ABSTRACT
Gastrointestinal stromal tumors (GISTs) are very uncommon in pediatric patients, and they are distinct clinical-pathological and molecular deviations from their adult counterparts. Most pediatric GISTs lack the c-kit or platelet-derived growth factor receptor alpha (PDGFRA) genes mutations. To date, there is no published standard guidelines available for the best treatment of pediatric GISTs, especially for infant GIST. Therefore, we report a case of 4-month-old infant with GIST of mesocolon without KIT/PDGFRA mutation. We also review the clinical, biological, and genetic features of pediatric GISTs and re-think several questions that could affect clinical practice.
Keywords: GIST, pediatric, young, adult, imatinib, CCND2, PTCH1
Introduction
Gastrointestinal stromal tumor (GIST) is a neoplasm of mesenchymal lineage originating from the interstitial cells of Cajal or their precursors of gastrointestinal tract and occur predominantly in adults.1 These tumors commonly show overexpression of the c-kit protein (CD117) and have mutations in the c-kit or platelet-derived growth factor receptor alpha (PDGFRA) genes.2 GISTs are usually observed in patients over the age of 50 and are extremely rare in pediatric populations.3 Patients under the age of 20 years comprise only about 0.4% of all patients with GIST.3 The median age of pediatric populations with GIST was 13 years.4
The majority of the pediatric GISTs lack the KIT and PDGFRA mutations that are typical of adult GISTs, which are considered wild-type GISTs (WT-GISTs).5 WT-GISTs have a female predominance, in contrast to adult GIST that occur at similar rates in men and women. The most common primary site for patients with WT-GISTs is the stomach. WT-GISTs are now known to be very different from its KIT/PDGFRA-mutated counterpart. Currently, the management of pediatric GISTs is not standardized, but it is widely accepted that surgery is the necessary treatment.6 Previous reports on GISTs in pediatric populations primarily described gastric tumors and, up to present, GIST of mesocolon in infant patient younger than one year without KIT/PDGFRA mutation has not been reported and its treatment modality remains unclear.
Therefore, we report a case of 4-month-old infant with GIST of mesocolon without KIT/PDGFRA mutation. We also entail the clinical, biological, and genetic features of WT-GISTs and re-think several practical questions that could affect clinical practice.
Presentation of case
A 4-month-old boy was admitted to the hospital with hematochezia and pale appearance in July 17, 2017. An abdominal ultrasonography detected a 7.4cm× 6.1cm× 4.6cm-sized tumor and peritoneal effusion. Blood routine examination revealed that hemoglobin and the red blood cell count was 63g/L (normal range: 130–175 g/L), 2.15 × 1012/L (normal range:4.3–5.8 × 1012/L), respectively. Volumic therapy and hemostatic treatment was administered immediately. Subsequently, a tumor (diameter, 4.6cm× 6.3cm) located in mesocolon was detected on an abdominal contrast-enhanced computed tomography (CT) scan (Figure 1). An exploratory laparotomy examination was performed and subsequently detected a tumor with clear margins. There was no evidence of metastatic lymph nodes, liver metastases, or peritoneal disseminations. Complete resection of tumor and clone (segmental bowel resection) was performed without regional lymphadenectomy.
Figure 1.
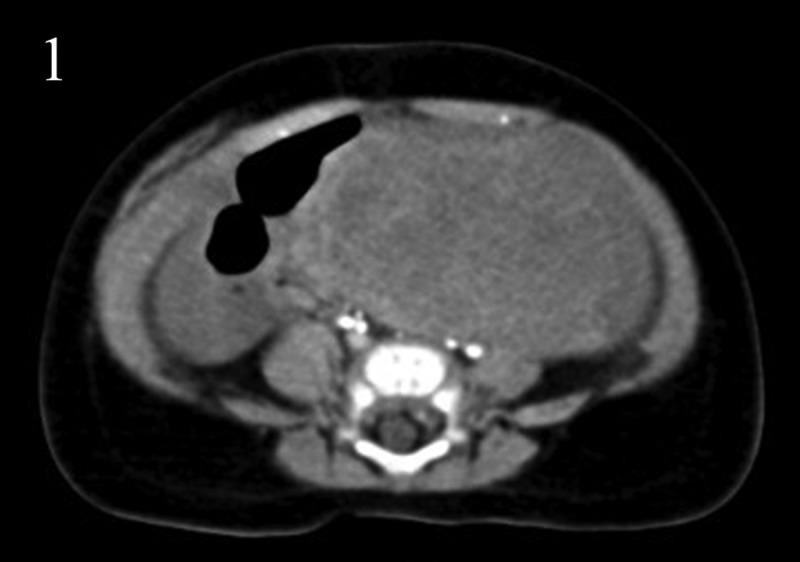
Abdominal contrast-enhanced computed tomography scan showed a tumor (diameter, 4.6cm× 6.3cm) located in mesocolon.
Hematoxylin–eosin staining showed spindle cell morphology (Figure 2). The findings of immunehistochemical analyses were positive for CD117 (Figure 3), PDGFR (Figure 4), Vimentin (Figure 5) and negative for the expression of succinate dehydrogenase B (SDHB) (Figure 6), DOG-1‚Desmin, CK, Syn‚ CgA and WT-1.The Ki-67 index was 30%. Moe than 10 mitoses were observed per 50 high-power fields (HPF). The diagnosis of a high-risk GIST was confirmed in accordance with the risk assessment classification using size and the mitotic index. Mutational analysis showed a wild type for KIT (exons 9, 11, 13, 17, 18) (Figure 7) and HER2 (Figure 8). Adjuvant anti-cancer treatment was not undertaken after operation on account of bad general condition.
Figure 2.
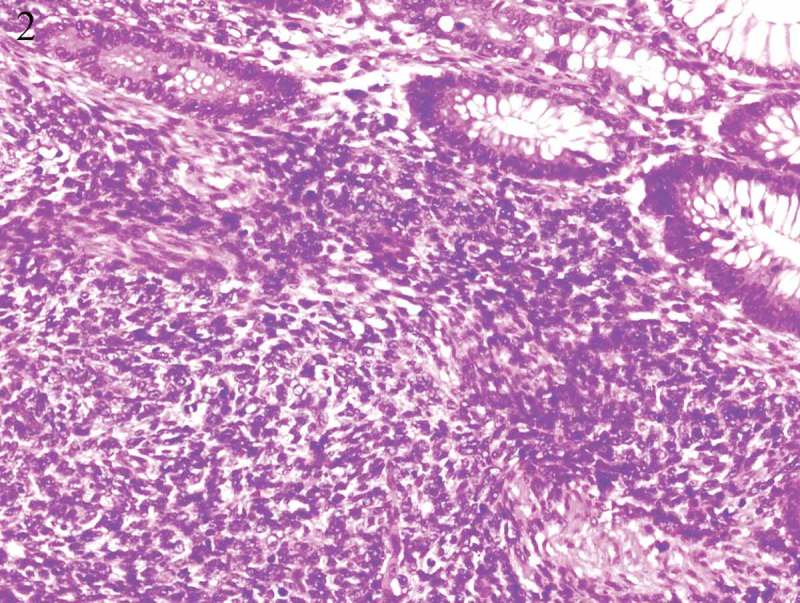
Hematoxylin–eosin staining showed spindle cell morphology.
Figure 3-5.
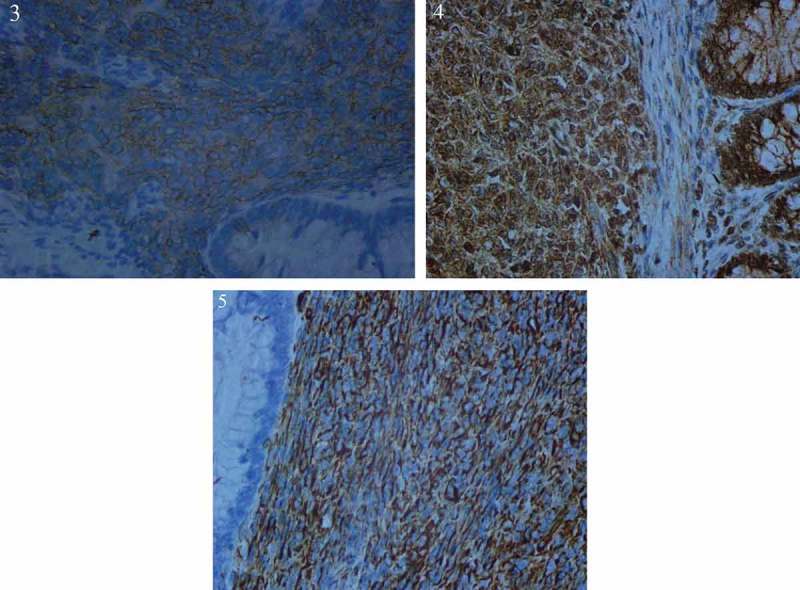
Immunehistochemical analyses were positive for CD117 (Fig.3), PDGFR (Fig.4), Vimentin (Fig.5).
Figure 6.
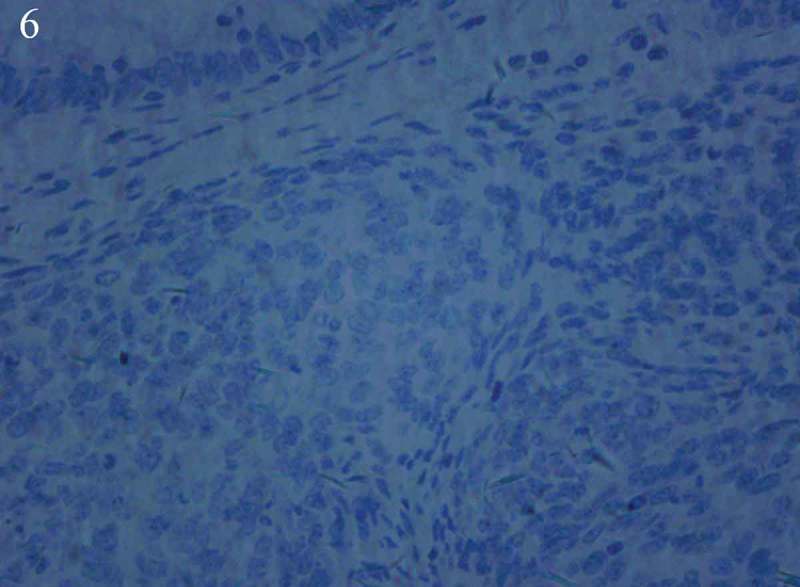
Immunehistochemical analyses showed negative for the expression of succinate dehydrogenase B (SDHB).
Figure 7-8.
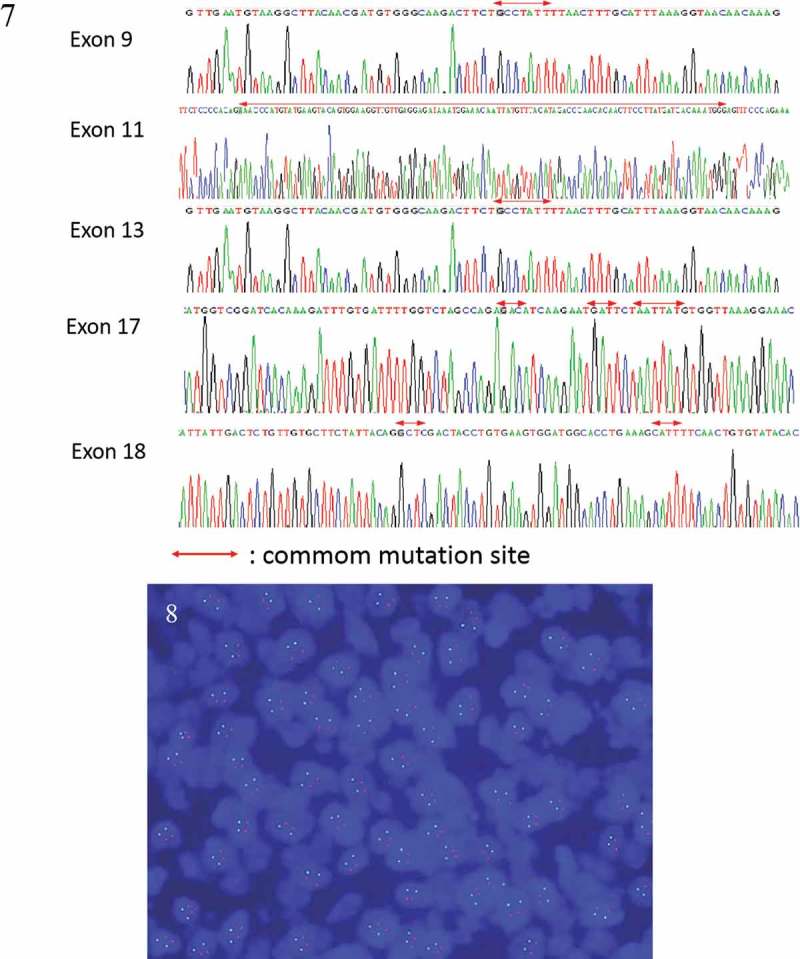
Mutational analysis showed a wild type for KIT (exons 9, 11, 13, 17, 18) (Fig.7) and HER2 (HER2/CEP17 1.0) (Fig.8).
Two months after surgery (September 22, 2017), re-image unfortunately found metastases of omentum, mesentery, retroperitoneum, inguinal lymph node and abdominal wall (Figure 9). Besides, pyoperitoneum was seen. Further examination of whole genome sequencing showed no known target-drug-related mutations (KIT, PDGFR, BRAF were all negative). The results suggested two mutated gene: CCND2 (copy number amplification, 2.6-times) and PTCH1 (polymorphism: exon 19; c.2794G> A; p.V932M; 0.78%). According to gene test, there was no target medicine for treatment.
Figure 9.
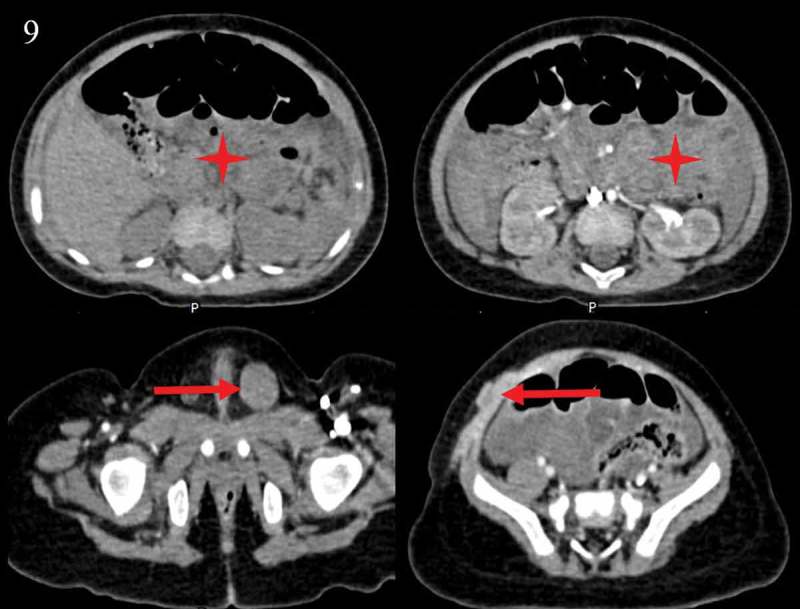
Re-image found metastases of omentum, mesentery, retroperitoneum, inguinal lymph node and abdominal wall two months after surgery.
After multidisciplinary discussion, Imatinib,100mg‚once daily was administered. Meanwhile, abdominal puncture drainage was introduced. After 22 days treatment of Imatinib, it was suspended because of severe diarrhea. Subsequently, his general condition became worse and worse and sadly he died in December 5, 2017.
The patient’s guardian has consented for the publication of the present case report.
Discussion
Gastrointestinal stromal tumor (GIST) is extremely rare entity in children that can be quite different from its adult counterpart. The Table 1 summarizes comparison of adult and pediatric GISTs. Due to their rarity and the lack of a standardized diagnostic protocol, the real incidence of pediatric GISTs is unknown. Accurate epidemiological data on pediatric GISTs stemming from the UK National Registry of Childhood Tumors indicate an annual incidence of 0.02 per million children below the age of 14 years2,4
Table 1.
Comparison of pediatric GIST with adult GIST.
| Characteristic | Pediatric GIST | Adult GIST |
|---|---|---|
| Incidence | Rare | Common |
| Median age (years) | 13 | 50 |
| Sex | Female predominance(70%) | Equal gender distribution |
| Histology | Epithelioid and mixed | Spindle |
| Gene profile | Wild Type KIT/PDGFR (85%) SDH mutation (85%) |
KIT mutation (85%) PDGFR mutation (5–10%) |
| Primary site | Stomach (85%) | Stomach (50%) |
| Lymph node metastasis | Common | Rare |
The median age of pediatric populations with GISTs was 13 years.4 The most common primary site for pediatric GISTs is the stomach, and to a far lesser degree in other gastrointestinal locations.7 Tumors have also been reported primarily in the omentum and abdominal wall, but this is exceedingly rare.4 Pediatric GIST have a female predominance, in contrast to adult GIST with equal gender distribution.
GISTs can have a spindle, epithelioid, or mixed morphology. GISTs occurring in children more often have an epithelioid or mixed morphology.8 Most adults GISTs are driven by activating mutations in the oncogenic KIT or platelet-derived growth factor alpha (PDGFRA) genes9,10, but 85% of GISTs in children are negative for KIT and PDGFRA mutations (wild-type GIST,WT-GIST).11
In present case, the patient was 4 months old when diagnosed. As far as we know, this is the youngest patient reported. Besides, the tumor located in mesocolon, which is a very rare location. This patient lack KIT, PDGFRA and BRAF mutation as other pediatric populations. Immunohistochemical analysis for succinate dehydrogenase (SDH) iron–sulfur subunit (subunit B) (SDHB) is negative, which is considered as SDH-deficient GIST.
Approximately half of WT GISTs are SDH-deficient and not driven by KIT/PDGFRA mutations.12 SDH-deficient GISTs have characteristic morphologic features including multi-nodular gastric wall involvement, multiple separate tumors, lymphovascular invasion, and lymph node metastases. Diagnostic is the loss of SDHB from the tumor cells and this can be practically assessed by IHC.13
SDH-deficient GISTs are associated with Carney’s triad syndrome, a non-heritable syndrome associated with presence of GIST, pulmonary chondroma, and paraganglioma, which is seen mainly in girls and young women.14 In this case, CT scans of chest, abdomen and pelvic showed no positive signs. Carney’s triad syndrome could not be diagnosed. The time interval from diagnosis of GIST to the appearance of other component/s of the CT averages may be more than 8 years but as long as three decades.15 Therefore, pediatric patients should receive regular clinical examination to timely detect possible extra-adrenal paraganglioma, pulmonary chondroma. Unfortunately, this case died 4 months after diagnosis.
A special notice is made regarding lymph node involvement in pediatric GISTs. Approximately 30% of the pediatric patients develop regional lymph node metastases16 contrasting with the rarity of lymph node metastasis in GISTs in adults (≤ 2%)2 Therefore, prophylactic regional dissection of lymph nodes is unnecessary given the low frequency of lymph node affectation or metastasis in adult GIST patients. In consideration of greater lymph node involvement for pediatric patients, whether prophylactic dissection is need or not is a baffling question in clinical practice and by now, there is no guideline for guiding treatment. In our case, absence of lymph node metastases was demonstrated before surgery. Unfortunately, lymph node metastases of omentum, mesentery, retroperitoneum, inguinal lymph node and abdominal wall were proved merely 2 months after operation. Accordingly, we assume that part of pediatric GIST (high-risk of lymph node metastases) may require an active dissection for lymph nodes. What we need to do afterwards is to precisely define the population of high-risk of lymph node metastases. Besides, dissection field should be discussed. In our case, metastasis inguinal lymph node was present, so, the field may be difficult to define and need to be individually tailored. All three practical questions are in the air and further research is warranted.
Pediatric GIST is a rare disease for which we have an incomplete understanding of biology and clinical features. Therefore, there is no published standard guidelines available for the best treatment of pediatric GISTs.17
Surgery is the primary modality of treatment in localized WT-GISTs, and it is the only known cure. Complete surgical resection is the initial treatment for primary and localized GISTs when the risk of morbidity is acceptable. As in adults the major goal is to achieve complete excision of the lesion with negative margins.3 These recommendations were not explicitly geared towards pediatric patients, but they are applicable nonetheless.18 The fact that GIST rarely has lymph node metastasis, routine dissection of regional lymph nodes is not necessary as discussed above. If enlarged lymph nodes were observed and lymphatic metastasis was suspected during surgery, SDH-deficient GIST should be considered and the enlarged lymph nodes should be dissected.19
Adjuvant imatinib is the only treatment for adult GISTs with results available from randomized trials. Individuals at intermediate or high risk of recurrence should be considered for adjuvant treatment.20,21 Adjuvant imatinib is not recommended in pediatric patients with WT GISTs because the available clinical data suggest that adjuvant imatinib might be less efficacious22 and has not been fully studied in this group of patients. In present case, patient with high risk for recurrence did not receive adjuvant imatinib therapy. However, recurrence-free survival in this case was merely two months, which is very short compared with adults after adjuvant imatinib. Besides, he suffered multiple locations metastasis. Therefore, we speculate that adjuvant imatinib may be feasible for special pediatric populations. What we next to do is to find out this eligible patients. Specific questions with regard to the timing of treatment, adequate dose levels, duration of therapy and timing of medicine discontinuance are supposed to afterwards address in order to optimize imatinib and minimize potential toxicity. In conclusion, the information regarding postoperative therapy should be shared with patients, and a multidisciplinary team should be involved in clinical decision-making.
In this case, the boy unfortunately suffered disease progression in multiple sites two months after operation. Whole genome sequencing suggested no identified target-drug-related mutations, but revealed CCND2 gene mutation (copy number amplification, 2.6-times) and PTCH1 gene mutation (polymorphism: exon 19; c.2794G> A; p.V932M; 0.78%). Gene test showed no mutation of CCND1 and CCNE1. Other cyclins were not detected. Unfortunately, no more tissues were used to determine the phosphorylation status or activity of CDK proteins, and the phosphorylation of pRB. Besides, IHC studies to define the functionality of the Hedgehog pathway and DNA methylation were not performed.
CCND2 is a crucial cyclin that forms a complex with CDK4 or CDK6, whose activity is required for cell cycle G1/S transition. High-level expression of this gene was observed in ovarian and testicular tumors.23,24
CCND2 gene amplification was reported in metastatic exon 11 mutant adult GIST.25 In our case CCND2 was found amplified with copy number 2.6 times. As far as we know, CCND2 was never reported in pediatric wild-type GISTs. However, because of low copy number of our case and unclear understanding of CCND2, there is no target medicine at present for GISTs treatment, especially pediatric GISTs.
PTCH1 gene, a tumor suppressor gene, is a vital component of Hedgehog signaling pathway that is critical for the development of the gastrointestinal tract.26 PTCH1 gene mutation is common in nevoid basal cell carcinoma syndrome, an autosomal-dominant inherited disorder.27 It is reported that PTCH1 gene mutation is associated with gastrointestinal stromal-like tumors in mice28 and contributes to the pathogenesis of human gastrointestinal stromal tumors via GLI-mediated activation of KIT expression.29 To the best of our knowledge, PTCH1 gene mutation is not recorded in pediatric GIST, therefore, we know little in pediatric populations and no corresponding medicine exists for GIST treatment.
Accordingly, multidisciplinary discussion was performed to optimize the treatment for our sad little boy. Sunitinib or imatinib can be selected as the first line therapy.
Sunitinib is 10 times more potent than imatinib in achieving of WT KIT inhibition.8 In adult patients with advanced imatinib-resistant GIST, sunitinib significantly prolongs time to tumor progression and survival.30 Adult patients with WT GIST achieves the greatest clinical benefit from sunitinib.31 Sunitinib has been reported to help achieve substantial antitumor activity with acceptable tolerability in adult and pediatric patients with imatinib-resistant GIST.32 Based on these findings, sunitinib might be considered as front-line treatment in WT GIST. Because of financial reason, sunitinib was not preferred.
Imatinib is the preferred first line therapy for inoperable, metastatic, or recurrent adults GIST because it prolongs the median survival.33 However, response to imatinib differs by tumor genotype. The objective response rate and median time to tumor progression with imatinib therapy in adult patients with WT GIST are significantly lower than in adult mutant patients.22 What’s more, the pediatric dosing and adverse event profile of imatinib are established in reference of Philadelphia chromosome-positive leukemia.34 Therefore, our patient received imatinib therapy. However, after 22 days treatment, imatinib was suspended because of severe diarrhea and the boy died after two weeks of drug withdrawal.
Conclusion
In conclusion, pediatric GISTs is an extremely rare entity, especially infant GISTs. Many aspects are different between pediatric GISTs and adult GISTs, such as KIT and PDGFRA mutations, treatment modality. We in detail review the clinical, biological, and genetic features of pediatric GISTs and re-think several practical questions that could affect clinical practice. This report suggests treatment should be tailored for some specific pediatric GIST after fully considering age, disease location, gene mutation, general condition. Efforts to better study this disease are urgently needed and will likely lead to new treatment approaches for these patients.
Funding Statement
This work is supported by the national natural science foundation of china (81472810), science and technology development plans of Shandong province (2014GSF118138).
Potential Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose
References
- 1.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459–465. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J.. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466–1478. [DOI] [PubMed] [Google Scholar]
- 3.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet (London, England). 2013;382(9896):973–983. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 4.Benesch M, Wardelmann E, Ferrari A, Brennan B, Verschuur A. Gastrointestinal stromal tumors (GIST) in children and adolescents: A comprehensive review of the current literature. Pediatric Blood & Cancer. 2009;53(7):1171–1179. doi: 10.1002/pbc.22123. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Broto J, Rubio L, Alemany R, Lopez-Guerrero JA. Clinical implications of KIT and PDGFRA genotyping in GIST. Clin Transl Oncol. 2010;12(10):670–676. doi: 10.1007/s12094-010-0576-7. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29(10):1373–1381. [DOI] [PubMed] [Google Scholar]
- 7.Scarpa M, Bertin M, Ruffolo C, Polese L, D’Amico DF, Angriman I. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. J Surg Oncol. 2008;98(5):384–392. doi: 10.1002/jso.21120. [DOI] [PubMed] [Google Scholar]
- 8.Agaram NP, Laquaglia MP, Ustun B, Guo T, Wong GC, Socci ND, Maki RG, DeMatteo RP, Besmer P, Antonescu CR. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14(10):3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science (New York, N.Y.).. 1998;279(5350):577–580. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen C-J, Joseph N, Singer S, Griffith DJ, Haley A, Town A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science (New York, N.Y.). 2003;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 11.Janeway KA, Liegl B, Harlow A, Le C, Perez-Atayde A, Kozakewich H, Corless CL, Heinrich MC, Fletcher JA. Pediatric KIT wild-type and platelet-derived growth factor receptor alpha-wild-type gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res. 2007;67(19):9084–9088. doi: 10.1158/0008-5472.CAN-07-1938. [DOI] [PubMed] [Google Scholar]
- 12.Jasek K, Buzalkova V, Minarik G, Stanclova A, Szepe P, Plank L, Lasabova Z. Detection of mutations in the BRAF gene in patients with KIT and PDGFRA wild-type gastrointestinal stromal tumors. Virchows Archiv. 2017;470(1):29–36. doi: 10.1007/s00428-016-2044-4. [DOI] [PubMed] [Google Scholar]
- 13.Poveda A. Garcia Del Muro X, Lopez-Guerrero JA, et al. GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treat Rev. 2017;55:107–119. doi: 10.1016/j.ctrv.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Carney JA, Sheps SG, Go VL, Gordon H. The triad of gastric leiomyosarcoma, functioning extra-adrenal paraganglioma and pulmonary chondroma. N Engl J Med. 1977;296(26):1517–1518. doi: 10.1056/NEJM197706302962609. [DOI] [PubMed] [Google Scholar]
- 15.Carney JA. Carney triad: a syndrome featuring paraganglionic, adrenocortical, and possibly other endocrine tumors. J Clin Endocrinol Metab. 2009;94(10):3656–3662. doi: 10.1210/jc.2009-1156. [DOI] [PubMed] [Google Scholar]
- 16.Agaimy A, Carney JA. Lymphatics and D2-40/podoplanin expression in gastrointestinal stromal tumours of the stomach with and without lymph node metastasis: an immunohistochemical study with special reference to the Carney triad. J Clin Pathol. 2010;63(3):229–234. doi: 10.1136/jcp.2009.074062. [DOI] [PubMed] [Google Scholar]
- 17.Janeway KA, Pappo A. Treatment guidelines for gastrointestinal stromal tumors in children and young adults. J Pediatr Hematol Oncol. 2012;34(Suppl 2):S69–72. doi: 10.1097/MPH.0b013e31824e3899. [DOI] [PubMed] [Google Scholar]
- 18.Janeway KA, Weldon CB. Pediatric gastrointestinal stromal tumor. Semin Pediatr Surg. 2012;21(1):31–43. doi: 10.1053/j.sempedsurg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Doyle LA, Nelson D, Heinrich MC, Corless CL, Hornick JL. Loss of succinate dehydrogenase subunit B (SDHB) expression is limited to a distinctive subset of gastric wild-type gastrointestinal stromal tumours: a comprehensive genotype-phenotype correlation study. Histopathology. 2012;61(5):801–809. doi: 10.1111/j.1365-2559.2012.04300.x. [DOI] [PubMed] [Google Scholar]
- 20.Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PWT, Demetri GD, Blackstein ME, Blanke CD, Von Mehren M, Brennan MF, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2009;373(9669):1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran S-E, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. Jama. 2012;307(12):1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 22.Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CDM, Ryan CW, Von Mehren M, Blanke CD, Rankin C, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 study by cancer and leukemia group B and Southwest oncology group. J Clinical Oncology: Official Journal Am Soc Clin Oncol. 2008;26(33):5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunn S, Reveles X, Weldon K, Barrera A, Ishaque M, Taylor D, McCaskill C, Kim J, Shah R, Mohammed M, et al. Molecular cytogenetics as a clinical test for prognostic and predictive biomarkers in newly diagnosed ovarian cancer. J Ovarian Res. 2013;6(1):2. doi: 10.1186/1757-2215-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Von Eyben FE. Chromosomes, genes, and development of testicular germ cell tumors. Cancer Genet Cytogenet. 2004;151(2):93–138. doi: 10.1016/j.cancergencyto.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Saponara M, Urbini M, Astolfi A, Indio V, Ercolani G, Del Gaudio M, Santini D, Pirini MG, Fiorentino M, Nannini M, et al. Molecular characterization of metastatic exon 11 mutant gastrointestinal stromal tumors (GIST) beyond KIT/PDGFRalpha genotype evaluated by next generation sequencing (NGS). Oncotarget. 2015;6(39):42243–42257. doi: 10.18632/oncotarget.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, Madison BB, Waghray M, Ferris JE, Hu C, Merchant JL, et al. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137(2):618–628. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponti G, Manfredini M, Pastorino L, Maccaferri M, Tomasi A, Pellacani G. PTCH1 germline mutations and the basaloid follicular hamartoma values in the tumor spectrum of basal cell carcinoma syndrome (NBCCS). Anticancer Res. 2018;38(1):471–476. doi: 10.21873/anticanres.12246. [DOI] [PubMed] [Google Scholar]
- 28.Pelczar P, Zibat A, Van Dop WA, Heijmans J, Bleckmann A, Gruber W, Nitzki F, Uhmann A, Guijarro MV, Hernando E, et al. Inactivation of Patched1 in mice leads to development of gastrointestinal stromal-like tumors that express Pdgfralpha but not kit. Gastroenterology. 2013;144(1):134–144.e136. doi: 10.1053/j.gastro.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang CM, Lee TE, Syed SA, Burgoyne AM, Leonard SY, Gao F, Chan JC, Shi E, Chmielecki J, Morosini D, et al. Hedgehog pathway dysregulation contributes to the pathogenesis of human gastrointestinal stromal tumors via GLI-mediated activation of KIT expression. Oncotarget. 2016;7(48):78226–78241. doi: 10.18632/oncotarget.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demetri GD, Van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet (London, England). 2006;368(9544):1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 31.Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, Town A, McKinley A, Ou W-B, Fletcher JA, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clinical Oncology: Official Journal Am Soc Clin Oncol. 2008;26(33):5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichardt P, Kang YK, Rutkowski P, Schuette J, Rosen LS, Seddon B, Yalcin S, Gelderblom H, Williams CC, Fumagalli E, et al. Clinical outcomes of patients with advanced gastrointestinal stromal tumors: safety and efficacy in a worldwide treatment-use trial of sunitinib. Cancer. 2015;121(9):1405–1413. doi: 10.1002/cncr.29220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demetri GD, Von Mehren M, Blanke CD, Van Den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 34.Champagne MA, Capdeville R, Krailo M, Qu W, Peng B, Rosamilia M, Therrien M, Zoellner U, Blaney SM, Bernstein M. Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: results from a Children’s Oncology Group phase 1 study. Blood. 2004;104(9):2655–2660. doi: 10.1182/blood-2003-09-3032. [DOI] [PubMed] [Google Scholar]


