Key Points
Cell-specific hypoxia-inducible factor 1 can regulate cancer-associated hypercoagulation and thrombus formation.
Abstract
Despite the increased risk of thrombosis in cancer patients compared with healthy individuals, mechanisms that regulate cancer-induced hypercoagulation are incompletely understood. The aim of this study was to investigate whether cell-specific hypoxia-inducible factor (HIF) 1α regulates cancer-associated hypercoagulation, using in vitro clotting assays and in vivo cancer models. In mouse lung and mammary tumor cells, hypoxia led to increases in cell adhesion, clotting, and fibrin deposition; these increases were eliminated in HIF1α null cells. Increased levels of HIF1α were also associated with increased tissue factor expression in human breast tumor samples. Conversely, deletion of endothelial (but not myeloid) cell-specific HIF1α doubled pulmonary fibrin deposition, and trebled thrombus formation compared with wildtype littermates in tumor-bearing mice. Our data suggest that tumor and endothelial cell-specific HIF1α may have opposing roles in cancer-associated coagulation and thrombosis. Off-target effects of manipulating the HIF1 axis in cancer patients should be carefully considered when managing thrombotic complications.
Introduction
Venous thromboembolism is a common cause of death in cancer patients, who are ∼7 times more likely to suffer from venous thrombosis compared with healthy individuals.1 The risk of thrombosis is increased in a variety of cancer types, including both lung and breast cancers.1 Despite substantial health care costs and post-thrombotic complications, current treatments for thrombosis in cancer patients have major limitations (including increased risk of bleeding). Novel antithrombotic treatments may arise from a better understanding of the mechanisms that regulate cancer-associated hypercoagulation.
Thrombi form under conditions of low blood flow and reduced oxygenation (hypoxia), and this is commonly seen within solid tumors.2-4 The vascular response that follows hypoxia is controlled in significant part by stabilization of the α-subunit of hypoxia-inducible factor (HIF) 1. Transcriptional targets of HIF1 include both anti- and procoagulant factors (the latter of which include tissue factor [TF] and plasminogen activator inhibitor 1).5 HIF1α expression is found in virtually all cell types, and this includes the tumor cells themselves as well as other cells in the tumor, including endothelial cells and myeloid cells (ie, neutrophils and macrophages). Hypoxic tumor cells can promote coagulation via release of procoagulant factors and TF-bearing microparticles,6 but the role of tumor and stromal-cell HIF1 in cancer-associated hypercoagulation is unknown. We aimed to determine whether HIF1 in tumor and stromal cells could regulate cancer-associated hypercoagulability.
Methods
Tumor cells and in vitro assays
Wild-type and HIF1α-suppressed or nullizygous Lewis lung cancer cells (LLCs) and mammary epithelial tumor cells (MECs)7 were maintained at 37°C, 5% CO2, and 21% or 1% O2 for 24 hours as described (n = 5 per group).8 Suppression of HIF1α in LLCs was achieved by lentiviral shRNA silencing as described.9 Wild-type control LLCs were infected with scrambled shRNA lentiviral particles. Deletion of HIF1α in MECs was achieved by isolating mammary tumor cells from HIF1α conditional deletion Polyoma Middle T (PyMT) mice by digestion with collagenase as described,7 followed by infection of plated cells at day 1 with either adenoviral Cre recombinase or a β-galactosidase–expressing adenovirus as a wild-type control (Vector BioLabs, Malvern, PA). Western blotting was used to confirm gene silencing as described.8
Cell adhesion onto fibrin was assessed by calculating the percentage of tumor cells that adhered onto fibrin-coated 48-well plates (Sigma Aldrich, UK) after incubation for 2 hours at 37°C, 5% CO2, and 21% O2. After incubation, nonadherent cells were immediately removed and adherent cells were resuspended (0.25% Trypsin, Gibco, UK) and then counted using an automatic cell counter (ADAM, Digital Bio, UK). Clotting times of the cells or their conditioned media were measured by recording the time to clot after introduction of calcium chloride to either cell suspension or conditioned media with citrated mouse plasma as described.10 Fibrin deposition was quantified by image analysis of cell monolayers stained with Martius Scarlet Blue as described.11 Clotting times were also assessed as described10 in conditioned media: (1) with or without monoclonal antibodies against TF (Abcam, UK) or FVII (Sigma Aldrich, UK); and (2) with or without secreted microvesicles, which were removed by centrifugation as described.12
Cell-specific HIF1α knockout mice, tumor establishment, tissue staining, and image analysis
Animal experiments were performed with local animal care committee approval under the Animals (Scientific Procedures) Act of 1986. Endothelial- and myeloid cell–specific HIF1α nullizygous mice (male, 8-10 weeks old) were generated on a C57BL/6J background as described8,13 and compared with wild-type littermates (n = 5-8 per group). Pulmonary tumors were established as described and lungs were excised, processed, and embedded in paraffin at 14 days post-LLC administration.8 Tissue cross-sections (7 μm) were taken at 200 μm intervals throughout the length of the tissue. Pulmonary fibrin deposition, microthrombi numbers, and tumor fibrin deposition were assessed by image analysis of Martius Scarlet Blue–stained tissue as described.11
Human tumor microarrays
Human investigations were performed with informed consent and approval by the institution ethics review board. Breast tumor microarray samples and plasma were obtained from a lymph node–negative population of breast cancer patients that have previously been described (n = 211).14 Levels of HIF1α and phosphorylated TF (pTF) were quantified in tumor samples by immunostaining and image analysis as described.15,16
Statistical analysis
Differences between normoxic and hypoxic cells, and between wild-type and cell-specific HIF1α knockout mice, were assessed using independent Student t tests. Correlations were assessed using Spearman’s correlation. P < .05 was considered significant and data were expressed as mean ± standard error.
Results
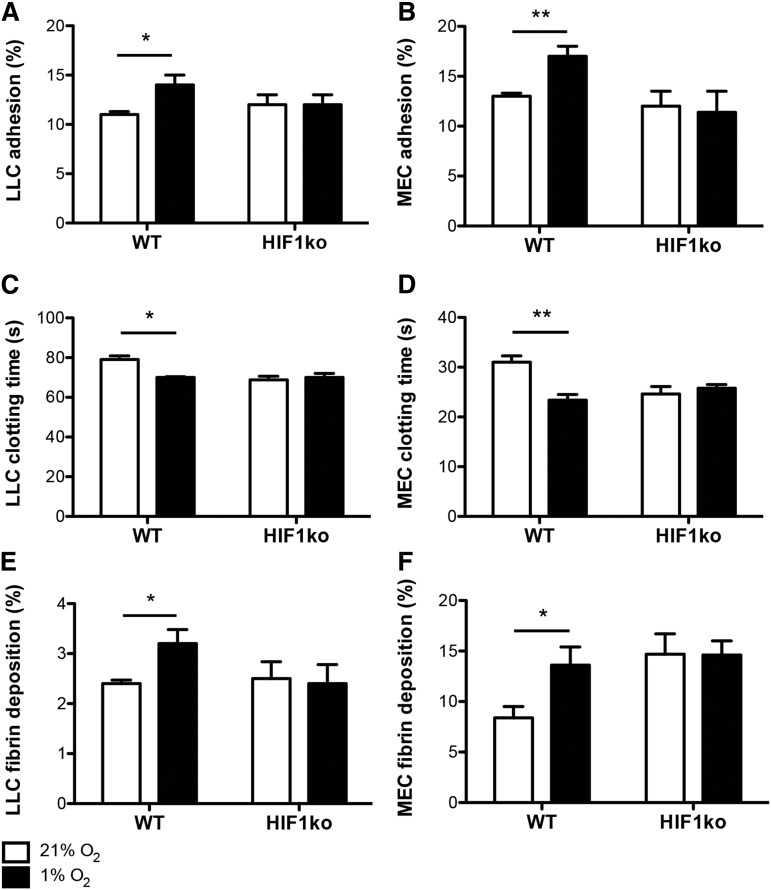
To first determine whether tumor cell–expressed HIF1 could mediate hypoxia-induced hypercoagulation, LLCs or MECs were cultured under ambient (21%) or reduced oxygen (1%) conditions, and their propensity to induce coagulation was assessed. In both wild-type LLCs and MECs, hypoxia led to increases in cell: (1) adhesion to fibrin (Figure 1A-B); (2) coagulability (as shown by shorter clotting times, Figure 1C-D); and (3) fibrin deposition (Figure 1E-F). When HIF1α was suppressed in LLCs or deleted in MECs, however, hypoxia-induced increases were absent compared with normoxic controls across all of the assays (Figure 1A-F).
Figure 1.
Hypoxia-induced increases in tumor cell coagulability are HIF1-dependent. Wild-type (WT) and HIF1α knockout (HIF1ko) LLCs and MECs were cultured under ambient (21%) and hypoxic (1%) oxygen conditions, after which (A-B) cell adhesion to fibrin, (C-D) cell coagulation times, and (E-F) fibrin deposition were assessed (n = 5 per group). *P < .05 and **P < .01.
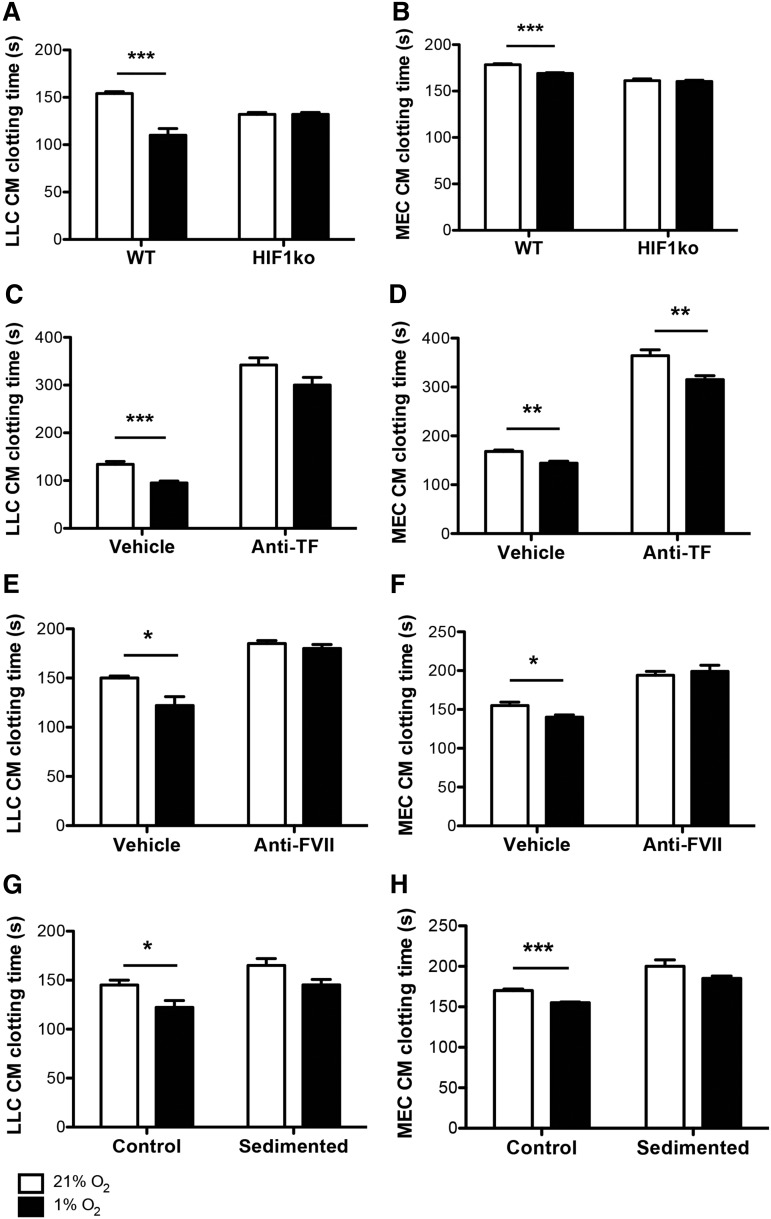
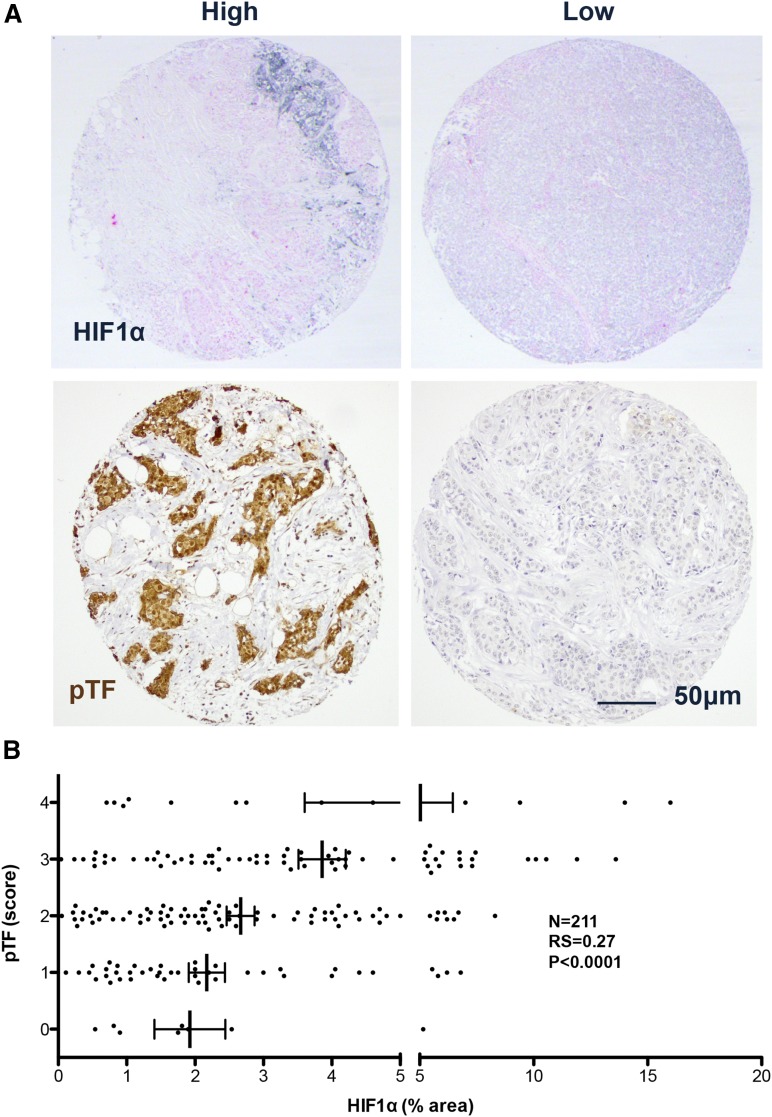
Hypoxia-induced reductions in the clotting times of the conditioned media taken from both wild-type LLC or MEC cultures were also observed, and these reductions were abolished when tumor cell HIF1α was suppressed or deleted, respectively (Figure 2A-B). Conditioned media clotting times of LLCs and MECs were extended under normoxia and hypoxia when either TF or FVII were inhibited (Figure 2C-F). Hypoxia-induced reductions in the clotting times of LLC-conditioned media were also attenuated when TF (Figure 2C) or FVII (Figure 2E) were inhibited, and absent when FVII was inhibited in MEC-conditioned media (Figure 2D,F). Hypoxia-induced reductions in the clotting times of both LLC- and MEC-conditioned media did not reach statistical significance when secreted microvesicles were lacking (Figure 2G-H). In primary human breast tumor samples, there was a strong positive correlation between levels of HIF1α and pTF (Figure 3A-B).
Figure 2.
Hypoxia-induced decreases in the clotting times of tumor cell–conditioned media are dependent on HIF1α and TF/FVII. Wild-type (WT) and HIF1α knockout (HIF1ko) LLCs and MECs were cultured under ambient (21%) and hypoxic (1%) oxygen conditions, after which (A-B) conditioned media (CM) coagulation times were assessed (n = 5 per group). Clotting times were also assessed in WT LLC- and MEC-conditioned media (n = 5 per group) with (Anti-) or without (Vehicle IgG) inhibition of TF (C-D) or FVII (E-F), and with (Control) or without (Sedimented) the presence of secreted microvesicles (G-H). *P < .05, **P < .01, ***P < .001.
Figure 3.
Correlation between HIF1α and phosphorylated TF in human breast tumors. (A) Representative images of human breast tumor microarrays stained for HIF1α (black, upper panels) and phosphorylated TF (pTF, brown, lower panels). (B) Scatterplot of HIF1α level vs pTF score in human breast tumor microarrays (n = 211). Spearman’s correlation: RS = 0.27, P < .0001.
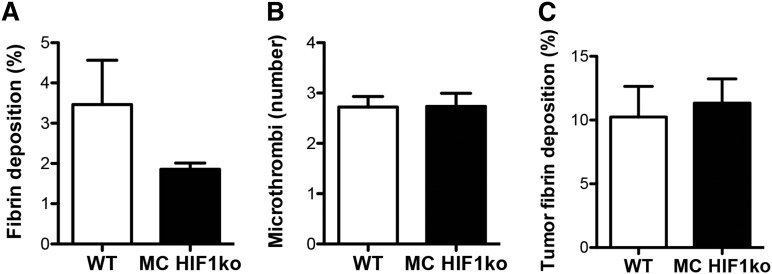
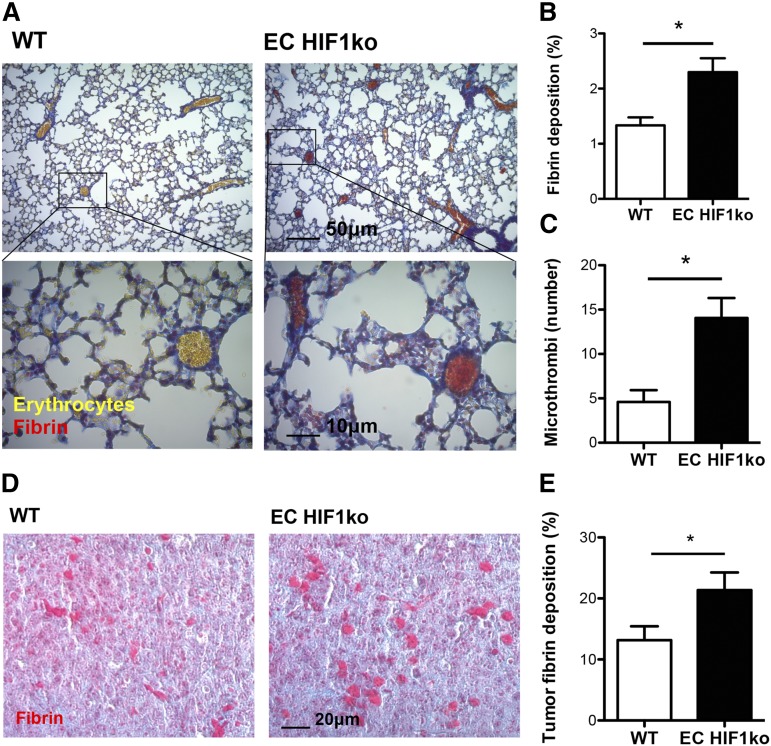
To study the roles of HIF1 in stromal cells during cancer-associated thrombosis, we assessed the effect of deleting myeloid (neutrophil and macrophage) or endothelial HIF1α on pulmonary thrombus formation in tumor-bearing mice. When we analyzed pulmonary thrombosis in tumor-bearing mice with or without myeloid HIF1α, there were no significant differences in pulmonary fibrin deposition, microthrombi number, or tumor fibrin deposition when these tissue-specific deletion mice were compared with wild-type littermates (Figure 4A-C). Endothelial HIF1α deletion, however, resulted in increases in fibrin deposition and microthrombi number in the pulmonary microvasculature, when compared with wild-types (Figure 5A-C). Similarly, fibrin deposition in the pulmonary tumors of endothelial HIF1α-deletion mice was greater than that observed in their wild-type littermates (Figure 5D-E).
Figure 4.
Cancer-associated thrombosis is unaltered in the pulmonary microvasculature and pulmonary tumors of myeloid HIF1α knockout mice. (A) Quantification of fibrin deposition and (B) microthrombi number in pulmonary microvasculature of tumor-bearing wild-type (WT) and myeloid HIF1α knockout (MC HIF1ko) mice (n = 8 per group). (C) Quantification of tumor fibrin deposition in WT and MC HIF1ko mice (n = 8 per group).
Figure 5.
Cancer-associated thrombosis is enhanced in the pulmonary microvasculature and pulmonary tumors of endothelial HIF1α knockout mice. (A) Tumor-bearing lung cross-sections of wild-type (WT) and endothelial HIF1α knockout (EC HIF1ko) mice stained with Martius Scarlet Blue for fibrin (red) and erythrocytes (yellow). (B) Quantification of fibrin deposition and (C) microthrombi number in pulmonary microvasculature of tumor-bearing WT and EC HIF1ko mice (n = 5/group). (D) Pulmonary tumors of WT and EC HIF1ko mice stained with Martius Scarlet Blue. (E) Quantification of tumor fibrin deposition in WT and EC HIF1ko mice (n = 5/group). Images are of representative tissue cross-sections. *P < .05.
Discussion
There is strong positive correlation between cancer and thrombosis.1 Our study suggests that HIF1 can regulate cancer-associated hypercoagulation in a cell-specific manner: tumor cell ablation of HIF1 eliminates the procoagulant response of these cells to hypoxia, whereas endothelial HIF1 deletion augments thrombosis in tumor-bearing mice. It was previously shown that hypoxia and PTEN expression regulate TF levels and coagulability in glioblastoma cells.10 Current data suggest that hypoxia-induced alterations in tumor cell coagulability are also HIF1-dependent and partly regulated through changes in the expression and release of coagulant factors including TF. The regulation of cancer-associated thrombosis through the expression and secretion of such factors (plus other hypoxia-responsive adhesion molecules) deserves attention in future studies.
We show here that hypoxia-induced decreases in the clotting times of tumor cell–conditioned media are dependent on HIF1 and TF/FVII, and that these times are extended by removal of tumor cell microvesicles. Malignancy promotes coagulation via release of hypoxia-responsive, tumor-derived microvesicles, and pro-coagulant factors such as TF.6,17 Chuvash polycythemia resulting from germline VHLR200W mutation is a condition characterized by stabilization of HIF1α (even under normoxia) and a high incidence of thrombosis.18 We show here that the HIF1-mediated response to tumorigenesis in distinct cell subsets can differentially regulate cancer-associated thrombosis—that is, HIF1 in tumor cells promotes hypoxic cell adhesion, clotting, and fibrin deposition, whereas HIF1 in endothelial cells prevents microthrombus formation and fibrin deposition after tumorigenesis. Nevertheless, HIF1 in other stromal cells (including fibroblasts and T cells) could also contribute to the regulation of coagulability in patients with hypercoagulable disorders.
It has previously been shown that endothelial HIF1 knockout reduces nitric oxide (NO) production.8 Given that endothelial NO inhibits platelet function and preserves endothelial anticoagulant function,19 endothelial HIF1 deletion could enhance thrombosis via reductions in the NO-mediated anticoagulant response. In other words, endothelial HIF1 deletion may reduce local NO levels, and in doing so, suppress the anticoagulant impact of NO on aggregating platelets and on the innermost vessel wall. The impact of endothelial HIF1/NO signaling on cancer-associated coagulation deserves attention in future studies, especially given that previous studies have identified a role for endothelial cells in the regulation of hypoxia-induced coagulation.20 Our data suggest that endothelial but not myeloid cell HIF1α could counteract the procoagulant response after the onset of cancer. Current data also support the possibility that oxidative stress could have advantageous as well as deleterious consequences during cancer progression.21-23
Previously we showed that systemic manipulation of the HIF1 signaling pathway through administration of HIF1/vascular endothelial growth factor receptor inhibitors could affect thrombotic incidence in a mouse model of venous thrombosis.24 Data herein suggest that the influence of HIF1 targeting on thrombotic potential in cancer patients could depend on cell type targeted, and that this should therefore be carefully considered when managing thrombotic events in these patients. Given that cancer is a prothrombotic disease, it could prove beneficial to these patients to be able to target cell-specific HIF signaling pathways that reduce both cancer progression and associated thrombosis, ideally without the potential side effects of systemic therapy. Further studies of multiple cancer types will be required to confirm the precise roles of cell-specific HIF1 in cancer-associated hypercoagulation. It should also be noted here that the contribution of the other major HIF isoform (HIF2) in cancer-associated hypercoagulation cannot be discounted.
In summary, we have shown that the hypoxia-responsive transcription factor, HIF1, can regulate cancer-associated coagulation and thrombosis in a cell-specific manner. Data suggest that further investigations of the effects of cell-specific HIFs on cancer-induced hypercoagulation will improve understanding of this pathological process.
Acknowledgments
This study was supported through a Wellcome Trust Principal Research Fellowship (092738MA) (R.S.J.); and Pump-Priming grants from the University of Cambridge British Heart Foundation Centre of Research Excellence and the British Society for Haematology (C.E.E.). Breast tumors were obtained from the South Swedish Breast Cancer Group with assistance from Professor Per Malström.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.E.E., M.B., and C.B. obtained the data; C.E.E. and P.-O.B. analyzed the data; and C.E.E., C.B., and R.S.J. interpreted the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Randall S. Johnson, Department of Physiology, Development and Neuroscience, University of Cambridge, Cambridge CB2 3EG, United Kingdom; e-mail: rsj33@cam.ac.uk.
References
- 1.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 2.Hamer JD, Malone PC, Silver IA. The PO2 in venous valve pockets: its possible bearing on thrombogenesis. Br J Surg. 1981;68(3):166–170. doi: 10.1002/bjs.1800680308. [DOI] [PubMed] [Google Scholar]
- 3.Yan SF, Mackman N, Kisiel W, Stern DM, Pinsky DJ. Hypoxia/hypoxemia-induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis. Arterioscler Thromb Vasc Biol. 1999;19(9):2029–2035. doi: 10.1161/01.atv.19.9.2029. [DOI] [PubMed] [Google Scholar]
- 4.Zerrouqi A, Pyrzynska B, Brat DJ, Van Meir EG. P14ARF suppresses tumor-induced thrombosis by regulating the tissue factor pathway. Cancer Res. 2014;74(5):1371–1378. doi: 10.1158/0008-5472.CAN-13-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Vascular responses to hypoxia and ischemia. Arterioscler Thromb Vasc Biol. 2010;30(4):648–652. doi: 10.1161/ATVBAHA.108.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svensson KJ, Belting M. Role of extracellular membrane vesicles in intercellular communication of the tumour microenvironment. Biochem Soc Trans. 2013;41(1):273–276. doi: 10.1042/BST20120248. [DOI] [PubMed] [Google Scholar]
- 7.Kim JW, Evans C, Weidemann A, et al. Loss of fibroblast HIF-1α accelerates tumorigenesis. Cancer Res. 2012;72(13):3187–3195. doi: 10.1158/0008-5472.CAN-12-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branco-Price C, Zhang N, Schnelle M, et al. Endothelial cell HIF-1α and HIF-2α differentially regulate metastatic success. Cancer Cell. 2012;21(1):52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai WB, Zhang Y, Cheng R, et al. Dual inhibition of plasminogen kringle 5 on angiogenesis and chemotaxis suppresses tumor metastasis by targeting HIF-1α pathway. PLoS One. 2012;7(12):e53152. doi: 10.1371/journal.pone.0053152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005;65(4):1406–1413. doi: 10.1158/0008-5472.CAN-04-3376. [DOI] [PubMed] [Google Scholar]
- 11.Saha P, Andia ME, Modarai B, et al. Magnetic resonance T1 relaxation time of venous thrombus is determined by iron processing and predicts susceptibility to lysis. Circulation. 2013;128(7):729–736. doi: 10.1161/CIRCULATIONAHA.113.001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svensson KJ, Kucharzewska P, Christianson HC, et al. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci USA. 2011;108(32):13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malmström P, Bendahl PO, Boiesen P, Brünner N, Idvall I, Fernö M South Sweden Breast Cancer Group. S-phase fraction and urokinase plasminogen activator are better markers for distant recurrences than Nottingham Prognostic Index and histologic grade in a prospective study of premenopausal lymph node-negative breast cancer. J Clin Oncol. 2001;19(7):2010–2019. doi: 10.1200/JCO.2001.19.7.2010. [DOI] [PubMed] [Google Scholar]
- 15.Ryden L, Grabau D, Schaffner F, Jonsson PE, Ruf W, Belting M. Evidence for tissue factor phosphorylation and its correlation with protease-activated receptor expression and the prognosis of primary breast cancer. Int J Cancer. 2010;126(10):2330–2340. doi: 10.1002/ijc.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans CE, Humphries J, Mattock K, et al. Hypoxia and upregulation of hypoxia-inducible factor 1alpha stimulate venous thrombus recanalization. Arterioscler Thromb Vasc Biol. 2010;30(12):2443–2451. doi: 10.1161/ATVBAHA.110.215038. [DOI] [PubMed] [Google Scholar]
- 17.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110(6):1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordeuk VR, Sergueeva AI, Miasnikova GY, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103(10):3924–3932. doi: 10.1182/blood-2003-07-2535. [DOI] [PubMed] [Google Scholar]
- 19.Irokawa M, Nishinaga M, Ikeda U, et al. Endothelial-derived nitric oxide preserves anticoagulant heparan sulfate expression in cultured porcine aortic endothelial cells. Atherosclerosis. 1997;135(1):9–17. doi: 10.1016/s0021-9150(97)00117-2. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa S, Shreeniwas R, Brett J, Clauss M, Furie M, Stern DM. The effect of hypoxia on capillary endothelial cell function: modulation of barrier and coagulant function. Br J Haematol. 1990;75(4):517–524. doi: 10.1111/j.1365-2141.1990.tb07792.x. [DOI] [PubMed] [Google Scholar]
- 21.Harris IS, Brugge JS. Cancer: The enemy of my enemy is my friend. Nature. 2015;527(7577):170–171. doi: 10.1038/nature15644. [DOI] [PubMed] [Google Scholar]
- 22.Le Gal K, Ibrahim MX, Wiel C, et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med. 2015;7(308):308re8. doi: 10.1126/scitranslmed.aad3740. [DOI] [PubMed] [Google Scholar]
- 23.Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527(7577):186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans CE, Grover SP, Humphries J, et al. Antiangiogenic therapy inhibits venous thrombus resolution. Arterioscler Thromb Vasc Biol. 2014;34(3):565–570. doi: 10.1161/ATVBAHA.113.302998. [DOI] [PubMed] [Google Scholar]