ABSTRACT
Cytokine-induced killer (CIK) cells are demonstrated to possess potent cytolytic effect against ovarian cancer cells in vitro and in vivo. However, the clinical efficacy of maintenance therapy of CIK cells in patients with epithelial ovarian cancer (EOC) after first-line treatment remains unclear. This retrospective study included 646 cases of postoperative EOC patients, 72 of which received chemotherapy and sequential immunotherapy (CIT group), and 574 of which received only chemotherapy (Control group). Patients in the CIT group received at least four cycles of CIK cell (range 8.0 × 109 – 1.3 × 1010 cells) transfusion, with the interval of each cycle being 2 weeks. Survival analysis showed a significantly higher overall survival (OS) rate in the CIT group compared with the control group, as well as a favorable progression-free survival (PFS). Univariate and multivariate analyses indicated that adjuvant CIT was an independent prognostic factor for the OS of patients with EOC. Furthermore, subgroup analyses showed that adjuvant CIT significantly improved the OS of patients older than 45 years, with CA125 ≤ 1000, or with moderate or poorly differentiated tumors, and prolonged the PFS of patients with residual disease > 1 cm. Additionally, Kaplan-Meier analyses revealed that a higher fraction of CD3+CD8+/CD3+CD56+ phenotypes or lower percentage of CD3+CD4+/CD3−CD56+ phenotypes in the infused CIK cells significantly associated with better survival of patients with EOC. Furthermore, across all processes of CIK cell immunotherapy in the CIT group, 12.5% (9/72) of patients developed self-limiting light fevers and shivering at grade 1 or 2. No immunotherapy-related serious reactions were recorded. These data indicate that adjuvant CIT with CIK cells is an effective therapeutic approach to prolonging the survival of EOC patients.
KEYWORDS: Adjuvant cellular immunotherapy, clinical outcome, epithelial ovarian cancer
Introduction
Ovarian cancer is the most lethal gynecologic malignancy and the fifth leading cause of cancer mortality in women worldwide,1,2 whose most common form is epithelial ovarian cancer (EOC).2 Despite improvements in surveillance and therapeutic approaches, the 5-year survival rate for EOC patients who receive a first-line regimen lingers at 35 – 40%, with said regimen consisting of both surgical cytoreduction and chemotherapy with a two-drug combination of paclitaxel and carboplatin. Furthermore, 85% of patients diagnosed as stage III–IV who have an initial complete response to surgery and chemotherapy, will ultimately experience disease progression and resistance to the first-line regimen.3–5 Therefore, the search for more effective and novel therapies for patients with EOC to prevent recurrence remains an imperative clinical challenge.
In recent years, malignant tumors have been established to be immunogenic, and accumulating evidence points to a potential linkage between anti-tumor immunity and cancer progression.6–8 In 1991, Schmidtwolf et al were the first to attempt using CIK cells for lymphoma treatment in SCID mice,9 while in 1994, Lu et al first identified the CD3+CD56+ cell subset as highly efficient cytotoxic effector cells.10 Later, Introna et al performed the first series of phase I clinical trials using allogeneic (donor’s) CIK cells in patients relapsing after allogeneic haematopoietic stem cell transplantation (HSCT).11 Presently, CIK cells have been proven to be safe and effective in cancer treatment. Our previous studies, alongside others, have demonstrated that adjuvant cellular immunotherapy (CIT) elicits favorable clinical responses in hematological diseases 11–13 and many solid tumors 14–17 . To date, several immune cell types have shown promise in cancer treatment, of which cytokine-induced killer (CIK) cells are the most widely used. CIK cells are a type of heterogeneous immune-active host effector cells, including CD3+CD56+ NKT-like cells, CD3–CD56+ NK cells and CD3+CD56− antitumor T cells. Among these, CD3+CD56+ cells have been identified as the main effectors of CIK cells.18–22 In comparison with other immune cells, CIK cells possess several distinctly superior aspects: They (1) proliferate rapidly and can be obtained quickly from cancer patients via in vitro culture; (2) exhibit strong antitumor activity and a broad spectrum of targeted tumors, up to and including ones that are non-susceptible to lymphokine-activated killer cells or NK cells; (3) have minimal toxicity and few graft-versus-host diseases. Although their significant antitumor capacity and potential efficacy against ovarian cancer has been identified in cell and mouse models, the clinical efficacy of CIK cells in ovarian cancer treatment remains unclear.8,23–27
Therefore in this study, we retrospectively assessed the clinical efficacy of adjuvant CIT with CIK cells combined with chemotherapy in EOC patients after surgery to provide supportive information on whether CIT could improve the clinical outcome in patients with EOC. Our data suggest that clinical CIT with CIK cells is able to significantly prolong the survival of EOC patients.
Results
Patient demographics and clinical characteristics
In total, 646 patients with EOC were retrospectively analyzed. The average age was 57.94 years (± 10.80 years), with a range of 34–89 years. Among them, 72 patients that underwent surgery/chemotherapy and received postoperative immunotherapy were enrolled as the CIT group, whereas 574 cases that underwent surgery/chemotherapy only were enrolled as the control group. The demographics and clinical characteristics of the patients in each group are presented herein, and no significant difference was present in the age, gender and clinical features of the two groups (Table 1).
Table 1.
Demographics and clinical characteristics of EOC patients.
| Variables | Control group (n = 574) |
CIT group (n = 72) |
p value* |
|---|---|---|---|
| Age (years) | 51.41 ± 10.82 | 50.62 ± 11.31 | 0.761 |
| CA125 (mean ± SD) | 2395.88 ± 5386.18 | 2448.2 ± 5033.1 | 0.935 |
| Pathologic grade | 0.376 | ||
| Well | 23 | 3 | |
| Moderate | 64 | 13 | |
| Poor | 364 | 46 | |
| missing cases | 123 | 10 | |
| FIGO tumor stage | 0.99 | ||
| Ⅰ/Ⅱ | 97 | 14 | |
| Ⅲ/Ⅳ | 466 | 58 | |
| missing data | 11 | 0 | |
| Histopathology | 0.244 | ||
| Serous carcinoma | 498 | 59 | |
| Mucinous carcinoma | 15 | 2 | |
| Clear cell carcinoma | 10 | 3 | |
| Endometrioid carcinoma | 20 | 3 | |
| Unspecified type | 31 | 5 | |
| Pelvic lymph node metastasis | 0.160 | ||
| Yes | 105 | 21 | |
| No | 163 | 20 | |
| missing data | 306 | 31 | |
| Abdominal aorta lymph node metastasis | 0.203 | ||
| Yes | 99 | 14 | |
| No | 91 | 7 | |
| missing data | 384 | 51 | |
|
Chemotherapy# CBP/CAP TP/TC Other Residual disease |
67 459 48 |
6 64 2 |
0.147 0.527 |
| < 1cm | 354 | 49 | |
| ≥ 1cm | 87 | 8 | |
| missing data | 133 | 15 |
*, Chi-Squared Tests;
#, CBP: Cyclophosphoramide + bleomycin + Cisplatin; CAP: Cyclophosphoramide + deoxymycin + Cisplatin; TP: paclitaxel + Cisplatin; TC: paclitaxel + Carborplatin.
Quality of cultured immune cells
To prepare the therapeutic cells, immune cells were isolated from the peripheral blood of post-chemotherapy patients and purified. After culturing and expansion, the final number of CIK cells was approximately 8.0 × 109 to 1.3 × 1010. The viability of immune cells exceeded 95% and the cells were without any bacterial, fungal and mycoplasma contamination. Moreover, the median percentage of CD3+CD56+ population in the CIK cells was 21.9% (range, 8.5% – 34.8%). Representative results from one of the patients enrolled in the CIT group are shown in Figure 1A.
Figure 1.
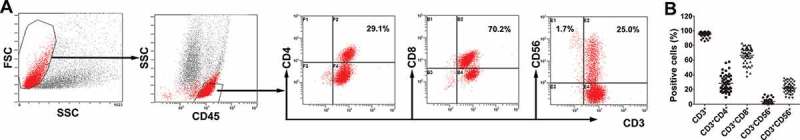
Characteristics of CIK cells. (A) Phenotypic analysis of immune cells after expansion. The percentage of CIK cells after 14-d induction from the patient. Numbers indicate the percentage of cells. (B) Characteristics of cultured CIK cells. The phenotype of the autologous CIK cells after culturing at day 14th from each patient was characterized by flow cytometry using four-color fluorescence. The positive rates of CD3+, CD3+CD4+, CD3+CD8+, CD3−CD56+, CD3+CD56+ are shown.
Following quality testing, all qualified immune cells were infused back into the patients. All patients in the CIT group completed at least four cycles of CIK infusion as scheduled by the protocol (Fig. S1).
Characteristics of cultured CIK cells
The total number of activated CIK cells at the time of infusion was, on average, 8.8 × 109 cells (range, 8.0 × 109–1.3 × 1010 cells). The CIK cells were primarily CD3+ T cells (median, 96.4%; range, 85.8% to 98.9%) and also comprised of CD4+ T cells (median, 25.4%; range, 9.4% to 57.9%), CD8+ T cells (median, 69.9%; range, 38.2% to 83.1%), NK cells (CD3−CD56+, median, 2.7%; range, 0.5% to 13.3%), and NKT-like cells (CD3+CD56+, median, 21.9%; range, 8.5% to 34.8%) (Figure1B).
Programmed death-1 (PD-1) is well known negative receptor on CIK cell activation, migration, proliferation and secretion of cytotoxic mediators. As such, the phenotype and PD-1 expression of the autologous CIK cells is characterized by flow cytometry. The ratio of PD-1+ cells among the populations of CIK cells ranged from 5.1 to 7.3% with a median value of 6.8%; the ratio of the CD4+PD-1+ subset ranged from 2.4 to 5.0% with a median of 3.9%; the ratio of the CD8+PD-1+ subset ranged from 0.8 to 2.6% with a median of 2.1%; the ratio of CD3−CD56+ PD-1+ subset ranged from 0.02 to 0.29% with a median of 0.15%; and the ratio of the CD3+CD56+ PD-1+ subset ranged from 0.13 to 0.93% with a median of 0.67% (Figure 2A-2E). These data collectively suggested that CIK cells exhibited a low PD-1 expression.
Figure 2.
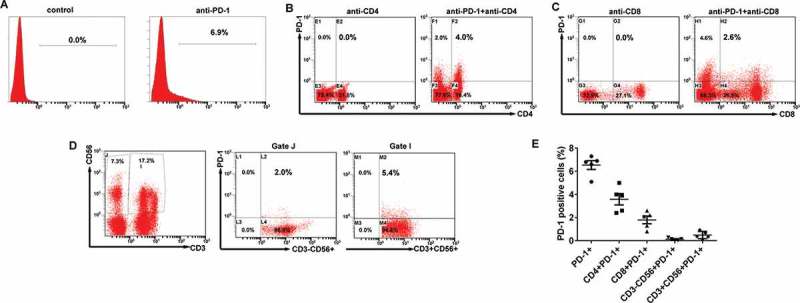
PD-1 expression on the CIK cells. (A) The percentages of PD-1 positive cells among the populations of CIK cells. (B) The percentages of CD4+PD-1+ CIK cells. (C) The percentages of CD8+PD-1+ CIK cells. (D) The percentages of CD3−CD56+ PD-1+ and CD3+CD56+ PD-1+ CIK cells. (E) PD-1 expression on each subgroup of CIK cells in five EOC patients.
Furthermore, CIK cells were prepared from three EOC patients and their cytotoxic activity on EOC cells was assessed by an LDH release assay. As shown in Fig. S2, the CIK cells displayed a strong cell killing activity on two EOC cell lines in a cell number-dependent manner. This data confirms that the CIK cells in our study demonstrate potent cytotoxic activity.
Complications and toxicity of CIK cell treatment
Across all processes of CIK cell immunotherapy in the CIT group, 12.5% (9/72) of patients developed self-limiting light fevers and shivering at grade 1 or 2. No patients exhibited pulmonary or renal symptoms, or any sign of infection, hepatic functional deterioration, or autoimmune disorders.
Overall survival (OS) and progression-free survival (PFS) analysis
All 646 patients enrolled in this study were firstly assessed for OS. Over a median follow-up of 33.0 months (range, 8 – 127 months), 47.1% (304/646) of patients died with a median post-surgery OS of 43.67 months (range 0.37–120.9 months). The OS rates at 1-, 3-, and 5-years were 87%, 63% and 47% for CIT patients, and 65%, 44%, and 31% for control group patients, respectively. Patients who received adjuvant CIT exhibited a significantly more favorable OS than control group patients (median OS, 63.6 vs. 39.6 months, p = 0.001, Figure 3A).
Figure 3.
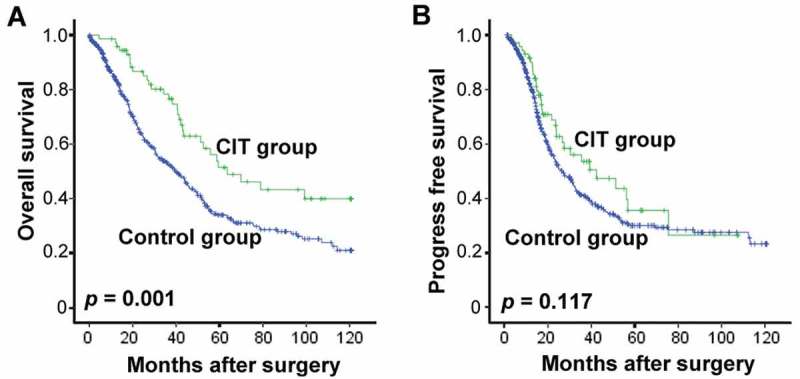
Survival analysis in patients with EOC. Overall survival curves (OS, A) and Progress-free survival curves (PFS, B) for EOC patients (n = 646) who received adjuvant cellular immunotherapy (CIT) combined with chemotherapy (CIT group, n = 72) or chemotherapy alone (Control group, n = 574).
To assess the survival benefit resulting from CIT, the patients’ PFS was also analyzed. The median PFS after surgery was 28.2 months (range 0.27 – 120.7 months), while the PFS rates at 1-, 3-, and 5-years were 63%, 48% and 36% in CIT patients, and 52%, 35%, and 30% in control group patients, respectively. Similarly, Kaplan-Meier curves showed association between CIT and favorable PFS (median PFS, 41.6 vs. 26.1 months, p = 0.117, Figure 3B), with the not-significant p-value.
To further evaluate the differences in CIT treatment-induced survival benefit, the association between OS or PFS of patients and the number of CIK cell injections has been analyzed. Interestingly, patients with no less than 10 instances of CIK cell injection displayed significantly better OS and PFS compared with those with less than 10 (Figure 4A-4B). This data further suggests that CIK treatment improves both OS and PFS of patients with EOC.
Figure 4.
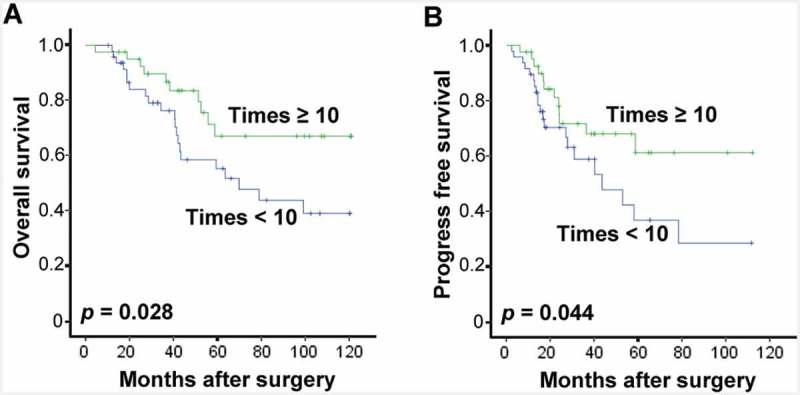
Benefits of CIT treatment times in patients with EOC. Overall survival curves (OS, A) and Progress-free survival curves (PFS, B) for EOC patients who received adjuvant cellular immunotherapy. Patients with CIK cell injections of no fewer than ten times (Times ≥ 10, n = 33) and patients with fewer than 10 injections (Times < 10, n = 39) were compared.
Univariate and multivariate analysis
The favorable OS outcome of the adjuvant CIT treatment for patients with EOC was further supported by univariate and multivariate Cox proportional hazards regression analyses. According to the univariate analysis (Table 2, middle panel), factors including: younger patient age (p = 0.011), CA125 ≤ 1000 (p = 0.021), better pathologic grade (p = 0.002), earlier stage (p = 0.0001), serous histopathology (p = 0.008), pelvic or abdominal aorta lymph node negativity (p = 0.001 or p = 0.0001), residual disease < 1 cm (p = 0.0001) and adjuvant CIT treatment (p = 0.001) were significantly associated with an improved OS of patients with EOC. Further multivariate survival analysis indicated that earlier stage (p = 0.002), less residual disease (p = 0.0001) and CIT treatment (p = 0.039) were associated with improved overall survival (Table 2, right panel). Together, these results suggest that adjuvant CIT was an independent prognostic factor for OS of patients with EOC, and CIT is efficient in prolonging OS of patients with EOC.
Table 2.
Univariate and multivariate analysis of overall survival in EOC patients.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Variables | HR (95 % CI) | p value* | HR (95 % CI) | p value* |
|
Age (≥ 45 vs. < 45) |
1.448 (1.087–1.928) | 0.011 | 1.215 (0.825–1.791) | 0.324 |
|
CA125 (> 1000 vs. ≤ 1000) |
1.313 (1.042–1.655) | 0.021 | 1.080 (0.803–1.454) | 0.610 |
|
Pathologic grade (1,2 vs. 3) |
0.681 (0.492–0.943) | 0.002 | 0.942 (0.637–1.392) | 0.764 |
|
FIGO tumor stage (I, II vs. III, IV) |
0.355 (0.229–0.489) | 0.0001 | 0.467 (0.287–0.760) | 0.002 |
|
Histopathology (Serous vs. other) |
0.606 (0.420–0.875) | 0.008 | 0.753 (0.421–1.348) | 0.341 |
|
Pelvic lymph node metastasis (Yes vs. No) |
1.783 (1.264–2.515) | 0.001 | NA | NA |
|
Abdominal aorta lymph node metastasis (Yes vs. No) |
2.401 (1.590–3.626) | 0.0001 | NA | NA |
|
Residual disease (< 1cm vs. ≥ 1cm) |
0.506 (0.375–0.683) | 0.0001 | 0.492 (0.348–0.694) | 0.0001 |
|
Treatment (CIT vs. Control) |
0.536 (0.369–0.778) | 0.001 | 0.600 (0.369–0.975) | 0.039 |
*, Cox regression;
HR: hazard ratio; CI: confidence interval; NA: not analyzed; CIT: cellular immunotherapy.
Identifying CIT treatment effect in EOC patient subgroups
To further identify whether a subgroup of EOC patients may potentially benefit to a greater degree from CIT treatment, the patients were firstly divided into different groups based on their age (≥ 45- or < 45-year), CA125 level (> 1000 or ≤ 1000), pathologic grade (1, 2 or 3), FIGO tumor stage (stages I–II or stages III–IV), histopathology (serous or other), and residual disease (< 1 cm or ≥ 1 cm). Analysis of OS of these subgroups revealed that patients in the Age ≥ 45 group tended to show greater improvement in OS in response to CIT treatment than patients younger than 45 (p, 0.001 vs. 0.341, Figure 5A and 5B). Moreover, for patients with CA125 ≤ 1000 or with moderate or poorly differentiated tumors, CIT significantly improved survival in comparison with control (Figure 5C; p = 0.011; Figure 5E and 5F, p = 0.001), while this trend was absent in patients with CA125 > 1000 (Figure 5D) or with well differentiated tumors (Figure 5G). However, neither FIGO tumor stage nor residual disease had any effect on the effectiveness of adjuvant CIT (data not shown).
Figure 5.
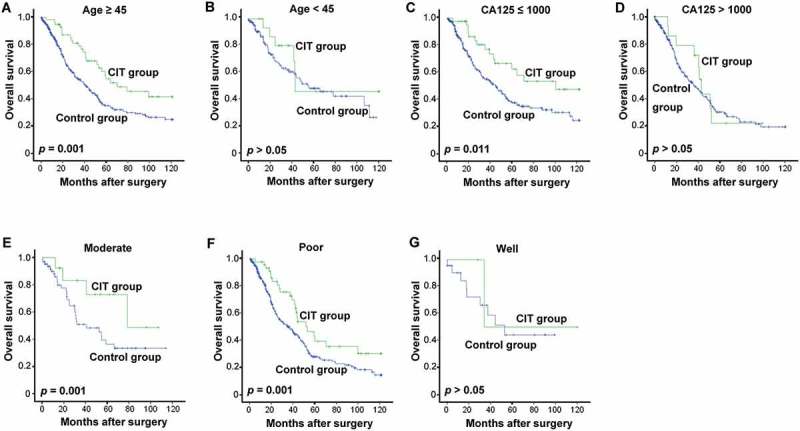
Subgroup analysis to estimate the benefits of OS from adjuvant CIT. (A-B) CIT significantly prolonged the OS of EOC patients more than 45-y-old. Patients older than 45-y (A, n = 484) or younger than 45-y (B, n = 162) were divided and were analyzed. (C-D) CIT significantly prolonged the OS of EOC patients with CA125 ≤ 1000. Patients with CA125 ≤ 1000 (C, n = 344) or CA125 > 1000 (D, n = 271) were divided and were analyzed. (E-G) CIT significantly prolonged the OS of EOC patients with moderate to poorly differentiated tumors. Patients with moderate (E, n = 410), poor (F, n = 77) or well differentiated tumors (G, n = 26) were divided and were analyzed. For (A-G), the OS rate was evaluated by the Kaplan–Meier method and compared by the stratified log-rank test.
To the same end, we also conducted an analysis of PFS in the patient subgroups which revealed that patients with residual disease (≥ 1 cm) benefited more from CIT (Figure 6A and 6B). Meanwhile, age, FIGO tumor stage, pathologic grade or CA125 did not pose any significant effect on the effectiveness of adjuvant CIT (data not shown).
Figure 6.
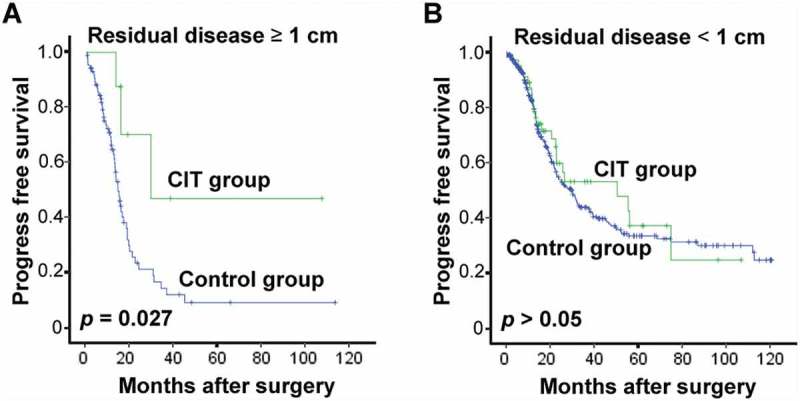
Subgroup analysis to estimate the benefits of PFS from adjuvant CIT. CIT significantly prolonged the PFS of EOC patients with residual tumors of no less than 1 cm. Patients with residual tumor ≥ 1 cm (A, n = 95) or residual tumor < 1 cm (B, n = 403) were divided and were analyzed. The PFS rate was evaluated by the Kaplan–Meier method and compared by the stratified log-rank test.
Collectively, our data demonstrated that adjuvant CIT is an effective therapeutic approach to prolong survival in EOC patients, especially patients older than 45 years, with CA125 ≤ 1000, with moderate or poorly differentiated tumors, or with residual disease≥ 1 cm.
Effect of various CIK cell phenotypes on patient survival
As variation was present in the phenotypes of the CIK cells that were transfused to each patient (Figure 1B), we sought to investigate whether these phenotype differences posed any impact on the patients’ prognoses. A survival analysis revealed that a higher percentage of CD3+ CD8+ T cells correlated significantly with improved rates of OS and PFS for patients in the CIK treatment group (Figure 7A; p = 0.005; Figure 7E, p = 0.027). As the main effectors of CIK cells, a higher percentage of transfused CD3+CD56+ correlated significantly with improved benefit to OS from CIK cell treatment in comparison with a low percentage population (Figure 7B; p = 0.042), and demonstrated a contributive tendency in PFS as well (Figure 7F). Unexpectedly, patients who underwent a transfusion of a lower percentage of CD3+ CD4+ T cells or CD3−CD56+ NK cells exhibited better OS and PFS than those with a transfusion of a higher percentage of CD3+ CD4+ or CD3−CD56+cells (Figure 7C and 7G, and Figure 7D and 7H).
Figure 7.
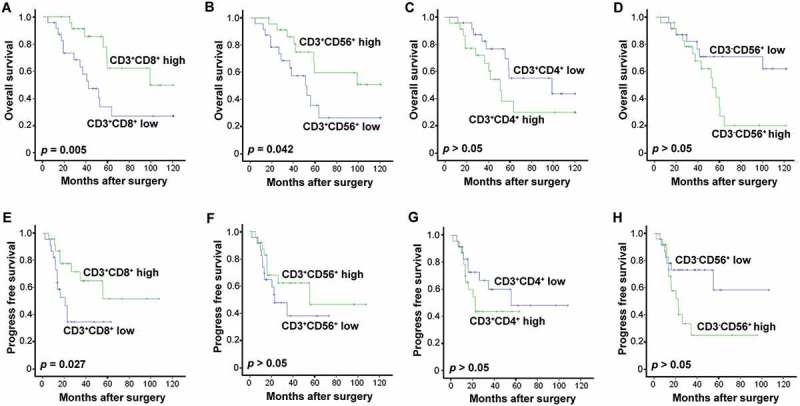
Effects of CIK cell phenotype on survival in the patients. (A-D) Association of CIK cell phenotype and the patients' OS. (E-H) Association of CIK cell phenotype and the patients' PFS. The phenotype of the autologous CIK cells after culturing at day 14th from each patient was characterized by flow cytometry. The median of positive rate of CD3+ CD8+ (A, E), CD3+ CD56+ (B, F), CD3+ CD4+ (C, G), and CD3-CD56+ (D, H) cells was chosen as the cut-off point for separating low and high groups. The OS and PFS rates were evaluated by the Kaplan–Meier method and compared by the stratified log-rank test.
Discussion
Innovative immunotherapeutic strategies offer the promise of improving clinical outcomes in women with EOC by enhancing host anti-tumor responses. While in vitro studies have demonstrated the potential induction of anti-tumor responses via the application of immunotherapeutic strategies, no clinical evidence and trials currently exist to approve immunotherapeutic viability for women afflicted with EOC.8,23,25,28,29 Therefore, in this study, through a retrospective analysis of 646 EOC patient cases, we sought to validate the survival benefit of maintenance immunotherapy with CIK cells in EOC patients after first-line cytoreduction and chemotherapy.
In this study, we established that EOC patients who received additional sequential CIT demonstrate significantly improved OS and prolonged PFS in comparison with patients in the control group, whom received postoperative chemotherapy alone. Previously, Liu et al showed that adjuvant CIK cell treatment improved the PFS of EOC patients, and marginally improved the OS of patients.28 The difference in effect of CIT on OS and PFS of patients in our and Liu’s studies may be due to limited sample size. Nonetheless, these data collectively suggest that immunotherapy with CIK cells improves the OS and PFS of patients with ovarian cancer after first-line treatment. CIT may be a promising new therapeutic strategy against EOC, and further endeavors involving larger sample sizes are desired.
The incidence rate of EOC increases with age. Our data together with others’ studies showed that advanced age in patients with EOC was associated with short survival duration.30,31 Furthermore, in the subgroup analyses, adjuvant CIT was found to be significantly associated with an improved overall survival rate in patients more than 45 years old, but this association was absent in EOC patients who were under the age of 45. This improvement or lack thereof may be explained by the fact that immune alteration is age dependent.32 Decreased antitumor immunity in elderly patients may be associated with the general decline in the performance of immune cells, since aging may severely affect chemokine production and the physical condition of immune cells.31,33 On a further note, thanks to advancement in new treatments, mortality caused by ovarian cancer has declined in the last decade. However, the decline in mortality rate is unevenly distributed across the age spectrum. While mortality in younger women has decreased by 21.7%, said figure was only 2.2% lower for elderly women.31 Our results revealed that adjuvant CIT achieved favorable clinical outcomes for older patients, suggesting its effectiveness as a treatment for older EOC patients.
Moreover, subgroup analyses of OS based on clinical features showed that patients from the CA125 ≤ 1000 group and the moderate-to-poor differential group attained more benefit from adjuvant CIT. Firstly, past studies have demonstrated that ovarian tumor cells utilized CA125 to attenuate the cytotoxicity of human NK cells and to consequently evade immune detection and attack.34,35 As such, ovarian tumors with lower CA125 level may be more susceptible to be attacked by CIK cells, which may be the cause for patients with lower CA125 to benefit to a greater degree from CIT. Secondly, our results showed that the OS rate in the group with moderate-to-poor differential was significantly better than that of the group with well differentiation. The reason for this discrepancy may be due to patients with well differentiation already possessing better prognosis,36 and hence might derive some benefit from adjuvant CIT, but the benefit would not be statistically significant. Conversely, the moderate-to-poor differential EOC patients exhibited worse OS rates, and adjuvant CIT could significantly improve the prognosis of this subset of patients. Therefore, our data suggest that EOC patients with lower CA125 or with moderate-to-poor differential are more recommended to undertake additional CIT after their completion of postoperative chemotherapy.
Residual post-surgery cancer cells have been considered a main origin of cancer recurrence, and some may even develop resistance to chemotherapy.37–39 Recent studies have shown that chemo-resistant cancer cells are sensitive to the cytotoxic effect of CIK cells,40,41 and thus, theoretically, CIK cells may be potent in eradicating residual tumor cells following surgery and chemotherapy. In this study, we showed that patients with residual tumor ≥ 1 cm yielded more favorable PFS rates from CIT. This result provides clinical evidence that residual cancer cells after first line treatment may be sensitive to CIK cells, and that residual tumor may be deemed a selection criterion for CIT in EOC.
CIK cells have been designated as a heterogeneous cell population, which includes T cells (CD3+CD4+ and CD3+CD8+), NKT-like cells (CD3+CD56+), NK cells (CD3−CD56+), and other phenotypes.19,42,43 However, there is ambiguity surrounding which phenotypes directly contribute to the clinical benefit from CIK-cell infusion. In this study, we found an unexpected correlation between survival benefit and certain infused CIK phenotypes, namely better OS and PFS in EOC patients who received CIK cell infusion that comprised of a higher proportion of CD8+ T or NKT-like cells. Although, these analyses are still somewhat preliminary for drawing conclusions in regards to direct functional impact, some indications warrant further investigation. Firstly, CD8+ cytotoxic T cells have long been proposed as the primary effector function necessary for tumor eradication and control via various antitumor mechanisms.44 As MHC-unrestricted direct cytotoxic effector cells, CD3+CD56+ NKT-like cells display a potent antitumor toxicity with granzyme and perforin-mediated tumor cell lysis after tumor recognition.22,45 It is therefore logical to assume that a high proportion of CD8+ T cells or NKT-like cells delivered via CIK cell infusion may beget potent antitumor effects in patients. On the other hand, we found that a higher proportion of CD4+ T cells or NK cells were associated with poor survival in patients, though more adequately powered samples are needed. Indeed, CD4+ T cells contain subsets of immunosuppressive populations such as regulatory T cells (Tregs), Th2 and Th17 cells, which could suppress the antitumor function of effector immune cells.46 Emerging reports suggest that NK cells can inhibit T cell–mediated antitumor immune responses in a variety of contexts in addition to promoting T cell responses.47 Consistent with our results, a previous study suggested that the presence of CD3−CD56+ cells in TIL cultures corresponded to a shorter recurrence-free survival (RFS) in high-grade serous cancer (HGSC) patients.47 However, whether there are specific phenotypes that exhibit negative regulation in CD4+ T cells or NK cells, as well as their precise mechanisms in relation to the regulation of CIK cell activity and antitumor responses, are topics warranting further investigation in our future work.
Moreover, consistently with our previously report,16 our findings showed that only approximately 6.8% of CIK cells exhibited PD-1 expression, indicating CIK cells would not be induced into exhaustion or anergy by PD-L1/PD-1 pathway.
Some limitations are present in this study due to its retrospective nature. Firstly, the clinical cohorts were retrospectively collected. Therefore, treatment selection bias is an inevitable consequence as patients were chosen to receive CIT for specific clinical reasons. As such, the uniformity of patients between CIT and control groups may not be thoroughly guaranteed. Secondly, the frequency of follow-up in the chemotherapy standalone group was lower than in the adjuvant CIT group. Despite these various impediments, our study demonstrates that a combination of CIT and postoperative chemotherapy is a safe and potential treatment modality for patients with EOC. With these data, we will further conduct randomized controlled trials of CIT on EOC patients and monitor the immune cell status of said patients following CIK therapy.
In conclusion, in this single-center retrospective study, we provided evidence that sequential CIT after surgery and chemotherapy results in survival improvement for EOC patients. Furthermore, patients whom were over 45 years of age, with CA125 of not more than 1000, with moderate or poorly differentiated tumors, or with residual disease ≥ 1 cm, may benefit to a greater degree from CIT. Prospective randomized studies are warranted to confirm the present findings and to further define optimal combinational treatment strategies for immunotherapy for EOC.
Patients and methods
Patient demographics and clinical characteristics
The Sun Yat-sen University Cancer Center Institutional Review Board approved this study of CIK cell-based immunotherapy, and written consent from each patient was obtained. From January 2001 to December 2015, 646 EOC patients who underwent surgery formed the cohort of this study. Among them, 574 cases received only postoperative chemotherapy (control group), while 72 cases received chemotherapy and sequential adjuvant CIK cell immunotherapy (CIT group) at the Sun Yat-sen University Cancer Center. There were no special selection criteria regarding whether patients would receive adjuvant CIK treatment. A multidisciplinary team of doctors from different departments, including surgeons, immunologists, and oncologists, made the treatment decisions. The clinicopathological features of the ovarian cancer cohort are outlined in Table 1.
Treatment schedule
All patients underwent completion surgery/surgical staging or comprehensive staging and cytoreduction. Following surgery, all patients in the control and CIT groups received three-six cycles of chemotherapy with a TP regimen (paclitaxel and cisplatin), TC regimen (paclitaxel and carboplatin), CAP (cyclophosphamide, epirubicin and cisplatin), or CBP regimen (cyclophosphamide, carboplatin, and Bleomycin). Beginning one month after completion of chemotherapy, CIT-group patients were subject to immune cell infusions every 2 weeks. The cell preparation and infusion processes are described as follows.
CIK cell preparation
CIK cells were prepared using a standard method. Briefly, 50 mL of heparinized peripheral blood was obtained from the EOC patient over 2 weeks after the patient had completed chemotherapy treatment and when routine blood examination results had normalized. Peripheral blood mononuclear cells were separated by Ficoll–Hypaque density centrifugation, resuspended at 2 × 106 cells/mL in fresh serum-free X-VIVO 15 medium (Lonza, Visp, Switzerland) containing 1,000 U/mL recombinant human IFN-γ (rhIFN-γ; ShangClone, Shanghai, China) and incubated at 37 ℃ in a humidified atmosphere containing 5% CO2 for 24 hours. Subsequently, 100 ng/mL mouse-anti-human CD3 monoclonal antibody (R&D Systems, MN, USA), 100 U/mL recombinant human IL-1 (rhIL-1, Life Technologies, CA, USA), and 1,000 U/mL recombinant human IL-2 (Life Technologies) were added to the media. Finally, 100 ng/mL mouse anti-human CD3 monoclonal antibody (R&D Systems), Fresh IL-2 and fresh medium were added every 2 days and the cell density was maintained at 2 × 106 cells/mL. The CIK cells were harvested on the 14th day. A fraction of harvested CIK cells were taken for viability and phenotype analysis, and the majority of fresh CIK cells were transfused intravenously (iv.) into the patients immediately upon harvesting.
To analyze the cell viability of CIK cells, trypan blue exclusion tests were employed. CIK cells were suspended in PBS containing 0.4% trypan blue for 3 min, and then examined under light microscope to determine the percentage of cells that had clear cytoplasm (viable cells) versus cells that had blue cytoplasm (nonviable cells).
The CIK cells were checked twice for possible contamination of bacteria, fungi and endotoxins to ensure that their safety for patients. Briefly, cell cultures were evaluated for possible contamination of bacteria or fungi by using BacT/ALERT® 3D instrument (Biomerieux, America). Endotoxins were quantified with Limulus Amebocyte Lysate PYROGENTTM-5000 Bulk Kit (Lonza, Basel, Switzerland) and ELx808™ Absorbance Microplate Reader (BioTek, Winooski, VT, USA). The result of the endotoxin test was less than 5 EU in the culture cells.
CIK cell phenotype analysis
Following culturing at day 14, the phenotype of the autologous CIK cells from each patient was characterized by flow cytometry (FC500, Beckman Coulter, CA, USA) using four-color fluorescence. The following mouse-anti-human monoclonal antibodies were used: anti-CD45, anti-CD3, anti-CD4, anti-CD8, and anti-CD56 (all from BD Bioscience, CA, USA).
PD-1 expression analysis of CIK cell
PD-1 expression of the autologous CIK cells from five ECO patients was characterized by flow cytometry using anti-CD3-PE-Cy5, anti-CD4-FITC, anti-CD8-PECF594, anti-CD56- PE-Cy7, and anti-PD-1-PE (BD Bioscicence CA, USA). The ratio of each subgroup was calculated based on the cell density of each gate.
Detection of cytotoxicity of CIK cells
The cytotoxicity of CIK cells was estimated by quantification of LDH activity in the culture medium by using the QuantiChromTM LDH Cytotoxicity Assay Kit (BioAssay Systems, Hayward, CA, USA). Briefly, cytotoxicity assays were carried out in round-bottomed 96-well plates with a final sample volume of 100 μl/well. Target cells (A780 or SKOV3, 2 × 105/ml cells) in 50 μl/well were co-cultured with effector cells (CIK cells) at various effector-to-target ratios (1:1, 3:1, 10:1 and 30:1) for 4h. In addition to the test samples, tumor cells without co-culture with CIK cells (Control) and cells treated with Triton X-100 (Total Lysis) were included. The released lactate dehydrogenase (LDH) was determined by measuring the absorbance of the samples at 490 nm using a spectrophotometer (Tecan Sunrise, Switzerland).
Cytotoxicity was calculated as the percentage of the maximum LDH release in the Total Lysis wells in the Sample wells, with the formula as follows: Cytotoxicity = (ODSample – ODControl)/(ODTotal Lysis – ODControl) × 100 (%), where ODSample, ODControl and ODTotal Lysis are absorbance values of the test sample, the no treatment Control, and the Triton X-100-treated cells, respectively.
CIK cell treatment
The CIK cell treatment for EOC patients were conducted as described previously.9,10,12 Patients received CIK (range 8.0 × 109 – 1.3 × 1010 cells) via intravenous infusion during each cycle. Patients received at least four cycles of CIK cell transfusion, and the interval of every cycle was 2 weeks. The patients were eligible for maintenance treatment if they were disease-stable.
Follow-up
All postoperative patients were followed up regularly. Postoperative follow-up included clinical and laboratory examinations or phone-call inquiry, and was conducted every 3 months for the first 2 years after surgery at either the outpatient department or follow-up center of the Sun Yat-sen University Cancer Center, every 6 months from years 3–5, and annually thereafter until at least 5 years after the operation or until patient death, whichever should occur prior. The follow-up deadline for this study was May 30, 2017. During each follow-up visit, the patient’s serum CA125, abdominal ultrasonography, and chest radiography data were obtained. Where tumor recurrence or metastases were suspected, further examinations involving computed tomography or positron emission computed tomography were performed. A second ovarian cancer cytoreductive surgery was performed if the recurrent tumor was resectable. For unresectable recurrent EOCs, chemotherapy was administered for as long as the tumor condition, liver function, and the general condition of the patient allowed. Otherwise, supportive treatment was provided. Supportive care was provided for all patients with distant metastasis.
Statistical analysis
For comparison of groups, the t-test, χ2-test, and the Fisher exact test were used where appropriate. Postoperative survival rates were evaluated by the Kaplan–Meier method and compared by the stratified log-rank test. For univariate analysis of risk factors for OS, survived patients were compared with deceased patients. The Cox stepwise regression model was used for multivariate analysis. A difference of 0.05 was considered significant. SPSS 19.0 (SPSS Inc., Chicago, IL, USA) was used for statistical calculations.
Funding Statement
This work was supported by the National Natural Science Foundation of China under grant numbers 81672863; the Project of State Key Laboratory of Oncology in South China under grant number 030041060004; the Project of Guangzhou Science and Technology Plan [2014J4100238]; and Science and Technology Plan Program of Guangdong Province [2016A020215080].
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Cho KR, Shih I. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach E, MBaergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Pfisterer J, Weber B, Reuss A, Kimmig R, Du Bois A, Wagner U, Bourgeois H, Meier W, Costa S, Blohmer JU, et al. Randomized phase III trial of topotecan following carboplatin and paclitaxel in first-line treatment of advanced ovarian cancer: a gynecologic cancer intergroup trial of the AGO-OVAR and GINECO. J Natl Cancer Inst. 2006;98:1036–1045. doi: 10.1093/jnci/djj296. [DOI] [PubMed] [Google Scholar]
- 5.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giraudo L, Gammaitoni L, Cangemi M, Rotolo R, Aglietta M, Sangiolo D. Cytokine-induced killer cells as immunotherapy for solid tumors: current evidence and perspectives. Immunotherapy. 2015;7:999–1010. doi: 10.2217/imt.15.61. [DOI] [PubMed] [Google Scholar]
- 7.Introna M. CIK as therapeutic agents against tumors. J Autoimmun. 2017;85:32–44. doi: 10.1016/j.jaut.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Odunsi K. Immunotherapy in ovarian cancer. Ann Oncol. 2017;28:i1–i7. doi: 10.1093/annonc/mdx444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 11.Introna M, Borleri G, Conti E, Franceschetti M, Barbui AM, Broady R, Dander E, Gaipa G, D'Amico G, Biagi E, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92:952–959. [DOI] [PubMed] [Google Scholar]
- 12.Rettinger E, Huenecke S, Bonig H, Merker M, Jarisch A, Soerensen J, Willasch A, Bug G, Schulz AK, lingebiel T, et al. Interleukin-15-activated cytokine-induced killer cells may sustain remission in leukemia patients after allogeneic stem cell transplantation: feasibility, safety and first insights on efficacy. Haematologica. 2016;101:e153–e156. doi: 10.3324/haematol.2015.138016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeck CL, Amberger DC, Doraneh-Gard F, Sutanto W, Guenther T, Schmohl J, Schuster F, Salih H, Babor F, Borkhardt A, et al. Significance of frequencies, compositions, and/or antileukemic activity of (DC-stimulated) Invariant NKT, NK and CIK cells on the outcome of patients with AML, ALL and CLL. J Immunother. 2017;40:224–248. doi: 10.1097/CJI.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 14.Xu L, Wang J, Kim Y, Shuang ZY, Zhang YJ, Lao XM, Li YQ, Chen MS, Pawlik TM, Xia JC, et al. A randomized controlled trial on patients with or without adjuvant autologous cytokine-induced killer cells after curative resection for hepatocellular carcinoma. Oncoimmunology. 2016;5:e1083671. doi: 10.1080/2162402X.2015.1083671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan QZ, Tang Y, Wang QJ, Li YQ, Zhang L, Li XD, Zhao JJ, Weng DS, Liu Q, Huang LX, et al. Adjuvant cellular immunotherapy in patients with resected primary non-small cell lung cancer. Oncoimmunology. 2015;4:e1038017. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CL, Pan QZ, Zhao JJ, Wang Y, Li YQ, Wang QJ, Pan K, Weng DS, Jiang SS, Tang Y, et al. PD-L1 expression as a predictive biomarker for cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. Oncoimmunology. 2016;5:e1176653. doi: 10.1080/2162402X.2016.1176653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ, et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol. 2013;20:4305–4311. doi: 10.1245/s10434-013-3144-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhu Y, Zhao E, He X, Zhao L, Wang Z, Fu X, Qi Y, Ma B, Song Y, et al. Autologous cytokine-induced killer cell immunotherapy may improve overall survival in advanced malignant melanoma patients. Immunotherapy. 2017;9:1165–1174. doi: 10.2217/imt-2017-0061. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Han W. Cytokine-induced killer (CIK) cells: from basic research to clinical translation. Chin J Cancer. 2015;34:99–107. doi: 10.1186/s40880-015-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang N, Qiao G, Wang X, Morse MA, Gwin WR, Zhou L, Song Y, Zhao Y, Chen F, Zhou X, et al. Dendritic Cell/Cytokine-Induced Killer Cell Immunotherapy Combined with S-1 in Patients with Advanced Pancreatic Cancer: A Prospective Study. Clin Cancer Res. 2017;23:5066–5073. doi: 10.1158/1078-0432.CCR-17-0492. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Mi Y, Guo N, Xu H, Xu L, Gou X, Jin W. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol. 2017;8:774. doi: 10.3389/fimmu.2017.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383–1391. doi: 10.1053/j.gastro.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 23.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian F, Villella J, Wallace PK, Mhawech-Fauceglia P, Tario JJ, Andrews C, Matsuzaki J, Valmori D, Ayyoub M, Frederick PJ, et al. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 2009;69:5498–5504. doi: 10.1158/0008-5472.CAN-08-2106. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odunsi K, Qian F, Matsuzaki J, Mhawech-Fauceglia P, Andrews C, Hoffman EW, Pan L, Ritter G, Villella J, Thomas B, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–12842. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang RY, Francois A, McGray AR, Miliotto A, Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology. 2017;6:e1249561. doi: 10.1080/2162402X.2016.1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Li H, Cao S, Zhang X, Yu J, Qi J, An X, Yu W, Ren X, Hao, X. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. J Immunother. 2014;37:115–122. doi: 10.1097/CJI.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 29.Santoiemma PP, Reyes C, Wang LP, McLane MW, Feldman MD, Tanyi JL, Powell DJ. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol Oncol. 2016;143:120–127. doi: 10.1016/j.ygyno.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 30.Lum C, Steer CB. Targeted therapies in the management of ovarian cancer: A focus on older patients. Drugs Aging. 2017;34:821–831. doi: 10.1007/s40266-017-0495-1. [DOI] [PubMed] [Google Scholar]
- 31.Tortorella L, Vizzielli G, Fusco D, Cho WC, Bernabei R, Scambia G, Colloca G. Ovarian cancer management in the oldest old: improving outcomes and tailoring treatments. Aging Dis. 2017;8:677–684. doi: 10.14336/AD.2017.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller L, Pawelec G. Aging and immunity - impact of behavioral intervention. Brain Behav Immun. 2014;39:8–22. doi: 10.1016/j.bbi.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Liu H, Liu H, He P, Li J, Liu X, Chen L, Wang M, Xi J, Wang H, et al. Dendritic cell-activated cytokine-induced killer cell-mediated immunotherapy is safe and effective for cancer patients >65 years old. Oncol Lett. 2016;12:5205–5210. doi: 10.3892/ol.2016.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP, et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer. 2010;9:11. doi: 10.1186/1476-4598-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A, Clark GF. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecol Oncol. 2005;99:704–713. doi: 10.1016/j.ygyno.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RJ, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15:668–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, Giassas S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22:1570–1575. doi: 10.1245/s10434-014-4157-9. [DOI] [PubMed] [Google Scholar]
- 38.Gadducci A, Cosio S, Zizioli V, Notaro S, Tana R, Panattoni A, Sartori E. Patterns of recurrence and clinical outcome of patients with stage IIIC to stage IV epithelial ovarian cancer in complete response after primary debulking surgery plus chemotherapy or neoadjuvant chemotherapy followed by interval debulking surgery: an italian multicenter retrospective study. Int J Gynecol Cancer. 2017;27:28–36. doi: 10.1097/IGC.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Du C, Wu L, Yu J, An X, Yu W, Cao S, Li H, Ren X. Cytokine-induced killer cells modulates resistance to cisplatin in the A549/DDP cell line. J Cancer. 2017;8:3287–3295. doi: 10.7150/jca.19426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Q, Zhang H, Li Y, Liu J, Hu X, Fan L. Anti-tumor effects of CIK combined with oxaliplatin in human oxaliplatin-resistant gastric cancer cells in vivo and in vitro. J Exp Clin Cancer Res. 2010;29:118. doi: 10.1186/1756-9966-29-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gammaitoni L, Giraudo L, Macagno M, Leuci V, Mesiano G, Rotolo R, Sassi F, Sanlorenzo M, Zaccagna A, Pisacane A, et al. Cytokine-induced killer cells kill chemo-surviving melanoma cancer stem cells. Clin Cancer Res. 2017;23:2277–2288. doi: 10.1158/1078-0432.CCR-16-1524. [DOI] [PubMed] [Google Scholar]
- 42.Linn YC, Lau SK, Liu BH, Ng LH, Yong HX, Hui KM. Characterization of the recognition and functional heterogeneity exhibited by cytokine-induced killer cell subsets against acute myeloid leukaemia target cell. Immunology. 2009;126:423–435. doi: 10.1111/j.1365-2567.2008.02910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Wang L, Wang Y, Zhang W, Cao Y. Phenotypic characterization and anticancer capacity of CD8+ cytokine-induced killer cells after antigen-induced expansion. PLoS One. 2017;12:e175704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waugh KA, Leach SM, Slansky JE. Targeting transcriptional regulators of CD8+ T cell dysfunction to boost anti-tumor immunity. Vaccines (Basel). 2015;3:771–802. doi: 10.3390/vaccines3030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118:3301–3310. doi: 10.1182/blood-2011-02-336321. [DOI] [PubMed] [Google Scholar]
- 46.Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, Hoyos BE, Putintseva EV, Chaudhry A, Dikiy S, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017;546:421–425. doi: 10.1038/nature22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crome SQ, Nguyen LT, Lopez-Verges S, Yang SY, Martin B, Yam JY, Johnson DJ, Nie J, Pniak M, Yen PH, et al. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat Med. 2017;23:368–375. doi: 10.1038/nm.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


