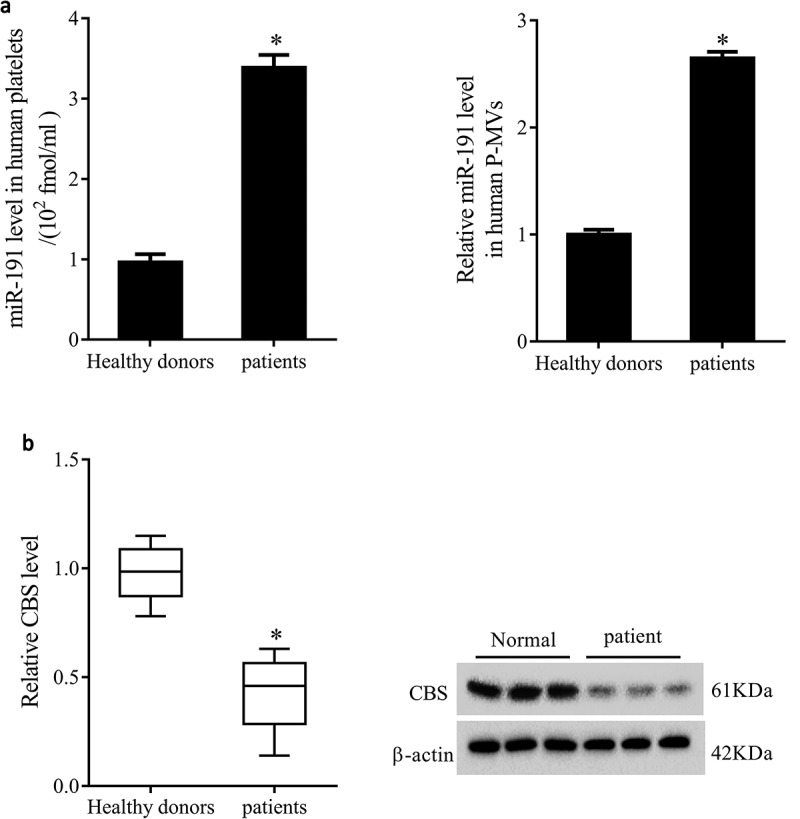
miR-191 and CBS expressions in platelets, P-MVs and renal tissues of patients with renal allograft rejection. Blood samples and renal tissures were collected from healthy volunteers (n = 30) and patients with renal allograft rejection (n = 30). (a) miR-191 expression in platelets and P-MVs of healthy volunteers and patients. (b) mRNA and protein levels of CBS in renal tissues of healthy volunteers and patients. *P < 0.05, compared with healthy donors.
miR-191 and CBS expressions in platelets, P-MVs and renal tissues of allograft renal transplantation rats
In order to make it easier to understand the changes of miR-191 and CBS expressions in health and illness, we established allograft renal transplantation rat model. We found miR-191 expression in platelets and P-MVs of allograft renal transplantation rats was significantly upregulated than that of sham rats (Figure 2(a)). mRNA and protein levels of CBS in renal tissues of allograft renal transplantation rats were significantly downregulated than that of sham rats (Figure 2(b)).
Figure 2.
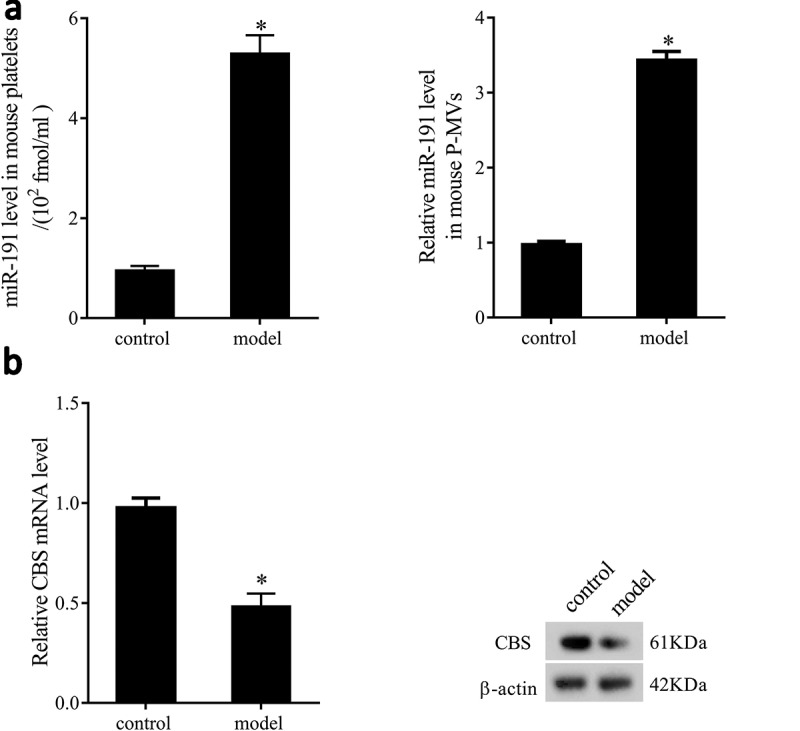
miR-191 and CBS expressions in platelets, P-MVs and renal tissues of allograft renal transplantation rats. (a) miR-191 expression in platelets and P-MVs of sham and allograft renal transplantation rats. (b) mRNA and protein levels of CBS in renal tissues of sham and allograft renal transplantation rats. *P < 0.05, compared with control group.
Patient P-MVs increased miR-191 expression of human tubular epithelial cell
To investigate the effect of P-MVs on miR-191 expression in HK-2 cells, P-MVs (healthy or patient) were co-cultured with HK-2 cells. After HK-2 cells co-cultured with healthy P-MVs, miR-191 expression was significantly higher than that of HK-2 cells without co-culture. And miR-191 expression in patient P-MVs group was significantly higher than that of healthy P-MVs group (Figure 3(a)). As shown in Figure 3(b), P-MVs isolated from patients were collected and transfected with negative control or miR-191 inhibitor. miR-191 expression was significantly upregulated after co-cultured with P-MVs, while miR-191 inhibitor reversed this effect. pre-miR-191 expression was slightly downregulated after co-cultured with P-MVs, and miR-191 inhibitor slightly upregulated pre-miR-191 expression.
Figure 3.
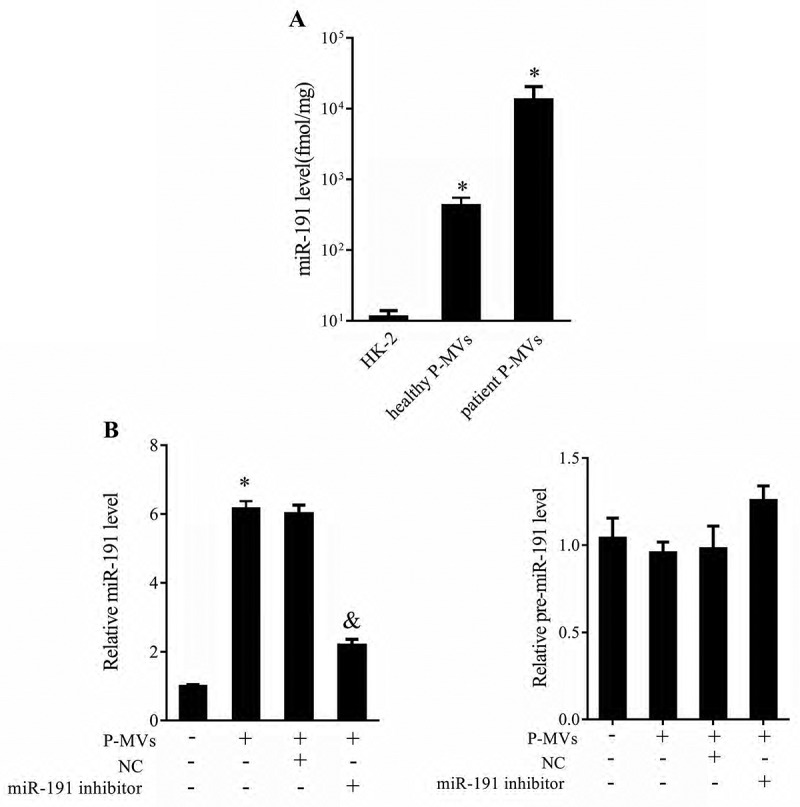
Patient P-MVs increased miR-191 expression of human tubular epithelial cell. A. P-MVs and tubular epithelial cell HK-2 were co-cultured and divided into three groups: HK-2, healthy P-MVs+HK-2, and patient P-MVs+HK-2. miR-191 expression was measured by qRT-PCR. B. P-MVs isolated from patients with renal allograft rejection were collected and transfected with negative control or miR-191 inhibitor. miR-191 and pre-miR-191 expressions measured by qRT-PCR. *P < 0.05, compared with HK goup or negative control group. &P < 0.05, compared with P-MVs+negative control group.
CBS was a direct target of miR-191
To confirm whether CBS was a direct target of miR-191, luciferase reporter gene was conducted. Online bioinformatics software (TargetScan and microrna.org) predicted that there were combination sites between miR-191 and 3’UTR of CBS (Figure 4(a)). Compared to HEK-293 transfected with pre-miR-NC, transfection of HEK-293 with pre-miR-191 remarkably increased miR-191 levels (Figure 4(b)). Moreover, the activity of CBS-WT 3’UTR was significantly decreased by pre-miR-191. There was no significant difference in the activity of CBS-MUT 3’UTR between pre-miR-control and pre-miR-191 groups. pre-miR-191 decreased mRNA and protein levels of CBS (Figure 4(c)). We also found miR-191 inhibitor significantly increased mRNA and protein levels of CBS (Figure 4(d)). These results suggested that miR-191 negatively regulated CBS.
Figure 4.
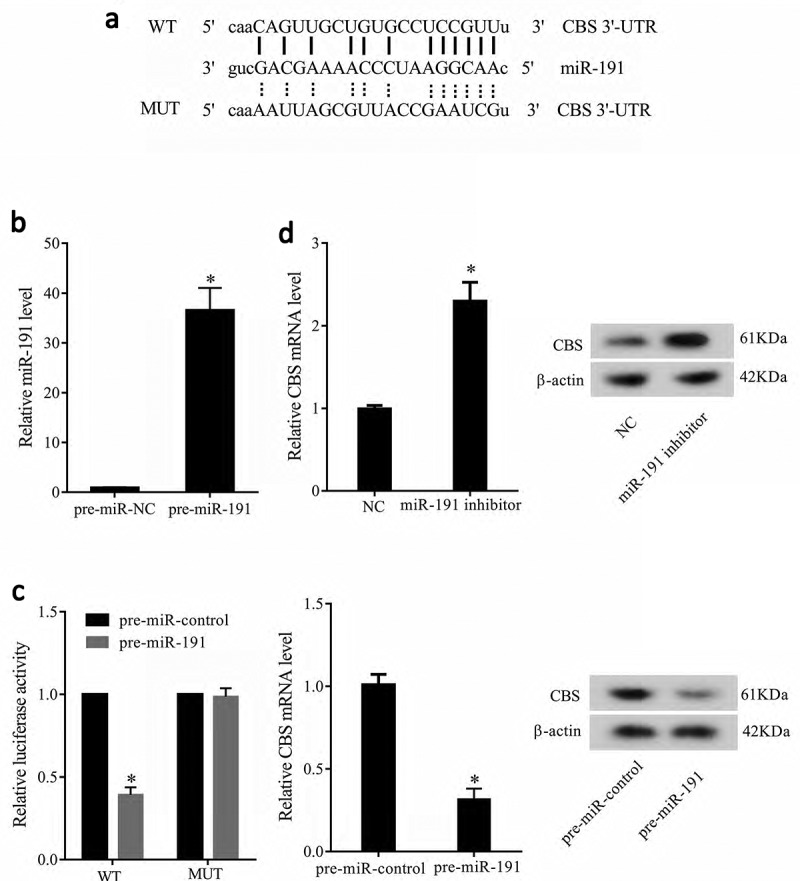
CBS was a direct target of miR-191. (a) Online bioinformatics software TargetScan and microrna.org predicted that there were combination sites between miR-191 and 3’UTR of CBS. (b) Compared to HEK-293 transfected with pre-miR-NC, transfection of HEK-293 with pre-miR-191 remarkably increased miR-191 levels. (c) The activity of CBS-WT 3’UTR was significantly decreased by pre-miR-191. There was no significant difference in the activity of CBS-MUT 3’UTR between pre-miR-control and pre-miR-191 groups. pre-miR-191 decreased mRNA and protein levels of CBS. (d) miR-191 inhibitor significantly increased mRNA and protein levels of CBS. *P < 0.05, compared with pre-miR-negative control group or pre-miR control group.
P-MVs-secreted miR-191 inhibited CBS expression
In order to understand the role of P-MVs-secreted miR-191 on CBS expression, HK-2 cells were transfected with miR-191 inhibitor after co-cultured with P-MVs. As shown in Figure 5, mRNA and protein levels of CBS were significantly downregulated after co-cultured with P-MVs of patients, while miR-191 inhibitor reversed these effects, indicating P-MVs-secreted miR-191 inhibited CBS expression.
Figure 5.
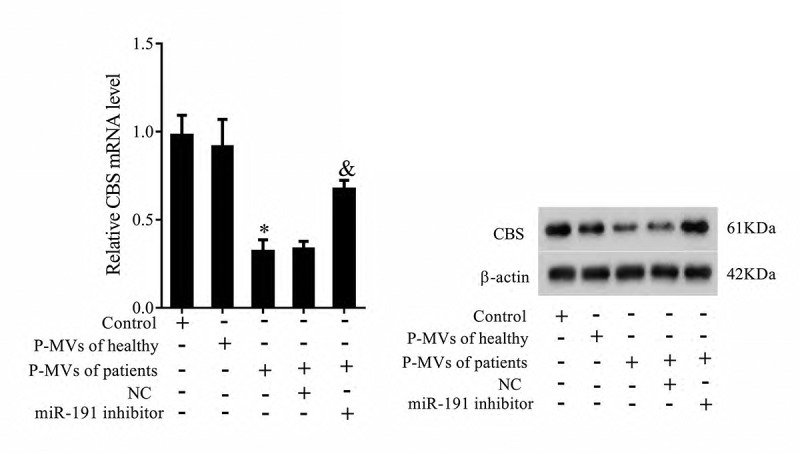
P-MVs-secreted miR-191 inhibited CBS expression. P-MVs isolated from patients with renal allograft rejection were co-cultured with HK-2 cells for 24 h, and divided into five groups: Control (HK-2), P-MVs of healthy, P-MVs of patients, P-MVs of patients+NC, and P-MVs of patients+miR-191 Inhibitor. mRNA and protein levels of CBS were detected by qRT-PCR and Western blotting. *P < 0.05, compared with control group; &P < 0.05, compared with P-MVs of patients+negative control group.
P-MVs-secreted miR-191 affected tubular epithelial cell apoptosis via CBS
To explore whether P-MVs-secreted miR-191 affected apoptosis of HK-2 cells via CBS, HK-2 cells were transfected with si-CBS. As shown in Figure 6(a), HK-2 cell apoptosis was significantly increased after co-cultured with P-MVs of patients, miR-191 inhibitor significantly decreased cell apoptosis, and si-CBS reversed the effect of miR-191 inhibitor on cell apoptosis. These results proved our assumption. In addition, apoptosis related proteins of Bax, Bcl-2, Caspase 3 were measured by Western blotting (Figure 6(b)), which further proved P-MVs-secreted miR-191 affected apoptosis of HK-2 cells via CBS.
Figure 6.
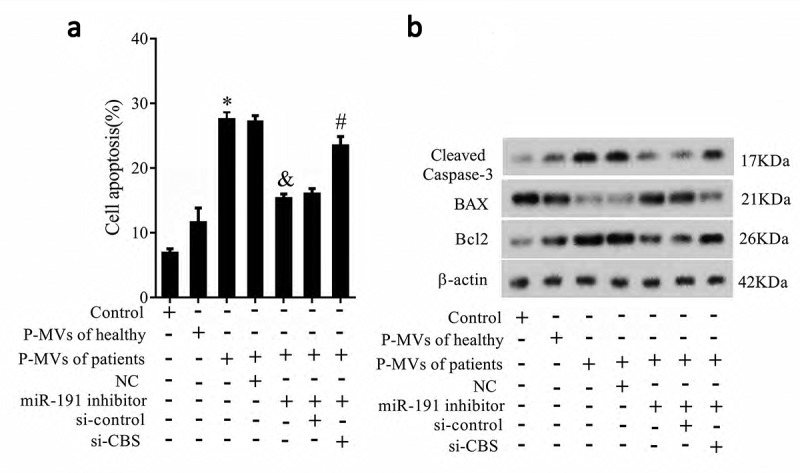
P-MVs-secreted miR-191 affected tubular epithelial cell apoptosis via CBS. P-MVs isolated from patients with renal allograft rejection were divided into seven groups: control, P-MVs of healthy, P-MVs of patients, P-MVs of patients+NC, P-MVs of patients+miR-191 Inhibitor, P-MVs of patients+miR-191 inhibitor+si-control, P-MVs of patients+miR-191 Inhibitor+si-CBS. A. HK-2 cell apoptosis was observed by flow cytometry. B. Apoptosis related proteins of Bax, Bcl-2, Caspase 3 were measured. *P < 0.05, compared with control group; &P < 0.05, compared with P-MVs of patients+negative control group; #P < 0.05, compared with P-MVs of patients+miR-191 inhibitor+si-control group.
Verification of the trends of CBS expression and HK-2 cell apoptosis in vivo
We established allograft renal transplantation rat model and autotransplantation rat model to verify the trends of CBS expression and HK-2 cell apoptosis in vivo were similar as the trends in vitro. In allograft renal transplantation rat model, rats received miR-191 inhibitor (800 pmol) by transrectal insufflations. We found that mRNA and protein levels of CBS were significantly downregulated than sham rats, while miR-191 inhibitor reversed this effect (Figure 7(a)). HK-2 cell apoptosis was significantly increased than sham rats, while miR-191 inhibitor reversed this effect (Figure 7(b)). Apoptosis related proteins of Bax, Bcl-2, Caspase 3 further proved the trends of cell apoptosis (Figure 7(c)).
Figure 7.
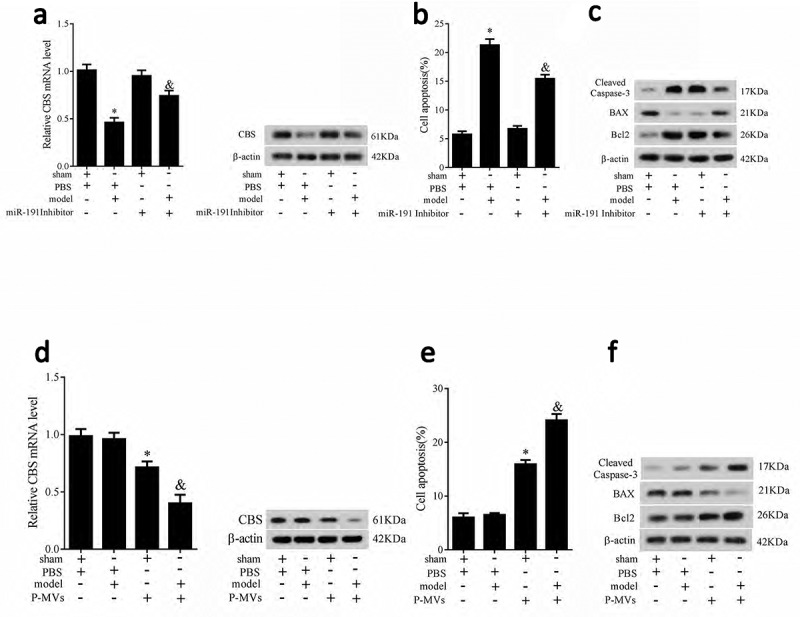
CBS expression, HK-2 cell apoptosis and apoptosis related proteins in allograft renal transplantation rat model. Rats were divided into four groups: sham+PBS, model+PBS, sham+miR-191 inhibitor (800 pmol), and model+miR-191 inhibitor (800 pmol). mRNA and protein levels of CBS (a), HK-2 cell apoptosis (b) apoptosis related proteins of Bax, Bcl-2, Caspase 3 (c) were measured. CBS expression, HK-2 cell apoptosis and apoptosis related proteins in renal autotransplantation rat model. Rats were divided into four groups: sham+PBS, model+PBS, sham+P-MVs (200 ug), and model+P-MVs (200 ug). mRNA and protein levels of CBS (d), HK-2 cell apoptosis (e) apoptosis related proteins of Bax, Bcl-2, Caspase 3 (f) were measured. *P < 0.05, compared with sham group; &P < 0.05, compared with sham+miR-191 inhibitor group or sham+P-MVs group.
In renal autotransplantation rat model, there were no significant differences in mRNA and protein levels of CBS between sham and model rats. P-MVs significantly downregulated CBS expression in sham and model rats, and the downregulation degree in model rat was greater (Figure 7(d)). HK-2 cell apoptosis was significantly increased in sham and model rats, and the increase degree in model rat was greater (Figure 7(e)). Apoptosis related proteins of Bax, Bcl-2, Caspase 3 further proved the trends of cell apoptosis (Figure 7(f)).
Discussion
Renal transplantation is a good treatment for patients with end stage renal disease, and renal IR injury is a common cause for delayed function of renal transplantation which is a relevant factor in determining high morbidity and mortality in renal allograft rejection [12,13]. Strategies to reduce renal IR injury can be focused on metabolic and anti-inflammatory strategies, antioxidant therapy, inhibition of innate inflammatory response, and anti-apoptosis treatment [14]. In this study, we mainly focused on the molecules that inducing HK-2 cell apoptosis, which showed that mIR-191/CBS pathway promoted apoptosis of HK-2 cells.
miRNAs are small, endogenous, non-coding RNAs that can bind to the 3’ UTR of targets and regulate protein levels [15]. Critical roles of miRNAs in IR injury have been discovered. For example, researchers found that miR-155 involved in renal IR injury via directly targeting FoxO3a [16]. Chen et al [17] reported that miR-204 regulateed epithelial-mesenchymal transition (EMT) via targeting SP1 in the tubular epithelial cells after acute kidney injury induced by IR. Several reports have showed that miR-191 could act as potential biomarker in renal cell carcinom, and it also abnormally expressed in kidney biopsies [18,19]. Moreover, miR-191 also reflected the changes of renal function and histopathology in dogs [8]. These reports showed that miR-191 was an important molecular in the function of renal, so we assumed that miR-191 may play a role in renal IR injury. Thus, we studied miR-191 in this research and explored the possible mechanism of miR-191 on renal IR injury. In this study, the findings shed light on the underlying mechanism of miR-191 derived from P-MVs in patients with renal allograft rejection, which is meaningful for providing targets for the treatment of IR injury.CBS played a critical role in mammalian sulfur metabolism. There are researches focused on the effects of CBS deficiency, and they mainly concerned about homocysteine management [20]. Importantly, CBS can act as an important contributor to the biogenesis of H2S [21]. The role of CBS in the production of H2S is believed to be especially vital in renal IR injury [4]. In addition, we proved CBS was a direct target of miR-191, and miR-191 could transfer to HK-2 cells via P-MVs to decrease the expression of CBS, thus to promote cell apoptosis and renal IR injury. No other studies have demonstrated the role of miR-191/CBS in the regulation of HK-2 cell apoptosis after renal IR injury, which can provide directions of the treatment of IR injury.
In conclusion, we first discovered that miR-191 and CBS acted as critical molecules in renal IR injury, and revealed the miR-191/CBS pathway as one of the mechanisms in renal IR injury, providing new targets for treating IR injury.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Xu Z, Prathapasinghe G, Wu N, et al. Ischemia-reperfusion reduces cystathionine-beta-synthase-mediated hydrogen sulfide generation in the kidney. Am J Physiol Renal Physiol. 2009;297(1):F27–F35. [DOI] [PubMed] [Google Scholar]
- [2].Martín-Solé O., Rodó J, García-Aparicio L, et al. Effects of platelet-rich plasma (PRP) on a model of renal ischemia-reperfusion in rats. PLoS One. 2016;11(8):e0160703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jung KJ, Jang H-S, Kim JI, et al. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim Biophys Acta. 2013;1832(12):1989–1997. [DOI] [PubMed] [Google Scholar]
- [4].Han SJ, Kim JI, Park J-W, et al. Hydrogen sulfide accelerates the recovery of kidney tubules after renal ischemia/reperfusion injury. Nephrol Dial Transplant. 2015;30(9):1497–1506. [DOI] [PubMed] [Google Scholar]
- [5].Venugopal P, Lavu V, Rao SR, et al. Association of microRNA-125a and microRNA-499a polymorphisms in chronic periodontitis in a sample south Indian population: a hospital-based genetic association study. Gene. 2017;631:10–15. [DOI] [PubMed] [Google Scholar]
- [6].Xu X., Jiao X, Song N, et al. Role of miR21 on vascular endothelial cells in the protective effect of renal delayed ischemic preconditioning. Mol Med Rep. 2017;16:2627–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shao H., Yang L, Wang L, et al. MicroRNA-34a protect myocardial cells against ischemia-reperfusion injury through inhibiting autophagy via regulating TNFalpha expression. Biochem Cell Biol. 201796:349–354. [DOI] [PubMed] [Google Scholar]
- [8].Ichii O, Ohta H, Horino T, et al. Urinary exosome-derived microRNAs reflecting the changes of renal function and histopathology in dogs. Sci Rep. 2017;7:40340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pan Y, Liang H, Liu H, et al. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol. 2014;192(1):437–446. [DOI] [PubMed] [Google Scholar]
- [10].Fuentes E, Palomo I, Alarcon M.. Platelet miRNAs and cardiovascular diseases. Life Sci. 2015;133:29–44. [DOI] [PubMed] [Google Scholar]
- [11].Zhang Y, Liu D, Chen X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39(1):133–144. [DOI] [PubMed] [Google Scholar]
- [12].Saat TC, EK den Akker, JN IJzermans, et al. Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med. 2016;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Granger DN, Kvietys PR.. Reperfusion injury and reactive oxygen species: the evolution of a concept(). Redox Biol. 2015;6:524–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Salvadori M, Rosso G, Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: pathogenesis and treatment. World J Transplant. 2015;5(2):52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng H, Li W, Wang Y, et al. miR-23a inhibits E-cadherin expression and is regulated by AP-1 and NFAT4 complex during Fas-induced EMT in gastrointestinal cancer. Carcinogenesis. 2014;35(1):173–183. [DOI] [PubMed] [Google Scholar]
- [16].Wu H, Huang T, Ying L, et al. MiR-155 is involved in renal ischemia-reperfusion injury via direct targeting of FoxO3a and regulating renal tubular cell pyroptosis. Cell Physiol Biochem. 2016;40(6):1692–1705. [DOI] [PubMed] [Google Scholar]
- [17].Chen S-J, Wu P, Sun L-J, et al. miR-204 regulates epithelial-mesenchymal transition by targeting SP1 in the tubular epithelial cells after acute kidney injury induced by ischemia-reperfusion. Oncol Rep. 2017;37(2):1148–1158. [DOI] [PubMed] [Google Scholar]
- [18].Hauser S, Wulfken LM, Holdenrieder S, et al. Analysis of serum microRNAs (miR-26a-2*, miR-191, miR-337-3p and miR-378) as potential biomarkers in renal cell carcinoma. Cancer Epidemiol. 2012;36(4):391–394. [DOI] [PubMed] [Google Scholar]
- [19].Lu M, Wang C, Yuan Y, et al. Differentially expressed microRNAs in kidney biopsies from various subtypes of nephrotic children. Exp Mol Pathol. 2015;99(3):590–595. [DOI] [PubMed] [Google Scholar]
- [20].Sacharow SJ, Picker JD, Levy HL. Homocystinuria caused by cystathionine beta-synthase deficiency, in GeneReviews(R). R.A. Pagon, et al., editors. Seattle: University of Washington; 1993. University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved.: Seattle (WA). [PubMed] [Google Scholar]
- [21].Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285(29):21903–21907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


