ABSTRACT
Malignant melanoma is an aggressive type of skin cancer whose incidence is increasing globally. Although surgery is effective in early stage melanoma, patients with advanced melanoma only have a 20% 5-year survival rate. Hence, combinations of existing and new immunotherapy technologies and immunotherapeutic agents are being evaluated. ONCOS-102 is an oncolytic adenovirus armed with human GM-CSF and an Ad5/3 chimeric capsid. It has shown to be well tolerated in phase I study (NCT01598129) wherein it induced antitumor immunity, infiltration of CD8 + T cells to tumors, and up-regulation of PD-L1. We propose that ONCOS-102 could serve as an immunosensitizer in combination therapies with checkpoint inhibitors. In this preclinical study, we investigated the cytotoxicity of ONCOS-102 and pembrolizumab, an anti-PD-1 antibody, in four human melanoma cell lines, A375, A2058, SK-Mel-2 and SK-Mel-28. Humanized mice engrafted with A2058 melanoma cells showed significant tumor volume reduction after ONCOS-102 treatment. Combination of pembrolizumab with ONCOS-102 reduced tumor volume to an even greater extent, while pembrolizumab (200 µg, or 400 µg) did not show any therapeutic benefit by itself. Body weight loss, and metastasis were not significantly affected by any treatment. These data support the scientific rationale for the ongoing clinical study of combination therapy of ONCOS-102 and pembrolizumab for the treatment of melanoma (NCT03003676).
KEYWORDS: ONCOS-102, Pembrolizumab, melanoma, NOG, immunogenic cell death, PD-1 inhibitor, antitumor, cancer vaccine, oncolytic adenovirus
Introduction
Melanoma is a highly immunogenic tumor as it is more sensitive to cell-based therapies and immunotherapies than chemotherapy and radiation.1 Checkpoint inhibitors have revolutionized treatment of advanced melanoma by releasing suppression of T-cell immunity in the tumor milieu, by inducing durable clinical antitumor immune response, and supporting long-term survival in patients.1 For example, pembrolizumab (Keytruda®) is a humanized antibody that binds to the programmed cell death-1 (PD-1) receptor on lymphocytes, and is recommended for cancers that express PD-L1.2 Pembrolizumab binds to PD-1 on T cells which blocks its interaction with PD-L1 on tumor cells or other cells in the tumor microenvironment, thereby blocking the inhibitory signal to T cells.3,4 Pembrolizumab was initially approved for metastatic melanoma.1 The 2-yr survival rates of metastatic melanoma patients treated with checkpoint inhibitors range from 43% to 55%,5,6 and suggest that additional treatment modalities of advanced melanoma warrant continued research, possibly involving combined treatment.1 Hence, combinations of new immunotherapy technologies and immunotherapeutic agents are being studied with a specific focus on exposing tumor antigens in an immunogenic milieu.1,7
ONCOS-102 (Ad5/3-Δ24-GM-CSF) is an engineered oncolytic adenovirus (Ad5/3) that expresses granulocyte-macrophage colony-stimulating factor (GM-CSF).8 Its chimeric 5/3 capsid contains the fiber knob derived from Ad serotype 3, so infection can occur through binding of the desmoglein 2 receptor, which is often expressed on tumor cells.9 Selective replication in tumor cells is provided by 24 bp deletion in the Rb binding site of the E1A gene restricting its replication to cells with p16-Rb pathway defects, such as most cancers.8 The local production of GM-CSF by ONCOS-102 ensures local concentration but minimizes systemic exposure and toxicity associated with GM-CSF.8 Thus, natural killer cells and tumor-specific CD8+ cytotoxic T-lymphocytes are recruited into tumors by ONCOS-102 in both animal models10 and human studies.8 In a phase I study, ONCOS-102 treatment of refractory and immune cell-poor solid tumors of different types showed disease control in 40% of patients and good tolerability of the treatment.11 ONCOS-102 also induced a transient prominent influx of tumor infiltrating lymphocytes into the treated tumor lesions and increased PD-L1 expression on two treated mesotheliomas.11 Vassilev et al.12 reported that ONCOS-102 treatment induced antitumor immune responses: increased of CD8 + T-cell infiltration, stimulated development of CD8 + T-cell response to four tumor-associated antigens, and supported a 31 month survival of a patient with refractory stage 3 ovarian cancer. Under the Advanced Therapy Access Program, nine patients received ONCOS-102 treatment for refractory melanoma and two of the four patients evaluable by modified RECIST 1.1 criteria had an extended survival of greater than 2149 days and 559 days post treatment.13 Furthermore, ONCOS-102 have shown to have significant cytotoxicity against five melanoma cell lines and one low passage human primary melanoma cells.13 These results led to the speculation that ONCOS-102 could be combined with other therapeutic strategies, especially those inhibiting the immune checkpoint12 for the treatment of melanoma.
Efficacy of checkpoint inhibitors are associated with tumors containing numerous novel tumor antigens due to mismatch repair deficiency14 and the percentage of PD-L1 expressing cells in the tumor milieu, in a dose dependent manner with 1% PD-L1 being considered positive.15 Cancer cell death can range from poorly immunogenic to immunogenic.16–18 Immunogenic cell death (ICD) involves cell surface structural changes and leads to release of proinflammatory cytokines, chemokines, and pro-immunogenic factors.17,18 Antigen-processing cells (APCs) are attracted to the dying cells where they take up tumor antigens, process them, and elicit antitumor immune responses.17,19 ICD biomarkers include pre-apoptotic calreticulin exposure in the outer plasma membrane, extracellular release of nonhistone chromatin high-mobility group box 1 protein (HMGB1), ATP secretion during the blebbing phase of apoptosis, and other processes.18 Induction of ICD by ONCOS-102 would generate a tumor microenvironment dominated by Th1 and Th17 cytokines.20,21 The co-administration of agents, such as anti-PD1 antibodies and ONCOS-102, may reactivate tumor infiltrating leukocytes against the numerous exposed tumor-associated antigens released by lysis as the final step of viral replication.21
The present study was designed and performed to examine the benefits of a combination of the oncolytic adenovirus ONCOS-102 with the immunotherapeutic anti-PD-1 antibody, pembrolizumab in human melanoma cells in vitro, and in A2058 melanoma-engrafted mice. To investigate the potential efficacy of this combination, we used the severely immunodeficient NOG (NOD/Shi-scid/IL-2Rγnull) mouse engrafted with human cord blood stem cells as an in vivo model for human immune responses. After subsequent engraftment with a tumor22,23 such as malignant melanoma cells, this model supports characterization of human immune responses to the tumor antigens. Our findings suggest that the combination showed varying in vitro cytotoxicity in the cell lines and showed antitumor effects in the melanoma-engrafted mouse model with a human reconstituted immune system.
Results
Receptor expression in melanoma cell lines
The first step in assessing combination therapy with two diverse anticancer treatments against melanoma is to assess the presence of their receptors on multiple human melanoma cell lines (e.g. A375, A2058, SK-Mel2 and SK-Mel28). The CD46, desmoglein-2,24 and CAR are receptors for Ad3, Ad3, and Ad5, respectively. As shown in Figure 1, the CD46 receptors for Ad3 fiber knobs, which should also bind the chimeric fiber protein in ONCOS-102 virions, were expressed on nearly all cells (≥ 98%) of the four cell lines. Desmoglein-2 proteins were expressed in 92.1% of A375 cells, 88.7% of SK-Mel2, and 99.1% of SK-Mel28 cells, but only 52% of A2058 cells (Figure 1). Thus, the four chosen melanoma cell lines express receptors for the attachment and replication of the ONCOS-102. The Ad5 receptor, CAR, was observed in 20% of A375 cells, 40% of A2058 cells, 54% of SK-Mel-2 and 27% of SK-Mel-28 (Figure 1). Pembrolizumab binds to the PD-1 protein expressed on activated T cells and blocks its interaction with PD-L1 expressed on many cancer cells.15 PD-L1 expression was detected on 39% of A375 cells, 11% of A2058, 56% of SK-Mel-2, and 67% of SK-Mel-28 cells (Figure 1). Thus, overall expression for all receptors of interest was in the order of SK-Mel-28 > SK-Mel-2 > A375 > A2058 (Figure 1).
Figure 1.
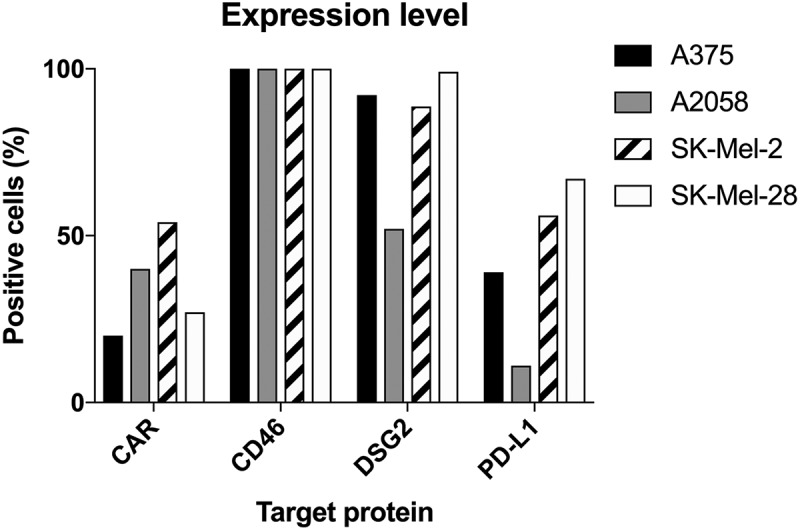
Expression of CAR, CD46, Desmoglein-2 and PD-L1 in human melanoma cells measured by flow cytometry (at least 104 cells/events were analyzed by flow cytometry in one replicate experiment). Data are expressed as percentage of cells positive for the marker.
Cytotoxicity of ONCOS-102 and pembrolizumab treatments
The effect of ONCOS-102, pembrolizumab, and combination of the two agents were assessed in vitro at 24 h, 48 h, and 72 h post treatment in the four melanoma cell lines A375, A2058, SK-Mel-2 and SK-Mel-28 (Supplementary Figure 1A, 1B, 1C, 1D, respectively). Addition of pembrolizumab along with ONCOS-102 did not significantly alter cell viability for any human melanoma cell line (Supplementary Figure 1) although cell viability in A2058 at 24 h was higher with combination therapy (Supplementary Figure 1). SK-Mel-2 and A375 melanoma cell lines were more sensitive to oncolytic activity of ONCOS-102 than the A2058 and SK-Mel-28 melanoma cell lines (Supplementary Figure 1).
Apoptosis and necrosis
Since necrosis can increase immunogenic activity of lyzed cells, the ONCOS-102 treated cells were stained with annexin V and propidium iodide (PI) to determine the predominant mechanism of cell death: annexin V-positive/PI-negative staining indicated early apoptosis or early necrosis whereas annexin V-positive/PI-positive staining indicated necrosis.25 Before ONCOS-102 treatment, SK-Mel-28 had a higher percentage of cells with annexin V staining than cells with PI staining. The kinetics of early apoptosis and necrosis in ONCOS-102 treated SK-Mel2 and SK-Mel-28 cells (1000 viral particles (VP)/cell) was cell line specific. After ONCOS-102 treatment, SK-Mel-2 showed a greater increase in early apoptotic cell death and necrotic cell death over time than SK-Mel-28 (Supplementary Figure 2). Oncolysis was significantly higher at 72 h and 96 h in SK-Mel-2 cells (Supplementary Figure 2).
Figure 2 shows the effect of various doses of ONCOS-102, with and without pembrolizumab, on both necrosis and apoptosis in the four cell lines. Pembrolizumab at 200 and 400 µg did not significantly increase early apoptosis or necrosis in these cell lines (Figure 2). ONCOS-102 treatment, with and without pembrolizumab, more frequently induced apoptosis in A375 (39% and 31%, respectively), A2058 (38% and 36%, respectively), and SK-Mel-2 (30% and 25%, respectively) cells (Figure 2A-2C). Although SK-MEL-28 cells had high spontaneous backgrounds in the assays (49% and 37.6%), ONCOS-102 treatment increased the apoptotic marker to 76.3% and the necrotic marker to 47.1% (Figure 2D). Treatment with ONCOS-102 induced a higher frequency of necrotic cell death in SK-MEL-28 cells (47%) than in A2058 (14%), A375 (5%), and SK-Mel-2 (3%) (Figure 2).
Figure 2.
Effects of ONCOS-102 and pembrolizumab on annexin V (A) and propidium iodide staining (B) in human melanoma cell lines after 72 h (A375, SK-Mel-2) and 96 h (A2058, SK-Mel-28). Data are expressed as percentage of cells staining positive. Analyses were performed in one replicate experiment.
Immunogenic cell death
To study the extent of immune stimulation by ONCOS-102 treatment, we chose to examine calreticulin (CRT) exposure, ATP secretion, and cell death-associated release of the nonhistone chromatin protein high-mobility group box 1 (HMGB1).17,18 In general, pembrolizumab co-treatment did not induce significantly higher CRT exposure in the cell lines (Figure 3A). As shown in Figure 3A, ONCOS-102 treatment induced moderate to high CRT exposure in percentage of the four melanoma cell lines, suggesting that endoplasmic stress resulting in CRT exposure was a part of mechanism of ONCOS-102 induced cell death.
Figure 3.
Effects of ONCOS-102 and pembrolizumab treatment on three indicators of immunogenic cell death in human melanoma cell lines after 72 treatment. A. Calreticulin exposure. B. ATP release in human melanoma cell lines after 72 treatment. C. Effects of ONCOS-102 and pembrolizumab on HMGB1 release in human melanoma cell lines after 72 treatment. Data are expressed as percentage of cells staining positive for specific marker. Analyses were performed in two replicates experiment.
ONCOS-102 treatment had different effects on ATP release from the four melanoma cell lines: While ATP release increased from SK-MEL-2 at higher ONCOS-102 inocula (100 and 1000 VP/cell), it was lower in SK-MEL-28 and A2058, and was not affected in A375 cells (Figure 3B). Pembrolizumab co-treatment showed no effects on ATP release in SK-MEL-2 and A375 but led to decreased ATP release in SK-Mel-28 cells (Figure 3B). However, the effects of pembrolizumab co-treatment on ATP release in A2058 cells varied with the dose of ONCOS-102 (Figure 3B). No significant HMGB1 release was seen after ONCOS-102 treatment, with or without pembrolizumab, in any of the four melanoma cell lines (Figure 3C). In summary, ONCOS-102 treatment augmented CRT exposure which may be one mechanism that contributes to the immunogenic properties of cell death by ONCOS-102 treatment.
Antitumor effects of ONCOS-102 and pembrolizumab in humanized NOG mice bearing A2058 tumors
The hu-NOG mice were characterized for sufficient engraftment of the human hematopoietic stem cells (mean range: 52% to 59%; individual range: 22% to 93%) after 14 weeks by assessing the blood cells for expression of human CD45 found on most hematopoietic cells.26 The humanized NOG mice were engrafted with A2058 melanoma cells on day 0, and the mice were randomized into eight groups on Day 14 with similar mean volumes of right and left tumors and mean human BM engraftment of 56% (Supplementary Figure 3). On first day of treatment (Day 16), the mean volumes of left tumors in the eight groups ranged from 39.5 mm3 to 59.2 mm3 and right tumors ranged from 50.8 mm3 to 85.1 mm3. ONCOS-102 (intratumor) and/or pembrolizumab (i.v.) were administered on days 15, 17, 19 post A2058 engraftment to the appropriate groups, and pembrolizumab iv treatment continued every 3–4 days until sacrifice.
Body weight and tumor volumes were measured three times per week. Body weight decreased in all groups with no significant difference in body weight loss between the groups (Supplementary Figure 4).
Right and left tumor volumes were compared on day 26 and day 40 among the groups. Volumes of the left tumors or the right tumors on day 26 or on day 40 were not significantly different between the groups (Figure 4A, B). Pooling the left and right tumor volume data for all groups revealed 51.5% tumor volume reduction (P = 0.0205) in the ONCOS-102 treated group relative to the vehicle-treated group on day 40 (Figure 4C, D). Pembrolizumab alone did not show any significant antitumor effects (Figure 4C, D). However, in the presence of ONCOS-102, 200 and 400 µg doses of pembrolizumab significantly reduced tumor volume by 61% (P = 0.0242) and 69% (P = 0.0047), respectively (Figure 5C, D). Tumor volume reduction with combined therapy (200 and 400 µg doses of pembrolizumab i.v. plus ONCOS-102 in the right tumor) was 49.7% (P = 0.013) and 41% (P > 0.05), respectively (Figure 4C, D).
Figure 4.
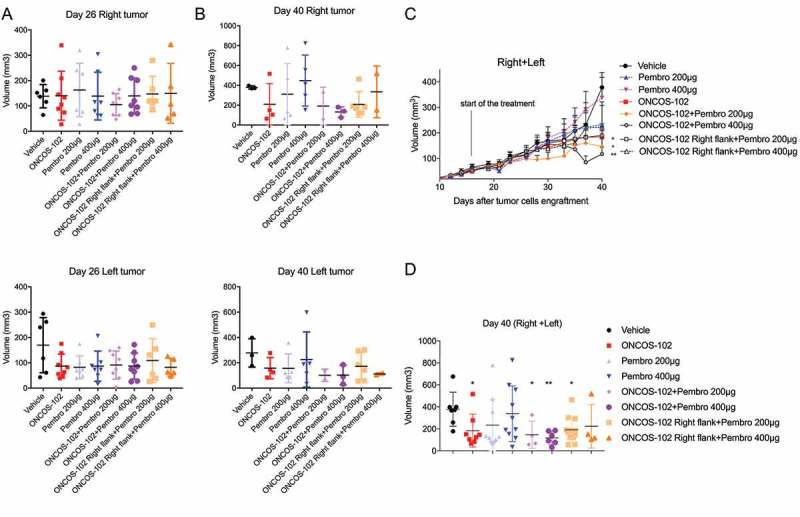
Effects of ONCOS-102 and pembrolizumab treatments on tumor volume of A2058 engrafted hu-mice. A. Tumor volume on day 26 for right and left tumor. B. Tumor volume on day 40 for right and left tumor. C. Tumor volume throughout the treatment (pooled for right and left tumors). D. Tumour volume on day 40 (pooled for right and left tumors). Data represent mean ± SEM. *P < 0.05, **P < 0.01 vs vehicle. Groups: 1–6 had 8 animals/group, groups: 7–8 had 6 animals/group.
Figure 5.
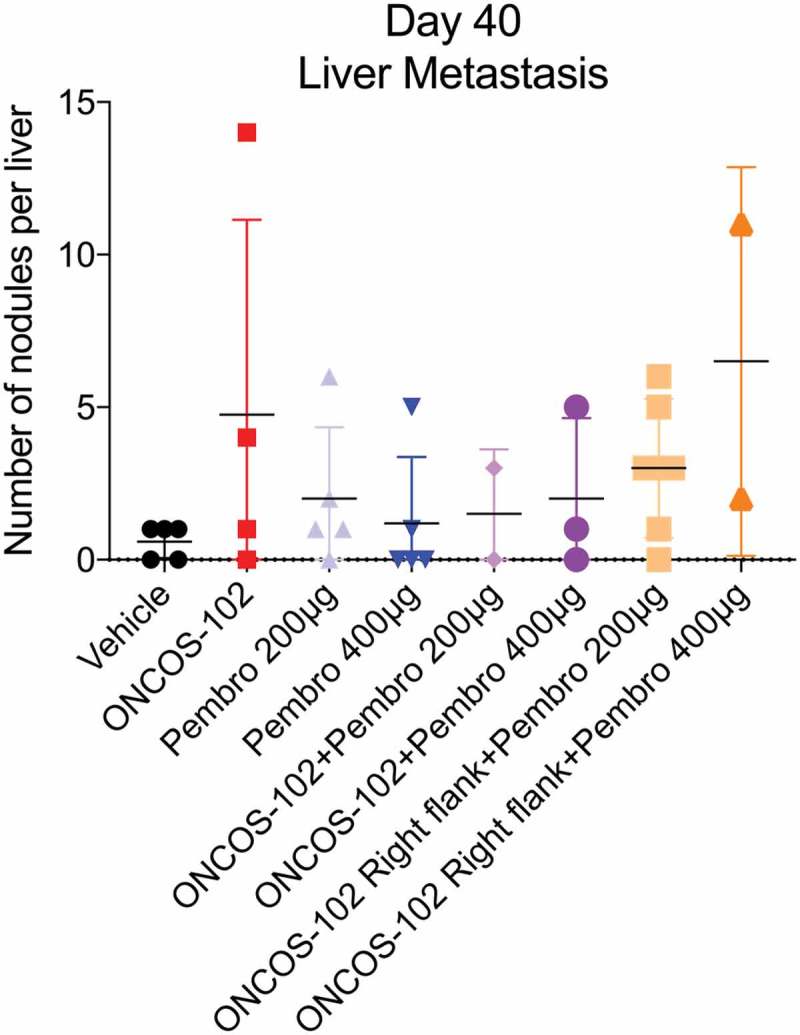
Effects of ONCOS-102 and pembrolizumab on number of liver nodules in each mouse at time of sacrifice (day 40). Data represent mean ± SEM. Groups: 1–6 had 8 animals/group, groups: 7–8 had 6 animals/group.
Subsequent FTV17 analysis was performed to assess the possibility of synergistic interaction between these two modalities (Table 1). Synergistic antitumor interactions were observed in group V (ONCOS-102 + pembrolizumab 200 µg) and group VI (ONCOS-102 + pembrolizumab 400 µg) on day 37.
Table 1.
Assessment of therapeutic synergism using the FTV calculation method. The expected FTV (i.e., the product of FTV values for monotherapies (ONCOS-102 or Pembrolizumab)) of the combination therapy (ONCOS-102 plus Pembrolizumab) is divided by the observed FTV of the combination, yielding a ratio that indicates the nature of the interaction (> 1 indicates synergy and < 1 indicates a less than additive effect). FTV foreach experimental group is obtained by dividing the mean tumor volume of the experimental group (ONCOS-102 or Pembrolizumab) by the mean tumor volume of the control group (mock).
| Day | FTV for monotherapy |
ONCOS-102 + Pembro 200 μg |
ONCOS-102 + Pembro 400 μg |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pembro 200 μg | Pembro 400 μg | ONCOS-102 | Expected FTV | Observed FTV | Ratio (Expected/ Observed) |
Expected FTV | Observed FTV | Ratio (Expected/ Observed) |
|
| 26 | 0,95 | 0,87 | 0,88 | 0,83 | 0,76 | 1,1 | 0,77 | 0,87 | 0,88 |
| 37 | 0,91 | 1,26 | 0,68 | 0,62 | 0,42 | 1,47 | 0,86 | 0,40 | 2,14 |
Effects of ONCOS-102 and pembrolizumab on liver metastases
On day 40, all animals were examined for the presence and degree of liver metastases after sacrifice. No significant differences were observed in numbers of liver nodules between the groups (Figure 5).
Immune cell infiltration of tumors
After tumors were harvested and weighed, tumor infiltrating lymphocytes were characterized by cell surface staining with the T-cell markers, human CD3,27 CD4 and CD8.28 Although no difference was observed in percentage of human CD45+ cells in the left tumor, the percentage of hCD45+ was higher in the ONCOS-102 + Pembrolizumab 200 µg group relative to the vehicle-treated group (P > 0.05) (Figure 6A). Percentage of hCD3 + T cells was higher in ONCOS-102-treated groups in left and right flanks relative to the vehicle-treated group (Figure 6B).
Figure 6.
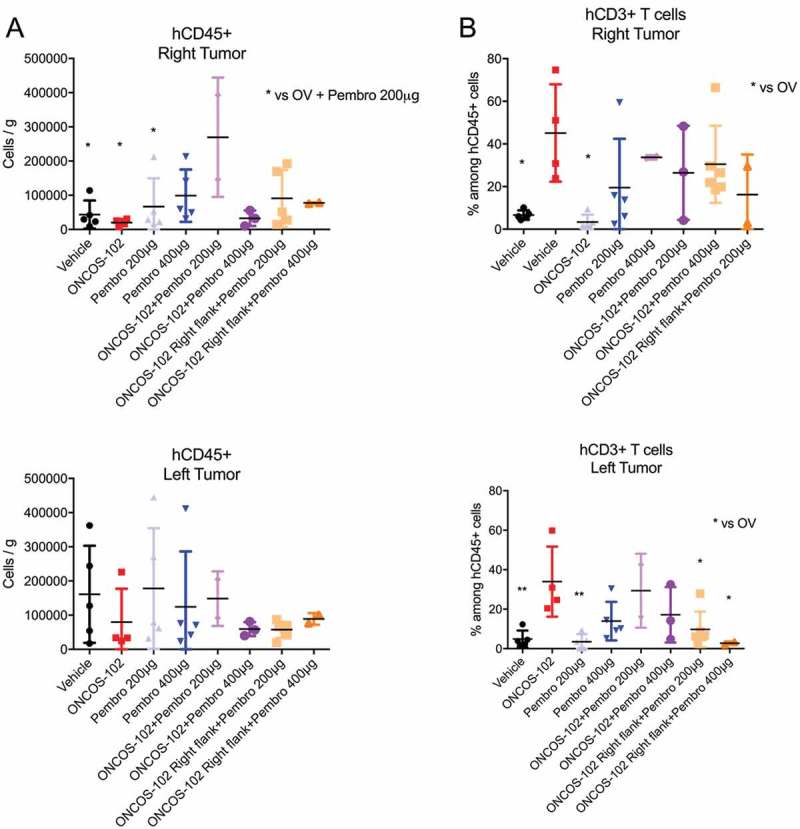
Effects of ONCOS-102 and pembrolizumab on number of hCD45+ leukocytes. A. Number of hCD45+ leukocytes per gram of tumor on left and right flank. B. Percentage of hCD3+ leukocytes among hCD45+ leukocytes in tumors on left and right flank. The populations were gated with forward and side scattering (FSC-A/SSC-A dot plot) in leukocytic regions (at least 104 cells/events were analyzed by flow cytometry, 6–8 tumors/experimental group have been analysed in one experimental replicate). Flow cytometry analysis was performed on FlowJo v10 software. Data represent mean ± SEM. n = 2–6, P* < 0.05, P** < 0.01 vs vehicle.
ONCOS-102 treatment, with and without pembrolizumab, induced a significant increase in numbers of intratumoral CD8 + T cells (Figure 7A). However, ONCOS-102 treatment was associated with a decrease in CD4 + T cells (Figure 7B) in both right and left tumors (Figure 7A). A significant increase in CD8 + T cells was seen in the right flank tumors of ONCOS-102 treated mice (Figure 7A). Pembrolizumab 200 µg and 400 µg groups showed no effects on CD8 + T cells relative to vehicle-treated mice (Figure 7A). No significant difference was seen in the PD-L1 expression of the tumor cells in any of the groups (Figure 7C).
Figure 7.
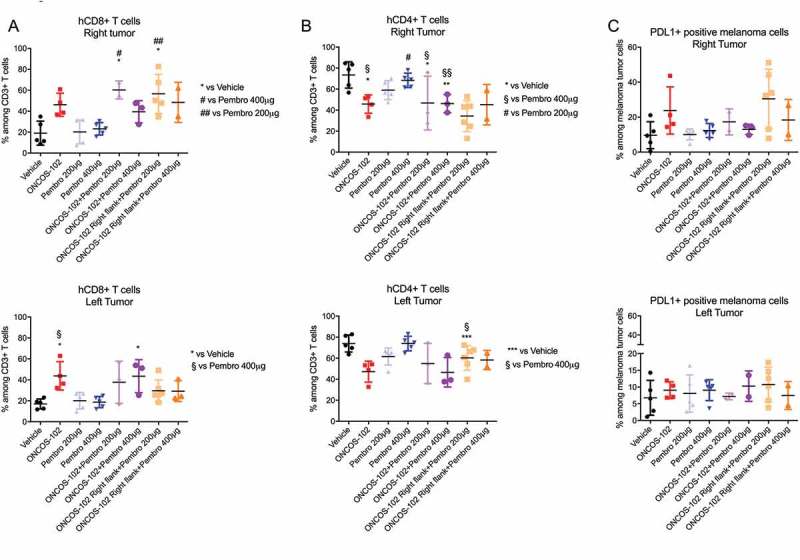
Effects of ONCOS-102 and pembrolizumab on subsets of leukocytes in tumors on left and right flank. A. Percentage of hCD8+ leukocytes among hCD3+ leukocytes. B. percentage of hCD4+ leukocytes among hCD3+ leukocytes. C. Percentage of PD-L1+ leukocytes in tumors. The populations were gated with forward and side scattering (FSC-A/SSC-A dot plot) in leukocytic regions (at least 104 cells/events were analyzed by flow cytometry, 6–8 tumors/experimental group have been analysed in one experimental replicate). Flow cytometry analysis was performed on FlowJo v10 software. Data represent mean ± SEM.
Discussion
Patients with metastatic melanoma have a 5-year survival rate of approximately 20%,29 which suggests that despite the effectiveness of checkpoint inhibitors in a minority of patients with metastatic melanoma,5,6 new therapeutic combinations and modalities are clearly warranted.30 Since metastatic melanoma is more sensitive to immunotherapeutic agents and adoptive cell therapies than treatment with chemotherapy and radiation, combining two agents that can induce and increase antitumor immune responses by distinct mechanisms may augment efficacy. The aims of this study were to characterize the in vitro interactions and use a humanized mouse model that mimics human immune responses to evaluate the antitumor effectiveness of the combination of two agents that augment immune responses: the oncolytic adenovirus ONCOS-102 and the checkpoint inhibitor, pembrolizumab, which blocks the PD1:PD-L1 inhibitory signalling pathway in T cells.
Oncolytic viruses have been tested in preclinical studies and in numerous clinical trials against various tumor types, have a tolerable safety profile, and show varying degrees of efficacy.8,10–12,31–40 Their advantages include lysis of infected tumor cells, release of tumor antigens in an immunogenic milieu, and viral infection of adjacent tumor cells. In addition, adenoviruses that bind to desmoglein 2 in epithelial cells enable the transient opening of intercellular junctions, improve access to receptors trapped in intercellular junctions, such as CD46,24 and facilitate viral spread and movement of drugs, antibodies, oncolytic viruses, and immune cells to tumor sites.24,41 This study investigated the anticancer effects of ONCOS-102 in combination with the PD-1 inhibitor, pembrolizumab, both in vitro in human melanoma cell lines and in a humanized mouse model bearing A2058 melanoma tumor nodules.
Human adenovirus serotype 5 is a species-specific virus. It does not replicate to the same extent as in humans in most animal models. Therefore, humanized animal model has been developed in order to assess both oncolytic properties of the virus and immunomodulatory assets of investigated agents in cancer treatment. The virus entry receptor DSG-2 has been found to be expressed by human A2058 cells, however not by NOG mouse. Therefore, the virus was not able to replicate in murine tissueONCOS-102 treatment reduced the viability of the four melanoma cell lines with varying kinetics: two cell lines (A375 and SK-Mel2) were more sensitive to ONCOS-102 than the A2058 and SK-Mel-28 cell lines. Responsiveness of the four melanoma cell lines to ONCOS-102 was SK-Mel28 > SK-Mel2 > A375 > A2058, consistent with the expression levels of the receptors for the chimeric Ad/5/3 virus, ONCOS-102. In agreement, ONCOS-102 also showed cytotoxicity on three additional melanoma cell lines (Mel624, C8161, and Mel888) and one primary early passage melanoma cells, pMelL.13 As expected, pembrolizumab did not significantly increase cytotoxicity of ONCOS-102 in any of the cell lines since it binds to T cells and not the tumor cells.
Oncolytic adenoviruses, after replication in the tumor cells, induce cell death and release progeny that can infect nearby tumor cells. Cell death by necrosis (annexin V/PI+) rather than apoptosis (annexin V+) is considered to be more immunogenic.25 Here, ONCOS-102 treatment induced higher levels of necrosis in SK-Mel-28 > A2058 cells > A375 > SKMel2 cells in a predominantly dose dependent manner, regardless of the status of pembrolizumab. The presence of these three biomarkers during cell death― calreticulin exposure on the cell surface, excess ATP release, and HMGB1 release― usually indicate a more immunogenic cell death18 than if they remain at baseline. ONCOS-102 treated melanoma cells (1000 vp, 72 hrs) induced CRT exposure in 115% to 516% of cells (A375 > SK-Mel-2> A2058 > SK-Mel-28), which was much higher in percent CRT exposure in ONCOS-102 treated mesothelioma cells (range: 6% to 23%).17 ONCOS-102 treated melanoma cells (72 hrs) showed higher ATP release in SK-Mel-2, no significant changes in A2058 and A375 and modest decline with higher inoculum in SK-Mel-28. Significant HMGB1 release was not observed after ONCOS-102 treatment, with or without pembrolizumab in these four melanoma cell lines, whereas ONCOS-102 treatment of three mesothelioma cell lines had increased HMGB1 release by 4 to 13 ng/ml compared to the uninfected cells.17 In general, pembrolizumab co-treatment did not induce significantly higher CRT exposure, ATP release, nor HMGB1 release in the melanoma cell lines. These data suggest that CRT exposure may contribute to the immunogenic nature of ONCOS-102 induced lysis of melanoma cell lines, but other mechanisms are probably involved.18
A2058 melanoma cells were chosen for engraftment of tumor xenografts in humanized NOG mice (designated as day 0); and mice were sacrificed on day 40 of the study. Approx. 11% of A2058 cells expressed PD-L1; thus, the A2058 melanoma cell line is considered PD-L1 positive and its PD-L1 expression level falls within the patient groups that had higher durable responses to checkpoint inhibitors,15 such as pembrolizumab. Although the treated groups lost weight, the weight loss was similar to the vehicle group, consistent with mild adverse events observed after ONCOS-102 treatment of humans.11 ONCOS-102 treatment and combination therapy, but not pembrolizumab alone, significantly reduced tumor volumes by 52% to 69% relative to the vehicle group. Although the group 7 (OV right tumor + pembrolizumab 200 µg) had no deaths, the mortality rates were not significantly different between the groups. Metastases were not significantly affected by any treatment. FTV analysis for synergism revealed that the combination therapy groups V and VI ([ONCOS-102 + pembrolizumab 200 µg] and [OV + pembrolizumab 400 µg]) showed synergistic interactions of antitumor efficacy on day 37.
ONCOS-102 therapy has induced an immune response in many tumor types.11–13 Treatment of the A2058 xenografts with ONCOS-102 and the combination therapy increased the percentage of CD3 + T cells (left and right tumors) and CD8 + T cells (right tumor), but recruited fewer CD4 + T cells in both flank tumors. In comparison, the ONCOS-102 treated tumors in the phase 1 study observed an increase of CD3 + T cells and CD8 + T cells,11 suggesting the predictive value of the hu-mouse model. However, the ONCOS-102 treated tumors in the phase I study also showed an increase in recruitment of CD4 + T cells to the injected tumors,11 which confirms that the hu-mouse model may not fully predict all aspects of the human antitumor immune responses during clinical trials. Although treatment with checkpoint inhibitors often is associated with lower PD-L1 expression in human studies, no significant difference was seen in PD-L1 expression in the tumors between the hu-mice groups.
Limitations of this study include probable MHC mismatches between the cord blood donor reconstituted human immune system in the hu-mice and the HLA type of the A2058 melanoma cell line. Although the hu-mice based melanoma model more closely mimics the immune responses of the diverse human population, these responses may not totally recapitulate the human antitumor immune responses in patients with melanoma. However, the hu-mouse models probably provide a better approximation to the clinical setting than syngeneic mouse models. Several studies suggest that transient hindering of the immune responses may allow wider spread of the oncolytic virus and increase efficacy.17,30 In contrast, other studies suggest that viral therapy can be enhanced by blockage of the PD-1:PD-L1 interaction and the combined therapy can increase antitumor efficacy.42
In summary, synergistic antitumor effects were observed in the humanized mice treated with the combination of ONCOS-102 and pembrolizumab as demonstrated by reduced tumor volumes. Our results lead us to hypothesize that ONCOS-102 induced cell death of the tumor cells by recruiting immune cells to the tumor environment. Pembrolizumab may then help to activate the T cells by suppressing the PD-1:PD-L1 mediated inhibition of T cell signalling. Our results show that the combination of ONCOS-102 and PD-1 inhibitors, such as pembrolizumab, could be beneficial in treatment of melanoma. These findings warrant the clinical testing of ONCOS-102 in combination with immune checkpoint inhibitors, including PD-1 inhibitors, for treatment of advanced melanoma.
Materials and methods
Cell lines
Melanoma cell lines, A2058 (ATCC® CRL-1147™), A375 (ATCC® CRL-1619™), SK-Mel-2 (ATCC® HTB-68™) and SK-Mel-28 (ATCC® HTB-72™), were cultured in DMEM high glucose medium supplemented with 10% FCS and penicillin/streptomycin. Trypsin-EDTA was used to detach and sub-culture cells at 80% confluence for in vitro assays. Phosphate-buffered (PBS)-EDTA (10 mM) was used to detach A2058 melanoma cells before suspension of 20 × 106 cells in isotonic PBS for subcutaneous injection. All cell lines were maintained in a humidified atmosphere containing 5% CO2 at 37ºC. Cells were plated in 96 well plates (104 cells/well) or, if indicated 24 well plates (5 × 104 cells/well), and incubated for 24 hrs before being treated as indicated in the following in vitro assays.
Receptor expression
The expression of CAR, CD46, desmoglein-2, and PD-L1 on the four untreated melanoma cell lines grown in tissue culture was detected by specific staining with rabbit polyclonal anti-CAR antibody (ThermoFisher Scientific, PA5-12476), recombinant human anti-CD46 antibody (Miltenyi Biotec, 130–104-559), mouse monoclonal anti-desmoglein antibody (ThermoFisher Scientific, 12–9159-42) and human anti-PD-L1 antibody (Biolegend, 329708), respectively by flow cytometry (at least 104 events were analysed for each marker and cell line). Alexa Fluor® 488 secondary antibodies were used after each primary antibody.
Cell viability
Cell viability was evaluated by using the MTS Cell Proliferation Assay kit (Abcam) according to the manufacturer’s instructions with the following modifications. MTS assay kit is a colorimetric method for the sensitive quantification of viable cells. It can be used to assess cell proliferation, cell viability and cytotoxicity. The MTS assay is based on the reduction of the MTS tetrazolium compound by viable mammalian cells to generate a colored formazan dye that is soluble in cell culture media. This conversion is thought to be carried out by NAD(P)H-dependent dehydrogenase enzymes in metabolically active cells. The formazan dye is quantified by measuring the absorbance at 490 nm. Cells were incubated with and without 0.1, 1, 10, 100 and 1000 viral particles (VP) of ONCOS-102/cell and/or 50 µg/mL pembrolizumab for 24, 48 and 72 h. After 20 μl MTS reagent was added to 200 µL of well contents, plates were incubated for 0.5–4 h at 37ºC in standard culture conditions. The plates were shaken briefly on a shaker and absorbance measured at 490 nm. Absorbance values for untreated cells were set to correspond to 100% viability, and viability of treated cells was expressed as a percentage of the untreated control absorbance value.
Apoptosis and necrosis
Induction of apoptosis by ONCOS-102 and pembrolizumab was measured by using an Alexa Fluor® 488 Annexin V/Dead Cell Apoptosis Kit (ThermoFisher Scientific). Briefly, cells in 24 well plates were treated with and without ONCOS-102 and/or pembrolizumab for either 72 h (A375 and SK-Mel-2 cells) or for 96 h (A2058 and SK-Mel-28 cells). The cells were harvested and washed in cold PBS. After centrifugation, the cell pellets were resuspended in 1X annexin-binding buffer and diluted to approximately 1 × 104 cells in 100 µL per assay. Five microliters Alexa Fluor® 488 Annexin V and 1 µL of 100 µg/mL PI solution were added to 100 µL of cell suspension. After incubation at room temperature for 15 min, 400 µL 1X annexin-binding buffer was added, mixed gently and the samples stored on ice until measurement of fluorescence emission at 530 nm and > 575 nm from stained cells by flow cytometry.
Immunogenic cell death
Calreticulin exposure was measured in cells that were grown in 24 well plates and treated for 72 hrs as indicated, harvested, washed twice with PBS and fixed in 0.25% paraformaldehyde in PBS for 5 min. After washing twice in cold PBS, cells were incubated with primary anti-calreticulin antibody (Abcam, Ab 83220) in cold blocking buffer. After washing and incubation with FITC-conjugated secondary antibody in blocking buffer for 30 min, samples were analyzed by flow cytometry to identify cell surface calreticulin (CRT). Secondary antibody alone was used as a control and gating of fluorescent intensity of stained cells was done on PI-negative cells.
ATP release was measured by using the ATP Assay Kit (Abcam Ab 83355) according to the manufacturer’s instructions. Briefly, A375 and SK-Mel-2 cells were incubated for 72 h and A2058 and SK-Mel-28 cells for 96 h with ONCOS-102 and/or Pembrolizumab as indicated. Cells were washed with cold PBS and resuspended in 100 µL ATP assay buffer. Cell suspension was homogenized and then centrifuged for 5 min at 4ºC at 13,000 g, and the supernatant was stored on ice. ATP reaction mix (50μl) was added to standard and sample wells, and 50 µL of background reaction mix was added to background control sample wells. After incubation at room temperature for 30 min in the dark, absorbance of the quinoneimine dye was measured at 570 nm.
For the HMGB-1 ELISA, cells in 24-well plates were treated as indicated. Supernatants were collected after 96 h for A2058 and SK-Mel-28 cells and after 72 h for A375 and SK-Mel-2 cells. The assay was performed using the HMGB1 ELISA kit (IBL International, ST51011) according to manufacturer’s instructions with the following modifications. Briefly, the plates containing the standards, positive control, and treated samples were incubated for 24 h at 37ºC. After well contents were discarded, wells were washed 5X with 400 µL diluted wash buffer. Anti-HMGB1-horseradish peroxidase-conjugated antibody was added to each well and incubated for 2 h at 25ºC. After plates were washed again, color solution (100 μL) containing 3,3ʹ,5,5ʹ-Tetramethylbenzidine (TMB) and hydrogen peroxide solution were added to each well and incubated for 30 min at 25ºC. Stop (0.35M sulphuric acid) solution (100 μL) was added to each well and the contents mixed by shaking the plates gently. Absorbance was measured at 450 nm and reference wavelength of 600–650 nm within 60 min.
Mice
Four week-old NOD/Shi-scid/IL-2Rynull immunodeficient (NOG) mice received chemical myoablative treatment and were reconstituted two days later with human stem cells by intravenous injection of one of six cord blood-derived CD34+ hematopoietic stem and progenitor cells (60,000 cells per mouse; French Blood Bank, anonymized). Fourteen weeks after these injections, flow cytometry (Atune, Life Technologies) was used to assess the composition of the human CD45+ leukocytes in the blood. Humanization rate was defined as the ratio of circulating CD45+/total CD45+ (mCD45 and hCD45).
Sixty humanized mice (24 males, 36 females) with mean humanization rate of 56% from six different cord blood samples were chosen for this study. On day 0, 60 humanized NOG mice were injected subcutaneously in each flank with 0.1 mL suspension containing 2 × 106 A2058 melanoma cells. Fourteen days after tumor challenge, the mice were randomized based on tumor size (left and right) and humanization rate. Treatment details have been described in Table 2. Tumor dimensions (length (L) and width (W)) were measured three times per week with calipers and volumes calculated by using the formula (L × W2/2). Body weight was also measured before the tumor engraftment and three times per week after engraftment. Weight loss of > 20%, tumor volume > 1000 cu.mm, and end of study on day 40 were endpoint criteria for sacrifice.
Table 2.
Treatment groups in antitumor study of ONCOS-102 and pembrolizumab.
| Administration of |
||||
|---|---|---|---|---|
| ONCOS-102 (2.5 × 106 VP/tumor) |
||||
| Group | Left | Right | Pembrolizumab (i.v.) per mouse |
Mode and Days of administration |
| I (Vehicle) |
-(PBS) | -(PBS) | -(PBS) | Days 15, 17, 19 intratumoral i.v. on days 15, 17, 19 and every 3–4 days throughout study |
| II (ONCOS-102) |
+ | + | - | Days 15, 17, 19 intratumoral |
| III (Pembro 200 µg) |
- | - | 200 µg | i.v. on days 15, 17, 19 and every 3–4 days throughout study |
| IV (Pembro 400 µg) |
- | - | 400 µg | i.v. on days 15, 17, 19 and every 3–4 days throughout study |
| V (ONCOS-102 + Pembro 200 µg) |
+ | + | 200 µg | OV: Days 15, 17, 19 intratumoral Pembrolizumab: i.v. on days 15, 17, 19 and every 3–4 days throughout study |
| VI (ONCOS-102 + Pembro 400 µg) |
+ | + | 400 µg | OV: Days 15, 17, 19 intratumoral Pembrolizumab: i.v. on days 15, 17, 19 and every 3–4 days throughout study |
| VII (ONCOS-102 Right flank + Pembro 200 µg) |
- | + | 200 µg | OV: Days 15, 17, 19 intratumoral Pembrolizumab: i.v. on days 15, 17, 19 and every 3–4 days throughout study |
| VIII (ONCOS-102 Right flank + Pembro 400 µg) |
- | + | 400 µg | OV: Days 15, 17, 19 intratumoral Pembrolizumab: i.v. on days 15, 17, 19 and every 3–4 days throughout study |
Eight mice per group in groups I – VI; six mice/group in groups VII and VIII. Abbreviations: OV: oncolytic virus, ONCOS-102
All animal studies were reviewed and approved by the local ethics committee (01_TransCureBioServices-AB-01). Three to five mice were housed in ventilated type II cages (16 x 19 × 35 cm, 500 cm2 floor area) at room temperature (22 ± 2ºC), 55 ± 10% humidity, 12/12-hour light-dark cycles (light 7 AM to 7 PM) and water and food ad libitum.
Immune cell infiltration and PD-L1 expression level
The percentage and absolute number of human immune cell populations and tumor cells were monitored by flow cytometry: human CD45+ (Miltenyi, Germany, 130–096-616) lymphocytes (Panel 1): whole T cells (hCD3+ (Miltenyi, Germany, 130–109-462)), CD4 + T cells (hCD3+ hCD4+ (Miltenyi, Germany, 130–092-373)), CD8 + T cells (hCD3+ hCD8+ (Miltenyi, Germany, 130–096-561)); human tumor cells expression (Panel 2): hCD146 (ThermoFisher Scientific, 12–1469-42) and hPD-L1 (BioLegend, 329708). Tumors were harvested, weighed and subsequently dissociated with human tumor dissociation kit on the gentleMACS TM Octo Dissociator according to the manufacturer’s instructions (Miltenyi, Germany). After dissociation, cells were washed and stained with antibodies 30 min at 4°C in the dark and then suspended in PBS. Samples were acquired on the Attune Nxt (Life technologies). The populations were gated with forward and side scattering (FSC-A/SSC-A dot plot) in leukocytic regions (Supplementary Figure 5). Flow cytometry analysis was performed on FlowJo v10 software.
Statistics
Data were reported as mean ± SEM or as indicated. Statistical analysis was performed with GraphPad Prism software version 7 (La Jolla, CA, USA). A Mann-Whitney test was used to compare two groups. Therapeutic synergy was assessed with the FTV calculation method. FTV (mean tumor volume experimental)/(mean tumor volume control) provided the expected outcome: (Mean FTV of Pembrolizumab) x (mean FTV of Virus) provided the observed outcome: (Expected FTV/observed FTV). A ratio of > 1 indicates a synergistic effect, and a ratio of < 1 indicates a less than additive effect.
Acknowledgments
We thank Tuuli Ranki and Antti Vuolanto for the help and assistance with the initial pilot study preparation. The authors also thank Katherine Molnar-Kimber, PhD of Kimnar Group LLC for her editorial assistance.
Disclosure of Potential Conflicts of Interest
LK, ASM, MJ are employees and/or shareholders in Targovax Oy in Finland and Targovax ASA in Norway.
Supplementary material
Supplemental data for this article can be accessed here.
References
- 1.Redman JM, Gibney GT, Atkins MB.. Advances in immunotherapy for melanoma. BMC Med. 2016;14:20. doi: 10.1186/s12916-016-0571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee for Medicinal Products for Human Use (CHMP) Assessment Report Keytruda International non-propriety name pembrolizumab Procedure No. EMEA/H/C/003820/0000; WC500190992 report. London UK: European Medicines Agency, 2015. [Google Scholar]
- 3.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R, Joshua AM, Patnaik A, Hwu W-J, Weber JS, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 6.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Cerdeira C, Carnero Gregorio M, Lopez-Barcenas A, Sanchez-Blanco E, Sanchez-Blanco B, Fabbrocini G, Bardhi B, Sinani A, Guzman RA. Advances in immunotherapy for melanoma: A comprehensive review. Mediators Inflamm. 2017;2017:3264217. doi: 10.1155/2017/3264217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koski A, Kangasniemi L, Escutenaire S, Pesonen S, Cerullo V, Diaconu I, Nokisalmi P, Raki M, Rajecki M, Guse K, et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerullo V, Vaha-Koskela M, Hemminki A. Oncolytic adenoviruses: A potent form of tumor immunovirotherapy. Oncoimmunology. 2012;1:979–981. doi: 10.4161/onci.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuryk L, Moller AW, Garofalo M, Cerullo V, Pesonen S, Alemany R, Jaderberg M. Antitumor-specific T-cell responses induced by oncolytic adenovirus ONCOS-102 (AdV5/3-D24-GM-CSF) in peritoneal mesothelioma mouse model. J Med Virol. 2018;90:1669–1673. doi: 10.1002/jmv.25229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranki T, Pesonen S, Hemminki A, Partanen K, Kairemo K, Alanko T, Lundin J, Linder N, Turkki R, Ristimäki A, et al. Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers. J Immunother Cancer. 2016;4:17. doi: 10.1186/s40425-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassilev L, Ranki T, Joensuu T, Jager E, Karbach J, Wahle C, Partanen K, Kairemo K, Alanko T, Turkki R, et al. Repeated intratumoral administration of ONCOS-102 leads to systemic antitumor CD8(+) T-cell response and robust cellular and transcriptional immune activation at tumor site in a patient with ovarian cancer. Oncoimmunology. 2015;4:e1017702. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bramante S, Kaufmann JK, Veckman V, Liikanen I, Nettelbeck DM, Hemminki O, Vassilev L, Cerullo V, Oksanen M, Heiskanen R, et al. Treatment of melanoma with a serotype 5/3 chimeric oncolytic adenovirus coding for GM-CSF: results in vitro, in rodents and in humans. Int J Cancer. 2015;137:1775–1783. doi: 10.1002/ijc.29536. [DOI] [PubMed] [Google Scholar]
- 14.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–4109. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 17.Kuryk L, Haavisto E, Garofalo M, Capasso C, Hirvinen M, Pesonen S, Ranki T, Vassilev L, Cerullo V. Synergistic anti-tumor efficacy of immunogenic adenovirus ONCOS-102 (Ad5/3-D24-GM-CSF) and standard of care chemotherapy in preclinical mesothelioma model. Int J Cancer. 2016;139:1883–1893. doi: 10.1002/ijc.30228. [DOI] [PubMed] [Google Scholar]
- 18.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 19.Hendrickx R, Stichling N, Koelen J, Kuryk L, Lipiec A, Greber UF. Innate immunity to adenovirus. Hum Gene Ther. 2014;25:265–284. doi: 10.1089/hum.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 22.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 23.Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr Protoc Immunol. 2008;21:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Moller T, Koyuncu D, Drescher MR, Strauss R, Zhang X-B, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawai H, Domae N. Discrimination between primary necrosis and apoptosis by necrostatin-1 in Annexin V-positive/propidium iodide-negative cells. Biochem Biophys Res Commun. 2011;411:569–573. doi: 10.1016/j.bbrc.2011.06.186. [DOI] [PubMed] [Google Scholar]
- 26.Ngo N, Patel K, Isaacson PG, Naresh KN. Leucocyte common antigen (CD45) and CD5 positivity in an “undifferentiated” carcinoma: a potential diagnostic pitfall. J Clin Pathol. 2007;60:936–938. doi: 10.1136/jcp.2006.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes RA, Shore DA, Vuong MT, Yu C, Zhu X, Pereira-Lopes S, Brouwer H, Fennelly JA, Jessup CM, Evans EJ, et al. T cell receptors are structures capable of initiating signaling in the absence of large conformational rearrangements. J Biol Chem. 2012;287:13324–13335. doi: 10.1074/jbc.M111.332783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez L, Strbo N, Podack ER. Humanized mice: novel model for studying mechanisms of human immune-based therapies. Immunol Res. 2013;57:326–334. doi: 10.1007/s12026-013-8471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Cancer Society Cancer facts and figures. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 30.Filley AC, Dey M. Immune system, friend or foe of oncolytic virotherapy? Front Oncol. 2017;7:106. doi: 10.3389/fonc.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemminki O, Parviainen S, Juhila J, Turkki R, Linder N, Lundin J, Kankainen M, Ristimäki A, Koski A, Liikanen I, et al. Immunological data from cancer patients treated with Ad5/3-E2F-Delta24-GMCSF suggests utility for tumor immunotherapy. Oncotarget. 2015;6:4467–4481. doi: 10.18632/oncotarget.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I, Tähtinen S, Oksanen M, Heiskanen R, Pesonen S, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res. 2013;19:2734–2744. doi: 10.1158/1078-0432.CCR-12-2546. [DOI] [PubMed] [Google Scholar]
- 33.Kuryk L, Vassilev L, Ranki T, Hemminki A, Karioja-Kallio A, Levalampi O, Vuolanto A, Cerullo V, Pesonen S, Kirchmair R. Toxicological and bio-distribution profile of a GM-CSF-expressing, double-targeted, chimeric oncolytic adenovirus ONCOS-102 - Support for clinical studies on advanced cancer treatment. PLoS One. 2017;12:e0182715. doi: 10.1371/journal.pone.0182715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zafar S, Parviainen S, Siurala M, Hemminki O, Havunen R, Tahtinen S, Bramante S, Vassilev L, Wang H, Lieber A, et al. Intravenously usable fully serotype 3 oncolytic adenovirus coding for CD40L as an enabler of dendritic cell therapy. Oncoimmunology. 2017;6:e1265717. doi: 10.1080/2162402X.2016.1265717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capasso C, Magarkar A, Cervera-Carascon V, Fusciello M, Feola S, Muller M, Garofalo M, Kuryk L, Tähtinen S, Pastore L, et al. A novel in silico framework to improve MHC-I epitopes and break the tolerance to melanoma. Oncoimmunology. 2017;6:e1319028. doi: 10.1080/2162402X.2017.1319028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirvinen M, Capasso C, Guse K, Garofalo M, Vitale A, Ahonen M, Kuryk L, Vähä-Koskela M, Hemminki A, Fortino V, et al. Expression of DAI by an oncolytic vaccinia virus boosts the immunogenicity of the virus and enhances antitumor immunity. Mol Ther Oncolytics. 2016;3:16002. doi: 10.1038/mto.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capasso C, Hirvinen M, Garofalo M, Romaniuk D, Kuryk L, Sarvela T, Vitale A, Antopolsky M, Magarkar A, Viitala T, et al. Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma. Oncoimmunology. 2016;5:e1105429. doi: 10.1080/2162402X.2015.1105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garofalo M, Iovine B, Kuryk L, Capasso C, Hirvinen M, Vitale A, Yliperttula M, Bevilacqua MA, Cerullo V. Oncolytic adenovirus loaded with L-carnosine as novel strategy to enhance the antitumor activity. Mol Cancer Ther. 2016;15:651–660. doi: 10.1158/1535-7163.MCT-15-0559. [DOI] [PubMed] [Google Scholar]
- 39.Lipiec A, Kuryk L. Onkolityczne wektory wirusowe w immunoterapii nowotworów. Immunoterapia PZWL. Warszawa: Wydawnictwo Lekarskie PZWL, 2018.
- 40.Garofalo M, Saari H, Somersalo P, Crescenti D, Kuryk L, Aksela L, Capasso C, Madetoja M, Koskinen K, Oksanen T, et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J Control Release. 2018;283:223–234. doi: 10.1016/j.jconrel.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Green SK, Karlsson MC, Ravetch JV, Kerbel RS. Disruption of cell-cell adhesion enhances antibody-dependent cellular cytotoxicity: implications for antibody-based therapeutics of cancer. Cancer Res. 2002;62:6891–6900. [PubMed] [Google Scholar]
- 42.Cervera-Carrascon V, Siurala M, Santos JM, Havunen R, Tahtinen S, Karell P, Sorsa S, Kanerva A, Hemminki A. TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. Oncoimmunology. 2018;7:e1412902. doi: 10.1080/2162402X.2017.1412902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


