ABSTRACT
Objective: To investigate the underlying mechanism of lncRNA myocardial infarction-associated transcript (MIAT) in hypoxic-ischemic (HI)-induced neonatal cerebral palsy.
Materials and methods: Neonatal rat model of HI injury was established to detect the motor function. LncRNA MIAT, miR-211, glial cell line-derived neurotrophic factor (GDNF) and caspase-3 expressions were measured by qRT-PCR or western blot. The apoptosis of Neuro2A cells was detected by flow cytometry. RNA immunoprecipitation (RIP) and RNA pull-down assays were performed to confirm the interaction between MIAT and miR-211.
Results: Compared with control group, lncRNA MIAT and GDNF were downregulated in striatal tissues of neonatal rats in HI group and oxygen glucose deprivation (OGD)-induced ischemic injury of Neuro2A cells, whereas miR-211 was up-regulated in striatal tissues of HI group and OGD-induced ischemic injury of Neuro2A cells. LncRNA MIAT interacted with miR-211, and lncRNA MIAT overexpression reduced neuron apoptosis through miR-211. Besides, GDNF expression was positively regulated by lncRNA MIAT and negatively regulated by miR-211 in Neuro2A cells. In vivo experiment proved MIAT promoted motor function and relieved HI injury.
Conclusion: MIAT overexpression reduced apoptosis of Neuro2A cells through miR-211/GDNF, which relieved HI injury of neonatal rats.
KEYWORDS: MIAT, miR-211, GDNF, neonatal rat, hypoxic-ischemic injury, cerebral palsy
Introduction
Cerebral palsy (Cp) is a chronic childhood disorder that can be caused by hypoxic-ischemic (HI) injury, germinal matrix hemorrhage, extreme prematurity, etc., which leads to neural behavior disorders, learning disorders, and cognitive disorder [1–3]. HI injury affects 1–3 per 1000 full-term infants, and is related with high mortality of Cp [4]. Currently, rat model of HI injury has been widely applied in brain injury in neonates [5,6]. Besides, more and more researchers have found neuronal apoptosis is important for the loss of neurons and involved in the evolution of HI injury in the brain of neonates [7,8]. However, the mechanism mediating apoptosis of neurons in HI-induced brain injury is still not fully understood.
Glial cell line-derived neurotrophic factor (GDNF) is a neurotrophic factor that protects and repairs dopaminergic neurons, which has the potential ability to treat Parkinson’s disease [9,10]. GDNF can also promote axonal regeneration in critical nerve injury and inhibit spinal microglia activation to relieve age-related neuromuscular dysfunction [11,12]. Decrease of GDNF contributed to the learning and cognitive disorders in neonatal rats [13]. GDNF injection into striatum reduced HI injury in neonatal rats, which suggested that GDNF microbubbles played a neuroprotective effect in neonatal rats with HI injury [14]. GDNF was down-regulated in rat model of HI injury, and acupuncture up-regulated GDNF expression in hippocampus which reduced the apoptosis of hippocampus cells [15]. Hence, GDNF plays an important role in HI injury.
Long non-coding RNAs (lncRNAs) are a kind of noncoding RNAs longer than 200 nucleotides that involve in the development and progression of many cancers, endocrine disorders, inflammatory response, etc [16,17]. LncRNA myocardial infarction-associated transcript (MIAT) was involved in regulating pathological angiogenesis [18], paranoid schizophrenia [19], myocardial infarction [20]. It has been reported that lncRNA MIAT (Gomafu) is important for retinal and brain development, and dysregulation of lncRNA MIAT may lead to neurological disorders [21]. However, the role of lncRNA MIAT in Cp or HI injury-induced brain injury is unclear. Numerous studies have shown that lncRNAs involve in biological or pathological process through regulating miRNAs [22,23]. miR-211 is a neuron-related miRNA that can inhibit neuronal differentiation in Alzheimer’s disease [24]. Xia et al found that GDNF was a target of miR-211, and GDNF was negatively regulated by miR-211 in retinal ganglion cells [25]. Moreover, bioinformatics software predicted the binding sites between MIAT and miR-211. Therefore, we assumed that lncRNA MIAT might regulate neuron apoptosis in a neonatal rat model of HI injury through miR-211/GDNF pathway.
In this study, we demonstrated lncRNA MIAT was downregulated in striatal tissues of neonatal rats with HI injury, and confirmed the interaction between lncRNA MIAT and miR-211. MIAT overexpression reduced apoptosis of Neuro2A cells and relieved HI injury of neonatal rats.
Materials and methods
Establishment of neonatal rat model of HI injury
Female Wistar neonatal rats were purchased from the laboratory animal center of Zhengzhou University. For establishment of neonatal rat model of HI injury, permanent unilateral carotid ligation was made and the time of surgery was less than 5 min according to previous report [26]. Under halothane anesthesia, the midline of the neck was incised and a 5–0 surgical silk was used to permanently ligate with the left carotid artery. After two hours’ recovery and feeding, rats were exposed to hypoxia environment (92% N2, 8% O2) for 2.5 h in airtight containers and kept in a 37°C water bath. During surgery, rats with bleeding during ligation or respiratory arrest were excluded from this study. This animal experiment was approved by the Ethics Committee of The Fifth Affiliated Hospital of Zhengzhou University. Twenty-eight days later, the muscle strength of the rat was tested by the grip-traction test. The rats were hung from a horizontal bar by their forepaws, and duration of time to falling (maximum 50 s) was recorded. Then, striatal tissues of neonatal rats were collected for further use.
Lentiviral injection
For observation the effect of MIAT overexpression on neonatal rats of HI injury, lentiviral vector lenti-NC or lenti-MIAT (purchased from GENECHEM, Shanghai, China) was injected into the cortex of the mice at three points by a stereotaxic instrument under the anesthesia of pentobarbital sodium (80 mg/kg, i.p.) according to the previous report [27]. Each point was injected with 1.0 μl lentivirus suspension containing 1.0 × 109 TU/ml at an injection rate of 0.2 μl/min. Three days after injection, neonatal rat model of HI injury was established.
OGD-induced ischemic injury of Neuro2A cells
Mouse neuroblastoma cell line Neuro2A was purchased from American Type Culture Collection (ATCC, USA), and cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) in a 37°C incubator under normoxia (5% CO2, 95% O2). To simulate HI injury in vitro, Neuro2A cells were exposed to oxygen glucose deprivation (OGD, glucose-free DMEM) and maintained in hypoxic condition (5% CO2, 1% O2, 94% N2) for 3 h. Then, Neuro2A cells were returned to glucose containing DMEM under normoxia for 24 h reoxygenation.
pcDNA-MIAT was constructed by inserting MIAT cDNA into pcDNA3.1 (Invitrogen, USA). Neuro2A cells were seeded at 3 × 105 cells/well in a 6-well plate and cultured until 50% confluence, then transfected with 30 nmol pcDNA-MIAT, miR-211 mimic or their negative controls using transfection reagent (Invitrogen, USA) according to the manufacturer’s instructions.
Quantitative real-time RCR (qRT-PCR)
Total RNAs were extracted from striatal tissues and Neuro2A cells using Trizol (Invitrogen, USA). Reverse transcription was performed using the High-Capacity RNA-to-cDNATM Kit (Applied Biosystems, USA). Amplification was carried out with the ABI 7300 PCR System (Applied Biosystems, USA) using SYBRTM Green Master Mix (Invitrogen, USA), primer and cDNA template. The relative expressions of MIAT and miR-211 were calculated using the 2−ΔΔCt method. Primers for MIAT and miR-211 were as follows: MIAT, F: 5ʹ-GAGGGAAGTTCTGAGCTTGG-3ʹ and R: 5ʹ-CCTTTCTTCTGGGCTGAGAC-3ʹ; miR-211, F: 5ʹ-GATCTTCCCTTTGTCATCC-3ʹ and R: 5ʹ- GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAGGCAA-3ʹ.
Western blot
Striatal tissues and Neuro2A cells were lysed in RIPA buffer (Beyotime Biotechnology, China) supplemented with phenylmethanesulfonyl fluoride Protease Inhibitor (PMSF, Applied Biosystems, USA). Protein samples was separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) followed by transferring to polyvinylidene fluoride (PVDF) membrane (Millipore, USA). The membrane was treated with primary antibodies against GDNF (1:1000; CAT. No: PA1-9524, Invitrogen, USA), caspase-3 (1:500; CAT. No: 43–7800, Invitrogen, USA), GAPDH (1:1000; CAT. No: MA5-15738-BTIN, Invitrogen, USA), β-tublin (1:5000; CAT. No: ab151318, Abcam, USA) at 4°C overnight following blocking with 5% skim milk for 1 h. Then, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:5000; CAT. No: ab6734, Abcam, USA) at room temperature for 2 h. The membrane was visualized by an enhanced chemiluminescence reagent (Bio-Rad, USA), and the intensity of band was quantified using image software (Bio-Rad, USA).
Flow cytometry
The apoptosis of Neuro2A cells was detected by a Annexin V-FITC detection kit (Beyotime Biotechnology, China). Neuro2A cells were collected and re-suspended in PBS at 1 × 105 cells/ml. After centrifuged at 1000 rpm for 5 min, 195 μl Annexin V-FITC binding buffer was added to re-suspended Neuro2A cells. Then, 5 μl Annexin V-FITC was added into the cells. Later, 10 μl propidium iodide (PI) was added into the cells. After incubation at room temperature for 20 min at dark, flow cytometry (FACSCanto II; BD, USA) was used to detect the apoptosis of Neuro2A cells, and the data were analyzed using CELLQuest software.
RNA immunoprecipitation (RIP) and RNA pull-down
The binding sites between MIAT and miR-211 were predicted by bioinformatics software DIANA tools (http://diana.imis.athena-innovation.gr/DianaTools/index.php).
RIP assay was conducted by Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Merck Millipore, USA). Neuro2A cells were rinsed with cold PBS and lysed in radioimmunoprecipitation buffer at 4°C. Cell lysate was prepared by 1 × 107 cells using 0.25 μl RNase inhibitor, 0.5 μl protease inhibitor and 100 μl RIP lysis buffer. The lysate was incubated with protein A/G sepharose beads conjugated to Ago2 antibody or IgG. Then, RNA-binding protein complex was obtained, and qRT-PCR was used to detect lncRNA MIAT and miR-211 in the RNA-binding protein complex and the whole cell lysate (input).
The interaction between MIAT and miR-211 was further confirmed by RNA pull-down assay. The biotin labelled MIAT was transcribed with the Biotin RNA Labeling Mix (Roche, Switzerland) and T7 RNA polymerase (Roche, Switzerland). Neuro2A cell lysate was prepared by 1 × 107 cells in 100 μl RIP buffer, and mixed with 50 pmol biotin-labeled MIAT and 50 μl streptavidin agarose magnetic beads for 1 h at 4℃. RNA-binding protein complex was eluted with biotin elution buffer and then boiled in SDS buffer for another 10 min. The retrieved Ago2 protein levels were detected by western blot, and qRT-PCR was used to detect miR-211 in MIAT pull-down complex using Loc as positive control.
Statistical analysis
All data were analyzed using SPSS (version 19.0, USA) and expressed as mean±standard deviation (SD). The differences were assessed by student t test or one-way analysis of variance (ANOVA). A p value less than 0.05 was considered statistically significant.
Results
LncRNA MIAT was decreased in striatal tissues of neonatal rats of HI injury
Neonatal rat model of HI injury was established to observe the abnormally expressed molecules in striatal tissues. At 28 d after the establishment of neonatal rat model of HI injury, motor function of rats was detected by grip-traction test. As shown in Figure 1(a), the duration of time falling in HI group was decreased compared with control group, which indicated that neonatal rat model of HI injury was successfully established. In addition, lncRNA MIAT was downregulated in striatal tissues of neonatal rats in HI group than control group, whereas miR-211 was up-regulated in striatal tissues of HI group (Figure 1(b)). GDNF expression was downregulated in striatal tissues of neonatal rats HI group than control group (Figure 1(c)).
Figure 1.
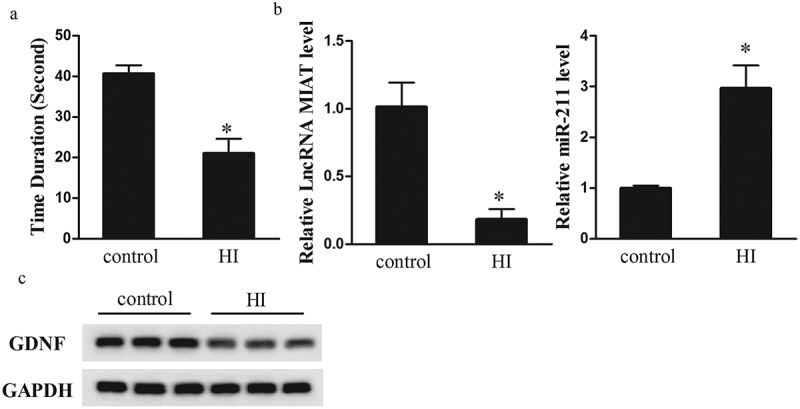
LncRNA MIAT was decreased in striatal tissues of neonatal rats of hypoxic-ischemic injury. Neonatal rats were divided into control group (n = 6) and HI group (n = 6). (a) At 28 d after HI, motor function of rats was detected by grip-traction test. Compared with control group, the duration of time falling in HI group was decreased. (b) qRT-PCR was performed to detect lncRNA MIAT and miR-211 expressions. Compared with control group, lncRNA MIAT was down-regulated in striatal tissues of neonatal rats in HI group, whereas miR-211 was up-regulated. (c) Western blot was performed to detect GDNF expression. Compared with control group, GDNF expression was downregulated in striatal tissues of neonatal rats HI group. *p < 0.05, compared with control.
LncRNA MIAT was decreased in OGD-induced ischemic injury of Neuro2A cells
OGD 3 h/reoxygenation 24 h treatment of Neuro2A cells was used to simulate HI injury in vitro [28], and lncRNA MIAT, miR-211 and GDNF expressions were measured in Neuro2A cells with normoxia or 3 h OGD followed by 24 h reoxygenation treatment. As shown in Figure 2(a), lncRNA MIAT was down-regulated in OGD/reoxygenation group than that of normoxia group, whereas miR-211 was up-regulated in OGD/reoxygenation group. GDNF expression was downregulated in OGD/reoxygenation group than that of normoxia group (Figure 2(b)). These findings indicated that in vitro HI injury model was successfully established.
Figure 2.
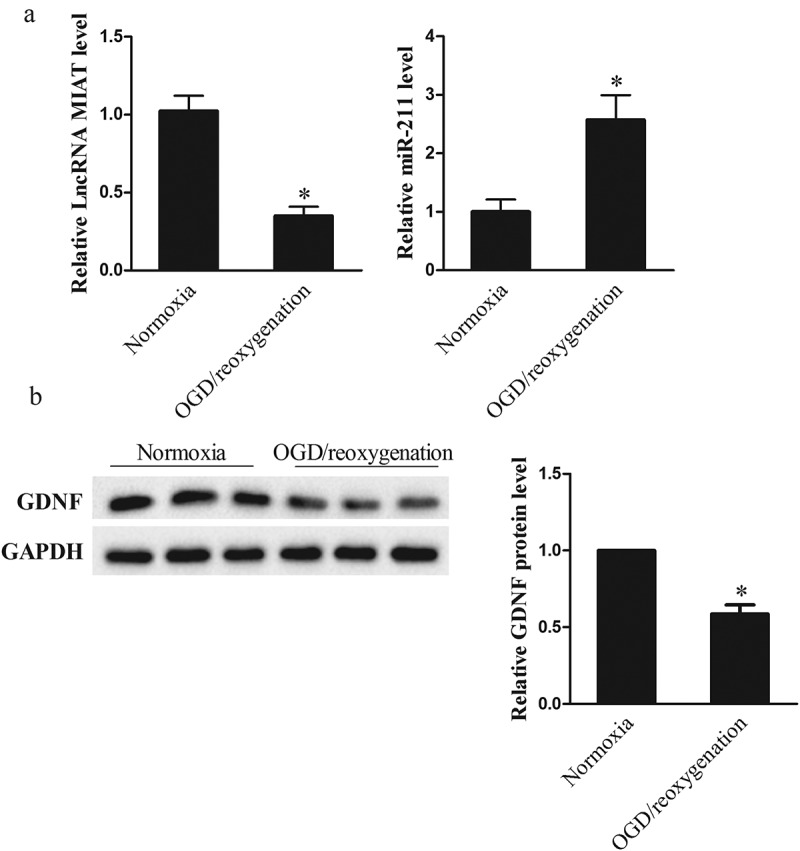
LncRNA MIAT was decreased in OGD-induced ischemic injury of Neuro2A cells. Neuro2A cells were divided into normoxia group and OGD/reoxygenation group. (a) lncRNA MIAT was down-regulated in OGD/reoxygenation group than that of normoxia group, whereas miR-211 was up-regulated. (b) GDNF expression was downregulated in OGD/reoxygenation group than that of normoxia group. *p < 0.05, compared with normoxia.
LncRNA MIAT overexpression reduced apoptosis of Neuro2A cells
To determine the effect of lncRNA MIAT on the apoptosis of Neuro2A cells, we used pcDNA-MIAT to overexpress MIAT level in Neuro2A cells. As shown in Figure 3(a), 3 h OGD/24 h reoxygenation significantly downregulated lncRNA MIAT expression, and pcDNA-MIAT upregulated lncRNA MIAT expression. We further found that OGD (3 h)/24 h reoxygenation significantly increased the apoptosis of Neuro2A cells, and pcDNA-MIAT reduced the apoptosis of Neuro2A cells (Figure 3(b)). OGD (3 h)/24 h reoxygenation significantly upregulated caspase-3 expression, and pcDNA-MIAT downregulated caspase-3 expression (Figure 3(c)).
Figure 3.
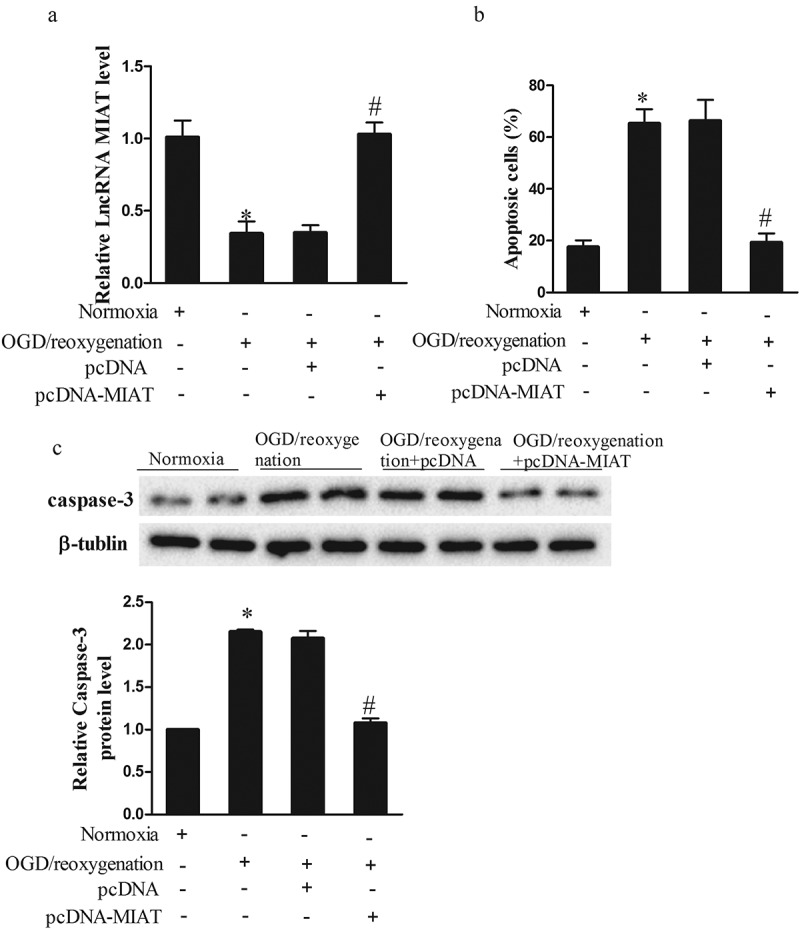
LncRNA MIAT overexpression reduced neuron apoptosis. Neuro2A cells were transfected with pcDNA or pcDNA-MIAT. After the treatment of OGD (3 h)/reoxygenation (24 h), Neuro2A cells were divided into Normoxia, OGD/reoxygenation, OGD/reoxygenation+pcDNA, and OGD/reoxygenation+pcDNA-MIAT groups. (a) LncRNA MIAT was upregulated in OGD/reoxygenation+pcDNA-MIAT group than OGD/reoxygenation+pcDNA group. (b) Compare with normoxia group, the apoptosis of Neuro2A cells was increased in OGD/reoxygenation group, whereas pcDNA-MIAT reduced the apoptosis of Neuro2A cells. (c) Compare with normoxia group, caspase-3 expression was upregulated in OGD/reoxygenation group, whereas pcDNA-MIAT downregulated caspase-3 expression. *p < 0.05, compared with normoxia; #p < 0.05, compared with OGD/reoxygenation+pcDNA.
The interaction between lncRNA MIAT and miR-211
According to bioinformatics software used to predict the potential binding sites between lncRNAs and miRNAs, we found the binding sites between MIAT and miR-211 (Figure 4(a)). To verify whether lncRNA MIAT can interact with miR-211, RIP and RNA pull-down assays were conducted. RIP was conducted using an antibody AGO2 from nuclear extracts of Neuro2A cells. We found significant enrichments of miR-211 and MIAT using AGO2 antibody than IgG antibody (Figure 4(b)). RNA pull-down was conducted to confirm the relationship between MIAT and miR-211. We found miR-211 level was higher in MIAT pull-down complex than that of beads or positive control Loc (Figure 4(c)).
Figure 4.
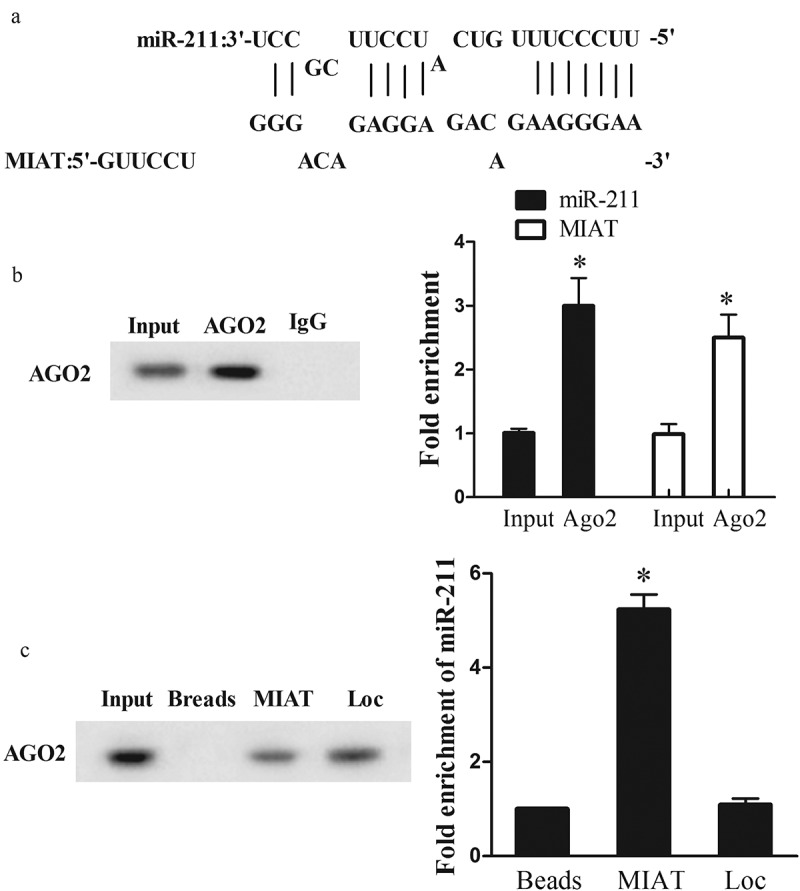
The interaction between lncRNA MIAT and miR-211. (a) Bioinformatics software predicted the binding sites between MIAT and miR-211. (b) AGO2 antibody was used for RIP, and miR-211 or MIAT expression in AGO2 complex was detected by qRT-PCR. (c) RNA pull-down assay was used to detect AGO2 expression in MIAT pull-down complex. Loc, positive control of MIAT. miR-211 expression in MIAT pull-down complex was detected by qRT-PCR.
LncRNA MIAT overexpression reduced neuron apoptosis through miR-211
To determine whether lncRNA MIAT regulate the apoptosis of Neuro2A cells through miR-211, we transfected pcDNA-MIAT or miR-211 mimic into Neuro2A cells. As shown in Figure 5(a), 3h OGD/24 h reoxygenation significantly upregulated miR-211 expression, pcDNA-MIAT downregulated miR-211 expression, and miR-211 mimic abolished the inhibition effect of pcDNA-MIAT. OGD (3 h)/24 h reoxygenation significantly decreased GDNF expression, pcDNA-MIAT increased GDNF expression, and miR-211 mimic abolished the promotion effect of pcDNA-MIAT (Figure 5(b)). In addition, 3 h OGD/24 h reoxygenation significantly promoted the apoptosis of Neuro2A cells, pcDNA-MIAT reduced the apoptosis of Neuro2A cells, and miR-211 mimic abolished the inhibition effect of pcDNA-MIAT (Figure 5(c)). OGD (3 h)/24 h reoxygenation upregulated caspase-3 expression, pcDNA-MIAT downregulated the expression of caspase-3, and miR-211 mimic abolished the inhibition effect of pcDNA-MIAT (Figure 5(d)). These findings indicated that miR-211 was negatively regulated by MIAT, and MIAT overexpression reduced Neuro2A cell apoptosis through miR-211.
Figure 5.
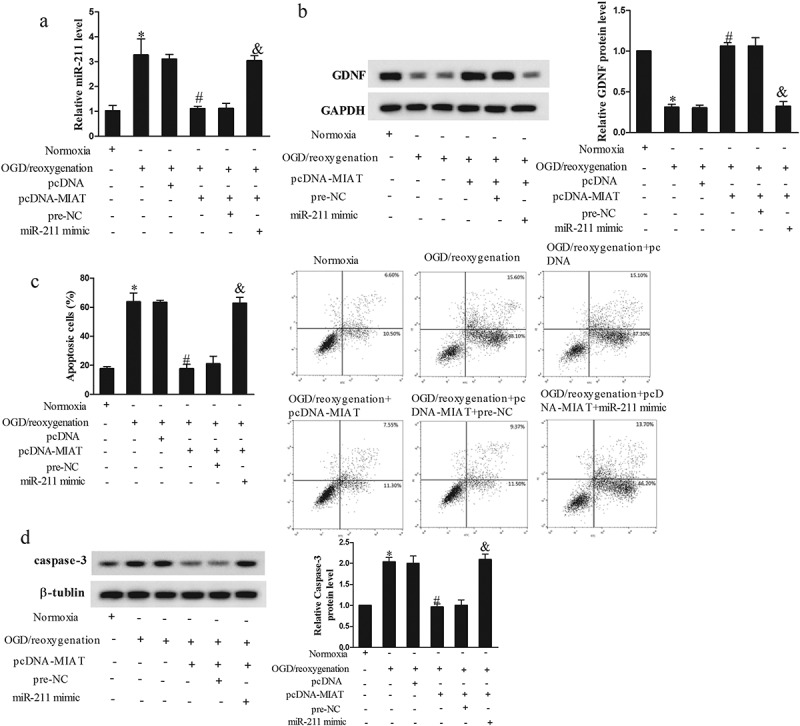
LncRNA MIAT overexpression reduced neuron apoptosis through miR-211. Neuro2A cells were transfected with pcDNA-MIAT or miR-211 mimic. After the treatment of OGD (3 h)/reoxygenation (24 h), Neuro2A cells were divided into Normoxia, OGD/reoxygenation, OGD/reoxygenation+pcDNA, OGD/reoxygenation+pcDNA-MIAT, OGD/reoxygenation+pcDNA-MIAT+pre-NC, OGD/reoxygenation+pcDNA-MIAT+miR-211 mimic groups. (a) Under the treatment of OGD/reoxygenation, pcDNA-MIAT downregulated miR-211 expression, and miR-211 mimic abolished the inhibition effect of pcDNA-MIAT. (b) pcDNA-MIAT increased GDNF expression, and miR-211 mimic abolished the promotion effect of pcDNA-MIAT. (c) pcDNA-MIAT reduced the apoptosis of Neuro2A cells, and miR-211 mimic abolished the inhibition effect of pcDNA-MIAT. (d) pcDNA-MIAT downregulated the expression of caspase-3, and miR-211 mimic abolished the inhibition effect of pcDNA-MIAT. *p < 0.05, compared with normoxia; #p < 0.05, compared with OGD/reoxygenation+pcDNA; &p < 0.05, compared with OGD/reoxygenation+pcDNA-MIAT+pre-NC.
Effect of MIAT overexpression on neonatal rats of HI injury
To evaluate the effect of MIAT in HI injury, we injected lenti-NC or lenti-MIAT into the cortex of neonatal rats and established neonatal rat model of HI injury after 3 d of injection. As shown in Figure 6(a), at 28 d after the establishment of neonatal rat model of HI injury, duration of time falling in lenti-MIAT group was prolonged than lenti-NC group, which indicated that MIAT overexpression improved motor function of rats with HI injury. Then, striatal tissues were taken from neonatal rat model of HI injury. Compared with lenti-NC group, lncRNA MIAT was upregulated in striatal tissues of lenti-MIAT group, whereas miR-211 was downregulated (Figure 6(b)). GDNF expression was up-regulated in striatal tissues of lenti-MIAT group than that of lenti-NC group (Figure 6(c)). These results indicated MIAT regulated miR-211 and GDNF in vivo and relieved HI injury.
Figure 6.
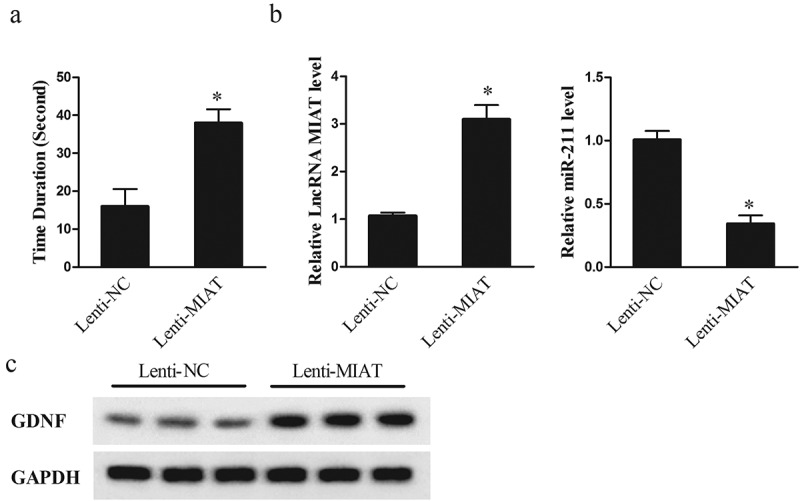
Effect of MIAT overexpression on neonatal rats of HI injury. Three days before conducting the neonatal rat model of HI injury, lentiviral vector lenti-NC or lenti-MIAT was injected into the cortex of neonatal rats. Then, neonatal rat model of HI injury was performed, and divided into lenti-NC (n = 6) and lenti-MIAT group (n = 6). (a) Twenty-eight days after the establishment of neonatal rat model of HI injury, motor function of rats was detected by grip-traction test. (b) Compared with lenti-NC group, lncRNA MIAT was upregulated in striatal tissues of lenti-MIAT group, whereas miR-211 was downregulated. (c) GDNF expression was up-regulated in striatal tissues of lenti-MIAT group than that of lenti-NC group. *p < 0.05, compared with leti-NC.
Discussion
Neuron apoptosis induced by HI injury plays a vital role in the progression of Cp, which contributes to death in neonates. HI enhances the proapoptosis balance of Bcl-2 protein in neonatal rats, and apoptosis-related protein caspase 3 is involved in the neurodegeneration after neonatal HI injury [29,30]. For decades, researchers dedicate to find effective ways to relieve the brain injury caused by HI. Treatment of levetiracetam reduced the number of apoptotic neurons thus to play a neuroprotective role in neonatal rats with HI injury [31]. Tert-butylhydroquinone post-treatment inhibited apoptosis and neuronal degeneration, suppressed inflammatory cytokines, and abated oxidative stress in the cerebral cortex of neonatal rat [32]. miR-592-5p overexpression decreased the apoptosis rate of hippocampal neuronal cells and protected against HI brain injury-induced hippocampal neuronal injury [33]. Until now, finding effective therapeutic method is still urgent and important for reducing the mortality of neonates.
LncRNAs exert an increasing important role in brain injury. For example, lncRNA FosDT promoted ischemic brain injury through interacting with REST-associated chromatin-modifying proteins in middle cerebral artery occlusion rat model [34]. LncRNA MALAT1 secreted from adipose-derived stem cells-derived exosomes improved motor impairment and reduced lesion volume in rats with controlled cortical impact injury [35]. LncRNA Meg3 was decreased in rat brain after ischemic stroke, and Meg3 knockdown reduced brain lesions and improved neurological outcome and promoted angiogenesis [36]. Inhibition of lncRNA GAS5 reduced brain infarct size and improved neurological function recovery in HI-induced neonatal brain injury [37]. This study firstly focused on the expression and the role of lncRNA MIAT in neonatal rat model of HI injury and OGD-induced ischemic injury of Neuro2A cells, which enriched the literature of HI induced neonatal brain injury-related lncRNAs.
The molecular mechanism involving lncRNA-miRNA-mRNA network underlying cancers, endocrine disease, lung disease, etc. has gained a lot of attention and many satisfied achievements, where lncRNAs act as ceRNA to interfere with miRNAs at posttranscriptional level [17,38,39]. This molecular mechanism also has been investigated in brain injury. LncRNA Meg3 was up-regulated in hypoxia-injured PC12 cells and overexpression of Meg3 enhanced hypoxia injuries by regulating miR-147/Sox2 [40]. LncRNA MALAT1 was upregulated in ischemia-reperfusion induced brain microvascular endothelial cell (BMEC) injury, and it enhanced BMEC autophagy under OGD/reoxygenation and promoted BMEC survival through miR-26b/ULK2 [41]. LncRNA Gm4419 contribute to inflammatory damage and trauma-induced astrocyte apoptosis through miR-4661/TNF-α during traumatic brain injury [42]. Studies have shown the role of lncRNA MIAT in the protection of neurovascular remodeling and in the regulation of the proliferation and migration of lens epithelial cells through miR-150-5p [43,44]. We detected the expression of miR-150 in striatal tissues from control and neonatal rats of HI injury, and found that miR-150 expression was up-regulated in HI group than control group. miR-150 expression was also up-regulated in OGD/reoxygenation group than normoxia group. In addition, the expression of lncRNA MIAT increased to 3.95 fold after miR-150 inhibitor transfection and decreased to 0.35 fold after miR-150 mimic transfection. However, there was no significant change in miR-150 expression after the treatment of si-MIAT or pcDNA-MIAT, indicating that lncRNA MIAT might be regulated by miR-150. In this study, we found lncRNA MIAT decreased in striatal tissues of neonatal rats of HI injury and OGD-induced ischemic injury of Neuro2A cells, whereas miR-211 was upregulated in striatal tissues and Neuro2A cells. We performed the RIP and RNA pull-down assays and firstly identified that MIAT interacted with miR-211. Moreover, we proved lncRNA MIAT overexpression reduced apoptosis of Neuro2A cells through miR-211, which determined the underlying mechanism of MIAT in regulating neuron apoptosis.
In conclusion, we demonstrated the decrease of lncRNA MIAT in striatal tissues of neonatal rats with HI injury and OGD-induced ischemic injury of Neuro2A cells, and confirmed the interaction between lncRNA MIAT and miR-211. MIAT overexpression reduced apoptosis of Neuro2A cells through miR-211/GDNF, which relieved HI injury of neonatal rats.
Funding Statement
This research was supported by Major Project of Synergistic Innovation in Zhengzhou (Zhengzhou University) (Grant No. 18XTZX12003), Special Project of Traditional Chinese Medicine in Henan Province (Grant No. 2018ZY3013), Henan Medical Science and Technology Research Project (Grant No. 2018020223), Key Scientific Research Projects of Colleges and Universities in Henan Province (Grant No. 17A320007 and 16B320026), Research Projects of Vocational Education Teaching Reform in Henan Province (Grant No. ZJC18051).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Ma S, Santhosh D, Kumar P, et al. A brain-region-specific neural pathway regulating germinal matrix angiogenesis. Dev Cell. 2017;41:366–381.e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nance E, Porambo M, Zhang F, et al. Systemic dendrimer-drug treatment of ischemia-induced neonatal white matter injury. J Control Release. 2015;214:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bennet L, Tan S, Van Den Heuij L, et al. Cell therapy for neonatal hypoxia-ischemia and cerebral palsy. Ann Neurol. 2012;71:589–600. [DOI] [PubMed] [Google Scholar]
- [4].Kurinczuk J, White-Koning M, Badawi N.. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–338. [DOI] [PubMed] [Google Scholar]
- [5].Chen A, Xu Y, Yuan J.. Ginkgolide B ameliorates NLRP3 inflammasome activation after hypoxic-ischemic brain injury in the neonatal male rat. Int J Dev Neurosci. 2018;69:106–111. [DOI] [PubMed] [Google Scholar]
- [6].Tucker L, Lu Y, Dong Y, et al. Photobiomodulation therapy attenuates hypoxic-ischemic injury in a neonatal rat model. J Mol Neurosci. 2018;65:514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li L, Klebe D, Doycheva D, et al. G-CSF ameliorates neuronal apoptosis through GSK-3β inhibition in neonatal hypoxia-ischemia in rats. Exp Neurol. 2015;263:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tu L, Wang Y, Chen D, et al. Protective effects of notoginsenoside R1 via regulation of the PI3K-Akt-mTOR/JNK pathway in neonatal cerebral hypoxic-ischemic brain injury. Neurochem Res. 2018;43:1210–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kumar A, Kopra J, Varendi K, et al. GDNF overexpression from the native locus reveals its role in the nigrostriatal dopaminergic system function. PLoS Genet. 2015;11:e1005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Meka D, Müller-Rischart A, Nidadavolu P, et al. Parkin cooperates with GDNF/RET signaling to prevent dopaminergic neuron degeneration. J Clin Invest. 2015;125:1873–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alsmadi N, Bendale G, Kanneganti A, et al. Glial-derived growth factor and pleiotrophin synergistically promote axonal regeneration in critical nerve injuries. Acta Biomater. 2018;78:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xie F, Zhang F, Min S, et al. Glial cell line-derived neurotrophic factor (GDNF) attenuates the peripheral neuromuscular dysfunction without inhibiting the activation of spinal microglia/monocyte. BMC Geriatr. 2018;18:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gui L, Lei X, Zuo Z. Decrease of glial cell-derived neurotrophic factor contributes to anesthesia- and surgery-induced learning and memory dysfunction in neonatal rats. J Mol Med. 2017;95:369–379. [DOI] [PubMed] [Google Scholar]
- [14].Wang X, Guo S, Lu S, et al. Ultrasound-induced release of GDNF from lipid coated microbubbles injected into striatum reduces hypoxic-ischemic injury in neonatal rats. Brain Res Bull. 2012;88:495–500. [DOI] [PubMed] [Google Scholar]
- [15].Zhang Y, Lan R, Wang J, et al. Acupuncture reduced apoptosis and up-regulated BDNF and GDNF expression in hippocampus following hypoxia-ischemia in neonatal rats. J Ethnopharmacol. 2015;172:124–132. [DOI] [PubMed] [Google Scholar]
- [16].Cho S, Xu J, Sun R, et al. Promoter of lncRNA Gene PVT1 Is a Tumor-Suppressor DNA Boundary Element. Cell. 2018;173:1398–1412.e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou K, Zhang C, Yao H, et al. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018;17:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yan B, Yao J, Liu J, et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116:1143–1156. [DOI] [PubMed] [Google Scholar]
- [19].Rao S, Hu H, Ye N, et al. Genetic variants in long non-coding RNA MIAT contribute to risk of paranoid schizophrenia in a Chinese Han population. Schizophr Res. 2015;166:125–130. [DOI] [PubMed] [Google Scholar]
- [20].Liao J, He Q, Li M, et al. LncRNA MIAT: myocardial infarction associated and more. Gene. 2016;578:158–161. [DOI] [PubMed] [Google Scholar]
- [21].Barry G, Briggs J, Vanichkina D, et al. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol Psychiatry. 2014;19:486–494. [DOI] [PubMed] [Google Scholar]
- [22].Fang Y, Hu J, Wang Z, et al. LncRNA H19 functions as an Aquaporin 1 competitive endogenous RNA to regulate microRNA-874 expression in LPS sepsis. Biomed Pharmacother. 2018;105:1183–1191. [DOI] [PubMed] [Google Scholar]
- [23].Lv S, Shan T, Pan X, et al. The lncRNA ZEB1-AS1 sponges miR-181a-5p to promote colorectal cancer cell proliferation by regulating Wnt/β-catenin signaling. Cell Cycle. 2018;17(10):1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Krol J, Busskamp V, Markiewicz I, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. [DOI] [PubMed] [Google Scholar]
- [25].Xia Z, Ding D, Zhang N, et al. MicroRNA-211 causes ganglion cell dysplasia in congenital intestinal atresia via down-regulation of glial-derived neurotrophic factor. Neurogastroenterol Motil. 2016;28:186–195. [DOI] [PubMed] [Google Scholar]
- [26].Yesilirmak D, Kumral A, Tugyan K, et al. Effects of activated protein C on neonatal hypoxic ischemic brain injury. Brain Res. 2008;1210:56–62. [DOI] [PubMed] [Google Scholar]
- [27].Chi W, Meng F, Li Y, et al. Downregulation of miRNA-134 protects neural cells against ischemic injury in N2A cells and mouse brain with ischemic stroke by targeting HSPA12B. Neuroscience. 2014;277:111–122. [DOI] [PubMed] [Google Scholar]
- [28].Zhang N, Zhong J, Han S, et al. MicroRNA-378 alleviates cerebral ischemic injury by negatively regulating apoptosis executioner caspase-3. Int J Mol Sci. 2016;17:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lok J, Martin L. Rapid subcellular redistribution of Bax precedes caspase-3 and endonuclease activation during excitotoxic neuronal apoptosis in rat brain. J Neurotrauma. 2002;19:815–828. [DOI] [PubMed] [Google Scholar]
- [30].Ness J, Harvey C, Washington J, et al. Differential activation of c-fos and caspase-3 in hippocampal neuron subpopulations following neonatal hypoxia-ischemia. J Neurosci Res. 2008;86:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Komur M, Okuyaz C, Celik Y, et al. Neuroprotective effect of levetiracetam on hypoxic ischemic brain injury in neonatal rats. Childs Nerv Syst. 2014;30:1001–1009. [DOI] [PubMed] [Google Scholar]
- [32].Zhang J, Tucker L, DongYan N, et al. Tert-butylhydroquinone post-treatment attenuates neonatal hypoxic-ischemic brain damage in rats. Neurochem Int. 2018;116:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun L, Guo G, Zhang S, et al. Effects of MicroRNA-592-5p on hippocampal neuron injury following hypoxic-ischemic brain damage in neonatal mice - Involvement of PGD2/DP and PTGDR. Cell Physiol Biochem. 2018;45:458–473. [DOI] [PubMed] [Google Scholar]
- [34].Mehta S, Kim T, Vemuganti R. Long noncoding RNA FosDT promotes ischemic brain injury by interacting with REST-associated chromatin-modifying proteins. J Neurosci. 2015;35:16443–16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Patel N, Moss L, Lee J, et al. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J Neuroinflammation. 2018;15:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu J, Li Q, Zhang K, et al. Downregulation of the long non-coding RNA Meg3 promotes angiogenesis after ischemic brain injury by activating notch signaling. Mol Neurobiol. 2017;54:8179–8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhao R, Zhu L, Shu J, et al. GAS5 silencing protects against hypoxia/ischemia-induced neonatal brain injury. Biochem Biophys Res Commun. 2018;497:285–291. [DOI] [PubMed] [Google Scholar]
- [38].Wang X, Peng C, Li J, et al. LncRNA MALAT1 is neuroprotective in a rat model of spinal cord ischemia-reperfusion injury through miR-204 regulation. Curr Neurovasc Res. 2018;15(3):211–219. [DOI] [PubMed] [Google Scholar]
- [39].Jiang H, Chen Y, Yu T, et al. Inhibition of lncRNA PFRL prevents pulmonary fibrosis by disrupting the miR-26a/Smad2 loop. Am J Physiol Lung Cell Mol Physiol. 2018;315:L563-L575. [DOI] [PubMed] [Google Scholar]
- [40].Han L, Dong Z, Liu N, et al. Maternally expressed gene 3 (MEG3) enhances PC12 cell hypoxia injury by targeting MiR-147. Cell Physiol Biochem. 2017;43:2457–2469. [DOI] [PubMed] [Google Scholar]
- [41].Li Z, Li J, Tang N. Long noncoding RNA Malat1 is a potent autophagy inducer protecting brain microvascular endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury by sponging miR-26b and upregulating ULK2 expression. Neuroscience. 2017;354:1–10. [DOI] [PubMed] [Google Scholar]
- [42].Yu Y, Cao F, Ran Q, et al. Long non-coding RNA Gm4419 promotes trauma-induced astrocyte apoptosis by targeting tumor necrosis factor α. Biochem Biophys Res Commun. 2017;491:478–485. [DOI] [PubMed] [Google Scholar]
- [43].Jiang Q, Shan K, Qun-Wang X, et al. Long non-coding RNA-MIAT promotes neurovascular remodeling in the eye and brain. Oncotarget. 2016;7:49688–49698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shen Y, Dong LF, Zhou RM, et al. Role of long non-coding RNA MIAT in proliferation, apoptosis and migration of lens epithelial cells: a clinical and in vitro study. J Cell Mol Med. 2016;20:537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


